SUMMARY
Previous studies have shown that VimA, an acetyltransferase, can modulate gingipain biogenesis in Porphyromonas gingivalis. Inactivation of the vimA gene resulted in isogenic mutants that showed a late onset of gingipain activity that only occurred during the stationary growth phase. To further elucidate the role and contribution of the gingipains in this VimA-dependent process, isogenic mutants defective in the gingipain genes in the vimA-deficient genetic background were evaluated. In contrast to the wild-type strain, RgpB and Kgp gingipain activities were absent in exponential phase in the ΔrgpA::tetQ-vimA::ermF mutant. However, these activities increased to 31% and 53%, respectively, of that of the wild-type during stationary phase. In the ΔrgpA::cat-Δkgp::tetQ-vimA::ermF mutant, the RgpB protein was observed in the extracellular fraction but no activity was present even at the stationary growth phase. There was no gingipain activity observed in the ΔrgpB::cat-Δkgp::tetQ-vimA::ermF mutant while Kgp activity in ΔrgpA::cat-ΔrgpB::tetQ-vimA::ermF mutant was 24% of the wild-type at late stationary phase. In contrast to RgpA, the glycosylation profile of the RgpB catalytic domain from both W83 and P. gingivalis FLL92 (vimA::ermF) showed similarity. Taken together, the results suggest multiple gingipain activation pathways in P. gingivalis. While the maturation pathways for RgpA and RgpB are different, the late onset gingipain activity in the vimA-defective mutant was due to activation/maturation of RgpB and Kgp. Moreover, unlike RgpA which is VimA-dependent, the maturation/activation pathways for RgpB and Kgp are interdependent in the absence VimA.
Keywords: Gingipain, Periodontal disease, VimA, Acetylation, Glycosylation
Introduction
Porphyromonas gingivalis is considered as an important etiological agent of periodontal disease and is associated with other systemic diseases including cardiovascular disease and rheumatoid arthritis (Darveau, 2010; Hajishengallis, 2009; Mikuls et al., 2009; Ogrendik, 2012). Among several proteolytic enzymes produced by P. gingivalis, cysteine proteinases, referred to as gingipains, have been recognized as one of the major virulence factors and play a significant role in the pathogenesis of the organism (Stafford et al., 2013; Yongqing et al., 2011).
The gingipain family in P. gingivalis consists of three members: two arginine-specific proteases (Rgp) and a lysine-specific protease (Kgp). These gingipains are encoded for by the rgpA, rgpB, and kgp, genes respectively. In contrast to the mature RgpB which only possesses a catalytic domain, RgpA and Kgp have homologous adhesin/hemagglutinin domains as part of their C-terminal extension (Li & Collyer, 2011). One of the functional properties of these domains involves targeting these gingipains to specific host substrates (Fitzpatrick et al., 2009; Rapala-Kozik et al., 2011). Common to the three members of the gingipain family is a conserved C-terminal domain (CTD; 70–80 amino acid residues in length) which is known to be involved in their maturation and translocation (Nguyen et al., 2009; Sato et al., 2013; Seers et al., 2006; Slakeski et al., 2011; Zhou et al., 2013). Gingipain biogenesis including secretion and activation is a complex process whose details are still emerging. In addition to their use of a novel secretion system [T9SS (PorSS)] to translocate the maturing enzyme through the outer membrane of the cell, post-translational cleavage and glycosylation is required for their anchorage and activation (Muthiah et al., 2013; Yamaguchi et al., 2010; Zhou et al., 2013). There is still a gap in our comprehensive understanding of the exact mechanism required for the activation of the gingipains.
The P. gingivalis vimA gene is part of the bcp-recA-vimA-vimE-vimF-aroG operon, and encodes a 39 kDa putative acetyltransferase protein (Vanterpool et al., 2006). Inactivation of the vimA gene resulted in a non-black pigmented strain designated P. gingivalis FLL92, which showed dramatically reduced levels of proteolytic, hemagglutination, hemolytic activities, and virulence in a mouse model. The gingipain activity, which had a late onset and was mostly soluble with little or no cell-associated activity, was predominantly observed in the stationary growth phase (Abaibou et al., 2001). Furthermore, compared to the wild-type strain, there were no observed changes in the expression profile of the gingipain genes in P. gingivalis FLL92 (vimA-defective mutant) (Vanterpool et al., 2004). Thus, the reduced proteolytic activity observed in the vimA-defective mutant was a result of a defect in the post-translational modification of the gingipains. This was further confirmed by the detection of the secreted proenzyme species (Osbourne et al., 2012; Vanterpool et al., 2006). Though the mechanism for the Vim proteins has not been thoroughly elucidated, we have demonstrated that VimA modulates the glycosylation and anchorages of several surface proteins including the gingipain proteases (Osbourne et al., 2010). In other studies we have shown that the vimA-defective mutant FLL92 has decreased acetyl-CoA transfer activity compared to the wild-type (Aruni et al., 2012). While VimA can interact with several proteins including the gingipains (Vanterpool et al., 2006), its impact on the activation of the specific gingipain is unknown.
To determine whether the late onset gingipain activity in P. gingivalis FLL92 (vimA-defective mutant) is due to RgpA or RgpB, isogenic mutants defective in the gingipain gene in various combinations in both W83 and FLL92 (vimA-defective) genetic backgrounds were constructed and characterized. The emerging observations suggest the presence of multiple gingipain activation pathways in P. gingivalis. While the maturation pathways for RgpA and RgpB are different, the late onset gingipains activity in the vimA-defective mutant was due to activation/maturation of RgpB and Kgp. Moreover, unlike RgpA which is VimA-dependent, the maturation and activation pathways for RgpB and Kgp are interdependent in the absence VimA.
Materials and Methods
Bacterial strains, plasmids, and culture conditions
Strains and plasmids used in this study are listed in Table 1. P. gingivalis strains were grown in Brain Heart Infusion (BHI) broth supplemented with 0.5% yeast extract (Difco Laboratories, Detroit, Mich.), hemin (5 μg/ml), vitamin K (0.5 μg/ml), and DL-cysteine (0.1%) (Sigma-Aldrich, St. Louis, MO). P. gingivalis strains were grown in an anaerobic chamber (Coy Manufacturing, Ann Arbor, Mich.) in 10% H2, 10% CO2, and 80% N2 at 37°C. Growth rates for P. gingivalis strains were determined spectrophotometrically by measuring optical density at 600 nm [OD600]. Antibiotics were used at the following concentrations: erythromycin 10 μg/ml; tetracycline 3 μg/ml; chloramphenicol 20 μg/ml.
Table 1.
