Abstract
Striatal cholinergic interneurons (ChIs) are central for the processing and reinforcement of reward-related behaviors that are negatively affected in states of altered dopamine transmission, such as in Parkinson’s disease or drug addiction. Nevertheless, the development of therapeutic interventions directed at ChIs has been hampered by our limited knowledge of the diverse anatomical and functional characteristics of these neurons in the dorsal and ventral striatum, combined with the lack of pharmacological tools to modulate specific cholinergic receptor subtypes. This review highlights some of the key morphological, synaptic, and functional differences between ChIs of different striatal regions and across species. It also provides an overview of our current knowledge of the cellular localization and function of cholinergic receptor subtypes. The future use of high-resolution anatomical and functional tools to study the synaptic microcircuitry of brain networks, along with the development of specific cholinergic receptor drugs, should help further elucidate the role of striatal ChIs and permit efficient targeting of cholinergic systems in various brain disorders, including Parkinson’s disease and addiction.
Keywords: accumbens, caudate nucleus, putamen, cocaine, Parkinson’s disease, drug addiction
Introduction
The basal ganglia are a group of tightly interconnected subcortical nuclei that regulate various aspects of sensorimotor, cognitive, and limbic functions.1 Disorders involving these brain structures encompass many neurodegenerative and neuropsychiatric syndromes.2–4 The striatum, which consists of the caudate nucleus, putamen, and nucleus accumbens, serves as the main entry point for highly topographic and functionally segregated cortical information to gain access to the basal ganglia circuitry.1, 5 Thus, the cerebral cortex imposes a functional compartmentalization of sensorimotor (postcommissural putamen), associative (caudate nucleus and precommissural putamen), and limbic (nucleus accumbens) processes upon the striatum, which are partly maintained throughout the basal ganglia–thalamocortical loops.1, 5 Complex intrastriatal microcircuits that involve two populations of GABAergic projection neurons (also called medium spiny neurons (MSNs)), various groups of GABAergic interneurons, and a single population of cholinergic interneurons integrate, process, and transmit this extrinsic information to other basal ganglia nuclei.6–8 Despite the low number of striatal interneurons,6, 9, 10 they prevail as essential nodes of normal basal ganglia function because of their neurochemical diversity, intricate connectivity with MSNs, and central role in modulating striatal afferents.6 This review will focus on the striatal cholinergic interneurons (ChIs), the largest cells in the striatum that are recognized for their key regulatory roles of striatal and basal ganglia function in normal and diseased states.11–19
Together with ventral midbrain dopaminergic neurons and their widespread striatal innervation, ChIs regulate aversive, attentional, motivational, and reward-related behaviors, as well as synaptic plasticity, conditioned learning, and action selection in the striatum.12, 14, 20–23 A striatal dopamine/acetylcholine imbalance has been discussed in numerous reviews as a key neurochemical substrate for basal ganglia disorders, such as Parkinson’s disease (PD) and drug addiction/abuse.11, 12, 16, 17, 20 Although significant advances have been made toward delineating ChI function in such disorders, the development of potential cholinergic drug therapies has been hindered by incomplete and controversial knowledge about the network connectivity of ChIs, along with a limited number of pharmacological tools that regulate specific subtypes of cholinergic receptors. In addition, striatal ChIs are often mistakenly assumed to represent a homogeneous population of cells with minor morphological and functional differences between humans and other animal species, and across different striatal regions.
This review discusses evidence for interspecies and regional differences in the morphology, abundance, distribution, connectivity, and physiological activity of ChIs in the caudate nucleus, putamen, and nucleus accumbens, along with some attributes of these neurons that are common to all mammals and striatal regions. The potential impact of striatal cholinergic dysfunction in diseased states, particularly PD and cocaine addiction/abuse, is then considered, and possible therapeutic targets for these disorders are examined. We conclude with a brief discussion about the limitations of the current cholinergic and dopaminergic therapies, and highlight the potential clinical relevance of new drugs aimed at specific cholinergic receptor subtypes, or receptors expressed on ChIs themselves, as potential therapeutics for PD and drug addiction.
Striatum: functional compartmentalization and afferent connections
Striatal compartmentalization
The striatum is the main entryway through which extrinsic afferents can influence functionally diverse basal ganglia circuits to generate context-dependent, goal-directed, and habitual behaviors.24–26 It is commonly divided into the dorsal and ventral striatum on the basis of gross anatomical localization and divergent connectivity. In primates, the dorsal striatum consists of the caudate nucleus and putamen, divided from each other by the internal capsule, whereas in rodents, it is a single mass of gray matter often referred to as the caudate–putamen complex.27 The ventral striatum consists of the nucleus accumbens and the striatal portion of the olfactory tubercle, along with the ventromedial extension of the caudate nucleus and putamen.28 The nucleus accumbens comprises a core and a shell subregion, two anatomically and functionally defined areas that are well-characterized in rodents, but less so in primates.28–32 In general, the rostral-most, medial, lateral, and ventral parts of the accumbens are referred to as the shell, while its dorsal and central portions constitute the core.28, 29
Another level of striatal compartmentalization is the patch (or striosomes)/matrix system, which is largely based on the heterogeneous distribution of neurochemical markers and differential afferent or efferent connections. The two compartments have been identified within the dorsal and ventral striatum of primates and nonprimates, but they are particularly evident in the caudate nucleus, anterior putamen, and core of the accumbens.29, 33–37 Although the functional significance of this patch/matrix dichotomy remains poorly understood, evidence that these two compartments are differentially affected in some basal ganglia disorders is of significant interest.38
Afferent connections to the striatum
Corticostriatal system
Extrinsic topographically and functionally organized projections from the cerebral cortex, thalamus, and ventral midbrain constitute the bulk of striatal afferents. Cortical glutamatergic projections terminate throughout the whole striatum in a functionally topographic fashion.1, 5 In primates, the postcommissural putamen (or dorsolateral striatum in rodents) receives its main cortical inputs from sensorimotor cortices, while the precommissural putamen and the caudate nucleus (or dorsomedial striatum in rodents) are the main targets of cognitive afferents from associative prefrontal, temporal, and parietal cortical regions.5 These sensorimotor and cognitive inputs terminate predominantly within the matrix sector of the caudate nucleus and putamen. In contrast, the striosomes of the dorsal striatum receive their main cortical innervation from limbic cortices (such as the orbitofrontal, anterior cingulate, and insular cortices) and the amygdala,33, 34, 38, 39 which, together with the hippocampus, are the main sources of cortical and subcortical inputs to the ventral striatum.40–42 In rodents, clear evidence exists for a differential origin of limbic-related cortical afferents to the core and shell of the accumbens.42–44 Although such a dichotomy also exists in primates, the less distinct borders between the accumbens core and shell in monkeys and humans makes the distinction between these two regions more difficult to establish.28, 45–49
Thalamostriatal system
The thalamus is the other major source of glutamatergic inputs to the striatum. Although the rostral intralaminar (mainly the centrolateral nucleus), ventral motor (ventral anterior/ventral lateral nuclei), associative (mediodorsal nucleus), and midline thalamic nuclei contribute to the thalamostriatal system, the main origin of these projections are the caudal intralaminar nuclei, namely the centre median (CM) and parafascicular (Pf) nuclei, that project primarily to the sensorimotor (CM inputs) and associative/limbic (Pf inputs) dorsal striatal territories in primates.50–52 In non-primates, the intralaminar (and most other thalamic nuclei) preferentially target the matrix region of the caudate–putamen complex, while the patch/striosomes receive their main thalamic innervation from the paraventricular (PV) nucleus.53, 54 The PV, parataenial, Pf, and mediodorsal thalamic nuclei constitute the main sources of thalamic inputs to the ventral striatum across species.50, 55–57 Within subregions of the monkey accumbens, the core (i.e., the dorsal centrolateral region) receives a dense innervation from the Pf with limited inputs from the midline thalamic nuclei, whereas the shell (i.e., medial region) is the recipient of afferents from both of these thalamic nuclear groups.56 Like the corticostriatal system, the thalamostriatal projections to the caudate nucleus, putamen, and nucleus accumbens are functionally topographic,52, 58 and in the case of CM/Pf, part of functionally-segregated basal ganglia–thalamostriatal loops that process sensorimotor, associative, and limbic information.52, 58, 59 However, a certain level of integration and convergence exists within and between the various structures involved in these complex circuits, particularly at the level of associative and limbic loops.5, 60, 61
Mesostriatal dopaminergic systems
In addition to glutamatergic inputs, the mammalian striatum is the target of strong dopaminergic afferents from the substantia nigra pars compacta (SNc), the ventral tegmental area (VTA), and the retrorubral area (RRA).44, 62–66 In general, the VTA and medial SNc mainly project to the limbic striatum, the lateral SNc innervates the associative and sensorimotor striatum, while the mid-central SNc and RRA send afferents to all three functional regions of the dorsal and ventral striatum in all species.64, 66, 67 In rats, the medial and lateral regions of the dorsal SNc innervate the matrix compartment of the associative and sensorimotor striatum, respectively, whereas the patches receive their main dopaminergic innervation from the medial and lateral aspects of the ventral SNc.66, 68, 69 Despite this general topographical arrangement, it is noteworthy that single dopaminergic axons often innervate neurons in both striatal subcompartments.70 It is currently unknown if similar relationships exist between the patch–matrix compartments and the mesostriatal dopaminergic systems in primates.71–75
Cholinergic systems
Although the bulk of acetylcholine in the striatum is released by intrinsic striatal ChIs, the brainstem pedunculopontine nucleus (PPN) and the laterodorsal tegmental (LDT) region also provide significant cholinergic inputs to the mammalian striatum.76 Early evidence for this projection from retrograde-tracing studies in various species62, 77–80 has recently been confirmed and expanded in choline acetyltransferase (ChAT)-Cre rats using AAV2-YFP as an anterograde tracer.76 Results of this study showed a significant, topographically organized projection from various subdivisions of the PPN/LDT complex to the sensorimotor and limbic regions of the rat striatum. This striatal projection originates from PPN/LDT neurons that also provide cholinergic innervation of the thalamus and ventral midbrain, suggesting that this ascending cholinergic system can modulate striatal activity through both direct striatal projections or indirectly via modulation of the thalamostriatal and mesostriatal systems. At the ultrastructural level, the majority of cholinergic terminals from PPN/LDT formed asymmetric synapses with spines and dendritic shafts of striatal projection neurons,76 a pattern of connectivity different from the symmetric synapses formed by terminals of intrastriatal ChIs.81–85 Future studies are needed to assess the functional significance of this extrinsic cholinergic innervation to the striatum.
Other striatal afferents
Additional inputs to the dorsal and ventral striatum originate from GABAergic neurons in the globus pallidus (GP)86–88 and the subcommissural ventral pallidum (VP),89–91 respectively, along with those from serotonergic neurons in the dorsal raphe,48, 62, 92 noradrenergic neurons in the locus coeruleus (mainly to the ventral striatum),62, 93–95 histaminergic neurons in the hypothalamus,96, 97 and glutamatergic neurons in the subthalamic nucleus.62, 98
Striatum: cellular organization and efferent connections
MSNs of the dorsal striatum: direct and indirect pathways
In rodents, 90–95% of dorsal and ventral striatal neurons are GABAergic projection MSNs, while the remaining striatal neuronal population consists of interneurons.9, 99, 100 A similar cellular organization exists within the primate dorsal striatum, although the proportion of interneurons is larger in primates than non-primates.6, 8–10, 101 Two populations of dorsal striatal MSNs have been categorized on the basis of their projection sites, neuropeptide expression, and dopamine receptor content. The MSNs that project directly to the basal ganglia output nuclei (i.e., the internal segment of the GP (GPi) and the substantia nigra pars reticulata, (SNr)), referred to as direct-pathway neurons, predominantly express substance P (SP), dynorphin, and D1 dopamine receptors. On the other hand, striatal MSNs that project to the external segment of the GP (GPe), referred to as indirect-pathway neurons, contain enkephalin (Enk) and preferentially express the D2 dopamine receptors.34, 101, 102 Although this segregation of striatal output neurons is the basis for functional models of information flow through the basal ganglia circuits, it is oversimplified because a small, but significant, subset of MSNs co-express D1 and D2 dopamine receptors (and possibly D3 and D4 receptors), and a number of striatal MSNs send axonal projections to both the GPe and GPi (or SNr) in rats and monkeys.20, 103–105
MSNs of the accumbens core and shell: overlapping efferent connectivity
Although the dorsal and ventral striatum are similar in many respects, the cellular composition of the ventral striatum tends to be more heterogeneous. For example, distinct clusters of neurons referred to as interfaced islands, consisting of the insula major of Calleja and the islands of Calleja, reside in the accumbens and olfactory tubercle, respectively, but not in the dorsal striatum.106, 107 In addition, the classification of accumbal MSNs into only two categories (i.e., direct and indirect) is not straightforward because of their overlapping efferent projection patterns and the complexity of the shell/core compartmentalization.108–110
Similarly to the efferents from the dorsal striatum, ventral striatal projections primarily target the pallidum and the ventral mesencephalon,44, 111 but projections from the accumbens shell also innervate non-basal ganglia structures, such as the lateral preoptic and hypothalamic areas, the mediodorsal thalamic nucleus, the pedunculopontine nucleus (PPN), the medial central gray, the bed nucleus of the stria terminalis, and the nucleus basalis.44, 111 In contrast to the dominant accumbal projection to the ventral pallidum in rodents, accumbal efferents in primates provide an equally large innervation of both the dorsal and ventral pallidum.111–113 In particular, accumbal MSNs topographically project to the rostral pole of the GPe, the rostromedial portion of the GPi, and the subcommissural VP, implying that the rostral and medial GP has a distinct association with the ventral striatum in monkeys.44, 111 The accumbal efferents to the nigra (i.e. both the SNc and SNr) and VTA follow a loose topographical organization that permits the integration of information from different accumbal regions across the entire ventral mesencephalon in primates.44, 67, 111 Overall, the accumbal projection to the SNc is more massive than to the VTA, most particularly in primates, suggesting that nigrostriatal neurons that innervate sensorimotor and associative striatal regions are tightly regulated by limbic-related signals from the ventral striatum.44, 67, 111 Conversely, the accumbal projections in rats follow a strict topography, consisting of core and shell MSNs that project either to the SNr or to the SNc, VTA, and retrorubral neurons, respectively.114
Striatal interneurons
GABAergic neurons that express parvalbumin, somatostatin/neuropeptide Y/nitric oxide synthase, calretinin or tyrosine hydroxylase (TH), and non-GABAergic cholinergic cells, represent the main populations of striatal interneurons in the dorsal and ventral striatum of primates and non-primates.10, 115–120 Overall, interneurons account for about 5–10% of the total neuronal population in rodents, but this proportion is significantly higher in primates.9 In the rat striatum, ChIs account for approximately 1% of the total neuronal population (absolute number is unknown),85 but the proportion of ChIs in the primate dorsal striatum has not been thoroughly quantified.121, 122 Similarly, the proportion of striatal neurons accounted for by ChIs in the rodent and primate nucleus accumbens remains to be determined using unbiased stereological methods.
Heterogeneous morphology of striatal cholinergic interneurons
ChIs in the dorsal striatum
Morphological, ultrastructural, and cytological features of ChIs
Although both ChAT immunoreactivity and acetylcholinesterase (AchE) staining have been used as markers of putative ChIs in primates and rodents, some interspecies differences in AchE expression are worth noting. In primates, there is a tight correlation between the two markers, with both being exclusively expressed in a population of large neurons with similar morphology and frequency.123 However, three populations of AchE-positive neurons have been identified in the rodent dorsal striatum, only one of those being immunoreactive for ChAT. 124–128
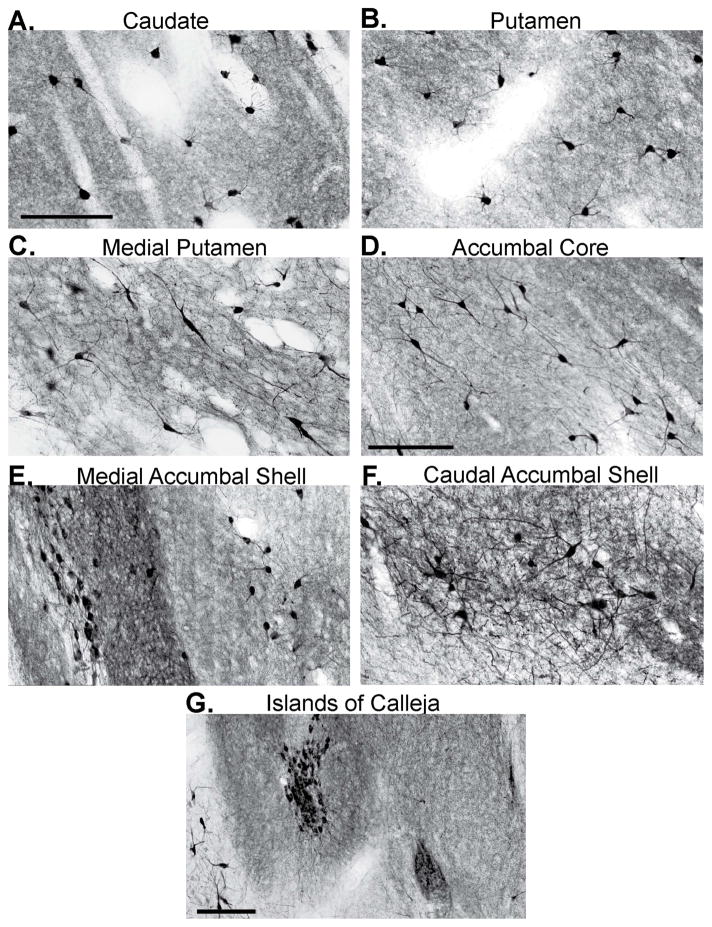
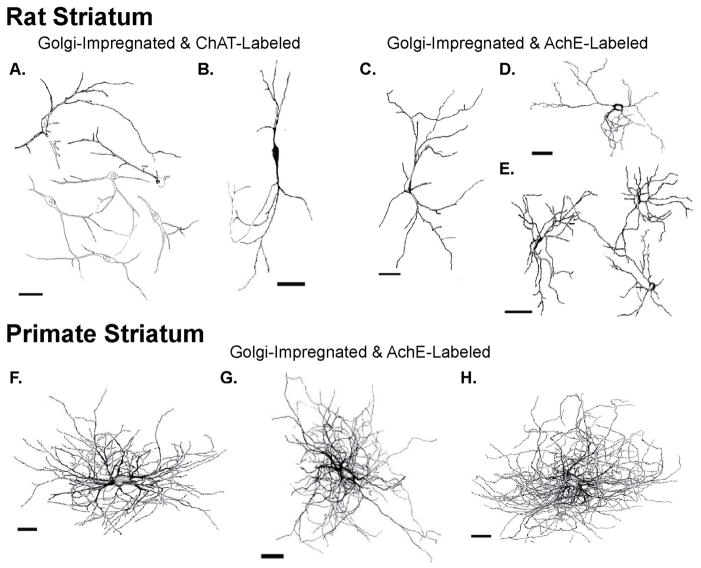
The morphology and size of ChIs in the dorsal striatum differs between primates and non-primates. Immunoreactive cell bodies for ChAT- or AchE-containing neurons in the primate dorsal striatum have an average diameter of 35 μm and display various shapes (Figs.1A–C and 2F–H),7, 82, 123, 129 whereas their cell bodies are smaller (around 25 μm) and mostly oval in rodents (Fig. 2A–E and Table 1).85, 120, 124, 126 In addition to these somatic differences, ChIs in the rat caudate–putamen complex have more somatic and dendritic spines, less primary dendrites, and a sparser ramification of their distal dendritic trees (Fig. 2A–E)21, 85, 120, 124–126, 130 compared with monkeys (Figs. 1A–C and 2G) and humans (Fig. 2F).7, 82, 123, 127, 129, 131–134 Through correlations between the morphology of Golgi-filled neurons and ChAT- (or AchE)-positive cells in the primate dorsal striatum, ChIs have been described as having a “spidery” appearance because of their large cell bodies from which emerge thick (up to 10 μm) primary dendrites that give rise to profuse “spider-like” dendritic trees and widespread intrastriatal axonal arborizations (Figs. 1A–C and 2F–H).7, 82, 123, 127, 129, 131–134 On the other hand, a similar type of analysis performed in the rodent striatum demonstrated that “spidery” cells correspond to only a small proportion of putative ChIs (Fig. 2C–E).124–128 In fact, even these apparent spidery neurons in the rodent striatum (Fig. 2C–E) display major morphological differences from ChIs in primates (Fig. 2F–H). For example, the spidery neurons in rats have a smaller number of dendritic tips (21–28 in rats versus over 100 in monkeys) and a significantly shorter total dendritic length (1,400–2,500 μm versus 23,400 μm) than in monkeys,127, 129 suggesting that the extent of synaptic innervation of, and extrinsic information processing by, ChIs is more complex in primates than in rodents.
Figure 1.
Morphological characterization of striatal cholinergic interneurons.
(A–G) Light micrographs of ChAT-positive interneurons in various dorsal and ventral striatal regions of rhesus monkeys. Note the differences in size and shape of labeled cell bodies, the extent of dendritic arborization, and intensity of neuropil immunostaining between the different sectors of the striatum. Scale bars = 200 μm in A (applies to B and C), in D (applies to E and F), and in G.
Figure 2.
Morphological differences of cholinergic interneurons between the rat and primate striatum.
(A, B) Reconstructions of the somatodendritic domain of Golgi-impregnated ChAT-immunopositive neurons in the rat striatum; taken from Refs. 124 (A) and 85 (B). (C, D) Reconstruction of the somatodendritic domain of Golgi-impregnated AchE-positive neurons in the rat striatum; taken from Refs. 124 (C) and 127 (D–E). (F–H) Reconstructions of the somatodendritic domain of three Golgi-impregnated AchE-positive neurons in the human (F), rhesus monkey (G), and baboon (H) striatum (from Ref. 129). Note the smaller cell body size and less profuse dendritic arborization of cholinergic interneurons in the rat versus the primate striatum. Scale bars = 50 μm.
Table 1.
Morphological and ultrastructural characteristics of ChIs in the dorsal and ventral striatum of rodents and primates
| ChI characteristics | Rodents | Primates |
|---|---|---|
| DS—morphology & distribution | ||
| Soma size (diameter) | 17–35 μm | 35–50 μm |
| Soma shape | Mainly oval | Highly diverse |
| Dendritic tree size | Moderate, infrequently branched | Large, highly branched |
| Somata densities | Highest rostrally | Highest caudally |
| Neuropil densities | Mostly homogeneous | Moderately patchy |
| Ultrastructure | Indented nucleus, organelle-rich cytoplasm, subsurface cisternae, and lipofuscin granules | Indented nucleus, organelle-rich cytoplasm, subsurface cisternae, and lipofuscin granules |
| NA—morphology & distribution | ||
| Soma size (diameter) | 10–21 μm | 20–40 μm |
| Soma shape | Mainly round or oval | Mainly round or oval |
| Dendritic tree size | Moderate, infrequently branched | Moderate, infrequently branched |
| Somata densities | Highest medially | Highest medially and in insula major of Calleja |
| Neuropil densities | Moderately patchy | Extensively patchy |
| Ultrastructure | Indented nucleus, organelle-rich cytoplasm, subsurface cisternae, and lipofuscin granules | Not available |
Abbreviations: DS, dorsal striatum; NA, nucleus accumbens
Evidence from our laboratory and others shows differences in the shape and size of cell bodies across functional striatal regions in primates. For instance, ChAT-labeled neurons in the ventromedial caudate nucleus and the entire extent of the medial portion of the putamen have round or elongated cell bodies, as well as sparsely-branched dendritic trees (Fig. 1C), while cholinergic neurons in the remainder of the caudate nucleus (i.e., dorsomedial, mid, and lateral regions) and the whole lateral putamen display the classical spidery appearance (Fig. 1A and B). In rats, one research group has recently shown that ChAT-immunoreactive neurons in the dorsolateral and ventromedial striatum exhibit morphologically similar characteristics,120 which resemble those of primate ChIs in the ventromedial caudate nucleus and medial putamen (Fig. 1C). In humans, the volume of ChI cell bodies differs between regions of the dorsal striatum.7 ChIs with the largest soma volumes reside in the striatal regions that have the lowest density of these neurons—the caudate gyrus and the precommissural caudate nucleus and putamen—suggesting that the increased size of ChIs perikarya in these areas may compensate for their lower neuronal densities.7
There is a general assumption that ChAT-positive neurons belong to a single cell population in the dorsal striatum of primates and non-primates because most ChIs display a non-GABAergic phenotype135, 136 and common subcellular features, such as deeply indented nuclei and a richly embedded cytoplasm that contains rough endoplasmic reticulum, subsurface cisternae, lipofuscin granules, and large dense bodies.82, 85, 124–126, 131, 137, 138 However, the morphological and neurochemical features discussed above suggest a much more diverse group of neurons with higher capabilities of information integration in primates than in non-primates. Another striking difference between ChIs in rodents and primates relates to their differential expression of the calcium binding protein, calretinin. A subset of striatal ChIs co-express calretinin in primates, but not in rats and mice.93, 122, 139 Although the functional significance of this colocalization remains to be established, it is noteworthy that the number of calretinin/ChAT–positive neurons is reduced in the striatum of Huntington’s disease patients.139 Thus, the significant interspecies and intrastriatal morphological differences of these neurons (Fig. 1) should be taken into account when anatomical and functional data gathered from rodent ChIs are translated to the primate striatum.
Relative density of ChIs in the dorsal striatum
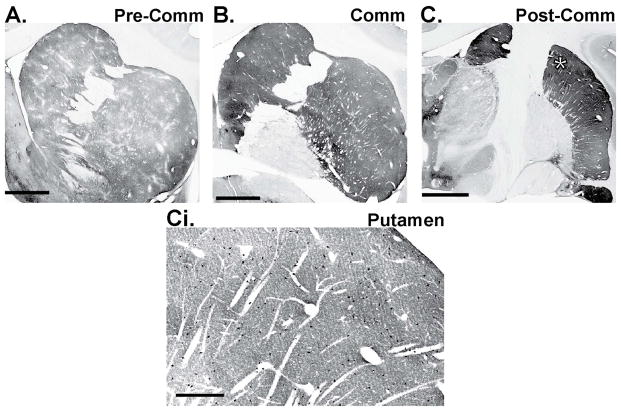
The most comprehensive stereological assessment of ChAT-immunoreactive neuron densities across all striatal territories has been performed by Bernacer et al. in the human caudate and putamen.7 According to this study, significant differences in the prevalence and pattern of distribution of ChI cell bodies are found between cortically defined functional territories of the dorsal striatum.7 The associative striatum (i.e., dorsomedial sector of the caudate head, body, and gyrus, and precommissural putamen) harbors a larger density of ChIs than the sensorimotor (i.e., dorsolateral caudate head, dorsal precommissural putamen, and postcommissural putamen excluding its ventral portion) and limbic (i.e., ventral caudate head and putamen) striatal regions.7 The density of ChIs was also shown to follow a positive rostrocaudal gradient in all functional areas of the human striatum in that the density of ChIs in precommissural striatal regions is lower than in postcommissural sectors of the striatum.7 Although similar rigorous quantitative analyses of ChI prevalence in the rodent striatum have not been performed, some authors have suggested that ChAT-labeled cell bodies are more densely distributed in the rostral than in the caudal regions of the caudate–putamen complex in rats.85 If this is the case, it would represent another striking difference in the anatomical organization of striatal ChIs between rodents and primates (Fig. 3).
