Abstract
Stress is present in everyday life in various forms and situations. Two stressors frequently investigated are physiological and psychosocial stress. Besides similar subjective and hormonal responses, it has been suggested that they also share common neural substrates. The current study used activation-likelihood-estimation meta-analysis to test this assumption by integrating results of previous neuroimaging studies on stress processing. Reported results are cluster-level FWE corrected.
The inferior frontal gyrus (IFG) and the anterior insula (AI) were the only regions that demonstrated overlapping activation for both stressors. Analysis of physiological stress showed consistent activation of cognitive and affective components of pain processing such as the insula, striatum, or the middle cingulate cortex. Contrarily, analysis across psychosocial stress revealed consistent activation of the right superior temporal gyrus and deactivation of the striatum. Notably, parts of the striatum appeared to be functionally specified: the dorsal striatum was activated in physiological stress, whereas the ventral striatum was deactivated in psychosocial stress. Additional functional connectivity and decoding analyses further characterized this functional heterogeneity and revealed higher associations of the dorsal striatum with motor regions and of the ventral striatum with reward processing.
Based on our meta-analytic approach, activation of the IFG and the AI seems to indicate a global neural stress reaction. While physiological stress activates a motoric fight-or-flight reaction, during psychosocial stress attention is shifted towards emotion regulation and goal-directed behavior, and reward processing is reduced. Our results show the significance of differentiating physiological and psychosocial stress in neural engagement. Furthermore, the assessment of deactivations in addition to activations in stress research is highly recommended.
Keywords: Achievement stress, social exclusion, pain, striatum, IFG, insula
1. Introduction
In everyday life we are confronted with social, cognitive or physiological stressors in various situations. Stress is a response to demands placed upon the body independent of the stressors' nature. Various stressor types that are associated with potential threat can induce stress (Selye, 1998; reprinted from 1936). The bodily stress reaction activates the hypothalamic-pituitary-adrenal gland (HPA) axis and subsequently the release of cortisol (Kirschbaum et al., 1993). The psychological homeostatic process is also altered by stress (Burchfield, 1979; Koob, 2009). Thus, the stress response is linked to a state of arousal and hypermobilization of the body's normal activation and emotion system (Hennessy and Levine, 1979; Koob, 2009). According to this view, two distinct types of stressors are physiological stress and psychosocial stress.
Physiological stress is indicated by an unpleasant sensoric, emotional and subjective experience that is associated with potential damage of body tissue and bodily threat (Peyron et al., 2000; Price, 2000; Tracey, 2005). Different bodily conditions may fulfill these criteria, e.g. pain, hunger, oxidative stress, etc. (see e.g., Colaianna et al., 2013). In the current study we will focus on pain processing as physiological stressor, for two main reasons. First, investigating pain as a physiological form of stress has a long lasting history (Lupien et al., 2007; Selye, 1998; Vachon-Presseau et al., 2013b). Second, pain processing is easily manipulated and therefore most frequently investigated in neuroimaging environments. Handling pain integrates sensory as well as affective processing (Price, 2000) and it has an arousing effect, increasing cortisol release and negative affect (Rainville, 2002; Vachon-Presseau et al., 2013a; Zubieta and Stohler, 2009). In neuroimaging environments, acute pain is induced by paradigms such as electric shocks or ice cold water which are known to increase cortisol and noradrenalin release.
Psychosocial stress is induced by situations of social threat including social evaluation, social exclusion and achievement situations claiming goal-directed performance (Dickerson and Kemeny, 2004; Pruessner et al., 2010). The need to be affiliated with others and to maintain the social-self are core psychological needs (Dickerson and Kemeny, 2004; Panksepp, 2003; Tossani, 2013). If the gratification of these needs is threatened, for example by a negative judgment of performance by others, then social threat and therefore stress is induced (Dickerson and Kemeny, 2004). Social evaluation as well as cognitive achievement with unpredictable outcome induce heightened cortisol responses, which are accompanied by increases in electrodermal activity, subjective stress reports and negative affect (Dedovic et al., 2009a; Dickerson and Kemeny, 2004; Eisenberger and Lieberman, 2004). Individuals having higher sensitivity towards social evaluation also express elevated cortisol response to acute stressors such as achievement tasks or social exclusion (Kirschbaum et al., 1995; Pruessner et al., 2008, 1999; Seidel et al., 2013; Somerville et al., 2010; Stroud et al., 2002).
Generally, neuroimaging studies refer to neural activations; however, studies investigating psychosocial stress also frequently report neural deactivations (Dagher et al., 2009; Dedovic et al., 2009a; Gradin et al., 2012; Pruessner et al., 2008). The interrelation between activated and deactivated neural areas is not well understood (Arsalidou et al., 2013b). Particularly, deactivations in limbic and cortical regions associated with emotion processing are reported (e.g., Critchley et al., 2000a; Moor et al., 2012; Onoda et al., 2009). However, some studies also report activations in these regions (e.g., Cacioppo et al., 2013; Eisenberger et al., 2003; Sebastian et al., 2011). Thus, inconsistent results regarding activation and deactivation have been reported, particularly in brain regions such as the hippocampus/amygdala, the anterior cingulate cortex (ACC) and prefrontal areas.
In contrast to psychosocial stress, the neural correlates of physiological stress are better characterized. Various meta-analyses of the neural correlates of pain processing identified a network of activated brain areas including primary and secondary motor and somatic regions, insula, dorsal ACC, thalamus, periaqueductal grey and prefrontal cortex (e.g., Apkarian et al., 2005; Friebel et al., 2011; Strigo et al., 2003). These regions process sensory-discriminative information as well as affective-cognitive pain properties (Tracey, 2005). Similar to psychosocial stress, specific deactivations during pain processing in emotion regulation areas such as the amygdala, nucleus accumbens and frontal regions, as well as in motor and sensoric-related areas have been reported (e.g., Aziz et al., 1997; Becerra et al., 2001; Derbyshire et al., 1997).
Taken together, pain as a physiological stressor and achievement situations and social exclusion as psychosocial stressors cause similar subjective, emotional and peripheral stress responses (e.g., Eisenberger et al., 2003; MacDonald and Leary, 2005; Mee et al., 2006; Meerwijk et al., 2013). Both psychosocial and physiological stress are associated with situations that threaten survival (Karremans et al., 2011), and both stressors alter the mesolimbic dopamine transmission in the striatum and the prefrontal cortex (Adler et al., 2000; Pruessner et al., 2008; Saal et al., 2003; Scott et al., 2006). Additionally, it has been argued that similar neural regions, such as the limbic-prefrontal circuit, are activated in processing psychosocial as well as physiological stress (Zubieta and Stohler, 2009). However, until now, this assumption has not been tested quantitatively. The primary interest of the current study lies in assessing the neural correlates of human stress responses to different stressors. In addition to neural activations, we wanted to further determine deactivations from both psychosocial and physiological stress. Therefore, the current meta-analysis set out to test whether psychosocial and physiological stress share overlapping and also distinct neural deactivations and/or activations. To do so, we used an activation-likelihood-estimation (ALE) meta-analysis approach (Eickhoff et al., 2012). Based on previous results, we expected to find overlaps in deactivations between psychosocial and physiological stress in the amygdala, prefrontal regions and distinct somatosensory areas. Contrarily, brain regions associated with peripheral arousal, emotion processing and avoidance (e.g. prefrontal regions, insula, ACC) were suspected to be activated during both psychosocial and physiological stress.
2. Material and Methods
2.1. Selection criteria for used data
Literature research was conducted using PubMed (www.pubmed.com) searching for combinations of the keywords: “fMRI”, “PET”, “neuroimaging”, “stress”, “achievement/cognitive stress”, “psychosocial stress”, “social exclusion”, “social stress”, “social rejection”, “ostracism”, “social pain”, “physiological stress”, “pain”, or “pain regulation”. Additional studies were identified by review articles, other meta-analyses and by tracing references from retrieved studies. Furthermore, in the case that a study did not sufficiently report the results, the corresponding authors were contacted and asked to provide more information on their data. In the following the term “experiment” refers to any single contrast analysis, and the term “study” refers to a scientific publication, usually reporting more “experiments” (Laird et al., 2011).
Only data of healthy adults (aged 18 and older) with no prior report of neurological, psychiatric or pain-related disorders were considered for the current meta-analysis, while results of patient or group effects (e.g., gender differences) were excluded. Furthermore, only neuroimaging studies which utilized either functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) on a whole-brain level and reported the coordinates of brain region activation or deactivation in standard anatomical reference space (Talairach/Tournoux; Montreal Neurological Institute [MNI]) were included. We excluded articles that conducted solely region-of-interest (ROI) analyses or did not report all significant peak-voxels at a specific threshold as well as receptor-PET studies. At last, we excluded studies in which any stress type served as an independent factor affecting further cognitive domains (e.g., fear conditioning, decision making), any pharmacological/placebo studies and correlation or resting-state analyses.
For psychosocial stress we included social exclusion and rejection studies as well as studies investigating cognitive achievement under time pressure or concurrent social evaluation. For physiological stress we included paradigms manipulating pain experience (e.g., extreme heat or cold, electrical stimulation, etc.). As we focused on both activation and deactivation of brain regions during a stressful event compared to a control or baseline condition, activation peaks were defined as brain regions more strongly activated during stress than during control or baseline (stress>control/baseline) and deactivation peaks as less activated during stress compared to control or baseline (control/baseline>stress). As of January 29th, 2014, this resulted in inclusion of 43 experiments for psychosocial (26 activation/17 deactivation; n=1130) and 82 experiments for physiological (69 activation/13 deactivation; n=967) stress (table 1).
Table 1.
Overview of included studies. A) Physiological stress. B) Psychosocial stress. (Included PET-studies are marked with an asterisk.)