Strains and plasmids used in this study
| Strains and plasmids | Relevant characteristics | References |
|---|---|---|
| Strains | ||
| P. gingivalis | ||
| W83 | Wild-type | Henry et al. (2008) |
| FLL92 | vimA::ermF | Abaibou et al. (2001) |
| FLL370 | ΔrgpB::ermF | This study |
| FLL371 | ΔrgpB::tetQ, vimA::ermF | This study |
| FLL372 | ΔrgpA::ermF | This study |
| FLL373 | ΔrgpA::tetQ, vimA::ermF | This study |
| FLL374 | Δkgp::ermF | This study |
| FLL375 | Δkgp::tetQ,vimA::ermF | This study |
| FLL376 | ΔrgpA::ermF, ΔrgpB::tetQ | This study |
| FLL377 | ΔrgpA::tetQ, Δkgp::ermF | This study |
| FLL378 | ΔrgpB::tetQ, Δkgp::ermF | This study |
| FLL379 | ΔrgpA::cat, Δkgp::tetQ,vimA::ermF | This study |
| FLL380 | ΔrgpB::cat, Δkgp::tetQ,vimA::ermF | This study |
| FLL381 | ΔrgpA::cat, ΔrgpB::tetQ,vimA::ermF | This study |
| Plasmids | ||
| pVA2198 | Spr, ermF | Fletcher et al. (1995) |
| pT-COW | Apr, tetQ | Gardner et al. (Gardner et al., 1996) |
| pCM7 | Apr, cat | ATCC37173 |
Construction of rgpA, rgpB and kgp deficient mutants in W83 and FLL92 (vimA-defective mutant)
Construction of isogenic mutants defective in the gingipain genes in the vimA-defective and wild-type strains was carried out by long PCR based fusion of several fragments using overlapping extension PCR as previously described (Dou et al., 2010). Primers used in this study are listed in Table 2. One kilo-base flanking fragments of both upstream and downstream of the gingipain genes were PCR amplified from chromosomal DNA of P. gingivalis W83. For mutants in W83 the ermF cassette was amplified from the plasmid pVA2198 (Fletcher et al., 1995) with oligonucleotide primers that contained overlapping nucleotides for the 1 kilo-base upstream and downstream fragments respectively. For the mutants in FLL92 (vimA-defective mutant), with the tetQ was used as the selectable marker. The promoterless tetQ cassette was amplified from plasmid pT-COW (Gardner et al., 1996). The upstream fragment, ermF or tetQ, and downstream fragments were fused together by using the forward primer for the upstream fragment and the reverse primer for the downstream fragment. The fusion PCR program consisted of 1 cycle of 5 min at 94 °C, followed by 30 cycles of 30 sec at 94 °C, 30 sec at 54°C, and 4 min at 68°C, with a final extension of 5 min at 68 °C. This PCR-fused fragment was used to electroporate P. gingivalis as previously described (Abaibou et al., 2001). The cells were plated on BHI agar containing 10 μg/ml of erythromycin or 3 μg/ml of tetracycline and incubated at 37 °C for 7 days. The correct gene replacement in the erythromycin or tetracycline resistant mutants was confirmed by PCR and DNA sequencing.
Table 2.
Primers used in this study
| Primer | Sequence (5′---3′) |
|---|---|
| rgpA_F1 | TCCTTCAGCCGGTCGTCTCGGAT |
| rgpA_R1 | TCATTTATTCCTCCTAGTTAGTCAGCAATCGAAACAAACTTGTTCAAGT |
| rgpA_F3 | TTCGTAGTACCTGGAGGGAATAATCCTCGCTATCAAGTAAATCTGTCTTG |
| rgpA_R3 | CCTATAGGCAACTTCTCTTGCAGCA |
| rgpA_TetQ_F3 | AAAATAATACCTGGAGGGAATAATCCTCGCTATCAAGTAAATCTGTCTTG |
| rgpB_F1 | TACGATCTGTACAAGTGGCAAATGCC |
| rgpB_R1 | TCATTTATTCCTCCTAGTTAGTCACTTTTTCATTTGCTTGAATTAGTTTTT |
| rgpB_F3 | TTCGTAGTACCTGGAGGGAATAATCAAGTAATTCACACTGCAATTCTCTAA |
| rgpB_R3 | TGCTACAAACTTCGGCTGGCTCCG |
| rgpB_TetQ_F3 | CAAAATAATACCTGGAGGGAATAATCAAGTAATTCACACTGCAATTCTCTAA |
| kgp_F1 | CGATCGGACAGGGAGATTACAGCTT |
| kgp_R1 | TCATTTATTCCTCCTAGTTAGTCACGATCAGCAATAATAATTTCCTCAT |
| kgp_F3 | TTCGTAGTACCTGGAGGGAATAATCACGTAGAGAAACTCGCTGTAAAGTA |
| kgp_R3 | GGGTGGCGGTAAGGAGTGAAAGTA |
| kgp_TetQ_F3 | AAATAATACCTGGAGGGAATAATCACGTAGAGAAACTCGCTGTAAAGTA |
| rgpA_RT_F | ATGAAAAACTTGAACAAGTTTGTTTC |
| rgpA_RT_R | CCGGTGTGTAACGCCCTG |
| rgpB_RT_F | AAGATATCTATAAGAGCGTCTTCA |
| rgpB_RT_R | CGACCGATGAAGACTTCG |
| kgp_RT_F | ATGAGGAAATTATTATTGCTGATCG |
| kgp_RT_R | TTGAAGAGCTGTTTATAAGCTGTTT |
| Erm_F_f(5) | TGACTAACTAGGAGGAATAAATGACAAAAAAGAAATTGCCCG |
| Erm_F_r(3) | GATTATTCCCTCCAGGTACTACGAAGGATGAAATTTTTCA |
| Tet_Q_F | TGACTAACTAGGAGGAATAAATGAATATTATAAATTTAGGAA |
| Tet_Q_R | GATTATTCCCTCCAGGTATTATTTTGATGACATTGATTTTTGGA |
| Cat_F | TGACTAACTAGGAGGAATAAATGGAGAAAAAAATCACTGGAT |
| Cat_R | GATTATTCCCTCCAGGTATTACGCCCCGCCCTGCCACT |
| rgpB_cat_R1 | CCATTTATTCCTCCTAGTTAGTCACTTTTTCATTTGCTTGAATTAGTTTTT |
| rgpB_cat_F3 | GGCGTAATACCTGGAGGGAATAATCAAGTAATTCACACTGCAATTCTCTAA |
| rgpA_cat_R1 | CCATTTATTCCTCCTAGTTAGTCAGCAATCGAAACAAACTTGTTCAAGT |
| rgpA_cat_F3 | GGCGTAATACCTGGAGGGAATAATCCTCGCTATCAAGTAAATCTGTCTTG |
Construction of rgpArgpB, rgpAkgp and rgpBkgp double mutants in W83
To create the ΔrgpA::ermF-ΔrgpB::tetQ and ΔrgpB::tetQ-Δkgp::ermF double mutants, 1-kb upstream and downstream fragments of rgpB were fused to the promoterless tetQ and then introduced into P. gingivalis FLL372 (ΔrgpA::ermF) or FLL374 (Δkgp::ermF) by electroporation. To make the ΔrgpA::tetQ-Δkgp::ermF double mutant, 1-kb upstream and downstream fragments of rgpA were fused to the promoterless tetQ, and electroporated into FLL374. The mutants were selected on BHI agar containing 10 μg/ml of erythromycin and 3 μg/ml of tetracycline. The correct gene replacement in the erythromycin and tetracycline resistant mutants was confirmed by PCR and DNA sequencing.
