Abstract
Human cell adhesion molecules (CAMs) are essential both for a) proper development, modulation and maintenance of interactions between cells and for b) cell-to-cell (and matrix-to-cell) communication about these interactions. CAMs are thus key to proper development and plasticity of organs and tissues that include the brain. Despite recognition of the existence of these dual CAM roles and appreciation of the differential functional significance of these roles, there have been surprisingly few systematic studies that have carefully enumerated the universe of CAMs, identified the preferred roles for specific CAMs in distinct types of cellular connections and communication, or related these issues to specific brain disorders or brain circuits. In this paper, we substantially update and review the set of human genes that are likely to encode CAMs based on searches of databases, literature reviews and annotations. We describe the likely CAMs and the functional CAM subclasses into which they fall. These include “iCAMs”, whose contacts largely mediate cell to cell communication, those involved in focal adhesions, CAM genes whose products are preferentially involved with stereotyped and morphologically-identifiable connections between cells (adherens junctions, gap junctions) and smaller numbers of genes in other classes. We discuss a novel proposed mechanism involving selective anchoring of the constituents of iCAM-containing lipid rafts in zones of close neuronal apposition to membranes expressing binding partners of these iCAMs. CAM data from genetic and genomic studies of addiction in humans and mouse models provide examples of the ways in which CAM variation is likely to contribute to a specific brain-based disorder. We discuss how differences in CAM splicing mediated by differences in the addiction-associated splicing regulator RBFOX1/A2BP1 could enrich this picture. CAM expression in dopamine neurons provides one of the ways in which variations in cell adhesion molecule genes could impact a specific set of circuits central to addiction and drug reward.
Keywords: cell adhesion molecules, addiction, dopamine, substance use disorders, lipid rafts, GWAS, connectome
Introduction
“Cell adhesion molecules” (CAMs) play central roles in much of the connection and communication between cells and their synapses (1–6). Cell adhesion-related communication is essential for many aspects of the proper development of a variety of organs and tissues. This cellular communication also plays substantial roles in the plasticity of cell recognition processes in developed, adult organisms.
Cell adhesion molecules are likely to be especially important in the brain. Proper brain development requires appropriate connection of perhaps 100 trillion synapses (7). Brain function requires substantial plasticity in many of these synapses, providing the bases for learning, memory, addiction and related phenotypes (8,9). Physiologic and cell biologic studies implicate CAM roles in properties that include synapse adhesion (10,11), neuronal connectivity and communication (11), signal transduction (10,12–14), and proper arrangement of pre-synaptic active zones and postsynaptic densities at classical synapses (15,16). We and others have advanced working hypotheses concerning the large contributions of cell adhesion molecules to the development and plasticities of the brain connectome (17), and the CAM “bar codes” that allow the proper connections of specific cell types (GRU and JD, in preparation).
Current genetic studies have linked and/or associated variants in cell adhesion molecule genes with a number of phenotypes based on variation in the brain and other organs. Vulnerabilities to addictions are associated with variants in CAM genes in studies of several independent samples (17–21). The importance of CAMs in learning and memory-associated disorders is also demonstrated in genome wide association (GWAS) data (22,23). Genetic variants of CAM genes have been associated with autism (9,24,25). Variants in neuregulin have been associated with vulnerability to schizophrenia (26,27). Variants in a CAM KIAA0319 have been associated with dyslexia (28–30).
Despite the importance of cell adhesion molecules in the normal physiologies of and in the disorders of brain and other organs, and our initial work in defining a set of these genes (2), there remains only a modest amount of updated, systematic work that: 1) enumerates the genes and gene families that function as CAMs; 2) delineates those more likely to function in proper development, modulation and maintenance of morphologically-visible sites for physical interactions between cells and between cells and matrix vs those “iCAMs” that appear to largely transmit information about cell-cell and cell-matrix interactions; 3) establishes the ways in which the patterns of CAM expression by any specific cell type might relate to these cells’ connectivities and functions; 4) documents the ways in which CAM variation, taken as a whole, might relate to individual differences in vulnerabilities to disease and 5) explores ways in which CAM expression by specific cell types might relate to disease vulnerabilities.
We now report compilation of an updated list of potential human genes annotated or otherwise identified as possible CAMs. We annotate the members of this list that are likely to be CAMs vs those that are questionable vs those unlikely to be CAMs. For the genes that are likely to encode bona fide CAMs, we describe those likely to play largely information transmission roles between cells (“iCAMs”) or between cellular elements and extracellular matrix (eg focal adhesions). We contrast these genes to those more likely to be involved in relatively stereotypical, morphologically-visible connections between cells (eg adherens junctions, gap junctions). As a specific example of involvement in a complex disorder, we focus on CAMs identified by genome-wide association (GWAS) signals for addiction phenotypes that are both reproducible and modest in individual samples. This list of genes includes many that are expressed in the dopaminergic neurons that play central roles in current models of the reward that can come from abused drugs of many pharmacological classes. These data allow specific hypotheses about the differential connectivities and architectures of dopaminergic neurons in individuals who may display higher vs lower expression of (and/or different versions of) interesting cell adhesion molecules. Possible novel roles for glycosylphosphatidyl inositol (GPI)-coupled and other lipid-raft associated CAMs in stabilizing raft contents near areas of close cell-cell apposition are described, providing additional testable hypotheses that flow from our current understanding of the roles for these CAMs. We underscore some of the ways in which understanding CAMs and their human variants is likely to aid understanding of both the brain connectome and a variety of human brain disorders, including addiction.
Identification of human CAM genes
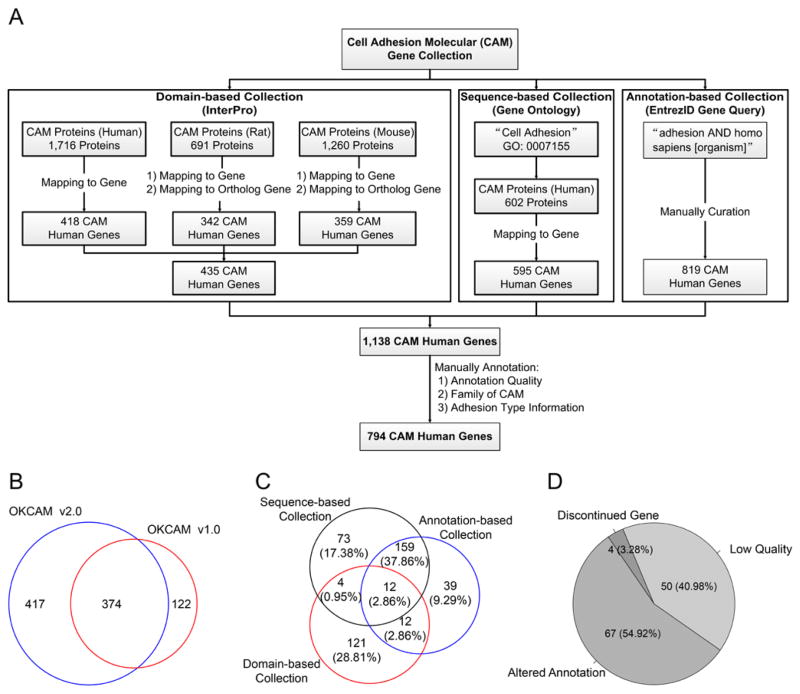
Human CAM gene candidates were identified based on compilation of data from several sources (Fig 1):
Figure 1. Cell adhesion molecule gene identification and annotation.
Identification of human cell adhesion molecules.
(A) Cell adhesion molecules were compiled by integrating Gene Ontology annotations, domain structure information and keyword queries against NCBI Entrez annotations. 794 unique human genes were identified after manual curation. (B) Overlap of the current version of OKCAM with the previous version. (C) Characteristics of the 417 newly-added CAMs. (D) Characteristics of the122 genes that were included in the prior CAM dataset but not included in the current set.
Entrez Gene query “cell adhesion molecule AND Homo sapiens [organism]”.
Interpro was searched for genes that encoded common protein domains for CAM families based on common motifs from cadherin, immunoglobulin, fibronectin, integrin, neurexin, neuroligin, cub/sushi and catenin families.
The Gene Ontology term “cell adhesion” (GO:0007155) (31) was searched.
Our previously-described OKCAM database (2,32) was searched.
We manually curated these candidate CAM gene lists. For each gene, we evaluated evidence from all NCBI data sources that its product(s) were likely to serve as cell adhesion molecule(s), could questionably play such a role, or were unlikely to be cell adhesion molecules. Many of the genes placed in the latter “unlikely” category received “cell adhesion” annotations in other databases due to the gene products’ abilities to interact with a cell adhesion molecule, by regulating its expression, for example. We assigned a category based on both the amount and nature of evidence available for each gene (data available at http://rhesusbase.org/OKCAM/).
Data annotations
To elucidate the functions of CAMs, further detailed annotations were assigned to each “likely” CAM gene (Table I). For these genes identified as likely CAMs, we sought evidence that might separate them into functional classes based on a) their involvement in relatively stereotypical and morphologically-recognizable cell-cell contacts, including tight junctions, gap junctions, desmosomes and adherens junctions; b) their predominant roles in axonal guidance; c) their apparently and/or likely greater roles in transmitting information about cell-cell or cell matrix (focal adhesions) contacts than in mediating physical cell-cell/cell-matrix contacts or their preferential roles in a number of other smaller categories (Table I; Fig 2). We term the products of the genes in the third group “iCAMs” to denote their preferential role in communication as opposed to the establishment of physical interconnections (but see below). Online annotations (http://rhesusbase.org/OKCAM/) provide information about expression, regulation, functions, gene structure, genetic variations, phenotype associations, disease associations and drug development for each gene. In Table II, we list the 39 likely CAMs for which SNPs are likely to knock out their expression.
Table I.
Genes judged “likely” to encode bona fide human cell adhesion molecules.
| symbol | #entrezID | CAM type | Inter Pro | GO | NCBI | Sum | name |
|---|---|---|---|---|---|---|---|
| CDH1 | 999 | i | 1 | 1 | 1 | 3 | cadherin 1 |