| Study | n | Deactivation/Activation | Task |
|---|---|---|---|
| A) Physiological Stress | |||
| Seminowicz and Davis (2007) | 23 | deactivation/activation | electrical stimulation |
| Aziz et al. (1997)* | 8 | deactivation/activation | esophageal distension |
| Torta et al. (2013) | 17 | deactivation/activation | mechanical stimulation |
| Carlsson et al. (2006) | 9 | deactivation/activation | electrical stimulation |
| Lui et al. (2008) | 14 | deactivation/activation | mechanical stimulation |
| Becerra et al. (2001) | 8 | deactivation/activation | thermal stimulation |
| Oshiro et al. (2009) | 12 | deactivation/activation | thermal stimulation |
| Coghill et al. (1994)* | 9 | deactivation/activation | thermal stimulation |
| Derbyshire et al. (1994)* | 6 | deactivation/activation | thermal stimulation |
| Derbyshire et al. (1997)* | 12 | deactivation/activation | thermal stimulation |
| Derbyshire and Jones (1998)* | 12 | deactivation/activation | thermal stimulation |
| Perini et al. (2013) | 18 | deactivation/activation | thermal stimulationthermal stimulation/ |
| Strigo et al. (2003) | 7 | activation | esophageal distension |
| Ladabaum et al. (2001)* | 15 | activation | gastric distension |
| Benson et al. (2012) | 30 | activation | rectal distension rectal distension/ |
| Dunckley et al. (2005) | 10 | activation | thermal stimulation |
| Niddam et al. (2002) | 10 | activation | electrical stimulation |
| Wiech et al. (2006) | 12 | activation | electrical stimulation |
| Singer et al. (2004) | 32 | activation | electrical stimulation |
| Ibinson et al. (2004) | 6 | activation | electrical stimulation |
| Xu et al. (1997)* | 6 | activation | mechanical stimulation |
| Pujol et al. (2009) | 9 | activation | mechanical stimulation |
| Farrell et al. (2006)* | 10 | activation | mechanical stimulation |
| Rolls et al. (2003) | 8 | activation | mechanical stimulation |
| Iadarola et al. (1998)* | 13 | activation | capsaicin pain |
| Mochizuki et al. (2007) | 14 | activation | thermal stimulation |
| Seifert and Maihöfner (2007) | 12 | activation | thermal stimulation |
| Botvinick et al. (2005) | 12 | activation | thermal stimulation |
| Lorenz et al. (2002)* | 14 | activation | thermal stimulation |
| de Leeuw et al. (2006) | 9 | activation | thermal stimulation |
| Valet et al. (2004) | 7 | activation | thermal stimulation |
| Bornhövd et al. (2002) | 10 | activation | thermal stimulation |
| Talbot et al. (1991)* | 8 | activation | thermal stimulation |
| Vachon-Presseau et al. (2013a) | 18 | activation | thermal stimulation |
| Hofbauer et al. (2001)* | 10 | activation | thermal stimulation |
| Kurata et al. (2002) | 5 | activation | thermal stimulation |
| Peyron et al. (1999)* | 12 | activation | thermal stimulation |
| Tracey et al. (2000) | 6 | activation | thermal stimulation |
| Svensson et al. (1998)* | 10 | activation | thermal stimulation |
| Coghill et al. (2001)* | 9 | activation | thermal stimulation |
| Brooks et al. (2002) | 18 | activation | thermal stimulation |
| Dubé et al. (2009) | 12 | activation | thermal stimulation |
| Kong et al. (2006) | 16 | activation | thermal stimulation |
| Oshiro et al. (2007) | 12 | activation | thermal stimulation |
| Coghill et al. (1999)* | 16 | activation | thermal stimulation |
| Derbyshire et al. (2002)* | 16 | activation | thermal stimulation |
| Geuze et al. (2007) | 12 | activation | thermal stimulation |
| Paulson et al. (1998)* | 10 | activation | thermal stimulation |
| Schmahl et al. (2006) | 12 | activation | thermal stimulation |
| Smith et al. (2002) | 8 | activation | thermal stimulation |
| Keltner et al. (2006) | 13 | activation | thermal stimulation |
| Becerra et al. (2004) | 9 | activation | thermal stimulation |
| B) Psychosocial stress | |||
| Dedovic et al. (2009b) | 28 | deactivation/activation | MIST |
| Dagher et al. (2009) | 15 | deactivation | MIST |
| Derntl et al. (submitted) | 80 | deactivation/activation | MIST |
| Kogler et al. (2015) | 43 | deactivation/activation | MISTMental arithmetic + |
| Critchley et al. (2000a)* | 6 | deactivation | isometric exercise |
| Kern et al. (2008)* | 14 | deactivation/activation | TSST |
| Moor et al. (2010) | 16 | deactivation | Social evaluation |
| Gradin et al., (2012) | 16 | deactivation/activation | Cyberball |
| Bolling et al. (2011) | 23 | deactivation/activation | Cyberball |
| Bolling et al. (2012) | 20 | deactivation/activation | Cyberball |
| Sebastian et al. (2011) | 16 | deactivation/activation | Cyberball |
| Seidel et al. (submitted) | 80 | deactivation/activation | Cyberball |
| Maurage et al. (2012) | 22 | deactivation/activation | Cyberball |
| Moor et al. (2012) | 15 | deactivation/activation | Cyberball |
| Lederbogen et al. (2011) | 32 | activation | MIST |
| Soliman et al. (2011) | 40 | deactivation/activation | MIST |
| Fechir et al. (2010) | 16 | activation | STROOP |
| Koric et al. (2012) | 15 | deactivation/activation | PASAT |
| Eisenberger et al. (2003) | 13 | activation | Cyberball |
| Kawamoto et al. (2012) | 22 | activation | Cyberball |
| Masten et al. (2011) | 18 | activation | Cyberball |
| DeWall et al. (2012) | 25 | activation | Cyberball |
| Karremans et al. (2011) | 15 | activation | Cyberball |
| Lelieveld et al. (2012) | 72 | activation | Cyberball |
| Onoda et al. (2010) | 26 | activation | Cyberball |
| Masten et al. (2012) | 21 | activation | Cyberball |
2.2. Activation-likelihood (ALE) estimation
All meta-analyses were performed according to the standard analysis method used in previous studies (cf. Bzdok et al., 2012; Langner and Eickhoff, 2013; Rottschy et al., 2012). In particular, analyses were based on the revised ALE algorithm for coordinate-based meta-analysis of neuroimaging results (Eickhoff et al., 2012). This algorithm aims at identifying topographic clusters of activation/deactivation that show significantly higher convergence across experiments than expected under random spatial distributions. Importantly, the reported foci are not treated as single points, but rather as centers of 3D Gaussian probability distributions. This acknowledges spatial uncertainty and reliability by weighting studies according to their sample sizes through the width of the 3D Gaussian probability distribution. Thus, larger sample sizes provide more reliable approximations of the true activation/deactivation effect and are therefore modeled by smaller Gaussian distributions (Eickhoff et al., 2009). The resulting probabilities of all reported foci in a given experiment are combined for each voxel yielding a modeled activation (MA) map (Turkeltaub et al., 2012). The union of all MA maps from all experiments included in the analysis then results in voxel-wise ALE scores, which describe the convergence of results at each particular location in the brain. These ALE scores are then compared to an empirical null-distribution reflecting a random spatial association between experiments' MA maps (Eickhoff et al., 2012). Hereby, a random-effects inference was invoked, focusing on inference on the above-chance convergence between studies, rather than clustering of foci within a particular study.
The null-hypothesis was derived by sampling a random voxel from each of the MA maps and taking the union of these values. The p-value of a “true” ALE score is given by the proportion of equal or higher values obtained under the null-distribution. The resulting non-parametric p-values were then thresholded at a cluster-level family-wise error (FWE) corrected threshold of p<.05 (cluster-forming threshold at voxel-level p<0.001) (Bzdok et al., 2012; Eickhoff et al., 2011; Rottschy et al., 2012). Additionally, we conducted contrast and conjunction analysis between the meta-analyses of psychosocial and physiological stress. Minimum conjunction analyses (Nichols et al., 2005) were computed in order to isolate the intersection of the thresholded z-maps of two separate meta-analyses. Thus, any voxel determined to be significant by the conjunction analysis constitutes a region in the brain which survived inference corrected on cluster-level FWE in each of the individual meta-analyses. Differences between psychosocial and physiological stress were tested by comparing the two ALEs to a random distribution. First, the true difference between two individual analyses was determined by computing the voxel-wise difference between the unthresholded ALE maps of each analysis (cf. Eickhoff et al., 2012). Second, we determined a null-distribution of differences. This was done by pooling all experiments contributing to either analysis and randomly dividing them into two groups of the same size as the two original sets of experiments. ALE-scores for these two randomly assembled groups were calculated and the difference between these ALE-scores was recorded for each voxel in the brain. Repeating this process 25000 times then yielded an expected distribution of ALE-score differences under the assumption of exchangeability. The “true” difference in ALE scores was then tested against this null-distribution yielding a probability that the true difference was not due to random noise in an exchangeable set of labels, based on the proportion of lower differences in the random exchange. The resulting probability values were thresholded at p>.95 (95% chance for true difference) and inclusively masked by the respective main effects, i.e., the significant effects of the ALE analysis for the particular condition. For both the conjunction and the contrast analyses only clusters larger than 10 voxels were considered. Anatomical labeling was conducted with SPM Anatomy Toolbox version 1.8 (Eickhoff et al., 2007, 2005).
2.3. Follow-up analyses
In order to specifically determine functional networks for regions of interest derived from the current meta-analyses, we additionally conducted resting-state functional connectivity analyses as well as functional characterization (e.g., Müller et al., 2014).
2.3.1. Resting-state functional connectivity analysis
Resting-state images were obtained from the Nathan Kline Institute “Rockland” sample (available online as part of the International Neuroimaging Datasharing Initiative; http://fcon_1000.projects.nitrc.org/indi/pro/nki.html), consisting of 132 healthy subjects (representing the U.S. population in key demographic measures; 18-85 years; mean age: 42.3 ± 18.08 years; 78 male, 54 female). 260 images were acquired on a Siemens 3T TrioTim scanner using BOLD contrast (gradient echo EPI pulse sequence, repetition time (TR)=2.5s, echo time (TE)=30ms, flip angle=80°, in-plane resolution=3.0×3.0 mm, 38 axial slices (3.0mm thickness) covering the entire brain). Data was processed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The first four scans were discarded from each subject prior to further analyses. EPI images were corrected for head movement by affine registration using a two-pass procedure. In a first step, images were aligned to the initial volumes and subsequently to the mean of all volumes. Next, the mean EPI image was spatially normalized to the MNI single-subject template for each subject (Holmes et al., 1998) using the “unified segmentation” approach (Ashburner and Friston, 2005). Ensuing deformation was applied to the individual EPI volumes. Images were smoothed by a 5mm full-width-at-half-maximum Gaussian kernel to improve signal-to-noise ratio and to compensate for residual anatomical variations. Time-series of each voxel were processed as follows (Müller et al., 2013): Spurious correlations were reduced by excluding variance which could be explained by the following variables: (1) the six motion parameters derived from image realignment; (2) their first derivatives; (3) mean gray-matter (GM), white-matter (WM), and cerebral blood flow (CBF) intensity (each tissue-signal-class related signal separately). All nuisance variables entered the model as first and second order terms. Finally, data was band-pass filtered (cut-off frequencies of 0.01 and 0.08Hz). The time-courses of all voxels within each seed of interest were extracted for each subject as the first eigenvariate of all GM voxels within the respective seed. Linear (Pearson) correlation coefficients were computed between the resulting characteristic time series of the seed and the time series of all other GM voxels of the brain to quantify resting-state functional connectivity. The voxel-wise correlation coefficients of each subject were transformed into Fisher's z-scores and fed into a second-level ANOVA including an appropriate non-sphericity correction implemented in SPM8. Results were again thresholded at a cluster-level FWE corrected threshold of p<.05 (cluster-forming threshold at voxel-level p<.001; k>10).
2.3.2. Functional characterization
Functional characterization of regions of interest derived from the meta-analyses was performed by using meta-data categories that classify each single experimental contrast from the BrainMap database according to the assessed “behavioral domain” (such as emotion, cognition or perception) and “paradigm class” (such as flanker task, mental rotation or reward task) (Turner and Laird, 2012; see http://brainmap.org/scribe/ for the complete list of behavioral domains and paradigm classes). For the analyses the forward and reverse inference approaches were calculated (Müller et al., 2013). The forward inference approach determines the probability of observing activity in a brain region when a mental process is present. We tested whether the conditional probability of activation given a particular task [P(Activation|Task)] was higher than the baseline probability of activation [P(Activation)]. The baseline denotes the probability of finding a (random) activation from BrainMap in the region of interest. Significance was tested using a binominal test (p<.05, corrected for multiple comparisons). Additionally, the reverse inference approach tests the probability of the presence of a mental process given knowledge of activation in a particular region of interest. This likelihood [P(Task|Activation)] can be derived from P(Activation|Task) as well as P(Task) and P(Activation) using Bayes' rule. Significance was assessed by means of a chi-square test (p<.05, corrected for multiple comparisons) (Amft et al., 2015).
3. Results
3.1. Activation
Physiol ogical stress
The analysis across experiments reporting activations during physiological stress revealed convergent activity in bilateral insula extending to the putamen (PUT), caudate nucleus (CN), pallidum (PA) and temporal pole. Additionally, activation of bilateral supramarginal cortex and rolandic operculum, bilateral thalamus, as well as the right supplementary motor cortex (SMA) extending to the left middle cingulate cortex (MCC), left cerebellum, and right middle frontal gyrus (MFG) emerged (for details see table 2).
Table 2.