Figure 3.
ChAT immunolabeling in the monkey dorsal striatum.
(A–C) Low-power light micrographs of ChAT immunostaining through the rostrocaudal extent of the monkey striatum. Note the patchy neuropil staining in the anterior striatum (A) and the positive rostrocaudal gradient in the intensity of ChAT-immunoreactive neuropil (compare A with C). Ci depicts a higher-power view of ChAT-labeled cells bodies in the dorsal part of the postcommissural putamen (asterisk in C). Scale bars = 3 mm in AC; 600 μm in Ci. Abbreviations: Pre-Comm, precommissural; Comm, commissural; Post-Comm, postcommissural.
A common feature of ChIs in rats, monkeys, and humans is their predominant localization within the striatal matrix compartment, often at the striosomal borders, where their axons and dendrites cross over the patch–matrix boundaries, providing them a unique position to facilitate cross-communication between MSNs of the different striatal subcompartments and functional territories.7, 38, 140–142 Furthermore, in primates, ChAT-labeled neurons often reside in the striatal tissue bridges that cross the internal capsule between the caudate nucleus and putamen.
Dorsal striatal cholinergic neuropil
In addition to ChAT-immunoreactive cell bodies and dendrites, a dense meshwork of fine ChAT-labeled processes that most likely represent thin axons, small distal dendritic processes, and axon terminals of ChIs and brainstem afferents occupies the entire striatum (Figs. 1 and 3).123, 134, 142 Three main features characterize this rich cholinergic neuropil in the monkey and human striatum. First, it displays a patchy appearance made up of pockets of light immunostaining embedded within a field of denser immunoreactivity, reminiscent of the striatal patch/striosome compartment (Fig. 3).7, 123, 140, 143 Second, it follows a positive rostrocaudal gradient in labeling intensity (Fig. 3A–C).123 Third, areas of denser ChAT immunostaining lay within the medial parts of the precommissural and commissural caudate and putamen (Fig. 3A and B) and the lateral borders of the postcommissural putamen (Fig. 3C).7, 142 In contrast, the rodent ChAT-immunolabeled neuropil is rather homogeneous,85, 124 except for an increased labeling intensity (of both somata and neuropil) in the lateral border and most rostral regions of the striatum.85
ChIs in the nucleus accumbens
Morphological, ultrastructural, and cytological features of accumbal ChIs
ChAT-labeled somata in the nucleus accumbens are either elongated or round in both primates and rodents with an average size smaller than those in the dorsal striatum (Table 1; Figs. 1 and 2).31, 49, 84, 123, 144 Similar to the dendritic trees of dorsal striatal ChIs (Fig. 1A–C), accumbal ChAT-labeled dendrites extend over long distances within the neuropil but are thinner and less profusely arborized than those in the dorsal striatum in both rodents and primates (Fig. 1D–G).31, 49, 84, 123, 144 At the ultrastructural level, ChIs in the ventral and dorsal striatum of rats display similar features,84, 85 but such comparisons remain to be made in primates.
ChIs neuronal density and cholinergic neuropil labeling in the accumbens
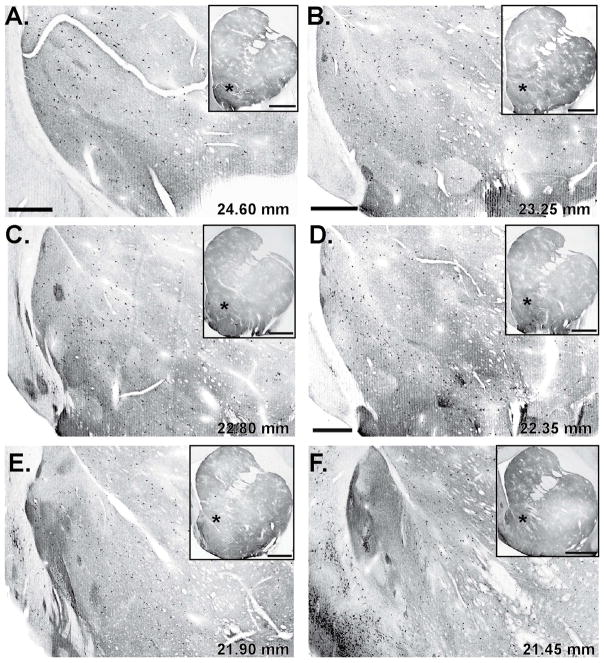
ChAT immunolabeling displays greater heterogeneity in the nucleus accumbens than in the dorsal striatum across species (Figs. 3 and 4). In particular, rodent studies have shown a larger density of ChAT-labeled neurons and a stronger level of ChAT immunoreactivity in the shell neuropil (mainly the medial part) than in the core of the nucleus accumbens.84, 144 Although differences have been suggested in the number of ChAT-labeled neurons between the shell and core of the accumbens in primates,145 these data must be confirmed using unbiased stereological quantification and a clearer delineation of the two accumbal regions in primates.31, 49, 121 The pattern of ChAT-labeled neuropil described in the rodent accumbens is reminiscent of that observed in primates (Figs. 1E and 4).31, 123, 142, 145 Furthermore, the insula major of Calleja and the islands of Calleja or the so-called “interface islands”106, 146 display the densest ChAT neuropil labeling in the ventral striatum of both primates (Figs. 1G and 4B, C, E, F) and non-primates.49, 84, 147 More specifically, ChAT-immunoreactive cell bodies reside at either the border or in the center of these strongly immunoreactive areas that become more complex in primates (Fig. 1G).
Figure 4.
ChAT immunolabeling in the monkey ventral striatum.
(A–F) Low-power views of ChAT immunoreactivity through the rostrocaudal extent of the monkey ventral striatum. The approximate interaural sterereotaxic levels of each section are indicated in the lower right corner of the different micrographs. The insets show low-power views of the striatal sections from which the accumbens micrographs were taken (labeled with an asterisk). Note the highly heterogeneous ChAT-immunoreactive neuropil along the entire extent of the rostrocaudal axis of the nucleus accumbens. Scale bar = 700 μm in A; 900 μm in B (applies to C, E–H); 800 μm in D. Scale bars for insets = 3 mm.
Physiological activity of striatal cholinergic interneurons
Tonically active neurons in the dorsal striatum
On the basis of common morphological, regional, and functional similarities, ChIs correspond to electrophysiologically characterized, tonically active neurons (TANs) in the dorsal striatum,19, 21, 148–151 although direct correspondence between these cell types remains to be fully established in primates.149 Two recent studies151, 152 that combined juxtacellular labeling and in vivo extracellular recordings in anesthetized rats revealed that striatal neurons with the electrophysiological features of TANs21, 148, 150, 153, 154 displayed immunoreactivity for ChAT and the characteristic large-sized soma of ChIs. In agreement with anatomical evidence for the existence of two morphologically distinct populations of ChIs (i.e., non-spidery and spidery) in the rodent dorsal striatum,21, 85, 120, 124–126, 130 TANs with long, sparsely-branched dendritic trees (Fig. 2A–C)148, 150–152, 155–158 or thick, moderately-branched dendrites that tapered into finer processes have been described (Fig. 2D and E).159–162
Besides their morphological characteristics, the firing properties of striatal TANs distinguish them from other neurons in the dorsal striatum. Striatal TANs in rodents and monkeys exhibit a large depolarized membrane potential (approximately –60 mV),148, 157, 163 tonic spike discharge around 2–10 spikes/s,148, 149, 163, 164 broad spike waveforms,148, 149, 157 and diverse spiking patterns (i.e., regular, irregular, and bursting).151, 158, 160, 164–167 In rodents, in vivo recording of TAN responses to current pulse injections or afferent stimulation have revealed that TANs display a strong spike-frequency adaptation and low-frequency oscillations (1–5 Hz) that are regulated by intrinsic mechanisms.148, 150, 157, 158, 165–170 Through ion channels located along their entire somatodendritic domain,171 a spike-induced, calcium-dependent afterhyperpolarization deters TAN rapid spiking, while a prominent Ih current–mediated sag conductance prevents sustained hyperpolarization of TANs.148, 150, 157, 165, 167, 169, 170 Indeed, the average firing rate of TANs is almost impossible to change using constant current injections or an artificial synaptic barrage.168 TANs fire spontaneously and maintain their diverse spiking patterns in the absence of current injections or signals from external inputs.148, 160, 165–167, 172, 173 The firing pattern of individual TANs also alternates between spiking patterns shaped by temporally-defined (< 5Hz) oscillatory mechanisms, afterhyperpolarization currents (slow, medium, and fast), and the release of neurotransmitters and/or neuromodulators from their synaptic inputs.164–166, 168, 169 Collectively, these data imply that both intrinsic membrane properties and synaptic afferents regulate the pattern of TAN spike timing and output.19, 168
While the mechanisms responsible for TAN spiking properties in the primate striatum remain to be fully established, in vivo extracellular recordings of striatal TANs in awake monkeys demonstrated that afferent activation induces changes in TAN firing activity similar to those observed during reward-related behavioral learning. For example, TANs in the dorsal striatum (mainly recorded from the precommissural level) respond to sensory salient events during behavioral conditioning,149, 164, 174–180 and to cortical (mainly the supplementary motor area) or thalamic (CM/Pf) stimulation,181, 182 with a triphasic response that includes an early excitation, a pause in activity, and a rebound excitation. However, the most common response of TANs to these events comprises only a pause and rebound excitation in spike discharge,149, 164, 183–187 which is reduced or abolished by the pharmacological blockade of thalamic (i.e., CM/Pf) afferents.179 In light of these findings and others, it appears that the firing activity patterns of primate TANs associated with behavioral learning is under the regulation of strong afferent connections from the CM/Pf and midbrain dopaminergic neurons, as is the case in the rodent dorsal striatum.19
Even though most TANs throughout the primate dorsal striatum have a similar response profile to behavioral conditioning, several key features of this response may be dependent on the location of TANs within a particular striatal territory. For instance, there is evidence that striatal TANs that respond to an individual stimulus scheme (i.e., to only one stimulus type or to multiple types of stimuli) during reward-associated learning are occasionally clustered together in specific functional regions of the striatum.149, 164, 188 For instance, TANs in the precommissural putamen display more synchronous firing activity during classical conditioning events than those in the caudate nucleus,184 while TANs in the ventromedial striatum (i.e., precommissural striatum dorsolateral to the nucleus accumbens) display slower average discharge rates and, possibly, larger responses to behavioral learning compared to those in other striatal regions in monkeys.183, 184 On the other hand, synchronized TANs in the monkey postcommissural putamen encode motivational instructions for goal-directed action learning, but respond to behavioral cues in a similar fashion as TANs in the precommissural striatum.189 In contrast to this heterogeneity of primate TANs, the firing rates and responses of TANs to behavioral conditioning in rats are similar between the dorsolateral and ventromedial striatal territories,190 in agreement with an analogous morphology of ChIs amid these regions in rodents.120
TANs in the nucleus accumbens
Albeit with slightly different firing properties, TANs also exist in the rodent nucleus accumbens. Accumbal TANs are often overlooked though, due to recordings of accumbal neurons being grouped together on the basis of their responses to various tasks and pharmacological manipulations, instead of their firing rate and pattern properties.191–198 In the instances of TAN characterization, the reported baseline firing rates of TANs in the accumbens were quite variable (0.6–12 spikes/s with a tendency to be in the lower range).190, 199–206 In those studies that utilized in vitro optogenetic201, 204 or juxtacellular single-cell filling203 approaches, recorded accumbal TANs corresponded to ChAT-immunoreactive neurons in ChAT-Cre or glutamic acid decarboxylase (GAD)-Cre mice, respectively. Collective data gathered from in vitro and in vivo electrophysiological studies in rodents have revealed that accumbal TANs exhibit variable spontaneous activity and firing patterns, Ih currents, and prolonged refractory periods.190, 200–204, 206
TANs in the ventral striatum play a role in the negative symptoms associated with depression, such as anhedonia and despair,207 suggesting their role in emotional control.205 In regard to their responses during behavioral conditioning and afferent stimulation, TANs simultaneously recorded with microarray electrodes in rat accumbal slices have non-synchronous firing activity, regular firing patterns, and display a decrease in firing activity in response to high-frequency intra-accumbal stimulation.206 During a simple instrumental task, several in vivo extracellularly recorded TANs in the rodent accumbal core respond to reward-related cues and an expected reward with attenuated firing activity, whereas an unexpected reward increases their spike discharge.190 TANs in the accumbens shell change their in vitro firing pattern to irregular and bursty following training for a contingent Pavlovian task in rodents.208, 209 Accumbal TANs display a pause in their activity followed by an excitation in response to in vitro and in vivo optogenetic activation of GABAergic VTA projections,203, 210 known to have a role in salience processing.211 However, the in vivo recordings of TANs in the ventromedial striatum (includes the ventromedial caudate–putamen complex and nucleus accumbens) during the acquisition of reward-related learning have revealed that these neurons have unique bidirectional outcome responses (i.e., excitation upon the learning and relearning of positive reward-related tasks, but inhibition after reward omission).205 These data demonstrate that TANs in both striatal regions can modify intrastriatal circuits for the learning of new associations, even though their responses to reward and other motivational-related stimuli highly differ.205 The physiological firing properties of accumbal TANs in monkeys and their responses during the learning of stimulus-outcome associations remain to be characterized.
Are striatal TANs exclusively ChIs?
Recent data have shown that other striatal interneurons, such as those that express parvalbumin or nitric oxide, may also display spontaneous tonic activity in the dorsal striatum,212–214 raising caution when identifying ChIs solely on the basis of their tonic discharge patterns. These findings were obtained from ChAT-Cre transgenic mice with GFP-labeled ChIs (or other striatal neurons) combined with optogenetic brain activation. Although these experimental tools are instrumental in relating the physiological properties of striatal neurons (including TANs) with their chemical phenotype and their respective role in basal ganglia function/dysfunction,215, 216 the fact that the Cre recombinase technology is exclusively applied in rodents makes findings obtained from these animals difficult to confirm in primates. Thus, without a reliable translation of the rapidly growing genetic technology from mice to primates, our understanding of circuit- and cellular-specific processes of chemically characterized neurons in the human brain may rely entirely on data gathered from the mouse brain.
Synaptic regulation of striatal cholinergic interneurons
In addition to the numerous studies that have provided valuable insights into the underlying regulatory mechanisms of striatal ChIs in primates and non-primates,13, 18–21, 217 the following discussion includes findings from our laboratory134 and others82, 131, 132 showing that GABAergic inputs represent a substantial source of synaptic innervation to primate ChIs. We propose that this GABAergic innervation may play a critical role in mediating communication between groups of ChIs or between ChIs and other striatal neurons or their extrinsic striatal afferents.
General synaptic innervation of cholinergic interneurons
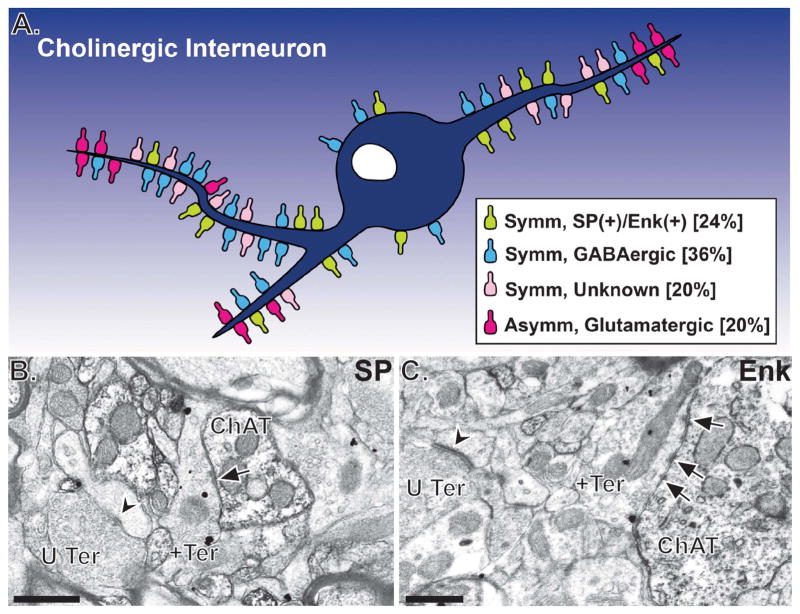
Qualitative electron microscopic observations of either Golgi-impregnated spidery neurons or ChAT-immunoreactive cells in the primate dorsal striatum have revealed that ChIs receive symmetric and asymmetric synaptic inputs from morphologically heterogeneous terminal boutons indicative of diverse sources (Fig. 6).82, 131, 132, 218–226 In a recent quantitative study, we demonstrated that 60% of all terminals in contact with ChIs in the monkey postcommissural putamen are GABAergic, while about 20% are putatively glutamatergic (asymmetric synapses/GABA-negative), and the remaining 20% are of unknown chemical phenotype (symmetric synapses/GABA-negative) (Fig. 5).134 While GABA-positive and GABA-negative terminals form symmetric synapses with the entire somatodendritic domain of ChIs, putative glutamatergic terminals form asymmetric synapses mainly with the distal (or thinner) dendrites of these neurons.134 Because such detailed quantitative assessment of rodent ChIs innervation has not been achieved, it is difficult to make direct comparisons between our findings and those reported in rodents. It appears though that terminals forming symmetric or asymmetric synapses innervate striatal ChIs in rats, with a predominance of inhibitory synapses onto their proximal parts.83–85, 124, 130, 152, 227–230 However, in contrast to monkeys, the proximal dendrites and cell bodies of ChIs receive substantial excitatory inputs in rats.152 The proportion and location of putative inhibitory and excitatory synaptic inputs to ChIs in the ventral striatum remain to be determined.
Figure 6.
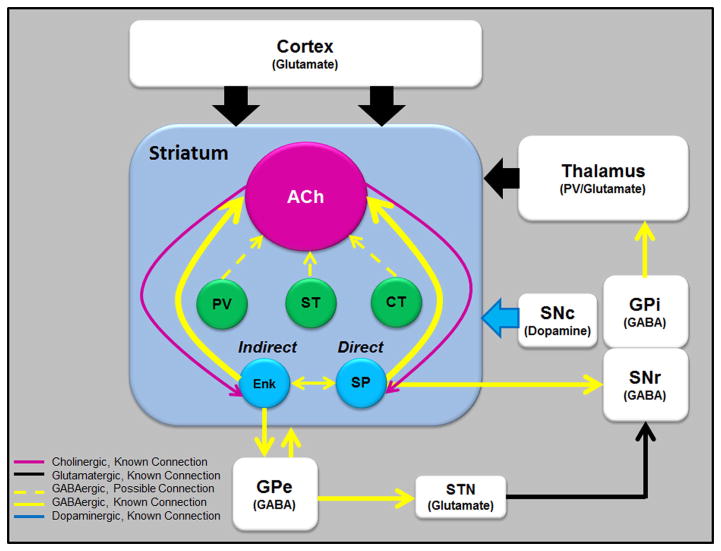
Synaptic inputs to striatal cholinergic interneurons. Schematic showing the main intrastriatal synaptic connections of striatal cholinergic interneurons. Note that the thalamus, cerebral cortex, GPe, and SNc also contribute, to varying degrees, direct synaptic inputs to ChIs. Some striatal afferents are not depicted because of the lack of detailed knowledge of their synaptic connections with ChIs. Full lines indicate connections shown by electron microscopy and hatched lines indicate putative connections that remain to be confirmed at the electron microscopic level. Abbreviations: Ach, cholinergic interneurons; CT, calretinin-positive interneurons; Enk, enkephalin-positive MSNs; SP, substance P–positive MSNs; ST, somatostatin-positive interneurons; PV, parvalbumin-positive interneurons. See Ref. 134 for more detail.
Figure 5.
Summary of the known sources and relative abundance of synaptic inputs on ChAT-positive neurons in the monkey putamen. (A) Schematic of the different synaptic inputs to ChIs in the monkey putamen; taken from Ref. 134. Note that most synaptic inputs to ChIs are from putative GABAergic terminals (including SP+ and ENK+ terminals) that form symmetric synapses (i.e., blue and green terminals in panel A). Putative glutamatergic inputs are sparse and predominantly localized on the distal dendrites of ChIs. B–C show examples of SP− (B; + Ter) or ENK− (C; + Ter) positive terminals in contact with ChAT-positive dendrites in the monkey putamen. In the material, SP and ENK were localized with pre-embedding immunogold, while ChAT was labeled with immunoperoxidase. Scale bars: 0.5 μm. Abbreviations: +Ter, substance P− or enkephalin-immunoreactive terminals; U Ter, unlabeled terminals.
Glutamatergic regulation of cholinergic interneurons
Glutamatergic inputs to ChIs
Although the cerebral cortex and thalamus are the two main sources of glutamatergic inputs to the striatum, thalamostriatal projections from the caudal intralaminar nuclei (i.e., the CM/Pf) are the predominant origin of glutamatergic terminals in contact with ChIs in the primate and non-primate dorsal striatum.229, 231 Various studies that aimed at assessing the synaptic relationships of ChIs have demonstrated that corticostriatal afferents (labeled with anterograde tracers from prefrontal and parietal cortices or vesicular glutamate transporter 1 (vGluT1) antibodies) provide a sparse innervation of the distal dendrites of ChAT-labeled neurons in rodents and monkeys.130, 152, 229, 232 These ultrastructural findings are difficult to reconcile with in vitro and in vivo electrophysiological and pharmacological studies showing that cortical stimulation elicits short-latency excitatory responses in TANs148, 152, 162, 182, 214, 233–235 and induces extracellular acetylcholine release in the striatum.236–238 Thus, it is likely that the unique intrinsic membrane properties of ChI dendrites allow them to respond to these sparse distal cortical inputs.160, 161, 165, 166, 168 On the other hand, despite clear anatomical evidence for a monosynaptic connection between CM/Pf thalamic terminals and striatal ChIs,229, 231 in vivo electrophysiological data have revealed that CM/Pf activation in monkeys results in complex multifaceted responses of combined increases and decreases in TAN firing152, 181 and a reduction of striatal acetylcholine levels.181 It remains to be determined whether these complex thalamic-induced response patterns result from the recruitment of intrastriatal GABAergic networks that impose strong inhibitory influences on ChIs (see below)162, 179, 181, 238, 239 and/or the recordings of non-ChI striatal TANs.213, 214
Currently, no studies have examined the physiological effects of neocortical or thalamic inputs to accumbal ChIs. However, tracing studies in rats have shown that hippocampal inputs from the subiculum provide only sparse and distal synaptic innervation of accumbal ChIs,240 as was found for neocortical inputs in the dorsal striatum.130, 229, 232 In regard to their thalamic innervation, tracing studies from two groups have resulted in contrasting findings. On one hand, some researchers have shown that inputs from the PV nucleus terminate onto the dendrites of accumbal ChIs in the medial shell,240 while another research group has demonstrated that terminals from PV, midline, and rostral intralaminar nuclei do not form direct synaptic contacts with accumbal ChIs.83 An explanation for this discrepancy could be that the anterograde tracer injection aimed at the PV nucleus in the former study slightly contaminated more posterior thalamic nuclei, such as the Pf,59, 229, 241 known to largely innervate dorsal striatal ChIs.229, 231
Thus, a prominent source of glutamatergic innervation to ChIs in the dorsal striatum stems from the CM/Pf complex in both primates and non-primates (Fig. 6), but the details of this thalamic innervation in the nucleus accumbens remain to be further clarified. Nevertheless, the exact proportions of cortical, thalamic, hippocampal, and amygdalar inputs at the level of single ChIs await further quantitative ultrastructural analyses in both the dorsal and ventral striatum.
Glutamatergic receptors on ChIs
In both the ventral and dorsal striatum, ChIs express various subtypes of ionotropic and metabotropic glutamate receptor protein and mRNA,242–250 which is consistent with a large number of pharmacological studies showing that direct or indirect activation of glutamatergic systems results in changes in acetylcholine release and/or the depolarization of dorsal striatal ChIs.21, 251–254 Prefrontal cortical inhibition and hippocampal activation induce N-methyl-D-aspartate (NMDA)-mediated release of acetylcholine in the accumbens, most likely through indirect routes that involve glutamatergic, GABAergic, and dopaminergic neurotransmitter systems.240, 255–259 Other pharmacological studies suggested that thalamic inputs from Pf regulate ChIs activity predominantly through NMDA receptor activation, while the effects of cortical afferents are mainly mediated through AMPA receptor activation.237, 238, 251, 260 Because of the limited information on the synaptic relationships between subtypes of glutamate receptors and their presynaptic afferent terminals from the cerebral cortex or thalamus, the underlying substrate for these specific glutamate receptor–mediated effects on ChIs is unknown. However, it is worth noting that a high NMDA/AMPA receptor ratio was found at thalamic synapses formed by Pf terminals in rat MSNs.261, 262
At the ultrastructural level, both group-I metabotropic glutamate receptors (mGluRs) (i.e., mGluR1 and mGluR5) are expressed extrasynaptically, or at the edges of glutamatergic synapses, on ChAT-labeled dendrites in the primate and rat accumbal core and shell regions,263 in agreement with data from the rodent dorsal striatum.264 Although direct evidence must be provided, the extrasynaptic glutamate spillover from cortical or thalamic terminals and/or the astrocytic release of glutamate are the most likely sources of activation of these receptors.265
GABAergic regulation of cholinergic interneurons
Striatal projection neurons: role of GABA and neuropeptides
Although the source(s) of GABAergic inputs to ChIs remains to be fully characterized, we have recently demonstrated that axon collaterals of GABAergic projection neurons provide major synaptic inputs to ChIs in the monkey putamen (Fig. 5).134 As much as one-third of intrastriatal GABAergic terminals from axon collaterals of direct-pathway neurons (Fig. 5B) and one-half of those from indirect-pathway neurons (Fig. 5C) form symmetric synapses with monkey ChIs.134 These findings are in striking contrast with rat data showing that ChIs receive GABAergic inputs from direct-, but not indirect-, pathway neurons in the dorsal266, 267 and ventral268 striatum.