| CDH10 | 1008 | i | 1 | 1 | 1 | 3 | cadherin 10 |
| CDH11 | 1009 | i | 1 | 1 | 1 | 3 | cadherin 11 |
| CDH13 | 1012 | i | 1 | 1 | 1 | 3 | cadherin 13 |
| CDH15 | 1013 | i | 1 | 1 | 1 | 3 | cadherin 15 |
| CDH16 | 1014 | i | 1 | 1 | 1 | 3 | cadherin 16 |
| CDH17 | 1015 | i | 1 | 1 | 1 | 3 | cadherin 17 |
| CDH2 | 1000 | i | 1 | 1 | 1 | 3 | cadherin 2 |
| CDH23 | 64072 | i | 1 | 1 | 1 | 3 | cadherin-related 23 |
| CDH24 | 64403 | i | 1 | 1 | 1 | 3 | cadherin 24 |
| CDH3 | 1001 | i | 1 | 1 | 1 | 3 | cadherin 3 |
| CDH4 | 1002 | i | 1 | 1 | 1 | 3 | cadherin 4 |
| CDH6 | 1004 | i | 1 | 1 | 1 | 3 | cadherin 6 |
| CDH7 | 1005 | i | 1 | 1 | 1 | 3 | cadherin 7 |
| CDH8 | 1006 | i | 1 | 1 | 1 | 3 | cadherin 8 |
| CDH9 | 1007 | i | 1 | 1 | 1 | 3 | cadherin 9 |
| CDHR2 | 54825 | i | 1 | 1 | 1 | 3 | cadherin-related family member 2 |
| CDHR5 | 53841 | i | 1 | 1 | 1 | 3 | cadherin-related family member 5 |
| CEACAM1 | 634 | i | 1 | 1 | 1 | 3 | carcinoembryonic antigen-related cell adhesion molecule 1 |
| CELSR2 | 1952 | i | 1 | 1 | 1 | 3 | cadherin EGF LAG seven-pass G-type receptor 2 |
| CLSTN1 | 22883 | i | 1 | 1 | 1 | 3 | calsyntenin 1 |
| CNTN4 | 152330 | i | 1 | 1 | 1 | 3 | contactin 4 |
| DCHS1 | 8642 | i | 1 | 1 | 1 | 3 | dachsous cadherin-related 1 |
| DSCAM | 1826 | i | 1 | 1 | 1 | 3 | Down syndrome cell adhesion molecule |
| DSCAML1 | 57453 | i | 1 | 1 | 1 | 3 | Down syndrome cell adhesion molecule like 1 |
| EPHA2 | 1969 | i | 1 | 1 | 1 | 3 | EPH receptor A2 |
| EPHA3 | 2042 | i | 1 | 1 | 1 | 3 | EPH receptor A3 |
| EPHA7 | 2045 | i | 1 | 1 | 1 | 3 | EPH receptor A7 |
| EPHA8 | 2046 | i | 1 | 1 | 1 | 3 | EPH receptor A8 |
| EPHB3 | 2049 | i | 1 | 1 | 1 | 3 | EPH receptor B3 |
| EPHB4 | 2050 | i | 1 | 1 | 1 | 3 | EPH receptor B4 |
| FAT1 | 2195 | i | 1 | 1 | 1 | 3 | FAT atypical cadherin 1 |
| FAT2 | 2196 | i | 1 | 1 | 1 | 3 | FAT atypical cadherin 2 |
| FAT4 | 79633 | i | 1 | 1 | 1 | 3 | FAT atypical cadherin 4 |
| L1CAM | 3897 | i | 1 | 1 | 1 | 3 | L1 cell adhesion molecule |
| LRRN2 | 10446 | i | 1 | 1 | 1 | 3 | leucine rich repeat neuronal 2 |
| NCAM2 | 4685 | i | 1 | 1 | 1 | 3 | neural cell adhesion molecule 2 |
| NLGN1 | 22871 | i | 1 | 1 | 1 | 3 | neuroligin 1 |
| NLGN2 | 57555 | i | 1 | 1 | 1 | 3 | neuroligin 2 |
| NLGN3 | 54413 | i | 1 | 1 | 1 | 3 | neuroligin 3 |
| NLGN4X | 57502 | i | 1 | 1 | 1 | 3 | neuroligin 4, X-linked |
| NLGN4Y | 22829 | i | 1 | 1 | 1 | 3 | neuroligin 4, Y-linked |
| NRCAM | 4897 | i | 1 | 1 | 1 | 3 | neuronal cell adhesion molecule |
| NRXN1 | 9378 | i | 1 | 1 | 1 | 3 | neurexin 1 |
| NRXN2 | 9379 | i | 1 | 1 | 1 | 3 | neurexin 2 |
| NRXN3 | 9369 | i | 1 | 1 | 1 | 3 | neurexin 3 |
| PCDHA1 | 56147 | i | 1 | 1 | 1 | 3 | protocadherin alpha 1 |
| PCDHA10 | 56139 | i | 1 | 1 | 1 | 3 | protocadherin alpha 10 |
| PCDHA11 | 56138 | i | 1 | 1 | 1 | 3 | protocadherin alpha 11 |
| PCDHA2 | 56146 | i | 1 | 1 | 1 | 3 | protocadherin alpha 2 |
| PCDHA3 | 56145 | i | 1 | 1 | 1 | 3 | protocadherin alpha 3 |
| PCDHA4 | 56144 | i | 1 | 1 | 1 | 3 | protocadherin alpha 4 |
| PCDHA5 | 56143 | i | 1 | 1 | 1 | 3 | protocadherin alpha 5 |
| PCDHA6 | 56142 | i | 1 | 1 | 1 | 3 | protocadherin alpha 6 |
| PCDHA7 | 56141 | i | 1 | 1 | 1 | 3 | protocadherin alpha 7 |
| PCDHA8 | 56140 | i | 1 | 1 | 1 | 3 | protocadherin alpha 8 |
| PCDHAC1 | 56135 | i | 1 | 1 | 1 | 3 | protocadherin alpha subfamily C1 |
| PCDHAC2 | 56134 | i | 1 | 1 | 1 | 3 | protocadherin alpha subfamily C2 |
| PCDHB10 | 56126 | i | 1 | 1 | 1 | 3 | protocadherin beta 10 |
| PCDHB11 | 56125 | i | 1 | 1 | 1 | 3 | protocadherin beta 11 |
| PCDHB12 | 56124 | i | 1 | 1 | 1 | 3 | protocadherin beta 12 |
| PCDHB13 | 56123 | i | 1 | 1 | 1 | 3 | protocadherin beta 13 |
| PCDHB14 | 56122 | i | 1 | 1 | 1 | 3 | protocadherin beta 14 |
| PCDHB15 | 56121 | i | 1 | 1 | 1 | 3 | protocadherin beta 15 |
| PCDHB16 | 57717 | i | 1 | 1 | 1 | 3 | protocadherin beta 16 |
| PCDHB2 | 56133 | i | 1 | 1 | 1 | 3 | protocadherin beta 2 |
| PCDHB3 | 56132 | i | 1 | 1 | 1 | 3 | protocadherin beta 3 |
| PCDHB4 | 56131 | i | 1 | 1 | 1 | 3 | protocadherin beta 4 |
| PCDHB5 | 26167 | i | 1 | 1 | 1 | 3 | protocadherin beta 5 |
| PCDHB6 | 56130 | i | 1 | 1 | 1 | 3 | protocadherin beta 6 |
| PCDHB9 | 56127 | i | 1 | 1 | 1 | 3 | protocadherin beta 9 |
| PCDHGB4 | 8641 | i | 1 | 1 | 1 | 3 | protocadherin gamma subfamily B 4 |
| PCDHGC3 | 5098 | i | 1 | 1 | 1 | 3 | protocadherin gamma subfamily C 3 |
| PTPRC | 5788 | i | 1 | 1 | 1 | 3 | receptor type protein tyrosine phosphatase C |
| PTPRD | 5789 | i | 1 | 1 | 1 | 3 | receptor type protein tyrosine phosphatase D |
| PTPRJ | 5795 | i | 1 | 1 | 1 | 3 | receptor type protein tyrosine phosphatase J |
| PTPRK | 5796 | i | 1 | 1 | 1 | 3 | receptor type protein tyrosine phosphatase K |
| PTPRM | 5797 | i | 1 | 1 | 1 | 3 | receptor type protein tyrosine phosphatase M |
| PTPRT | 11122 | i | 1 | 1 | 1 | 3 | receptor type protein tyrosine phosphatase T |
| PTPRU | 10076 | i | 1 | 1 | 1 | 3 | receptor type protein tyrosine phosphatase U |
| FN1 | 2335 | m | 1 | 1 | 1 | 3 | fibronectin 1 |
| ITGA11 | 22801 | m | 1 | 1 | 1 | 3 | integrin alpha 11 |
| ITGA2 | 3673 | m | 1 | 1 | 1 | 3 | integrin alpha 2 |
| ITGA2B | 3674 | m | 1 | 1 | 1 | 3 | integrin alpha 2b |
| ITGA4 | 3676 | m | 1 | 1 | 1 | 3 | integrin alpha 4 |
| ITGA5 | 3678 | m | 1 | 1 | 1 | 3 | integrin alpha 5 |
| ITGA7 | 3679 | m | 1 | 1 | 1 | 3 | integrin alpha 7 |
| ITGA8 | 8516 | m | 1 | 1 | 1 | 3 | integrin alpha 8 |
| ITGA9 | 3680 | m | 1 | 1 | 1 | 3 | integrin alpha 9 |
| ITGAL | 3683 | m | 1 | 1 | 1 | 3 | integrin alpha L |
| ITGAM | 3684 | m | 1 | 1 | 1 | 3 | integrin alpha M |
| ITGAV | 3685 | m | 1 | 1 | 1 | 3 | integrin alpha V |
| ITGB1 | 3688 | m | 1 | 1 | 1 | 3 | integrin beta 1 |
| ITGB2 | 3689 | m | 1 | 1 | 1 | 3 | integrin beta 2 |
| ITGB3 | 3690 | m | 1 | 1 | 1 | 3 | integrin beta 3 |
| ITGB4 | 3691 | m | 1 | 1 | 1 | 3 | integrin beta 4 |
| ITGB6 | 3694 | m | 1 | 1 | 1 | 3 | integrin beta 6 |
| ITGB8 | 3696 | m | 1 | 1 | 1 | 3 | integrin beta 8 |
| TEK | 7010 | m | 1 | 1 | 1 | 3 | TEK tyrosine kinase, endothelial |
| THY1 | 7070 | m | 1 | 1 | 1 | 3 | Thy-1 cell surface antigen |
| TNR | 7143 | m | 1 | 1 | 1 | 3 | tenascin R |
| TNXB | 7148 | m | 1 | 1 | 1 | 3 | tenascin XB |
| ROBO1 | 6091 | ag | 1 | 1 | 1 | 3 | roundabout axon guidance receptor homolog 1 |
| ROBO2 | 6092 | ag | 1 | 1 | 1 | 3 | roundabout axon guidance receptor homolog 2 |
| SEMA4D | 10507 | ag | 1 | 1 | 1 | 3 | semaphorin 4D |
| PVRL2 | 5819 | aj | 1 | 1 | 1 | 3 | poliovirus receptor-related 2 |
| CDON | 50937 | c | 1 | 1 | 1 | 3 | cell adhesion associated oncogene regulated |
| VCAM1 | 7412 | c | 1 | 1 | 1 | 3 | vascular cell adhesion molecule 1 |
| DSC2 | 1824 | fa | 1 | 1 | 1 | 3 | desmocollin 2 |
| DSC3 | 1825 | fa | 1 | 1 | 1 | 3 | desmocollin 3 |
| DSG1 | 1828 | fa | 1 | 1 | 1 | 3 | desmoglein 1 |
| DSG2 | 1829 | fa | 1 | 1 | 1 | 3 | desmoglein 2 |
| AMICA1 | 120425 | i | 0 | 1 | 1 | 2 | adhesion molecule, interacts with CXADR antigen 1 |
| AMIGO1 | 57463 | i | 0 | 1 | 1 | 2 | adhesion molecule with Ig-like domain 1 |
| AMIGO2 | 347902 | i | 0 | 1 | 1 | 2 | adhesion molecule with Ig-like domain 2 |
| AMIGO3 | 386724 | i | 0 | 1 | 1 | 2 | adhesion molecule with Ig-like domain 3 |
| ASTN1 | 460 | i | 1 | 0 | 1 | 2 | astrotactin 1 |
| BCAM | 4059 | i | 0 | 1 | 1 | 2 | basal cell adhesion molecule |
| BOC | 91653 | i | 1 | 0 | 1 | 2 | BOC cell adhesion associated oncogene regulated |
| CADM1 | 23705 | i | 0 | 1 | 1 | 2 | cell adhesion molecule 1 |
| CADM3 | 57863 | i | 0 | 1 | 1 | 2 | cell adhesion molecule 3 |
| CD151 | 977 | i | 0 | 1 | 1 | 2 | CD151 molecule |
| CDH12 | 1010 | i | 1 | 0 | 1 | 2 | cadherin 12 |
| CDH18 | 1016 | i | 1 | 0 | 1 | 2 | cadherin 18 |
| CDH19 | 28513 | i | 1 | 0 | 1 | 2 | cadherin 19 |
| CDH20 | 28316 | i | 1 | 0 | 1 | 2 | cadherin 20 |
| CDH22 | 64405 | i | 1 | 0 | 1 | 2 | cadherin 22 |
| CDH26 | 60437 | i | 1 | 0 | 1 | 2 | cadherin 26 |
| CDH5 | 1003 | i | 1 | 0 | 1 | 2 | cadherin 5 |
| CDHR1 | 92211 | i | 1 | 0 | 1 | 2 | cadherin-related family member 1 |
| CDHR3 | 222256 | i | 1 | 0 | 1 | 2 | cadherin-related family member 3 |
| CDHR4 | 389118 | i | 1 | 0 | 1 | 2 | cadherin-related family member 4 |
| CELSR1 | 9620 | i | 1 | 0 | 1 | 2 | cadherin, EGF LAG seven-pass G-type receptor 1 |
| CELSR3 | 1951 | i | 1 | 0 | 1 | 2 | cadherin, EGF LAG seven-pass G-type receptor 3 |
| CERCAM | 51148 | i | 0 | 1 | 1 | 2 | cerebral endothelial cell adhesion molecule |
| CHL1 | 10752 | i | 1 | 0 | 1 | 2 | cell adhesion molecule L1-like |
| CLSTN2 | 64084 | i | 1 | 0 | 1 | 2 | calsyntenin 2 |
| CLSTN3 | 9746 | i | 1 | 0 | 1 | 2 | calsyntenin 3 |
| CNTN1 | 1272 | i | 1 | 0 | 1 | 2 | contactin 1 |
| CNTN2 | 6900 | i | 1 | 0 | 1 | 2 | contactin 2 |
| CNTN3 | 5067 | i | 1 | 0 | 1 | 2 | contactin 3 |
| CNTN5 | 53942 | i | 1 | 0 | 1 | 2 | contactin 5 |
| CNTN6 | 27255 | i | 1 | 0 | 1 | 2 | contactin 6 |
| CTNNA1 | 1495 | i | 0 | 1 | 1 | 2 | catenin alpha 1 |
| CTNNA2 | 1496 | i | 0 | 1 | 1 | 2 | catenin alpha 2 |
| CTNNA3 | 29119 | i | 0 | 1 | 1 | 2 | catenin alpha 3 |
| CTNNB1 | 1499 | i | 0 | 1 | 1 | 2 | catenin beta 1 |
| CTNND1 | 1500 | i | 0 | 1 | 1 | 2 | catenin delta 1 |
| CTNND2 | 1501 | i | 0 | 1 | 1 | 2 | catenin delta 2 |
| DCHS2 | 54798 | i | 1 | 0 | 1 | 2 | dachsous cadherin-related 2 |
| DDR1 | 780 | i | 0 | 1 | 1 | 2 | discoidin domain receptor tyrosine kinase 1 |
| EFNA1 | 1942 | i | 0 | 1 | 1 | 2 | ephrin A1 |
| EFNA5 | 1946 | i | 0 | 1 | 1 | 2 | ephrin A5 |
| EFNB1 | 1947 | i | 0 | 1 | 1 | 2 | ephrin B1 |
| EFNB2 | 1948 | i | 0 | 1 | 1 | 2 | ephrin B2 |
| EMR1 | 2015 | i | 0 | 1 | 1 | 2 | egf-like module containing mucin-like hormone receptor-like 1 |
| EMR2 | 30817 | i | 0 | 1 | 1 | 2 | egf-like module containing mucin-like hormone receptor-like 2 |
| EPHA1 | 2041 | i | 1 | 1 | 0 | 2 | eph tyrosine kinase 1 |
| EPHA4 | 2043 | i | 1 | 0 | 1 | 2 | EPH receptor A4 |
| EPHB1 | 2047 | i | 1 | 1 | 0 | 2 | eph tyrosine kinase 2 |
| FAT3 | 120114 | i | 1 | 0 | 1 | 2 | FAT atypical cadherin 3 |
| FEZ1 | 9638 | i | 0 | 1 | 1 | 2 | fasciculation and elongation protein zeta 1 |
| FLRT1 | 23769 | i | 1 | 0 | 1 | 2 | fibronectin leucine rich transmembrane protein 1 |
| FLRT2 | 23768 | i | 1 | 0 | 1 | 2 | fibronectin leucine rich transmembrane protein 2 |
| FLRT3 | 23767 | i | 1 | 0 | 1 | 2 | fibronectin leucine rich transmembrane protein 3 |
