Abstract
An emerging aspect of neuronal–glial interactions is the connection glial cells have to synapses. Mounting research now suggests a far more intimate relationship than previously recognized. Moreover, the current evidence implicating synapse loss in neurodegenerative disease etiology is overwhelming, but the role of glia in the process of synaptic degeneration has only recently been considered in earnest. Each main class of glial cell, including astrocytes, oligodendrocytes, and microglia, performs crucial and multifaceted roles in the maintenance of synaptic function and excitability. As such, aging and/or neuronal stress from disease-related misfolded proteins may involve disruption of multiple non-cell-autonomous synaptic support systems that are mediated by neighboring glia. In addition, glial cell activation induced by injury, ischemia, or neurodegeneration is thought to greatly alter the behavior of glial cells toward neuronal synapses, suggesting that neuroinflammation potentially contributes to synapse loss primarily mediated by altered glial functions. The present review discusses recent evidence highlighting novel roles for glial cells at neuronal synapses and in the maintenance of neuronal connectivity, focusing primarily on their implications for neurodegenerative disease research.
Keywords: synapse, glia, neurodegeneration
Introduction
The functions of the mammalian central nervous system (CNS) depend on appropriate cell signaling mediated by neuronal synapses. This observation has been a cornerstone of brain research since the neuroanatomical work of Santiago Ramon y Cajal led to the development of the neuronal doctrine over a century ago. Synapses are highly specialized structures that have evolved to allow neurons to communicate with each other via the electrochemical release of neurotransmitter molecules. This gives rise to the formation of neuronal circuits and networks that cooperate, support, and actively maintain one another by means of continued signaling events.1 As such, synapses represent arguably the most important ultrastructural elements in the CNS. In support of this assertion is the well-established observation that in several neurodegenerative disorders, including Alzheimer's disease (AD) and Huntington's disease (HD), cognitive decline correlates strongly with synapse loss.2,3 Consequently, there has been a great deal of research interest into the underlying mechanisms of synapse loss, largely focusing on processes that occur within or between axons and dendrites, the neuronal components of synapses. More recently, major roles for glial cells—including astrocytes, microglia, and oligodendrocytes—at synapses during development and adulthood have been elucidated.4,5,52 Coupled to the reported role of glial cells as essential cellular arbitrators in neurological diseases through non-cell-autonomous mechanisms,6 these discoveries are provoking interest into the notion that synapse integrity and loss may be less cell autonomous than are currently recognized. In this review we highlight recent insights into how glia influence neuronal circuits and synapses in the developing and adult brain. We also discuss evidence for how each glial cell type might influence synaptic changes observed in neurodegenerative disease.
Synapses and astrocytes
Astrocytes have long been thought of merely as passive support cells. But over the past decade, data emerged demonstrating that astrocytes possess fundamental roles in mediating synapse formation and function.4 This concept first gained prominence after reports that purified retinal ganglion cell (RGC) cultures that lack glia form tenfold fewer excitatory synapses than RGCs cultured on an astrocyte feeder layer or with astrocyte-conditioned medium.7 Later work recapitulated these findings in spinal motor neurons,8 cerebellar Purkinje cells,9 hippocampal neurons,10 cerebral cortex neurons,11 and human pluripotent stem cells (hPSCs).12 Moreover, a flurry of studies identified astrocyte-secreted factors that may underpin this phenomenon, including apolipoprotein E bound to cholesterol,13 thrombospondin 1 (TSP-1) and 2 (TSP-2),14 the matricellular glycoproteins proteins hevin and SPARC (SPARC being an anti-synaptogenic protein that antagonizes the function of hevin),15 and the heparan sulfate proteoglycans Gpc-4 and Gpc-6.16 Astrocyte-secreted signals are hypothesized to operate via a number of spatiotemporal-specific mechanisms during development that have been extensively discussed elsewhere.4 In addition, one study recently reported that developing astrocytes engulf synapses in an activity-dependent manner via the MEGF10 and MERTK phagocytic pathways. Mice deficient in both molecules displayed an 85% reduction in relative engulfment activity, leading to the retention of excess synapses.17