In addition to GABA, the release of neuropeptides from axon collaterals of MSNs may also affect striatal cholinergic activity. Dorsal striatal ChIs display mRNA and protein expression for μ-opioid (daytime sensitive and mainly in the striosomes and the ventromedial caudate–putamen complex) and δ-opioid receptors in rats,269–271 as well as neurokinin (NK1) receptors in rats and humans.272–277 The cell bodies and dendritic processes of ChIs in the rodent accumbens shell also express μ- and δ-opioid receptor and NK1 receptor immunoreactivity.208, 271, 277–279 At the functional level, SP and Enk exert opposite effects on striatal ChI activity in rodents. While the striatal release or bath application of SP depolarizes ChIs and increases acetylcholine release in the dorsal striatum,277, 280–283 opposite effects are elicited following the endogenous Enk-mediated activation or in vitro administration of μ- or δ-opioid receptors in the dorsal284–290 and ventral291, 292 striatum. Furthermore, a recent study revealed that μ-opioid receptor activation inhibits the in vitro spontaneous activity of ChIs in the mouse dorsal striatum (functional territory unknown), independently of GABA(A) or NMDA receptor activation.293 The systemic administration of SP, however, decreases extracellular acetylcholine concentrations in the dorsal striatum and accumbens of freely moving rats,294 but most likely through the activation of multisynaptic pathways. At the level of the ventral striatum, in vitro activation of μ- and δ-opioid receptors decreases acetylcholine release,291 similar to effects described in the caudate–putamen complex,284–290 and increases putative ChI responses to contingent Pavlovian training in rodents.208, 209
Altogether, it appears that ChIs in the dorsal striatum are under tight regulation by intrastriatal GABAergic afferents that originate, in large part, from axon collaterals of GABAergic direct and indirect striatofugal neurons in primates (Figs. 5 and 6). In both primates and non-primates, the neuropeptides ENK and SP, which co-exist with GABA in MSN axon collaterals, also regulate ChI activity. Further studies must be performed to determine if this peptidergic modulation occurs in concert or in parallel with that mediated by the GABAergic system.
Other sources of GABAergic inputs to ChIs
GABAergic afferents from various populations of striatal interneurons, the globus pallidus, and the VTA (mainly to the nucleus accumbens)203 may also contribute to the GABAergic regulation of ChIs (Fig. 6),295, 296 thereby providing additional substrates through which various sources of GABA may regulate striatal ChI activity.
GABA receptors on ChIs
Consistent with their strong GABAergic synaptic innervation,134 ChIs in the dorsal striatum are enriched in both GABA(A) and GABA(B) receptor subunits in rats and primates,297–300 and electrophysiological evidence from in vitro and in vivo preparations in rodents have demonstrated that activation of either receptor subtype elicits inhibitory synaptic responses in ChIs161, 233, 297 and reduces in vivo acetylcholine release.301–303 However, conflicting results have been published about the GABAergic regulation of ChIs in the rodent ventral striatum. In rodent accumbal slices, GABA application alters acetylcholine output and turnover, but not as strongly as in the caudate–putamen complex.301, 304, 305 On the other hand, the local application of GABA and GABA(A) or GABA(B) receptor agonists resulted in extracellular in vivo acetylcholine release in the accumbens of freely moving rats.306 A recent in vitro slice study demonstrated that high-frequency intra-accumbal stimulation produces GABA(B)-mediated TAN inhibition in rats.206 Overall, it appears that accumbal ChIs are endowed with GABA receptors and display functional properties necessary for GABAergic modulation, although the functional context and mechanisms by which these inhibitory networks are recruited and involved in the regulation of accumbal ChIs activity remain to be characterized.
Dopaminergic regulation of cholinergic interneurons
A substantial amount of pharmacological, electrophysiological, and neurochemical data has suggested close functional interactions between the mesostriatal dopaminergic systems and striatal ChIs in normal and diseased states.11–16, 19–23, 38, 217, 307, 308 However, the exact synaptic mechanisms that mediate these interactions are complex and remain poorly understood. At the ultrastructural level, Kubota et al. showed that ChAT-immunoreactive soma and proximal dendrites receive direct synaptic inputs from TH-labeled terminals in the rat caudate putamen,309 while other studies in both the dorsal and ventral striatum of rodents demonstrated close appositions with only scarce direct synaptic connections between putative dopaminergic terminals and ChIs.224, 310–315
Despite this paucity of synaptic contacts, it is clear that ChIs activity is highly sensitive to dopamine receptor modulation, albeit to varying degrees between the dorsal and ventral striatum. In both striatal regions, D2 dopamine receptor mRNA is strongly expressed in ChIs,20, 316–318 with the greatest densities found in the dorsolateral caudate–putamen complex of rodents.319 The majority of ChIs in the dorsal and ventral striatum also express moderate to high levels of D5 dopamine receptors.20, 297, 319–323 On the other hand, D1 dopamine receptors are found on ChIs in the ventral, but not the dorsal, striatum in primates.317, 321 Although D3 dopamine receptor expression is undetectable in ChIs of the rodent dorsal striatum,297 ChIs in the ventral striatum, particularly those in the insula major of Calleja of the nucleus accumbens, are enriched in this dopamine receptor subtype.324–327 Thus, despite limited direct synaptic connections between dopamine terminals and ChIs, the strong dopamine receptor expression of ChIs allows them to be highly sensitive to extracellular dopamine.
In rodents and monkeys, in vitro and in vivo electrophysiological and pharmacological studies have revealed that cholinergic activity in both the dorsal and ventral striatum is significantly altered by manipulation of the midbrain dopaminergic systems, resulting in a wide range of receptor subtype–specific responses (i.e., no effect, increased or decreased cholinergic activity).11, 14, 19, 21, 22, 174, 217, 297, 328–335 Additionally, dopamine modulates cholinergic function in the caudate–putamen complex by regulating intrinsic cellular properties of ChIs and their synaptic afferents, particularly those from the glutamatergic corticostriatal system.19, 150, 161, 328, 336–340 Although such interactions most likely exist throughout the whole striatum, they have been mainly studied in dorsal striatal regions.
Functions of the intrastriatal cholinergic network in the dorsal striatum and nucleus accumbens are significantly altered following acute or chronic chemical disruption of dopaminergic inputs from the SNc11, 13, 15, 19 or the VTA,16, 38 respectively. Thus, it is reasonable to conclude that the ascending dopaminergic systems are major regulators of ChIs activity, but that such control is largely mediated by diffusion of non-synaptic dopamine that can exert opposing and/or synergistic effects through the activation of specific dopamine receptors (D2, D3, and D5) expressed by ChIs, as well as through the manipulation of ChI synaptic afferents and intrinsic conductances.341–344
Cholinergic regulation of striatal activity
Cholinergic receptor expression in the dorsal and ventral striatum
Muscarinic receptor expression of striatal neurons
ChIs modulate the activity of striatal neurons through direct and indirect mechanisms via a wide range of pre- and postsynaptic cholinergic receptors.14, 251, 345 The G protein–coupled muscarinic cholinergic receptors (mAChRs) and ionotropic nicotinic cholinergic receptors (nAChRs) are located on the surface of striatal neurons and their various synaptic afferents.344–350 Five types of mAChRs have been genetically identified (M1–M5) and categorized into two groups on the basis of their distinct pharmacological properties upon activation: the Gq/11-coupled M1-like receptors (M1, M3, and M5) that enhance internal calcium release through stimulation of phospholipases, and the Gi/o-coupled M2-like receptors (M2 and M4) that block calcium channel activity by reducing cyclic AMP formation through the inhibition of adenylyl cyclase.349, 351, 352
The highly heterogeneous distributions of M1–M5 receptor mRNA, protein, and binding sites in the dorsal and ventral striatum, and within single striatal cell populations, contribute to the multifaceted distinguishing features of the striatal cholinergic system. The patterns of striatal mAChR expression have been studied using autoradiography, reverse transcription-polymerase chain reactions (RT-PCR), in situ hybridization, and immunohistochemistry.353–367 Significant M1 mAChR mRNA and binding sites are heterogeneously expressed (i.e., patchy appearance) throughout the dorsal and ventral striatum of rats359, 360, 364, 366, 368 and primates.354, 357, 362, 367 The striosomes in the caudate nucleus of cats, monkeys, and humans display the highest level of M1 receptor binding, while the lowest striatal expression is found in the insula major of Calleja.353, 354 M2 receptor mRNAs are moderately expressed in the dorsal regions of the caudate–putamen complex369 and the core of the accumbens366 in adult rats. In cats and primates,362, 365, 370 M2 expression in the dorsal striatum is comparable to that in rodents,369 but the medial shell of the accumbens and the insula major of Calleja in primates also contain dense M2 receptor binding sites.354 The expression pattern of striatal M3 receptor mRNA and binding sites significantly differs from that of M1 and M2 receptors (i.e., they are mainly enriched in the mid-ventral and ventral regions of the dorsal striatum in rats and monkeys)362, 365, 370 and in the core and medial shell of the rodent accumbens.365, 370 The M4 mAChRs mRNA and binding sites are prevalent in all regions of the rodent striatum,360 although some studies have shown lower levels of M4 mRNA in the nucleus accumbens of rodents.359, 364 In rats, the expression of M4 receptor mRNA and protein exhibits a dense, patchy-like pattern (not clear if correlated to striosomal boundaries) in the caudate–putamen complex,359, 371, 372 but a more homogeneous expression in the nucleus accumbens.373, 374 Although displacement radioligand binding experiments have illustrated the likeliness of M3 and M4 receptor expression in the primate caudate nucleus and putamen,362, 367 a full characterization of these expression patterns using more specific markers must be established in the dorsal and ventral striatum of nonhuman and human primates.
At the cellular level, M1 mAChR mRNA is highly expressed by direct- (labeled with SP or D1 dopamine receptors) and indirect- (labeled with Enk or D2 dopamine receptors) pathway MSNs, neuropeptide Y (NPY)/somatostatin–containing interneurons, and ChIs in the dorsal striatum.375–382 A moderate number of Enk-containing projection neurons359, 375, 378 and ChAT-labeled neurons376, 380, 383 also contain M4 mAChRs. It is noteworthy that SP/D1 direct-pathway MSNs display a five-fold higher M4 mRNA expression level than that in Enk-labeled indirect-pathway neurons in rats.375, 378, 384 At the light microscopic level, M2 mAChRs are expressed in ChAT-labeled neurons and NPY/NADPH–containing interneurons in the rodent and monkey dorsal striatum.372, 375, 377, 385–387 Single-cell RT-PCR findings have suggested that mRNA for M3 receptors and the neuropeptide Enk coincide within around 10% of the same neurons in the rat dorsal striatum.378 Comparative data for M1, M2, M3, and M4 expression in specific cell populations are currently unavailable for the nucleus accumbens of all species, as well as for the primate dorsal striatum.
With regard to M5 mAChRs, immunocytochemical and in situ hybridization studies have shown minimal staining in the striatum,359, 364, 378, 388 most likely due to the low, undetectable level of M5 receptor mRNA expression in striatal neurons and the lack of sensitive M5 receptor antibodies to detect immunolabeling in striatal neurons and their afferents. Interestingly, activation of M5 receptors in the striatum inhibits dopamine release, while M5 receptor stimulation at the cell body level in the SNc increases the firing activity of dopaminergic neurons (see below for details).
Nicotinic receptor expression in striatal neurons
Nicotinic receptors form pentameric ion (Na+, K+, and Ca2+) channels that consist of either homomeric (α7 in the mammalian brain) or heteromeric combinations of α (α2–α10) and β (β2–β4) subunits, resulting in functional receptors with highly diverse pharmacological and functional properties.347, 389–393 The α4 and β2 mRNA and protein are the predominant subunits expressed in the dorsal and ventral striatum of rodents and primates.390, 392, 394 In general, a similar homogeneous distribution pattern for nAChR subunit mRNA exists in the dorsal striatum of rodents and primates.395–406 However, the expression patterns for α4 and α7 mRNA are different from each other in the monkey and human dorsal striatum, but similar in rats. In addition, the α5, α6, and β3 subunits are more strongly expressed in the putamen than in the caudate nucleus of primates.390, 392, 394, 395, 399, 401, 402, 404, 406 At the cellular level, α7 and β2 nAChR subunits are co-expressed on ChIs and GABAergic axons of unknown sources in the rat dorsal striatum.407–409 As specific antibodies, receptor ligands, and pharmacological drugs are developed,393 a clear map of the cellular localization of nAChRs will be established across different neuronal populations, striatal regions, and species.
Muscarinic and nicotinic receptor expression in striatal afferents
Although there is no detailed quantitative assessment of mAChRs expression in striatal afferents, qualitative ultrastructural findings have suggested that putative glutamatergic terminals from either the cerebral cortex or thalamus express M1, M2, M3, and/or M4 mAChRs in the dorsal striatum.371, 372, 377, 410 On the other hand, M5 receptors, which occasionally colocalize with α4β2 nicotinic receptors, are mainly expressed in midbrain dopaminergic axon/terminal processes.374, 410–413 These findings are consistent with the expression of M5 receptor mRNA in SNc and VTA dopaminergic neurons359, 414 and the physiological effects of M5 allosteric modulators on striatal dopamine release.415
Several populations of striatal afferents express nicotinic receptors. Attempts at characterizing nAChR expression in striatal dopaminergic axons/terminals have been ongoing for decades by means of in situ hybridization, autoradiography, and immunoprecipitation techniques in the SNc, VTA, and striatum,416–418 along with striatal dopamine release studies in receptor subunit knock-out mice.347, 391, 419 Currently, the general consensus is that α3 (mainly in primates), α4, α5, α6, β2, and β3 subunits are expressed in highly diverse and complex subunit compositions on dopaminergic axons/terminals in the striatum.373, 390, 418, 420–422 At the ultrastructural level, one study utilizing double-label electron microscopy has revealed the co-expression of TH and β2 nicotinic receptors on dopamine terminals in the rat dorsal striatum.373 On the other hand, a combination of immunoprecipitation and radioligand binding studies performed on the monkey dorsal striatum have suggested that non-dopaminergic axons, possibly of glutamatergic origin,423 express nicotinic receptors with α7 subunits.406 Double-label confocal microscopy has demonstrated the co-expression of 5-hydroxytryptamine (5HT3) serotonin receptors and α4 nAChRs on terminals of an unknown chemical phenotype in the rat dorsal striatum.424 Nicotinic receptor expression on striatal (especially accumbal) afferents and their postsynaptic targets remains to be fully characterized in primates and non-primates.
Electron microscopic localization of cholinergic receptors
Electron microscopic studies of cholinergic receptor localization have only been carried out in the caudate–putamen complex of rats and mice and the monkey caudate nucleus. According to these studies, spines are the main striatal neuronal elements that express M1 mAChRs in rats and monkeys.371, 372, 377 With regard to presynaptic localization, M1 mAChRs are located on terminals forming asymmetric or symmetric synapses in the monkey dorsal striatum, while in rats, only terminal boutons that form asymmetric synapses express the M1 receptor subtype.371, 372, 377 By means of double-labeling experiments at the light microscopic level, M1 receptor expression has been found in calbindin-labeled, but not PV- or NADPH-labeled neurons, suggesting a preferential M1 expression in MSNs over GABAergic interneurons.377
On the other hand, striatal M2 mAChRs are expressed on soma, dendritic shafts, and terminals that typically form symmetric axodendritic and axospinous synapses in rats and monkeys.371, 372, 377, 386 Interestingly, M2 receptor immunoreactivity is also found at putative dendrodendritic synapses.372 In the rodent dorsal striatum, M3 receptors are located on spines and, like M4 receptors,372, 410, 425 on terminals that formed asymmetric axospinous synapses.371, 372 In normal states, the plasma membrane of ChAT-labeled soma and dendrites displays immunoreactivity for M2 mAChRs, whereas M4 receptor immunostaining is mainly found intracellularly in the endoplasmic reticulum of ChIs or extrasynaptically on the plasma membrane of striatal MSNs.386, 425 After acute oxotremorine (i.e., a nonselective muscarinic agonist) treatment, M2 receptors are trafficked from the plasma membrane of ChAT-labeled neurons to internally located endosomes, while M4 receptor expression is unaffected,386, 425 suggesting different sensitivities and trafficking properties of these two receptor subtypes in response to increase cholinergic activation.
In line with evidence for presynaptic nAChRs in various brain regions,426–429 extrasynaptic β2 subunit immunoreactivity is found in TH-labeled nigrostriatal terminals forming axospinous symmetric synapses and in unlabeled terminals forming symmetric or asymmetric synapses with dendritic shafts in the rat dorsal striatum.373, 430 Additional ultrastructural studies are needed to characterize the subcellular and subsynaptic location of other subunits of nicotinic receptors in the dorsal and ventral striatum of rodents and primates.
Cholinergic regulation of striatal neurons and their afferents
Autoregulation of cholinergic excitability in the striatum
Spike firing by ChIs sets a regulatory tone in the dorsal striatum through the tonic release of acetylcholine, acting on diverse muscarinic and nicotinic receptors located on striatal GABAergic neurons and their afferents.346–350 Additionally, cholinergic receptors influence endogenous cholinergic activity through both muscarinic autoreceptors (M2, M4) on ChIs themselves and nicotinic receptors on non-cholinergic synaptic inputs to ChIs.103, 359, 372, 375 However, because of the limited availability of specific pharmacological tools and detailed assessments of cholinergic receptor expression, significant controversy exists about the relative roles of mAChRs and nAChRs in their autoregulatory function of ChIs in the dorsal striatum.165, 172, 359, 383, 431–437 Information about the cholinergic receptor subtypes that regulate ChIs activity in the nucleus accumbens is minimal.199 However, the fact that low M2, but significant M4, receptor expression is found in the nucleus accumbens suggests a different role for the muscarinic receptor modulation of ChIs between the dorsal and ventral striatum.
Nicotinic receptor modulation of ChIs has been reported in the dorsal and ventral striatum of rodents. In slices from the caudate–putamen complex, ChIs inhibit their own activity through nicotinic receptor–mediated activation of their GABAergic afferents (i.e., neurogliaform/NPY–containing interneurons and other GABAergic striatal neurons), resulting in a synchronized pause in cholinergic activity.19, 438, 439 Similarly, cholinergic tone in accumbal slices is also regulated by nAChRs, most likely located presynaptically on GABAergic afferents to ChIs,199 as in the dorsal striatum.
Muscarinic and nicotinic modulation of GABAergic striatal neurons
Because of their diverse G protein coupling, multifarious neuronal and synaptic expression, and close proximity to nAChRs,199, 201, 349, 434, 440–442 acetylcholine-induced activation of mAChRs facilitates or suppresses the activity of striatal neurons.13, 346, 349 In striatal slices, the general activation of mAChRs by oxotremorine or carbachol decreases spontaneous inhibitory postsynaptic current (sIPSC) frequency and amplitude in MSNs of the caudate–putamen complex or nucleus accumbens in rodents.199, 434, 443, 444 M1 receptor activation directly contributes to MSN depolarization and dendritic excitability through the coordinated modulation of calcium and potassium channels, or indirectly by regulating the suppression of the endocannabinoid system in the rodent dorsal and ventral striatum.349, 381, 441, 444–448 On the other hand, MSN inhibition is also indirectly mediated through the M2- or M4-induced suppression of neurotransmitter release from cholinergic and/or glutamatergic terminals,15, 239, 377, 410 and for the case of M4, similar effects were found for both striatonigral and striatopallidal MSNs.410 M4 receptor signaling was also shown to shape the firing activity and up- and down-state transitions of MSNs.380, 449, 450 In contrast to M4 regulation of both MSN populations,451 M1 receptor activation modulates the basal dendritic excitability of striatopallidal, but not striatonigral, neurons via the downregulation of their Kir2 potassium channels,446 or by the facilitation of their dopamine/DARP-32 signaling,410 in genetically-modified mice. At this time, it is unknown whether these concepts uphold in the primate dorsal and ventral striatum.
On the contrary to muscarinic receptors, there is limited evidence for nAChR expression in MSNs, suggesting that MSN excitability undergoes indirect nicotinic regulation through presynaptic receptors on GABAergic, glutamatergic, serotonergic, and/or dopaminergic striatal afferents.199, 345, 349, 409, 423, 443, 444, 452–454 In patch-clamp recordings from rodent caudate–putamen slices, nicotinic receptor agonists directly activate GABAergic interneurons that express PV, TH, or NPY (neurogliaform and non-neurogliaform), but not D1- or D2-containing MSNs.409, 454 However, in this particular environment, only the GABAergic responses induced in MSNs by neurogliaform/NPY– and TH-containing interneurons respond to nAChRs (i.e., carbachol plus atropine).409 In whole-cell recordings from rat dorsal striatal slices, acetylcholine has a dual effect on fast-spiking (putative PV-containing) interneurons (FSIs). On one hand, it depolarizes FSIs by acting on non-desensitizing somatodendritic nAChRs, while it attenuates FSI-mediated GABAergic inhibition through activation of presynaptic mAChRs.345 Collectively, these findings suggest that in vitro muscarinic modulation may overpower nicotinic regulation of PV-containing interneurons in the dorsal striatum of rats, an effect that could be dependent on the basal ACh levels and firing activity of ChIs at the time of recordings.345
Acetylcholine also modulates GABA release in the striatum through complex interactions between presynaptic mAChRs and nAChRs on the same GABAergic terminals.199, 409, 440, 442, 455 In synaptosomes from the dorsal striatum, atropine and the presumed M4 antagonist MT3 facilitate depolarization-evoked release of GABA, likely from presynaptic terminals.440, 442 On the other hand, nicotine and a variety of nAChR agonists evoke GABA release in striatal synaptosomes (and in other experimental configurations) that could be counteracted by muscarinic activation and/or antagonism of α4β2 nicotinic receptor subunits.407, 442 On the basis of these observations, M4 mAChRs and α4β2 nAChRs are thought to co-exist on the same GABAergic terminals (of unknown origin) in the dorsal striatum.442 As specific drugs for cholinergic receptor subtypes are developed and techniques optimized, the existence of similar types of interactions between other muscarinic and nicotinic receptors, as well as the chemical phenotype and source(s) of the terminals on which they reside, will be determined in the dorsal and ventral striatum.
Muscarinic and nicotinic modulation of striatal glutamatergic transmission
Cholinergic modulation of glutamatergic transmission at excitatory synapses is mediated through the activation of M1–M4 mAChRs in the rodent dorsal striatum. Muscarine and a putative M1 receptor agonist enhance NMDA (but not AMPA) receptor–induced depolarization of MSNs through postsynaptic mechanisms.451, 456 On the other hand, presynaptic M2/M3 mAChR activation decreases the probability of multivesicular release from glutamatergic terminals in striatal slices, thereby reducing corticostriatal glutamatergic transmission and the induction phase of long-term potentiation (LTP) in MSNs.457, 458 Likewise, cholinergic single spikes depress cortically evoked excitatory postsynaptic currents (EPSCs) in one-third of MSNs located within 100 μm of the spiking ChI,459 while oxotremorine or a burst in ChI firing (induced by thalamic activation) inhibits both corticostriatal and thalamostriatal excitation of D1- and D2-expresssing MSNs.239 These data suggest that striatal ChIs finely regulate glutamatergic transmission at cortical and thalamic synapses on MSNs through temporal release of acetylcholine and activation of different pre- and postsynaptic mAChR subtypes. Presynaptic nAChRs activation also intricately modulates glutamate release from excitatory synapses, which often coincides with nigrostriatal dopamine release in the rodent dorsal striatum.340, 423, 460–462
In the nucleus accumbens, muscarine application inhibits glutamate release, possibly via M3 receptors, in rat brain slices.455 In addition, general activation of mAChRs by oxotremorine reduces evoked EPSCs in MSNs, likely through the presynaptic inhibition of excitatory synapses.199 With the development of highly selective cholinergic receptor drugs, the mAChRs and nAChRs underlying cholinergic regulation of glutamatergic synapses in the nucleus accumbens will be delineated, along with those in the primate dorsal and ventral striatum.
Muscarinic regulation of striatal dopamine transmission
ChIs may directly and indirectly regulate dopaminergic transmission in the striatum through presynaptic activation of various subtypes of cholinergic receptors on dopaminergic, GABAergic, glutamatergic, and cholinergic axons.371, 372, 377, 463 There is evidence that this presynaptic regulation involves different cholinergic receptor subtypes in the dorsal versus ventral striatum.14, 19, 239, 377, 441, 446, 457, 459, 464 For example, muscarinic and nicotinic regulation of dopamine release in the rodent caudate–putamen complex requires M2/M4 mAChRs and nAChRs with α4β2 and α4α5β2 subunits, whereas M4 mAChRs and α6β2 nAChRs mediate these effects in the nucleus accumbens.14, 346, 465–468 These rodent data are at odds with nonhuman primate studies that show a strong α6β2 and α3β2 nicotinic receptor–mediated modulation of dopamine release in the monkey dorsal striatum.406, 467, 469, 470
Findings from slices of muscarinic receptor knock-out mice have shown that acetylcholine-mediated (i.e., oxotremorine-induced) dopamine release is enhanced by activation of M4 mAChRs, but inhibited by M3 receptor activation.374, 411 M4 mAChRs likely mediate their effects through the regulation of neurotransmitter release from intrastriatal GABAergic terminals.374, 411 On the other hand, on the basis of data showing that M3 mAChRs are expressed in terminals forming asymmetric synapses (i.e., putatively glutamatergic),371, 372 it is unclear how these receptors inhibit dopamine release. Interestingly, although strong anatomical evidence is still needed, a recent study using fast-scan cyclic voltammetry has revealed that M5 receptor potentiation with a positive allosteric modulator (PAM) inhibits dopamine release in the rat dorsal striatum, opposing its excitatory effects when applied at the level of SNc neuronal cell bodies.415 Dopamine release in the caudate–putamen complex and nucleus accumbens is also indirectly regulated by activation of the pedunculopontine (PPN) and laterodorsal (LDT) tegmental nucleus, respectively, by means of ACh activation of mAChRs on dopaminergic neurons in the SNc and VTA. M5-mediated excitation of dopamine neurons in the SNc and VTA and/or471–475 M3-mediated inhibition of dopamine release from the SNc have been suggested as mechanisms underlying these effects.415, 472, 475
Nicotinic regulation of striatal dopamine transmission
The nicotinic regulation of mesostriatal dopaminergic systems is altered in multiple neurological and psychiatric disorders.14, 180, 239, 346, 347, 391, 463, 476–481 Recent evidence indicates that nAChRs regulate striatal dopamine release by acting as “presynaptic filters.”463 They utilize both dopamine and cholinergic neuron spiking activity (i.e., frequency and pattern) to adjust the probability of neurotransmitter release from dopaminergic axons/terminals in the striatum, independently from action potentials at dopaminergic soma.14, 347, 463, 481 For example, in vitro optogenetic stimulation of ChIs in the dorsolateral striatum, or of their thalamostriatal afferents, triggers nAChR-mediated, frequency-independent (i.e., single action potentials or synchronized activity of ChIs) dopamine release from dopaminergic axons severed from their parent cell bodies.481 Thus, synchronized ChI-driven activation of dopaminergic axons, but not dopamine neuron firing, was proposed to mediate the role of dopamine in salience- or attention-related events.481
The nicotinic modulation of striatal dopamine release driven by dopamine neuron activity is frequency-dependent and differs between striatal regions in rodents and monkeys14, 347, 405, 464, 470, 482–485 Because of their higher probability of dopamine release in response to a single stimulus,482, 486, 487 dopaminergic terminals in the dorsolateral striatum respond more robustly (i.e., release more neurotransmitter) to an arriving single action potential than those in the accumbens shell.347, 464, 481, 482, 484, 485 In both regions, dopamine release is suppressed by nicotinic (partially, β2) receptor antagonism and/or desensitization204, 464, 481, 482, 484, 485, 487 via possible changes in their presynaptic nAChR-mediated calcium regulation.452, 464, 488, 489 In contrast, pulse-train stimulation alone, or in combination with nicotine application, induces a larger enhancement of dopamine release in the accumbens shell than in the dorsolateral striatum.347, 464, 482, 484, 485 These data indicate that the facilitation of dopamine release in the nucleus accumbens is greater than in the dorsal striatum, perhaps due to the initial low probability of dopamine release in the ventral striatum.484, 485 Interestingly, dopamine transporter (DAT) density differences (i.e., lower DAT levels in the accumbens shell than in the dorsal striatum490) do not entirely account for the variable dopaminergic signaling found between these striatal regions.484, 485 In monkey slices, antagonism of α3α6β2 nAChRs, along with a small contribution by α4β2 subunits, decreases single-pulse electrically stimulated dopamine release in both the dorsal and ventral putamen.405, 470, 483 However, when dopamine release is induced by burst stimulations or chronic nicotine administration, the effect is seen only in the ventral putamen.470 Thus, these findings demonstrate that nicotinic modulation of dopamine release is dependent on the striatal subregion and dopaminergic firing frequency in the primate striatum,470, 486 as observed in the rodent dorsal striatum482, 485, 491 and nucleus accumbens.485, 487
Nicotinic modulation of striatal serotonin release
In the rat dorsal striatum, confounding data have been reported about the nicotinic receptor–mediated regulation of serotonin release. In general, the activation of colocalized α4 (but not α3 and α5) nAChR and 5HT3 receptors increases presynaptic intracellular calcium in rat striatal synaptosomes,424, 492 indicating possible modulation of neurotransmitter release and/or other presynaptic events by both nicotinic and serotonergic receptors located on the same terminals.390, 493–495 In superfused rat slices and synaptosomes from the dorsal striatum, nicotine increases serotonin release in a dose-dependent manner,496, 497 whereas locally applied acetylcholine or nicotine (but not mAChR agonists) decreases in vivo serotonin release in the feline caudate nucleus.498 Interestingly, in the latter study, the nicotine receptor–mediated effects on serotonin release are blocked by application of a GABA receptor antagonist, implying that these effects are perhaps indirectly mediated (via unknown mechanisms) by striatal GABAergic interneurons, but not projection neurons.498 Although discrepancies exist between results of in vitro and in vivo studies, there is agreement that serotonin release in the dorsal striatum is under modulation of the nicotinic cholinergic system. It remains to be established whether this occurs in the primate dorsal striatum, as well as in the rodent and primate nucleus accumbens.
vGluT3 expression in ChIs: role in cholinergic striatal regulation
While vGluT1 and 2 are commonly found in known glutamatergic neurons, such is not the case for vGluT3 in the dorsal striatum.499, 500 In ChAT-Cre mice, some striatal ChIs have terminals enriched in vGluT3,499, 500 and activation of ChIs mediates fast vGluT3-dependent glutamatergic transmission at synapses with FSIs.500, 501 At the ultrastructural level, vGluT3 and vAChT co-exist on the surface of some synaptic vesicles, suggesting the possible co-release of glutamate and acetylcholine from single vesicles.499 In line with these observations, the disruption of the vGluT3 gene causes a hypocholinergic striatal phenotype.500, 502 The significance of these findings in the primate striatum remains to be established.