| GAS6 | 2621 | i | 0 | 1 | 1 | 2 | growth arrest-specific 6 |
| GPR56 | 9289 | i | 0 | 1 | 1 | 2 | G protein coupled receptor 56 |
| ICAM1 | 3383 | i | 0 | 1 | 1 | 2 | intercellular adhesion molecule 1 |
| IL1RAPL1 | 11141 | i | 0 | 1 | 1 | 2 | interleukin 1 receptor accessory protein-like 1 |
| KIRREL2 | 84063 | i | 0 | 1 | 1 | 2 | kin of IRRE like 2 |
| LPHN1 | 22859 | i | 0 | 1 | 1 | 2 | latrophilin 1 |
| LRFN3 | 79414 | i | 1 | 0 | 1 | 2 | leucine rich repeat and fibronectin type III domain containing 3 |
| MAEA | 10296 | i | 0 | 1 | 1 | 2 | macrophage erythroblast attacher |
| MCAM | 4162 | i | 1 | 0 | 1 | 2 | melanoma cell adhesion molecule |
| MEGF10 | 84466 | i | 0 | 1 | 1 | 2 | multiple EGF-like-domains 10 |
| MEGF11 | 84465 | i | 0 | 1 | 1 | 2 | multiple EGF-like-domains 11 |
| MIA3 | 375056 | i | 0 | 1 | 1 | 2 | melanoma inhibitory activity family, member 3 |
| NCAM1 | 4684 | i | 1 | 0 | 1 | 2 | neural cell adhesion molecule 1 |
| NEO1 | 4756 | i | 1 | 0 | 1 | 2 | neogenin 1 |
| NFASC | 23114 | i | 1 | 0 | 1 | 2 | neurofascin |
| NINJ2 | 4815 | i | 0 | 1 | 1 | 2 | ninjurin 2 |
| NPHS1 | 4868 | i | 1 | 0 | 1 | 2 | nephrin |
| NPTN | 27020 | i | 0 | 1 | 1 | 2 | neuroplastin |
| NRG1 | 3084 | i | 0 | 1 | 1 | 2 | neuregulin 1 |
| NRP2 | 8828 | i | 0 | 1 | 1 | 2 | neuropilin 2 |
| OPCML | 4978 | i | 1 | 0 | 1 | 2 | opioid binding protein |
| PCDH1 | 5097 | i | 1 | 0 | 1 | 2 | protocadherin 1 |
| PCDH10 | 57575 | i | 1 | 0 | 1 | 2 | protocadherin 10 |
| PCDH11X | 27328 | i | 1 | 0 | 1 | 2 | protocadherin 11 X-linked |
| PCDH11Y | 83259 | i | 1 | 0 | 1 | 2 | protocadherin 11 Y-linked |
| PCDH12 | 51294 | i | 1 | 0 | 1 | 2 | protocadherin 12 |
| PCDH15 | 65217 | i | 1 | 0 | 1 | 2 | protocadherin-related 15 |
| PCDH17 | 27253 | i | 1 | 0 | 1 | 2 | protocadherin 17 |
| PCDH18 | 54510 | i | 1 | 0 | 1 | 2 | protocadherin 18 |
| PCDH19 | 57526 | i | 1 | 0 | 1 | 2 | protocadherin 19 |
| PCDH20 | 64881 | i | 1 | 0 | 1 | 2 | protocadherin 20 |
| PCDH7 | 5099 | i | 1 | 0 | 1 | 2 | protocadherin 7 |
| PCDH8 | 5100 | i | 1 | 0 | 1 | 2 | protocadherin 8 |
| PCDH9 | 5101 | i | 1 | 0 | 1 | 2 | protocadherin 9 |
| PCDHA12 | 56137 | i | 1 | 0 | 1 | 2 | protocadherin alpha 12 |
| PCDHA13 | 56136 | i | 1 | 0 | 1 | 2 | protocadherin alpha 13 |
| PCDHA9 | 9752 | i | 1 | 0 | 1 | 2 | protocadherin alpha 9 |
| PCDHB1 | 29930 | i | 1 | 0 | 1 | 2 | protocadherin beta 1 |
| PCDHB18 | 54660 | i | 1 | 0 | 1 | 2 | protocadherin beta 18 |
| PCDHB7 | 56129 | i | 1 | 0 | 1 | 2 | protocadherin beta 7 |
| PCDHB8 | 56128 | i | 1 | 0 | 1 | 2 | protocadherin beta 8 |
| PCDHGA1 | 56114 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 1 |
| PCDHGA10 | 56106 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 10 |
| PCDHGA11 | 56105 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 11 |
| PCDHGA12 | 26025 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 12 |
| PCDHGA2 | 56113 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 2 |
| PCDHGA3 | 56112 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 3 |
| PCDHGA4 | 56111 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 4 |
| PCDHGA5 | 56110 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 5 |
| PCDHGA6 | 56109 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 6 |
| PCDHGA7 | 56108 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 7 |
| PCDHGA8 | 9708 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 8 |
| PCDHGA9 | 56107 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily A, 9 |
| PCDHGB1 | 56104 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily B, 1 |
| PCDHGB2 | 56103 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily B, 2 |
| PCDHGB3 | 56102 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily B, 3 |
| PCDHGB5 | 56101 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily B, 5 |
| PCDHGB6 | 56100 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily B, 6 |
| PCDHGB7 | 56099 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily B, 7 |
| PCDHGC4 | 56098 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily C, 4 |
| PCDHGC5 | 56097 | i | 1 | 0 | 1 | 2 | protocadherin gamma subfamily C, 5 |
| POSTN | 10631 | i | 0 | 1 | 1 | 2 | periostin |
| PTPRF | 5792 | i | 1 | 0 | 1 | 2 | receptor type protein tyrosine phosphatase F |
| PTPRO | 5800 | i | 1 | 1 | 0 | 2 | receptor-type tyrosine-protein phosphatase O |
| PTPRS | 5802 | i | 1 | 0 | 1 | 2 | receptor type protein tyrosine phosphatase S |
| SDK1 | 221935 | i | 1 | 0 | 1 | 2 | sidekick cell adhesion molecule 1 |
| SDK2 | 54549 | i | 1 | 0 | 1 | 2 | sidekick cell adhesion molecule 2 |
| SSPN | 8082 | i | 0 | 1 | 1 | 2 | sarcospan |
| CD36 | 948 | m | 0 | 1 | 1 | 2 | CD36 molecule |
| CD44 | 960 | m | 0 | 1 | 1 | 2 | CD44 molecule |
| FREM2 | 341640 | m | 1 | 0 | 1 | 2 | FRAS1 related extracellular matrix protein 2 |
| FREM3 | 166752 | m | 0 | 1 | 1 | 2 | FRAS1 related extracellular matrix 3 |
| ITGA10 | 8515 | m | 1 | 1 | 0 | 2 | integrin alpha-10 |
| ITGA3 | 3675 | m | 1 | 1 | 0 | 2 | integrin alpha-3 |
| ITGA6 | 3655 | m | 1 | 0 | 1 | 2 | integrin alpha 6 |
| ITGAD | 3681 | m | 1 | 0 | 1 | 2 | integrin alpha D |
| ITGAE | 3682 | m | 1 | 0 | 1 | 2 | integrin alpha E |
| ITGAX | 3687 | m | 1 | 0 | 1 | 2 | integrin alpha X |
| ITGB1BP1 | 9270 | m | 0 | 1 | 1 | 2 | integrin beta 1 binding protein 1 |
| ITGB3BP | 23421 | m | 0 | 1 | 1 | 2 | integrin beta 3 binding protein |
| ITGB5 | 3693 | m | 1 | 0 | 1 | 2 | integrin beta 5 |
| ITGB7 | 3695 | m | 1 | 0 | 1 | 2 | integrin beta 7 |
| ITGBL1 | 9358 | m | 0 | 1 | 1 | 2 | integrin beta-like 1 |
| KAL1 | 3730 | m | 1 | 0 | 1 | 2 | Kallmann syndrome 1 sequence |
| LAMA5 | 3911 | m | 0 | 1 | 1 | 2 | laminin alpha 5 |
| LAMB1 | 3912 | m | 0 | 1 | 1 | 2 | laminin beta 1 |
| LAMC1 | 3915 | m | 0 | 1 | 1 | 2 | laminin gamma 1 |
| NID2 | 22795 | m | 0 | 1 | 1 | 2 | nidogen 2 |
| OLFM4 | 10562 | m | 0 | 1 | 1 | 2 | olfactomedin 4 |
| PVR | 5817 | m | 1 | 0 | 1 | 2 | poliovirus receptor |
| REG3A | 5068 | m | 0 | 1 | 1 | 2 | regenerating islet-derived 3 alpha |
| TGFBI | 7045 | m | 0 | 1 | 1 | 2 | transforming growth factor, beta-induced, 68kDa |
| THBS1 | 7057 | m | 0 | 1 | 1 | 2 | thrombospondin 1 |
| THBS4 | 7060 | m | 0 | 1 | 1 | 2 | thrombospondin 4 |
| TINAG | 27283 | m | 0 | 1 | 1 | 2 | tubulointerstitial nephritis antigen |
| TNC | 3371 | m | 1 | 0 | 1 | 2 | tenascin C |
| WNT1 | 7471 | m | 0 | 1 | 1 | 2 | wingless-type MMTV integration site family, member 1 |
| PLXNC1 | 10154 | ag | 0 | 1 | 1 | 2 | plexin C1 |
| RGMB | 285704 | ag | 0 | 1 | 1 | 2 | repulsive guidance molecule family member b |
| SEMA3E | 9723 | ag | 1 | 1 | 0 | 2 | semaphorin 3E |
| SEMA5A | 9037 | ag | 0 | 1 | 1 | 2 | semaphorin 5A |
| DLG1 | 1739 | aj | 0 | 1 | 1 | 2 | discs large homolog 1 |
| DLG5 | 9231 | aj | 0 | 1 | 1 | 2 | discs large homolog 5 |
| PVRL3 | 25945 | aj | 0 | 1 | 1 | 2 | poliovirus receptor-related 3 |
| PVRL4 | 81607 | aj | 1 | 0 | 1 | 2 | poliovirus receptor-related 4 |
| BYSL | 705 | c | 0 | 1 | 1 | 2 | bystin-like |
| CD2 | 914 | c | 0 | 1 | 1 | 2 | CD2 molecule |
| CD24 | 1E+08 | c | 0 | 1 | 1 | 2 | CD24 molecule |
| CD33 | 945 | c | 1 | 0 | 1 | 2 | CD33 molecule |
| CD40LG | 959 | c | 0 | 1 | 1 | 2 | CD40 ligand |
| CD47 | 961 | c | 0 | 1 | 1 | 2 | CD47 molecule |
| CD58 | 965 | c | 0 | 1 | 1 | 2 | CD58 molecule |
| CD72 | 971 | c | 0 | 1 | 1 | 2 | CD72 molecule |
| CD84 | 8832 | c | 0 | 1 | 1 | 2 | CD84 molecule |
| CD9 | 928 | c | 0 | 1 | 1 | 2 | CD9 molecule |
| CD93 | 22918 | c | 0 | 1 | 1 | 2 | CD93 molecule |
| CD96 | 10225 | c | 1 | 0 | 1 | 2 | CD96 molecule |
| SELE | 6401 | c | 0 | 1 | 1 | 2 | selectin E |
| SELP | 6403 | c | 0 | 1 | 1 | 2 | selectin P |
| SELPLG | 6404 | c | 0 | 1 | 1 | 2 | selectin P ligand |
| TRO | 7216 | c | 0 | 1 | 1 | 2 | trophinin |
| DSC1 | 1823 | fa | 1 | 0 | 1 | 2 | desmocollin 1 |
| DSG3 | 1830 | fa | 1 | 0 | 1 | 2 | desmoglein 3 |
| DSG4 | 147409 | fa | 1 | 0 | 1 | 2 | desmoglein 4 |
| LPXN | 9404 | fa | 0 | 1 | 1 | 2 | leupaxin |
| PKP1 | 5317 | fa | 0 | 1 | 1 | 2 | plakophilin 1 |
| PKP2 | 5318 | fa | 0 | 1 | 1 | 2 | plakophilin 2 |
| PKP4 | 8502 | fa | 0 | 1 | 1 | 2 | plakophilin 4 |
| BVES | 11149 | tj | 0 | 1 | 1 | 2 | blood vessel epicardial substance |
| CLDN1 | 9076 | tj | 0 | 1 | 1 | 2 | claudin 1 |
| CLDN10 | 9071 | tj | 0 | 1 | 1 | 2 | claudin 10 |
| CLDN11 | 5010 | tj | 0 | 1 | 1 | 2 | claudin 11 |
| CLDN12 | 9069 | tj | 0 | 1 | 1 | 2 | claudin 12 |
| CLDN14 | 23562 | tj | 0 | 1 | 1 | 2 | claudin 14 |
| CLDN15 | 24146 | tj | 0 | 1 | 1 | 2 | claudin 15 |
| CLDN16 | 10686 | tj | 0 | 1 | 1 | 2 | claudin 16 |
| CLDN17 | 26285 | tj | 0 | 1 | 1 | 2 | claudin 17 |
| CLDN18 | 51208 | tj | 0 | 1 | 1 | 2 | claudin 18 |
| CLDN19 | 149461 | tj | 0 | 1 | 1 | 2 | claudin 19 |
| CLDN2 | 9075 | tj | 0 | 1 | 1 | 2 | claudin 2 |
| CLDN20 | 49861 | tj | 0 | 1 | 1 | 2 | claudin 20 |
| CLDN22 | 53842 | tj | 0 | 1 | 1 | 2 | claudin 22 |
| CLDN23 | 137075 | tj | 0 | 1 | 1 | 2 | claudin 23 |
| CLDN4 | 1364 | tj | 0 | 1 | 1 | 2 | claudin 4 |
| CLDN5 | 7122 | tj | 0 | 1 | 1 | 2 | claudin 5 |
| CLDN6 | 9074 | tj | 0 | 1 | 1 | 2 | claudin 6 |
| CLDN7 | 1366 | tj | 0 | 1 | 1 | 2 | claudin 7 |
| CLDN8 | 9073 | tj | 0 | 1 | 1 | 2 | claudin 8 |
| CLDN9 | 9080 | tj | 0 | 1 | 1 | 2 | claudin 9 |
| CYTH1 | 9267 | tj | 0 | 1 | 1 | 2 | cytohesin 1 |
| CYTH2 | 9266 | tj | 0 | 1 | 1 | 2 | cytohesin 2 |
| CYTH3 | 9265 | tj | 0 | 1 | 1 | 2 | cytohesin 3 |
| CYTH4 | 27128 | tj | 0 | 1 | 1 | 2 | cytohesin 4 |
| CYTIP | 9595 | tj | 0 | 1 | 1 | 2 | cytohesin 1 interacting protein |
| JAM2 | 58494 | tj | 0 | 1 | 1 | 2 | junctional adhesion molecule 2 |
| JUP | 3728 | tj | 0 | 1 | 1 | 2 | junction plakoglobin |
| PVRL1 | 5818 | tj | 0 | 1 | 1 | 2 | poliovirus receptor-related 1 |
| ALCAM | 214 | i | 0 | 0 | 1 | 1 | activated leukocyte cell adhesion molecule |
| ASTN2 | 23245 | i | 1 | 0 | 0 | 1 | astrotactin 2 |
| BAI1 | 575 | i | 0 | 0 | 1 | 1 | brain-specific angiogenesis inhibitor 1 |
| CADM2 | 253559 | i | 0 | 0 | 1 | 1 | cell adhesion molecule 2 |
| CADM4 | 199731 | i | 0 | 0 | 1 | 1 | cell adhesion molecule 4 |
| CD200 | 4345 | i | 1 | 0 | 0 | 1 | CD200 antigen |
| CD48 | 962 | i | 1 | 0 | 0 | 1 | CD48 antigen |
| CD8A | 925 | i | 1 | 0 | 0 | 1 | CD8 antigen alpha polypeptide |
| CNTNAP1 | 8506 | i | 0 | 0 | 1 | 1 | contactin associated protein 1 |