Construction of rgpArgpB, rgpAkgp and rgpBkgp mutants in FLL92 (vimA-defective mutant)
To make ΔrgpA::cat-Δkgp::tetQ-vimA::ermF and ΔrgpB::cat-Δkgp::tetQ-vimA::ermF mutants, a chloramphenicol resistant gene cat was used. The cat gene was amplified from plasmid pCM7 (ATCC37173) and then fused to the upstream and downstream fragments of rgpA and rgpB respectively. All the primers used are listed in Table 2. The fused fragments were then transformed into FLL375 by electroporation. To make the ΔrgpA::cat-ΔrgpB::tetQ-vimA::ermF mutant, a DNA fragment carrying the upstream and downstream sequences of rgpA fused to the promoterless cat, was electroporated into FLL371. The mutants were selected on BHI agar containing 10 μg/ml of erythromycin, 3 μg/ml of tetracycline, and 20 μg/ml of chloramphenicol. The correct gene replacement in the erythromycin, tetracycline and chloramphenicol resistant mutants was confirmed by PCR and DNA sequencing.
Reverse transcription (RT)-PCR analysis
Total RNA from P. gingivalis strains was extracted by using the SV Total RNA Isolation System (Promega Corp. Madison, WI) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized by using a Transcriptor High Fidelity cDNA Synthesis Kit (Roche Corp., Indianapolis, IN) according to the manufacturer’s instructions. The primers used to amplify gingipain genes are listed in Table 2.
Gingipain activity assays
The presence of Arg-X- and Lys-X-specific cysteine protease (Rgp and Kgp respectively) activity was determined as previously reported (Dou et al., 2010). In brief, activity of arginine gingipains was measured with 1mM BAPNA (Nα-benzoyl-DL-arginine-p-nitroanilide) in an activated protease buffer (0.2 M Tris-HCl, 0.1 M NaCl, 5 mM CaCl2, 10 mM L-cysteine, pH 7.6). Lysine gingipain activity was measured with 1mM ALNA (Ac-Lys-p-nitroanilide HCl). The O.D. at 405 nm was then measured against a blank BHI broth sample containing no bacteria.
Preparation of P. gingivalis extracellular fractions
Three-liter cultures of P. gingivalis strains were grown to stationary phase. Preparations of whole-cell culture, cell-free medium, and cell suspension were made as previously reported (Vanterpool et al., 2004). Cells were harvested by spinning at 10,000 ×g for 30 min at 4 °C. The cell-free culture fluid was precipitated by using 80% of ammonium sulfate, and the protein pellet was resuspended in 5 ml of 100 mM Tris-HCl buffer (pH 7.4), dialyzed for 24 h against the same buffer, and then stored on ice or at 0 °C. The presence of Arg-X- and Lys-X-specific cysteine protease activities was determined by microplate reader (Bio-Rad).
Purification of the gingipains
The gingipains were purified according to Potempa and Nguyen (Potempa & Nguyen, 2007) with some modifications. Ammonium sulfate instead of acetone precipitation was used to precipitate the gingipains from the culture supernatant of P. gingivalis FLL92 (vimA-defective mutant) or W83 grown to OD600 of 0.8~1.0. In addition, four columns were used in the following order: Hi Load 16/60 Superdex 200, DEAE cellulose anion exchange column, arginine sepharose column, followed by the Superdex 200HR 10/30 column.
Determining monosaccharide composition
Glycoprotein samples (100 ug) were dried and the monosaccharide composition was determined by methanolysis and silylation followed by GC-MS analysis of trimethylsilyl (TMS)-methyl glycosides according to Hu Fu et al (Fu et al., 2007), with the addition of a reacetylation step just prior to silylation using 25 ul of methanol, 25 ul of pyridine, and 25 ul of acetic anhydride at room temperature for 15 minutes, in order to detect amino sugars.
Outer membrane protein isolation
P. gingivalis cells were grown and the outer membrane fraction was extracted using the Sarkosyl method as previously described (Osbourne et al., 2010).
Immunoblot analysis
The proteins were separated and analyzed by regular SDS-PAGE and then transferred the protein bands from polyacrylamide gel to Bio-trace nitrocellulose membranes (Pall Corporation, Ann Arbor, Mich.) using a Semi-Dry Trans-blot apparatus (Bio-Rad) at 15 V for 30 min as described previously (Dou et al., 2014). The blots were probed with antibodies against specific protease domains and species specific secondary antibodies conjugated to horseradish peroxidase (Zymed Laboratories). Immunoreactive proteins were detected by the procedure described in the Western Lightning Chemiluminescence Reagent Plus kit (Perkin-Elmer Life Sciences, Boston, Mass.). For the lectin experiment, the membranes were blocked with PBS/2% Tween-20 for 1 hour and then rinsed twice, 15 min each in PBS. The blots were then incubated at room temperature overnight with 1 ug/ml of lectin-peroxidase (Erythrina cristagalli lectin [ECA] specific for galactose (beta 1,4) N-acetylglucosamine) in PBS containing 0.05% (v/v) Tween-20, 1 mM CaCl2, 1 mM MnCl2, and 1 mM MgCl2. The membranes were then rinsed twice, 15 min each in PBS. Blots were developed using the DAB staining kit (Vector Laboratories).
Tryptic digestion of proteins bound to nitrocellulose
Membrane containing band(s) of interest were cut into 2 mm squares and wet with 50 mM ammonium bicarbonate (ABC). Proteins were reduced with 200 mM TCEP (tris (2-carboxyethyl) phosphine) and alkylated with 0.4 M acrylamide and incubated for 90 minutes at room temperature. Excess acrylamide was inactivated with 200 mM DTT. Protease MAX™ Surfactant (PMax) was added to a final concentration of 0.02% and 0.2 ug of trypsin in 50 mM ABC was added for digestion of proteins at 37 °C for 3 hours. The membranes were removed and washed further with 25 ul 0.01% PMax/50 mM ABC to remove additional peptides and was pooled with the previous reaction. The trypsin and PMax were inactivated with 5 ul 20% acetic acid and dried down to 15 ul with a Speed Vacuum Concentrator (SpeedVac). Peptides were then cleaned with C18 zip tips (Thermo-Fisher Scientific), dried down to dryness with a SpeedVac and then resuspended in 15 ul 0.25% trifluoroacetic acid (TFA).
LC-MS/MS & data analysis
Peptides from each lectin experiment were evaluated by LC-MS/MS on a Thermo LTQ Orbitrap Velos Pro mass spectrometer (http://www.thermo.com) (Thermo-Fisher Scientific). Peptides were separated by capillary reverse phase on a 10 cm × 75 um analytical column using MicroMagic RP-18AQ resin (http://www.michrom.com) (Michrom Bioresources, Inc., Auburn, CA). MS analyses were accomplished using the Standard Top10 CID Instrument Method supplied with the Thermo MS-Software. Run times of 2 hours were alternated with a standard wash. A trypsin-digested hemoglobin standard prepared in-house from Sigma Aldrich Human Hemoglobin (http://www.sigmaaldrich.com) (Sigma Aldrich, St Louis, MO) was run before and after each set of samples. Runs were analyzed first by Mascot (http://www.matrixscience.com) using the Uniprot P. gingivalis FASTA database, which had been modified by combining it with a Uniprot Yeast FASTA database so as to minimize false discoveries. Then for further analyses with X! Tandem and ease of comparison as well as data manipulation, the Mascot files were moved into Scaffold (http://www.proteomesoftware.com). In this way, several runs could be combined into a single biosample so as to maximize true protein discoveries.