Less well understood, however, is how astrocyte-controlled synapse regulation occurs in the adult CNS and the significance this has for age-related neurodegenerative disorders where synapse loss is a hallmark feature. Early studies demonstrated that astrocytes communicate with neurons at the synapse and can modulate neurotransmission by astrocyte-mediated calcium and glutamate signaling.18,19,20 It is known that astrocytes can receive and respond to the synaptic information produced by neuronal activity, owing to their expression of a wide range of neurotransmitter receptors.21 Astrocytes can detect and regulate synaptic activity induced by single synaptic stimulation via spontaneous and rapid calcium transients at functional compartments in the astrocyte processes.22 Furthermore, the close proximity of fine astrocytic processes to synapses suggests an ongoing relationship throughout life. It has been shown that astrocytes contact 60% of synapses in the hippocampus as well as demonstrating cooperative and dynamic motility with their associated dendritic spines.23 More recently, a forward genetic screen in Caenorhabditis elegans identified a mutation in CIMA-1, an SLC17 transporter protein secreted by epidermal cells that modulated synaptic connectivity between neurons in the nematode nerve ring. Interestingly, CIMA-1 was found to function as a synaptic maintenance signal by ensuring the appropriate positioning of astrocyte processes alongside axons during growth. This process was subsequently shown to be crucial for the correct distribution of synapses during adulthood.24 In addition, it was recently reported that adult mouse astrocytes contain internalized material from both excitatory and inhibitory synapses,17 suggesting that astrocytes influence synaptic structures through phagocytic activity in addition to modulating synaptic signaling. Indeed, research has shown that Drosophila larval astrocytes acquire phagocytic properties during neuronal circuit remodeling and actively engulf axons via the Draper and Crk/Mbc/dCed-12 signaling pathways.25 Further supporting the significance of these findings is that the mammalian homologue for Draper is MEGF10. Thus, the molecular mechanisms underlying the potential for astrocytes to actively phagocytose synapses may belong to an evolutionarily conserved pathway.
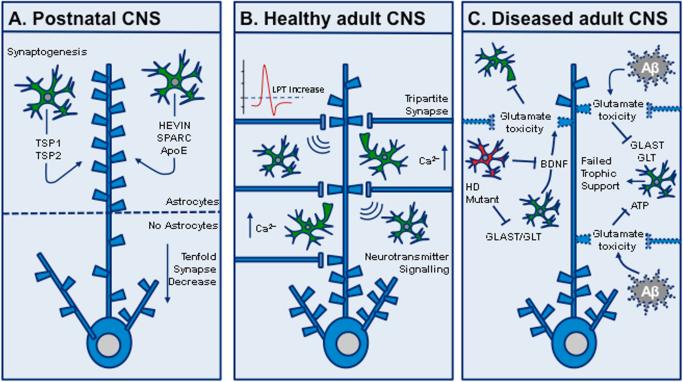
Studies such as these suggest a paradigm shift in our understanding of the extent to which synapse dysfunction and/or loss in neurodegenerative diseases may be mediated by non-cell-autonomous mechanisms involving astrocytes (Fig. 1). A number of studies on AD and HD are now elucidating previously underappreciated roles for astrocytes as causative agents in synaptic pathophysiology in these disorders. Studies of AD for example, have demonstrated that the accumulation of soluble oligomeric Aβ forms may cause a loss of excitatory synapses but spare gamma-aminobutyric acid (GABA)ergic synapses, thereby weakening synapse function and impairing synaptic plasticity.26 Oligomeric Aβ may directly influence astrocyte–synapse signaling by inducing astrocytic glutamate release, which in turn activates extrasynaptic N-methyl-D-aspartate (NMDA) receptors, leading to synaptic depression and degeneration.27 Furthermore, enhanced secretion of interferon (IFN)-γ by inflamed microglia may induce synapse-enwrapping astrocytes to express β-secretase (BACE-1) genes and produce and secrete Aβ to the detriment of synapses.28 Aβ plaque deposition may also stimulate increased astrocyte GABA synthesis, leading to enhanced survival of GABAergic synapses,29 which in turn may promote maladaptive plasticity and a “quad-partite” depression in the activity of glutamatergic synapses.30 In aggregate, these findings suggest that astrocytes actively participate in the pathogenesis of synapse loss in AD.
Figure 1.
Astrocyte–synapse interactions. (A) In the developing CNS, astrocytes secrete molecules that promote synapse formation and function, including HEVIN, SPARC (anti-synaptogenic), apolipoprotein E bound to cholesterol (ApoE), thrombospondin 1 (TSP-1) and 2 (TSP-2), and the heparan sulfate proteoglycans Gpc-4 and Gpc-6. Retinal ganglion cell neurons lacking astrocyte support form tenfold fewer excitatory synapses than neurons with astrocytes or neurons cultured in astrocyte-conditioned medium. (B) In the normal adult CNS, astrocytes “listen” to neuronal synapses and form the tripartite synapse to regulate neurotransmission, synaptic plasticity, and long-term potentiation (LTP), mediated by internal astrocytic calcium signaling. (C) In CNS disease models, astrocyte and/or neuronal dysfunction, resulting from disease-associated insults such as amyloid beta (Aβ) deposition, 6-OHDA toxicity, and mutant huntingtin expression, may lead to failed neurotrophic support and glutamate excitotoxicity. Glutamate aspartate transporter, GLAST; glutamate transporter, GLT; adenosine triphosphate, ATP.