| CNTNAP2 | 26047 | i | 0 | 0 | 1 | 1 | contactin associated protein-like 2 |
| CNTNAP3 | 79937 | i | 0 | 0 | 1 | 1 | contactin associated protein-like 3 |
| CNTNAP3B | 728577 | i | 0 | 0 | 1 | 1 | contactin associated protein-like 3B |
| CNTNAP4 | 85445 | i | 0 | 0 | 1 | 1 | contactin associated protein-like 4 |
| CNTNAP5 | 129684 | i | 0 | 0 | 1 | 1 | contactin associated protein-like 5 |
| CTNNAL1 | 8727 | i | 0 | 0 | 1 | 1 | catenin alpha-like 1 |
| DAB1 | 1600 | i | 0 | 0 | 1 | 1 | disabled homolog 1 |
| DCC | 1630 | i | 1 | 0 | 0 | 1 | deleted in colorectal cancer protein |
| EDIL3 | 10085 | i | 0 | 0 | 1 | 1 | EGF-like repeats and discoidin I-like domains 3 |
| ELFN1 | 392617 | i | 1 | 0 | 0 | 1 | extracellular leucine-rich repeat and fibronectin type III containing 1 |
| EPCAM | 4072 | i | 0 | 1 | 0 | 1 | epithelial glycoprotein-2 |
| EPHA10 | 284656 | i | 1 | 0 | 0 | 1 | ephrin type-A receptor 10 |
| EPHA5 | 2044 | i | 1 | 0 | 0 | 1 | ephrin type-A receptor 5 |
| EPHA6 | 285220 | i | 1 | 0 | 0 | 1 | ephrin receptor EphA6 |
| EPHB2 | 2048 | i | 1 | 0 | 0 | 1 | ephrin type-B receptor 2 |
| EPHB6 | 2051 | i | 1 | 0 | 0 | 1 | ephrin type-B receptor 6 |
| GPR116 | 221395 | i | 1 | 0 | 0 | 1 | G-protein coupled receptor 116 |
| HEPACAM | 220296 | i | 0 | 0 | 1 | 1 | hepatic and glial cell adhesion molecule |
| ICAM2 | 3384 | i | 0 | 0 | 1 | 1 | intercellular adhesion molecule 2 |
| ICAM3 | 3385 | i | 0 | 0 | 1 | 1 | intercellular adhesion molecule 3 |
| ICAM4 | 3386 | i | 0 | 0 | 1 | 1 | intercellular adhesion molecule 4 |
| ICAM5 | 7087 | i | 0 | 0 | 1 | 1 | intercellular adhesion molecule 5 |
| ICOS | 29851 | i | 0 | 0 | 1 | 1 | inducible T-cell co-stimulator |
| IGSF11 | 152404 | i | 0 | 0 | 1 | 1 | immunoglobulin superfamily, member 11 |
| IGSF5 | 150084 | i | 0 | 0 | 1 | 1 | immunoglobulin superfamily, member 5 |
| IGSF9 | 57549 | i | 1 | 0 | 0 | 1 | immunoglobulin superfamily member 9A |
| IGSF9B | 22997 | i | 1 | 0 | 0 | 1 | immunoglobulin superfamily member 9B |
| IL1RAP | 3556 | i | 1 | 0 | 0 | 1 | IL-1 receptor accessory protein |
| KIR2DL1 | 3802 | i | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor 2DL1 |
| KIR2DL3 | 3804 | i | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor 2DL3 |
| KIR2DL4 | 3805 | i | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor 2DL4 |
| KIR2DL5A | 57292 | i | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor 2DL5A |
| KIR2DL5B | 553128 | i | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor 2DL5B |
| KIR2DS1 | 3806 | i | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor 2DS1 |
| KIR2DS2 | 1E+08 | i | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor 2DS2 |
| KIR2DS3 | 3808 | i | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor 2DS3 |
| KIR2DS4 | 3809 | i | 1 | 0 | 0 | 1 | killer Ig receptor|killer cell immunoglobulin-like receptor 2DS4 |
| KIR2DS5 | 3810 | i | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor 2DS5 |
| KIR3DL1 | 3811 | i | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor 3DL1 |
| LRFN1 | 57622 | i | 1 | 0 | 0 | 1 | leucine-rich repeat and fibronectin type III domain-containing protein 1 |
| LRFN2 | 57497 | i | 1 | 0 | 0 | 1 | leucine-rich repeat and fibronectin type-III domain-containing protein 2 |
| LRFN4 | 78999 | i | 1 | 0 | 0 | 1 | leucine-rich repeat and fibronectin type-III domain-containing protein 4 |
| LRFN5 | 145581 | i | 1 | 0 | 0 | 1 | leucine-rich repeat and fibronectin type-III domain-containing protein 5 |
| LRRN1 | 57633 | i | 1 | 0 | 0 | 1 | leucine-rich repeat neuronal protein 1 |
| LRRN3 | 54674 | i | 1 | 0 | 0 | 1 | leucine-rich repeat neuronal protein 3 |
| LRRN4 | 164312 | i | 1 | 0 | 0 | 1 | leucine-rich repeat neuronal protein 4 |
| LRRN4CL | 221091 | i | 1 | 0 | 0 | 1 | LRRN4 C-terminal-like protein |
| LSAMP | 4045 | i | 0 | 0 | 1 | 1 | limbic system-associated membrane protein |
| MADCAM1 | 8174 | i | 0 | 0 | 1 | 1 | mucosal vascular addressin cell adhesion molecule 1 |
| MLLT4 | 4301 | i | 0 | 0 | 1 | 1 | myeloid lymphoid or mixed-lineage leukemia translocated to 4 |
| MPL | 4352 | i | 1 | 0 | 0 | 1 | thrombopoietin receptor |
| NINJ1 | 4814 | i | 0 | 0 | 1 | 1 | ninjurin 1 |
| NOTCH1 | 4851 | i | 0 | 1 | 0 | 1 | Notch homolog 1 |
| NRP1 | 8829 | i | 0 | 0 | 1 | 1 | neuropilin 1 |
| NTM | 50863 | i | 0 | 0 | 1 | 1 | neurotrimin |
| PODXL2 | 50512 | i | 0 | 1 | 0 | 1 | podocalyxin-like protein 2 |
| PRPH2 | 5961 | i | 0 | 0 | 1 | 1 | peripherin 2 |
| PRTG | 283659 | i | 1 | 0 | 0 | 1 | protogenin homolog |
| PTPRB | 5787 | i | 1 | 0 | 0 | 1 | receptor-type tyrosine-protein phosphatase beta |
| PTPRG | 5793 | i | 1 | 0 | 0 | 1 | receptor type protein tyrosine phosphatase gamma |
| PTPRH | 5794 | i | 1 | 0 | 0 | 1 | receptor-type tyrosine-protein phosphatase H |
| PTPRQ | 374462 | i | 1 | 0 | 0 | 1 | receptor type protein-tyrosine phosphatase Q |
| PTPRZ1 | 5803 | i | 1 | 0 | 0 | 1 | receptor type protein tyrosine phosphatase zeta 1 |
| SDC1 | 6382 | i | 1 | 0 | 0 | 1 | syndecan 1 |
| SDC2 | 6383 | i | 1 | 0 | 0 | 1 | syndecan 2 |
| SDC3 | 9672 | i | 1 | 0 | 0 | 1 | syndecan 3 |
| SDC4 | 6385 | i | 1 | 0 | 0 | 1 | syndecan 4 |
| ABI3BP | 25890 | m | 1 | 0 | 0 | 1 | ABI gene family member 3-binding protein |
| AGER | 177 | m | 1 | 0 | 0 | 1 | RAGE isoform NtRAGE-delta |
| ANTXR1 | 84168 | m | 0 | 1 | 0 | 1 | tumor endothelial marker 8 |
| AXL | 558 | m | 1 | 0 | 0 | 1 | tyrosine-protein kinase receptor UFO |
| FBLN5 | 10516 | m | 0 | 1 | 0 | 1 | fibulin 5 |
| FBLN7 | 129804 | m | 0 | 0 | 1 | 1 | fibulin 7 |
| FREM1 | 158326 | m | 1 | 0 | 0 | 1 | FRAS1-related extracellular matrix protein 1 |
| HSPG2 | 3339 | m | 1 | 0 | 0 | 1 | basement membrane-specific heparan sulfate proteoglycan core protein |
| IBSP | 3381 | m | 0 | 0 | 1 | 1 | integrin-binding sialoprotein |
| ITGA1 | 3672 | m | 1 | 0 | 0 | 1 | integrin alpha 1 |
| LAMA1 | 284217 | m | 0 | 0 | 1 | 1 | laminin alpha 1 |
| LAMA2 | 3908 | m | 0 | 0 | 1 | 1 | laminin alpha 2 |
| LAMA3 | 3909 | m | 0 | 0 | 1 | 1 | laminin alpha 3 |
| LAMA4 | 3910 | m | 0 | 0 | 1 | 1 | laminin alpha 4 |
| LAMB2 | 3913 | m | 0 | 0 | 1 | 1 | laminin beta 2 |
| LAMB3 | 3914 | m | 0 | 0 | 1 | 1 | laminin beta 3 |
| LAMB4 | 22798 | m | 0 | 0 | 1 | 1 | laminin beta 4 |
| LAMC2 | 3918 | m | 0 | 0 | 1 | 1 | laminin gamma 2 |
| LAMC3 | 10319 | m | 0 | 0 | 1 | 1 | laminin gamma 3 |
| LYVE1 | 10894 | m | 0 | 1 | 0 | 1 | lymphatic vessel endothelial hyaluronic acid receptor 1 |
| MFAP4 | 4239 | m | 0 | 0 | 1 | 1 | microfibrillar-associated protein 4 |
| NCAN | 1463 | m | 0 | 0 | 1 | 1 | neurocan |
| RELN | 5649 | m | 0 | 0 | 1 | 1 | reelin |
| RPSA | 3921 | m | 0 | 0 | 1 | 1 | ribosomal protein SA |
| SGCE | 8910 | m | 0 | 1 | 0 | 1 | epsilon-sarcoglycan |
| THBS2 | 7058 | m | 0 | 0 | 1 | 1 | thrombospondin 2 |
| THBS3 | 7059 | m | 0 | 1 | 0 | 1 | thrombospondin-3 |
| TMEM8B | 51754 | m | 0 | 1 | 0 | 1 | nasopharyngeal carcinoma expressed 6 |
| TNN | 63923 | m | 1 | 0 | 0 | 1 | tenascin-N |
| UMODL1 | 89766 | m | 1 | 0 | 0 | 1 | olfactorin |
| USH2A | 7399 | m | 1 | 0 | 0 | 1 | usher syndrome type-2A protein |
| VCAN | 1462 | m | 0 | 0 | 1 | 1 | versican |
| WNT3A | 89780 | m | 0 | 0 | 1 | 1 | wingless-type MMTV integration site family member 3A |
| KIi0319L | 79932 | ag | 1 | 0 | 0 | 1 | dyslexia-associated protein KIi0319-like protein |
| MDGA1 | 266727 | ag | 1 | 0 | 0 | 1 | MAM domain-containing glycosylphosphatidylinositol anchor protein 1 |
| MDGA2 | 161357 | ag | 1 | 0 | 0 | 1 | MAM domain-containing glycosylphosphatidylinositol anchor protein 2 |
| NTN1 | 9423 | ag | 0 | 0 | 1 | 1 | netrin 1 |
| PLXNB1 | 5364 | ag | 0 | 1 | 0 | 1 | plexin B1 |
| PLXNB3 | 5365 | ag | 0 | 1 | 0 | 1 | plexin B3 |
| ROBO3 | 64221 | ag | 1 | 0 | 0 | 1 | roundabout homolog 3 |
| ROBO4 | 54538 | ag | 1 | 0 | 0 | 1 | roundabout homolog 4 |
| SEMA3G | 56920 | ag | 1 | 0 | 0 | 1 | semaphorin 3G |
| SLIT2 | 9353 | ag | 0 | 0 | 1 | 1 | slit homolog 2 |
| VEZT | 55591 | aj | 0 | 0 | 1 | 1 | vezatin |
| CD226 | 10666 | c | 0 | 0 | 1 | 1 | CD226 molecule |
| CD6 | 923 | c | 0 | 0 | 1 | 1 | CD6 molecule |
| KIR3DL3 | 1E+08 | c | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor three domains long cytoplasmic tail 3 |
| KIR3DS1 | 3813 | c | 1 | 0 | 0 | 1 | killer cell immunoglobulin-like receptor 3DS1 |
| PECAM1 | 5175 | c | 0 | 0 | 1 | 1 | platelet endothelial cell adhesion molecule 1 |
| SELL | 6402 | c | 0 | 0 | 1 | 1 | selectin L |
| SPAM1 | 6677 | c | 0 | 0 | 1 | 1 | sperm adhesion molecule 1 |
| FERMT2 | 10979 | fa | 0 | 1 | 0 | 1 | fermitin family homolog 2 |
| LIMS2 | 55679 | fa | 0 | 0 | 1 | 1 | LIM and senescent cell antigen-like domains 2 |
| LPP | 4026 | fa | 0 | 0 | 1 | 1 | LIM domain containing preferred translocation partner in lipoma |
| NEDD9 | 4739 | fa | 0 | 0 | 1 | 1 | neural precursor cell expressed, developmentally down-regulated 9 |
| PEAK1 | 79834 | fa | 0 | 1 | 0 | 1 | NKF3 kinase family member |
| PKP3 | 11187 | fa | 0 | 0 | 1 | 1 | plakophilin 3 |
| TSC1 | 7248 | fa | 0 | 1 | 0 | 1 | tuberous sclerosis 1 protein |
| ZYX | 7791 | fa | 0 | 0 | 1 | 1 | zyxin |
| MAG | 4099 | my | 0 | 0 | 1 | 1 | myelin associated glycoprotein |
| OMG | 4974 | my | 0 | 0 | 1 | 1 | oligodendrocyte myelin glycoprotein |
| AJAP1 | 55966 | tj | 0 | 0 | 1 | 1 | adherens junctions associated protein 1 |
| AJUBA | 84962 | tj | 0 | 0 | 1 | 1 | ajuba LIM protein |
| CLDN3 | 1365 | tj | 0 | 0 | 1 | 1 | claudin 3 |
| DSP | 1832 | tj | 0 | 0 | 1 | 1 | desmoplakin |
| DST | 667 | tj | 0 | 0 | 1 | 1 | dystonin |
| ESAM | 90952 | tj | 0 | 0 | 1 | 1 | endothelial cell adhesion molecule |
| F11R | 50848 | tj | 0 | 0 | 1 | 1 | F11 receptor |
| JAM3 | 83700 | tj | 0 | 0 | 1 | 1 | junctional adhesion molecule 3 |
Columns: Gene symbol, ENTREZ gene ID number, annotated CAM type (see text), sources that identify this CAM and sum of the number of sources, and gene name. Genes are sorted by number of sources, CAM type, then alphabetically by gene. CAM types: i: information predominant CAM, m: primarily involved in interactions with cell matrix, ag: primary roles in axonal guidance, aj: primary role in adherens junctions, c: primary roles in cell/cell interactions, principally in immune system, fa: primary involvement in focal adhesions, tj: primary involvement in tight junctions. Please note likely involvement of many of the products of these CAM genes in multiple functions (esp cadherins).