Brain regions and activation peaks showing convergence in activation for the main effects for psychosocial and physiological stress, for the contrasts activation in psychosocial vs. physiological and physiological vs. psychosocial stress and for the conjunction of psychosocial and physiological stress.
| Contrasts | Cluster | Macroanatomical location | Cytoarchitectonic location | X | Y | Z | t value |
|---|---|---|---|---|---|---|---|
| Activation Physiological | |||||||
|
| |||||||
| Cluster 1 (k=2181) | Right Insula Lobe | 38 | 18 | 0 | = 8.25 | ||
| Superior Temporal Gyrus | 52 | 12 | -4 | = 6.90 | |||
| Right Temporal Pole | 60 | 6 | 2 | = 6.40 | |||
| Right Pallidum | 22 | 0 | -4 | = 5.59 | |||
| Right Putamen | 22 | 8 | -2 | = 4.75 | |||
|
|
|||||||
| Cluster 2 (k=2011) | Left Insula Lobe | -38 | 14 | 4 | = 7.92 | ||
| Left Rolandic Operculum | OP 4 | -58 | 0 | 6 | = 5.69 | ||
| Left Putamen | -20 | 6 | 2 | = 5.07 | |||
| Left Caudate | -14 | 8 | 6 | = 3.61 | |||
|
|
|||||||
| Cluster 3 (k=1683) | Right SMA | Area 6 | 4 | 6 | 46 | = 7.97 | |
| Left Middle Cingulate Cortex | 0 | 14 | 36 | = 7.81 | |||
|
|
|||||||
| Cluster 4 (k=934) | Left Rolandic Operculum | OP 3 | -42 | -18 | 18 | = 6.62 | |
| Left SupraMarginal Gyrus | IPC (PFop) | -54 | -24 | 24 | = 6.50 | ||
| Left Insula Lobe | Insula (Ig2) | -36 | -20 | 2 | = 4.07 | ||
|
|
|||||||
| Cluster 5 (k=916) | Left Thalamus | Th-Prefrontal | -14 | -12 | 10 | = 8.25 | |
| Right Thalamus | Th-Prefrontal | 10 | -18 | 4 | = 7.20 | ||
| 10 | -16 | -8 | = 3.43 | ||||
| Th-Temporal | 2 | -6 | 6 | = 3.25 | |||
| Left Thalamus | -20 | -16 | -2 | = 3.88 | |||
|
|
|||||||
| Cluster 6 (k=893) | Right SupraMarginal Gyrus | IPC (PFop) | 56 | -24 | 24 | = 7.86 | |
| Right Rolandic Operculum | OP 3 | 44 | -14 | 16 | = 4.91 | ||
|
|
|||||||
| Cluster 7 (k=277) | Right Middle Frontal Gyrus | 38 | 50 | 12 | = 5.83 | ||
|
|
|||||||
| Cluster 8 (k=131) | Left Cerebellum | Lobule VI (Hem) | -24 | -66 | -26 | = 4.18 | |
| Lobule VIIa Crus I (Hem) | -26 | -70 | -28 | = 4.04 | |||
|
|
|||||||
| Activation Psychosocial | |||||||
|
| |||||||
| Cluster 1 (k=126) | Right Inferior Frontal Gyrus (p. Triangularis) | 38 | 22 | 8 | = 4.91 | ||
| Right Inferior Frontal Gyrus (p. Triangularis) | 42 | 18 | 4 | = 4.05 | |||
| Right Insula Lobe | 38 | 26 | -4 | = 3.61 | |||
|
|
|||||||
| Cluster 2 (k=120) | Right Superior Temporal | ||||||
| Gyrus | IPC (PF) | 62 | -40 | 22 | = 5.22 | ||
| Contrast Activation Physiological>Psychosocial | |||||||
|
| |||||||
| Cluster 1 (k=1558) | Left Insula Lobe | -36 | 6 | 6 | = 5.77 | ||
| Left Rolandic Operculum | OP 4 | -58 | 0 | 6 | = 5.69 | ||
| Left Insula Lobe | -38 | 4 | -4 | = 5.26 | |||
| Left Inferior Frontal Gyrus (p. Opercularis) | -50 | 8 | 8 | = 4.92 | |||
| Left Putamen | -24 | 0 | 4 | = 4.58 | |||
| Left Rolandic Operculum | -62 | 2 | 2 | = 3.94 | |||
| Left Putamen | -24 | 8 | 6 | = 3.81 | |||
| -30 | 10 | 2 | = 3.80 | ||||
| -20 | 6 | 8 | = 3.77 | ||||
| -26 | 2 | -10 | = 3.25 | ||||
| Left Pallidum | -18 | -2 | 0 | = 3.23 | |||
|
|
|||||||
| Cluster 2 (k=1401) | Right Insula Lobe | 36 | 10 | 6 | = 7.84 | ||
| Superior Temporal Gyrus | 54 | 14 | -4 | = 6.65 | |||
| Right Pallidum | 26 | -2 | -2 | = 4.74 | |||
| Right Putamen | 24 | 8 | -2 | = 4.57 | |||
| Right Pallidum | Amyg (CM) | 24 | -8 | -6 | = 3.02 | ||
| 18 | -4 | -4 | = 2.54 | ||||
| Right Insula Lobe | 40 | 18 | -4 | = 2.11 | |||
|
|
|||||||
| Cluster 3 (k=1169) | Left SMA | 2 | 12 | 46 | = 6.75 | ||
| Left Middle Cingulate Cortex | 0 | 12 | 32 | = 6.65 | |||
| Right Middle Cingulate Cortex | 4 | 12 | 34 | = 6.44 | |||
| Left Middle Cingulate Cortex | -8 | 8 | 38 | = 5.60 | |||
| Right Middle Cingulate Cortex | 4 | 16 | 30 | = 3.94 | |||
| Left Middle Cingulate Cortex | -12 | 6 | 40 | = 3.94 | |||
| Right Middle Cingulate Cortex | 6 | 12 | 40 | = 3.67 | |||
|
|
|||||||
| Cluster 4 (k=767) | Left SupraMarginal Gyrus | IPC (PFop) | -54 | -24 | 24 | = 6.50 | |
| Left Postcentral Gyrus | IPC (PFop) | -50 | -22 | 30 | = 3.94 | ||
| Left Heschls Gyrus | Insula (Ig2) | -38 | -18 | 8 | = 3.80 | ||
| Left Insula Lobe | Insula (Ig2) | -36 | -18 | 2 | = 3.78 | ||
| Left Heschls Gyrus | TE 1.0 | -40 | -22 | 10 | = 3.49 | ||
| Left SupraMarginal Gyrus | IPC (PF) | -58 | -32 | 28 | = 3.35 | ||
| IPC (PFt) | -56 | -28 | 34 | = 3.02 | |||
|
|
|||||||
| Cluster 5 (k=695) | Right SupraMarginal Gyrus | IPC (PFop) | 58 | -18 | 24 | = 5.57 | |
| IPC (PFop) | 58 | -22 | 30 | = 4.87 | |||
| IPC (PFop) | 54 | -20 | 28 | = 4.30 | |||
| IPC (PF) | 58 | -26 | 32 | = 3.94 | |||
| Right Rolandic Operculum | OP 1 | 52 | -20 | 18 | = 3.86 | ||
| OP 4 | 52 | -16 | 16 | = 3.81 | |||
| Right SupraMarginal Gyrus | IPC (PF) | 60 | -30 | 32 | = 3.78 | ||
| IPC (PF) | 56 | -32 | 34 | = 3.62 | |||
| IPC (PFop) | 66 | -18 | 24 | = 3.60 | |||
| Right Insula Lobe | OP 3 | 36 | -12 | 8 | = 3.13 | ||
| Insula (Ig2) | 34 | -14 | 6 | = 3.07 | |||
|
|
|||||||
| Cluster 6 (k=583) | Left Thalamus | Th-Prefrontal | -12 | -12 | 8 | = 7.05 | |
| -20 | -16 | -2 | = 3.49 | ||||
| Right Thalamus | Th-Prefrontal | 16 | -12 | 6 | = 3.45 | ||
| Th-Premotor | 16 | -14 | 2 | = 3.38 | |||
| Th-Prefrontal | 10 | -10 | 0 | = 3.33 | |||
| Th-Temporal | 8 | -8 | 8 | = 3.28 | |||
| Th-Temporal | 2 | -6 | 6 | = 3.24 | |||
| Th-Parietal | 16 | -20 | 10 | = 2.67 | |||
| Th-Somatosensory | 16 | -20 | -2 | = 2.66 | |||
| 12 | -18 | -8 | = 2.63 | ||||
| Th-Prefrontal | 4 | -12 | 0 | = 2.31 | |||
|
|
|||||||
| Cluster 7 (k=224) | Right Middle Frontal Gyrus | 44 | 44 | 14 | = 3.26 | ||
| 46 | 52 | 14 | = 3.19 | ||||
|
|
|||||||
| Cluster 8 (k=61) | Left Cerebellum | Lobule VI (Hem) | -26 | -64 | -28 | = 3.16 | |
| Lobule VIIa Crus I (Hem) | -28 | -68 | -30 | = 2.07 | |||
|
|
|||||||
| Contrast Activation Psychosocial>Physiological | |||||||
|
| |||||||
| Cluster 1 (k=96) | Right Superior Temporal | ||||||
| Gyrus | IPC (PFm) | 62 | -44 | 18 | = 3.13 | ||
| IPC (PF) | 60 | -34 | 16 | = 2.20 | |||
|
|
|||||||
| Conjunction Activation Physiological & Psychosocial | |||||||
|
| |||||||
| Cluster 1 (k=120) | Right Inferior Frontal Gyrus (p. T riangularis) | 38 | 22 | 8 | = 4.91 | ||
| 42 | 18 | 4 | = 4.05 | ||||
| Right Insula Lobe | 38 | 26 | -4 | = 3.61 | |||
Note. Coordinates x, y, z of local maxima refer to Montreal Neurological Institute space (MNI) (k>10). k=number of voxels in cluster.
References for histological assignments: Amyg (CM): Amunts et al. (2005); Insula lg2: Kurth et al. (2010a); IPC (PF, PFop): Caspers et al. (2006); Lobules VI (Hem), Lobule VIIa Crus I (Hem): Diederichsen et al. (2009); Thalamus (connectivity zones): Behrens et al. (2003); TE 1.0: Morosan et al. (2001); OP1, OP 3, OP 4: Eickhoff et al. (2006a; 2006b).
Psychosocial stress
Investigation of consistent activation across experiments assessing psychosocial stress revealed activation of the right superior temporal gyrus (STG) and the right inferior frontal gyrus (IFG) (pars triangularis) extending to the insula (table 2).
Physiological vs. psychosocial stress
This direct comparison revealed stronger convergence of activation for physiological stress in the bilateral insula extending to PUT, PA and IFG, bilateral supramarginal gyrus extending to the right rolandic operculum, bilateral MCC, bilateral thalamus, right MFG, and left cerebellum (figure 1 and table 2).
Figure 1. Activations for physiological and psychosocial stress.

Contrasts showing stronger convergence in activation in psychosocial stress than in physiological stress (red) and stronger convergence in activation in physiological stress than in psychosocial stress (blue). (Abbreviations: L=left; R=right; BIL=bilateral; INS=insula; IFG=inferior frontal gyrus; ROP=rolandic operculum; MCC=middle cingulate gyrus; MFG=middle frontal gyrus; TH=thalamus; CEREB=cerebellum; PUT=putamen; SMG=supramarginal gyrus; STG=superior temporal gyrus.) Results are cluster-level FWE corrected (p<.05).
Psychosocial vs. physiological stress
For psychosocial compared to physiological stress stronger convergence of activation for psychosocial stress emerged in the right STG (figure 1 and table 2).
Physiological and psychosocial stress
The conjunction analysis revealed common activation for both stressor types in the right IFG (pars triangularis) extending into the insula lobe (figure 2 and table 2).
Figure 2. Conjunction of activations.

Conjunction of the results of the meta-analysis on activation in psychosocial stress and the one on activation in physiological stress revealing a cluster in the right inferior frontal gyrus (IFG) extending into the anterior insula (AI). Results are cluster-level FWE corrected (p<.05).
3.2. Deactivation
Physiol ogical st ress
The meta-analysis across experiments reporting deactivation upon physiological stress revealed significant convergence in the right paracentral lobule (table 3).
Table 3.
