Abstract
Stress resilience is mediated, in part, by our ability to predict and control threats within our environment. Therefore, determining the neural mechanisms that regulate the emotional response to predictable and controllable threat may provide important new insight into the processes that mediate resilience to emotional dysfunction and guide the future development of interventions for anxiety disorders. To better understand the effect of predictability and controllability on threat-related brain activity in humans, two groups of healthy volunteers participated in a yoked Pavlovian fear conditioning study during functional magnetic resonance imaging (fMRI). Threat predictability was manipulated by presenting an aversive unconditioned stimulus (UCS) that was either preceded by a conditioned stimulus (i.e., predictable) or by presenting the UCS alone (i.e., unpredictable). Similar to animal model research that has employed yoked fear conditioning procedures, one group (Controllable Condition; CC), but not the other group (Uncontrollable Condition; UC) was able to terminate the UCS. The fMRI signal response within the dorsolateral prefrontal cortex (PFC), dorsomedial PFC, ventromedial PFC, and posterior cingulate was diminished during predictable compared to unpredictable threat (i.e., UCS). In addition, threat-related activity within the ventromedial PFC and bilateral hippocampus was diminished only to threats that were both predictable and controllable. These findings provide insight into how threat predictability and controllability affects the activity of brain regions (i.e., ventromedial PFC and hippocampus) involved in emotion regulation, and may have important implications for better understanding neural processes that mediate emotional resilience to stress.
Keywords: prefrontal cortex, hippocampus, controllability, predictability, stress resilience
Introduction
Resilience to stress is mediated, in part, by our ability to predict and control threats in our surroundings. For example, chronic exposure to unpredictable and uncontrollable threat is an important trigger in the development of anxiety-related disorders (Chorpita and Barlow, 1998; Foa et al., 1992; Maier and Seligman, 1976). Therefore, determining the impact of threat predictability and controllability on brain regions that regulate the emotional response is necessary for a better understanding of the factors that promote resilience to emotional dysfunction. Prior human neuroimaging work has examined threat predictability (Dunsmoor et al., 2008; Knight et al., 2010; Wood et al., 2012; 2013) and controllability (Kerr et al., 2012; Salomons et al., 2004; 2007; Wiech et al., 2006) independently, however, important questions regarding how threat predictability and threat controllability interact within the human brain remain unanswered. Thus, there is a critical gap in our understanding of the impact that predictability and controllability have on the neural response to threat in humans. Determining the neural response to predictable and controllable threat may provide important new insights into processes that mediate resilience to emotional dysfunction and guide the development of future interventions for anxiety disorders.
Although the predictability and controllability of aversive events have been previously studied, most prior work as only focused on one of these two factors (i.e., predictability or controllability) (Dunsmoor et al., 2008; Kerr et al., 2012; Salomons et al., 2004; 2007; Wiech et al., 2006; Wood et al., 2012; 2013). For example, prior investigations of controllability have generally not included unpredictable stimulus presentations that were both controllable and uncontrollable (Kerr et al., 2012; Salomons et al., 2004; 2007; Wiech et al., 2006). Thus, research that compares both predictability and controllability is necessary for a complete understanding of these processes and how they interact. Further, predictability and controllability have often been tightly linked in prior work on this topic (Foa et al., 1992; Maier and Seligman, 1976; Mineka and Kihlstrom, 1978; Mineka and Hendersen, 1985). For example, the termination of a controllable threat can be predicted, whereas the termination of an uncontrollable threat cannot be predicted (Amat et al., 1998; 2008; Baratta et al., 2007; 2008; Rozeske et al., 2011). However, threat predictability can be operationalized in terms of whether or not a warning signal precedes the threat, which would permit a more independent assessment of controllability (i.e., controllable vs. uncontrollable threat) and predictability (i.e., presentations of both predictable and unpredictable threat), as well as, the interaction of these processes.
Human anxiety disorders are characterized by emotional behavior that resembles Pavlovian conditioned fear responses (Davis et al., 2009; Grillon, 2002; Nitschke et al., 2006; 2009). Therefore, fear conditioning has become a popular paradigm for the study of emotion expression and regulation. The conditioned response (CR) is often the primary focus of conditioning studies, and there are a number of interesting issues related to the impact controllability has on the CR. However, the response to the threat itself is of utmost biological relevance (Domjan, 2005), and recent work from our lab has demonstrated important emotion and learning-related differences in threat-elicited brain and behavioral responses (Dunsmoor et al., 2008; Knight et al., 2010; 2011; Wood et al., 2012; 2013; 2014). Therefore, determining the neural processes that mediate the influence predictability and controllability have on the threat-elicited response is vital to understanding emotional behavior.
The prefrontal cortex (PFC), hippocampus, and amygdala are important components of the neural circuit that mediates expression and regulation of the conditioned emotional response (Davis, 1992; Fanselow, 1994; Hartley and Phelps, 2010). In particular, the ventromedial PFC (vmPFC) and hippocampus support learning-related processes that appear to inhibit conditioned and unconditioned fear expression in humans (Milad et al., 2007; 2009; Rauch et al., 2006; Schiller et al., 2013; Wood et al., 2012). Further, animal model studies indicate the vmPFC and hippocampus mediate stress resilience to predictable and controllable threat (Amat et al., 1998; Baratta et al., 2007; 2008; Franklin et al., 2012; Russo et al., 2012). Therefore, the vmPFC and hippocampus may support processes that regulate the emotional response to predictable and controllable threat in humans.
Research using functional magnetic resonance imaging (fMRI) and psychophysiological recording often employ within-subject designs to account for inter-subject differences and reduce error variance. However, within-subject designs can introduce confounds (e.g., carry-over effects) and interfere with the interpretation of the results. For example, confounds related to habituation and/or prior associative learning prevent assessing control using a within-subject design in separate scanning sessions, or separate blocks in the same scanning session, because participants must have control in the first session to match for threat duration in the second session. In contrast, a yoked Pavlovian fear conditioning paradigm, similar to prior animal model research (Amat et al., 1998; 2008; Baratta et al., 2008; 2009; Maier and Seligman, 1976; Maier, 1986; Maier and Watkins, 2010; Rozeske et al., 2011), can be used to investigate the effect that threat predictability and controllability have on human brain activity while limiting confounds related to habituation and prior conditioning. Yoked conditioning studies typically consist of one group that has the ability to control (i.e., terminate) the threat and a second group that is “yoked” to the first group, and thus receives the same stimuli, but cannot control the threat. Finally, yoked conditioning paradigms ensure the order, onset, duration, and intensity of stimuli is consistent across groups that receive varying degrees of predictable and controllable threat (i.e., between-subject design).
Prior human neuroimaging studies that have employed a within-subject design to investigate controllability have typically given participants control on some trials, but not on other trials (Kerr et al., 2012; Wiech et al., 2006). Thus, these manipulations do not simply compare control vs. no control, but control vs. loss of control. The distinction between not having control vs. losing control may seem subtle, but is an important issue in the learned helplessness literature (Maier and Seligman, 1976). Other human neuroimaging studies have primarily focused on the perception of control, instead of actual behavioral control over a threat (Salomons et al., 2004; 2007). Each of these studies are valuable contributions to the field, however, the current study focused on important questions specifically related to controllable vs. uncontrollable threat.
The present study investigated the effect of predictability and controllability on the threat-elicited neurophysiological response to better understand the underlying processes that support emotional resilience. In addition, this study includes psychophysiological, cognitive, and self-assessment measures to identify relationships that may influence stress resilience. Similar to prior animal model research, this study employed a yoked Pavlovian conditioning procedure to assess threat-elicited brain activity to predictable-controllable, predictable-uncontrollable, unpredictable-controllable, and unpredictable-uncontrollable presentations of threat. Given that the PFC and hippocampus support emotion regulation, we hypothesized that predictability and controllability would modulate the amplitude of the threat-elicited response within these brain areas. Although previous research has demonstrated greater activity within the vmPFC and hippocampus during the anticipation of threat (Kerr et al., 2012; Milad et al., 2007), our prior work has demonstrated anticipatory and threat-related activity are inversely related (Wood et al., 2012). Thus, we expected diminished PFC and hippocampal activity to predictable and controllable threats.