Cholinergic systems in transgenic ChAT-Cre mice
It is noteworthy that several of the aforementioned studies used optogenetic methods in dorsal and ventral striatal slices of ChAT-Cre and ChAT-ChR2-EYFP Bac transgenic mice.204, 481 However, the recent evidence of vesicular acetylcholine transporter (vAChT) overexpression, the subsequent amplified cholinergic tone and behavioral deficits (i.e., amplified drug-induced stereotypies), and the decreased density of ChAT-labeled neurons and neuropil in these mice,215, 503 raise caution about data interpretation and translation from these animals to primates.503 More information should be gathered from other transgenic mice Cre lines to determine if the baseline transmission of the neurotransmitter system under study is altered in these animals.
Striatal cholinergic system dysfunction in Parkinson’s disease and cocaine addiction
Dysfunction of the nigrostriatal and mesolimbic dopaminergic systems prompts a number of neurochemical, physiological, and behavioral changes in basal ganglia disorders, such as PD and drug dependency.307, 504–508 However, in contrast to the significant dopaminergic nigrostriatal degeneration in PD, stimulant addiction partly depends on hyperdopaminergic functions that “hijack” normal learning processes to reinforce their own acquisition, gradually leading to habitual drug seeking, loss of self-control, and relapse susceptibility.504–507, 509 At the circuit level, both disorders manifest abnormal dopaminergic regulation of striatal principal neurons, intrastriatal circuits, and striatal afferents.17, 58, 510, 511
The abnormal activity of multiple neurotransmitter systems, including the cholinergic systems, precedes or coincides with dopaminergic dysfunction in parkinsonism and drug addiction,17, 58, 510–516 suggesting that a striatal dopamine–acetylcholine imbalance underlies some aspects of the pathophysiology of parkinsonism and substance abuse.11–16, 22, 38, 217, 308, 517 However, the time point and mechanisms of this cholinergic dysfunction remain poorly understood, and the development of new therapeutic approaches aimed at cholinergic systems is limited for these disorders. Contributing factors to this shortfall likely result from the complex and diverse regulatory functions, physiological properties, and behavioral correlates of striatal ChIs discussed above, along with the limited availability of reliable and specific drugs to regulate cholinergic transmission. In the following discussion, we review and compare the changes to the striatal cholinergic systems that have been described in PD animal models (i.e., 6-hydroxydopamine (6-OHDA)–lesioned or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated rodents and monkeys) and in animals acutely or chronically exposed to cocaine. The suitability of cholinergic therapeutic interventions for PD and cocaine addiction/abuse will be considered. It is noteworthy that striatal cholinergic dysfunction has also been reported in many other brain disorders, such as Huntington’s disease, dystonia, Alzheimer’s disease).
Striatal cholinergic dysfunction in Parkinson’s disease
Because the complex interplay between the nigrostriatal dopamine and intrastriatal cholinergic transmission involves both direct and indirect routes that use various transmitter systems, the interpretation of changes in the dopamine–acetylcholine balance that occur in various animal models of PD, may be difficult. For example, although increased in vitro extracellular levels of acetylcholine380, 518, 519 and decreased AchE activity520–524 have been shown in the dorsal striatum of rodent and monkey PD models, respectively, neither the spontaneous firing rate of TANs (i.e., putative ChIs) or the level of synchronization of TAN activity is significantly altered in MPTP-treated monkeys.174, 525, 526 However, TANs display an increase in their oscillatory activity (~10–20 Hz),525–527 along with decreased sensory responsiveness to rewards,174 in the dopamine-denervated monkey dorsal striatum. In rodent models of PD, TANs exhibit an apparent reduced after-hyperpolarization current, possibly contributing to their reduced spike frequency adaptation under these conditions.519 It is not fully understood how these changes in cholinergic activity affect overall striatal function and output in parkinsonism, although a recent review considered the idea of whether an enhanced cholinergic tone is sufficient to sustain synchronous oscillatory activity of striatal projection neurons in the parkinsonian state.528
Another layer of complexity revolves around the network connectivity of ChIs in the dorsal striatum. As mentioned above, ChIs express D2 and D5 dopamine receptors.20, 297, 316–323 In parkinsonian rodent models, striatal dopamine receptors become hypersensitive, often resulting in enhanced cholinergic responsiveness to their activation in the dopamine-depleted state.11, 13, 217, 307 Thus, the altered inhibitory (via D2 receptors) and excitatory (via D5 receptors) actions of dopamine on ChIs activity might be partly responsible for striatal cholinergic changes in parkinsonism.174, 519, 525, 526 Striatal ChIs also express receptors that are co-expressed with dopamine receptors and negatively affected in PD, such as the adenosine A2A receptors, which are currently the target of multiple clinical studies.529, 530 Furthermore, anatomical and functional changes in non-dopaminergic afferents and efferents of ChIs must also be considered. For example, ChIs increase their connectivity with striatopallidal (D2-expressing) projection neurons, but decrease their interactions with striatonigral (D1-expressing) neurons after striatal dopamine depletion.531, 532 The abnormal activity of MSNs in the parkinsonian state533–535 may also indirectly contribute to changes in striatal cholinergic function536 because of the substantial connection between MSN axon collaterals and ChIs in the dorsal striatum.134 The substantial cell loss in the CM/Pf thalamic nuclei of MPTP-treated monkeys537 and PD patients538–541 and the subsequent degeneration of the thalamostriatal system, along with impaired GluN2D NMDA receptor signaling in parkinsonian rodents,542 might significantly impact the glutamatergic regulation of ChIs in parkinsonism.
Thus, the functional alterations of ChI activity in the dopamine-depleted state further highlight the importance of targeting the striatal cholinergic system in PD. However, a more complete description of the expression and function of specific cholinergic receptor subtypes in the striatum and other basal ganglia nuclei, as well as the plastic changes ChIs undergo in parkinsonism, are necessary to identify therapeutically relevant targets for motor and non-motor symptoms of PD. In addition, therapeutic agents that avoid the unwanted cognitive and autonomic side effects of the currently available, broad-spectrum cholinergic drugs, must be developed to achieve that goal.
Striatal cholinergic dysfunction in cocaine addiction
Although dopaminergic systems are antagonistically altered in PD and cocaine abuse, the self-administration of cocaine in rodents increases striatal acetylcholine release, as seen in parkinsonism. However, this effect occurs primarily in the accumbal shell (and to a lesser extent, in the core of the accumbens and the dorsal striatum), and is D1, but not D2 receptor dependent.331, 334, 543 In agreement, ChIs activation has also been reported in the accumbens shell and the ventromedial caudate putamen after acute self-administration of cocaine in rats.544 In contrast, ChIs ablation in the rodent nucleus accumbens enhances long-lasting behavioral changes, such as hyperlocomotion, associated with cocaine administration, whereas accumbal acetylcholine enhancement through AchE inhibition suppresses these cocaine-induced behaviors.545–547 Moreover, hypercholinergic rodent models of depression exhibit a reduction in their cocaine-induced responses to locomotion and dynorphin neuroadaptations.548 As recently described, the effects of cocaine abuse on cholinergic systems are highly dependent on physiological and experimental variables, such as receptor subtypes, brain region localization, drug dosage, form of administration (i.e., voluntary versus involuntary), length of use (i.e., acute versus chronic), and phase of addiction (i.e., initiation, reinforcement, withdrawal, and relapse),543, 549–553 providing possible explanations for the complex responses of the striatal cholinergic system to cocaine use. Further contribution to this complexity may be accounted for by the specific effects of dopamine on accumbal ChIs which, in contrast to dorsal striatal ChIs, also heavily express D3 dopamine receptors.324–327
The role of the striatal cholinergic system in the reward responses induced by short- and long-term use of addictive substances, such as cocaine, has not been fully delineated at this time.308, 554, 555 However, data from the dorsal striatum revealed that TANs (putative ChIs) play a role in various aspects of reward behavior associated with goal-directed and habitual learning, reinforcement, and motivation19, 149, 164, 174, 179, 180, 187, 188, 556 through their actions on dopamine spike discharge and release.204, 481 Additionally, dorsal striatal ChIs respond to sensory cues associated with reward (and non-rewards) with an initial excitation and pause in activity, followed by a rebound excitation.19 In contrast, TANs in the core or shell of the nucleus accumbens respond to specific reward parameters with either an increase or decrease in firing activity, respectively.190, 208, 209 For instance, accumbal TANs display bidirectional reinforcement responses during reward learning (i.e., increased firing after reward appearance, but paused firing upon reward omission).205 Furthermore, nigral and ventral tegmental dopaminergic afferents to the striatum respond to both the reward itself and reward-predicted stimuli with a burst in their activity.180, 505 These studies strongly suggest that accumbal and dorsal striatal ChIs likely contribute to cocaine-induced reward responses.
Ensembles of accumbal neurons with different functional properties respond to natural and unnatural rewards (including cocaine) in a variety of ways. For example, distinct temporal firing patterns of striatal neuron populations were recorded in the rodent and monkey accumbens (and dorsal striatum) in response to both cocaine and water reinforcement, implying that cocaine utilizes a similar brain reward circuitry and temporal encoding as natural rewards.197, 556–559 Currently, it is unclear if cholinergic cells are represented in these various striatal neuron populations, but on the basis of the physiological properties of dorsal striatal ChIs, their well-characterized responses to reward, and their cocaine-induced inhibition in the dorsal striatum, it seems likely that they may be involved in these responses.164, 175, 179, 180, 187, 189, 239 In line with this possibility, recent data from the medial nucleus accumbens of BAC transgenic ChAT-Cre mice demonstrated that TAN photoinhibition during cocaine place conditioning (20 mg/kg) significantly decreased preference for the cocaine-affiliated chamber, but did not alter cocaine conditioning itself.201 Furthermore, acute cocaine administration increased the in vitro spontaneous activity of ChIs.201
Electrophysiological recordings of ChIs in various sub-compartments of the nucleus accumbens must be performed to better define the firing properties of these neurons in the ventral striatum under normal conditions and after acute and chronic cocaine self-administration in rats and non-human primates. Additionally, the responses of ChIs to reward-associated cues and stimuli (unnatural versus natural) must be further characterized across subregions of the nucleus accumbens.560–562 Because neural activity changes throughout the entire striatum have been described in response to cocaine,563, 564 it may be useful for future studies to directly compare changes in TAN (putative ChIs) activity between the dorsal and ventral striatum, so that the participation of striatal TANs in the neural circuits of cocaine reward can be better understood.
Cholinergic therapies for Parkinsonism and cocaine dependency: A major challenge?
Cholinergic therapy in Parkinson’s disease
Muscarinic receptor–related drugs
Conflicting data from previous studies, along with the limited understanding of the dorsal and ventral striatal cholinergic systems and the lack of specific drugs, have hindered the progress of developing cholinergic drug therapies for the treatment of PD and drug dependency. Anti-cholinergic drugs, used alone or in conjunction with dopaminergic therapies, were utilized as one of the first treatments for PD,565–567 although their use was limited because of significant cognitive and autonomic side effects.568 More than a century later, the use of anti-cholinergic agents as PD therapeutics still remains a major challenge. Anticholinergic drugs are sometimes utilized as a supplement to dopamine-replacement therapy for PD to alleviate some of the non-motor and motor symptoms, such as bladder dysfunction and tremor.12, 565, 568 However, the long-term use of dopaminergic drugs that involves the progressive ramping up of dosages eventually leads to abnormal, involuntary movements (dyskinesias) and psychiatric complications in PD patients, while aversive cognitive and autonomic side effects are the consequences of the non-selectivity of current anti-cholinergic therapies.12, 569 As a result, the search for selective cholinergic receptor drugs that regulate striatal cholinergic activity are currently being investigated as alternative monotherapies or combination therapies in conjunction with dopaminergic agonists for PD.17,431, 432, 569–573
In that regard, selective M1 mAChR antagonists have weaker anti-parkinsonian properties than the non-selective muscarinic drugs.15, 569 In mice, the genetic deletion of M1 mAChRs partially prevents the loss of glutamatergic innervation of MSN dendritic spines in models of PD,446 while M1 receptor antagonists or ChI ablation reduce L-DOPA–induced dyskinesias in mice with striatal dopaminergic denervation.574, 575 However, their effects must be interpreted cautiously, because the M1 receptor antagonists used in these studies also partly inhibit M4 mAChRS.569 In fact, it may be more practical to regulate M2/M4 muscarinic autoreceptors of striatal ChIs to inhibit acetylcholine release from these neurons and thereby promote dopaminergic neurotransmission.11, 14, 576 On the other hand, because M4 is expressed postsynaptically in dendrites and spines of MSNs372, 410 and presynaptically in glutamatergic striatal afferents, it could be beneficial to also regulate these interactions for the attenuation of PD symptoms. In agreement, studies have demonstrated that M4 mAChR mRNA expression and autoreceptor signaling that normally regulate ChIs spiking and acetylcholine release are significantly reduced in the dorsal striatum of dopamine-depleted rodents,380, 577 further supporting the need for preclinical testing of these drugs in reliable animal models of PD. Thus, the development of highly specific M1- and M4-related drugs is necessary to directly test these hypotheses.
Nicotinic receptor–related drugs
Another potential cholinergic target for PD are the nAChRs expressed by striatal neurons (data inconclusive for ChIs) and nigrostriatal terminals.516 Drugs aimed at α4β2 and α6β2 nicotinic receptors, indeed, exposed potential neuroprotective (for midbrain dopaminergic neurons), anti-parkinsonian, and anti-dyskinetic effects in animal models of PD.12, 346, 467, 469, 480, 578 Numerous studies have demonstrated a drastic loss of α6β2 nicotinic receptors (primarily responsible for acetylcholine-induced dopamine release in primates) in parkinsonian animal models and PD patients, along with a less severe reduction in α4β2 receptor subtypes.346, 467, 469, 579, 580 Because of their localization on dopaminergic terminals, striatal α4α6β2-expressing nAChRs are significantly reduced after nigrostriatal damage,469 leaving only α4β2- and α6β2-expressing non-dopaminergic neurons to regulate acetylcholine-induced dopamine release in the striatum of late-stage parkinsonism. In partially dopamine-depleted parkinsonian rodents and monkeys, nicotine synergistically acting on α4β2 and α6β2 nAChRs decrease abnormal, involuntary movements induced by commonly used dopaminergic therapies.516 Therefore, PD therapy aimed at nAChRs could be beneficial, particularly if used as an early treatment strategy at a time when nicotinic agonists acting at the α6β2 (and possibly α4β2) subtype could still induce dopamine release from intact dopaminergic axons. Further electrophysiological and pharmacological studies using specific nicotinic-related drugs are needed to help clarify these uncertainties.
Cholinergic therapy for cocaine addiction
As for parkinsonism, studies of therapeutic interventions for stimulant addiction have revolved mainly around dopaminergic therapies, because of the compelling evidence that excessive dopamine release and abnormal mesolimbic activity occur with cocaine use and addiction.507, 581–588 However, therapies aimed solely at the dopaminergic system in cocaine-addicted individuals were not found to be successful,589–591 possibly due to the fact that cocaine dependency affects diverse neurotransmitter systems beyond the dopaminergic mesostriatal projection.24, 505, 592 Due to the tight dopamine–acetylcholine relationship and abnormal cholinergic activity associated with cocaine abuse in the nucleus accumbens, research on possible cholinergic interventions has also been undertaken. As in PD, anti-cholinergic drugs were first assessed for their efficacy in alleviating stimulant addiction in animal models, on the basis of their potential to possibly reverse increased striatal acetylcholine release after cocaine use. However, because many anti-cholinergic drugs actually enhance the effects of cocaine,471, 593–598 the effectiveness of cholinergic agonists/partial agonists on the abuse-related effects of cocaine are being examined.393
The expression of M1-like and M2-like cholinergic receptors is either upregulated, downregulated, or unchanged in response to cocaine use, mirroring the complexity of D1-like and D2-like dopamine receptor changes observed with cocaine self-administration.16 On the basis that acetylcholine may enhance the abuse-related properties of cocaine, pharmacological studies first attempted to assess the effects of anti-muscarinic and anti-nicotinic drugs on cocaine abuse in animals.16 These studies revealed that mAChR antagonists dose-dependently decrease cocaine self-administration and reinstatement in rats and monkeys599, 600 and that cocaine conditioned place preference is significantly reduced in M5 mAChR knock-out mice.601
Similarly, nicotinic receptor blockade prevents the escalation of cocaine self-administration in rats with extended daily access, but does not block the actual drug intake.602 In humans, a nicotine receptor antagonist, mecamylamine, reduces cocaine-induced craving,603 supporting preclinical findings of decreased nicotine and cocaine self-administration in mice treated with this drug.604, 605 The co-administration of α7 and β2 nicotinic receptor antagonists or mecamylamine also prevents the development of cocaine-induced increases in dopamine release on repetitive cocaine injections (i.e., sensitization) in mice.554 In addition, nicotine application in cocaine-dependent rats enhances cocaine-seeking behavior and potentiates cocaine reinforcement,606 while stimulating cue-elicited craving in humans.607
Because the systemic administration of nonselective muscarinic antagonists enhances cocaine-induced effects,471, 593–598, 608 systemically-injected muscarinic agonists/partial agonists were also assessed for their ability to minimize cocaine dependency. Nonselective muscarinic agonists, such as oxotremorine and pilocarpine, xanomeline, as well as M1-specific muscarinic agonists, block chronic cocaine self-administration and decrease cocaine discrimination in mice.608 Furthermore, an M4 positive allosteric agonist blocks cocaine-induced dopamine release in both the dorsal and ventral striatum of wild-type mice.609 However, dissimilar findings between the dorsal and ventral striatum were recently observed in studies examining cocaine-induced effects in M4 muscarinic receptor knock-out mice. Cocaine injections increase dopamine release in the nucleus accumbens, but not in the dorsal striatum, of mice with an M4 receptor gene deletion.609, 610 It was hypothesized that M2 receptors uniquely located on ChIs in the dorsal, but not the ventral, striatum compensate for changes in dopamine levels observed after cocaine injections in M4 receptor knock-out mice.609, 610 Thus, M4 receptors expressed by ChIs in the nucleus accumbens may have a greater role in regulating dopamine transmission than those in the dorsal striatum,576, 609 and striatal ChIs may regulate dopaminergic activity via M2 in the dorsal striatum but M4 in the ventral striatum.
Altogether, these unexpected neuroadaptations in the cholinergic system in response to cocaine use may possibly be due to the complex heterogeneity of the dopamine–acetylcholine interactions in the nucleus accumbens, along with different accumbal regions examined in these studies. Alternatively, ChIs may undergo plastic changes (i.e., increased synapses per neuron (including glutamatergic asymmetric synapses)) and larger dendritic branching, after repeated cocaine self-administration, which has been extensively demonstrated for spiny and aspiny neurons in the core and shell of the accumbens.611–614
Although still poorly characterized, the potential for the use of cholinergic therapy in addiction remains of interest, particularly with the development of more selective muscarinic and nicotinic receptor agonists/antagonists.392, 393, 432, 573, 608, 615, 616 Specific positive allosteric modulators for M1, M2, M4, and M5 have now been developed and proven to be highly selective for their receptor subtypes.570, 571, 617, 618 The therapeutic assessment of these drugs alone, or in combination with dopaminergic receptor antagonists, should be explored in normal and cocaine-dependent animal models to further define the underlying roles and therapeutic relevance of specific cholinergic receptor subtypes in cocaine (or other psychostimulants) abuse and dependency.