Brain regions and activation peaks showing convergence in deactivation for the main effects for physiological and psychosocial stress as well as for the contrasts deactivation in physiological vs. psychosocial and psychosocial vs. physiological stress.
| Contrasts | Cluster | Macroanatomical location | Cytoarchitectonic location | X | Y | Z | t value |
|---|---|---|---|---|---|---|---|
| Deactivation Physiological | |||||||
|
| |||||||
| Cluster 1 (k=107) | Right Paracentral Lobule | Area 4a | 2 | -28 | 64 | = 5.29 | |
|
|
|||||||
| Deactivation Psychosocial | |||||||
|
| |||||||
| Cluster 1 (k=148) | Left Caudate Nucleus | -10 | 18 | -6 | = 4.73 | ||
|
|
|||||||
| Left Putamen | -18 | 14 | -4 | = 3.99 | |||
| Contrast Deactivation Physiological>Psychosocial | |||||||
|
| |||||||
| Cluster 1 (k=45) | Medial Frontal Gyrus | 0 | -30 | 58 | = 2.96 | ||
| Right Paracentral Lobule | Area 4a | 2 | -30 | 62 | = 2.30 | ||
| Left Paracentral Lobule | 0 | -26 | 62 | = 1.90 | |||
| Right SMA | 4 | -24 | 62 | = 1.81 | |||
| Right Paracentral Lobule | Area 4a | 4 | -30 | 66 | = 1.73 | ||
|
|
|||||||
| Contrast Deactivation Psychosocial>Physiological | |||||||
|
| |||||||
| Cluster 1 (k=67) | Left Caudate Nucleus | -14 | 20 | -4 | = 2.30 | ||
| -10 | 20 | -8 | = 1.99 | ||||
| -6 | 20 | -4 | = 1.94 | ||||
Note. Coordinates x, y, z of local maxima refer to Montreal Neurological Institute space (MNI) (k>10). k=number of voxels in cluster.
References for histological assignments: Area 4a: Geyer et al. (1996).
Psychosocial st ress
Convergent deactivation across experiments of psychosocial stress was found in one cluster extending from the left CN to the PUT (table 3).
Physiological vs. psychosocial stress
Physiological stress directly compared to psychosocial stress showed stronger convergence of deactivation in the right paracentral lobule (see figure 3 and table 3).
Figure 3. Deactivations for physiological and psychosocial stress.
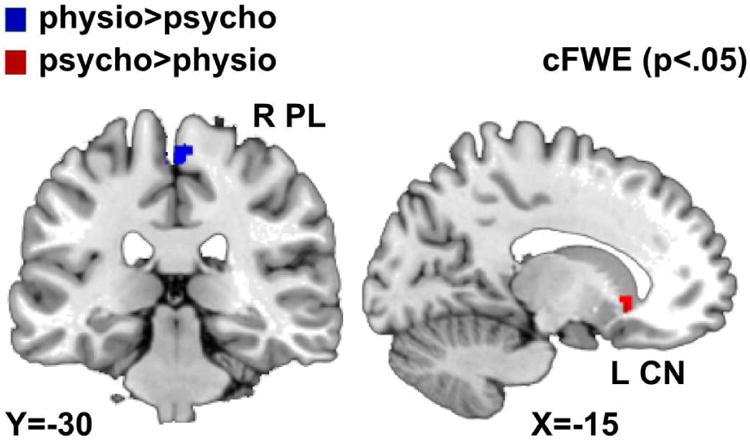
Contrasts showing stronger convergence in deactivation in psychosocial stress than in physiological stress (red) and stronger convergence in deactivation in physiological stress than in psychosocial stress (blue). (Abbreviations: L=left; R=right; CN=caudate nucleus; PL=paracentral lobule.) Results are cluster-level FWE corrected (p<.05).
Psychosocial vs. physiological stress
The direct comparison of psychosocial and physiological stress revealed significantly stronger convergence of deactivations for psychosocial stress in the left CN (see figure 3 and table 3).
Physiological and psychosocial stress
Conjunction analysis did not reveal any common deactivations for both stressor types.
3.3. The striatum in physiological and psychosocial stress
Interestingly, engagement of the striatum was found for both stress conditions: for physiological stress activation was reported while for psychosocial stress deactivation emerged. Notably, this activation-deactivation pattern engaged distinct parts of the striatum. Therefore, we additionally compared the results of activation during physiological stress with the results of deactivation during psychosocial stress. Contrast analysis revealed stronger convergence of activation during physiological stress compared to deactivation during psychosocial stress in the dorsal striatum [maximum peak: -20 2 8], while deactivation during psychosocial stress compared to activation during physiological stress showed significantly more convergence in the ventral part of the striatum [maximum peak: -12 20 -8] (figure 4A).
Figure 4. Dorsal and ventral striatum in stress processing.

A) Contrasts showing convergence of deactivation in psychosocial in the ventral striatum (red) and activation in physiological stress in the dorsal striatum (blue). B) Regions showing functional resting-state connectivity with the ventral (red) and the dorsal (blue) striatum. C) Likelihood ratio for significant behavioral domains (graphs in the upper panel) and paradigm classes (graphs in the lower panel) for the ventral (red) and the dorsal (blue) striatum for the forward inference approach.
From clusters that derived from the comparison of convergent activation of physiological and deactivation of psychosocial stress, we extracted ROIs according to in-house cytoarchitectonic maps of the striatum (Ludwig-Zahl et al., 2014) implemented in SPM Anatomy Toolbox (Eickhoff et al., 2007, 2005). Both ROIs were then further investigated with regard to their functional connectivity profile as well as their functional properties.
Comparison of resting-state functional connectivity between dorsal and ventral striatum
The left dorsal striatum showed higher functional connectivity than the ventral striatum with numerous regions such as precentral areas and middle cingulate gyrus, supramarginal gyrus extending to the STG, left middle and inferior temporal gyrus or right middle frontal gyrus (see figure 4B and table 4). The left ventral striatum demonstrated higher functional connectivity than the dorsal striatum with regions such as mid orbital gyrus and ACC or angular and supramarginal gyri (see figure 4B and table 4).
Table 4.
Comparison of resting-state functional connectivity between dorsal and ventral striatum. Note. Coordinates x, y, z of local maxima refer to Montreal Neurological Institute space (MNI) (k>10). k=number of voxels in cluster.
| Contrasts | Cluster | Macroanatomical location | Cytoarchitectonic location | X | Y | Z | t value |
|---|---|---|---|---|---|---|---|
| dorsal>ventral | |||||||
|
| |||||||
| Cluster 1 (k=21190) | Left Putamen | -24 | 2 | 6 | = 35.46 | ||
| Right Putamen | 26 | 0 | 6 | = 18.97 | |||
| 28 | -4 | 8 | = 18.57 | ||||
| 28 | -10 | 8 | = 17.03 | ||||
| 28 | 2 | 0 | = 15.99 | ||||
| 30 | -8 | 0 | = 15.99 | ||||
| Left Putamen | -28 | -16 | 6 | = 15.83 | |||
| -18 | 0 | 14 | = 14.79 | ||||
| -28 | 2 | -14 | = 12.51 | ||||
| 18 | 0 | 14 | = 12.05 | ||||
| Left Inferior Frontal Gyrus (p. Opercularis) | Area 44 | -54 | 8 | 6 | = 12.02 | ||
|
|
|||||||
| Cluster 2 (k=1481) | Right SupraMarginal Gyrus | IPC (PF) | 62 | -40 | 24 | = 10.33 | |
| IPC (PF) | 62 | -32 | 30 | = 9.77 | |||
| IPC (PF) | 60 | -40 | 34 | = 9.53 | |||
| IPC (PF) | 60 | -32 | 36 | = 9.52 | |||
| OP 1 | 60 | -24 | 24 | = 7.53 | |||
| Right Superior Temporal Gyrus | IPC (PFcm) | 48 | -32 | 22 | = 7.28 | ||
| Right SupraMarginal Gyrus | IPC (PFt) | 66 | -20 | 34 | = 5.97 | ||
| Right Superior Temporal Gyrus | 68 | -42 | 12 | = 5.71 | |||
| 60 | -30 | 12 | = 4.91 | ||||
|
|
|||||||
| Cluster 3 (k=398) | Right Cerebellum | Lobule VI (Hem) | 34 | -58 | -24 | = 5.64 | |
| Lobule VI (Hem) | 26 | -64 | -22 | = 5.49 | |||
| Lobule VI (Hem) | 38 | -46 | -32 | = 5.06 | |||
| Lobule VI (Hem) | 12 | -76 | -20 | = 4.89 | |||
| Lobule VI (Hem) | 12 | -70 | -18 | = 4.52 | |||
| Lobule VI (Hem) | 16 | -68 | -26 | = 4.12 | |||
| Right Lingual Gyrus | Lobule VI (Hem) | 10 | -64 | -10 | = 3.76 | ||
|
|
|||||||
| Cluster 4 (k=361) | Left Cerebellum | Lobule VIIIa (Hem) | -30 | -60 | -56 | = 5.79 | |
| Lobule VIIIa (Hem) | -24 | -66 | -52 | = 5.41 | |||
| 14 | -56 | -34 | = 5.10 | ||||
| Left Cerebellum | Lobule VIIb (Hem) | -38 | -54 | -54 | = 4.99 | ||
| -14 | -50 | -36 | = 4.91 | ||||
| Cerebellar Vermis | Lobule IX (Vermis) | 2 | -54 | -32 | = 4.73 | ||
| Left Cerebellum | -26 | -52 | -44 | = 4.70 | |||
| -18 | -48 | -40 | = 4.60 | ||||
| Lobule VIIb (Hem) | -38 | -50 | -52 | = 4.23 | |||
| -12 | -56 | -34 | = 3.91 | ||||
| -8 | -56 | -32 | = 3.87 | ||||
|
|
|||||||
| Cluster 5 (k=282) | Left Cerebellum | Lobule VI (Hem) | -32 | -60 | -26 | = 6.24 | |
| Lobule VI (Hem) | -10 | -76 | -20 | = 5.44 | |||
| Lobule VI (Hem) | -26 | -66 | -22 | = 5.37 | |||
| Lobule VI (Hem) | -36 | -46 | -36 | = 5.14 | |||
| Lobule VI (Hem) | -18 | -70 | -20 | = 4.75 | |||
| Lobule VI (Hem) | -36 | -48 | -30 | = 4.35 | |||
|
|
|||||||
| Cluster 6 (k=248) | -8 | -38 | -42 | = 6.84 | |||
| 8 | -38 | -44 | = 5.51 | ||||
| 4 | -28 | -40 | = 5.15 | ||||
| 2 | -18 | -38 | = 4.26 | ||||
| -10 | -28 | -34 | = 3.69 | ||||
| -6 | -28 | -38 | = 3.61 | ||||
|
|
|||||||
| Cluster 7 (k=239) | Left Middle Temporal Gyrus | -62 | -52 | 2 | = 7.94 | ||
| Left Inferior Temporal Gyrus | -58 | -54 | -6 | = 7.00 | |||
| Left Middle Temporal Gyrus | -60 | -62 | 0 | = 6.80 | |||
| -50 | -46 | 10 | = 5.07 | ||||
| Left Superior Temporal Gyrus | -62 | -60 | 8 | = 4.85 | |||
|
|
|||||||
| Cluster 8 (k=204) | Right Cerebellum | 24 | -70 | -50 | = 6.01 | ||
| 24 | -64 | -52 | = 5.44 | ||||
| 26 | -62 | -50 | = 5.17 | ||||
| 16 | -62 | -50 | = 4.28 | ||||
|
|
|||||||
| Cluster 9 (k=89) | Left Cerebellum | Lobules I-IV (Hem) | -4 | -46 | -12 | = 4.98 | |
| Cerebellar Vermis | Lobule V | 6 | -48 | -12 | = 4.89 | ||
|
|
|||||||
| Cluster 10 (k=85) | Right Middle Frontal Gyrus | 40 | 50 | 24 | = 4.70 | ||
| 44 | 50 | 16 | = 3.28 | ||||
| ventral>dorsal | |||||||
|
| |||||||
| Cluster 1 (k=26469) | Left Caudate Nucleus | -12 | 18 | -6 | = 56.36 | ||
| Right Caudate Nucleus | 10 | 18 | -6 | = 23.18 | |||
| Left Anterior Cingulate Cortex | -12 | 38 | -4 | = 13.79 | |||
| Left Mid Orbital Gyrus | 0 | 50 | -10 | = 13.54 | |||
| Right Mid Orbital Gyrus | 6 | 42 | -12 | = 13.39 | |||
| Left Mid Orbital Gyrus | -2 | 58 | -4 | = 13.38 | |||
| -10 | 50 | -8 | = 13.31 | ||||
| Left Anterior Cingulate Cortex | -18 | 44 | -4 | = 13.24 | |||
| -6 | 42 | -4 | = 13.22 | ||||
| Left Mid Orbital Gyrus | -10 | 42 | -6 | = 13.13 | |||
| Right Mid Orbital Gyrus | 10 | 40 | -4 | = 12.98 | |||
|
|
|||||||
| Cluster 2 (k=629) | Right Cerebellum | Lobule VIIa Crus I (Hem) | 52 | -64 | -40 | = 6.98 | |
| Lobule VIIa Crus I (Hem) | 48 | -60 | -46 | = 6.03 | |||
| Lobule VIIa Crus I (Hem) | 42 | -56 | -44 | = 5.76 | |||
| Lobule VIIa Crus I (Hem) | 30 | -84 | -34 | = 4.05 | |||
| Lobule VIIa Crus I (Hem) | 38 | -62 | -36 | = 3.74 | |||
| Lobule VIIa Crus I (Hem) | 30 | -76 | -34 | = 3.56 | |||
|
|
|||||||
| Cluster 3 (k=597) | Left Angular Gyrus | IPC (PFm) | -42 | -52 | 34 | = 5.92 | |
| Left SupraMarginal Gyrus | -36 | -50 | 30 | = 5.48 | |||
| Left Angular Gyrus | IPC (PFm) | -44 | -56 | 42 | = 5.30 | ||
| Left Inferior Parietal Lobule | -38 | -48 | 26 | = 5.21 | |||
| IPC (PGa) | -50 | -56 | 36 | = 5.13 | |||
| Left Angular Gyrus | IPC (PGa) | -50 | -64 | 36 | = 4.83 | ||
| IPC (PFm) | -50 | -64 | 46 | = 4.44 | |||
| -42 | -74 | 48 | = 4.38 | ||||
| -36 | -58 | 32 | = 4.34 | ||||
| IPC (PGa) | -42 | -64 | 34 | = 4.22 | |||
| Left Inferior Parietal Lobule | -40 | -44 | 26 | = 3.99 | |||
|
|
|||||||
| Cluster 4 (k=579) | Left Cerebellum | -48 | -64 | -46 | = 7.78 | ||