Figure 2. Functional classes of genes encoding likely CAMs.
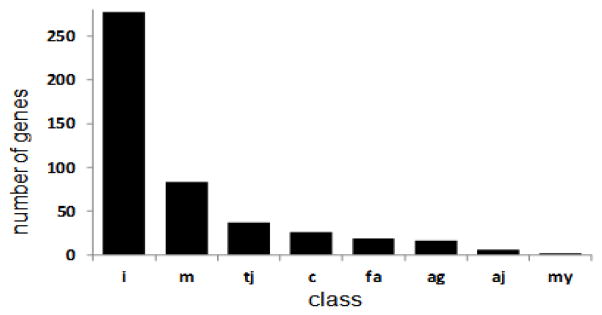
Distribution of likely CAM genes into classes annotated here. CAM types: i: information predominant CAM, m: primarily involved in interactions with cell matrix, tj: primary involvement in tight junctions c: primary roles in cell/cell interactions, principally in immune system, fa: primary involvement in focal adhesions, ag: primary roles in axonal guidance, aj: primary role in adherens junctions, my: primarily involved in myelin interactions. Please note likely involvement of many of the products of these CAM genes in multiple functions (esp cadherins).
Table II.
Likely CAMs for which single nucleotide polymorphisms are likely to provide partial or complete human “knockout” (79).
| gene | chr:bp | Ref | Alt | Af | frequency | ||
|---|---|---|---|---|---|---|---|
| EuAm | As | Eu | |||||
| CDH19 | 18:64235709 | C | A | 0 | 0.28 | 0 | 0 |
| CDHR2 | 5:175998270 | C | T | 0 | 0 | 0.2 | 0 |
| CEACAM1 | 19:43031354 | G | A | 0 | 0.28 | 0 | 0 |
| CLDN20 | 6:155597004 | G | A | 0 | 0 | 0.2 | 0.8 |
| CNTN6 | 3:1415319 | G | T | 0 | 0 | 0 | 0.1 |
| DSG4 | 18:28993525 | C | T | 0.4 | 0 | 0 | 0 |
| 18:28979436 | T | G | 0 | 0 | 0.2 | 0 | |
| 18:28993216 | C | T | 0 | 0 | 0 | 0.1 | |
| DSP | 6:7584223 | T | A | 0 | 0.28 | 0 | 0 |
| 6:7583371 | G | A | 0 | 0 | 0.2 | 0 | |
| EPHB1 | 3:134885826 | C | T | 0 | 0.28 | 0 | 0 |
| FAT1 | 4:187518227 | C | A | 0.2 | 0 | 0 | 0 |
| 4:187630007 | C | T | 0.2 | 0 | 0 | 0 | |
| FAT2 | 5:150923303 | G | A | 0 | 0.28 | 0 | 0 |
| ICAM3 | 19:10446525 | C | T | 0 | 0.28 | 0 | 0 |
| 19:10445374 | G | A | 0 | 0 | 0.2 | 0 | |
| ITGA10 | 1:145542272 | C | T | 0 | 0.28 | 0 | 0.1 |
| 1:145539790 | C | T | 0 | 0 | 0.2 | 0 | |
| 1:145533485 | C | T | 0 | 0 | 0 | 0.1 | |
| ITGA11 | 15:68641223 | C | A | 0 | 0 | 0 | 0.1 |
| ITGA7 | 12:56092316 | G | A | 0 | 0 | 0.2 | 0 |
| ITGAD | 16:31425869 | C | T | 0 | 0 | 0 | 0.1 |
| ITGB6 | 2:160994697 | C | A | 0 | 0.28 | 0 | 0 |
| 2:160993947 | C | A | 0.2 | 0 | 0 | 0 | |
| KIR2DL1 | 19:55286866 | C | G | 0 | 0 | 0 | 0.5 |
| 19:55331238 | C | T | 0 | 0 | 0.2 | 0 | |
| LAMA2 | 6:129475705 | A | T | 0 | 0 | 0.5 | 0 |
| 6:129636987 | C | T | 0 | 0 | 0 | 0.1 | |
| LAMC3 | 9:133954628 | C | T | 0.2 | 0 | 0 | 0 |
| LRRN3 | 7:110764535 | C | T | 0 | 0 | 0.2 | 0 |
| OLFM4 | 13:53617308 | C | T | 0 | 0 | 0 | 2.6 |
| 13:53624743 | T | A | 0 | 0 | 0.2 | 0 | |
| PCDH18 | 4:138449706 | G | A | 0 | 0.28 | 0 | 0 |
| PCDH7 | 4:30725762 | A | T | 0 | 0 | 0 | 0.1 |
| PCDHB1 | 5:140432438 | C | T | 0 | 0 | 0.2 | 0 |
| PCDHB4 | 5:140503823 | C | G | 0 | 0 | 0 | 0.3 |
| PCDHB7 | 5:140554309 | G | T | 0 | 1.66 | 0.2 | 0.3 |
| 5:140553499 | G | T | 0.4 | 0 | 0 | 0 | |
| PCDHB8 | 5:140558148 | T | G | 0.2 | 0 | 0 | 0 |
| 5:140558623 | C | T | 0 | 0 | 0.2 | 0 | |
| 5:140559627 | C | A | 0 | 0 | 0.2 | 0 | |
| PCDHGA10 | 5:140794929 | C | T | 0.2 | 1.1 | 0 | 0.4 |
| PCDHGA8 | 5:140773284 | C | G | 0 | 0 | 0 | 0.1 |
| PCDHGA9 | 5:140783659 | C | T | 0 | 0 | 0.5 | 0 |
| PCDHGC5 | 5:140869257 | C | T | 0.2 | 0 | 0 | 0 |
| 5:140870361 | C | T | 0 | 0 | 0 | 0.1 | |
| 5:140870415 | C | T | 0 | 0 | 0 | 0.1 | |
| PTPRH | 19:55697711 | G | A | 0 | 0.28 | 0 | 0.5 |
| PTPRU | 1:29650188 | G | A | 0.2 | 0 | 0 | 0 |
| ROBO2 | 3:77611841 | C | A | 0.2 | 0 | 0 | 0 |
| SDK1 | 7:4213906 | C | A | 0 | 0 | 0.2 | 0 |
| SELL | 1:169676573 | C | T | 0.2 | 0 | 0 | 0 |
| SEMA5A | 5:9197325 | G | T | 0 | 0 | 0.2 | 0 |
| SPAM1 | 7:123599667 | G | A | 0 | 0 | 0 | 0.1 |
| THBS4 | 5:79372855 | C | T | 0.2 | 0 | 0 | 0 |
| 5:79366570 | C | T | 0 | 0 | 0 | 0.1 | |
| TNN | 1:175052986 | C | T | 0 | 0 | 0.2 | 0 |
Columns list: gene symbol, chromosome and basepair of variant, major/reference allele, minor/mutant allele, and minor allele frequencies in 1000 genomes data from African, US individuals of European ancestry, Asian and European samples. Note that many CAM genes are also sites for copy number and other variation that can also provide knockouts.
Comparison: human addiction phenotype association GWAS dataset
Data from 500000 – 1M single nucleotide polymorphism (SNP) genome wide association studies for addiction-related phenotypes allowed ranking of genes based on the consistency of their identification by modest genome wide association (GWAS) signals (17). These GWAS signals were provided by clusters of at least 4 SNPs that lay within 10kb of each other and displayed 10−2 > p > 10−8 nominal significance for assessments of case vs control allele frequency differences. Nine hundred seventy nine genes contained clusters of SNPs that displayed such nominally-significant case control differences in at least three independent samples were identified. In classical genetic (eg twin) studies, the addiction-related phenotypes examined in these studies display substantial evidence for genetic overlap (17). The fraction of the genome (within genes) identified in this way in each independent study provides a basis for identifying the extent to which multiple independent samples would identify the gene by chance. GWAS samples available for ranking these CAM genes include data from eight samples for dependence described in (21,33–37) and five samples studying individual differences in ability to quit smoking (which displays strongly overlapping genetic influences in twin data) in (35,38–42).
RESULTS
1138 candidate CAM genes were identified by one or more of the approaches used here (Fig 1). The Entrez Gene query “cell adhesion molecule AND Homo sapiens” identified 819 gene records. Interpro searches for genes that encoded common CAM protein motifs from the cadherin, immunoglobulin, fibronectin, integrin, neurexin, neuroligin, cub/sushi, and catenin families identified 1716 human proteins, which mapped to 418 human genes. The Gene Ontology term “cell adhesion” (GO:0007155) identified 595 gene records. For comparison, our previously-described OKCAM database (2) identified 424 gene records. There were thus 1138 candidate human CAM genes available for annotation.
Annotation of the records of each of these 1138 candidate CAM genes and relevant literature revealed 474 of these genes that were judged likely to encode bona fide cell adhesion molecules (Table I). Three hundred forty four were judged to be unlikely to encode cell adhesion molecules, and 320 were questionable (http://rhesusbase.org/OKCAM/; Fig 1). On average, the genes judged to be “likely” cell adhesion molecules were identified by almost 2 of the four current annotation methods, while other genes were supported by less evidence. Genes classed as “unlikely” to encode cell adhesion molecules often encoded enzymes or transcription factors whose annotations appeared to arise due to their interactions with cell adhesion molecules. Many “questionable” genes were identified only by electronic annotations that provided insufficient data to provide even moderate confidence in bona fide roles in cell adhesion processes or in the absence of such roles. Products of other “questionable” genes displayed ambiguous functions. Such ambiguity is prominent for the large family of collagen genes, whose products contribute to the extracellular matrix which, usually when studded with more specific cell adhesion molecules, can play roles in cell/matrix interactions.
We class 283 of the members of the set of 474 “likely” CAM genes as iCAMs. This subset provided the largest subgroup of the likely cell adhesion molecules. iCAMs were judged to be more involved in providing information about the cell’s environment than in participating in a stereotyped contact or axonal guidance. These iCAMs contain protein motifs from a number of classes. We placed most cadherins in this class after some internal debate, though we acknowledge that several cadherins also participate in stereotypical cell adhesions (see below).
The remaining 191 genes are distributed into several groups. Eighty six genes’ products are likely to be involved with interactions between cells and the adjacent extracellular matrix; many of these are secreted and likely to be available in the extracellular space. Thirty six genes are involved in tight junctions. Products of 22 genes are identified primarily with cell-cell recognition for eg immune cells, though they are likely to play other roles as well. Eighteen genes, largely expressed on cell surfaces, are involved with focal adhesions. Products of 16 genes are so identified with axonal guidance that they are categorized in this way. The products of “axonal guidance” genes are likely to play other roles. Similarly, products of other cell adhesion molecule genes not annotated in this way are also likely to play roles in axonal guidance. Six are involved selectively in adherens junctions.