Materials and Methods
Experimental Design
Volunteers participated in a differential fear conditioning procedure during fMRI that consisted of yoked pairs of subjects. To examine the effect of threat controllability, one group received a controllable unconditioned stimulus (UCS) (Controllable Condition; CC) and the second group received an uncontrollable UCS (Uncontrollable Condition; UC). CC participants had the ability to terminate the UCS, whereas UC participants could not terminate the UCS. Instead, the timing, duration, and order of stimuli presented to each CC participant were recorded and used to determine stimulus presentation for their yoked match in the UC group. In this way, CC participants controlled the duration of the UCS for their matched counterpart in the UC group. Threat predictability was assessed by comparing presentations of the unconditioned stimulus (UCS; threat) that was paired with the conditioned stimulus (i.e., CS+UCS) to presentations of the UCS alone.
Participants
A total of fifty-four (27 CC and 27 UC) healthy right-handed volunteers participated in this study [28 female, 26 male; age = 23.39 ± 0.77 years (mean ± SEM); range = 18–38 years]. Participants in the two groups were matched on gender, ethnicity, age, and level of education (Table 1). There were no significant differences between the two groups based on these factors. All subjects provided written informed consent as approved by the University of Alabama at Birmingham Institutional Review Board.
Table 1.
Demographics and group characteristics.
| Measures | Controllable Condition | Uncontrollable Condition | t | p |
|---|---|---|---|---|
| Male/Female | 13/14 | 13/14 | ||
| Age | 23.37 ± 1.10 | 23.41 ± 1.11 | −0.03 | 0.97 |
| range | 18–38 | 18–38 | ||
| Education (years) | 14.89 ± 0.49 | 15.26 ± 0.59 | −0.68 | 0.50 |
| range | 12–22 | 12–21 | ||
| State Anxiety | 33.30 ± 1.47 | 35.30 ± 1.49 | −1.05 | 0.30 |
| Trait Anxiety | 36.93 ± 1.71 | 38.63 ± 1.23 | −0.81 | 0.42 |
There were no differences in gender, age, education, or anxiety level between the groups.
State-Trait Anxiety Inventory
Participants completed the State-Trait Anxiety Inventory (STAI; Form Y) for Adults (Spielberger, 1983) after the conditioning session. The STAI consists of a self-assessment measure of state and trait anxiety in terms of general negative affect (Grös et al., 2007). Scores on the state scale reflect anxiety level at the current moment, whereas trait anxiety scores reflect a relatively long-term predisposition for anxiety (Spielberger, 1983).
Conditioned and unconditioned stimuli
Two tones (700 and 1300 Hz; 10 s duration; 20 s ITI) served as the CSs and a loud (100 dB) white-noise served as the UCS (duration: 0.5–6.0 s in 0.5 s increments) and were presented through magnetic resonance (MR) compatible pneumatic headphones. UCS onset occurred 0.5 s before termination of one tone (CS+) and the second tone was presented alone (CS−) (Figure 1). There were a total of 24 CS+, 24 CS−, and 24 UCS alone trials presented across the two 960 s blocks of conditioning. The stimuli were counterbalanced and presented in a pseudorandom order such that no more than two trials of the same stimulus were consecutively presented.
Figure 1.
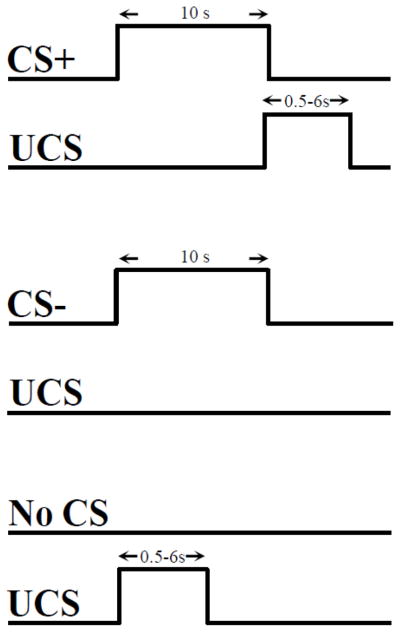
Conditioning Procedure. Participants received a total of 24 presentations of each stimulus (24 CS+, 24 CS−, and 24 UCS alone). UCS duration was determined by participants in the CC group and varied between 0.5–6.0 s in 0.5 s increments. Timing, duration, and stimulus order for each CC participant was recorded and used to determine the stimulus presentation for their yoked match in the UC group.
UCS duration
CC participants were instructed that the UCS would last between 0.5–6.0 s, and that they had the ability to control the duration of the UCS. They were instructed that they could terminate the UCS by making a single button press on the joystick. CC participants were also informed that if no button press was made the UCS would last the entire 6.0 s. In doing so, CC participants determined the duration of the UCS for themselves as well as their matched UC counterpart. UC participants were also informed that the UCS would last between 0.5–6.0 s. Given that UC participants did not have the ability to control the UCS, they were instructed to make a button press when the UCS ended, to control for motor activity associated with the button presses made by their match in the CC group.
UCS unpleasantness
Participants rated the unpleasantness of the UCS following each conditioning scan. Unpleasantness was rated on a 0 (not unpleasant) to 10 (unpleasant) scale. Separate ratings were acquired for each block to assess change over time. Repeated measures ANOVA was conducted to investigate change over time and group differences.
UCS expectancy
UCS expectancy was used as a measure of conscious expectation of the UCS. The present analysis focused on conditioned UCR diminution. Therefore, contrasts were limited to predictable (i.e., CS+UCS) and unpredictable (i.e., UCS alone) presentations of the UCS. Presentation software (Neurobehavioral Systems, Inc.; Albany, CA) was used to present a UCS expectancy rating scale on an IFIS-SA LCD (Invivo Corp.; Gainesville, FL) video screen located above the subject’s head and viewed through a mirror attached to the RF coil. An MR-compatible joystick (Current Designs; Philadelphia, PA) was used to monitor subjects’ expectancy of receiving the UCS. The joystick controlled a rating bar that was presented throughout the conditioning session on the video screen. Subjects were instructed to rate their UCS expectancy from moment to moment using a continuous scale from 0 to 100 (0 = certain the UCS would not be presented, 50 = uncertain whether the UCS would be presented, 100 = certain the UCS would be presented) to reflect their current UCS expectancy. UCS expectancy was calculated as the average response (1 s sample) at UCS onset. Additional details on this methodology have been published previously (Knight and Wood, 2011).
Skin conductance response
An MR-compatible physiological monitoring system (Biopac Systems; Goleta, CA) was used to collect skin conductance response (SCR) data. SCR was sampled (10 kHz) with a pair of disposable radio-translucent dry electrodes (EL509, Biopac Systems; Goleta, CA). Isotonic recording electrode gel (Gel101, Biopac Systems; Goleta, CA) was applied to the electrodes which were then affixed to the thenar and hypothenar eminences of the left palm. SCR data were processed using Biopac AcqKnowledge 4.1 software. A 1 Hz low pass digital filter was applied and SCR data were resampled at 250 Hz. Unconditioned SCRs were limited to those that occurred within 10 s following the UCS presentation. Unconditioned SCRs smaller than 0.05 μSiemens were scored as 0. Data were then square root transformed prior to statistical analyses.
Electromyography
The MR-compatible physiological monitoring system (Biopac Systems; Goleta, CA) was also used to collect eye-blink electromyography (EMG) data. EMG was sampled (10 kHz) with a pair of disposable radio-translucent electrodes (1 cm diameter, Biopac Systems; Goleta, CA) from the orbicularis oculi muscle below the left eye. The first electrode was placed directly below the left pupil while the second was placed laterally to the first electrode as per previous committee report guidelines (Blumenthal et al., 2005). EMG data were processed using Biopac AcqKnowledge 4.1 software. Following guidelines for digital filtering (Cook and Miller, 1992) and EMG denoising (Blumenthal et al., 2005) a Fast Fourier Transform was used to assess and remove frequency domains where noise occurred (Comb Band Stop filter at fMRI fundamental frequency ≈ 17.0 Hz, 60 Hz Notch filter, 28–400 Hz Kaiser-Bessel Band Pass filter). The EMG signal was resampled at 1000 Hz then rectified and integrated (20 ms time constant) for scoring. Responses were scored as the peak-valley difference with the valley (average EMG response) occurring in the first 20 ms prior to the UCS and the peak occurring within the 21–150 ms window following the UCS (Blumenthal et al., 2005). Negative responses were scored as a zero.