Results
Construction of gingipain deficient mutants in P. gingivalis FLL92 (vimA-defective mutant)
To construct gingipain defective mutants in P. gingivalis, a PCR based method was used as described above. To the promoter region upstream of the ATG start codon of ermF, cat, and tetQ, we added a 20-nt length oligonucleotide 5′-TGACTAACTAGGAGGAATAA-3′ containing three stop codons separated by one nucleotide, and the ribosome binding site (rbs) sequence. An 18-nt oligonucleotide 5′-TACCTGGAGGGAATAATC-3′ containing rbs sequence was added to the downstream of the stop codon to avoid polar mutation of downstream genes. We were able to delete the rgpA, rgpB, and kgp genes in W83 and FLL92 (vimA-defective mutant) genetic backgrounds. These mutants were confirmed by PCR, RT-PCR (Fig. 1A), and DNA sequencing (data not shown). Similar to the wild-type strain, FLL370 (ΔrgpB::ermF) and FLL372 (ΔrgpA::ermF) showed black colonies on blood agar plate while FLL374 (Δkgp::ermF) showed white colonies which is consistent with previous reports which showed that Kgp plays a role in acquisition of FePPIX from hemoglobin (Lewis et al., 1999). Moreover only the kgp isogenic mutant (FLL374) showed significantly decreased hemagglutination and hemolytic activities compared to the wild-type as previously reported (Lewis et al., 2009; Okamoto et al., 1998) [data not shown]. Similar to FLL92, the isogenic mutants FLL371 (ΔrgpB::tetQ-vimA::ermF), FLL373 (ΔrgpA::tetQ-vimA::ermF), FLL375 (Δkgp::tetQ-vimA::ermF), FLL379 (ΔrgpA::cat-Δkgp::tetQ-vimA::ermF), FLL380 (ΔrgpB::cat-Δkgp::tetQ-vimA::ermF), and FLL381 (ΔrgpA::cat-ΔrgpB::tetQ-vimA::ermF) displayed white colonies on BHI blood agar plates.
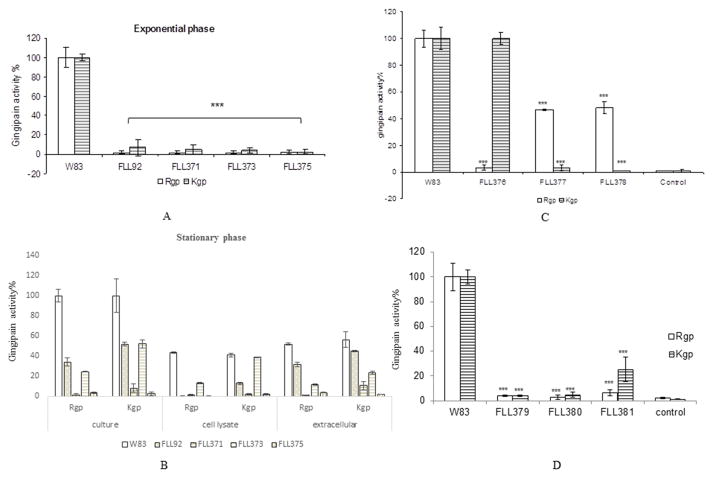
Fig. 1.

(A) RT-PCR confirmation of gingipain mutants. 1, rgpA in FLL373; 2, rgpA in W83; 3, rgpB in FLL371; 4, rgpB in W83; 5, kgp in FLL375; 6, kgp in W83. (B) FLL375 showed a decreased growth rate than other strains. Overnight cultures were adjusted to same value of OD600 and 1% inoculation into fresh BHI broth and samples were taken every three hours and the OD600 were measured by using a spectrophotometer. Error bars represent the standard deviation.
The growth of P. gingivalis FLL92 (vimA-defective mutant) is attenuated with the inactivation of RgpA and Kgp
Inactivation of rgpB in W83 resulted in a slight increase in doubling time in comparison to the wild-type strain while inactivation of rgpA or kgp in W83 did not show any variation in growth rate of P. gingivalis (Fig. 1B). Deletion of rgpA or kgp in FLL92 (vimA-defective mutant) showed a lower growth rate. The doubling time for FLL373 and FLL375 was shown to be between 5 and 6 hrs in comparison to other double mutant strains in W83 genetic background or mutant in FLL92 (vimA-defective) genetic backgrounds.
FLL373 (ΔrgpA::tetQ-vimA::ermF mutant) showed late onset gingipain activity
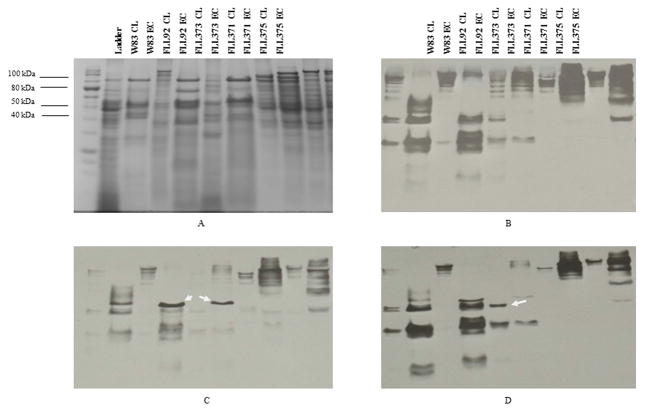
Previous reports have shown that the inactivation of vimA results in a decrease in gingipain activity of more than 95% at exponential phase but has late onset gingipain activity at stationary growth phase (Olango et al., 2003). In order to identify if the late onset activation is due to RgpA, or RgpB, and/or Kgp in FLL92 (vimA-defective mutant), the gingipain activities of FLL371, FLL373, and FLL375 were evaluated (Table 3; Fig. 2A,B). In FLL92, the Rgp activity was 9% of that of wild-type while the Kgp activity was 15% of that of wild-type at exponential phase. After 5 days incubation, the Rgp activity increased to 30% and Kgp activity increased to 60% (Table 3). In the wild-type genetic background, the rgpB deletion mutant FLL370 showed 50% of Arg-X activity and no change in Lys-X activity. In P. gingivalis FLL371 (ΔrgpB::tetQ-vimA::ermF), Arg-X or Lys-X specific gingipain activities were not detectable in either exponential or stationary growth phase (Table 3). In Western-blot analyses using anti-RgpA and/or anti-Kgp antibody, high molecular weight immunoreactive bands similar to the size of the proenzyme forms were observed (Fig. 3B,D). Also there were no detectable 50 kDa immunoreactive band representing the catalytic domain for both Rgp and Kgp in the cell lysate or extracellular part of FLL371 (Fig. 3).
Table 3.