We can seek patterns whereby the likely cell adhesion molecule genes are identified in genome wide association data for specific brain disorders. These patterns are likely to provide insights into the chemical coding of connectivities by cell types involved in the circuitries that underlie these disorders. We provide an example for dopaminergic neuronal expression from semiquantitative Allen Brain Atlas data for mice. These data are interpreted in the context of our working hypothesis: for specific types of neurons, the patterns of cell adhesion molecule expression provide principal determinants of the ways in which a neuron’s processes contact other cells and contact itself. These patterns of cell adhesion molecule expression also regulate ways in which a neuron’s processes are contacted by processes of other neurons. These genes’ products thus provide the basic building blocks, or “bar code” (GRU and JD, in preparation) for the specificity of the brain connectome. These genes’ variants provide basic underpinnings for the circuitry differences that contribute to brain disorders. In disorders in which brains do not display striking gross neuropathological abnormalities, we anticipate that the variants in these genes contribute prominently to individual differences in vulnerability.
Several specifics help to make these points:
Example: representation of CAMs of different classes among genes identified by modest GWAS signals in multiple addiction phenotype case vs control series
Substantial genetic contributions to both dependence and ability to quit smoking have strong support from classical genetic approaches that include twin studies (17). However, genome wide association studies for dependence on illegal or legal addictive substances provide few consistent signals that reach p < 10−8 Bonferroni-corrected levels of statistical significance (21). Similar conclusions come from studies of individual differences in the abilities to quit smoking, another addiction-related phenotype (39).
In one approach to the conundrum that this GWAS data raises, phenotypes that include the number of cigarettes smoked/day have been studied, identifying acetylcholine receptor and nicotine metabolizing gene variants that reach or approach these high levels of statistical significance in the large samples that are available for this relatively simple phenotype (43–45).
We have also studied genes that are identified in multiple independent addiction case vs control samples by clusters of nearby SNPs that display 10−2 > p > 10−8 levels of statistical significance (5,7,17). We have assembled the lists of genes identified by at least 3–4 such SNPs that lie within 10kb of each other in eight studies of dependence and five studies of ability to quit smoking for which we have complete data (Uhl et al, unpublished observations and (17)). Nine hundred seventy nine genes are identified by at least three independent samples in this way. One hundred forty six of these genes are identified in at least 6 of these studies and 16 genes are identified in at least 9 of these independent studies.
One of the initially-unanticipated results of these GWAS datasets has been the overrepresentation of cell adhesion molecules, as we have reported using earlier compilations of the lists of these genes (17). We now find 83 genes on each of two lists: 1) the “likely” cell adhesion molecules that we annotate here and 2) lists of genes identified by at least three GWAS case-control comparisons for addiction phenotypes, dependence or ability to quit smoking (Table III).
Table III.
Candidate addiction-related CAMs. Genes listed both a) encode “likely” bona fide human cell adhesion molecules and b) are identified by at least 3 independent case vs control GWAS studies of addiction-related phenotypes by clusters of SNPs with 10−2 > p >10−8. Genes are arranged by the number of addiction-related case-control sample pairs in which they are identified, then alphabetical order.
| gene | type | #samp | structure | ec motifs |
|---|---|---|---|---|
| CDH13 | iCAM | 13 | GPI | Cdh |
| CSMD1 | iCAM | 12 | 1TM | Cub/sushi |
| PTPRD | iCAM | 12 | 1TM | Ig/Fn |
| CLSTN2 | iCAM | 10 | 1TM | Cdh/laminin |
| DAB1 | iCAM | 10 | cyt/sec | pTyr-bind dom |
| CNTNAP2 | iCAM | 8 | 1TM | EGF/laminin |
| CTNNA2 | iCAM | 8 | cyt | Vinculin |
| PTPRM | iCAM | 8 | 1TM | Ig/Fn/MAM |
| ASTN1 | iCAM | 7 | 1TM | Mem-attack comp/Fn/EGF |
| CNTNAP5 | iCAM | 7 | 1TM | EGF/laminin |
| CTNNA3 | aj | 7 | cyt | Vinculin |
| DSCAM | iCAM | 7 | 1TM | Ig/Fn |
| NRG1 | iCAM | 7 | 1TM | Ig/EGF |
| OPCML | iCAM | 7 | GPI | Ig |
| CHL1 | iCAM | 6 | 1TM | Ig/Fn |
| CNTN4 | iCAM | 6 | GPI | Ig/Fn |
| CNTN5 | iCAM | 6 | GPI | Ig/Fn |
| CTNND2 | iCAM | 6 | cyt | Arm/β-catenin-l |
| EPHB1 | iCAM | 6 | 1TM | SAM/Fn/TNFR |
| LAMA1 | m | 6 | EC | Lam |
| NLGN1 | iCAM | 6 | 1TM | Esterase |
| NRXN3 | iCAM | 6 | 1TM | EGF/laminin |
| PTPRT | iCAM | 6 | 1TM | Ig/Fn/MAM |
| USH2A | m | 6 | EC | EGF/lam/Fn |
| CDH11 | iCAM | 5 | 1TM | Cdh |
| CSMD2 | iCAM | 5 | 1TM | Cub/sushi |
| ITGA9 | m | 5 | TM-assoc | Int |
| ITGB8 | m | 5 | 1TM | Int |
| LAMA2 | m | 5 | EC | EGF/lam |
| MDGA2 | ag | 5 | GPI | Ig/MAM |
| PLXNC1 | ag | 5 | 1TM | Sema/plexin |
| TEK | m | 5 | 1TM | Ig/Fn/EGF |
| ASTN2 | iCAM | 4 | 1TM | Mem-attack comp/Fn/EGF |
| CDH2 | iCAM | 4 | 1TM | Cdh |
| CDH4 | iCAM | 4 | 1TM | Cdh |
| CDH6 | iCAM | 4 | 1TM | Cdh |
| CDH7 | iCAM | 4 | 1TM | Cdh |
| DSCAML1 | iCAM | 4 | 1TM | Ig/Fn |
| FAT3 | iCAM | 4 | 1TM | Cdh/EGF/lam |
| FLRT2 | iCAM | 4 | 1TM | LRR/Fn |
| FREM1 | m | 4 | EC | C lectin/Calxbeta/CSPG |
| FREM2 | m | 4 | 1TM | C lectin/Calxbeta/CSPG |
| ITGA1 | m | 4 | TM-assoc | Int |
| LPP | fa | 4 | cyt/nuc | LIM/GKAP |
| LRRN2 | iCAM | 4 | 1TM | LRR/Ig |
| NCAM1 | iCAM | 4 | 1TM | Ig/Fn |
| PCDH15 | iCAM | 4 | 1TM | Cdh |
| PCDH9 | iCAM | 4 | 1TM | Cdh |
| PTPRB | iCAM | 4 | 1TM | Fn |
| PTPRG | iCAM | 4 | 1TM | Fn |
| PTPRK | iCAM | 4 | 1TM | Ig/Fn/MAM |
| RELN | m | 4 | EC | Rln/EGF |
| ROBO2 | ag | 4 | 1TM | Ig/Fn |
| SELE | c | 4 | 1TM | CCP/Clect/EGF |
| SELL | c | 4 | 1TM | CCP/Clect/EGF |
| SEMA5A | ag | 4 | 1TM | Thrombosp/Plxn/Sema |
| TNR | m | 4 | EC | Fn/FReD/EGF |
| AJAP1 | tj | 3 | 1TM | AJAP1/PANP C-term |
| ANTXR1 | m | 3 | 1TM | Anth_Ig/vWA_ATR |
| CDH12 | iCAM | 3 | 1TM | Cdh |
| CDH18 | iCAM | 3 | 1TM | Cdh |
| CDHR3 | iCAM | 3 | 1TM | Cdh |
| CELSR1 | iCAM | 3 | 7TM | Cdh/EGF/lam |
| DCC | iCAM | 3 | 1TM | Ig/Fn |
| DSC3 | fa | 3 | 1TM | Cdh |
| DSG4 | fa | 3 | 1TM | Cdh |
| DSP | tj | 3 | cyt | PLEC/SPEC/ApoLp-III_like |
| EFNA5 | iCAM | 3 | GPI | Ephrin-A |
| FREM3 | m | 3 | EC | Calxbeta/CSPG |
| GPR116 | iCAM | 3 | 7TM | Ig/SEA/lathrophilin |
| ITGA6 | m | 3 | TM-assoc | Int |
| ITGBL1 | m | 3 | 1TM | Int |
| LAMC2 | m | 3 | EC | Lam/EGF |
| LAMC3 | m | 3 | EC | Lam/EGF |
| LRFN5 | iCAM | 3 | 1TM | LRR/Fn/Ig |
| LSAMP | iCAM | 3 | GPI | Ig |
| MEGF11 | iCAM | 3 | 1TM | EGF |
| NFASC | iCAM | 3 | 1TM | Ig/Fn |
| NINJ2 | iCAM | 3 | 1TM | Ninj |
| NRP1 | iCAM | 3 | 1TM | MAM/Cub/FA58C |
| SDK1 | iCAM | 3 | 1TM | Ig/Fn |
| SELP | c | 3 | 1TM | CCP/Clect/EGF |
Columns: Gene symbol, CAM type, number of samples out of 15 total that identify this gene, structural elements of protein, protein motifs. CAM types: iCAM: information predominant CAM, m: primarily involved in interactions with cell matrix, ag: primary roles in axonal guidance, aj: primary role in adherens junctions, c: primary roles in cell/cell interactions, principally in immune system, fa: primary involvement in focal adhesions, tj: primary involvement in tight junctions. Structural elements: GPI: glycosylphosphatidyl inositol modified, cyt: cytoplasmic; sec: secreted; EC: extracellular. Motifs: Cdh: cadherin; Ig: immunoglobulin; Fn: fibronectin; lam: laminin; EGF epidermal growth factor; pTyr bind dom: phosphor tyrosine binding domain; MAM: ; Mem-attack comp: membrane attack complex; Armad b-catenin-l: Armadillo and beta catenin like; SAM: sterile alpha motif; TNFR: tumor necrosis factor receptor; Int: integrin; sema: semaphorin domain; LRR: leucine-rich repeat; C lectin: C-type lectin; Calxbeta: Calx beta domain; CSPG: chondroitin sulfate proteoglycan; LIM: LIM interacting protein; GKAP: guanylate-kinase-associated protein;CCP: complement control protein; Thrombosp: thrombospondin; FReD: Fibrinogen-related domains. Samples for addiction- or quit success-related GWAS are described in (21,33,35–39,41,42,44,80,81); more details of analyses are available on request.
Based on the 979/20474 fraction of all genes identified in these GWAS datasets (0.0478) and the 474/20474 fraction of all genes identified in the likely cell adhesion molecule dataset (0.02315), we would expect that 22 genes would be identified in both ways by chance. We actually identify 83. Likely cell adhesion molecules, taken as a group, are thus substantially overrepresented among the genes identified by this approach to analyses of GWAS data (p= 3.17 × 10−25, hypergeometric test). What CAM subclasses do these 83 genes fall into? Most of the 83 cell adhesion molecules that are implicated in addiction phenotypes in this fashion are annotated as “iCAMs” (53 of 83; 63% of the total) or involved in focal adhesion/extracellular matrix interactions (20 of 83 or another 25% of the total).
Individual differences in brains predispose to addiction and altered likelihood of success in quitting smoking. One of the most significant overall contributions to such individual differences appears to come from variation in interactions between cells, likely largely neurons, that derive from individual differences in products of genes that encode CAMs. CAMs that play especial roles in these differences are largely those whose products do not typically form stereotypic, morphologically-identifiable connections. Quantitative differences in CAM “bar codes” (GRU and JD, in preparation) thus alter human addiction vulnerabilities.
Example: overall fit between CAMs identified by modest GWAS signals in multiple addiction phenotype case vs control series and those expressed by dopaminergic neurons
Semiquantitative information from Alan Brain Atlas in situ hybridization images (46) allows us to confirm dopaminergic expression for several of the addiction- associated genes that encode “likely” CAMs. For PTPRD, CLSTN2, CNTNAP2, ASTN1, CNTNAP5, CHL1, CNTN4, CNTN5, CTNND2, EPHB1 and NRXN3, there is relatively high levels of expression in neurons that are highly likely, based on locations and appearance, to be largely dopaminergic. For DAB1, CTNNA2, PTPRM, CTNNA3, DSCAM, NRG1, OPCML and NLGN1, there is substantial but more modest neuronal expression in these Allen Brain Atlas in situ hybridization images.
Example: candidate dopaminergic connection differences arising from variations in genes that encode dopaminergically-expressed cell adhesion molecules identified frequently in addiction GWAS
There is increasing information about the influences of common human haplotypes on several of the likely cell adhesion molecule genes that are both identified in at least 6 addiction case-control GWAS datasets by signals of at least modest magnitude and expressed by dopaminergic neurons at moderate or high levels (Table II). For CDH13 and PTPRD, we have identified 60 – 80% individual differences in expression in postmortem human brains that are associated with common 5′ haplotypes in these genes, as well as haplotypes associated with smaller ca. 20% differences in CSMD1 expression (JD, GRU et al, in preparation). For NRXN3, we and others have identified common mid-to-3′ haplotypes that alter patterns of splicing of key exons, levels of expression and physiologies of NRXN3-expressing circuits (47,48).
One way to focus on the influences of quantitative and/or qualitative differences in expression of these genes is to focus on connections from and to dopaminergic neurons.
Dopaminergic efferents: connections to striatal/accumbens cholinergic interneurons and cortical neurons
CDH13 and PTPRD mRNAs are both expressed in large, presumable cholinergic striatal neurons and in subsets of deeper cerebral cortical neurons that are most abundant in infralimbic, cingulate and entorhinal cortices in mouse (49). Current in vitro data supports homophilic CDH13-CDH13 interactions that inhibit process outgrowth and homophilic PTPRD-PTPRD interactions that foster richer process outgrowth (50,51). Dopaminergic connections with subsets of CDH13-expressing ventral striatal neurons, subsets of CDH13-expressing cortical neurons and perhaps the striatal terminals of these subsets of cortical neurons could all be different in individuals with differences in levels of expression of CDH13 and PTPRD. Interestingly, mice with deletion of CDH13 do display selective cerebral cortical differences in dopamine, its metabolites, and ratios between dopamine and its metabolites (JD, GRU et al, in preparation). These results are consistent with the idea that cortical dopaminergic projections are differentially wired in the absence of CDH13.