| -48 | -70 | -36 | = 5.43 | ||||
| -28 | -84 | -32 | = 4.79 | ||||
| -34 | -82 | -34 | = 4.54 | ||||
| -40 | -76 | -40 | = 4.21 | ||||
| -16 | -88 | -40 | = 4.19 | ||||
| -58 | -58 | -42 | = 4.02 | ||||
| -46 | -46 | -50 | = 3.89 | ||||
| -54 | -46 | -50 | = 3.85 | ||||
| -26 | -86 | -38 | = 3.71 | ||||
| -46 | -52 | -48 | = 3.56 | ||||
|
|
|||||||
| Cluster 5 (k=537) | Right Angular Gyrus | IPC (PGa) | 50 | -58 | 28 | = 6.47 | |
| 40 | -62 | 30 | = 5.81 | ||||
| Right Middle Temporal gyrus | 36 | -62 | 28 | = 5.78 | |||
| Right Angular Gyrus | IPC (PGp) | 44 | -70 | 30 | = 5.61 | ||
| Right SupraMarginal Gyrus | hIP1 | 36 | -52 | 28 | = 5.25 | ||
| Right Angular Gyrus | IPC (PGp) | 48 | -68 | 44 | = 4.60 | ||
| Right Angular Gyrus | IPC (PGp) | 46 | -72 | 44 | = 4.23 | ||
| Right Angular Gyrus | IPC (PGa) | 46 | -66 | 50 | = 3.95 | ||
| Right Angular Gyrus | IPC (PGp) | 44 | -74 | 36 | = 3.65 | ||
| Right Inferior Parietal Lobule | IPC (PGa) | 56 | -60 | 42 | = 3.59 | ||
References for histological assignments: Areas 44: Amunts et al. (1999); IPC (PF, PFcm, PFm, PFop, PFt, PGa): Caspers et al. (2006); Thalamus (connectivity zones): Behrens et al. (2003); OP 1, 4: Eickhoff et al. (2006a; 2006b); Lobules I-IV (Hem), V, VI (Hem), VIIa Crus I (Hem): Diederichsen et al. (2009); Intraparietal sulcus (hlP1): Choi et al. (2006).
Functional characterization of dorsal and ventral striatum
In addition, the derived clusters of dorsal and ventral striatum were functionally characterized using the “behavioral domain” and “paradigms class” meta-data of the BrainMap database.
Dorsal striatum
Based on the forward inference approach, activation of this cluster was particularly related to behavioral domains of action execution, perception of pain, and action imagination. Paradigm classes significantly associated with dorsal striatum were finger tapping, imagined movement, pain monitor/discrimination, and flexion/tension (figure 4C). Similar results (additionally including speech execution and overt recitation/repetition) were observed when calculating reverse inference.
Ventral striatum
Activation of the ventral striatum was significantly associated with the behavioral domains of cognition and emotion as well as the paradigm class reward using the forward inference approach (figure 4C). The same results emerged when applying the reverse inference.
Dorsal vs. ventral striatum
These findings were further supported by direct contrast analysis revealing significantly stronger association of the behavioral domains of emotion and cognition and the paradigm class reward with the ventral than the dorsal striatum. Additionally, the behavioral domains of vision and motion perception, action execution and imagination, speech execution, cognition of language and speech as well as the paradigm classes finger tapping, overt reading, saccades, tone monitor/discrimination, and overt recitation/repetition showed significantly stronger associations with the dorsal than with the ventral striatum.
4. Discussion
The current study used a meta-analytic approach for quantitatively summarizing activations and deactivations of fMRI and PET studies on psychosocial and physiological stress processing. For this purpose we included studies on achievement stress and social exclusion as indicators for psychosocial stress. Pain processing was used as an indicator for physiological stress. Our results show that physiological and psychosocial stressors deactivate as well as activate distinct neural regions. Furthermore, the striatum appeared to be especially involved in physiological and psychosocial stress. The results are discussed in detail in the following.
4.1. Activation in physiological and psychosocial stress
Physiol ogical stress
The meta-analysis of activation during physiological stress revealed convergence in regions typically activated during pain processing (e.g., Peyron et al., 2000), so our findings are in concordance with existing literature on pain experience. The ACC, prefrontal cortex and thalamic nuclei belong to the affective-cognitive-evaluative pain system whereas motor and somatosensory cortices are part of the discriminative-sensoric pain system (Friebel et al., 2011; Iannetti and Mouraux, 2010). The insula seems to mediate systems that are coding intensity and lateralization as well as emotional processing of pain, making it a suspect for coordinating emotional and sensoric properties during physiological stress processing (Friebel et al., 2011). Therefore, the detection of sensoric qualities, the handling of affective information and the integration of those sensoric and affective-emotional sensations are particularly significant in physiological stress processing.
Stronger convergence of activation in physiological than in psychosocial stress
Stronger convergence of activation in physiological compared to psychosocial stress in regions such as the posterior insula, dorsal striatum, IFG (pars opercularis) or MCC indicate sensory-motoric processing (Arsalidou et al., 2013a; Friebel et al., 2011; Kurth et al., 2010b). This specific part of the IFG is reported to be involved in action control (Binkofski and Buccino, 2006), indicating motoric processing and preparation of behavioral tendencies. Additionally, rostral MFG activation is associated with control of negative as well as self-referential processing and episodic working memory (e.g., Gilbert et al., 2006; Yang et al., 2013). The observed activation of regions involved in self-referential working memory and action control indicates a preparation of motoric and behavioral patterns that were acquired in previous situations of bodily threat. This specific induction of a “fight-or-flight” response in situations of physiological stress (Cannon, 1932; Taylor et al., 2000) should be taken into account when using this approach to induce stress for research purposes. Furthermore, people suffering from physiological stress complaints may benefit specifically from targeting these sensoric processing and “fight-or-flight” reactions in stress coping interventions.
Psychosocial stress
Analysis of consistent activation across experiments of psychosocial stress revealed convergence in the right IFG and the right posterior STG. The posterior STG cluster of the current meta-analyses widely overlaps with the anterior temporo-parietal junction, which is strongly involved in attentional processes and shows negative connectivity with a network involved in social cognition (Bzdok et al., 2013). In association with early life and social stress, this region shows decreased resting-state activity and greater gray matter volume (De Bellis et al., 2002; Philip et al., 2013). A recent meta-analysis on emotion regulation (Kohn et al., 2014) indicates the involvement of the right STG in cognitive regulation of emotion. In terms of psychosocial stress, the activation of the right posterior STG indicates enhanced attention processing and regulation of emotional arousal, probably resulting in focused and (ego-centric) goal-directed behavior in situations of psychosocial stress.
Stronger convergence of activation in psychosocial than in physiological stress
Here, the right STG showed stronger convergence in activation for psychosocial stress than for physiological stress. Given its role in attention processing during emotion regulation and goal-directed behavior (Bzdok et al., 2013; Kohn et al., 2014) as well as negative connectivity to networks involved in social cognition (Bzdok et al. 2013), our results indicate that during psychosocial stress attention is focused towards ego-centric emotional arousal while social processing is concurrently reduced. In contrast to pain experience, the competitive nature of challenging tasks in psychosocial stress may reduce social processing but increase attention as well as emotion control, which is subserving the goal-directed orientation when performing the task.
Common activation in physiological and psychosocial stress
A cluster in the right IFG (pars triangularis) extending to the insula showed convergence of activation in the meta-analyses of both stressor types. This part of the IFG, often referred to as VLPFC, is essential for action and cognitive control such as the inhibition of behavior (Aron et al., 2014) as well as for suppressing emotions and emotional memory (Depue et al., 2007; Quirk and Beer, 2006). In addition, IFG activity is associated with processing and regulating particularly negative affective states (e.g., negative affect/emotion processing during negative experiences: Eisenberger et al. (2003); Wang et al. (2005); cognitive emotion regulation: Lieberman et al. (2007); Ochsner and Gross (2005); social rejection: Cacioppo et al. (2013)). IFG activation during stress processing may indicate processing negative, subjective experiences, which result from and/or are accompanied by the inhibition of behavioral impulses such as a potential flight reaction.
Besides IFG, activation of the ventral, anterior part of the insula (AI) emerged in the analyses of both psychosocial and physiological stress. The insula merges nociceptive, thermoregulatory, and cardiovascular-related activation, and regulates peripheral activation and autonomic arousal (Critchley et al., 2000b; Rainville, 2002). It is therefore suspected to mediate sensoric and affective processing (Critchley, 2004; Critchley et al., 2000b; Rainville, 2002). The ventral anterior part in particular is engaged during reliving and processing strong emotions (Kober et al., 2008; Kurth et al., 2010b; Touroutoglou et al., 2012), and it is assumed to encode the affective and autonomic features of the current state, i.e. how unpleasant or aversive one feels in a certain situation (Singer and Lamm, 2009; Singer et al., 2004). Therefore, the engagement of the AI during physiological and psychosocial stress may reflect mapping and evaluation of emotions.
Hence, activation of the cluster spanning from IFG to AI found for both stressor types may reflect a global, neural stress reaction. This cluster may be a potential target for stress regulation trainings. Modulating its activation via neurofeedback or non-invasive stimulation and investigating the effect on stress reaction may be the focus of future research (Bauer et al., 2011; Linden et al., 2012; Votinov et al., 2013).
4.2. Deactivations in physiological and psychosocial stress
While most neuroimaging studies focus on neural activation, some studies additionally report neural deactivations during stress processing compared to a control condition or baseline activity.