Gingipain activity comparison between different strains at both exponential growth phase and stationary phase. The number showed are percentage to that of the wild-type W83, the data are the average of three biological replicates with three technical replicates each.
| Strain | Genotype | Gingipain activity (%)
|
|||
|---|---|---|---|---|---|
| Exponential phase | Stationary phase | ||||
| Rgp | Kgp | Rgp | Kgp | ||
| W83 | Wild-type | 100 | 100 | 100 | 100 |
| FLL92 | vimA::ermF | 9 | 15 | 29 | 60 |
| FLL370 | ΔrgpB::ermF | 60 | 86 | 56 | 100 |
| FLL371 | ΔrgpB::tetQ-vimA::ermF | 1 | 3 | 7 | 1 |
| FLL372 | ΔrgpA::ermF | 40 | 100 | 39 | 100 |
| FLL373 | ΔrgpA::tetQ-vimA::ermF | 1 | 3 | 31 | 53 |
| FLL374 | Δkgp::ermF | 100 | 0 | 97 | 2 |
| FLL375 | Δkgp::tetQ-vimA::ermF | 2 | 2 | 0 | 2 |
| FLL376 | ΔrgpA::ermF- ΔrgpB::tetQ | 2 | 100 | 2 | 100 |
| FLL377 | ΔrgpA::tetQ- Δkgp::ermF | 46 | 2 | 53 | 1 |
| FLL378 | ΔrgpB::tetQ- Δkgp::ermF | 47 | 0 | 47 | 0 |
| FLL379 | ΔrgpA::cat- Δkgp::tetQ-vimA::ermF | 4 | 3 | 3 | 3 |
| FLL380 | ΔrgpB::cat- Δkgp::tetQ-vimA::ermF | 2 | 4 | 2 | 4 |
| FLL381 | ΔrgpA::cat- ΔrgpB::tetQ-vimA::ermF | 3 | 6 | 5 | 24 |
Fig. 2.
Proteolytic activity of gingipain mutants. Activities against Rgp or Kgp were tested in 2 whole cell culture, and the gingipain activities were normalized to W83 being 100% and the 3 mutants reported as a percentage thereof. Asterisks indicate results significantly different from 4 those of W83 (P<0.005). Error bars represent the standard deviation. (A) Gingipain activity of W83, 5 FLL92, FLL371, FLL373 and FLL375 at exponential phase. (B) Gingipain activity of W83, 6 FLL92, FLL371, FLL373 and FLL375 at stationary phase. (C) Gingipain activity of FLL376, 7 FLL377, and FLL378. (D) FLL379 and FLL380 showed no gingipain activity, but FLL381 showed Kgp activity at stationary phase.
Fig. 3.
Immunoblot analysis showed that RgpB and Kgp were activated in FLL373 at stationary phase. The blots were probed with antibodies against specific protease domains and species specific secondary antibodies conjugated to horseradish peroxidase. (A) SDS-PAGE of W83, FLL92, and gingipain mutants. (B) Blot by RgpA antibody. (C) Blot by RgpB antibody. (D) Blot by Kgp antibody. CL: Cell Lysate; EC: Extracellular. Arrow point the catalytic domain signal band.
The Rgp activity in the rgpA deletion mutant FLL372 was around 40% of that of the wild-type but the Kgp was the same as the wild-type at early stationary phase (Table 3). The ΔrgpA::tetQ-vimA::ermF double mutant FLL373 showed almost no gingipain activity after 2 days at the early stationary phase. After 7 days the Rgp activity increased to 32% of that of the wild-type, and the Kgp activity increased to 53% in FLL373 (Table 3). Western-blot analysis using anti-RgpB antibody and anti-Kgp antibody showed the presence of the catalytic domain for RgpB and Kgp in both FLL92 and FLL373 extracellular preparations (Fig. 3C,D).
In the kgp deletion mutant (FLL374), the Rgp activity was similar to the wild-type; however there was no Kgp activity (Table 3). The Δkgp::tetQ-vimA::ermF double mutant (FLL375) did not show any gingipain activity after 7 days (Table 3; Fig. 2). In a western-blot using anti-RgpA antibody and anti-RgpB antibody, the catalytic domain of Rgp was not present in the cell lysate or the extracellular part of FLL375 (Fig. 3). This data suggests that the process of RgpA maturation is dependent on VimA, and the processes of RgpB and Kgp maturation are dependent on each other when the VimA is absent.
The gingipain activity showed a significant reduction in strain FLL373 (ΔrgpA::tetQ-vimA::ermF), and no gingipain activity in FLL375 (Δkgp::tetQ-vimA::ermF) which showed a decrease in growth. This is consistent with what is known about P. gingivalis as an asaccharolytic organism, which relies on peptides from its environment for all carbon and nitrogen needs. The results suggest that the activation of RgpA is dependent on VimA, with the processing of RgpB and Kgp associated with each other. To test this hypothesis, three mutants were constructed: ΔrgpA::ermF-ΔrgpB::tetQ (FLL376), ΔrgpA::tetQ-Δkgp::ermF (FLL377), and ΔrgpB::tetQ-Δkgp::ermF (FLL378). The gingipain activity assays showed that there was no Rgp activity in FLL376, and the Kgp activity was the same as wild-type (Table 3; Fig. 2C); there was no Kgp activity in both FLL377 and FLL378, and the Rgp activity was around 50% of wild-type in these two mutants. The results suggest that the processing and activation of RgpA, RgpB and Kgp is independent of each other in the W83 genetic background with VimA presence. It is noteworthy that most of the gingipain activity in FLL92 (vimA-defective mutant) at the late stationary phase was extracellular which is in contrast to FLL373 where less than half of those activities were found secreted into the medium (Fig. 2B).
FLL381 (ΔrgpA::cat-ΔrgpB::tetQ-vimA::ermF) showed Kgp activity
The maturation of RgpB and Kgp is putatively associated. To test this hypothesis, three triple mutants were constructed and evaluated for gingipain activity: FLL379 (deletion of rgpA and kgp in FLL92), FLL380 (deletion of rgpB and kgp in FLL92), and FLL381 (deletion of rgpA and rgpB in FLL92). The gingipain activity assay results showed that there is no gingipain activity recovered at stationary phase in FLL379 and FLL380 (Table 3; Fig. 2D). The Kgp activity in FLL381 was 24% that of the wild-type while there was no Rgp activity after 5 to 7 days incubation, which indicated that the maturation of Kgp has two pathways, one of which is dependent on VimA. The western-blot of FLL381 using anti-Kgp antibody showed that the proenzyme of Kgp containing both the catalytic and the adhesion domains existed both in the cell lysate and the extracellular fraction (Fig. 4B). These results suggested that the RgpB activation recovered in FLL373 was dependent on Kgp maturation. However in FLL379, RgpB was present as a 70 kDa protein in the extracellular medium (Fig. 4D), which suggested that the RgpB was secreted without cleavage of the C-terminal domain in FLL379 (ΔrgpA::cat-Δkgp::tetQ-vimA::ermF) (Chen et al., 2011).
Fig. 4.
Kgp proenzyme was shown in both cell lysate and culture supernatant of FLL381 (A,B), and RgpB in culture supernatant of FLL379 at late exponential phase (C,D). The blots were probed with antibodies against Kgp or RgpB catalytic domain, and species specific secondary antibodies conjugated to horseradish peroxidase. (A) SDS-PAGE of FLL381. (B) Kgp antibody western-blot of FLL381. (A) and (B): L, Protein Ladder; 1, Extracellular with 60%+80% ammonium sulfate precipitation; 2, Extracellular with 80% ammonium sulfate precipitation; 3, Cell lysate. (C) SDS-PAGE of FLL379 and FLL380. (D) Western-blot by RgpB antibody. CL: Cell Lysate; Ex: Extracellular. Arrow point the 70 kDa RgpB band.