Functional MRI
Structural and functional imaging was completed on a 3 Tesla Siemens Allegra scanner. High-resolution anatomical images (MPRAGE) were obtained in the sagittal plane using a T1 weighted series (TR=2300 ms, TE=3.9 ms, flip angle=12°, FOV=25.6 cm, matrix=256 × 256, slice thickness=1 mm, 0.5 mm gap) to serve as an anatomical reference. Blood oxygen level dependent fMRI of the entire brain was conducted using a gradient-echo echoplanar pulse sequence in an oblique-axial orientation (TR=2000 ms, TE=30 ms, flip angle=70°, FOV=24 cm, matrix=64 × 64, slice thickness=4 mm, no gap) during each block of stimulus presentations. Functional image processing was performed with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Echo-planar time series data were corrected for slice timing offset, motion corrected, concatenated, reregistered to the fifth volume of the first imaging block, and spatially blurred using a 4 mm full-width-at-half-maximum Gaussian filter.
Functional MRI data were analyzed at the individual subject level using the input from all stimuli in a multiple linear regression using a gamma variate hemodynamic response function. Regressors to account for brain activity not related to the UCR included reference waveforms for the CS+ and CS−, joystick movement, button presses, and head motion parameters. Head movement during the scanning session was assessed prior to any movement correction to the fMRI data. On average, less than one millimeter of movement occurred during the scanning session (mean ± SEM, 0.86 ± .05). Time points where 3% or more of the voxels across the brain were beyond the voxel-wise trend and median absolute deviation (i.e., outliers) of the time series were censored from the individual subject level analysis. On average, 1109 ± 2.1 of the 1124 total volumes acquired were included in the analysis. The regressors of interest used for the analyses in this study modeled the unconditioned fMRI signal response to UCS presentations during the CS+UCS and UCS alone. Analyses were restricted to voxels with sufficient signal quality by assigning a value of 0 to voxels with tSNR values below 30. Percent signal change was used as an index of the amplitude of the unconditioned fMRI signal response produced by the UCS. Functional maps reflecting percent signal change were converted to the Talairach and Tournoux stereotaxic coordinate system for group analyses (Talairach and Tournoux, 1988).
Given a priori hypotheses based on prior work (Dunsmoor et al., 2008; Kerr et al., 2012; Knight et al., 2010; Salomons et al., 2004; Wiech et al., 2006; Wood et al., 2012; 2013), the AFNI Talairach and Tournoux atlas was used to restrict group level analyses to the PFC, cingulate cortex, inferior parietal lobule (IPL), insula, amygdala, and hippocampus to reduce the number of voxel-wise comparisons. We conducted a repeated-measures ANOVA to test for a main effect of predictability (CS+UCS vs. UCS alone) and controllability (CC vs. UC), as well as a predictability x controllability interaction. Cluster threshold criteria were determined by Monte Carlo simulations which resulted in family-wise error (FWE) corrected significance threshold of p < 0.05 by rejecting small clusters of activation that are more likely to be produced by chance alone (Forman et al., 1995; Saad et al., 2006). The actual smoothness of the data was determined using AFNI’s 3dFWHMx for each participant, then calculating the mean FWHM value across participants. Criteria used for the AlphaSim program included the FWHM values (x, y, and z axes), voxel dimensions (in the acquired native space 3.75 × 3.75 × 4.00 mm3), anatomical ROI mask, and an uncorrected threshold of p < 0.005 for the volume correction simulations. Follow-up analyses consisted of paired t-test comparisons on the main effects revealed by the ANOVA. Fisher’s least significant difference (LSD) t-tests were conducted to assess the interaction effects revealed by the ANOVA. All follow-up contrasts were performed in SPSS on the mean percent signal change activation passing the significance threshold (p < 0.05 corrected) for the ANOVA.
A total of ten multiple linear regression analyses were completed to investigate the relationship between the unconditioned fMRI signal response from brain regions identified by the ANOVA (i.e., functional regions of interest; ROI) and behavior (i.e., UCS expectancy), psychophysiology, (SCR and EMG), and self-reported negative affected (i.e., state and trait). The regression analyses evaluated these relationships at p < 0.05 in SPSS using the averaged (CS+UCS and UCS alone) unconditioned fMRI signal response, as well as the difference (UCS alone – CS+UCS) in activation from the functional ROI as predictors for each outcome measure (i.e., behavior, psychophysiology, and negative affect). The analyses included all thirteen functional ROI (Tables 2 and 3) and were modeled separately for each outcome measure (i.e., state anxiety, trait anxiety, UCS expectancy, SCR, and EMG).
Table 2.
Regions that showed a diminished response to predictable threat.
| Region | Vol (mm3) | Talairach coordinates | CS+UCS – UCS alone | ||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | mean difference | ||
| Dorsolateral PFC | |||||
| Right | 647 | 31.7 | 0.2 | 57.3 | −0.14* |
| Left | 2970 | −33.6 | −0.3 | 52.6 | −0.12* |
| Left | 748 | −26.4 | 45.4 | 31.7 | −0.11* |
| Dorsomedial PFC | 11906 | 3.1 | 13.4 | 39.9 | −0.11* |
| Ventromedial PFC | |||||
| Right | 1922 | 7.5 | 38.8 | 7.0 | −0.09* |
| Left | 736 | −3.0 | 36.1 | 8.1 | −0.10* |
| Posterior Cingulate | 4948 | 2.1 | −33.7 | 29.8 | −0.09* |
Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass of areas of activation. Significance criteria: Brain areas revealed by the repeated measures ANOVA (F[1,52] > 8.60, p < 0.05, corrected). Follow-up paired t-tests were conducted on the mean fMRI signal (t[53] > 2.01, p < 0.05). Mean difference scores are shown, asterisk denotes significance (p < 0.0005).
Table 3.
Regions that showed a predictability x controllability interaction.
| Talairach coordinates | CS+UCS – UCS alone | CC – UC | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| mean difference | mean difference | |||||||
|
| ||||||||
| Region | Vol (mm3) | x | y | z | CC Group | UC Group | CS+UCS | UCS alone |
| Ventromedial PFC | ||||||||
| Bilateral | 364 | 2.0 | 39.6 | −12.7 | −0.14* | 0.07 | −0.13* | 0.07 |
| Hippocampus | ||||||||
| Right† | 46 | 31.1 | −26.8 | −8.8 | −0.08* | 0.03 | −0.07* | 0.04 |
| Left | 244 | −30.9 | −20.7 | −10.3 | −0.08* | 0.06 | −0.08* | 0.05 |
| Insula | ||||||||
| Right | 502 | 38.6 | −24.2 | 18.7 | −0.08 | 0.07 | −0.09 | 0.05 |
| Left | 488 | −40.1 | −13.9 | 15.9 | −0.08 | 0.03 | −0.06 | 0.05 |
| Left | 321 | −40.9 | −15.7 | −3.1 | −0.12 | 0.02 | −0.12 | 0.03 |
Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass of areas of activation.
Region met the ANOVA criteria for significance threshold (p < 0.005), but not volume threshold (112 mm3). Significance criteria: Brain areas revealed by the repeated measures ANOVA (F[1,52] > 8.60, p < 0.05, corrected). Fisher’s LSD t-tests were conducted on the mean fMRI signal to assess the interaction effect. Mean difference scores are shown, asterisk denotes significance (p < 0.05).
Results
UCS duration
Given that CC and UC participants were yoked, there were no differences in UCS duration between the groups. In addition, there were no differences in UCS duration between the CS+UCS (mean ± SEM: 3.51 ± 0.41; range = 0.5 – 6.0 s) and UCS alone (3.62 ± 0.40; range = 0.5 – 6.0 s; t[26] = −1.38, p = 0.18) trials.
UCS unpleasantness
Both the CC group (5.30 ± 0.39) and the UC group (5.80 ± 0.40) rated the UCS as unpleasant. There was no change in unpleasantness across the two conditioning blocks (F[1,52] = 1.87, p = .18). No group differences were observed (F[1,52] = 1.20, p = .28).
UCS expectancy
Repeated measures ANOVA revealed significant differences in UCS expectancy. Results showed a main effect for predictability (F[1,52] = 32.17, p < 0.001), but no main effect for controllability (F[1,52] = 0.03, p = 0.86) or predictability x controllability interaction (F[1,52] = 0.43, p = 0.51). UCS expectancy was greater on CS+UCS (75.55 ± 2.93) than on UCS alone (60.47 ± 3.56) trials for the CC group (t[26] = 3.22, p = 0.003). The UC group also showed greater UCS expectancy on CS+UCS (76.95 ± 2.95) than UCS alone (57.92 ± 2.70; t[26] = 5.04, p < 0.001) trials (Figure 2a).
Figure 2.