Dopaminergic afferents: connections from glutamatergic neurons
Several of the group of addiction-associated dopaminergic cell adhesion molecules defined above appear to be expressed postsynaptically by neurons that receive glutamatergic afferents. These include the products of the NLGN1 and CLSTN2 genes as well as the LRRTM3 gene product of the combined CTNNA3_ LRRTM3 locus (52–54). Double labeling experiments have identified glutamatergic synapses on dopamine neurons that come from VTA afferents of neurons whose cell bodies lie in a number of regions (55). These include regions of the prefrontal cortex, lateral and medial hypothalamic and preoptic areas, ventral pallidum, lateral habenula, dorsal and median raphe, mesopontine central gray and reticular formation, pedunculopontine and laterodorsal tegmental nuclei, parabrachial nucleus, cuneiform nucleus and medial septum/diagonal band of Broca. The LRRTM3 and NLGN1 gene products expressed by dopaminergic neurons are thus themselves likely to interact with neurexins and neurexin homologs that include CNTNAPs that are expressed in neurons in several of these zones of origin of glutamatergic VTA afferents. mRNAs for NRXN3 and CNTNAP2 are expressed robustly in neurons in most of these regions, though there are only low levels of CNTNAP5 expression (56). Conceivably, some of the influences of addiction-associated variation in genes encoding neurexin family proteins could come from variation in their expression by glutamatergic VTA afferents that arise from cell bodies in these areas.
Example: candidate addiction-associated splicing differences that could be mediated by RBFOX1 allelic variants
The RBFOX1 (or A2BP1) gene product serves as a regulator of splicing of the primary RNA transcripts of genes whose sequences include its canonical recognition motif (U)GCAUG and at sites that do not display this cis-acting element (57). We have identified modest associations of RBFOX1 variants with substance dependence or ability to quit smoking in 13 independent datasets (17,21,33–35,37–39,42,58,59). RBFOX1 variation has also been associated with individual differences in smoking quantity/frequency (44). Genomic markers within or quite near RBFOX1 have displayed linkage to substance dependence related phenotypes (60–62). Mice with RBFOX1 expression deleted from neurons display altered expression of splice variants from twenty genes. These genes include the NRCAM and NRXN3 iCAM genes that display association with substance dependence in several samples (47,63), as well as the iCAM PTPRO.
Shen and colleagues have recently used a variety of approaches to identify nominal high significance for RBFOX1 binding overlaps with epigenetic marks in a set of genes that includes many CAMs (64). These workers identify immunoprecipitation of RBFOX1 with antibodies that recognize the H3K4me3 modified histone (64). These workers and our laboratory have identified changes in cocaine reward with local and brain-wide knockdown/knockout of A2BP1 activities (64) (JD, GRU et al, in preparation).
Taken together, these genetic, epigenetic and behavioral results support the working ideas that splicing differences mediated by RBFOX1 allelic variants, likely to include variation in expression of CAMs, contribute to vulnerabilities to addiction.
DISCUSSION
The present review provides substantial updates to the list of human “cell adhesion” molecules, identifying likely, questionable and unlikely candidates from among the longer lists of candidate CAM genes. It seeks, for the first time, to assign cell adhesion molecule gene products to categories that signify more relevance to their adhesive properties vs those that appear more relevant to information-transmitting properties that many of these gene products mediate. We apply each of these labels based on reviews of currently published literature and annotations for each gene. These annotations substantially update our prior work with this gene set. As information about many of the “questionable” cell adhesion molecules increases, additional genes may well be recognized as “likely” cell adhesion molecules. As more information about the properties of more of these cell adhesion molecules becomes available, the assignment of the encoded products to “more adhesion” vs “more information” will also change. We welcome readers’ comments and will use these comments to update this list and its annotations.
The annual number of PubMed citations for “cell adhesion molecules” grew more than 6 fold between the late 1980s and 1990’s, but has grown by less than 1/3 since then. Cell adhesion molecules are likely to play roles in development and adult function of virtually every cell and tissue. Brain expression of many of these molecules appears to have especial relevance for some of the most challenging problems in normal and pathological biologies: How are the ca.100 trillion neuronal connections in the brain established appropriately? How are these connections modified by exposures to different patterns of activity that result from experiences and from exposure to drugs that act on the nervous system? How do differences in these connections and their modification with experience result in brain disorders? This review posits central roles for cell adhesion molecules in the answers to each of these questions. While the overall complexity of the influences of CAMs on the connectome is great, this complexity may be more manageable as we focus on specific cell types, specific circuits that involve these cell types and specific disease processes. Here, we thus focus on the subsets of cell adhesion molecule genes that are identified by modest GWAS signals in multiple independent studies of addiction-related phenotypes. This approach has limits. The lists of genes identified by modest signals in multiple addiction-related GWAS studies are likely to contain both false positives and false negatives in identifying CAM genes whose variations alter individuals’ vulnerabilities to express addiction-related phenotypes. The great diversity of genes identified here is often accompanied by great diversity of splicing variants (48,65), which can both play large roles in information provided by the CAMs expression. Splicing variation introduces complexity that we have tackled from the perspective of RBFOX1, but we are likely to have omitted discussion of other important splicing events. The focus on CAMs in the current review should not obscure roles for other mechanisms that have been postulated to aid in the long-term storage of information about prior drug experiences that provides the key feature of addiction, including changes in cyclic nucleotides (66), G and RGS proteins (67,68), neurotropic factors (69), patterns of protein phosphorylation (70), transcription factor expression (71), and histone modifications (72).
The set of genes identified here by both dopaminergic expression and addiction phenotype GWAS in multiple samples does provide evidence for biological plausibility. The plausibility of identification of specific sets of cell adhesion molecules, such as those associated with glutamatergic synapse formation, is enhanced by the relatively recent general recognition of the role of glutamate as cotransmitter for subsets of ventral midbrain dopaminergic neurons and the mapping of substantial numbers of glutamatergic neurons among the afferents to these dopaminergic neurons (73). These anatomical relationships are fit by the patterns of expression of cell adhesion molecules attributed to glutamatoceptive and glutamatergic neurons in the Alan Brain Atlas data for dopaminergic neurons, and for recipients of the efferent connections from dopamine neurons and the sources of many of their afferents.
GPI-anchored cell adhesion molecules are similarly expressed in targets of midbrain dopaminergic efferents and by neurons in regions that provide afferents to midbrain dopamine neurons. Localization of the GPI-anchored (and, likely, other) cell adhesion molecules to lipid raft domains supports an idea that has not been stated clearly previously, to our knowledge: that one of the roles of interactions between cell adhesion molecule binding partners located on adjacent neuronal processes might be to stabilize the lipid rafts that contain them in proximity to each other (Fig 3). Lipid rafts contain not only cell adhesion molecules but also transporters, G-protein coupled receptors, channels and G proteins (74,75). We can think about “presynaptic” and “postsynaptic” lipid raft pairs that are likely to be stabilized when the cell membrane elements that express them are close enough to allow interactions between the GPI-anchored and other cell adhesion molecules contained in each raft in the pair. Proximities that could facilitate such interactions can often be found in perisynaptic regions adjacent to classical synaptic specializations. Biochemical evidence supports localization of biochemically- and morphologically-defined lipid rafts that contain cell adhesion molecules next to the classical synaptic specializations (76). Other sites could also display important CAM recognition/paired raft stabilization. Monoaminergic projections to cortex are characterized by varicosities that contain clusters of apparent synaptic vesicles (77). These varicosities are often found in regions of close apposition of membranes that do not display electron densities after the heavy metal salt stains that characterize classical synapses. These likely nonclassical “synapses” also appear to be strong candidates for stabilization by CAMs expressed in lipid raft pairs from the monoaminergic projections and the cortical neuronal recipients of this innervation. The roles that GPI-anchored, and other cell adhesion molecules could play in stabilizing raft pairs at classical and nonclassical “synapses” imply their indirect roles in stabilizing the other contents of these raft pairs, (eg transporters, channels etc) in proximity to each other. To the extent that individual differences in CDH13, CNTN4 and CNTN5 expression alter the abundance of such closely-approximated “pre/perisynaptic-post/perisynaptic” lipid raft pairs in neurons that express them, the other contents of these rafts could be assembled in different abundance with significant impacts on dopaminergic functions.
Figure 3.
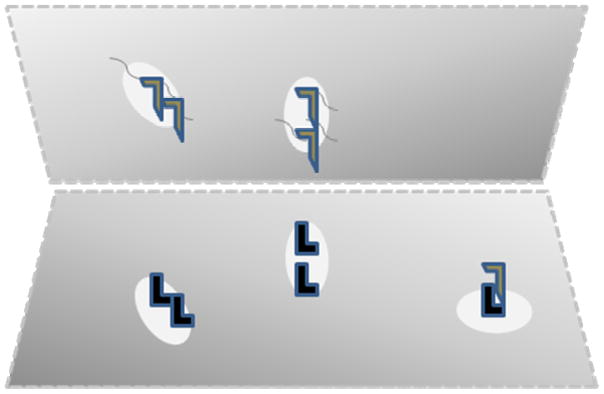
Schematic of pre (eg top) and post (eg bottom) membranes pulled away from each other to illustrate the way in which CAMs (L shapes) anchored to lipid rafts (ovals) might stabilize the constituents of the lipid rafts (squiggles) by allowing binding between “pre” and ”postsynaptic” CAM-containing lipid raft pairs. Bottom right: Lipid raft containing CAM bound to soluble CAM, blocking possible participation in stabilization of a lipid raft pair.
Other GPI-anchored cell adhesion molecules are likely to decorate lipid rafts that are not stabilized due to expression in closely-approximated membrane domains. These cell adhesion molecules could still recognize the soluble fragments that are produced from many cell adhesion molecule genes, including interesting soluble fragment products of a CDH13 splicing variant (JD, GRU et al, in preparation).
The cell adhesion molecules identified and categorized here thus have functions beyond just “cell glue” in ways that can generate specific testable hypotheses about their roles in specific cell types and specific circuits, and may even provide substrates for novel therapeutics that can modify brain connections (78). These hypotheses should be assessed in light of the strengths and limitations of the approaches used here, and the strengths and limitations of the underlying datasets employed for these analyses. Cell adhesion molecules and mechanisms remain fascinating and understudied ways in which the body, and the brain in particular, assembles and changes its assemblies during its development and through its interactions with the environment.
Acknowledgments
We acknowledge support by the NIH IRP (NIDA) (GRU), from the National Key Basic Research Program of China [2013CB531202] (CYL), from each of the investigators and subjects of the GWAS studies whose data is summarized here, and helpful comments on the manuscript from D Martinelli.