GC-MS analysis of oligosaccharides present on W83 catalytic domain, FLL92 catalytic domain, and FLL92 proenzyme
The monosaccharide composition of RgpB catalytic domain (active protease from exponential phase) from wild-type W83 was compared with that of the catalytic domain (active, stationary phase) of a vimA mutant FLL92 (Olango et al., 2003), and the inactive RgpB proenzyme of FLL92 using methanolysis and silylation followed by GC-MS analysis of the TMS-methyl glycosides. As shown in Table 4, there were differences in the oligosaccharide profile of the inactive form of RgpB compared to the active form in either wild-type or FLL92, and the acetylated sugars (N-Ac-galactosamine and N-Ac-glucosamine) were missing in the inactive form of RgpB proenzyme.
Table 4.
Monosaccharide composition of W83 RgpB catalytic domain (CD), FLL92 RgpB CD and FLL92 RgpB proenzyme (Unit: mol%). Glycosyl composition of RgpB CD from W83 during exponential phase, RgpB CD from FLL92 during stationary phase, and the RgpB proenzyme from FLL92 during late exponential phase for GC-MS analysis of TMS-methyl glycosides. Experiment was done three times, table is representative of typical data obtained.
| Source | Rib | Fuc | Man | Gal | Glc | GalNAc | GlcNAc |
|---|---|---|---|---|---|---|---|
| W83 RgpB-CD | 7.07 | 0 | 70.42 | 7.66 | 13.64 | 0.35 | 0.86 |
| FLL92 RgpB-CD | 9.40 | 1.12 | 61.33 | 5.97 | 16.70 | 1.92 | 3.55 |
| FLL92 proenzyme | 14.50 | 0 | 69.18 | 2.06 | 14.27 | 0 | 0 |
Differential glycosylation of missing proteins in VimA-defective mutant
Several outer membrane proteins were missing in the vimA-defective mutant compared to the parent strain (Osbourne et al., 2010). Further, there were observed differences in the glycosylation profile of the outer membrane proteins of the vimA-defective mutant compared to the parent strain however, the identity of these proteins were unclear (Osbourne et al., 2010). The ECA lectin specific for (beta 1,4) N-acetylglucosamine was used to identify the glycosylated proteins present in the membrane parent strain but missing in the vimA-defective mutant. LC-MS of the purified proteins showed that RgpA and several proteins annotated as hypothetical had differences in glycosylation and/or missing in FLL92 when compared to W83 (Table 5).
Table 5.
MS identification of outer-membrane proteins whose glycosylation is affected by the vimA mutation as determined by the ECA lectin.
| Identified protein | GenBank accession no. | MW of protein (kDa) | Putative role/function |
|---|---|---|---|
| Hypothetical protein | PG0217 | 33 | Unknown |
| Immunoreactive 42 kDa antigen | PG0694 | 42 | Porin activity and peptidoglyan binding |
| Immunoreactive 43 kDa antigen | PG0695 | 43 | Porin activity and peptidoglycan binding |
| Hypothetical protein | PG1341 | 21 | Contains sporulation related domain |
| Glutamate dehydrogenase | PG1232 | 49 | Degradation of glutamate |
| Hypothetical protein | PG1823 | 24 | Unknown |
| Arg-gingipain RgpA | PG2024 | 186 | Virulence factor |
| Lipoprotein PG3 | PG2054 | 23 | Peptidoglycan-associated |
Discussion
The regulation of gingipain activity can occur at multiple levels including expression of the gingipain genes, secretion, processing of an inactive secreted precursor to its active form, and the glycosylation or other modification of the proteins. Despite the full acceptance of post-translational processing during maturation and activation of the gingipains in P. gingivalis, the underlying mechanisms of biogenesis are still not clear and is under investigation. Furthermore, because the secretion of these proteins in P. gingivalis uses a novel type IX secretion system (Nakayama, 2015), the intricacy of the mechanism(s) for secretion/translocation and maturation/activation is now being uncovered. We have previously demonstrated that the vimA gene product can modulate gingipain activity in P. gingivalis (Abaibou et al., 2001; Olango et al., 2003). While there was decreased gingipain activity in the vimA-defective mutant, its activation did not occur until the stationary growth phase (Olango et al., 2003). Since the VimA protein is a putative acetyltransferase, this raised the possibility that a functional homologue that might be upregulated in the stationary growth phase may play a role in the late onset of gingipain activity in the vimA-defective mutant. Moreover it is likely that there could be a redundant mechanism(s) for gingipain activation in P. gingivalis. Hence, in the present study we have evaluated the components of the gingipain activity observed in the isogenic mutant defective in the vimA gene product.
We have demonstrated in this study, that there may indeed be multiple mechanisms for gingipain activation in P. gingivalis. A late onset of gingipain activity similar to that observed in P. gingivalis FLL92 (vimA::ermF) (Olango et al., 2003) was shown in this study to be present in an isogenic mutant deficient in the rgpA gene in the vimA-defective genetic background. Because Rgp activity was observed in an rgpB-defective mutant in the wild-type W83 genetic background, suggests that RgpA activity can be activated in the absence of RgpB. While this is in contrast to previous observations which suggest that RgpA/maturation/activation is RgpB and Kgp dependent (Rangarajan et al., 2005; Veith et al., 2002), it is likely that differences in the genetic background (P. gingivalis W50 versus W83) may influence the gingipain activation mechanism. Both gingipain RgpB and Kgp showed a similar pattern of activation in the vimA-deficient genetic background (Fig. 3). However, in the absence of Kgp activity there was no RgpB activity thus indicating that in the absence of VimA, the full activation of RgpB is Kgp dependent. Taken together, these observations suggest that the source of the late onset of Arg-X protease activity observed in P. gingivalis FLL92 (vimA::ermF) may be due to RgpB activation.
The activation of RgpB can involve autolytic processing. Mikolajczyk et al. (Mikolajczyk et al., 2003) previously showed that full in vitro activation of the recombinant RgpB required three sequential autolytic processing steps. The first phase which occurs in 2 steps, involves the removal of the N-terminal propeptide. This is sequentially followed in a second phase where the C-terminal extension removed. The involvement of RgpA or Kgp can also play a role in the first phase (Kadowaki et al., 1998; Veith et al., 2002). This is consistent with this study, where in in the absence of VimA, RgpB-dependent Arg-X activity is Kgp dependent. The appropriate glycosylation of the gingipains is also a requirement for full enzyme activation (Muthiah et al., 2013; Rangarajan et al., 2005; Vanterpool et al., 2005; Yamaguchi et al., 2010). A comparison of the RgpB active enzyme isolated at the stationary growth phase in the vimA-defective isogenic mutant showed a similar glycosylation profile as the wild-type. In contrast, the RgpB proenzyme which is present in the vimA-defective mutant during the exponential growth phase appeared to be missing the acetylated sugars. The molecular events of glycosylation include linking monosaccharides together, transferring sugars from one substrate to another and trimming sugars from the glycan structures (Spiro, 2002). Since glycosylation is a non-templated modification, these steps do not necessarily occur during every glycosylation event and rely on other enzymes to add, remove or modify sugars from one molecule to another, generating a diverse range of glycoproteins and modifications. It is unclear what specific role VimA may play in the generation of the acetylated sugars and is currently under further investigation in the laboratory.