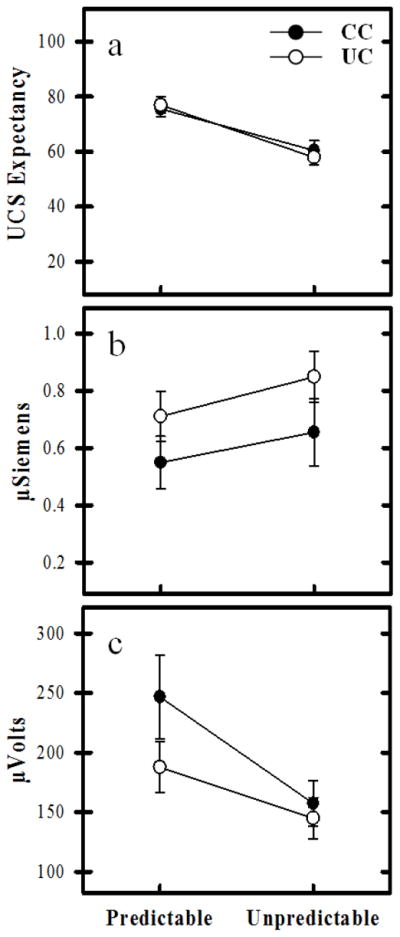
UCS expectancy, unconditioned SCR, and EMG response. a) Both the CC and UC groups demonstrated learning-related differences in UCS expectancy. UCS expectancy was higher to predictable (i.e., CS+UCS) than unpredictable (i.e., UCS alone) trials. b) Learning-related changes in unconditioned SCR expression were also observed for both the CC and UC groups. Unconditioned SCRs were diminished on predictable vs. unpredictable trials. c) Both groups demonstrated learning-related changes in the EMG response to the UCS. The EMG response was enhanced on predictable trials compared to unpredictable trials. No group differences were observed in UCS expectancy, unconditioned SCR, or EMG.
Skin conductance
Repeated measures ANOVA also revealed significant learning-related differences in unconditioned SCR expression. There was a main effect for predictability (F[1,52] = 15.71, p < 0.001), but no main effect for controllability (F[1,52] = 1.75, p = 0.19) or a predictability x controllability interaction (F[1,52] = 0.42, p = 0.52). T-test comparisons revealed a significantly diminished unconditioned SCR for CS+UCS trials (0.55 ± 0.09) compared to UCS alone trials (0.65 ± 0.12; t[26] = −2.23, p = 0.04) for CC participants. The same pattern was observed for UC participants. Unconditioned SCRs were diminished on CS+UCS trials (0.71 ± 0.09) compared to UCS alone trials (0.85 ± 0.09; t[26] = −3.44; p = 0.002) (Figure 2b).
Electromyography
Repeated measures ANOVA also revealed significant differences in the EMG response. There was a main effect for predictability (F[1,52] = 12.06, p = 0.001), but no main effect for controllability (F[1,52] = 1.53, p = 0.22) or predictability x controllability interaction (F[1,52] = 1.50, p = 0.23). T-test comparisons revealed a significantly enhanced EMG response on CS+UCS trials (246.70 ± 35.40) compared to UCS alone trials (157.25 ± 19.08; t[26] = 2.74, p = 0.01) for CC participants. The same pattern was also observed for the UC participants on CS+UCS trials (187.76 ± 21.69) compared to UCS alone trials (144.93 ± 17.27; t[26] = 2.18, p = 0.04) (Figure 2c).
Functional MRI
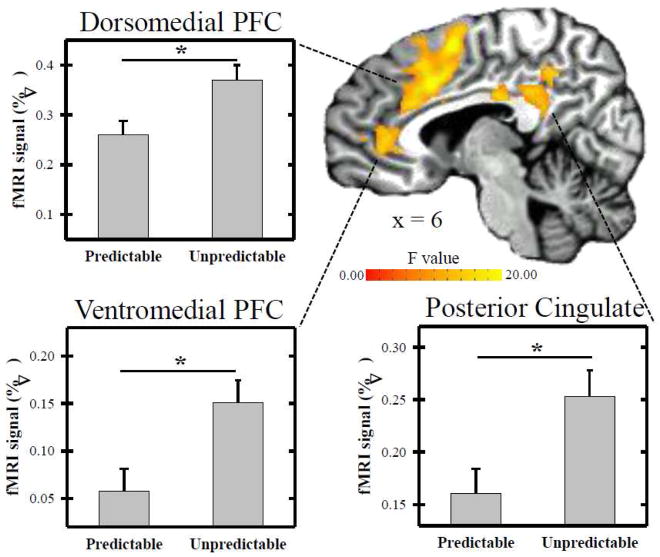
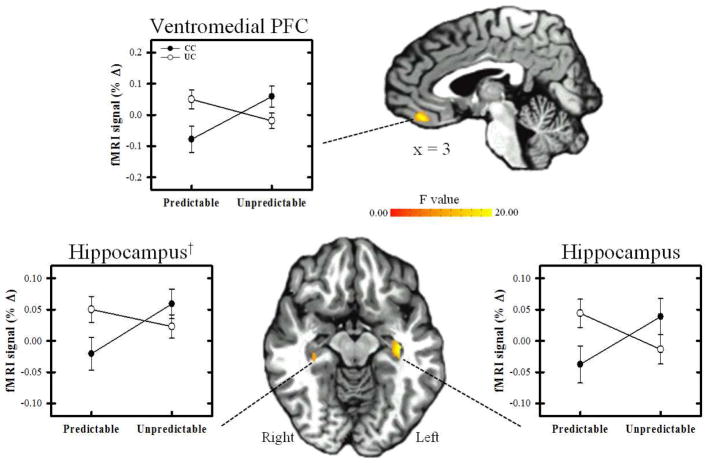
Repeated measures ANOVA revealed significant differences in the amplitude of the unconditioned fMRI signal response within several brain regions (Tables 2–3, Figures 3–4). A main effect for predictability was observed within the dorsolateral prefrontal cortex (PFC), dorsomedial PFC (dmPFC), vmPFC, and posterior cingulate cortex (PCC) (F[1,52] > 8.60; p < 0.05 FWE corrected). No clusters of activation were revealed for the main effect for controllability (F[1,52] < 8.60). A predictability x controllability interaction (F[1,52] > 8.60; p < 0.05 FWE corrected) was observed within the vmPFC, insula, and hippocampus. Specifically, the interaction effect was demonstrated within bilateral vmPFC, bilateral insula, and left hippocampus. The right hippocampus also showed an interaction effect that met the uncorrected significance threshold (p < 0.005) but did not meet the volume criteria for the corrected significance threshold (Figure 4). Because it is often difficult to acquire good EPI signal quality from ventral brain regions, we assessed tSNR values from the functional vmPFC activation (shown in Figure 4). On average, 96.1 ± 1.71% of the vmPFC activation (shown in Figure 4) had sufficient signal quality (i.e., tSNR values > 30). Thus, approximately 4% of the vmPFC activity contained voxels with low tSNR (< 30) and had been assigned a value of 0 prior to group level analyses. Follow-up contrasts were conducted in SPSS on the mean fMRI signal from each volume of activation revealed by the repeated measures ANOVA. Paired t-test comparisons were conducted for the main effect of stimulus type because these were within-subject contrasts. All regions showed a diminished UCR on CS+UCS vs. UCS alone trials (Table 2). Fisher’s LSD t-tests were used to assess the interaction effect and were completed for each volume of activation that showed a predictability x controllability interaction. These contrasts revealed a diminished UCR on CS+UCS vs. UCS alone trials for the CC group within the vmPFC, and hippocampus (Table 3). The fMRI signal response on CS+UCS vs. UCS alone trials for the UC group was not significantly different within in any of the ROI. In addition, the CC group showed a diminished UCR on CS+UCS trials compared to the UC group within the vmPFC and hippocampus (Table 3). No group differences were observed between the CC and UC group on UCS alone trials. No significant results were observed within the amygdala or IPL.
Figure 3.
Neural response to predictable and unpredictable threat. The fMRI signal response was diminished to predictable vs. unpredictable threat within several brain regions (see Table 2) including prefrontal cortex (PFC) and posterior cingulate. UCR amplitude within each of these brain areas was reduced on predictable (i.e., CS+UCS) compared to unpredictable (i.e., UCS alone) trials. Brain activation reflects the main effect for predictability revealed by the repeated-measures ANOVA. Graphs reflect the mean amplitude (% signal change) of all voxels within volumes of activation. Asterisk indicates significant difference.
Figure 4.
Regions that showed a predictability x controllability interaction. The unconditioned fMRI signal response within the ventromedial PFC and hippocampi were diminished on predictable trials compared to unpredictable trials for the CC group, but not the UC group. The unconditioned fMRI signal response was diminished on predictable trials for the CC group compared to the UC group (see Table 3). Graphs reflect the mean amplitude (% signal change) of all voxels within the volume of activation. †Right hippocampus met significance threshold (p < 0.005, uncorrected), but not volume threshold (volume > 112mm3) for multiple comparison correction.