Physiological stress
Convergent deactivation during physiological stress was found in a region covering the right paracentral lobule. The paracentral lobule is especially engaged during activation of inner body organs (Blok et al., 1997; Seseke et al., 2006; Zhang et al., 2005). Additionally, subjective pain sensitivity modulates activation of this region, with stronger activation in subjects who experience pain as more intense (Coghill et al., 2003). Deactivation of the paracentral lobule during physiological stress may cease the acute functioning of essential body organs, suggesting an expedient reaction to situations of bodily threat, which requires the prevention of potentially threatened resources and the preparation of fast motoric reactions.
Stronger convergence of deactivation in physiological than in psychosocial stress
It follows that physiological stress showed stronger convergence in deactivation than psychosocial stress in the paracentral lobule. Again we speculate that the ceasing of acute functioning of essential body organs seems to be more significant for processing sensoric information in the threatening situations of physiological stress compared to situations of psychosocial stress.
Psychosocial stress
Psychosocial stress resulted in consistent deactivations in one cluster within the striatum extending from the left CN to the PUT. In line with our results, Nikolova and colleagues (2012) reported a negative association between recent life stress and a concomitant decrease in striatal activation which in turn was associated with lower positive affect. In general, the CN is associated with various behavioral and cognitive domains, including reward processing and motivation (Arsalidou et al., 2013a). With regard to the latter function, Kumar and colleagues (2014) showed that psychosocial stress influences reward processing, with decreased CN activation during reward consumption. This taken together with our results indicates that psychosocial stress induces an anhedonic behavior and decreases processing of positive reinforcement as well as motivation (Wang et al., 2007). This is also consistent with reports that emotional complaints such as lack of motivation are associated with stress experience (e.g., American Stress Report, American Psychological Association, 2010; German Stress Report, Lohmann-Haislah, 2012). The PUT, in contrast to the CN, is classically assigned to motor processes and control (Arsalidou et al., 2013a; Leisman and Melillo, 2013). It was also shown to be directly related to pain sensation (Davis et al., 2002; Favilla et al., 2014) and intensity discrimination (Oshiro et al., 2009). At first glance, motor as well as pain related properties seem to be diminished, and reward processing and the capacity of cognitive resources seems to be reduced during psychosocial stress processing.
Stronger convergence of deactivation in psychosocial than in physiological stress
The CN cluster also showed stronger convergence in deactivation during psychosocial stress than during physiological stress. The deactivation of reward related areas may be associated with task engagement particularly during psychosocial stress. Paradigms used to elicit psychosocial stress instructed participants to engage in either a demanding achievement or a social goal-directed task. Contrarily, physiological stress tasks were mainly passive without immediate overt response or cognitive engagement. The effortful nature of the demanding task and the self-relevant evaluation of a situation seem to induce anhedonic mood and decrease processing of positive reinforcements (Pizzagalli et al., 2009; Wang et al., 2007). The engagement of cognitive resources should be taken into account when using different induction methods to assess stress reaction.
4.3. The role of the striatum in psychosocial and physiological stress
The striatum appeared to be involved in both psychosocial and physiological stress processing, with activation during physiological stress and deactivation during psychosocial stress. This result may be explained by the assumption that physiological stress engages motor preparation and sensory processing, whereas psychosocial stress down-regulates these processing states. The distinct involvement of the striatum displayed by the current results led us to examine this region in more detail. We conducted additional exploratory analysis, which revealed functionally divided sub-regions of the left striatum: While physiological stress activated dorsal parts, psychosocial stress deactivated ventral parts of the striatum.
Dorsal striatum
The current analyses revealed consistent engagement of the left dorsal striatum during physiological stress. Further functional connectivity analysis revealed that this cluster was functionally connected to other nuclei of the basal ganglia as well as parietal and frontal areas. The functional characterization of this region showed significant association with action execution, pain and sensory processing. Our finding of consistent activation of the dorsal striatum across experiments of physiological stress indicates a dominant role of this region for motor and sensory processes. This, together with results of other studies (Arsalidou et al., 2013a), suggests increased sensory processing and supports our assumption of the preparation of motor programs during physiological stress.
Ventral striatum
In contrast, during psychosocial stress the ventral striatum showed consistent deactivation. Functional connectivity analysis of this region revealed a network involved in emotion processing including other striatal and frontal regions. Additionally, functional characterization of the ventral striatum showed significant associations with cognition, emotion and reward. Therefore, psychosocial stress seems to correlate with deactivation of the ventral striatum, which is involved in processing reinforcement, motivation and executive functioning (Arsalidou et al., 2013a).
Dorsal vs. ventral striatum
Functional division of the striatum has already been suggested by some authors, in particular into three different subzones: a dorsal sensorimotor, a medial cognitive-associative, and a ventral limbic and emotional-motivational striatum (e.g., Lehéricy et al., 2004; Middleton and Strick, 2000; Postuma and Dagher, 2006). The ventral striatum is classically assigned to reward processing (Arsalidou et al., 2013a), and the current results as well as previous literature indicate that it has strong connections with regions associated with emotion processing and executive functioning (Lehéricy et al., 2004; Postuma and Dagher, 2006). Deactivation of the ventral striatum during psychosocial stress points to suppression of functions important for cognitive and emotion processing, in particular reward processing. In contrast, previous and current result show that the dorsal striatum is connected to motor and premotor areas as well as sensory processing brain regions (Postuma and Dagher, 2006). Therefore, activation of the dorsal striatum and its connectivity to motor regions in situations of physiological stress may indicate the preparation of musculoskeletal systems to induce either a fight or a flight response in life threatening situations (Cannon, 1932).
4.4. Limitations and suggestions for future studies
The current study has some limitations that might influence data interpretation.
First, we focused on acute stress reactivity without considering chronic psychosocial or physiological stress. Chronic stress is known to have long-lasting effects that are manifested on neural levels such as anatomical volume changes and even neural reorganization (e.g., Admon et al., 2013; Birbaumer et al., 1997). So far, the amount of neuroimaging studies on processing chronic stress is modest, but this factor should be taken into account in future research.
Second, it has to be noted that emotion-induction may induce negative affect and mood states as well, blurring the borders between emotional arousal and stress processing. However, it has been shown that mere emotion-induction studies do not trigger stress responses (Dickerson and Kemeny, 2004). Thus, in the current meta-analyses studies using stress-induction by emotional triggers (e.g., viewing of emotional pictures) were excluded to avoid confusion of mere emotional arousal and stress processing. Third, the current study focused on pain manipulation as a physiological stressor. Investigating similarities with further possible operationalization of physiological stress, such as hunger or oxidative stress, is of high interest to broaden the knowledge on the neural correlates of stress processing. Fourth, it may be argued that physiological stress possesses limited variance, whereas psychosocial stress may have more variability. One of the reasons to conduct the current analyses was to define regions that are involved in these diversified stress constructs of physiological and psychosocial stress. The term “stress” is often used without specifically differentiating the induction methods. We hope that the current analyses contribute to a better methodological separation of the different possibilities of stress induction in stress research.
Fifth, it is intriguing that regions often reported to be involved in stress reaction, such as the ACC or the amygdala, did not appear to be relevant for stress processing in the current meta-analyses. Inclusion of studies reporting whole-brain analyses was one precondition of the current study. We therefore excluded a fair amount of studies due to region-of-interest analyses or small-volume corrections. This may be a factor explaining the missing effects within these regions.
Sixth, the amount of studies in the field of psychosocial stress is modest and in both psychosocial and physiological stress only a few studies report deactivations. This is the first meta-analysis on deactivation and we hope that reports on psychosocial stress and deactivations will accumulate within coming years to enable more robust results on these data.
At last, future studies may deal with the neural correlates of different stressors when a cortisol reaction was observed vs. when there was no accompanying cortisol reaction. The specific contribution of the HPA axis on the neural deactivations and activations of stress processing is of great interest and may be addressed via this comparison.
5. Summary and conclusion
The current meta-analyses provide new insights into the neural correlates of stress processing which is strongly dependent on stressor type. Both physiological and psychosocial stress share activation in a cluster extending from the IFG into the AI; therefore, processing and regulation of negative subjective feelings is crucial for both stressor types. Besides that, rather distinct regions underlie the processing of both stress types. During the life threatening nature of physiological stress, the brain adapts by ceasing the functioning of essential body organs. It also engages motoric-sensoric processing and self-referential working memory to prepare a fight-or-flight reaction. Contrarily, the demanding character of psychosocial stress shifts attention to cognitive control of emotion and serves a goal-directed behavior. The effortful nature of psychosocial stress additionally deactivates reward processing and induces anhedonia. Overlaps in deactivations for physiological and psychosocial stress are missing. Our results have several implications as daily stress varies from health concerns to social and emotional complaints. Increases in prevalence rates in a variety of stress-related disorders have been reported (Keller et al., 2012; World Health Organization, 2001), which demonstrates the importance of investigating reactions to different stressors in more detail. The current analyses show the importance of differentiating physiological and psychosocial stress for specific conclusions on neural stress processing. Furthermore, the assessment of deactivations in addition to activations in stress research is highly recommended.
Highlights.
We meta-analytically analyzed physiological and psychosocial stress
IFG and insula are convergently activated in physiological and psychosocial stress
Physiological stress activates regions included in pain processing
Psychosocial stress activates the right STG and deactivates the left striatum
Dorsal and ventral striatum differ functionally in stress processing
Acknowledgments
We thank Anja Ludwig-Zahl for her support in characterizing the basal ganglia and particularly the dorsal and ventral striatum. We thank Dr. Catherine Sebastian (University College London and Royal Holloway University of London, London, UK), Dr. Keiichi Onoda (Shimane University, Izumo, Japan), Dr. Johan C. Karremans (Radboud University, Nijmegen, NL) and Dr. Dirk J. Heslenfeld (VU University, Amsterdam, NL), Dr. Pierre Maurage (Catholic University of Louvain, Louvain-la-Neuve, BE), Dr. Carrie Masten (University of California, Los Angeles, USA), Dr. Berna Guroglu and Prof. Eveline Crone (Universiteit Leiden, Leiden, NL), Dr. Eva Seidel (University of Vienna, Vienna, AUT), Prof. Jens Pruessner and Dr. Katarina Dedovic (McGill University, Montreal, CAN), Prof. Richard J. Davidson and Regina Lapate (University of Wisconsin-Madison, Madison, USA), Dr. Danny J.J. Wang (University of California, Los Angeles, USA), Dr. Etienne Vachon-Presseau (Université de Montréal, Montreal, CAN), Dr. Diana Torta (University of Turin, Turin, IT), Prof. Kenneth Casey (University of Michigan, Ann Arbor, USA) and Dr. Sven Benson and Mr. V. Kotsis (University of Duisburg-Essen, Essen, GER) for providing additional information on their work.
This study was supported by the Deutsche Forschungsgemeinschaft (DFG; IRTG-1328 [LK]; EI 816/4-1 [SBE]; EI 816/6-1 [SBE]; LA 3071/3-1 [SBE]), Jülich-Aachen-Research-Alliance, Translational Brain Medicine (LK and BD), the National Institute of Mental Health (R01-MH074457) (SBE) and the European EFT program (Human Brain Project) (SBE). The funding sources had no involvement in study design, collection, analysis or interpretation of the data, in writing the article and in decision for publication.
Footnotes
Preliminary results of parts of this study were presented at the annual Meeting of the Organization of Human Brain Mapping (OHBM) in Hamburg 2014.