Concluding remarks
In conclusion, the findings discussed in this review highlight the morphological heterogeneity of ChIs between species and functional regions of the striatum. Thus, in contrast to the common view that ChIs represent a functionally uniform population of neurons, in vivo and in vitro evidence clearly indicates that dorsal and ventral striatal regions contain different populations of ChIs. In addition to striking morphological differences, these neurons are differently regulated by extrinsic afferents and mediate some of their effects through different cholinergic receptor subtypes. Remarkably, striatal acetylcholine release increases in parkinsonism380, 518, 519 and after cocaine self-administration,331, 334, 543 although the dopaminergic mesostriatal system undergoes contrasting damage in these disorders. However, broad-spectrum anti-cholinergic drugs often induce negative side effects in parkinsonism and exacerbate the addictive behavioral properties of cocaine. Therefore, alternative therapeutic interventions that selectively target muscarinic or nicotinic receptors and their receptor subunits may be more effective in providing some relief for these disorders. In addition, because other neurotransmitter systems, such as those that utilize glutamate, opioids, endocannabinoids, or neuropeptides, also undergo significant alterations in parkinsonism and stimulant addiction,529, 619–621 the future development of drugs that preferentially target these non-dopaminergic systems could also allow for the indirect regulation of ChI activity in the dorsal and ventral striatum.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Rebrferences
- 1.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 2.Wichmann T, Delong MR. Anatomy and physiology of the basal ganglia: relevance to Parkinson’s disease and related disorders. Handb Clin Neurol. 2007;83:1–18. doi: 10.1016/S0072-9752(07)83001-6. [DOI] [PubMed] [Google Scholar]
- 3.Leisman G, Melillo R. The basal ganglia: motor and cognitive relationships in a clinical neurobehavioral context. Rev Neurosci. 2013;24:9–25. doi: 10.1515/revneuro-2012-0067. [DOI] [PubMed] [Google Scholar]
- 4.Post RM, Kalivas P. Bipolar disorder and substance misuse: pathological and therapeutic implications of their comorbidity and cross-sensitisation. Br J Psychiatry. 2013;202:172–176. doi: 10.1192/bjp.bp.112.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 6.Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Bernacer J, Prensa L, Gimenez-Amaya JM. Cholinergic interneurons are differentially distributed in the human striatum. PLoS ONE. 2007;2:e1174. doi: 10.1371/journal.pone.0001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernácer J, Prensa L, Giménez-Amaya JM. Distribution of GABAergic interneurons and dopaminergic cells in the functional territories of the human striatum. PLoS One. 2012;7:e30504. doi: 10.1371/journal.pone.0030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327:307–311. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Parent A. Striatal interneurons expressing calretinin, parvalbumin or NADPH-diaphorase: a comparative study in the rat, monkey and human. Brain Res. 2000;863:182–191. doi: 10.1016/s0006-8993(00)02135-1. [DOI] [PubMed] [Google Scholar]
- 11.Pisani A, et al. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Lester DB, Rogers TD, Blaha CD. Acetylcholine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:137–162. doi: 10.1111/j.1755-5949.2010.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonsi P, et al. Centrality of striatal cholinergic transmission in Basal Ganglia function. Front Neuroanat. 2011;5:6. doi: 10.3389/fnana.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Threlfell S, Cragg SJ. Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Syst Neurosci. 2011;5:11. doi: 10.3389/fnsys.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg JA, Ding JB, Surmeier DJ. Muscarinic modulation of striatal function and circuitry. Handb Exp Pharmacol. 2012:223–241. doi: 10.1007/978-3-642-23274-9_10. [DOI] [PubMed] [Google Scholar]
- 16.Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–1797. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brichta L, Greengard P, Flajolet M. Advances in the pharmacological treatment of Parkinson’s disease: targeting neurotransmitter systems. Trends Neurosci. 2013;36:543–554. doi: 10.1016/j.tins.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Scarr E, et al. Cholinergic connectivity: it’s implications for psychiatric disorders. Front Cell Neurosci. 2013;7:55. doi: 10.3389/fncel.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz JM, Reynolds JN. Pause and rebound: sensory control of cholinergic signaling in the striatum. Trends Neurosci. 2013;36:41–50. doi: 10.1016/j.tins.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg JA, Reynolds JN. Spontaneous firing and evoked pauses in the tonically active cholinergic interneurons of the striatum. Neuroscience. 2011;198:27–43. doi: 10.1016/j.neuroscience.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 22.Mark GP, et al. Cholinergic modulation of mesolimbic dopamine function and reward. Physiol Behav. 2011;104:76–81. doi: 10.1016/j.physbeh.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenz JD, Lobo MK. Optogenetic insights into striatal function and behavior. Behav Brain Res. 2013;255:44–54. doi: 10.1016/j.bbr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Redgrave P, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parent A. Comparative Neurobiology of the Basal Ganglia. Wiley-Interscience; New York: 1986. [Google Scholar]
- 28.Heimer L, et al. The human basal forebrain. Part II. In: Bloom FE, Bjorkland A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Elsevier; Amsterdam: 1999. pp. 57–226. [Google Scholar]
- 29.Meredith GE, et al. Shell and core in monkey and human nucleus accumbens identified with antibodies to calbindin-D28k. J Comp Neurol. 1996;365:628–639. doi: 10.1002/(SICI)1096-9861(19960219)365:4<628::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Voorn P, et al. Densitometrical analysis of opioid receptor ligand binding in the human striatum--I. Distribution of mu opioid receptor defines shell and core of the ventral striatum. Neuroscience. 1996;75:777–792. doi: 10.1016/0306-4522(96)00271-0. [DOI] [PubMed] [Google Scholar]
- 31.Prensa L, Richard S, Parent A. Chemical anatomy of the human ventral striatum and adjacent basal forebrain structures. J Comp Neurol. 2003;460:345–367. doi: 10.1002/cne.10627. [DOI] [PubMed] [Google Scholar]
- 32.Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- 33.Graybiel AM, Ragsdale CW. Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci U S A. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- 35.Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: immunohistochemical distribution of enkephalin, substance P, dopamine, and calcium-binding protein. J Comp Neurol. 1989;289:189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- 36.Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- 37.Mikula S, et al. Complete 3D visualization of primate striosomes by KChIP1 immunostaining. J Comp Neurol. 2009;514:507–517. doi: 10.1002/cne.22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci. 1995;15:5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russchen FT, et al. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 1985;329:241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- 41.Fudge JL, et al. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110:257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- 42.Voorn P, et al. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Groenewegen HJ, et al. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 44.Haber S. Neuroanatomy of reward: a view from the ventral striatum. In: Gottfried J, editor. Neurobiology of Sensation and Reward. CRC Press; Boca Raton, FL: 2011. [PubMed] [Google Scholar]
- 45.Voorn P, et al. Evidence for two neurochemical divisions in the human nucleus accumbens. Eur J Neurosci. 1994;6:1913–1916. doi: 10.1111/j.1460-9568.1994.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 46.Haber SN, et al. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ikemoto K, et al. Neurochemical heterogeneity of the primate nucleus accumbens. Exp Brain Res. 1995;104:177–190. doi: 10.1007/BF00242004. [DOI] [PubMed] [Google Scholar]
- 48.Ikemoto K, et al. The distribution of noradrenaline, serotonin and gamma-aminobutyric acid in the monkey nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:1403–1412. doi: 10.1016/s0278-5846(96)00135-2. [DOI] [PubMed] [Google Scholar]
- 49.Brauer K, et al. The core-shell dichotomy of nucleus accumbens in the rhesus monkey as revealed by double-immunofluorescence and morphology of cholinergic interneurons. Brain Res. 2000;858:151–162. doi: 10.1016/s0006-8993(00)01938-7. [DOI] [PubMed] [Google Scholar]
- 50.Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol. 1990;299:187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- 51.McFarland NR, Haber SN. Organization of thalamostriatal terminals from the ventral motor nuclei in the macaque. J Comp Neurol. 2001;429:321–336. doi: 10.1002/1096-9861(20000108)429:2<321::aid-cne11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 52.Smith Y, et al. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Herkenham M, Pert CB. Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature. 1981;291:415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- 54.Ragsdale CW, Graybiel AM. Compartmental organization of the thalamostriatal connection in the cat. J Comp Neurol. 1991;311:134–167. doi: 10.1002/cne.903110110. [DOI] [PubMed] [Google Scholar]
- 55.Berendse HW, et al. Nuclear origin of thalamic afferents of the ventral striatum determines their relation to patch/matrix configurations in enkephalin-immunoreactivity in the rat. J Chem Neuroanat. 1988;1:3–10. [PubMed] [Google Scholar]
- 56.Giménez-Amaya JM, et al. Organization of thalamic projections to the ventral striatum in the primate. J Comp Neurol. 1995;354:127–149. doi: 10.1002/cne.903540109. [DOI] [PubMed] [Google Scholar]
- 57.Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508:212–237. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- 58.Galvan A, Smith Y. The primate thalamostriatal systems: Anatomical organization, functional roles and possible involvement in Parkinson’s disease. Basal Ganglia. 2011;1:179–189. doi: 10.1016/j.baga.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith Y, et al. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009;78:60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63:363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- 61.Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus) Neuroscience. 1986;18:347–371. doi: 10.1016/0306-4522(86)90159-4. [DOI] [PubMed] [Google Scholar]
- 63.Lynd-Balta E, Haber SN. The organization of midbrain projections to the striatum in the primate: sensorimotor-related striatum versus ventral striatum. Neuroscience. 1994;59:625–640. doi: 10.1016/0306-4522(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 64.Lynd-Balta E, Haber SN. The organization of midbrain projections to the ventral striatum in the primate. Neuroscience. 1994;59:609–623. doi: 10.1016/0306-4522(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 65.Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol. 1997;11:323–342. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- 66.Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- 67.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jimenez-Castellanos J, Graybiel AM. Subdivisions of the dopamine-containing A8-A9-A10 complex identified by their differential mesostriatal innervation of striosomes and extrastriosomal matrix. Neuroscience. 1987;23:223–242. doi: 10.1016/0306-4522(87)90285-5. [DOI] [PubMed] [Google Scholar]
- 70.Matsuda W, et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jimenez-Castellanos J, Graybiel AM. Evidence that histochemically distinct zones of the primate substantia nigra pars compacta are related to patterned distributions of nigrostriatal projection neurons and striatonigral fibers. Exp Brain Res. 1989;74:227–238. doi: 10.1007/BF00248855. [DOI] [PubMed] [Google Scholar]
- 72.Jimenez-Castellanos J, Graybiel AM. Subdivisions of the primate substantia nigra pars compacta detected by acetylcholinesterase histochemisty. Brain Res. 1987;437:349–354. doi: 10.1016/0006-8993(87)91650-7. [DOI] [PubMed] [Google Scholar]
- 73.Langer LF, Jiménez-Castellanos J, Graybiel AM. The substantia nigra and its relations with the striatum in the monkey. Prog Brain Res. 1991;87:81–99. doi: 10.1016/s0079-6123(08)63048-4. [DOI] [PubMed] [Google Scholar]
- 74.Langer LF, Graybiel AM. Distinct nigrostriatal projection systems innervate striosomes and matrix in the primate striatum. Brain Res. 1989;498:344–350. doi: 10.1016/0006-8993(89)91114-1. [DOI] [PubMed] [Google Scholar]
- 75.Prensa L, Parent A. The nigrostriatal pathway in the rat: A single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J Neurosci. 2001;21:7247–7260. doi: 10.1523/JNEUROSCI.21-18-07247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dautan D, et al. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci. 2014;34:4509–4518. doi: 10.1523/JNEUROSCI.5071-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saper CB, Loewy AD. Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. Brain Res. 1982;252:367–372. doi: 10.1016/0006-8993(82)90404-8. [DOI] [PubMed] [Google Scholar]
- 78.Hallanger AE, Wainer BH. Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;274:483–515. doi: 10.1002/cne.902740403. [DOI] [PubMed] [Google Scholar]
- 79.Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- 80.Nakano K, et al. Topographical projections from the thalamus, subthalamic nucleus and pedunculopontine tegmental nucleus to the striatum in the Japanese monkey, Macaca fuscata. Brain Res. 1990;537:54–68. doi: 10.1016/0006-8993(90)90339-d. [DOI] [PubMed] [Google Scholar]
- 81.Wainer BH, et al. Cholinergic synapses in the rat brain: a correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Res. 1984;308:69–76. doi: 10.1016/0006-8993(84)90918-1. [DOI] [PubMed] [Google Scholar]
- 82.DiFiglia M. Synaptic organization of cholinergic neurons in the monkey neostriatum. J Comp Neurol. 1987;255:245–258. doi: 10.1002/cne.902550208. [DOI] [PubMed] [Google Scholar]
- 83.Ligorio M, Descarries L, Warren RA. Cholinergic innervation and thalamic input in rat nucleus accumbens. J Chem Neuroanat. 2009;37:33–45. doi: 10.1016/j.jchemneu.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 84.Phelps PE, Vaughn JE. Immunocytochemical localization of choline acetyltransferase in rat ventral striatum: a light and electron microscopic study. J Neurocytol. 1986;15:595–617. doi: 10.1007/BF01611860. [DOI] [PubMed] [Google Scholar]
- 85.Phelps PE, Houser CR, Vaughn JE. Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J Comp Neurol. 1985;238:286–307. doi: 10.1002/cne.902380305. [DOI] [PubMed] [Google Scholar]
- 86.Beckstead RM. A pallidostriatal projection in the cat and monkey. Brain Res Bull. 1983;11:629–632. doi: 10.1016/0361-9230(83)90003-5. [DOI] [PubMed] [Google Scholar]
- 87.Kita H, Tokuno H, Nambu A. Monkey globus pallidus external segment neurons projecting to the neostriatum. Neuroreport. 1999;10:1467–1472. doi: 10.1097/00001756-199905140-00014. [DOI] [PubMed] [Google Scholar]
- 88.Mallet N, et al. Dichotomous organization of the external globus pallidus. Neuron. 2012;74:1075–1086. doi: 10.1016/j.neuron.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hakan RL, Berg GI, Henriksen SJ. Electrophysiological evidence for reciprocal connectivity between the nucleus accumbens septi and ventral pallidal region. Brain Res. 1992;581:344–350. doi: 10.1016/0006-8993(92)90730-w. [DOI] [PubMed] [Google Scholar]
- 90.Kuo H, Chang HT. Ventral pallido-striatal pathway in the rat brain: a light and electron microscopic study. J Comp Neurol. 1992;321:626–636. doi: 10.1002/cne.903210409. [DOI] [PubMed] [Google Scholar]
- 91.Brog JS, et al. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 92.Parent M, et al. Serotonin innervation of basal ganglia in monkeys and humans. J Chem Neuroanat. 2011;41:256–265. doi: 10.1016/j.jchemneu.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 93.Petryszyn S, et al. Distribution and morphological characteristics of striatal interneurons expressing calretinin in mice: a comparison with human and nonhuman primates. J Chem Neuroanat. 2014;59–60:51–61. doi: 10.1016/j.jchemneu.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Delfs JM, et al. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- 95.Tong J, Hornykiewicz O, Kish SJ. Inverse relationship between brain noradrenaline level and dopamine loss in Parkinson disease: a possible neuroprotective role for noradrenaline. Arch Neurol. 2006;63:1724–1728. doi: 10.1001/archneur.63.12.1724. [DOI] [PubMed] [Google Scholar]
- 96.Köhler C, et al. The cytoarchitecture, histochemistry and projections of the tuberomammillary nucleus in the rat. Neuroscience. 1985;16:85–110. doi: 10.1016/0306-4522(85)90049-1. [DOI] [PubMed] [Google Scholar]
- 97.Ellender TJ, et al. Differential modulation of excitatory and inhibitory striatal synaptic transmission by histamine. J Neurosci. 2011;31:15340–15351. doi: 10.1523/JNEUROSCI.3144-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Groenewegen HJ, Berendse HW. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. J Comp Neurol. 1990;294:607–622. doi: 10.1002/cne.902940408. [DOI] [PubMed] [Google Scholar]
- 99.Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. The Journal of comparative neurology. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- 100.Oorschot D. The percentage of interneurons in the dorsal striatum of the rat, cat, monkey and human: A critique of the evidence. Basal Ganglia. 2013;3:19–24. [Google Scholar]
- 101.Gerfen CR, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science (New York, NY) 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 102.Aubert I, et al. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. The Journal of comparative neurology. 2000;418:22–32. [PubMed] [Google Scholar]
- 103.Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res. 1997;27:1–8. doi: 10.1016/s0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- 105.Lévesque M, et al. Novel aspects of the chemical anatomy of the striatum and its efferents projections. J Chem Neuroanat. 2003;26:271–281. doi: 10.1016/j.jchemneu.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 106.Talbot K, Woolf NJ, Butcher LL. Feline islands of Calleja complex: I. Cytoarchitectural organization and comparative anatomy. J Comp Neurol. 1988;275:553–579. doi: 10.1002/cne.902750406. [DOI] [PubMed] [Google Scholar]
- 107.Meyer G, et al. Aggregations of granule cells in the basal forebrain (islands of Calleja): Golgi and cytoarchitectonic study in different mammals, including man. J Comp Neurol. 1989;284:405–428. doi: 10.1002/cne.902840308. [DOI] [PubMed] [Google Scholar]
- 108.Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 109.Gangarossa G, et al. Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits. 2013;7:22. doi: 10.3389/fncir.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith RJ, et al. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haber SN, et al. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. J Comp Neurol. 1990;293:282–298. doi: 10.1002/cne.902930210. [DOI] [PubMed] [Google Scholar]
- 112.Heimer L, et al. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 113.Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- 114.Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- 115.Betarbet R, et al. Dopaminergic neurons intrinsic to the primate striatum. J Neurosci. 1997;17:6761–6768. doi: 10.1523/JNEUROSCI.17-17-06761.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- 117.Mazloom M, Smith Y. Synaptic microcircuitry of tyrosine hydroxylase-containing neurons and terminals in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkeys. J Comp Neurol. 2006;495:453–469. doi: 10.1002/cne.20894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ibáñez-Sandoval O, et al. Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum. J Neurosci. 2010;30:6999–7016. doi: 10.1523/JNEUROSCI.5996-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tepper JM, et al. Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat. 2010;4:150. doi: 10.3389/fnana.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma Y, et al. Morphological diversity of GABAergic and cholinergic interneurons in the striatal dorsolateral and ventromedial regions of rats. Cell Mol Neurobiol. 2014;34:351–359. doi: 10.1007/s10571-013-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Selden N, et al. Human striatum: chemoarchitecture of the caudate nucleus, putamen and ventral striatum in health and Alzheimer’s disease. Neuroscience. 1994;60:621–636. doi: 10.1016/0306-4522(94)90491-x. [DOI] [PubMed] [Google Scholar]
- 122.Cicchetti F, Beach TG, Parent A. Chemical phenotype of calretinin interneurons in the human striatum. Synapse. 1998;30:284–297. doi: 10.1002/(SICI)1098-2396(199811)30:3<284::AID-SYN6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 123.Mesulam MM, et al. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience. 1984;12:669–686. doi: 10.1016/0306-4522(84)90163-5. [DOI] [PubMed] [Google Scholar]
- 124.Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- 125.Bolam JP, Ingham CA, Smith AD. The section-Golgi-impregnation procedure--3. Combination of Golgi-impregnation with enzyme histochemistry and electron microscopy to characterize acetylcholinesterase-containing neurons in the rat neostriatum. Neuroscience. 1984;12:687–709. doi: 10.1016/0306-4522(84)90164-7. [DOI] [PubMed] [Google Scholar]
- 126.Woolf NJ, Butcher LL. Cholinergic neurons in the caudate-putamen complex proper are intrinsically organized: a combined Evans blue and acetylcholinesterase analysis. Brain Res Bull. 1981;7:487–507. doi: 10.1016/0361-9230(81)90004-6. [DOI] [PubMed] [Google Scholar]
- 127.Yelnik J, et al. Cholinergic neurons of the rat and primate striatum are morphologically different. Prog Brain Res. 1993;99:25–34. doi: 10.1016/s0079-6123(08)61336-9. [DOI] [PubMed] [Google Scholar]
- 128.Takagi H, Somogyi P, Smith AD. Aspiny neurons and their local axons in the neostriatum of the rat: a correlated light and electron microscopic study of Golgi-impregnated material. J Neurocytol. 1984;13:239–265. doi: 10.1007/BF01148118. [DOI] [PubMed] [Google Scholar]
- 129.Yelnik J, et al. Morphological taxonomy of the neurons of the primate striatum. J Comp Neurol. 1991;313:273–294. doi: 10.1002/cne.903130207. [DOI] [PubMed] [Google Scholar]
- 130.Dimova R, et al. Ultrastructural features of the choline acetyltransferase-containing neurons and relationships with nigral dopaminergic and cortical afferent pathways in the rat striatum. Neuroscience. 1993;53:1059–1071. doi: 10.1016/0306-4522(93)90489-3. [DOI] [PubMed] [Google Scholar]
- 131.DiFiglia M, Carey J. Large neurons in the primate neostriatum examined with the combined Golgi-electron microscopic method. J Comp Neurol. 1986;244:36–52. doi: 10.1002/cne.902440104. [DOI] [PubMed] [Google Scholar]
- 132.Difiglia M, Pasik T, Pasik P. Ultrastructure of Golgi-impregnated and gold-toned spiny and aspiny neurons in the monkey neostriatum. J Neurocytol. 1980;9:471–492. doi: 10.1007/BF01204837. [DOI] [PubMed] [Google Scholar]
- 133.Graveland GA, Williams RS, DiFiglia M. A Golgi study of the human neostriatum: neurons and afferent fibers. J Comp Neurol. 1985;234:317–333. doi: 10.1002/cne.902340304. [DOI] [PubMed] [Google Scholar]
- 134.Gonzales KK, et al. GABAergic inputs from direct and indirect striatal projection neurons onto cholinergic interneurons in the primate putamen. J Comp Neurol. 2013 doi: 10.1002/cne.23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kosaka T, Tauchi M, Dahl JL. Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res. 1988;70:605–617. doi: 10.1007/BF00247609. [DOI] [PubMed] [Google Scholar]
- 136.Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:375–386. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Satoh K, et al. Ultrastructural observations of the cholinergic neuron in the rat striatum as identified by acetylcholinesterase pharmacohistochemistry. Neuroscience. 1983;10:1121–1136. doi: 10.1016/0306-4522(83)90103-3. [DOI] [PubMed] [Google Scholar]
- 138.Contant C, et al. Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience. 1996;71:937–947. doi: 10.1016/0306-4522(95)00507-2. [DOI] [PubMed] [Google Scholar]
- 139.Massouh M, et al. The fate of the large striatal interneurons expressing calretinin in Huntington’s disease. Neurosci Res. 2008;62:216–224. doi: 10.1016/j.neures.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 140.Hirsch EC, et al. Striosomes and extrastriosomal matrix contain different amounts of immunoreactive choline acetyltransferase in the human striatum. Neurosci Lett. 1989;96:145–150. doi: 10.1016/0304-3940(89)90048-7. [DOI] [PubMed] [Google Scholar]
- 141.Kubota Y, Kawaguchi Y. Spatial distributions of chemically identified intrinsic neurons in relation to patch and matrix compartments of rat neostriatum. J Comp Neurol. 1993;332:499–513. doi: 10.1002/cne.903320409. [DOI] [PubMed] [Google Scholar]
- 142.Holt DJ, Hersh LB, Saper CB. Cholinergic innervation in the human striatum: a three-compartment model. Neuroscience. 1996;74:67–87. doi: 10.1016/0306-4522(96)00094-2. [DOI] [PubMed] [Google Scholar]
- 143.Graybiel AM, Baughman RW, Eckenstein F. Cholinergic neuropil of the striatum observes striosomal boundaries. Nature. 1986;323:625–627. doi: 10.1038/323625a0. [DOI] [PubMed] [Google Scholar]
- 144.Meredith GE, Blank B, Groenewegen HJ. The distribution and compartmental organization of the cholinergic neurons in nucleus accumbens of the rat. Neuroscience. 1989;31:327–345. doi: 10.1016/0306-4522(89)90377-1. [DOI] [PubMed] [Google Scholar]
- 145.Lehéricy S, et al. Selective loss of cholinergic neurons in the ventral striatum of patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:8580–8584. doi: 10.1073/pnas.86.21.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heimer L. Basal forebrain in the context of schizophrenia. Brain Res Brain Res Rev. 2000;31:205–235. doi: 10.1016/s0165-0173(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 147.Talbot K, Woolf NJ, Butcher LL. Feline islands of Calleja complex: II. Cholinergic and cholinesterasic features. J Comp Neurol. 1988;275:580–603. doi: 10.1002/cne.902750407. [DOI] [PubMed] [Google Scholar]
- 148.Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;73:1234–1252. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- 150.Reynolds JN, Hyland BI, Wickens JR. Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J Neurosci. 2004;24:9870–9877. doi: 10.1523/JNEUROSCI.3225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Inokawa H, et al. Juxtacellular labeling of tonically active neurons and phasically active neurons in the rat striatum. Neuroscience. 2010;168:395–404. doi: 10.1016/j.neuroscience.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 152.Doig NM, et al. Cortical and thalamic excitation mediate the multiphasic responses of striatal cholinergic interneurons to motivationally salient stimuli. J Neurosci. 2014;34:3101–3117. doi: 10.1523/JNEUROSCI.4627-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kawaguchi Y, et al. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 154.Pisani A, et al. Role of tonically-active neurons in the control of striatal function: cellular mechanisms and behavioral correlates. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:211–230. doi: 10.1016/s0278-5846(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 155.Plenz D, Aertsen A. Neural dynamics in cortex-striatum co-cultures--I. anatomy and electrophysiology of neuronal cell types. Neuroscience. 1996;70:861–891. doi: 10.1016/0306-4522(95)00406-8. [DOI] [PubMed] [Google Scholar]
- 156.Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kawaguchi Y. Large aspiny cells in the matrix of the rat neostriatum in vitro: physiological identification, relation to the compartments and excitatory postsynaptic currents. J Neurophysiol. 1992;67:1669–1682. doi: 10.1152/jn.1992.67.6.1669. [DOI] [PubMed] [Google Scholar]
- 158.Schulz JM, Oswald MJ, Reynolds JN. Visual-induced excitation leads to firing pauses in striatal cholinergic interneurons. J Neurosci. 2011;31:11133–11143. doi: 10.1523/JNEUROSCI.0661-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bishop GA, Chang HT, Kitai ST. Morphological and physiological properties of neostriatal neurons: an intracellular horseradish peroxidase study in the rat. Neuroscience. 1982;7:179–191. doi: 10.1016/0306-4522(82)90159-2. [DOI] [PubMed] [Google Scholar]