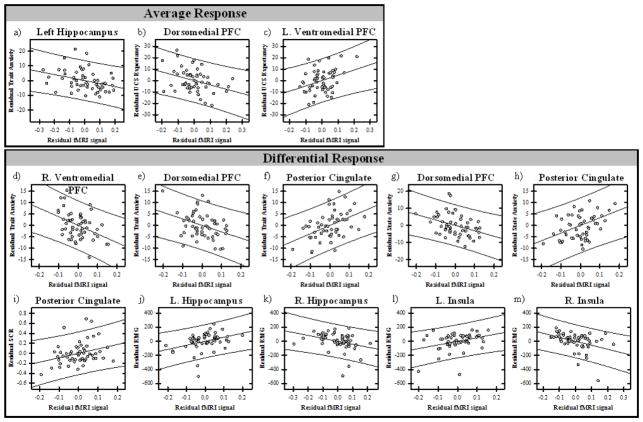
Multiple linear regression analyses were conducted on the averaged (CS+UCS and UCS alone) as well as the difference (UCS alone – CS+UCS) in unconditioned fMRI signal response. These analyses were restricted to the thirteen functional ROI identified from the ANOVA (Tables 2 and 3). The linear regression modeled all functional ROI as predictors for each outcome measure (i.e. state anxiety, trait anxiety, UCS expectancy, SCR, and EMG). The relationships were modeled separately for each measure with the averaged (i.e., CS+UCS and UCS alone) and difference scores (i.e., UCS alone – CS+UCS) for UCS expectancy, SCR, and EMG. Collinearity statistics indicated that multicollinearity was not an issue for the models (tolerance > .1; variance inflation factor < 10). The multiple linear regression analyses of the averaged responses revealed a significant negative relationship between left hippocampal activity and trait anxiety. Dorsomedial PFC showed a negative relationship to UCS expectancy, whereas the vmPFC showed a positive relationship to UCS expectancy (Table 4, Figure 5). The multiple linear regression analyses using difference scores (i.e., UCS alone – CS+UCS) revealed that differential dmPFC and vmPFC activity were negatively related to trait anxiety. In contrast, a positive relationship was observed between PCC and trait anxiety. A negative relationship was observed between differential dmPFC activity and state anxiety, whereas differential activation within the PCC was positively related to state anxiety. In addition, there was a positive relationship between differential PCC activity and SCR. Finally, the insula and hippocampal activity within the right hemisphere showed a negative relationship with the EMG response, whereas the insula and hippocampal activity within the left hemisphere showed a positive relationship to the EMG response (Table 4, Figure 5).
Table 4.
Regions that showed relationships with state anxiety, trait anxiety, and psychophysiology.
| FUNCTIONAL ROI | AVERAGE (CS+UCS and UCS alone) | DIFFERENCE (UCS alone – CS+UCS) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Region | State Anxiety | Trait Anxiety | UCS Expectancy | SCR | EMG | State Anxiety | Trait Anxiety | UCS Expectancy | SCR | EMG |
|
| ||||||||||
| β(p) | β(p) | β(p) | β(p) | β(p) | β(p) | β(p) | β(p) | β(p) | β(p) | |
| Dorsolateral PFC | ||||||||||
| Right | −.20(.32) | −.32(.14) | −.07(.75) | .19(.40) | .03(.90) | .02(.92) | −.23(.27) | −.36(.19) | −.08(.77) | −.30(.23) |
| Left | −.17(.55) | −.18(.55) | .39(.18) | −.11(.72) | .18(.45) | −.31(.34) | −.12(.72) | −.01(.98) | −.13(.73) | .07(.84) |
| Left | .11(.62) | .18(.44) | .15(.51) | .17(.48) | .05(.82) | .27(.19) | .24(.19) | .04(.88) | .00(.99) | .07(.74) |
| Dorsomedial PFC | 0.6(.85) | −.01(.96) | −.66(.03*) | −.53(.09) | .12(.70) | −.69(.02*) | −.61(.02*) | .25(.46) | −.29(.38) | .03(.92) |
| Ventromedial PFC | ||||||||||
| Right | .43(.29) | −.16(.70) | −.45(.28) | −.09(.84) | .00(.99) | −.02(.94) | −.72(.007*) | .34(.32) | −.06(.86) | −.06(.83) |
| Left | −.24(.48) | .08(.81) | .76(.03*) | .00(.99) | −.10(.79) | .28(.28) | .38(.09) | −.38(.21) | .06(.83) | .07(.78) |
| Bilateral | .01(.96) | .11(.48) | −.02(.89) | −.13(.41) | −.26(.11) | −.27(.10) | −.26(.07) | −.09(.62) | .17(.38) | −.19(.27) |
| Insula | ||||||||||
| Right | .27(.14) | .00(.99) | −.18(.33) | −.24(.21) | .19(.32) | −.97(.34) | .02(.90) | .10(.67) | −.23(.37) | −.61(.009*) |
| Left | −.29(.17) | −.23(.20) | .41(.06) | .24(.28) | .16(.48) | .05(.79) | .01(.94) | −.11(.60) | .02(.92) | .43(.03*) |
| Left | −.12(.58) | .06(.78) | .24(.26) | −.02(91) | −.37(.11) | .38(.10) | .08(.69) | .19(.48) | −.29(.28) | −.33(18) |
| Posterior Cingulate | .51(.12) | .50(.15) | −.21(.53) | .22(.53) | −.21(.56) | .63(.009*) | .68(.002*) | −.01(.97) | .55(.05*) | .49(.052) |
| Hippocampus | ||||||||||
| Right | .23(.24) | .36(.08) | −.24(.23) | −.08(.70) | −.06(.77) | −.09(.56) | −.10(.48) | .18(.33) | −.13(.49) | −.37(.03*) |
| Left | −.17(.30) | −.38(.03*) | −.05(.74) | .09(.60) | −.02(.89) | −.26(.17) | −.02(.92) | −.43(.06) | .26(.26) | .49(.02*) |
Figure 5.
Partial regression plots of relationships between threat-elicited brain activity, psychophysiology, and state and trait anxiety. Multiple linear regression analyses between the data from functional ROI and each behavioral measure were assessed using the average (CS+UCS and UCS alone) and differential (UCS alone – CS+UCS) response (Table 4). Graphs depict brain (x axis) and behavioral (y axis) residuals resulting from the regression analyses. Average response: Hippocampal activity varied with trait anxiety (a), while both dmPFC and vmPFC activity varied with UCS expectancy (b and c). Differential Response: dmPFC, vmPFC, and PCC activity varied with trait anxiety (d – f), the dmPFC and PCC also varied with state anxiety (g and h). PCC activity also varied with SCR (i), while hippocampal and insula activity varied with EMG (j–m).
Discussion
Resilience to stress is an important aspect of healthy emotional functioning, thus determining the neural mechanisms of stress resilience may provide important insights for improving emotional well-being. Resilience to stress is mediated, in part, by our ability to predict and control impending threats in our environment (Chorpita and Barlow, 1998; Foa et al., 1992; Maier and Seligman, 1976). Therefore, determining the human brain response to threats that vary in their predictability and controllability may provide important insights into neural processes that mediate resilience to stress. Findings from the present study demonstrate vmPFC and hippocampal activity varies with the predictability and controllability of threats. Given that the vmPFC and hippocampus are central components of the neural circuit that regulates emotion (Amat et al., 1998; Baratta et al., 2007; 2008; Hartley and Phelps, 2010; Milad et al., 2007; 2009; Rauch et al., 2006; Schiller et al., 2013), these findings suggest the ability to predict and control threat has an important impact on vmPFC and hippocampal function that may play a key role in emotional resilience.