The inability for activation of RgpA, and the activation of RgpB and Kgp in the vimA-defective mutant raises questions on the possibility for multiple mechanisms for gingipain activation in P. gingivalis. The activation of RgpA-dependent Arg-X activity is VimA dependent in P. gingivalis. Because VimA is an acetyltransferase and in other experiments have been shown to interact with the gingipains, it is likely that other types of post-translational modification including acetylation may also play a role in gingipains’ activation. It is postulated that the regulation of VimA on gingipains is modulated via a post-translational mechanism involving acetylation [reviewed in (Aruni et al., 2013)]. Preliminary studies in the laboratory have shown the acetylation of multiple sites in the gingipains (Fletcher, personal communication). Acetylation has been shown to affect protein function including protein-protein interaction (Jones & O’Connor, 2011). We cannot rule out the possibility that the acetylation of the gingipains may facilitate the appropriate tertiary conformation and/or protein-protein interaction resulting in its glycosylation/activation. As shown in Fig. 5, our previous studies have shown that VimA (Aruni et al., 2013), VimE (Vanterpool et al., 2005), and VimF (Muthiah et al., 2013) can affect the acetylation and glycosylation of the gingipains. The impact of protein acetylation of gingipain activation is unclear and is the subject for further investigation in the laboratory.
Fig. 5.
Proposed mechanistic role of VimA in gingipain modulation. VimA is an acetyl transferase and is involved in acetylation of Arg-gingipains (RgpA & RgpB) and Lys–gingipain (Kgp) leading to important processes such as gingipain activation/maturation and transport/anchorage. Other than the direct role of VimA on RgpA activation, unknown mechanisms involving acetylation through other acetyltransferase could also probably play an indirect role in gingipain maturation processes. The blue boxes show identified important processes in gingipain modulation. The green boxes show the post translational modifications. The pink boxes show the role of Vim proteins in gingipain modulation. The hatched lines show unknown mechanism under study.
In conclusion, VimA can regulate the maturation process of the gingipains and likely other proteins in P. gingivalis. Based on the observation from this study, the maturation/activation pathways for RgpA and RgpB are different. The late onset of Arg-X gingipain activity in the P. gingivalis vimA-defective mutant was due to activation/maturation of RgpB. Moreover, unlike RgpA which is VimA-dependent, we propose that the level of acetylation may impact RpgB maturation and activation pathway(s) and needs further studies.
Acknowledgments
This work was supported by Public Health Services Grants R56-DE13664, DE019730, DE019730 04S1, DE022508, DE022724 from NIDCR (to H.M.F).
Reference List
- Abaibou H, Chen Z, Olango GJ, Liu Y, Edwards J, Fletcher HM. vimA gene downstream of recA is involved in virulence modulation in Porphyromonas gingivalis W83. Infect Immun. 2001;69:325–335. doi: 10.1128/IAI.69.1.325-335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruni AW, Lee J, Osbourne D, Dou Y, Roy F, Muthiah A, Boskovic DS, Fletcher HM. VimA-dependent modulation of acetyl coenzyme A levels and lipid A biosynthesis can alter virulence in Porphyromonas gingivalis. Infect Immun. 2012;80:550–564. doi: 10.1128/IAI.06062-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruni AW, Robles A, Fletcher HM. VimA mediates multiple functions that control virulence in Porphyromonas gingivalis. Mol Oral Microbiol. 2013;28:167–180. doi: 10.1111/omi.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Peng B, Yang Q, Glew MD, Veith PD, Cross KJ, Goldie KN, Chen D, O’Brien-Simpson N, et al. The outer membrane protein LptO is essential for the O-deacylation of LPS and the co-ordinated secretion and attachment of A-LPS and CTD proteins in Porphyromonas gingivalis. Mol Microbiol. 2011;79:1380–1401. doi: 10.1111/j.1365-2958.2010.07530.x. [DOI] [PubMed] [Google Scholar]
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- Dou Y, Aruni W, Luo T, Roy F, Wang C, Fletcher HM. Involvement of PG2212 a Zinc-finger protein in the regulation of oxidative stress resistance in Porphyromonas gingivalis W83. J Bacteriol. 2014;196:4057–4070. doi: 10.1128/JB.01907-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Y, Osbourne D, McKenzie R, Fletcher HM. Involvement of extracytoplasmic function sigma factors in virulence regulation in Porphyromonas gingivalis W83. FEMS Microbiol Lett. 2010;312:24–32. doi: 10.1111/j.1574-6968.2010.02093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RE, Aprico A, Wijeyewickrema LC, Pagel CN, Wong DM, Potempa J, Mackie EJ, Pike RN. High molecular weight gingipains from Porphyromonas gingivalis induce cytokine responses from human macrophage-like cells via a nonproteolytic mechanism. J Innate Immun. 2009;1:109–117. doi: 10.1159/000181145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher HM, Schenkein HA, Morgan RM, Bailey KA, Berry CR, Macrina FL. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect Immun. 1995;63:1521–1528. doi: 10.1128/iai.63.4.1521-1528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Yadav MP, Nothnagel EA. Physcomitrella patens arabinogalactan proteins contain abundant terminal 3-O-methyl-L: -rhamnosyl residues not found in angiosperms. Planta. 2007;226:1511–1524. doi: 10.1007/s00425-007-0587-y. [DOI] [PubMed] [Google Scholar]
- Gardner RG, Russell JB, Wilson DB, Wang GR, Shoemaker NB. Use of a modified Bacteroides-Prevotella shuttle vector to transfer a reconstructed beta-1,4-D-endoglucanase gene into Bacteroides uniformis and Prevotella ruminicola B(1)4. Appl Environ Microbiol. 1996;62:196–202. doi: 10.1128/aem.62.1.196-202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect. 2009;11:637–645. doi: 10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, O’Connor CD. Protein acetylation in prokaryotes. Proteomics. 2011;11:3012–3022. doi: 10.1002/pmic.201000812. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Nakayama K, Yoshimura F, Okamoto K, Abe N, Yamamoto K. Arg-gingipain acts as a major processing enzyme for various cell surface proteins in Porphyromonas gingivalis. J Biol Chem. 1998;273:29072–29076. doi: 10.1074/jbc.273.44.29072. [DOI] [PubMed] [Google Scholar]
- Lewis JP, Dawson JA, Hannis JC, Muddiman D, Macrina FL. Hemoglobinase activity of the lysine gingipain protease (Kgp) of Porphyromonas gingivalis W83. J Bacteriol. 1999;181:4905–4913. doi: 10.1128/jb.181.16.4905-4913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JP, Iyer D, naya-Bergman C. Adaptation of Porphyromonas gingivalis to microaerophilic conditions involves increased consumption of formate and reduced utilization of lactate. Microbiology. 2009;155:3758–3774. doi: 10.1099/mic.0.027953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Collyer CA. Gingipains from Porphyromonas gingivalis - Complex domain structures confer diverse functions. Eur J Microbiol Immunol (Bp) 2011;1:41–58. doi: 10.1556/EuJMI.1.2011.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk J, Boatright KM, Stennicke HR, Nazif T, Potempa J, Bogyo M, Salvesen GS. Sequential autolytic processing activates the zymogen of Arg-gingipain. J Biol Chem. 2003;278:10458–10464. doi: 10.1074/jbc.M210564200. [DOI] [PubMed] [Google Scholar]