- 160.Bennett BD, Wilson CJ. Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci. 1999;19:5586–5596. doi: 10.1523/JNEUROSCI.19-13-05586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bennett BD, Wilson CJ. Synaptic regulation of action potential timing in neostriatal cholinergic interneurons. J Neurosci. 1998;18:8539–8549. doi: 10.1523/JNEUROSCI.18-20-08539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Suzuki T, et al. Dopamine-dependent synaptic plasticity in the striatal cholinergic interneurons. J Neurosci. 2001;21:6492–6501. doi: 10.1523/JNEUROSCI.21-17-06492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schulz JM, et al. Enhanced high-frequency membrane potential fluctuations control spike output in striatal fast-spiking interneurones in vivo. J Physiol. 2011;589:4365–4381. doi: 10.1113/jphysiol.2011.212944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aosaki T, et al. Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wilson CJ. The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron. 2005;45:575–585. doi: 10.1016/j.neuron.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 167.Goldberg JA, Wilson CJ. Control of spontaneous firing patterns by the selective coupling of calcium currents to calcium-activated potassium currents in striatal cholinergic interneurons. J Neurosci. 2005;25:10230–10238. doi: 10.1523/JNEUROSCI.2734-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beatty JA, Song SC, Wilson CJ. Cell-type-specific resonances shape the responses of striatal neurons to synaptic input. J Neurophysiol. 2015;113:688–700. doi: 10.1152/jn.00827.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wilson CJ, Goldberg JA. Origin of the slow afterhyperpolarization and slow rhythmic bursting in striatal cholinergic interneurons. J Neurophysiol. 2006;95:196–204. doi: 10.1152/jn.00630.2005. [DOI] [PubMed] [Google Scholar]
- 170.Oswald MJ, et al. IH current generates the afterhyperpolarisation following activation of subthreshold cortical synaptic inputs to striatal cholinergic interneurons. J Physiol. 2009;587:5879–5897. doi: 10.1113/jphysiol.2009.177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Goldberg JA, et al. Nonequilibrium calcium dynamics regulate the autonomous firing pattern of rat striatal cholinergic interneurons. J Neurosci. 2009;29:8396–8407. doi: 10.1523/JNEUROSCI.5582-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Calabresi P, et al. Muscarinic IPSPs in rat striatal cholinergic interneurones. J Physiol. 1998;510(Pt 2):421–427. doi: 10.1111/j.1469-7793.1998.421bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee K, et al. Identification of an ATP-sensitive potassium channel current in rat striatal cholinergic interneurones. J Physiol. 1998;510(Pt 2):441–453. doi: 10.1111/j.1469-7793.1998.441bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265:412–415. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- 175.Kimura M, Rajkowski J, Evarts E. Tonically discharging putamen neurons exhibit set-dependent responses. Proc Natl Acad Sci U S A. 1984;81:4998–5001. doi: 10.1073/pnas.81.15.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Graybiel AM, et al. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 177.Apicella P, Legallet E, Trouche E. Responses of tonically discharging neurons in the monkey striatum to primary rewards delivered during different behavioral states. Exp Brain Res. 1997;116:456–466. doi: 10.1007/pl00005773. [DOI] [PubMed] [Google Scholar]
- 178.Apicella P, Legallet E, Trouche E. Responses of tonically discharging neurons in monkey striatum to visual stimuli presented under passive conditions and during task performance. Neurosci Lett. 1996;203:147–150. doi: 10.1016/0304-3940(96)12328-4. [DOI] [PubMed] [Google Scholar]
- 179.Matsumoto N, et al. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J Neurophysiol. 2001;85:960–976. doi: 10.1152/jn.2001.85.2.960. [DOI] [PubMed] [Google Scholar]
- 180.Morris G, et al. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 181.Nanda B, et al. Effects of stimulation of the centromedian nucleus of the thalamus on the activity of striatal cells in awake rhesus monkeys. Eur J Neurosci. 2009;29:588–598. doi: 10.1111/j.1460-9568.2008.06598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nambu A, et al. Organization of corticostriatal motor inputs in monkey putamen. J Neurophysiol. 2002;88:1830–1842. doi: 10.1152/jn.2002.88.4.1830. [DOI] [PubMed] [Google Scholar]
- 183.Apicella P, Scarnati E, Schultz W. Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp Brain Res. 1991;84:672–675. doi: 10.1007/BF00230981. [DOI] [PubMed] [Google Scholar]
- 184.Adler A, et al. Encoding by synchronization in the primate striatum. J Neurosci. 2013;33:4854–4866. doi: 10.1523/JNEUROSCI.4791-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Adler A, et al. Different correlation patterns of cholinergic and GABAergic interneurons with striatal projection neurons. Front Syst Neurosci. 2013;7:47. doi: 10.3389/fnsys.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Adler A, et al. Temporal convergence of dynamic cell assemblies in the striato-pallidal network. J Neurosci. 2012;32:2473–2484. doi: 10.1523/JNEUROSCI.4830-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Joshua M, et al. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J Neurosci. 2008;28:11673–11684. doi: 10.1523/JNEUROSCI.3839-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kimura M. Behaviorally contingent property of movement-related activity of the primate putamen. J Neurophysiol. 1990;63:1277–1296. doi: 10.1152/jn.1990.63.6.1277. [DOI] [PubMed] [Google Scholar]
- 189.Yamada H, Matsumoto N, Kimura M. Tonically active neurons in the primate caudate nucleus and putamen differentially encode instructed motivational outcomes of action. J Neurosci. 2004;24:3500–3510. doi: 10.1523/JNEUROSCI.0068-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Benhamou L, Kehat O, Cohen D. Firing pattern characteristics of tonically active neurons in rat striatum: context dependent or species divergent? J Neurosci. 2014;34:2299–2304. doi: 10.1523/JNEUROSCI.1798-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Williams GV, et al. Neuronal responses in the ventral striatum of the behaving macaque. Behav Brain Res. 1993;55:243–252. doi: 10.1016/0166-4328(93)90120-f. [DOI] [PubMed] [Google Scholar]
- 192.Peoples LL, et al. Tonic inhibition of single nucleus accumbens neurons in the rat: a predominant but not exclusive firing pattern induced by cocaine self-administration sessions. Neuroscience. 1998;86:13–22. doi: 10.1016/s0306-4522(98)00116-x. [DOI] [PubMed] [Google Scholar]
- 193.Peoples LL, Cavanaugh D. Differential changes in signal and background firing of accumbal neurons during cocaine self-administration. J Neurophysiol. 2003;90:993–1010. doi: 10.1152/jn.00849.2002. [DOI] [PubMed] [Google Scholar]
- 194.Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol. 2004;14:763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 195.Shidara M, Richmond BJ. Differential encoding of information about progress through multi-trial reward schedules by three groups of ventral striatal neurons. Neurosci Res. 2004;49:307–314. doi: 10.1016/j.neures.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 196.Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–222. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Deadwyler SA. Electrophysiological correlates of abused drugs: relation to natural rewards. Ann N Y Acad Sci. 2010;1187:140–147. doi: 10.1111/j.1749-6632.2009.05155.x. [DOI] [PubMed] [Google Scholar]
- 198.Krause M, et al. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci. 2010;30:4746–4756. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.de Rover M, et al. Cholinergic modulation of nucleus accumbens medium spiny neurons. Eur J Neurosci. 2002;16:2279–2290. doi: 10.1046/j.1460-9568.2002.02289.x. [DOI] [PubMed] [Google Scholar]
- 200.Britt JP, McGehee DS. Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens. J Neurosci. 2008;28:1672–1681. doi: 10.1523/JNEUROSCI.4275-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Witten IB, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yarom O, Cohen D. Putative cholinergic interneurons in the ventral and dorsal regions of the striatum have distinct roles in a two choice alternative association task. Front Syst Neurosci. 2011;5:36. doi: 10.3389/fnsys.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Brown MT, et al. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012 doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- 204.Cachope R, et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Atallah HE, et al. Neurons in the ventral striatum exhibit cell-type-specific representations of outcome during learning. Neuron. 2014;82:1145–1156. doi: 10.1016/j.neuron.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Xie Y, et al. High-frequency electrical stimulation suppresses cholinergic accumbens interneurons in acute rat brain slices through GABA(B) receptors. Eur J Neurosci. 2014;40:3653–3662. doi: 10.1111/ejn.12736. [DOI] [PubMed] [Google Scholar]
- 207.Warner-Schmidt JL, et al. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A. 2012;109:11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bertran-Gonzalez J, et al. Learning-related translocation of δ-opioid receptors on ventral striatal cholinergic interneurons mediates choice between goal-directed actions. J Neurosci. 2013;33:16060–16071. doi: 10.1523/JNEUROSCI.1927-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Laurent V, et al. δ-Opioid and Dopaminergic Processes in Accumbens Shell Modulate the Cholinergic Control of Predictive Learning and Choice. J Neurosci. 2014;34:1358–1369. doi: 10.1523/JNEUROSCI.4592-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tan KR, et al. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cohen JY, et al. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Berke JD. Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. J Neurosci. 2008;28:10075–10080. doi: 10.1523/JNEUROSCI.2192-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Beatty JA, et al. Complex autonomous firing patterns of striatal low-threshold spike interneurons. J Neurophysiol. 2012;108:771–781. doi: 10.1152/jn.00283.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sharott A, et al. Relationships between the firing of identified striatal interneurons and spontaneous and driven cortical activities in vivo. J Neurosci. 2012;32:13221–13236. doi: 10.1523/JNEUROSCI.2440-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kolisnyk B, et al. ChAT-ChR2-EYFP mice have enhanced motor endurance but show deficits in attention and several additional cognitive domains. J Neurosci. 2013;33:10427–10438. doi: 10.1523/JNEUROSCI.0395-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Herman AM, et al. Cell type-specific and time-dependent light exposure contribute to silencing in neurons expressing Channelrhodopsin-2. Elife. 2014;3:e01481. doi: 10.7554/eLife.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Aosaki T, et al. Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr Gerontol Int. 2010;10(Suppl 1):S148–157. doi: 10.1111/j.1447-0594.2010.00588.x. [DOI] [PubMed] [Google Scholar]
- 218.Haber SN. Neurotransmitters in the human and nonhuman primate basal ganglia. Hum Neurobiol. 1986;5:159–168. [PubMed] [Google Scholar]
- 219.Semba K, Fibiger HC, Vincent SR. Neurotransmitters in the mammalian striatum: neuronal circuits and heterogeneity. Can J Neurol Sci. 1987;14:386–394. doi: 10.1017/s0317167100037781. [DOI] [PubMed] [Google Scholar]
- 220.Gerfen CR. Synaptic organization of the striatum. Journal of electron microscopy technique. 1988;10:265–281. doi: 10.1002/jemt.1060100305. [DOI] [PubMed] [Google Scholar]
- 221.Pasik P, et al. GABAergic elements in the neuronal circuits of the monkey neostriatum: a light and electron microscopic immunocytochemical study. The Journal of comparative neurology. 1988;270:157–170. doi: 10.1002/cne.902700202. [DOI] [PubMed] [Google Scholar]
- 222.Ribak CE, Roberts RC. GABAergic synapses in the brain identified with antisera to GABA and its synthesizing enzyme, glutamate decarboxylase. Journal of electron microscopy technique. 1990;15:34–48. doi: 10.1002/jemt.1060150105. [DOI] [PubMed] [Google Scholar]
- 223.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends in neurosciences. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 224.Smith Y, et al. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. 1994;344:1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- 225.Roberts RC, et al. Synaptic organization of the human striatum: a postmortem ultrastructural study. J Comp Neurol. 1996;374:523–534. doi: 10.1002/(SICI)1096-9861(19961028)374:4<523::AID-CNE4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 226.Bolam JP, et al. Synaptic organisation of the basal ganglia. J Anat. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chang HT, Kitai ST. Large neostriatal neurons in the rat: an electron microscopic study of gold-toned Golgi-stained cells. Brain Res Bull. 1982;8:631–643. doi: 10.1016/0361-9230(82)90091-0. [DOI] [PubMed] [Google Scholar]
- 228.Meredith GE, Wouterlood FG, Pattiselanno A. Hippocampal fibers make synaptic contacts with glutamate decarboxylase-immunoreactive neurons in the rat nucleus accumbens. Brain Res. 1990;513:329–334. doi: 10.1016/0006-8993(90)90476-r. [DOI] [PubMed] [Google Scholar]
- 229.Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51:533–545. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- 230.Sizemore RJ, Reynolds JN, Oorschot DE. Number and type of synapses on the distal dendrite of a rat striatal cholinergic interneuron: a quantitative, ultrastructural study. J Anat. 2010;217:223–235. doi: 10.1111/j.1469-7580.2010.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sidibe M, Smith Y. Thalamic inputs to striatal interneurons in monkeys: synaptic organization and co-localization of calcium binding proteins. Neuroscience. 1999;89:1189–1208. doi: 10.1016/s0306-4522(98)00367-4. [DOI] [PubMed] [Google Scholar]
- 232.Thomas TM, et al. Cortical inputs to m2-immunoreactive striatal interneurons in rat and monkey. Synapse. 2000;37:252–261. doi: 10.1002/1098-2396(20000915)37:4<252::AID-SYN2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 233.Pisani A, et al. Activation of D2-like dopamine receptors reduces synaptic inputs to striatal cholinergic interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:RC69. doi: 10.1523/JNEUROSCI.20-07-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Reynolds JN, Wickens JR. The corticostriatal input to giant aspiny interneurons in the rat: a candidate pathway for synchronising the response to reward-related cues. Brain Res. 2004;1011:115–128. doi: 10.1016/j.brainres.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 235.Sciamanna G, et al. Cholinergic dysfunction alters synaptic integration between thalamostriatal and corticostriatal inputs in DYT1 dystonia. J Neurosci. 2012;32:11991–12004. doi: 10.1523/JNEUROSCI.0041-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Taber MT, Fibiger HC. Cortical regulation of acetylcholine release in rat striatum. Brain Res. 1994;639:354–356. doi: 10.1016/0006-8993(94)91754-x. [DOI] [PubMed] [Google Scholar]
- 237.Baldi G, et al. Trans-synaptic modulation of striatal ACh release in vivo by the parafascicular thalamic nucleus. Eur J Neurosci. 1995;7:1117–1120. doi: 10.1111/j.1460-9568.1995.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 238.Consolo S, et al. The cerebral cortex and parafascicular thalamic nucleus facilitate in vivo acetylcholine release in the rat striatum through distinct glutamate receptor subtypes. Eur J Neurosci. 1996;8:2702–2710. doi: 10.1111/j.1460-9568.1996.tb01565.x. [DOI] [PubMed] [Google Scholar]
- 239.Ding JB, et al. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Meredith GE, Wouterlood FG. Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J Comp Neurol. 1990;296:204–221. doi: 10.1002/cne.902960203. [DOI] [PubMed] [Google Scholar]
- 241.Xu ZC, Wilson CJ, Emson PC. Restoration of thalamostriatal projections in rat neostriatal grafts: an electron microscopic analysis. J Comp Neurol. 1991;303:22–34. doi: 10.1002/cne.903030104. [DOI] [PubMed] [Google Scholar]
- 242.Landwehrmeyer GB, et al. NMDA receptor subunit mRNA expression by projection neurons and interneurons in rat striatum. J Neurosci. 1995;15:5297–5307. doi: 10.1523/JNEUROSCI.15-07-05297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Testa CM, et al. Differential expression of mGluR5 metabotropic glutamate receptor mRNA by rat striatal neurons. J Comp Neurol. 1995;354:241–252. doi: 10.1002/cne.903540207. [DOI] [PubMed] [Google Scholar]
- 244.Chen Q, Veenman CL, Reiner A. Cellular expression of ionotropic glutamate receptor subunits on specific striatal neuron types and its implication for striatal vulnerability in glutamate receptor-mediated excitotoxicity. Neuroscience. 1996;73:715–731. doi: 10.1016/0306-4522(96)00011-5. [DOI] [PubMed] [Google Scholar]
- 245.Standaert DG, et al. Expression of NMDA glutamate receptor subunit mRNAs in neurochemically identified projection and interneurons in the striatum of the rat. Brain Res Mol Brain Res. 1999;64:11–23. doi: 10.1016/s0169-328x(98)00293-9. [DOI] [PubMed] [Google Scholar]
- 246.Küppenbender KD, et al. Expression of NMDA receptor subunit mRNAs in neurochemically identified projection and interneurons in the human striatum. J Comp Neurol. 2000;419:407–421. doi: 10.1002/(sici)1096-9861(20000417)419:4<407::aid-cne1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 247.Bell MI, Richardson PJ, Lee K. Functional and molecular characterization of metabotropic glutamate receptors expressed in rat striatal cholinergic interneurones. J Neurochem. 2002;81:142–149. doi: 10.1046/j.1471-4159.2002.00815.x. [DOI] [PubMed] [Google Scholar]
- 248.Bloomfield C, et al. Cholinergic neurons of the adult rat striatum are immunoreactive for glutamatergic N-methyl-d-aspartate 2D but not N-methyl-d-aspartate 2C receptor subunits. Neuroscience. 2007;150:639–646. doi: 10.1016/j.neuroscience.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Deng YP, et al. Differential localization of the GluR1 and GluR2 subunits of the AMPA-type glutamate receptor among striatal neuron types in rats. J Chem Neuroanat. 2007;33:167–192. doi: 10.1016/j.jchemneu.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Deng YP, Shelby E, Reiner AJ. Immunohistochemical localization of AMPA-type glutamate receptor subunits in the striatum of rhesus monkey. Brain Res. 2010;1344:104–123. doi: 10.1016/j.brainres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 252.Bonsi P, et al. Distinct roles of group I mGlu receptors in striatal function. Neuropharmacology. 2008;55:392–395. doi: 10.1016/j.neuropharm.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 253.Pisani A, et al. Targeting striatal cholinergic interneurons in Parkinson’s disease: focus on metabotropic glutamate receptors. Neuropharmacology. 2003;45:45–56. doi: 10.1016/s0028-3908(03)00137-0. [DOI] [PubMed] [Google Scholar]
- 254.Pisani A, et al. Metabotropic glutamate 2 receptors modulate synaptic inputs and calcium signals in striatal cholinergic interneurons. J Neurosci. 2002;22:6176–6185. doi: 10.1523/JNEUROSCI.22-14-06176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 256.Totterdell S, Smith AD. Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J Chem Neuroanat. 1989;2:285–298. [PubMed] [Google Scholar]
- 257.Johnson LR, et al. Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience. 1994;61:851–865. doi: 10.1016/0306-4522(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 258.Kraus MM, Prast H. The nitric oxide system modulates the in vivo release of acetylcholine in the nucleus accumbens induced by stimulation of the hippocampal fornix/fimbria-projection. Eur J Neurosci. 2001;14:1105–1112. doi: 10.1046/j.0953-816x.2001.01735.x. [DOI] [PubMed] [Google Scholar]
- 259.Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 260.Consolo S, et al. Role of the parafascicular thalamic nucleus and N-methyl-D-aspartate transmission in the D1-dependent control of in vivo acetylcholine release in rat striatum. Neuroscience. 1996;71:157–165. doi: 10.1016/0306-4522(95)00421-1. [DOI] [PubMed] [Google Scholar]
- 261.Ellender TJ, et al. Heterogeneous properties of central lateral and parafascicular thalamic synapses in the striatum. J Physiol. 2013;591:257–272. doi: 10.1113/jphysiol.2012.245233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Smith Y, et al. The thalamostriatal system in normal and diseased states. Front Syst Neurosci. 2014;8:5. doi: 10.3389/fnsys.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- 264.Tallaksen-Greene SJ, et al. Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain Res. 1998;780:210–217. doi: 10.1016/s0006-8993(97)01141-4. [DOI] [PubMed] [Google Scholar]
- 265.Galvan A, Kuwajima M, Smith Y. Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience. 2006;143:351–375. doi: 10.1016/j.neuroscience.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bolam JP, et al. Substance P-containing terminals in synaptic contact with cholinergic neurons in the neostriatum and basal forebrain: a double immunocytochemical study in the rat. Brain Res. 1986;397:279–289. doi: 10.1016/0006-8993(86)90629-3. [DOI] [PubMed] [Google Scholar]
- 267.Martone ME, et al. Ultrastructural examination of enkephalin and substance P input to cholinergic neurons within the rat neostriatum. Brain Res. 1992;594:253–262. doi: 10.1016/0006-8993(92)91132-x. [DOI] [PubMed] [Google Scholar]
- 268.Meredith GE, Chang HT. Synaptic relationships of enkephalinergic and cholinergic neurons in the nucleus accumbens of the rat. Brain Res. 1994;667:67–76. doi: 10.1016/0006-8993(94)91714-0. [DOI] [PubMed] [Google Scholar]
- 269.Pasquini F, et al. Electron microscopic localization of photoaffinity-labelled delta opioid receptors in the neostriatum of the rat. J Comp Neurol. 1992;326:229–244. doi: 10.1002/cne.903260206. [DOI] [PubMed] [Google Scholar]
- 270.Le Moine C, et al. Delta-opioid receptor gene expression in the mouse forebrain: localization in cholinergic neurons of the striatum. Neuroscience. 1994;62:635–640. doi: 10.1016/0306-4522(94)90464-2. [DOI] [PubMed] [Google Scholar]
- 271.Jabourian M, et al. Functional mu opioid receptors are expressed in cholinergic interneurons of the rat dorsal striatum: territorial specificity and diurnal variation. Eur J Neurosci. 2005;21:3301–3309. doi: 10.1111/j.1460-9568.2005.04154.x. [DOI] [PubMed] [Google Scholar]
- 272.Gerfen CR, McGinty JF, Young WS., 3rd Dopamine differentially regulates dynorphin, substance P, and enkephalin expression in striatal neurons: in situ hybridization histochemical analysis. J Neurosci. 1991;11:1016–1031. doi: 10.1523/JNEUROSCI.11-04-01016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kaneko T, et al. Substance P receptor-immunoreactive neurons in the rat neostriatum are segregated into somatostatinergic and cholinergic aspiny neurons. Brain Res. 1993;631:297–303. doi: 10.1016/0006-8993(93)91548-7. [DOI] [PubMed] [Google Scholar]
- 274.Aubry JM, et al. NK1 receptor expression by cholinergic interneurones in human striatum. Neuroreport. 1994;5:1597–1600. doi: 10.1097/00001756-199408150-00014. [DOI] [PubMed] [Google Scholar]
- 275.Parent A, Cicchetti F, Beach TG. Striatal neurones displaying substance P (NK1) receptor immunoreactivity in human and non-human primates. Neuroreport. 1995;6:721–724. doi: 10.1097/00001756-199503270-00004. [DOI] [PubMed] [Google Scholar]
- 276.Richardson PJ, et al. Correlating physiology with gene expression in striatal cholinergic neurones. J Neurochem. 2000;74:839–846. doi: 10.1046/j.1471-4159.2000.740839.x. [DOI] [PubMed] [Google Scholar]
- 277.Perez S, et al. Tachykinin regulation of cholinergic transmission in the limbic/prefrontal territory of the rat dorsal striatum: implication of new neurokinine 1-sensitive receptor binding site and interaction with enkephalin/mu opioid receptor transmission. J Neurochem. 2007;103:2153–2163. doi: 10.1111/j.1471-4159.2007.04944.x. [DOI] [PubMed] [Google Scholar]
- 278.Pickel VM, et al. Neurokinin 1 receptor distribution in cholinergic neurons and targets of substance P terminals in the rat nucleus accumbens. J Comp Neurol. 2000;423:500–511. [PubMed] [Google Scholar]
- 279.Svingos AL, Colago EE, Pickel VM. Vesicular acetylcholine transporter in the rat nucleus accumbens shell: subcellular distribution and association with mu-opioid receptors. Synapse. 2001;40:184–192. doi: 10.1002/syn.1041. [DOI] [PubMed] [Google Scholar]
- 280.Aosaki T, Kawaguchi Y. Actions of substance P on rat neostriatal neurons in vitro. J Neurosci. 1996;16:5141–5153. doi: 10.1523/JNEUROSCI.16-16-05141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bell MI, Richardson PJ, Lee K. Characterization of the mechanism of action of tachykinins in rat striatal cholinergic interneurons. Neuroscience. 1998;87:649–658. doi: 10.1016/s0306-4522(98)00187-0. [DOI] [PubMed] [Google Scholar]
- 282.Govindaiah G, Wang Y, Cox CL. Substance P selectively modulates GABA(A) receptor-mediated synaptic transmission in striatal cholinergic interneurons. Neuropharmacology. 2010;58:413–422. doi: 10.1016/j.neuropharm.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 283.Arenas E, et al. Neurokinin receptors differentially mediate endogenous acetylcholine release evoked by tachykinins in the neostriatum. J Neurosci. 1991;11:2332–2338. doi: 10.1523/JNEUROSCI.11-08-02332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mulder AH, et al. Kappa- and delta-opioid receptor agonists differentially inhibit striatal dopamine and acetylcholine release. Nature. 1984;308:278–280. doi: 10.1038/308278a0. [DOI] [PubMed] [Google Scholar]
- 285.De Vries TJ, et al. Selective effects of [D-Ser2(O-t-butyl),Leu5]enkephalyl-Thr6 and [D-Ser2(O-t-butyl),Leu5]enkephalyl-Thr6 (O-t-butyl), two new enkephalin analogues, on neurotransmitter release and adenylate cyclase in rat brain slices. Eur J Pharmacol. 1989;170:137–143. doi: 10.1016/0014-2999(89)90534-7. [DOI] [PubMed] [Google Scholar]
- 286.Lapchak PA, Araujo DM, Collier B. Regulation of endogenous acetylcholine release from mammalian brain slices by opiate receptors: hippocampus, striatum and cerebral cortex of guinea-pig and rat. Neuroscience. 1989;31:313–325. doi: 10.1016/0306-4522(89)90376-x. [DOI] [PubMed] [Google Scholar]
- 287.Izquierdo I. Acetylcholine release is modulated by different opioid receptor types in different brain regions and species. Trends Pharmacol Sci. 1990;11:179–180. doi: 10.1016/0165-6147(90)90108-k. [DOI] [PubMed] [Google Scholar]
- 288.Jiang ZG, North RA. Pre- and postsynaptic inhibition by opioids in rat striatum. J Neurosci. 1992;12:356–361. doi: 10.1523/JNEUROSCI.12-01-00356.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lendvai B, Sandor NT, Sandor A. Influence of selective opiate antagonists on striatal acetylcholine and dopamine release. Acta Physiol Hung. 1993;81:19–28. [PubMed] [Google Scholar]
- 290.Miura M, et al. Compartment-specific modulation of GABAergic synaptic transmission by mu-opioid receptor in the mouse striatum with green fluorescent protein-expressing dopamine islands. J Neurosci. 2007;27:9721–9728. doi: 10.1523/JNEUROSCI.2993-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Heijna MH, et al. Opioid receptor-mediated inhibition of dopamine and acetylcholine release from slices of rat nucleus accumbens, olfactory tubercle and frontal cortex. Eur J Pharmacol. 1990;181:267–278. doi: 10.1016/0014-2999(90)90088-n. [DOI] [PubMed] [Google Scholar]
- 292.Jabourian M, et al. Mu opioid control of the N-methyl-D-aspartate-evoked release of [3H]-acetylcholine in the limbic territory of the rat striatum in vitro: diurnal variations and implication of a dopamine link. Neuroscience. 2004;123:733–742. doi: 10.1016/j.neuroscience.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 293.Ponterio G, et al. Powerful inhibitory action of mu opioid receptors (MOR) on cholinergic interneuron excitability in the dorsal striatum. Neuropharmacology. 2013;75C:78–85. doi: 10.1016/j.neuropharm.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 294.Boix F, et al. Substance P decreases extracellular concentrations of acetylcholine in neostriatum and nucleus accumbens in vivo: possible relevance for the central processing of reward and aversion. Behav Brain Res. 1994;63:213–219. doi: 10.1016/0166-4328(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 295.Chang HT, Kita H. Interneurons in the rat striatum: relationships between parvalbumin neurons and cholinergic neurons. Brain Res. 1992;574:307–311. doi: 10.1016/0006-8993(92)90830-3. [DOI] [PubMed] [Google Scholar]
- 296.Vuillet J, et al. Ultrastructural relationships between choline acetyltransferase- and neuropeptide y-containing neurons in the rat striatum. Neuroscience. 1992;46:351–360. doi: 10.1016/0306-4522(92)90057-9. [DOI] [PubMed] [Google Scholar]
- 297.Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron. 1997;19:1115–1126. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 298.Waldvogel HJ, et al. Comparative cellular distribution of GABAA and GABAB receptors in the human basal ganglia: immunohistochemical colocalization of the alpha 1 subunit of the GABAA receptor, and the GABABR1 and GABABR2 receptor subunits. J Comp Neurol. 2004;470:339–356. doi: 10.1002/cne.20005. [DOI] [PubMed] [Google Scholar]
- 299.Waldvogel HJ, et al. Regional and cellular localisation of GABA(A) receptor subunits in the human basal ganglia: An autoradiographic and immunohistochemical study. J Comp Neurol. 1999;415:313–340. doi: 10.1002/(sici)1096-9861(19991220)415:3<313::aid-cne2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 300.Yung KK, Ng TK, Wong CK. Subpopulations of neurons in the rat neostriatum display GABABR1 receptor immunoreactivity. Brain Res. 1999;830:345–352. doi: 10.1016/s0006-8993(99)01442-0. [DOI] [PubMed] [Google Scholar]
- 301.Stoof JC, Den Breejen EJ, Mulder AH. GABA modulates the release of dopamine and acetylcholine from rat caudate nucleus slices. Eur J Pharmacol. 1979;57:35–42. doi: 10.1016/0014-2999(79)90101-8. [DOI] [PubMed] [Google Scholar]
- 302.Anderson JJ, et al. GABAA and GABAB receptors differentially regulate striatal acetylcholine release in vivo. Neurosci Lett. 1993;160:126–130. doi: 10.1016/0304-3940(93)90395-2. [DOI] [PubMed] [Google Scholar]