Findings from the present study demonstrate diminished vmPFC and hippocampal activity to threats that were both predictable and controllable. In contrast, greater vmPFC and hippocampal activity was observed to threats that were not predictable or were not controllable (i.e., unpredictable-controllable, unpredictable-uncontrollable, and predictable-uncontrollable threats). Specifically, we observed diminished neural activity within the vmPFC and hippocampus to controllable threats that were predictable compared to controllable threats that were unpredictable. Further, these brain regions showed a diminished response to predictable threats that were controllable compared to predictable threats that were uncontrollable (Figure 4). In contrast to predictable trials, controllability did not influence threat-elicited activity within the vmPFC or hippocampus when threats were unpredictable. More specifically, no differences were observed in vmPFC and hippocampal activity to unpredictable threats regardless of whether the threat could be controlled or not. Further, the response to uncontrollable threat was similar on predictable and unpredictable trials. Taken together, these findings indicate controllability modulates vmPFC and hippocampal activity to predictable, but not unpredictable threat. Thus, the impact of threat controllability on the fMRI signal response was specific to predictable threat in the present study. Although an interaction effect was revealed within the vmPFC and hippocampus, a similar result was not observed in our psychophysiological data. The lack of a psychophysiological correlate to the activity observed within the vmPFC and hippocampus complicates the interpretation of the experimental manipulation of control. More specifically, the current results suggest controllability did not impact the peripheral emotional response (i.e., SCR and EMG) in the same manner that controllability modulated vmPFC and hippocampal activity. Although, SCR and EMG did show a main effect for threat predictability that was similar to the effects seen within other regions of the PFC (Table 2), the fact that these psychophysiological measures did not show an interaction effect leaves unanswered questions regarding the impact of vmPFC and hippocampal activation on the peripheral emotional response in the present study.
The current findings indicate threat predictability and controllability interact to affect brain activity within the vmPFC and hippocampus. These results are consistent with animal model research that suggests behavioral control over a threat is encoded within the vmPFC (Amat et al., 2008; Baratta et al., 2007; Maier et al., 2006). For example, prior research has demonstrated behavioral control over threat interferes with subsequent fear conditioning, whereas exposure to an uncontrollable threat results in an enhanced fear response (Baratta et al., 2007; Maier et al., 2006). This prior work indicates the vmPFC (Baratta et al., 2009) and hippocampus (Amat et al., 1998) mediate the stress-reducing effects that benefit an organism that has control over a threat. For example, prior experience with controllable threat alters vmPFC function and, in turn, regulates the amygdala-dependent emotional response to future threats (Amat et al., 2008; Baratta et al., 2007; 2008; Maier et al., 2006). Thus, the vmPFC and hippocampus appear to mediate the emotional resilience to stress (e.g., reduced emotional response to threat) that develops when we can control an impending threat.
Prior animal model research suggests that increased vmPFC activity is required to inhibit the emotional response to controllable threat (Amat et al., 2005; Baratta et al., 2008). However, we observed a lower fMRI signal response within the vmPFC on predictable-controllable trials in the present study. Thus, the current findings may initially appear somewhat inconsistent with prior animal model work. However, formal learning theory suggests that as an association between the CS and UCS is learned, the UCR (i.e., the threat-elicited response in the current study) should decrease (Rescorla and Wagner, 1972; Rescorla, 1988). More specifically, the CS acquires properties of the UCS, and as the CS-UCS association is learned, the CR increases, while the UCR decreases (see also Domjan, 2005). Therefore, the threat-elicited response in the present study would be expected to show an opposite pattern to the CR elicited by the CS. This phenomenon is generally referred to as conditioned UCR diminution, and prior work from our lab has demonstrated that as the anticipatory CR increases within the dmPFC, the UCR (i.e., threat-elicited response) within the vmPFC decreases (Wood et al., 2012). The present study suggests that this effect is mediated to some degree by an individual’s ability to control the threat. Although, prior animal model work suggests an increase in vmPFC activity inhibits the emotional response to controllable threat, this prior work was not focused on identifying UCR specific processes. More specifically, prior animal model research on threat controllability has either focused on vmPFC function in anticipation of threat (Baratta et al., 2008) or was not designed to disentangle anticipatory (i.e., the CR) from threat-specific (i.e., the UCR) responses (Amat et al., 2005). Thus, the increased vmPFC activity in prior work may reflect behavioral control processes elicited by a context or CS presentation (i.e., the CR), rather than a discrete response to the threat itself (i.e., the UCR). In contrast, the present study specifically evaluated the threat-elicited response (i.e., the UCR), and demonstrates that the response is diminished when threats are both predictable and controllable.
Dysfunction of vmPFC and hippocampal circuitry may mediate symptoms of disinhibition that characterize anxiety-related disorders. Post-traumatic stress disorder (PTSD), for example, is characterized by symptoms that include re-experiencing, hyperarousal, and avoidance behavior triggered by a life threatening traumatic event beyond one’s control (American Psychiatric Association, 2000). The hyperarousal often observed in PTSD appears to be due to insufficient top-down regulatory control that results in hypersensitivity of subcortical brain areas (e.g., the amygdala) (Briscione et al., 2014; Jovanovic and Ressler, 2010; Milad et al., 2009; Rauch et al., 2006; Robison-Andrew et al., 2014). Further, PTSD is often associated with hypoactivation of the vmPFC and hippocampus (Bremner et al., 2004; Haas et al., 2010; Milad et al., 2009; Rauch et al., 2006). Thus, dysfunction of top-down regulatory processes that are supported by vmPFC and hippocampal projections to the amygdala appear to mediate key symptoms of PTSD (Birn et al., 2014; Gilmartin et al., 2014; Hartley and Phelps, 2010; Sripada et al., 2012). In addition, PTSD symptom severity is greater among individuals that believe they lack control over daily life stressors (Benight and Bandura, 2004; Bolstad and Zinbarg, 1997; Freh et al., 2013; Palyo and Beck, 2005; Tsay et al., 2001). Given the findings from the present study, the perceived lack of control PTSD patients experience may further disrupt vmPFC and hippocampal function, and result in susceptibility to stress (Birn et al., 2014; Etkin and Wager, 2007; Koch et al., 2014; Maier and Watkins, 2010). Thus, learning-related processes that normally support healthy emotion regulation may instead disrupt regulatory functions and increase susceptibility to stress in anxiety disorder patients.
In the current study, both negative and positive relationships were observed between the differential EMG response and threat-elicited responses within the insula and hippocampus. More specifically, negative relationships were observed within the right hemisphere, and positive relationships were observed within the left hemisphere. Although, we did not expect to observe hemispheric differences in these relationships, there is prior research that suggests that emotion-related processes are asymmetrically represented within the human brain. For example, emotion studies have demonstrated a relationship between right hemisphere activity and unpleasant emotion, whereas left hemisphere activity varied with pleasant emotion (Canli et al., 1998; Craig, 2009; Maxwell and Davidson, 2007; Rutherford and Lindell, 2011; Wager et al., 2003). Further, hemisphere specific relationships previously observed have been associated with SCR (Cheng et al., 2003; Critchley et al., 2000; 2005; Dunsmoor and Labar, 2012; Knight et al., 2005; Ohira et al., 2006; Phelps et al., 2001), startle EMG (Anders et al., 2004; Klumpers et al., 2010; Kumari et al., 2008), pain (Brooks et al., 2002; Craig, 2002, 2003; Watson et al., 2009), and the cardiac response (Critchley et al., 2000; Critchley, 2005). Prior electroencephalograph (EEG) research has also demonstrated hemisphere specific relationships (Avram et al., 2010; Goodman et al., 2013; Jackson et al., 2003; Master et al., 2009; Wiedemann et al., 1999). These converging lines of evidence posit that the right hemisphere is associated with arousal and aversive behavior, whereas the left hemisphere is associated with rest and appetitive behavior (Craig, 2002; Critchley et al., 2000; 2001; Goodman et al., 2013; Jackson et al., 2003; Maxwell and Davidson, 2007). However, these prior studies did not show differentially signed relationships within the left compared to right hemisphere. While the current data are generally consistent with prior work showing hemisphere specific relationships, we must also take into consideration that the current relationships were observed with EMG while taking activity from other brain areas into account in the statistical model. More specifically, the startle EMG relationships with the bilateral hippocampus and insula were the result of a multiple linear regression that included activity from all other active brain regions (Tables 2 and 3). In contrast, prior work has primarily utilized simple correlation to assess relationships between brain activity and the physiological response (Burghy et al., 2012; Cheng et al., 2003; Craig, 2002, 2003; Critchley et al., 2000; Critchley, 2005; Dunsmoor and Labar, 2012; Jackson et al., 2003; Klumpers et al., 2010; Napadow et al., 2013; Nugent et al., 2011). However, in the present study, the observed effects do not appear with simple correlation analyses. Instead, the relationships between startle EMG and threat-elicited brain activity within the hippocampus and insula were only observed when included in a multiple regression analysis, suggesting these relationships may be disguised when activation from other brain regions is not taken into account. In summary, the present findings are generally in line with research that suggests emotion processes are asymmetrically represented within the human brain.