All authors declare no conflict of interest in relation to the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler CM, Elman I, Weisenfeld N, Kestler L, Pickar D, Breier A. Effects of Acute Metabolic Stress on Striatal Dopamine Release in Healthy Volunteers. Neuropsychopharmacology. 2000;22:545–550. doi: 10.1016/S0893-133X(99)00153-0. [DOI] [PubMed] [Google Scholar]
- Admon R, Leykin D, Lubin G, Engert V, Andrews J, Pruessner J, Hendler T. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum Brain Mapp. 2013;34:2808–2816. doi: 10.1002/hbm.22100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. Stress in America findings. Washington, D.C.: American Psychological Association; 2010. [Google Scholar]
- Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB. Definition and characterization of an extended social-affective default network. Brain, Struct Funct. 2015;220:1031–1049. doi: 10.1007/s00429-013-0698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210 doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comput Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Arsalidou M, Duerden EG, Taylor MJ. The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum Brain Mapp. 2013a;34:3031–3054. doi: 10.1002/hbm.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M, Pascual-Leone J, Johnson J, Morris D, Taylor MJ. A balancing act of the brain: activations and deactivations driven by cognitive load. Brain Behav. 2013b;3:273–285. doi: 10.1002/brb3.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Andersson JLR, Valind S, Sundin A, Hamdy S, Jones AKP, Foster ER, Långström B, Thompson DG. Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology. 1997;113:50–59. doi: 10.1016/s0016-5085(97)70079-9. [DOI] [PubMed] [Google Scholar]
- Bauer H, Pllana A, Sailer U. The EEG-based local brain activity (LBA-) feedback training. Act Nerv Super Rediviva. 2011;53:107–113. [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Becerra L, Iadarola M, Borsook D. CNS activation by noxious heat to the hand or foot: site-dependent delay in sensory but not emotion circuitry. J Neurophysiol. 2004;91:533–541. doi: 10.1152/jn.00326.2003. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Benson S, Kotsis V, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER, Elsenbruch S. Behavioural and neural correlates of visceral pain sensitivity in healthy men and women: does sex matter? Eur J pain. 2012;16:349–358. doi: 10.1002/j.1532-2149.2011.00027.x. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G. The role of ventral premotor cortex in action execution and action understanding. J Physiol Paris. 2006;99:396–405. doi: 10.1016/j.jphysparis.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Lutzenberger W, Montoya P, Larbig W, Unertl K, Grodd W, Taub E, Flor H. Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci. 1997;17:5503–5508. doi: 10.1523/JNEUROSCI.17-14-05503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok BFM, Sturms LM, Holstege G. A PET study on cortical and subcortical control of pelvic floor musculature. J Comp Neurol. 1997;389:535–544. [PubMed] [Google Scholar]
- Bolling DZ, Pelphrey KA, Vander Wyk BC. Differential brain responses to social exclusion by one's own versus opposite-gender peers. Soc Neurosci. 2012;7:331–346. doi: 10.1080/17470919.2011.623181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Mayes LC, Pelphrey KA. Dissociable brain mechanisms for processing social exclusion and rule violation. Neuroimage. 2011;54:2462–2471. doi: 10.1016/j.neuroimage.2010.10.049.Dissociable. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornhövd K, Quante M, Glauche V, Bromm B, Weiller C, Büchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Brooks JCW, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Burchfield SR. The stress response: a new perspective. Psychosom Med. 1979;41:661–672. doi: 10.1097/00006842-197912000-00008. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, Laird AR, Fox PT, Zilles K, Eickhoff SB. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage. 2013;81:381–392. doi: 10.1016/j.neuroimage.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct. 2012;217:783–796. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, Cacioppo JT. A quantitative meta-analysis of functional imaging studies of social rejection. Sci Rep. 2013;3:2027. doi: 10.1038/srep02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. The wisdom of the body. Norton; New York, NY: 1932. [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32:1804–1814. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol. 2006;459:53–69. doi: 10.1002/cne.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, Gilron I, Iadarola MJ. Hemispheric lateralization of somatosensory processing. J Neurophysiol. 2001;85:2602–2612. doi: 10.1152/jn.2001.85.6.2602. [DOI] [PubMed] [Google Scholar]
- Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. 2003;100:8538–8542. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14:4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianna M, Schiavone S, Zotti M, Tucci P, Morgese MG, Bäckdahl L, Holmdahl R, Krause KH, Cuomo V, Trabace L. Neuroendocrine profile in a rat model of psychosocial stress: relation to oxidative stress. Antioxid Redox Signal. 2013;18:1385–1399. doi: 10.1089/ars.2012.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci U S A. 2004;101:6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol. 2000a;523:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: A functional magnetic resonance imaging study. J Neurosci. 2000b;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D. An acute psychosocial stress enhances the neural response to smoking cues. Brain Res. 2009;1293:40–48. doi: 10.1016/j.brainres.2009.07.048.An. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Pope GE, Crawley AP, Mikulis DJ. Neural correlates of prickle sensation: a percept-related fMRI study. Nat Neurosci. 2002;5:1121–1122. doi: 10.1038/nn955. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, Hall J. Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biol Psychiatry. 2002;51:544–552. doi: 10.1016/s0006-3223(01)01374-9. [DOI] [PubMed] [Google Scholar]
- De Leeuw R, Davis CE, Albuquerque R, Carlson CR, Andersen AH. Brain activity during stimulation of the trigeminal nerve with noxious heat. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:750–757. doi: 10.1016/j.tripleo.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009a;47:864–71. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, Lue SD, Lord C, Engert V, Pruessner JC. Neural correlates of processing stressful information: an event-related fMRI study. Brain Res. 2009b;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317:215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- Derbyshire SWG, Jones AKP. Cerebral responses to a continual tonic pain stimulus measured using positron emission tomography. Pain. 1998;76:127–135. doi: 10.1016/s0304-3959(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Derbyshire SWG, Jones AKP, Devani P, Friston KJ, Feinmann C, Harris M, Pearce S, Watson JDG, Frackowiak RSJ. Cerebral responses to pain in patients with atypical facial pain measured by positron emission tomography. J Neurol Neurosurg psychiatry. 1994;57:1166–1172. doi: 10.1136/jnnp.57.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SWG, Jones AKP, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Derbyshire SWG, Nichols TE, Firestone L, Townsend DW, Jones AKP. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J pain. 2002;3:401–411. doi: 10.1054/jpai.2002.126788. [DOI] [PubMed] [Google Scholar]
- Derntl B, Metzler H, Thaler H, Boubela R, Kogler L, Pruessner JC, Kryspin-Exner I, Moser E, Habel U, Seidel EM. Gender, menstrual cycle and testosterone modulate neural stress reactions submitted. [Google Scholar]
- DeWall CN, Masten CL, Powell C, Combs D, Schurtz DR, Eisenberger NI. Do neural responses to rejection depend on attachment style? An fMRI study. Soc Cogn Affect Neurosci. 2012;7:184–192. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diederichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dubé AA, Duquette M, Roy M, Lepore F, Duncan G, Rainville P. Brain activity associated with the electrodermal reactivity to acute heat pain. Neuroimage. 2009;45:169–180. doi: 10.1016/j.neuroimage.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Dunckley P, Wise RG, Aziz Q, Painter D, Brooks J, Tracey I, Chang L. Cortical processing of visceral and somatic stimulation: differentiating pain intensity from unpleasantness. Neuroscience. 2005;133:533–542. doi: 10.1016/j.neuroscience.2005.02.041. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Laird A, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based ALE meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718.Coordinate-based. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage. 2011;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021.Co-activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006a;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras M, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006b;16:254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Egan GF, Zamarripa F, Shade R, Blair-West J, Fox P, Denton DA. Unique, common, and interacting cortical correlates of thirst and pain. Proc Natl Acad Sci U S A. 2006;103:2416–2421. doi: 10.1073/pnas.0511019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favilla S, Huber A, Pagnoni G, Lui F, Facchin P, Cocchi M, Baraldi P, Porro CA. Ranking brain areas encoding the perceived level of pain from fMRI data. Neuroimage. 2014;90:153–162. doi: 10.1016/j.neuroimage.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Fechir M, Gamer M, Blasius I, Bauermann T, Breimhorst M, Schlindwein P, Schlereth T, Birklein F. Functional imaging of sympathetic activation during mental stress. Neuroimage. 2010;50:847–854. doi: 10.1016/j.neuroimage.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Friebel U, Eickhoff SB, Lotze M. Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. Neuroimage. 2011;58:1070–1080. doi: 10.1016/j.neuroimage.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, Westenberg HGM, Jochims A, de Kloet CS, Bohus M, Vermetten E, Schmahl C. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76–85. doi: 10.1001/archpsyc.64.1.76. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Bürgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gradin VB, Waiter G, Kumar P, Stickle C, Milders M, Matthews K, Reid I, Hall J, Steele JD. Abnormal neural responses to social exclusion in schizophrenia. PLoS One. 2012;7:e42608. doi: 10.1371/journal.pone.0042608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy JW, Levine S. Stress, arousal, and the pituitary-adrenal system: a psychoendocrine hypothesis. In: Sprague JM, Epstein AN, editors. Progress in Psychobiology and Physiological Psychology. Acadamic press; New York: 1979. pp. 133–178. [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain cortical representation of the sensory dimension of pain. J Neurophysiol. 2001;86:402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG, Gracely RH, Max MB, Bennett GJ. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain. 1998;121:931–947. doi: 10.1093/brain/121.5.931. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back) Exp brain Res. 2010;205:1–12. doi: 10.1007/s00221-010-2340-1. [DOI] [PubMed] [Google Scholar]
- Ibinson JW, Small RH, Algaze A, Roberts CJ, Clark DL, Schmalbrock P. Functional magnetic resonance imaging studies of pain an investigation of signaldecay during and across sessions. Anesthesiology. 2004;101:960–969. doi: 10.1097/00000542-200410000-00022. [DOI] [PubMed] [Google Scholar]
- Karremans JC, Heslenfeld DJ, van Dillen LF, Van Lange PAM. Secure attachment partners attenuate neural responses to social exclusion: an fMRI investigation. Int J Psychophysiol. 2011;81:44–50. doi: 10.1016/j.ijpsycho.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Onoda K, Nakashima K, Nittono H, Yamaguchi S, Ura M. Is dorsal anterior cingulate cortex activation in response to social exclusion due to expectancy violation? An fMRI study. Front Evol Neurosci. 2012;4:11. doi: 10.3389/fnevo.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Litzelman K, Wisk L, Maddox T, Cheng ER, Creswell PD, Witt WP. Does the perception that stress affects health matter? The association with health and mortality. Heal Psychol. 2012;31:677–684. doi: 10.1037/a0026743.Does. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: a functional ragnetic resonance imaging study. J Neurosci. 2006;26:4437–4443. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a social stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010.Glucose. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059.Functional. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Gur RC, Derntl B. Sex differences in cognitive regulation of psychosocial achievement stress: Brain and behavior. Hum Brain Mapp. 2015;36:1028–1042. doi: 10.1002/hbm.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation-an ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;721:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038.Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koric L, Volle E, Seassau M, Bernard FA, Mancini J, Dubois B, Pelissolo A, Levy R. How cognitive performance-induced stress can influence right VLPFC activation: an fMRI study in healthy subjects and in patients with social phobia. Hum Brain Mapp. 2012;33:1973–1986. doi: 10.1002/hbm.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Berghorst LH, Nickerson LD, Dutra SJ, Goer FK, Greve DN, Pizzagalli DA. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience. 2014;266:1–12. doi: 10.1016/j.neuroscience.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata J, Thulborn KR, Gyulai FE, Firestone LL. Early decay of pain-related cerebral activation in functional magnetic resonance imaging. Comparison with visual and motor tasks. Anesthesiology. 2002;96:35–44. doi: 10.1097/00000542-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Kurth F, Eickhoff SB, Schleicher A, Hoemke L, Zilles K, Amunts K. Cytoarchitecture and probabilistic maps of the human posterior insular cortex. Cereb Cortex. 2010a;20:1448–1461. doi: 10.1093/cercor/bhp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010b;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladabaum U, Minoshima S, Hasler WL, Cross D, Chey WD, Owyang C. Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology. 2001;120:369–376. doi: 10.1053/gast.2001.21201. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, McKay DR, Bzdok D, Laird RW, Robinson JL, Turner JA, Turkeltaub PE, Lancaster JL, Fox PT. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes. 2011;4:349. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull. 2013;139:870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wüst S, Pruessner JC, Rietschel M, Deuschle M, Meyer-Lindenberg A. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Leisman G, Melillo R. The basal ganglia: motor and cognitive relationships in a clinical neurobehavioral context. Rev Neurosci. 2013;24:9–25. doi: 10.1515/revneuro-2012-0067. [DOI] [PubMed] [Google Scholar]