- 303.DeBoer P, Westerink BH. GABAergic modulation of striatal cholinergic interneurons: an in vivo microdialysis study. J Neurochem. 1994;62:70–75. doi: 10.1046/j.1471-4159.1994.62010070.x. [DOI] [PubMed] [Google Scholar]
- 304.Scatton B, Bartholini G. gamma-Aminobutyric acid (GABA) receptor stimulation. IV. Effect of progabide (SL 76002) and other GABAergic agents on acetylcholine turnover in rat brain areas. J Pharmacol Exp Ther. 1982;220:689–695. [PubMed] [Google Scholar]
- 305.Scatton B, Bartholini G. Modulation by GABA of cholinergic transmission in the striatum. Brain Res. 1980;183:211–216. doi: 10.1016/0006-8993(80)90132-8. [DOI] [PubMed] [Google Scholar]
- 306.Rada PV, Mark GP, Hoebel BG. In vivo modulation of acetylcholine in the nucleus accumbens of freely moving rats: II. Inhibition by gamma-aminobutyric acid. Brain Res. 1993;619:105–110. doi: 10.1016/0006-8993(93)91601-n. [DOI] [PubMed] [Google Scholar]
- 307.Smith Y, Villalba R. Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov Disord. 2008;23(Suppl 3):S534–547. doi: 10.1002/mds.22027. [DOI] [PubMed] [Google Scholar]
- 308.Sofuoglu M, Mooney M. Cholinergic functioning in stimulant addiction: implications for medications development. CNS Drugs. 2009;23:939–952. doi: 10.2165/11310920-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kubota Y, et al. Neostriatal cholinergic neurons receive direct synaptic inputs from dopaminergic axons. Brain Res. 1987;413:179–184. doi: 10.1016/0006-8993(87)90167-3. [DOI] [PubMed] [Google Scholar]
- 310.Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- 311.Chang HT. Dopamine-acetylcholine interaction in the rat striatum: a dual-labeling immunocytochemical study. Brain Res Bull. 1988;21:295–304. doi: 10.1016/0361-9230(88)90244-4. [DOI] [PubMed] [Google Scholar]
- 312.Aoki C, Pickel VM. Neuropeptide Y-containing neurons in the rat striatum: ultrastructure and cellular relations with tyrosine hydroxylase- containing terminals and with astrocytes. Brain Res. 1988;459:205–225. doi: 10.1016/0006-8993(88)90637-3. [DOI] [PubMed] [Google Scholar]
- 313.Pickel VM, Chan J. Spiny neurons lacking choline acetyltransferase immunoreactivity are major targets of cholinergic and catecholaminergic terminals in rat striatum. Journal of neuroscience research. 1990;25:263–280. doi: 10.1002/jnr.490250302. [DOI] [PubMed] [Google Scholar]
- 314.Pickel VM, et al. Gamma-aminobutyric acid in the medial rat nucleus accumbens: ultrastructural localization in neurons receiving monosynaptic input from catecholaminergic afferents. J Comp Neurol. 1988;272:1–14. doi: 10.1002/cne.902720102. [DOI] [PubMed] [Google Scholar]
- 315.Pickel VM, Joh TH, Chan J. Substance P in the rat nucleus accumbens: ultrastructural localization in axon terminals and their relation to dopaminergic afferents. Brain Res. 1988;444:247–264. doi: 10.1016/0006-8993(88)90934-1. [DOI] [PubMed] [Google Scholar]
- 316.Le Moine C, Tison F, Bloch B. D2 dopamine receptor gene expression by cholinergic neurons in the rat striatum. Neurosci Lett. 1990;117:248–252. doi: 10.1016/0304-3940(90)90671-u. [DOI] [PubMed] [Google Scholar]
- 317.Berendse HW, Richfield EK. Heterogeneous distribution of dopamine D1 and D2 receptors in the human ventral striatum. Neurosci Lett. 1993;150:75–79. doi: 10.1016/0304-3940(93)90112-x. [DOI] [PubMed] [Google Scholar]
- 318.Alcantara AA, et al. Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain Res. 2003;986:22–29. doi: 10.1016/s0006-8993(03)03165-2. [DOI] [PubMed] [Google Scholar]
- 319.Jongen-Rêlo AL, et al. Differential localization of mRNAs encoding dopamine D1 or D2 receptors in cholinergic neurons in the core and shell of the rat nucleus accumbens. Brain Res Mol Brain Res. 1995;28:169–174. doi: 10.1016/0169-328x(94)00239-b. [DOI] [PubMed] [Google Scholar]
- 320.Le Moine C, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci U S A. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Bergson C, et al. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Rivera A, et al. Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. Eur J Neurosci. 2002;16:2049–2058. doi: 10.1046/j.1460-9568.2002.02280.x. [DOI] [PubMed] [Google Scholar]
- 323.Berlanga ML, Simpson TK, Alcantara AA. Dopamine D5 receptor localization on cholinergic neurons of the rat forebrain and diencephalon: a potential neuroanatomical substrate involved in mediating dopaminergic influences on acetylcholine release. J Comp Neurol. 2005;492:34–49. doi: 10.1002/cne.20684. [DOI] [PubMed] [Google Scholar]
- 324.Landwehrmeyer B, Mengod G, Palacios JM. Differential visualization of dopamine D2 and D3 receptor sites in rat brain. A comparative study using in situ hybridization histochemistry and ligand binding autoradiography. Eur J Neurosci. 1993;5:145–153. doi: 10.1111/j.1460-9568.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 325.Landwehrmeyer B, Mengod G, Palacios JM. Dopamine D3 receptor mRNA and binding sites in human brain. Brain Res Mol Brain Res. 1993;18:187–192. doi: 10.1016/0169-328x(93)90188-u. [DOI] [PubMed] [Google Scholar]
- 326.Piggott MA, et al. Dopaminergic activities in the human striatum: rostrocaudal gradients of uptake sites and of D1 and D2 but not of D3 receptor binding or dopamine. Neuroscience. 1999;90:433–445. doi: 10.1016/s0306-4522(98)00465-5. [DOI] [PubMed] [Google Scholar]
- 327.Andreoli M, et al. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- 328.Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- 329.Damsma G, et al. Dopaminergic regulation of striatal acetylcholine release: importance of D1 and N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1991;259:1064–1072. [PubMed] [Google Scholar]
- 330.Henselmans JM, Stoof JC. Regional differences in the regulation of acetylcholine release upon D2 dopamine and N-methyl-D-aspartate receptor activation in rat nucleus accumbens and neostriatum. Brain Res. 1991;566:1–7. doi: 10.1016/0006-8993(91)91673-o. [DOI] [PubMed] [Google Scholar]
- 331.Imperato A, et al. Cocaine releases limbic acetylcholine through endogenous dopamine action on D1 receptors. Eur J Pharmacol. 1992;229:265–267. doi: 10.1016/0014-2999(92)90565-l. [DOI] [PubMed] [Google Scholar]
- 332.DeBoer P, Heeringa MJ, Abercrombie ED. Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. Eur J Pharmacol. 1996;317:257–262. doi: 10.1016/s0014-2999(96)00761-3. [DOI] [PubMed] [Google Scholar]
- 333.Abercrombie ED, DeBoer P. Substantia nigra D1 receptors and stimulation of striatal cholinergic interneurons by dopamine: a proposed circuit mechanism. J Neurosci. 1997;17:8498–8505. doi: 10.1523/JNEUROSCI.17-21-08498.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Consolo S, et al. Different sensitivity of in vivo acetylcholine transmission to D1 receptor stimulation in shell and core of nucleus accumbens. Neuroscience. 1999;89:1209–1217. doi: 10.1016/s0306-4522(98)00309-1. [DOI] [PubMed] [Google Scholar]
- 335.Straub C, et al. Multiphasic modulation of cholinergic interneurons by nigrostriatal afferents. J Neurosci. 2014;34:8557–8569. doi: 10.1523/JNEUROSCI.0589-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Aosaki T, Kiuchi K, Kawaguchi Y. Dopamine D1-like receptor activation excites rat striatal large aspiny neurons in vitro. J Neurosci. 1998;18:5180–5190. doi: 10.1523/JNEUROSCI.18-14-05180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Maurice N, et al. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Salgado H, et al. A reconfiguration of CaV2 Ca2+ channel current and its dopaminergic D2 modulation in developing neostriatal neurons. J Neurophysiol. 2005;94:3771–3787. doi: 10.1152/jn.00455.2005. [DOI] [PubMed] [Google Scholar]
- 339.Deng P, Zhang Y, Xu ZC. Involvement of I(h) in dopamine modulation of tonic firing in striatal cholinergic interneurons. J Neurosci. 2007;27:3148–3156. doi: 10.1523/JNEUROSCI.5535-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wang W, et al. Acetylcholine encodes long-lasting presynaptic plasticity at glutamatergic synapses in the dorsal striatum after repeated amphetamine exposure. J Neurosci. 2013;33:10405–10426. doi: 10.1523/JNEUROSCI.0014-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294:366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- 342.Bertorello AM, et al. Inhibition by dopamine of (Na(+)+K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature. 1990;347:386–388. doi: 10.1038/347386a0. [DOI] [PubMed] [Google Scholar]
- 343.Svenningsson P, et al. Co-stimulation of D(1)/D(5) and D(2) dopamine receptors leads to an increase in c-fos messenger RNA in cholinergic interneurons and a redistribution of c-fos messenger RNA in striatal projection neurons. Neuroscience. 2000;98:749–757. doi: 10.1016/s0306-4522(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 344.Fetsko LA, Xu R, Wang Y. Alterations in D1/D2 synergism may account for enhanced stereotypy and reduced climbing in mice lacking dopamine D2L receptor. Brain Res. 2003;967:191–200. doi: 10.1016/s0006-8993(02)04277-4. [DOI] [PubMed] [Google Scholar]
- 345.Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Zhou FM, Wilson C, Dani JA. Muscarinic and nicotinic cholinergic mechanisms in the mesostriatal dopamine systems. Neuroscientist. 2003;9:23–36. doi: 10.1177/1073858402239588. [DOI] [PubMed] [Google Scholar]
- 347.Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl 1):S283–297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Havekes R, Abel T, Van der Zee EA. The cholinergic system and neostriatal memory functions. Behav Brain Res. 2011;221:412–423. doi: 10.1016/j.bbr.2010.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Oldenburg IA, Ding JB. Cholinergic modulation of synaptic integration and dendritic excitability in the striatum. Curr Opin Neurobiol. 2011;21:425–432. doi: 10.1016/j.conb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Wevers A. Localisation of pre- and postsynaptic cholinergic markers in the human brain. Behav Brain Res. 2011;221:341–355. doi: 10.1016/j.bbr.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 351.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 352.Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol Sci. 2003;24:414–420. doi: 10.1016/S0165-6147(03)00195-0. [DOI] [PubMed] [Google Scholar]
- 353.Nastuk MA, Graybiel AM. Patterns of muscarinic cholinergic binding in the striatum and their relation to dopamine islands and striosomes. J Comp Neurol. 1985;237:176–194. doi: 10.1002/cne.902370204. [DOI] [PubMed] [Google Scholar]
- 354.Nastuk MA, Graybiel AM. Autoradiographic localization and biochemical characteristics of M1 and M2 muscarinic binding sites in the striatum of the cat, monkey, and human. J Neurosci. 1988;8:1052–1062. doi: 10.1523/JNEUROSCI.08-03-01052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Brann MR, Buckley NJ, Bonner TI. The striatum and cerebral cortex express different muscarinic receptor mRNAs. FEBS Lett. 1988;230:90–94. doi: 10.1016/0014-5793(88)80648-3. [DOI] [PubMed] [Google Scholar]
- 356.Cortés R, Palacios JM. Muscarinic cholinergic receptor subtypes in the rat brain. I. Quantitative autoradiographic studies. Brain Res. 1986;362:227–238. doi: 10.1016/0006-8993(86)90448-8. [DOI] [PubMed] [Google Scholar]
- 357.Cortés R, et al. Muscarinic cholinergic receptor subtypes in the human brain. II. Quantitative autoradiographic studies. Brain Res. 1986;362:239–253. doi: 10.1016/0006-8993(86)90449-x. [DOI] [PubMed] [Google Scholar]
- 358.Cortés R, Probst A, Palacios JM. Quantitative light microscopic autoradiographic localization of cholinergic muscarinic receptors in the human brain: forebrain. Neuroscience. 1987;20:65–107. doi: 10.1016/0306-4522(87)90006-6. [DOI] [PubMed] [Google Scholar]
- 359.Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Vilaró MT, et al. Muscarinic cholinergic receptors in the rat caudate-putamen and olfactory tubercle belong predominantly to the m4 class: in situ hybridization and receptor autoradiography evidence. Neuroscience. 1991;40:159–167. doi: 10.1016/0306-4522(91)90181-m. [DOI] [PubMed] [Google Scholar]
- 361.Vilaró MT, et al. Muscarinic M2 receptor mRNA expression and receptor binding in cholinergic and non-cholinergic cells in the rat brain: a correlative study using in situ hybridization histochemistry and receptor autoradiography. Neuroscience. 1992;47:367–393. doi: 10.1016/0306-4522(92)90253-x. [DOI] [PubMed] [Google Scholar]
- 362.Flynn DD, Mash DC. Distinct kinetic binding properties of N-[3H]-methylscopolamine afford differential labeling and localization of M1, M2, and M3 muscarinic receptor subtypes in primate brain. Synapse. 1993;14:283–296. doi: 10.1002/syn.890140406. [DOI] [PubMed] [Google Scholar]
- 363.Levey AI, et al. Light and electron microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J Comp Neurol. 1995;351:339–356. doi: 10.1002/cne.903510303. [DOI] [PubMed] [Google Scholar]
- 364.Levey AI. Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 365.Zubieta JK, Frey KA. Autoradiographic mapping of M3 muscarinic receptors in the rat brain. J Pharmacol Exp Ther. 1993;264:415–422. [PubMed] [Google Scholar]
- 366.Aubert I, et al. Comparative ontogenic profile of cholinergic markers, including nicotinic and muscarinic receptors, in the rat brain. J Comp Neurol. 1996;369:31–55. doi: 10.1002/(SICI)1096-9861(19960520)369:1<31::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 367.Rodríguez-Puertas R, et al. Autoradiographic distribution of M1, M2, M3, and M4 muscarinic receptor subtypes in Alzheimer’s disease. Synapse. 1997;26:341–350. doi: 10.1002/(SICI)1098-2396(199708)26:4<341::AID-SYN2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 368.Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988;8:4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Vilaró MT, et al. Muscarinic M2-selective ligands also recognize M4 receptors in the rat brain: evidence from combined in situ hybridization and receptor autoradiography. Synapse. 1992;11:171–183. doi: 10.1002/syn.890110302. [DOI] [PubMed] [Google Scholar]
- 370.Levey AI, et al. Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience. 1994;63:207–221. doi: 10.1016/0306-4522(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 371.Hersch SM, Levey AI. Diverse pre- and post-synaptic expression of m1-m4 muscarinic receptor proteins in neurons and afferents in the rat neostriatum. Life Sci. 1995;56:931–938. doi: 10.1016/0024-3205(95)00030-a. [DOI] [PubMed] [Google Scholar]
- 372.Hersch SM, et al. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Jones IW, Bolam JP, Wonnacott S. Presynaptic localisation of the nicotinic acetylcholine receptor beta2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurones. J Comp Neurol. 2001;439:235–247. doi: 10.1002/cne.1345. [DOI] [PubMed] [Google Scholar]
- 374.Zhang W, et al. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 2002;22:6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Sugaya K, et al. mRNA for the m4 muscarinic receptor subtype is expressed in adult rat brain cholinergic neurons. Brain Res Mol Brain Res. 1997;50:305–313. doi: 10.1016/s0169-328x(97)00199-x. [DOI] [PubMed] [Google Scholar]
- 377.Alcantara AA, et al. Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol. 2001;434:445–460. doi: 10.1002/cne.1186. [DOI] [PubMed] [Google Scholar]
- 378.Yan Z, Flores-Hernandez J, Surmeier DJ. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience. 2001;103:1017–1024. doi: 10.1016/s0306-4522(01)00039-2. [DOI] [PubMed] [Google Scholar]
- 379.Santiago MP, Potter LT. Biotinylated m4-toxin demonstrates more M4 muscarinic receptor protein on direct than indirect striatal projection neurons. Brain Res. 2001;894:12–20. doi: 10.1016/s0006-8993(00)03170-x. [DOI] [PubMed] [Google Scholar]
- 380.Ding J, et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 381.Narushima M, et al. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Uchigashima M, et al. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 385.Vilaró MT, Palacios JM, Mengod G. Multiplicity of muscarinic autoreceptor subtypes? Comparison of the distribution of cholinergic cells and cells containing mRNA for five subtypes of muscarinic receptors in the rat brain. Brain Res Mol Brain Res. 1994;21:30–46. doi: 10.1016/0169-328x(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 386.Bernard V, et al. Subcellular redistribution of m2 muscarinic acetylcholine receptors in striatal interneurons in vivo after acute cholinergic stimulation. J Neurosci. 1998;18:10207–10218. doi: 10.1523/JNEUROSCI.18-23-10207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Smiley JF, Levey AI, Mesulam MM. m2 muscarinic receptor immunolocalization in cholinergic cells of the monkey basal forebrain and striatum. Neuroscience. 1999;90:803–814. doi: 10.1016/s0306-4522(98)00527-2. [DOI] [PubMed] [Google Scholar]
- 388.Yasuda RP, et al. Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: distribution of m4 and m5 receptors in rat brain. Mol Pharmacol. 1993;43:149–157. [PubMed] [Google Scholar]
- 389.Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 390.Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 391.Grady SR, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther. 2013;137:22–54. doi: 10.1016/j.pharmthera.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 393.Papke RL. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol. 2014;89:1–11. doi: 10.1016/j.bcp.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Gotti C, et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 395.Rubboli F, et al. Distribution of neuronal nicotinic receptor subunits in human brain. Neurochem Int. 1994;25:69–71. doi: 10.1016/0197-0186(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 396.Hellström-Lindahl E, et al. Regional distribution of nicotinic receptors during prenatal development of human brain and spinal cord. Brain Res Dev Brain Res. 1998;108:147–160. doi: 10.1016/s0165-3806(98)00046-7. [DOI] [PubMed] [Google Scholar]
- 397.Terzano S, et al. Expression of the alpha3 nicotinic receptor subunit mRNA in aging and Alzheimer’s disease. Brain Res Mol Brain Res. 1998;63:72–78. doi: 10.1016/s0169-328x(98)00260-5. [DOI] [PubMed] [Google Scholar]
- 398.Tohgi H, et al. Alterations with aging and ischemia in nicotinic acetylcholine receptor subunits alpha4 and beta2 messenger RNA expression in postmortem human putamen. Implications for susceptibility to parkinsonism. Brain Res. 1998;791:186–190. doi: 10.1016/s0006-8993(98)00093-6. [DOI] [PubMed] [Google Scholar]
- 399.Hellström-Lindahl E, et al. Regional distribution of nicotinic receptor subunit mRNAs in human brain: comparison between Alzheimer and normal brain. Brain Res Mol Brain Res. 1999;66:94–103. doi: 10.1016/s0169-328x(99)00030-3. [DOI] [PubMed] [Google Scholar]
- 400.Martin-Ruiz CM, et al. Alpha and beta nicotinic acetylcholine receptors subunits and synaptophysin in putamen from Parkinson’s disease. Neuropharmacology. 2000;39:2830–2839. doi: 10.1016/s0028-3908(00)00110-6. [DOI] [PubMed] [Google Scholar]
- 401.Han ZY, et al. Localization of [3H]nicotine, [3H]cytisine, [3H]epibatidine, and [125I]alpha-bungarotoxin binding sites in the brain of Macaca mulatta. J Comp Neurol. 2003;461:49–60. doi: 10.1002/cne.10659. [DOI] [PubMed] [Google Scholar]
- 402.Han ZY, et al. Localization of nAChR subunit mRNAs in the brain of Macaca mulatta. Eur J Neurosci. 2000;12:3664–3674. doi: 10.1046/j.1460-9568.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- 403.Graham AJ, et al. Human brain nicotinic receptors, their distribution and participation in neuropsychiatric disorders. Curr Drug Targets CNS Neurol Disord. 2002;1:387–397. doi: 10.2174/1568007023339283. [DOI] [PubMed] [Google Scholar]
- 404.Guan ZZ, et al. Selective changes in the levels of nicotinic acetylcholine receptor protein and of corresponding mRNA species in the brains of patients with Parkinson’s disease. Brain Res. 2002;956:358–366. doi: 10.1016/s0006-8993(02)03571-0. [DOI] [PubMed] [Google Scholar]
- 405.Quik M, McIntosh JM. Striatal alpha6* nicotinic acetylcholine receptors: potential targets for Parkinson’s disease therapy. J Pharmacol Exp Ther. 2006;316:481–489. doi: 10.1124/jpet.105.094375. [DOI] [PubMed] [Google Scholar]
- 406.Quik M, et al. Subunit composition of nicotinic receptors in monkey striatum: effect of treatments with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine or L-DOPA. Mol Pharmacol. 2005;67:32–41. doi: 10.1124/mol.104.006015. [DOI] [PubMed] [Google Scholar]
- 407.Lu Y, et al. Pharmacological characterization of nicotinic receptor-stimulated GABA release from mouse brain synaptosomes. J Pharmacol Exp Ther. 1998;287:648–657. [PubMed] [Google Scholar]
- 408.Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience. 2003;119:965–977. doi: 10.1016/s0306-4522(03)00220-3. [DOI] [PubMed] [Google Scholar]
- 409.Luo R, et al. Direct and GABA-mediated indirect effects of nicotinic ACh receptor agonists on striatal neurones. J Physiol. 2013;591:203–217. doi: 10.1113/jphysiol.2012.241786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Pancani T, et al. M4 mAChR-mediated modulation of glutamatergic transmission at corticostriatal synapses. ACS Chem Neurosci. 2014 doi: 10.1021/cn500003z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Yamada M, et al. Novel insights into M5 muscarinic acetylcholine receptor function by the use of gene targeting technology. Life Sci. 2003;74:345–353. doi: 10.1016/j.lfs.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 412.Grilli M, et al. Release-enhancing pre-synaptic muscarinic and nicotinic receptors co-exist and interact on dopaminergic nerve endings of rat nucleus accumbens. J Neurochem. 2008;105:2205–2213. doi: 10.1111/j.1471-4159.2008.05307.x. [DOI] [PubMed] [Google Scholar]
- 413.Bendor J, et al. AGAP1/AP-3-dependent endocytic recycling of M5 muscarinic receptors promotes dopamine release. EMBO J. 2010;29:2813–2826. doi: 10.1038/emboj.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Levey AI, et al. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Foster DJ, et al. M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor’s location. J Neurosci. 2014;34:3253–3262. doi: 10.1523/JNEUROSCI.4896-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Klink R, et al. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Azam L, et al. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- 418.Zoli M, et al. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Luetje CW. Getting past the asterisk: the subunit composition of presynaptic nicotinic receptors that modulate striatal dopamine release. Mol Pharmacol. 2004;65:1333–1335. doi: 10.1124/mol.65.6.1333. [DOI] [PubMed] [Google Scholar]
- 420.Salminen O, et al. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- 421.Salminen O, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 422.Gotti C, et al. Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Mol Pharmacol. 2005;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- 423.Kaiser S, Wonnacott S. alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- 424.Nayak SV, et al. Nicotinic receptors co-localize with 5-HT(3) serotonin receptors on striatal nerve terminals. Neuropharmacology. 2000;39:2681–2690. doi: 10.1016/s0028-3908(00)00109-x. [DOI] [PubMed] [Google Scholar]
- 425.Bernard V, Levey AI, Bloch B. Regulation of the subcellular distribution of m4 muscarinic acetylcholine receptors in striatal neurons in vivo by the cholinergic environment: evidence for regulation of cell surface receptors by endogenous and exogenous stimulation. J Neurosci. 1999;19:10237–10249. doi: 10.1523/JNEUROSCI.19-23-10237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Léna C, Changeux JP, Mulle C. Evidence for “preterminal” nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J Neurosci. 1993;13:2680–2688. doi: 10.1523/JNEUROSCI.13-06-02680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Brumwell CL, Johnson JL, Jacob MH. Extrasynaptic alpha 7-nicotinic acetylcholine receptor expression in developing neurons is regulated by inputs, targets, and activity. J Neurosci. 2002;22:8101–8109. doi: 10.1523/JNEUROSCI.22-18-08101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Poisik OV, et al. Functional alpha7-containing nicotinic acetylcholine receptors localize to cell bodies and proximal dendrites in the rat substantia nigra pars reticulata. J Physiol. 2008;586:1365–1378. doi: 10.1113/jphysiol.2008.149963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Hill JA, et al. Immunocytochemical localization of a neuronal nicotinic receptor: the beta 2-subunit. J Neurosci. 1993;13:1551–1568. doi: 10.1523/JNEUROSCI.13-04-01551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Digby GJ, Shirey JK, Conn PJ. Allosteric activators of muscarinic receptors as novel approaches for treatment of CNS disorders. Mol Biosyst. 2010;6:1345–1354. doi: 10.1039/c002938f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Digby GJ, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32:8532–8544. doi: 10.1523/JNEUROSCI.0337-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Galarraga E, et al. Cholinergic modulation of neostriatal output: a functional antagonism between different types of muscarinic receptors. J Neurosci. 1999;19:3629–3638. doi: 10.1523/JNEUROSCI.19-09-03629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Calabresi P, et al. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- 435.Zhang W, et al. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci. 2002;22:1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Bloch B, Bernard V, Dumartin B. “In vivo” intraneuronal trafficking of G protein coupled receptors in the striatum: regulation by dopaminergic and cholinergic environment. Biol Cell. 2003;95:477–488. doi: 10.1016/s0248-4900(03)00080-7. [DOI] [PubMed] [Google Scholar]
- 437.Van der Zee EA, Keijser JN. Localization of pre- and postsynaptic cholinergic markers in rodent forebrain: a brief history and comparison of rat and mouse. Behav Brain Res. 2011;221:356–366. doi: 10.1016/j.bbr.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 438.Sullivan MA, Chen H, Morikawa H. Recurrent inhibitory network among striatal cholinergic interneurons. J Neurosci. 2008;28:8682–8690. doi: 10.1523/JNEUROSCI.2411-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.English DF, et al. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci. 2012;15:123–130. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Raiteri M, et al. Muscarinic receptors mediating inhibition of gamma-aminobutyric acid release in rat corpus striatum and their pharmacological characterization. J Pharmacol Exp Ther. 1990;254:496–501. [PubMed] [Google Scholar]
- 441.Wang Z, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 442.Grilli M, et al. Nicotinic and muscarinic cholinergic receptors coexist on GABAergic nerve endings in the mouse striatum and interact in modulating GABA release. Neuropharmacology. 2009;56:610–614. doi: 10.1016/j.neuropharm.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 443.De Rover M, et al. Intermittent morphine treatment induces a long-lasting increase in cholinergic modulation of GABAergic synapses in nucleus accumbens of adult rats. Synapse. 2005;55:17–25. doi: 10.1002/syn.20087. [DOI] [PubMed] [Google Scholar]
- 444.Yamamoto K, et al. Reciprocal regulation of inhibitory synaptic transmission by nicotinic and muscarinic receptors in rat nucleus accumbens shell. J Physiol. 2013;591:5745–5763. doi: 10.1113/jphysiol.2013.258558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Hsu KS, et al. Carbachol induces inward current in neostriatal neurons through M1-like muscarinic receptors. Neuroscience. 1996;73:751–760. doi: 10.1016/0306-4522(96)00066-8. [DOI] [PubMed] [Google Scholar]
- 446.Shen W, et al. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci. 2007;10:1458–1466. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- 447.Shen W, et al. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25:7449–7458. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Ebihara K, et al. Cholinergic interneurons suppress action potential initiation of medium spiny neurons in rat nucleus accumbens shell. Neuroscience. 2013;236:332–344. doi: 10.1016/j.neuroscience.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 449.Howe AR, Surmeier DJ. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J Neurosci. 1995;15:458–469. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Perez-Rosello T, et al. Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J Neurophysiol. 2005;93:2507–2519. doi: 10.1152/jn.00853.2004. [DOI] [PubMed] [Google Scholar]
- 451.Barral J, Galarraga E, Bargas J. Muscarinic presynaptic inhibition of neostriatal glutamatergic afferents is mediated by Q-type Ca2+ channels. Brain Res Bull. 1999;49:285–289. doi: 10.1016/s0361-9230(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 452.Wonnacott S, et al. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol. 2000;393:51–58. doi: 10.1016/s0014-2999(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 453.Marchi M, et al. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem. 2002;80:1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 454.Liu Z, et al. Action-potential-independent GABAergic tone mediated by nicotinic stimulation of immature striatal miniature synaptic transmission. J Neurophysiol. 2007;98:581–593. doi: 10.1152/jn.00768.2006. [DOI] [PubMed] [Google Scholar]