A dissociation between the SCR and EMG response was observed in the current study, however prior work suggests that changes in these psychophysiological responses measure distinct aspects of emotional behavior (Anders et al., 2004; Benedek and Kaernbach, 2010; Davis et al., 1993; 2006; Grillon, 2002; Knight et al., 2010; 2011; Lang et al., 1998; Neuner et al., 2010; Piché et al., 2010). Thus, it is not unusual to observe different patterns or even opposite patterns between these psychophysiological measures (Piché et al., 2010; Weike et al., 2005). For example, the enhanced EMG response observed to predictable threat in the current study is similar to fear-potentiated startle findings (Grillon et al., 1993; 2004; 2011; van Well et al., 2012), whereas the diminished threat-elicited SCR to predictable threat is consistent with prior conditioned UCR diminution research (Dunsmoor et al., 2008; Knight et al., 2010; 2011; Wood et al., 2012; 2013). Thus, the present findings are consistent with prior conditioning research that has demonstrated different response patterns in SCR and EMG responses (Grillon and Ameli, 2001; Weike et al., 2005).
In the present study, the comparison of both predictability and controllability is an important strength. In addition, we have operationalized predictability in terms of whether or not a signal preceded the threat and assessed the threat-elicited response to both controllable and uncontrollable threat. Further, participants were exposed to controllable compared to uncontrollable threat. For example, participants in the CC group controlled the duration of the UCS, whereas participants in the UC group were unable to control the duration of threat. Although the CC group was informed that they could control (i.e., terminate) the UCS with a single button press, they were not given explicit instructions regarding if or when to terminate the UCS. Participants that asked for further instruction about the duration of the UCS (i.e., whether or not they should terminate the UCS; or when to terminate the UCS) were told to decide for themselves, and were reminded the UCS would last 6 seconds if not terminated. Participants were not instructed to terminate the UCS at a specific time given the effect those instructions could have on the manipulation of control. As a result, UCS duration varied across the entire possible range of 0.5 – 6.0 seconds. On average, UCS duration was 3.59 seconds and did not differ between predictable (3.51 seconds) and unpredictable (3.62 seconds) trials. These data suggest participants were generally motivated to terminate the UCS, and indicate the decision to terminate was not influenced by UCS predictability. However, participants’ motivation to terminate the UCS certainly varied from person-to-person. It is possible that a more aversive UCS (e.g., greater intensity or duration) may have motivated participants’ to terminate the UCS more quickly. However, unpleasantness ratings did not vary with UCS duration in the present study, suggesting the decision of when to terminate was not simply based on the perceived aversiveness of the UCS.
In summary, the ability to predict and control threats within one’s environment is a critical aspect of emotional resilience (Chorpita and Barlow, 1998; Foa et al., 1992; Kerr et al., 2012; Maier and Seligman, 1976; McNally et al., 2011; Wood et al., 2012), and converging lines of evidence suggest the vmPFC and hippocampus play a key role in the emotion regulation process (Franklin et al., 2012; Gilmartin et al., 2014; Hartley and Phelps, 2010; Maren, 2014; Milad et al., 2007; Russo et al., 2012). Thus, these brain regions appear to mediate functions that are important for stress resilience. Therefore, determining how the vmPFC and hippocampus respond to the predictability and controllability of threat provides important insight into neural processes that mediate emotion regulation and stress resilience. To our knowledge, this is the first neuroimaging study to investigate the impact of threat predictability and controllability on human brain activity using methods translated from traditional animal model behavioral neuroscience research. In this study, vmPFC and hippocampal activity was only diminished when threats were both predictable and controllable. In contrast, the response within these brain regions was relatively enhanced when threats were unpredictable or uncontrollable. Given that chronic exposure to unpredictable and uncontrollable threat is an important trigger for anxiety disorders (Chorpita and Barlow, 1998; Foa et al., 1992; Maier and Seligman, 1976), the present findings suggest the vmPFC and hippocampus are important components of the neural circuitry that mediates stress resilience and healthy emotional functioning.
Highlights.
Neural correlates of threat predictability and controllability studied using fMRI.
vmPFC and hippocampal activity decreased during predictable-controllable threat.
vmPFC and hippocampus may mediate important aspects of stress resilience.
Acknowledgments
This research was supported by National Institutes of Health grant MH098348 (DCK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res. 1998;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N. Brain activity underlying emotional valence and arousal: a response-related fMRI study. Hum Brain Mapp. 2004;23:200–209. doi: 10.1002/hbm.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram J, Balteş FR, Miclea M, Miu AC. Frontal EEG activation asymmetry reflects cognitive biases in anxiety: evidence from an emotional face Stroop task. Appl Psychophysiol Biofeedback. 2010;35:285–292. doi: 10.1007/s10484-010-9138-6. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Lucero TR, Amat J, Watkins LR, Maier SF. Role of the ventral medial prefrontal cortex in mediating behavioral control-induced reduction of later conditioned fear. Learn Mem. 2008;15:84–87. doi: 10.1101/lm.800308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur J Neurosci. 2009;30:1111–1116. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Kaernbach C. Decomposition of skin conductance data by means of nonnegative deconvolution. Psychophysiology. 2010;47:647–658. doi: 10.1111/j.1469-8986.2009.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benight CC, Bandura A. Social cognitive theory of posttraumatic recovery: the role of perceived self-efficacy. Behav Res Ther. 2004;42:1129–1148. doi: 10.1016/j.brat.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress Anxiety. 2014;31:880–892. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bolstad BR, Zinbarg RE. Sexual victimization, generalized perception of control, and posttraumatic stress disorder symptom severity. J Anxiety Disord. 1997;11:523–540. doi: 10.1016/s0887-6185(97)00028-5. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Briscione MA, Jovanovic T, Norrholm SD. Conditioned fear associated phenotypes as robust, translational indices of trauma-, stressor-, and anxiety-related behaviors. Frontiers in psychiatry. 2014;5:1–9. doi: 10.3389/fpsyt.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JCW, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, Birn RM. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JD. Hemispheric asymmetry for emotional stimuli detected with fMRI. Neuroreport. 1998;9:3233–3239. doi: 10.1097/00001756-199810050-00019. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: Stimulus processing versus response expression. Behav Neurosci. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Barlow DH. The development of anxiety: the role of control in the early environment. Psychol Bull. 1998;124:3–21. doi: 10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- Cook EW, 3rd, Miller AM. Methodology Digital Filtering: Background and Tutorial for Psychophysiologists. Psychophysiology. 1992;29:350–367. doi: 10.1111/j.1469-8986.1992.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Brain activity during biofeedback relaxation: a functional neuroimaging investigation. Brain. 2001;124:1003–1012. doi: 10.1093/brain/124.5.1003. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2009;35:1–31. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domjan M. Pavlovian conditioning: a functional perspective. Annu Rev Psychol. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage. 2008;40:811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Labar KS. Brain activity associated with omission of an aversive event reveals the effects of fear learning and generalization. Neurobiol Learn Mem. 2012;97:301–312. doi: 10.1016/j.nlm.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Freh FM, Chung MC, Dallos R. In the shadow of terror: posttraumatic stress and psychiatric co-morbidity following bombing in Iraq: the role of shattered world assumptions and altered self-capacities. J Psychiatr Res. 2013;47:215–225. doi: 10.1016/j.jpsychires.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Balderston NL, Helmstetter FJ. Prefrontal cortical regulation of fear learning. Trends Neurosci. 2014:1–10. doi: 10.1016/j.tins.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RN, Rietschel JC, Lo L-C, Costanzo ME, Hatfield BD. Stress, emotion regulation and cognitive performance: the predictive contributions of trait and state relative frontal EEG alpha asymmetry. Int J Psychophysiol. 2013;87:115–123. doi: 10.1016/j.ijpsycho.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Merikangas K, Woods SW, Davis M. Measuring the time course of anticipatory anxiety using the fear-potentiated startle reflex. Psychophysiology. 1993;30:340–346. doi: 10.1111/j.1469-8986.1993.tb02055.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R. Conditioned inhibition of fear-potentiated startle and skin conductance in humans. Psychophysiology. 2001;38:807–815. [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Heller R, Hirschhorn E, Kling Ma, Pine DS, Schulkin J, Vythilingam M. Acute hydrocortisone treatment increases anxiety but not fear in healthy volunteers: a fear-potentiated startle study. Biol Psychiatry. 2011;69:549–555. doi: 10.1016/j.biopsych.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grös DF, Antony MM, Simms LJ, McCabe RE. Psychometric properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA): Comparison to the State-Trait Anxiety Inventory (STAI) Psychol Assess. 2007;19:369–381. doi: 10.1037/1040-3590.19.4.369. [DOI] [PubMed] [Google Scholar]