- Lelieveld GJ, Moor BG, Crone EA, Karremans JC, van Beest I. A penny for your pain? The financial compensation of social pain after exclusion. Soc Psychol Personal Sci. 2012;4:206–214. doi: 10.1177/1948550612446661. [DOI] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words. Affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Linden DEJ, Habes I, Johnston SJ, Linden S, Tatineni R, Subramanian L, Sorger B, Healy D, Goebel R. Real-time self-regulation of emotion networks in patients with depression. PLoS One. 2012;7:e38115. doi: 10.1371/journal.pone.0038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann-Haislah A. Stressreport Deutschland 2012. Psychische Anforderungen, Ressourcen und Befinden Dortmund 2012 [Google Scholar]
- Lorenz J, Cross DJ, Minoshima S, Morrow TJ, Paulson PE, Casey KL. A unique representation of heat allodynia in the human brain. Neuron. 2002;35:383–393. doi: 10.1016/s0896-6273(02)00767-5. [DOI] [PubMed] [Google Scholar]
- Ludwig-Zahl A, Hoffstaedter F, Eickhoff SB, Mohlberg H, Zilles K, Amunts K. Cytoarchitecture, probability maps and functional differentiation of the human dorsal striatum. 20th Annu Meet Organ Hum Brain Mapp (OHBM); Hamburg, Ger. June 8-12, 2014.2014. [Google Scholar]
- Lui F, Duzzi D, Corradini M, Serafini M, Baraldi P, Porro CA. Touch or pain? Spatio-temporal patterns of cortical fMRI activity following brief mechanical stimuli. Pain. 2008;138:362–374. doi: 10.1016/j.pain.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull. 2005;131:202–223. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Eisenberger NI. An fMRI investigation of attributing negative social treatment to racial discrimination. J Cogn Neurosci. 2011;23:1042–1051. doi: 10.1162/jocn.2010.21520. [DOI] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Soc Cogn Affect Neurosci. 2012;7:106–14. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Philippot P, Heeren A, Vermeulen N, Mahau P, Delperdange C, Corneille O, Luminet O, de Timary P. Disrupted regulation of social exclusion in alcohol-dependence: an fMRI study. Neuropsychopharmacology. 2012;37:2067–2075. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee S, Bunney BG, Reist C, Potkin SG, Bunney WE. Psychological pain: a review of evidence. J Psychiatr Res. 2006;40:680–690. doi: 10.1016/j.jpsychires.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Meerwijk EL, Ford JM, Weiss SJ. Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain. Brain Imaging Behav. 2013;7:1–14. doi: 10.1007/s11682-012-9179-y. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Sadato N, Saito DN, Toyoda H, Tashiro M, Okamura N, Yanai K. Neural correlates of perceptual difference between itching and pain: A human fMRI study. Neuroimage. 2007;36:706–717. doi: 10.1016/j.neuroimage.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Moor BG, Güroğlu B, Op de Macks ZA, Rombouts SARB, Van der Molen MW, Crone EA. Social exclusion and punishment of excluders: neural correlates and developmental trajectories. Neuroimage. 2012;59:708–717. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Moor BG, van Leijenhorst L, Rombouts SARB, Crone EA, Van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc Neurosci. 2010;5:461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K. Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. Neuroimage. 2001;13:684–701. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Eickhoff SB. Dysregulated left inferior parietal activity in schizophrenia and depression: functional connectivity and characterization. Front Hum Neurosci. 2013;7:268. doi: 10.3389/fnhum.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Langner R, Cieslik EC, Rottschy C, Eickhoff SB. Interindividual differences in cognitive flexibility: influence of gray matter volume, functional connectivity and trait impulsivity. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Niddam DM, Yeh T, Wu Y, Lee P, Ho LT, Arendt-Nielsen L, Chen ACN, Hsieh JC. Event-related functional MRI study on central representation of acute muscle pain induced by electrical stimulation. Neuroimage. 2002;17:1437–1450. doi: 10.1006/nimg.2002.1270. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biol Psychiatry. 2012;72:157–163. doi: 10.1016/j.biopsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc Neurosci. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Yoshimura S, Yamawaki S, Yamaguchi S, Ura M. Does low self-esteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Soc Cogn Affect Neurosci. 2010;5:385–391. doi: 10.1093/scan/nsq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting discrimination of sensory features of pain: a new model. J Neurosci. 2009;29:14924–14931. doi: 10.1523/JNEUROSCI.5538-08.2009.Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro Y, Quevedo AS, Mchaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting spatial discrimination of pain. J Neurosci. 2007;27:3388–3394. doi: 10.1523/JNEUROSCI.5128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Neuroscience. Feeling the pain of social loss. Science. 2003;302:237–239. doi: 10.1126/science.1091062. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Minoshima S, Morrow TJ, Casey KL. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain. 1998;76:223–229. doi: 10.1016/s0304-3959(98)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini I, Bergstrand S, Morrison I. Where pain meets action in the human brain. J Neurosci. 2013;33:15930–15939. doi: 10.1523/JNEUROSCI.3135-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R, García-Larrea L, Grégoire MC, Costes N, Convers P, Lavenne F, Mauguière F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans sensory and attentional networks. Brain. 1999;122:1765–1779. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Philip NS, Kuras YI, Valentine TR, Sweet LH, Tyrka AR, Price LH, Carpenter LL. Regional homogeneity and resting state functional connectivity: associations with exposure to early life stress. Psychiatry Res neuroimaging. 2013;214:247–253. doi: 10.1016/j.pscychresns.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated subjects with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201.Reduced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Dagher A, Lupien SJ. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–91. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Low self-esteem, induced failure and the adrenocortical stress response. Pers Individ Dif. 1999;27:477–489. doi: 10.1016/S0191-8869(98)00256-6. [DOI] [Google Scholar]
- Pujol J, López-Solà M, Ortiz H, Vilanova JC, Harrison BJ, Yücel M, Soriano-Mas C, Cardoner N, Deus J. Mapping brain response to pain in fibromyalgia patients using temporal analysis of fMRI. PLoS One. 2009;4:e5224. doi: 10.1371/journal.pone.0005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Doherty JO, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13:308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050.Modelling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Schmahl C, Bohus M, Esposito F, Treede RD, Salle FDi, Greffrath W, Ludaescher P, Jochims A, Lieb K, Scheffler K, Hennig J, Seifritz E. Neural correlates of antinociception in borderline personality disorder. Arch Gen Psychiatry. 2006;63:659–667. doi: 10.1001/archpsyc.63.6.659. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, Tan GCY, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuroimage. 2011;57:686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Seidel E, Silani G, Metzler H, Thaler H, Lamm C, Gur R, Kryspin-Exner I, Habel U, Derntl B. The impact of social exclusion vs. inclusion on subjective and hormonal reactions in females and males. Psychoneuroendocrinology. 2013;38:2925–2932. doi: 10.1016/j.psyneuen.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel EM, Boubela R, Thaler H, Metzler H, Kryspin-Exner I, Moser E, Habel U, Derntl B. Gender differences in hormonal and neural responses to social exclusion. doi: 10.1016/j.psyneuen.2018.04.005. submitted. [DOI] [PubMed] [Google Scholar]
- Seifert F, Maihöfner C. Representation of cold allodynia in the human brain - a functional MRI study. Neuroimage. 2007;35:1168–1180. doi: 10.1016/j.neuroimage.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatry Clin Neurosci. 1998;10:230–231. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Interactions of pain intensity and cognitive load: the brain stays on task. Cereb Cortex. 2007;17:1412–1422. doi: 10.1093/cercor/bhl052. [DOI] [PubMed] [Google Scholar]
- Seseke S, Baudewig J, Kallenberg K, Ringert RH, Seseke F, Dechent P. Voluntary pelvic floor muscle control-an fMRI study. Neuroimage. 2006;31:1399–1407. doi: 10.1016/j.neuroimage.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Ann N Y Acad Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Smith KA, Ploghaus A, Cowen PJ, McCleery JM, Guy M, Smith S, Tracey I, Matthews PM. Cerebellar responses during anticipation of noxious stimuli in subjects recovered from depression: Functional magnetic resonance imaging study. Br J psychiatry. 2002;181:411–415. doi: 10.1192/bjp.181.5.411. [DOI] [PubMed] [Google Scholar]
- Soliman A, O'Driscoll GA, Pruessner J, Joober R, Ditto B, Streicker E, Goldberg Y, Caro J, Rekkas PV, Dagher A. Limbic response to psychosocial stress in schizotypy: a functional magnetic resonance imaging study. Schizophr Res. 2011;131:184–191. doi: 10.1016/j.schres.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF. Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cereb Cortex. 2010;20:3005–3013. doi: 10.1093/cercor/bhq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Duncan GH, Boivin M, Bushnell MC. Differentiation of visceral and cutaneous pain in the human brain. J Neurophysiol. 2003;89:3294–3303. doi: 10.1152/jn.01048.2002. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Svensson P, Johannsen P, Jensen TS, Arendt-Nielsen L, Nielsen J, Stodkilde-Jorgensen H, Gee AD, Hansen SB, Gjedde A. Cerebral blood-flow changes evoked by two levels of painful heat stimulation: a positron emission tomography study in humans. Eur J pain. 1998;2:95–107. doi: 10.1016/s1090-3801(98)90001-5. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple Representations of Pain in Human Cerebral Cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Torta DM, Diano M, Costa T, Gallace A, Duca S, Geminiani GC, Cauda F. Crossing the line of pain: fMRI correlates of crossed-hands analgesia. J Pain. 2013;14:957–965. doi: 10.1016/j.jpain.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Tossani E. The concept of mental pain. Psychother Psychosom. 2013;82:67–73. doi: 10.1159/000343003. [DOI] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Barrett LF. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60:1947–1958. doi: 10.1016/j.neuroimage.2012.02.012.Dissociable. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I. Nociceptive processing in the human brain. Curr Opin Neurobiol. 2005;15:478–487. doi: 10.1016/j.conb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Tracey I, Becerra L, Chang I, Breiter H, Jenkins L, Borsook D, González RG. Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2000;288:159–162. doi: 10.1016/s0304-3940(00)01224-6. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J, Laird A. The cognitive paradigm ontology: design and application. Neuroinformatics. 2012;10:57–66. doi: 10.1007/s12021-011-9126-x.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon-Presseau E, Martel MO, Roy M, Caron E, Albouy G, Marin MF, Plante I, Sullivan MJ, Lupien SJ, Rainville P. Acute stress contributes to individual differences in pain and pain-related brain activity in healthy and chronic pain patients. J Neurosci. 2013a;33:6826–6833. doi: 10.1523/JNEUROSCI.4584-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ, Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013b;136:815–827. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain — an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Votinov M, Aso T, Koganemaru S, Fukuyama H, Mima T. Transcranial direct current stimulation changes human endowment effect. Neurosci Res. 2013;76:251–256. doi: 10.1016/j.neures.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA. Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci U S A. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ. Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci. 2006;26:11501–11509. doi: 10.1523/JNEUROSCI.2568-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Mental Health: New Understanding, New Hope. Geneva: World Health Organization; 2001. The world health report 2001. [Google Scholar]
- Xu X, Fukuyama H, Yazawa S, Mima T, Hanakawa T, Magata Y, Kanda M, Fujiwara N, Shindo K, Nagamine T, Shibasaki H. Functional localization of pain perception in the human brain studied by PET. Neuroreport. 1997;8:555–559. doi: 10.1097/00001756-199701200-00035. [DOI] [PubMed] [Google Scholar]
- Yang W, Liu P, Cui Q, Wei D, Li W, Qiu J, Zhang Q. Directed forgetting of negative self-referential information is difficult: an FMRI study. PLoS One. 2013;8:e75190. doi: 10.1371/journal.pone.0075190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Reitz A, Kollias S, Summers P, Curt A, Schurch B. An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. Neuroimage. 2005;24:174–180. doi: 10.1016/j.neuroimage.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