- 455.Sugita S, et al. Distinct muscarinic receptors inhibit release of gamma-aminobutyric acid and excitatory amino acids in mammalian brain. Proc Natl Acad Sci U S A. 1991;88:2608–2611. doi: 10.1073/pnas.88.6.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Calabresi P, et al. Endogenous ACh enhances striatal NMDA-responses via M1-like muscarinic receptors and PKC activation. Eur J Neurosci. 1998;10:2887–2895. doi: 10.1111/j.1460-9568.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- 457.Calabresi P, et al. Blockade of M2-like muscarinic receptors enhances long-term potentiation at corticostriatal synapses. Eur J Neurosci. 1998;10:3020–3023. doi: 10.1111/j.1460-9568.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- 458.Higley MJ, Soler-Llavina GJ, Sabatini BL. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat Neurosci. 2009;12:1121–1128. doi: 10.1038/nn.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Pakhotin P, Bracci E. Cholinergic interneurons control the excitatory input to the striatum. J Neurosci. 2007;27:391–400. doi: 10.1523/JNEUROSCI.3709-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.McGehee DS, et al. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 461.Pakkanen JS, Jokitalo E, Tuominen RK. Up-regulation of beta2 and alpha7 subunit containing nicotinic acetylcholine receptors in mouse striatum at cellular level. Eur J Neurosci. 2005;21:2681–2691. doi: 10.1111/j.1460-9568.2005.04105.x. [DOI] [PubMed] [Google Scholar]
- 462.Bamford NS, et al. Repeated exposure to methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron. 2008;58:89–103. doi: 10.1016/j.neuron.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–137. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 465.Drenan RM, et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Grady SR, et al. Mouse striatal dopamine nerve terminals express alpha4alpha5beta2 and two stoichiometric forms of alpha4beta2*-nicotinic acetylcholine receptors. J Mol Neurosci. 2010;40:91–95. doi: 10.1007/s12031-009-9263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Quik M, Perez XA, Grady SR. Role of α6 nicotinic receptors in CNS dopaminergic function: relevance to addiction and neurological disorders. Biochem Pharmacol. 2011;82:873–882. doi: 10.1016/j.bcp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Exley R, et al. Striatal α5 nicotinic receptor subunit regulates dopamine transmission in dorsal striatum. J Neurosci. 2012;32:2352–2356. doi: 10.1523/JNEUROSCI.4985-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Quik M, Wonnacott S. α6β2* and α4β2* nicotinic acetylcholine receptors as drug targets for Parkinson’s disease. Pharmacol Rev. 2011;63:938–966. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Perez XA, et al. Prominent role of alpha3/alpha6beta2* nAChRs in regulating evoked dopamine release in primate putamen: effect of long-term nicotine treatment. Mol Pharmacol. 2009;75:938–946. doi: 10.1124/mol.108.053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Chapman CA, et al. Increased striatal dopamine efflux follows scopolamine administered systemically or to the tegmental pedunculopontine nucleus. Neuroscience. 1997;76:177–186. doi: 10.1016/s0306-4522(96)00358-2. [DOI] [PubMed] [Google Scholar]
- 472.Miller AD, Blaha CD. Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic transmission in the rat. Eur J Neurosci. 2005;21:1837–1846. doi: 10.1111/j.1460-9568.2005.04017.x. [DOI] [PubMed] [Google Scholar]
- 473.Lester DB, et al. Midbrain acetylcholine and glutamate receptors modulate accumbal dopamine release. Neuroreport. 2008;19:991–995. doi: 10.1097/WNR.0b013e3283036e5e. [DOI] [PubMed] [Google Scholar]
- 474.Schmidt LS, et al. Increased amphetamine-induced locomotor activity, sensitization, and accumbal dopamine release in M5 muscarinic receptor knockout mice. Psychopharmacology (Berl) 2010;207:547–558. doi: 10.1007/s00213-009-1685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Steidl S, et al. M5 muscarinic receptors mediate striatal dopamine activation by ventral tegmental morphine and pedunculopontine stimulation in mice. PLoS One. 2011;6:e27538. doi: 10.1371/journal.pone.0027538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- 477.Calabresi P, et al. A convergent model for cognitive dysfunctions in Parkinson’s disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol. 2006;5:974–983. doi: 10.1016/S1474-4422(06)70600-7. [DOI] [PubMed] [Google Scholar]
- 478.Janhunen S, Ahtee L. Differential nicotinic regulation of the nigrostriatal and mesolimbic dopaminergic pathways: implications for drug development. Neurosci Biobehav Rev. 2007;31:287–314. doi: 10.1016/j.neubiorev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 479.Livingstone PD, Wonnacott S. Nicotinic acetylcholine receptors and the ascending dopamine pathways. Biochem Pharmacol. 2009;78:744–755. doi: 10.1016/j.bcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 480.Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27:947–957. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Threlfell S, et al. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 482.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 483.McCallum SE, et al. Increases in alpha4* but not alpha3*/alpha6* nicotinic receptor sites and function in the primate striatum following chronic oral nicotine treatment. J Neurochem. 2006;96:1028–1041. doi: 10.1111/j.1471-4159.2005.03646.x. [DOI] [PubMed] [Google Scholar]
- 484.Zhang L, et al. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 2009;76:396–404. doi: 10.1124/mol.109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Zhang T, et al. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29:4035–4043. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Cragg SJ. Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum. J Neurosci. 2003;23:4378–4385. doi: 10.1523/JNEUROSCI.23-10-04378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- 488.Grady SR, et al. Pharmacological comparison of transient and persistent [3H]dopamine release from mouse striatal synaptosomes and response to chronic L-nicotine treatment. J Pharmacol Exp Ther. 1997;282:32–43. [PubMed] [Google Scholar]
- 489.Rathouz MM, Berg DK. Synaptic-type acetylcholine receptors raise intracellular calcium levels in neurons by two mechanisms. J Neurosci. 1994;14:6935–6945. doi: 10.1523/JNEUROSCI.14-11-06935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Coulter CL, Happe HK, Murrin LC. Postnatal development of the dopamine transporter: a quantitative autoradiographic study. Brain Res Dev Brain Res. 1996;92:172–181. doi: 10.1016/0165-3806(96)00004-1. [DOI] [PubMed] [Google Scholar]
- 491.Exley R, et al. Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc Natl Acad Sci U S A. 2011;108:7577–7582. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Dougherty JJ, Nichols RA. Cross-regulation between colocalized nicotinic acetylcholine and 5-HT3 serotonin receptors on presynaptic nerve terminals. Acta Pharmacol Sin. 2009;30:788–794. doi: 10.1038/aps.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 494.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 495.Vizi ES, Lendvai B. Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res Brain Res Rev. 1999;30:219–235. doi: 10.1016/s0165-0173(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 496.Yu ZJ, Wecker L. Chronic nicotine administration differentially affects neurotransmitter release from rat striatal slices. J Neurochem. 1994;63:186–194. doi: 10.1046/j.1471-4159.1994.63010186.x. [DOI] [PubMed] [Google Scholar]
- 497.Reuben M, Clarke PB. Nicotine-evoked [3H]5-hydroxytryptamine release from rat striatal synaptosomes. Neuropharmacology. 2000;39:290–299. doi: 10.1016/s0028-3908(99)00147-1. [DOI] [PubMed] [Google Scholar]
- 498.Becquet D, Faudon M, Hery F. In vivo evidence for acetylcholine control of serotonin release in the cat caudate nucleus: influence of halothane anaesthesia. Neuroscience. 1988;27:819–826. doi: 10.1016/0306-4522(88)90185-6. [DOI] [PubMed] [Google Scholar]
- 499.Gras C, et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Higley MJ, et al. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Nelson AB, et al. Striatal cholinergic neurotransmission requires VGLUT3. J Neurosci. 2014;34:8772–8777. doi: 10.1523/JNEUROSCI.0901-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Gras C, et al. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- 503.Crittenden JR, et al. Severe drug-induced repetitive behaviors and striatal overexpression of VAChT in ChAT-ChR2-EYFP BAC transgenic mice. Front Neural Circuits. 2014;8:57. doi: 10.3389/fncir.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 505.Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.DeLong M, Wichmann T. Changing views of basal ganglia circuits and circuit disorders. Clin EEG Neurosci. 2010;41:61–67. doi: 10.1177/155005941004100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Surmeier DJ, Sulzer D. The pathology roadmap in Parkinson disease. Prion. 2013;7:85–91. doi: 10.4161/pri.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov Disord. 2013;28:715–724. doi: 10.1002/mds.25187. [DOI] [PubMed] [Google Scholar]
- 510.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 511.Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 513.Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Luijten M, Field M, Franken IH. Pharmacological interventions to modulate attentional bias in addiction. CNS Spectr. 2014;19:239–246. doi: 10.1017/S1092852913000485. [DOI] [PubMed] [Google Scholar]
- 516.Quik M, et al. Role for the nicotinic cholinergic system in movement disorders; therapeutic implications. Pharmacol Ther. 2014;144:50–59. doi: 10.1016/j.pharmthera.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Britt JP, Bonci A. Optogenetic interrogations of the neural circuits underlying addiction. Curr Opin Neurobiol. 2013;23:539–545. doi: 10.1016/j.conb.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Fino E, Glowinski J, Venance L. Effects of acute dopamine depletion on the electrophysiological properties of striatal neurons. Neurosci Res. 2007;58:305–316. doi: 10.1016/j.neures.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 519.Sanchez G, et al. Reduction of an afterhyperpolarization current increases excitability in striatal cholinergic interneurons in rat parkinsonism. J Neurosci. 2011;31:6553–6564. doi: 10.1523/JNEUROSCI.6345-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Sirviö J, et al. Different forms of brain acetylcholinesterase and muscarinic binding in Parkinson’s disease. J Neurol Sci. 1989;90:23–32. doi: 10.1016/0022-510x(89)90042-7. [DOI] [PubMed] [Google Scholar]
- 521.Zang LY, Misra HP. Inactivation of acetylcholinesterase by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride. Mol Cell Biochem. 2003;254:131–136. doi: 10.1023/a:1027376303043. [DOI] [PubMed] [Google Scholar]
- 522.Zang LY, Misra HP. Inhibition of acetylcholinesterase by the neurotoxicant, 1-methyl-4-phenyl-2,3-dihydropyridinium ion. Arch Biochem Biophys. 1996;336:147–150. doi: 10.1006/abbi.1996.0542. [DOI] [PubMed] [Google Scholar]
- 523.Zang LY, Misra HP. Acetylcholinesterase inhibition by 1-methyl-4-phenylpyridinium ion, a bioactivated metabolite of MPTP. Mol Cell Biochem. 1993;126:93–100. doi: 10.1007/BF00925686. [DOI] [PubMed] [Google Scholar]
- 524.Hadjiconstantinou M, et al. Enhanced MPTP neurotoxicity after treatment with isoflurophate or cholinergic agonists. J Pharmacol Exp Ther. 1994;270:639–644. [PubMed] [Google Scholar]
- 525.Raz A, et al. Activity of pallidal and striatal tonically active neurons is correlated in mptp-treated monkeys but not in normal monkeys. J Neurosci. 2001;21:RC128. doi: 10.1523/JNEUROSCI.21-03-j0006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Raz A, et al. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. J Neurophysiol. 1996;76:2083–2088. doi: 10.1152/jn.1996.76.3.2083. [DOI] [PubMed] [Google Scholar]
- 527.Goldberg JA, et al. Spike synchronization in the cortex/basal-ganglia networks of Parkinsonian primates reflects global dynamics of the local field potentials. J Neurosci. 2004;24:6003–6010. doi: 10.1523/JNEUROSCI.4848-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Belluscio M, et al. Oscillations in the basal ganglia in Parkinson’s disease: Role of the striatum. Basal Ganglia. 2014;3:203–212. [Google Scholar]
- 529.Hung AY, Schwarzschild MA. Treatment of Parkinson’s disease: what’s in the non-dopaminergic pipeline? Neurotherapeutics. 2014;11:34–46. doi: 10.1007/s13311-013-0239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Silkis I. The reasons for the preferable use of A2A receptor antagonists for improvement of locomotor activity and learning. Neurochemical Journal. 2014;8:247–258. [Google Scholar]
- 531.Salin P, et al. Changes to interneuron-driven striatal microcircuits in a rat model of Parkinson’s disease. Neurobiol Dis. 2009;34:545–552. doi: 10.1016/j.nbd.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 532.Gittis AH, et al. Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron. 2011;71:858–868. doi: 10.1016/j.neuron.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Albin RL, et al. The functional anatomy of basal ganglia disorders. Trends in neurosciences. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 534.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 535.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Obeso JA, Lanciego JL. Past, present, and future of the pathophysiological model of the Basal Ganglia. Front Neuroanat. 2011;5:39. doi: 10.3389/fnana.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Villalba RM, Wichmann T, Smith Y. Neuronal loss in the caudal intralaminar thalamic nuclei in a primate model of Parkinson’s disease. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Henderson JM, et al. Degeneration of the centré median-parafascicular complex in Parkinson’s disease. Ann Neurol. 2000;47:345–352. [PubMed] [Google Scholar]
- 539.Henderson JM, et al. Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson’s disease: clinical and therapeutic implications. Brain. 2000;123(Pt 7):1410–1421. doi: 10.1093/brain/123.7.1410. [DOI] [PubMed] [Google Scholar]
- 540.Halliday GM. Thalamic changes in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S152–155. doi: 10.1016/S1353-8020(09)70804-1. [DOI] [PubMed] [Google Scholar]
- 541.Halliday GM, Macdonald V, Henderson JM. A comparison of degeneration in motor thalamus and cortex between progressive supranuclear palsy and Parkinson’s disease. Brain. 2005;128:2272–2280. doi: 10.1093/brain/awh596. [DOI] [PubMed] [Google Scholar]
- 542.Zhang X, Feng ZJ, Chergui K. GluN2D-containing NMDA receptors inhibit neurotransmission in the mouse striatum through a cholinergic mechanism: implication for Parkinson’s disease. J Neurochem. 2014;129:581–590. doi: 10.1111/jnc.12658. [DOI] [PubMed] [Google Scholar]
- 543.Mark GP, et al. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- 544.Berlanga ML, et al. Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine. Neuroscience. 2003;120:1149–1156. doi: 10.1016/s0306-4522(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 545.Hikida T, et al. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci U S A. 2003;100:6169–6173. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Kitabatake Y, et al. Impairment of reward-related learning by cholinergic cell ablation in the striatum. Proc Natl Acad Sci U S A. 2003;100:7965–7970. doi: 10.1073/pnas.1032899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Grasing K, He S, Yang Y. Long-lasting decreases in cocaine-reinforced behavior following treatment with the cholinesterase inhibitor tacrine in rats selectively bred for drug self-administration. Pharmacol Biochem Behav. 2009;94:169–178. doi: 10.1016/j.pbb.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 548.Fagergren P, et al. Blunted response to cocaine in the Flinders hypercholinergic animal model of depression. Neuroscience. 2005;132:1159–1171. doi: 10.1016/j.neuroscience.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 549.Imperato A, et al. Evidence that neuroleptics increase striatal acetylcholine release through stimulation of dopamine D1 receptors. J Pharmacol Exp Ther. 1993;266:557–562. [PubMed] [Google Scholar]
- 550.Zocchi A, Pert A. Alterations in striatal acetylcholine overflow by cocaine, morphine, and MK-801: relationship to locomotor output. Psychopharmacology (Berl) 1994;115:297–304. doi: 10.1007/BF02245069. [DOI] [PubMed] [Google Scholar]
- 551.Day JC, et al. Cocaine-induced increase in cortical acetylcholine release: interaction with the hypothalamo-pituitary-adrenal axis. Eur J Neurosci. 1997;9:1130–1136. doi: 10.1111/j.1460-9568.1997.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 552.Smith JE, Vaughn TC, Co C. Acetylcholine turnover rates in rat brain regions during cocaine self-administration. J Neurochem. 2004;88:502–512. doi: 10.1046/j.1471-4159.2003.02222.x. [DOI] [PubMed] [Google Scholar]
- 553.Zanetti L, et al. Inhibition of both alpha7* and beta2* nicotinic acetylcholine receptors is necessary to prevent development of sensitization to cocaine-elicited increases in extracellular dopamine levels in the ventral striatum. Psychopharmacology (Berl) 2006;187:181–188. doi: 10.1007/s00213-006-0419-y. [DOI] [PubMed] [Google Scholar]
- 554.Ena S, de Kerchove d’Exaerde A, Schiffmann SN. Unraveling the differential functions and regulation of striatal neuron sub-populations in motor control, reward, and motivational processes. Front Behav Neurosci. 2011;5:47. doi: 10.3389/fnbeh.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Prast JM, et al. Acetylcholine, drug reward and substance use disorder treatment: intra- and interindividual striatal and accumbal neuron ensemble heterogeneity may explain apparent discrepant findings. Pharmacology. 2012;90:264–273. doi: 10.1159/000342636. [DOI] [PubMed] [Google Scholar]
- 556.Carelli RM. Nucleus accumbens cell firing and rapid dopamine signaling during goal-directed behaviors in rats. Neuropharmacology. 2004;47(Suppl 1):180–189. doi: 10.1016/j.neuropharm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 557.Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysiol. 1996;75:1061–1073. doi: 10.1152/jn.1996.75.3.1061. [DOI] [PubMed] [Google Scholar]
- 558.Deadwyler SA, et al. Reward, memory and substance abuse: functional neuronal circuits in the nucleus accumbens. Neurosci Biobehav Rev. 2004;27:703–711. doi: 10.1016/j.neubiorev.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 559.Opris I, Hampson RE, Deadwyler SA. The encoding of cocaine vs. natural rewards in the striatum of nonhuman primates: categories with different activations. Neuroscience. 2009;163:40–54. doi: 10.1016/j.neuroscience.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Pratt WE, Spencer RC, Kelley AE. Muscarinic receptor antagonism of the nucleus accumbens core causes avoidance to flavor and spatial cues. Behav Neurosci. 2007;121:1215–1223. doi: 10.1037/0735-7044.121.6.1215. [DOI] [PubMed] [Google Scholar]
- 561.Pratt WE, Kelley AE. Nucleus accumbens acetylcholine regulates appetitive learning and motivation for food via activation of muscarinic receptors. Behav Neurosci. 2004;118:730–739. doi: 10.1037/0735-7044.118.4.730. [DOI] [PubMed] [Google Scholar]
- 562.Crespo JA, et al. Activation of muscarinic and nicotinic acetylcholine receptors in the nucleus accumbens core is necessary for the acquisition of drug reinforcement. J Neurosci. 2006;26:6004–6010. doi: 10.1523/JNEUROSCI.4494-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Pierce RC, Vanderschuren LJ. Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 565.Duvoisin RC. Cholinergic-anticholinergic antagonism in parkinsonism. Arch Neurol. 1967;17:124–136. doi: 10.1001/archneur.1967.00470260014002. [DOI] [PubMed] [Google Scholar]
- 566.Barbeau A. The pathogenesis of Parkinson’s disease: a new hypothesis. Can Med Assoc J. 1962;87:802–807. [PMC free article] [PubMed] [Google Scholar]
- 567.Bartholini G, et al. GABA receptor agonists and extrapyramidal motor function: therapeutic implications for Parkinson’s disease. Adv Neurol. 1987;45:79–83. [PubMed] [Google Scholar]
- 568.Katzenschlager R, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003:CD003735. doi: 10.1002/14651858.CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Xiang Z, et al. Roles of the M1 muscarinic acetylcholine receptor subtype in the regulation of basal ganglia function and implications for the treatment of Parkinson’s disease. J Pharmacol Exp Ther. 2012;340:595–603. doi: 10.1124/jpet.111.187856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Foster DJ, et al. Activation of M1 and M4 muscarinic receptors as potential treatments for Alzheimer’s disease and schizophrenia. Neuropsychiatr Dis Treat. 2014;10:183–191. doi: 10.2147/NDT.S55104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14:413–420. [PMC free article] [PubMed] [Google Scholar]
- 572.Smith GS, Li X, Conn PJ. Neurotherapeutics. Neuropsychopharmacology. 2012;37:1–3. doi: 10.1038/npp.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Thomsen M, et al. Contribution of both M1 and M4 receptors to muscarinic agonist-mediated attenuation of the cocaine discriminative stimulus in mice. Psychopharmacology (Berl) 2012;220:673–685. doi: 10.1007/s00213-011-2516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Lim SA, Kang UJ, McGehee DS. Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci. 2014;6:22. doi: 10.3389/fnsyn.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Ding Y, et al. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci U S A. 2011;108:840–845. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Threlfell S, et al. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Kayadjanian N, et al. Cortical and nigral deafferentation and striatal cholinergic markers in the rat dorsal striatum: different effects on the expression of mRNAs encoding choline acetyltransferase and muscarinic m1 and m4 receptors. Eur J Neurosci. 1999;11:3659–3668. doi: 10.1046/j.1460-9568.1999.00788.x. [DOI] [PubMed] [Google Scholar]
- 578.Perez XA, et al. α6β2* and α4β2* nicotinic receptors both regulate dopamine signaling with increased nigrostriatal damage: relevance to Parkinson’s disease. Mol Pharmacol. 2010;78:971–980. doi: 10.1124/mol.110.067561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Zhang D, et al. ABT-089 and ABT-894 reduce levodopa-induced dyskinesias in a monkey model of Parkinson’s disease. Mov Disord. 2014;29:508–517. doi: 10.1002/mds.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Bordia T, et al. Nigrostriatal damage preferentially decreases a subpopulation of alpha6beta2* nAChRs in mouse, monkey, and Parkinson’s disease striatum. Mol Pharmacol. 2007;72:52–61. doi: 10.1124/mol.107.035998. [DOI] [PubMed] [Google Scholar]
- 581.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Bradberry CW, et al. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Ito R, et al. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Ito R, et al. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Letchworth SR, et al. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Porrino LJ, et al. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Porrino LJ, et al. The expanding effects of cocaine: studies in a nonhuman primate model of cocaine self-administration. Neurosci Biobehav Rev. 2004;27:813–820. doi: 10.1016/j.neubiorev.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 588.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 589.Kosten TR, George TP, Kosten TA. The potential of dopamine agonists in drug addiction. Expert Opin Investig Drugs. 2002;11:491–499. doi: 10.1517/13543784.11.4.491. [DOI] [PubMed] [Google Scholar]
- 590.Kleber HD. Pharmacologic treatments for heroin and cocaine dependence. Am J Addict. 2003;12(Suppl 2):S5–S18. [PubMed] [Google Scholar]
- 591.Pierce RC, et al. Rational development of addiction pharmacotherapies: successes, failures, and prospects. Cold Spring Harb Perspect Med. 2012;2:a012880. doi: 10.1101/cshperspect.a012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30:15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Wilson MC, Schuster CR. Cholinergic influence on intravenous cocaine self-administration by rhesus monkeys. Pharmacol Biochem Behav. 1973;1:643–649. doi: 10.1016/0091-3057(73)90027-0. [DOI] [PubMed] [Google Scholar]
- 594.Dewey SL, et al. Effects of central cholinergic blockade on striatal dopamine release measured with positron emission tomography in normal human subjects. Proc Natl Acad Sci U S A. 1993;90:11816–11820. doi: 10.1073/pnas.90.24.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Acri JB, Siedleck BK, Witkin JM. Effects of benztropine on behavioral and toxic effects of cocaine: comparison with atropine and the selective dopamine uptake inhibitor 1-[2-(diphenylmethoxy)ethyl]-4-(3-phenyl-propyl)-piperazine. J Pharmacol Exp Ther. 1996;277:198–206. [PubMed] [Google Scholar]
- 596.Katz JL, et al. Novel 3alpha-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. J Pharmacol Exp Ther. 1999;288:302–315. [PubMed] [Google Scholar]
- 597.Tanda G, et al. Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther. 2007;321:334–344. doi: 10.1124/jpet.106.118067. [DOI] [PubMed] [Google Scholar]
- 598.Tanda G, Katz JL. Muscarinic preferential M(1) receptor antagonists enhance the discriminative-stimulus effects of cocaine in rats. Pharmacol Biochem Behav. 2007;87:400–404. doi: 10.1016/j.pbb.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Ranaldi R, Woolverton WL. Self-administration of cocaine: scopolamine combinations by rhesus monkeys. Psychopharmacology (Berl) 2002;161:442–448. doi: 10.1007/s00213-002-1069-3. [DOI] [PubMed] [Google Scholar]
- 600.Yee J, et al. Muscarinic acetylcholine receptors in the nucleus accumbens core and shell contribute to cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2011;650:596–604. doi: 10.1016/j.ejphar.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Fink-Jensen A, et al. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J Neurosci Res. 2003;74:91–96. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- 602.Hansen ST, Mark GP. The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access. Psychopharmacology (Berl) 2007;194:53–61. doi: 10.1007/s00213-007-0822-z. [DOI] [PubMed] [Google Scholar]
- 603.Reid MS, et al. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- 604.Zachariou V, et al. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–589. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 605.Blokhina EA, et al. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. Eur Neuropsychopharmacol. 2005;15:219–225. doi: 10.1016/j.euroneuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 606.Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology (Berl) 2002;162:178–185. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Reid MS, et al. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 608.Thomsen M, et al. Attenuation of cocaine’s reinforcing and discriminative stimulus effects via muscarinic M1 acetylcholine receptor stimulation. J Pharmacol Exp Ther. 2010;332:959–969. doi: 10.1124/jpet.109.162057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Dencker D, et al. An allosteric enhancer of M4 muscarinic acetylcholine receptor function inhibits behavioral and neurochemical effects of cocaine. Psychopharmacology (Berl) 2012;224:277–287. doi: 10.1007/s00213-012-2751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Schmidt LS, et al. Increased cocaine self-administration in M4 muscarinic acetylcholine receptor knockout mice. Psychopharmacology (Berl) 2011;216:367–378. doi: 10.1007/s00213-011-2225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Robinson TE, et al. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 612.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 613.Alcantara AA, et al. Cocaine- and morphine-induced synaptic plasticity in the nucleus accumbens. Synapse. 2011;65:309–320. doi: 10.1002/syn.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Li J, et al. Cocaine-induced dendritic remodeling occurs in both D1 and D2 dopamine receptor-expressing neurons in the nucleus accumbens. Neurosci Lett. 2012;517:118–122. doi: 10.1016/j.neulet.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 615.Bridges TM, et al. Chemical lead optimization of a pan G(q) mAChR M(1), M(3), M(5) positive allosteric modulator (PAM) lead. Part I: Development of the first highly selective M(5) PAM. Bioorg Med Chem Lett. 2010;20:558–562. doi: 10.1016/j.bmcl.2009.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Bridges TM, et al. Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part II: development of a potent and highly selective M1 PAM. Bioorg Med Chem Lett. 2010;20:1972–1975. doi: 10.1016/j.bmcl.2010.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Sidique S, et al. Orally active metabotropic glutamate subtype 2 receptor positive allosteric modulators: structure-activity relationships and assessment in a rat model of nicotine dependence. J Med Chem. 2012;55:9434–9445. doi: 10.1021/jm3005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Tarr JC, et al. Targeting selective activation of M(1) for the treatment of Alzheimer’s disease: further chemical optimization and pharmacological characterization of the M(1) positive allosteric modulator ML169. ACS Chem Neurosci. 2012;3:884–895. doi: 10.1021/cn300068s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Yoo JH, Kitchen I, Bailey A. The endogenous opioid system in cocaine addiction: what lessons have opioid peptide and receptor knockout mice taught us? Br J Pharmacol. 2012;166:1993–2014. doi: 10.1111/j.1476-5381.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Bossert JM, et al. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Giuliano C, et al. Attenuation of cocaine and heroin seeking by μ-opioid receptor antagonism. Psychopharmacology (Berl) 2013;227:137–147. doi: 10.1007/s00213-012-2949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]