- Haas BW, Garrett A, Song S, Reiss AL, Carrio VG. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an fMRI study. J Pediatr Psychol. 2010;35:559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, Rosenkranz Ma, Ryff CD, Singer BH, Davidson RJ. Now you feel it, now you don’t: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol Sci. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DL, McLaren DG, Mathy RM, Nitschke JB. Controllability modulates the anticipatory response in the human ventromedial prefrontal cortex. Front Psychol. 2012;3:1–11. doi: 10.3389/fpsyg.2012.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Raemaekers MAHL, Ruigrok ANV, Hermans EJ, Kenemans JL, Baas JMP. Prefrontal mechanisms of fear reduction after threat offset. Biol Psychiatry. 2010;68:1031–1038. doi: 10.1016/j.biopsych.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, King MK, Bandettini PA. Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage. 2010;49:843–848. doi: 10.1016/j.neuroimage.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Lewis EP, Wood KH. Conditioned diminution of the unconditioned skin conductance response. Behav Neurosci. 2011;125:626–631. doi: 10.1037/a0024324. [DOI] [PubMed] [Google Scholar]
- Knight DC, Wood KH. Investigating the neural mechanisms of aware and unaware fear memory with FMRI. Journal of visualized experiments. 2011:1–6. doi: 10.3791/3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: salience processing and fear inhibition processes. Psychoneuroendocrinology. 2014;40:242–256. doi: 10.1016/j.psyneuen.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Kumari V, Fannon D, Geyer MA, Premkumar P, Antonova E, Simmons A, Kuipers E. Cortical grey matter volume and sensorimotor gating in schizophrenia. Cortex. 2008;44:1206–1214. doi: 10.1016/j.cortex.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biol Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman ME. Learned helplessness: Theory and evidence. J Exp Psychol Gen. 1976;105:3–46. [Google Scholar]
- Maier SF. Stressor controllability and stress-induced analgesia. Ann N Y Acad Sci. 1986;467:55–72. doi: 10.1111/j.1749-6632.1986.tb14618.x. [DOI] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin Neurosci. 2006;8:397–407. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Fear of the unexpected: hippocampus mediates novelty-induced return of extinguished fear in rats. Neurobiol Learn Mem. 2014;108:88–95. doi: 10.1016/j.nlm.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master SL, Amodio DM, Stanton AL, Yee CM, Hilmert CJ, Taylor SE. Neurobiological correlates of coping through emotional approach. Brain Behav Immun. 2009;23:27–35. doi: 10.1016/j.bbi.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JS, Davidson RJ. Emotion as motion: asymmetries in approach and avoidant actions. Psychol Sci. 2007;18:1113–1119. doi: 10.1111/j.1467-9280.2007.02033.x. [DOI] [PubMed] [Google Scholar]
- McNally GP, Johansen JP, Blair HT. Placing prediction into the fear circuit. Trends Neurosci. 2011;34:283–292. doi: 10.1016/j.tins.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Kihlstrom JF. Unpredictable and uncontrollable events: a new perspective on experimental neurosis. J Abnorm Psychol. 1978;87:256–271. doi: 10.1037//0021-843x.87.2.256. [DOI] [PubMed] [Google Scholar]
- Mineka S, Hendersen RW. Controllability and predictability in acquired motivation. Annual Reviews of Psychology. 1985;36:495–529. doi: 10.1146/annurev.ps.36.020185.002431. [DOI] [PubMed] [Google Scholar]
- Napadow V, Lee J, Kim J, Cina S, Maeda Y, Barbieri R, Harris RE, Kettner N, Park K. Brain correlates of phasic autonomic response to acupuncture stimulation: an event-related fMRI study. Hum Brain Mapp. 2013;34:2592–2606. doi: 10.1002/hbm.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner I, Stöcker T, Kellermann T, Ermer V, Wegener HP, Eickhoff SB, Schneider F, Shah NJ. Electrophysiology meets fMRI: neural correlates of the startle reflex assessed by simultaneous EMG-fMRI data acquisition. Hum Brain Mapp. 2010;31:1675–1685. doi: 10.1002/hbm.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin NH. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Bain EE, Thayer JF, Sollers JJ, Drevets WC. Sex differences in the neural correlates of autonomic arousal: a pilot PET study. Int J Psychophysiol. 2011;80:182–191. doi: 10.1016/j.ijpsycho.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, Fukuyama S, Nakajima T, Yamada J. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Palyo SA, Beck JG. Post-traumatic stress disorder symptoms, pain, and perceived life control: associations with psychosocial and physical functioning. Pain. 2005;117:121–127. doi: 10.1016/j.pain.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Connor KJO, Gatenby JC, Gore JC, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Piché M, Arsenault M, Rainville P. Dissection of perceptual, motor and autonomic components of brain activity evoked by noxious stimulation. Pain. 2010;149:453–462. doi: 10.1016/j.pain.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rescorla R, Wagner A. In: A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. Black AH, Prokasy WF, editors. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Robison-Andrew EJ, Duval ER, Nelson CB, Echiverri-Cohen A, Giardino N, Defever A, Norrholm SD, Jovanovic T, Rothbaum BO, Liberzon I, Rauch SAM. Changes in trauma-potentiated startle with treatment of posttraumatic stress disorder in combat Veterans. J Anxiety Disord. 2014;28:358–362. doi: 10.1016/j.janxdis.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF. Uncontrollable, but not controllable, stress desensitizes 5-HT1A receptors in the dorsal raphe nucleus. J Neurosci. 2011;31:14107–14115. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han M-H, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJV, Lindell AK. Thriving and Surviving: Approach and Avoidance Motivation and Lateralization. Emot Rev. 2011;3:333–343. [Google Scholar]
- Saad ZS, Chen G, Reynolds RC, Christidis PP, Hammett KR, Bellgowan PSF, Cox RW. Functional imaging analysis contest (FIAC) analysis according to AFNI and SUMA. Hum Brain Mapp. 2006;27:417–424. doi: 10.1002/hbm.20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja M-M, Davidson RJ. Perceived controllability modulates the neural response to pain. J Neurosci. 2004;24:7199–7203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja M-M, Shackman AJ, Davidson RJ. Individual differences in the effects of perceived controllability on pain perception: critical role of the prefrontal cortex. J Cogn Neurosci. 2007;19:993–1003. doi: 10.1162/jocn.2007.19.6.993. [DOI] [PubMed] [Google Scholar]
- Schiller D, Kanen JW, LeDoux JE, Monfils M-H, Phelps EA. Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc Natl Acad Sci U S A. 2013;110:20040–20045. doi: 10.1073/pnas.1320322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory for Adults. Redwood City, CA: Mind Garden; 1983. [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I. Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Tsay SL, Halstead MT, McCrone S. Predictors of coping efficacy, negative moods and post-traumatic stress syndrome following major trauma. Int J Nurs Pract. 2001;7:74–83. doi: 10.1046/j.1440-172x.2001.00257.x. [DOI] [PubMed] [Google Scholar]
- van Well S, Visser RM, Scholte HS, Kindt M. Neural substrates of individual differences in human fear learning: Evidence from concurrent fMRI, fear-potentiated startle, and US-expectancy data. Cogn Affect Behav Neurosci. 2012;12:499–512. doi: 10.3758/s13415-012-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt Ba, Nadeau V, Jones AKP. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145:24–30. doi: 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weike AI, Hamm AO, Schupp HT, Runge U, Schroeder HWS, Kessler C. Fear conditioning following unilateral temporal lobectomy: dissociation of conditioned startle potentiation and autonomic learning. J Neurosci. 2005;25:11117–11124. doi: 10.1523/JNEUROSCI.2032-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ. Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci. 2006;26:11501–11509. doi: 10.1523/JNEUROSCI.2568-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, Buchkremer G. Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Arch Gen Psychiatry. 1999;56:78–84. doi: 10.1001/archpsyc.56.1.78. [DOI] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, Knight DC. Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. Neuroimage. 2012;60:787–799. doi: 10.1016/j.neuroimage.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Wood KH, Kuykendall D, Ver Hoef LW, Knight DC. Neural substrates underlying learning-related changes of the unconditioned fear response. The Open Neuroimaging Journal. 2013;7:41–52. doi: 10.2174/1874440001307010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, Knight DC. The amygdala mediates the emotional modulation of threat-elicited skin conductance response. Emotion. 2014;14:693–700. doi: 10.1037/a0036636. [DOI] [PMC free article] [PubMed] [Google Scholar]