References
- 1.Winograd-Katz SE, Fassler R, Geiger B, Legate KR. The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol. 2014;15:273–288. doi: 10.1038/nrm3769. [DOI] [PubMed] [Google Scholar]
- 2.Li CY, Liu QR, Zhang PW, Li XM, Wei L, Uhl GR. OKCAM: an ontology-based, human-centered knowledgebase for cell adhesion molecules. Nucleic Acids Res. 2009;37:D251–260. doi: 10.1093/nar/gkn568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke RA, Eapen V. Balance within the Neurexin Trans-Synaptic Connexus Stabilizes Behavioral Control. Front Hum Neurosci. 2014;8:52. doi: 10.3389/fnhum.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker MW, Macagno ER. Control of neuronal morphology and connectivity: emerging developmental roles for gap junctional proteins. FEBS Lett. 2014;588:1470–1479. doi: 10.1016/j.febslet.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Sheng L, Leshchyns’ka I, Sytnyk V. Cell adhesion and intracellular calcium signaling in neurons. Cell Commun Signal. 2013;11:94. doi: 10.1186/1478-811X-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada S, Nelson WJ. Synapses: sites of cell recognition, adhesion, and functional specification. Annu Rev Biochem. 2007;76:267–294. doi: 10.1146/annurev.biochem.75.103004.142811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJ, Nyengaard JR, Regeur L. Aging and the human neocortex. Exp Gerontol. 2003;38:95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- 8.Hishimoto A, Liu QR, Drgon T, Pletnikova O, Walther D, Zhu XG, Troncoso JC, Uhl GR. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Hum Mol Genet. 2007;16:2880–2891. doi: 10.1093/hmg/ddm247. [DOI] [PubMed] [Google Scholar]
- 9.Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro L, Love J, Colman DR. Adhesion molecules in the nervous system: structural insights into function and diversity. Annu Rev Neurosci. 2007;30:451–474. doi: 10.1146/annurev.neuro.29.051605.113034. [DOI] [PubMed] [Google Scholar]
- 11.Yamada S, Nelson WJ. Synapses: sites of cell recognition, adhesion, and functional specification. Annu Rev Biochem. 2007;76:267–294. doi: 10.1146/annurev.biochem.75.103004.142811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoker AW. Protein tyrosine phosphatases and signalling. J Endocrinol. 2005;185:19–33. doi: 10.1677/joe.1.06069. [DOI] [PubMed] [Google Scholar]
- 13.Salinas PC, Price SR. Cadherins and catenins in synapse development. Curr Opin Neurobiol. 2005;15:73–80. doi: 10.1016/j.conb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Hirano S, Suzuki ST, Redies C. The cadherin superfamily in neural development: diversity, function and interaction with other molecules. Front Biosci. 2003;8:d306–355. doi: 10.2741/972. [DOI] [PubMed] [Google Scholar]
- 15.Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dityatev A, Dityateva G, Schachner M. Synaptic strength as a function of post- versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron. 2000;26:207–217. doi: 10.1016/s0896-6273(00)81151-4. [DOI] [PubMed] [Google Scholar]
- 17.Uhl GR, Drgon T, Johnson C, Li CY, Contoreggi C, Hess J, Naiman D, Liu QR. Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify “connectivity constellation” and drug target genes with pleiotropic effects. Ann N Y Acad Sci. 2008;1141:318–381. doi: 10.1196/annals.1441.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson C, Drgon T, Liu QR, Walther D, Edenberg H, Rice J, Foroud T, Uhl GR. Pooled association genome scanning for alcohol dependence using 104,268 SNPs: validation and use to identify alcoholism vulnerability loci in unrelated individuals from the collaborative study on the genetics of alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:844–853. doi: 10.1002/ajmg.b.30346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu QR, Drgon T, Johnson C, Walther D, Hess J, Uhl GR. Addiction molecular genetics: 639,401 SNP whole genome association identifies many “cell adhesion” genes. Am J Med Genet B Neuropsychiatr Genet. 2006;141:918–925. doi: 10.1002/ajmg.b.30436. [DOI] [PubMed] [Google Scholar]
- 20.Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, David SP, Niaura R, Lerman C. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65:683–693. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson C, Drgon T, Walther D, Uhl GR. Genomic regions identified by overlapping clusters of nominally-positive SNPs from genome-wide studies of alcohol and illegal substance dependence. PLoS One. 2011;6:e19210. doi: 10.1371/journal.pone.0019210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butcher LM, Meaburn E, Dale PS, Sham P, Schalkwyk LC, Craig IW, Plomin R. Association analysis of mild mental impairment using DNA pooling to screen 432 brain-expressed single-nucleotide polymorphisms. Mol Psychiatry. 2005;10:384–392. doi: 10.1038/sj.mp.4001589. [DOI] [PubMed] [Google Scholar]
- 23.Hargreaves A, Anney R, O’Dushlaine C, Nicodemus KK, Gill M, Corvin A, Morris D, Donohoe G. The one and the many: effects of the cell adhesion molecule pathway on neuropsychological function in psychosis. Psychol Med. 2013:1–11. doi: 10.1017/S0033291713002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girirajan S, Dennis MY, Baker C, Malig M, Coe BP, Campbell CD, Mark K, Vu TH, Alkan C, Cheng Z, et al. Refinement and discovery of new hotspots of copy-number variation associated with autism spectrum disorder. Am J Hum Genet. 2013;92:221–237. doi: 10.1016/j.ajhg.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munafo MR, Attwood AS, Flint J. Neuregulin 1 genotype and schizophrenia. Schizophr Bull. 2008;34:9–12. doi: 10.1093/schbul/sbm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernstein HG, Bogerts B. Neuregulin-1 alpha, the underestimated molecule: emerging new roles in normal brain function and the pathophysiology of schizophrenia? Genome. 2013;56:703–704. doi: 10.1139/gen-2013-0171. [DOI] [PubMed] [Google Scholar]
- 28.Velayos-Baeza A, Toma C, da Roza S, Paracchini S, Monaco AP. Alternative splicing in the dyslexia-associated gene KIAA0319. Mamm Genome. 2007;18:627–634. doi: 10.1007/s00335-007-9051-3. [DOI] [PubMed] [Google Scholar]
- 29.Paracchini S, Thomas A, Castro S, Lai C, Paramasivam M, Wang Y, Keating BJ, Taylor JM, Hacking DF, Scerri T, et al. The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319, a novel gene involved in neuronal migration. Hum Mol Genet. 2006;15:1659–1666. doi: 10.1093/hmg/ddl089. [DOI] [PubMed] [Google Scholar]
- 30.Mascheretti S, Riva V, Giorda R, Beri S, Lanzoni LF, Cellino MR, Marino C. KIAA0319 and ROBO1: evidence on association with reading and pleiotropic effects on language and mathematics abilities in developmental dyslexia. J Hum Genet. 2014;59:189–197. doi: 10.1038/jhg.2013.141. [DOI] [PubMed] [Google Scholar]
- 31.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhl GR, Drgon T, Liu QR, Johnson C, Walther D, Komiyama T, Harano M, Sekine Y, Inada T, Ozaki N, et al. Genome-wide association for methamphetamine dependence: convergent results from 2 samples. Arch Gen Psychiatry. 2008;65:345–355. doi: 10.1001/archpsyc.65.3.345. [DOI] [PubMed] [Google Scholar]
- 34.Drgon T, Johnson CA, Nino M, Drgonova J, Walther DM, Uhl GR. “Replicated” genome wide association for dependence on illegal substances: genomic regions identified by overlapping clusters of nominally positive SNPs. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2011;156:125–138. doi: 10.1002/ajmg.b.31143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhl GR, Walther D, Musci R, Fisher C, Anthony JC, Storr CL, Behm FM, Eaton WW, Ialongo N, Rose JE. Smoking quit success genotype score predicts quit success and distinct patterns of developmental involvement with common addictive substances. Mol Psychiatry. 2014;19:50–54. doi: 10.1038/mp.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson C, Drgon T, Liu QR, Zhang PW, Walther D, Li CY, Anthony JC, Ding Y, Eaton WW, Uhl GR. Genome wide association for substance dependence: convergent results from epidemiologic and research volunteer samples. BMC Med Genet. 2008;9:113. doi: 10.1186/1471-2350-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drgon T, Montoya I, Johnson C, Liu QR, Walther D, Hamer D, Uhl GR. Genome-wide association for nicotine dependence and smoking cessation success in NIH research volunteers. Mol Med. 2009;15:21–27. doi: 10.2119/molmed.2008.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drgon T, Johnson C, Walther D, Albino AP, Rose JE, Uhl GR. Genome-wide association for smoking cessation success: participants in a trial with adjunctive denicotinized cigarettes. Mol Med. 2009;15:268–274. doi: 10.2119/molmed.2009.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, David SP, Niaura R, Lerman C. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65:683–693. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhl GR, Walther D, Musci R, Fisher C, Anthony JC, Storr CL, Behm FM, Eaton WW, Ialongo N, Rose JE. Smoking quit success genotype score predicts quit success and distinct patterns of developmental involvement with common addictive substances. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhl GR, Drgon T, Johnson C, Ramoni MF, Behm FM, Rose JE. Genome-wide association for smoking cessation success in a trial of precessation nicotine replacement. Mol Med. 2010;16:513–526. doi: 10.2119/molmed.2010.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uhl GR, Drgon T, Johnson C, Walther D, David SP, Aveyard P, Murphy M, Johnstone EC, Munafo MR. Genome-wide association for smoking cessation success: participants in the Patch in Practice trial of nicotine replacement. Pharmacogenomics. 2010;11:357–367. doi: 10.2217/pgs.09.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, Sulem P, Rafnar T, Esko T, Walter S, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bierut LJ. Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24-25. Trends Pharmacol Sci. 2010;31:46–51. doi: 10.1016/j.tips.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.http://mouse.brain-map.org/
- 47.Hishimoto A, Liu QR, Drgon T, Pletnikova O, Walther D, Zhu XG, Troncoso JC, Uhl GR. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Hum Mol Genet. 2007;16:2880–2891. doi: 10.1093/hmg/ddm247. [DOI] [PubMed] [Google Scholar]
- 48.Aoto J, Martinelli DC, Malenka RC, Tabuchi K, Sudhof TC. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell. 2013;154:75–88. doi: 10.1016/j.cell.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.mouse.brain-map.org
- 50.Ciatto C, Bahna F, Zampieri N, VanSteenhouse HC, Katsamba PS, Ahlsen G, Harrison OJ, Brasch J, Jin X, Posy S, et al. T-cadherin structures reveal a novel adhesive binding mechanism. Nat Struct Mol Biol. 2010;17:339–347. doi: 10.1038/nsmb.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Bixby JL. Receptor tyrosine phosphatase-delta is a homophilic, neurite-promoting cell adhesion molecular for CNS neurons. Mol Cell Neurosci. 1999;14:370–384. doi: 10.1006/mcne.1999.0789. [DOI] [PubMed] [Google Scholar]
- 52.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uvarov P, Kajander T, Airaksinen MS. Origin and loss of nested LRRTM/alpha-catenin genes during vertebrate evolution. PLoS One. 2014;9:e89910. doi: 10.1371/journal.pone.0089910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pettem KL, Yokomaku D, Luo L, Linhoff MW, Prasad T, Connor SA, Siddiqui TJ, Kawabe H, Chen F, Zhang L, et al. The specific alpha-neurexin interactor calsyntenin-3 promotes excitatory and inhibitory synapse development. Neuron. 2013;80:113–128. doi: 10.1016/j.neuron.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.mouse.brain-map.org
- 57.Gehman LT, Stoilov P, Maguire J, Damianov A, Lin CH, Shiue L, Ares M, Jr, Mody I, Black DL. The splicing regulator Rbfox1 (A2BP1) controls neuronal excitation in the mammalian brain. Nat Genet. 2011;43:706–711. doi: 10.1038/ng.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu QR, Drgon T, Johnson C, Walther D, Hess J, Uhl GR. Addiction molecular genetics: 639,401 SNP whole genome association identifies many “cell adhesion” genes. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2006;141B:918–925. doi: 10.1002/ajmg.b.30436. [DOI] [PubMed] [Google Scholar]
- 59.Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE. Molecular genetics of nicotine dependence and abstinence: whole genome association using 520,000 SNPs. BMC Genet. 2007;8:10. doi: 10.1186/1471-2156-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, Bennett PH, Goldman D. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81:216–221. doi: 10.1002/(sici)1096-8628(19980508)81:3<216::aid-ajmg2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 61.Ehlers CL, Gilder DA, Wall TL, Phillips E, Feiler H, Wilhelmsen KC. Genomic screen for loci associated with alcohol dependence in Mission Indians. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:110–115. doi: 10.1002/ajmg.b.30057. [DOI] [PubMed] [Google Scholar]
- 62.Morley KI, Medland SE, Ferreira MA, Lynskey MT, Montgomery GW, Heath AC, Madden PA, Martin NG. A possible smoking susceptibility locus on chromosome 11p12: evidence from sex-limitation linkage analyses in a sample of Australian twin families. Behav Genet. 2006;36:87–99. doi: 10.1007/s10519-005-9004-0. [DOI] [PubMed] [Google Scholar]
- 63.Ishiguro H, Liu QR, Gong JP, Hall FS, Ujike H, Morales M, Sakurai T, Grumet M, Uhl GR. NrCAM in addiction vulnerability: positional cloning, drug-regulation, haplotype-specific expression, and altered drug reward in knockout mice. Neuropsychopharmacology. 2006;31:572–584. doi: 10.1038/sj.npp.1300855. [DOI] [PubMed] [Google Scholar]
- 64.Feng J, Wilkinson M, Liu X, Purushothaman I, Ferguson D, Vialou V, Maze I, Shao N, Kennedy P, Koo J, et al. Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Biol. 2014;15:R65. doi: 10.1186/gb-2014-15-4-r65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Treutlein B, Gokce O, Quake SR, Sudhof TC. Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing. Proc Natl Acad Sci U S A. 2014;111:E1291–1299. doi: 10.1073/pnas.1403244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- 67.Nestler EJ, Erdos JJ, Terwilliger R, Duman RS, Tallman JF. Regulation of G proteins by chronic morphine in the rat locus coeruleus. Brain Res. 1989;476:230–239. doi: 10.1016/0006-8993(89)91243-2. [DOI] [PubMed] [Google Scholar]
- 68.Zachariou V, Georgescu D, Sanchez N, Rahman Z, DiLeone R, Berton O, Neve RL, Sim-Selley LJ, Selley DE, Gold SJ, et al. Essential role for RGS9 in opiate action. Proc Natl Acad Sci U S A. 2003;100:13656–13661. doi: 10.1073/pnas.2232594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berhow MT, Russell DS, Terwilliger RZ, Beitner-Johnson D, Self DW, Lindsay RM, Nestler EJ. Influence of neurotrophic factors on morphine- and cocaine-induced biochemical changes in the mesolimbic dopamine system. Neuroscience. 1995;68:969–979. doi: 10.1016/0306-4522(95)00207-y. [DOI] [PubMed] [Google Scholar]
- 70.Guitart X, Nestler EJ. Second messenger and protein phosphorylation mechanisms underlying opiate addiction: studies in the rat locus coeruleus. Neurochem Res. 1993;18:5–13. doi: 10.1007/BF00966918. [DOI] [PubMed] [Google Scholar]
- 71.Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trudeau LE, Hnasko TS, Wallen-Mackenzie A, Morales M, Rayport S, Sulzer D. The multilingual nature of dopamine neurons. Prog Brain Res. 2014;211:141–164. doi: 10.1016/B978-0-444-63425-2.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu H, Wakim B, Li M, Halligan B, Tint GS, Patel SB. Quantifying raft proteins in neonatal mouse brain by ‘tube-gel’ protein digestion label-free shotgun proteomics. Proteome Sci. 2007;5:17. doi: 10.1186/1477-5956-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williamson R, Thompson AJ, Abu M, Hye A, Usardi A, Lynham S, Anderton BH, Hanger DP. Isolation of detergent resistant microdomains from cultured neurons: detergent dependent alterations in protein composition. BMC Neurosci. 2010;11:120. doi: 10.1186/1471-2202-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suzuki T, Zhang J, Miyazawa S, Liu Q, Farzan MR, Yao WD. Association of membrane rafts and postsynaptic density: proteomics, biochemical, and ultrastructural analyses. J Neurochem. 2011;119:64–77. doi: 10.1111/j.1471-4159.2011.07404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carr DB, O’Donnell P, Card JP, Sesack SR. Dopamine terminals in the rat prefrontal cortex synapse on pyramidal cells that project to the nucleus accumbens. J Neurosci. 1999;19:11049–11060. doi: 10.1523/JNEUROSCI.19-24-11049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uhl GR, Drgonova J. Cell adhesion molecules: druggable targets for modulating the connectome and brain disorders? Neuropsychopharmacology. 2014;39:235. doi: 10.1038/npp.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang H, He B, Ma H, Tsaur SC, Ma C, Wu Y, Ting CT, Zhang YE. Expression profile and gene age jointly shaped the genome-wide distribution of premature termination codons in a Drosophila melanogaster population. Mol Biol Evol. 2014 doi: 10.1093/molbev/msu299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drgon T, Johnson CA, Nino M, Drgonova J, Walther DM, Uhl GR. “Replicated” genome wide association for dependence on illegal substances: genomic regions identified by overlapping clusters of nominally positive SNPs. Am J Med Genet B Neuropsychiatr Genet. 2011;156:125–138. doi: 10.1002/ajmg.b.31143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, Maier W, Moessner R, Gaebel W, Dahmen N, et al. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66:773–784. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]