- Mikuls TR, Payne JB, Reinhardt RA, Thiele GM, Maziarz E, Cannella AC, Holers VM, Kuhn KA, O’Dell JR. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol. 2009;9:38–42. doi: 10.1016/j.intimp.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthiah AS, Aruni W, Robles AG, Dou Y, Roy F, Fletcher HM. In Porphyromonas gingivalis VimF is involved in gingipain maturation through the transfer of galactose. PLoS One. 2013;8:e63367. doi: 10.1371/journal.pone.0063367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K. Porphyromonas gingivalis and related bacteria: from colonial pigmentation to the type IX secretion system and gliding motility. J Periodontal Res. 2015;50:1–8. doi: 10.1111/jre.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KA, Zylicz J, Szczesny P, Sroka A, Hunter N, Potempa J. Verification of a topology model of PorT as an integral outer-membrane protein in Porphyromonas gingivalis. Microbiology. 2009;155:328–337. doi: 10.1099/mic.0.024323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrendik M. Does periodontopathic bacterial infection contribute to the etiopathogenesis of the autoimmune disease rheumatoid arthritis? Discov Med. 2012;13:349–355. [PubMed] [Google Scholar]
- Okamoto K, Nakayama K, Kadowaki T, Abe N, Ratnayake DB, Yamamoto K. Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J Biol Chem. 1998;273:21225–21231. doi: 10.1074/jbc.273.33.21225. [DOI] [PubMed] [Google Scholar]
- Olango GJ, Roy F, Sheets SM, Young MK, Fletcher HM. Gingipain RgpB is excreted as a proenzyme in the vimA-defective mutant Porphyromonas gingivalis FLL92. Infect Immun. 2003;71:3740–3747. doi: 10.1128/IAI.71.7.3740-3747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbourne D, Aruni AW, Dou Y, Perry C, Boskovic DS, Roy F, Fletcher HM. VimA-dependent modulation of the secretome in Porphyromonas gingivalis. Mol Oral Microbiol. 2012;27:420–435. doi: 10.1111/j.2041-1014.2012.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbourne D, Aruni W, Roy F, Perry C, Sandberg L, Muthiah A, Fletcher HM. Role of vimA in cell surface biogenesis in Porphyromonas gingivalis. Microbiology. 2010;156:2180–2193. doi: 10.1099/mic.0.038331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J, Nguyen KA. Purification and characterization of gingipains. Curr Protoc Protein Sci. 2007;Chapter 21(Unit 21.20) doi: 10.1002/0471140864.ps2120s49. [DOI] [PubMed] [Google Scholar]
- Rangarajan M, Hashim A, Aduse-Opoku J, Paramonov N, Hounsell EF, Curtis MA. Expression of Arg-Gingipain RgpB is required for correct glycosylation and stability of monomeric Arg-gingipain RgpA from Porphyromonas gingivalis W50. Infect Immun. 2005;73:4864–4878. doi: 10.1128/IAI.73.8.4864-4878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapala-Kozik M, Bras G, Chruscicka B, Karkowska-Kuleta J, Sroka A, Herwald H, Nguyen KA, Eick S, Potempa J, et al. Adsorption of components of the plasma kinin-forming system on the surface of Porphyromonas gingivalis involves gingipains as the major docking platforms. Infect Immun. 2011;79:797–805. doi: 10.1128/IAI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Yukitake H, Narita Y, Shoji M, Naito M, Nakayama K. Identification of Porphyromonas gingivalis proteins secreted by the Por secretion system. FEMS Microbiol Lett. 2013;338:68–76. doi: 10.1111/1574-6968.12028. [DOI] [PubMed] [Google Scholar]
- Seers CA, Slakeski N, Veith PD, Nikolof T, Chen YY, Dashper SG, Reynolds EC. The RgpB C-terminal domain has a role in attachment of RgpB to the outer membrane and belongs to a novel C-terminal-domain family found in Porphyromonas gingivalis. J Bacteriol. 2006;188:6376–6386. doi: 10.1128/JB.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slakeski N, Seers CA, Ng K, Moore C, Cleal SM, Veith PD, Lo AW, Reynolds EC. C-terminal domain residues important for secretion and attachment of RgpB in Porphyromonas gingivalis. J Bacteriol. 2011;193:132–142. doi: 10.1128/JB.00773-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- Stafford P, Higham J, Pinnock A, Murdoch C, Douglas CW, Stafford GP, Lambert DW. Gingipain-dependent degradation of mammalian target of rapamycin pathway proteins by the periodontal pathogen Porphyromonas gingivalis during invasion. Mol Oral Microbiol. 2013;28:366–378. doi: 10.1111/omi.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanterpool E, Roy F, Fletcher HM. The vimE gene downstream of vimA is independently expressed and is involved in modulating proteolytic activity in Porphyromonas gingivalis W83. Infect Immun. 2004;72:5555–5564. doi: 10.1128/IAI.72.10.5555-5564.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanterpool E, Roy F, Fletcher HM. Inactivation of vimF, a putative glycosyltransferase gene downstream of vimE, alters glycosylation and activation of the gingipains in Porphyromonas gingivalis W83. Infect Immun. 2005;73:3971–3982. doi: 10.1128/IAI.73.7.3971-3982.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanterpool E, Roy F, Sanberg L, Fletcher HM. Altered gingipain maturation in vimA- and vimE-defective isogenic mutants of Porphyromonas gingivalis. Infect Immun. 2005;73:1357–1366. doi: 10.1128/IAI.73.3.1357-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanterpool E, Roy F, Zhan W, Sheets SM, Sangberg L, Fletcher HM. VimA is part of the maturation pathway for the major gingipains of Porphyromonas gingivalis W83. Microbiology. 2006;152:3383–3389. doi: 10.1099/mic.0.29146-0. [DOI] [PubMed] [Google Scholar]
- Veith PD, Talbo GH, Slakeski N, Dashper SG, Moore C, Paolini RA, Reynolds EC. Major outer membrane proteins and proteolytic processing of RgpA and Kgp of Porphyromonas gingivalis W50. Biochem J. 2002;363:105–115. doi: 10.1042/0264-6021:3630105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Sato K, Yukitake H, Noiri Y, Ebisu S, Nakayama K. A Porphyromonas gingivalis mutant defective in a putative glycosyltransferase exhibits defective biosynthesis of the polysaccharide portions of lipopolysaccharide, decreased gingipain activities, strong autoaggregation, and increased biofilm formation. Infect Immun. 2010;78:3801–3812. doi: 10.1128/IAI.00071-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongqing T, Potempa J, Pike RN, Wijeyewickrema LC. The lysine-specific gingipain of Porphyromonas gingivalis: importance to pathogenicity and potential strategies for inhibition. Adv Exp Med Biol. 2011;712:15–29. doi: 10.1007/978-1-4419-8414-2_2. [DOI] [PubMed] [Google Scholar]
- Zhou XY, Gao JL, Hunter N, Potempa J, Nguyen KA. Sequence-independent processing site of the C-terminal domain (CTD) influences maturation of the RgpB protease from Porphyromonas gingivalis. Mol Microbiol. 2013;89:903–917. doi: 10.1111/mmi.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]