In HD, recent research has shown that expression of mutant huntingtin (mHtt) in astrocytes can impair their homeostatic functions, leading to extracellular excitotoxicity and neuronal circuit damage. Studies in mice have shown that astrocytes expressing mHtt have decreased expression of the glutamate transporters GLAST and GLT-1, leading to reduced glutamate uptake and subsequent neuronal dysfunction.31 Moreover, transgenic mice expressing an N-terminal mHtt fragment specifically in astrocytes display an age-dependent HD neurological phenotype.32 Interestingly, recent work using a combination of the R6/2 and the more slowly progressing Q175 HD mouse models revealed that astrocyte Kir4.1 expression is downregulated, leading to increased extracellular potassium and striatal medium spiny neuron (MSN) excitability.33 Reduced Kir4.1 expression was also seen in the striatum of HD patients.33 Whether a dysregulation of extracellular potassium drives HD pathogenesis or is secondary to mHtt aggregation in astrocytes remains unclear.
Synapses and microglia
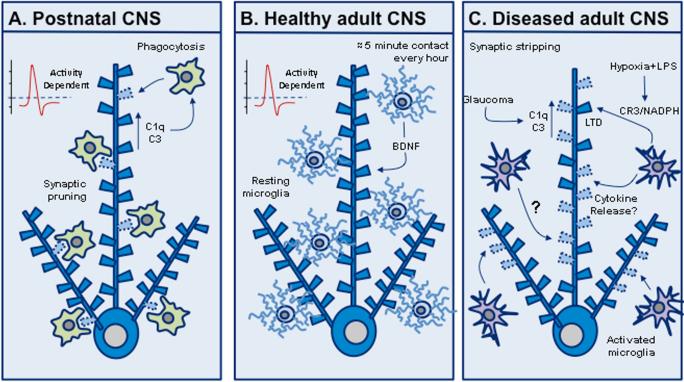
Microglia are the resident immunocompetent cells of the CNS, comprising approximately 10% of the total glial cell population in the human brain and up to 20% in the rodent brain.34 Inflammation is now widely recognized as an important underlying component of a diverse variety of neurodegenerative diseases, with microglia playing a crucial causative role in this process.35,36,37 An emerging aspect of neuronal–microglial interactions is the relationship microglia have with synapses. Early electrophysiological studies showed that microglia can modulate synaptic plasticity, long-term potentiation (LTP), and long-term depression (LTD) and that this effect may be mediated by tumor necrosis factor (TNF)-α.38,39 In the developing CNS it has been demonstrated that microglia can internalize synaptic material.40 During the first wave of embryonic synaptogenesis at E14–E15 in rodents, microglia promote synaptogenesis via the secretion of growth factors.41 During late-stage prenatal and early postnatal development, microglia actively engulf synapses, a process known as synaptic pruning.5 Mounting evidence implicates the complement cascade in this process whereby weak synapses are tagged for elimination via expression of the complement factors C1q and C3 in neurons and the subsequent phagocytosis of the synapse by microglia with abundant expression of receptors for C1q and C3.42,43 In the postnatal retinogeniculate system, microglia were observed to phagocytose presynaptic inputs in an activity-dependent and CR3/C3-specific manner during a well-defined period of synapse elimination.44
Recent studies have also begun to shed light on microglial–synapse interactions in the adult brain. Microglia make brief contact with synaptic structures at a frequency of approximately once per hour in basal in vivo conditions.45 This behavior was found to be activity-dependent, and microglia remained in contact with the synapse for roughly 5 min before retracting their processes and moving on. Building on this, researchers observed that microglia processes contact both pre- and postsynaptic compartments.46 The molecular cues that attract microglial processes to neuronal synapses are unknown and the function played by microglia–synapse interactions in the healthy brain have not been elucidated. Some postulate a role in synaptic plasticity and experience-dependent modification of synaptic circuits.47,48 A recent study showed that microglia promote learning-related glutamatergic synapse formation in the mouse hippocampus and cortex and that this effect is mediated by microglial brain-derived neurotrophic factor (BDNF). Microglial BDNF depletion did not alter synapse density in these brain regions, but instead led to a reduced level of synaptic GluN2B and VGluT1.49 The close association between microglia and synapses has also raised the question of how microglia influence synaptic anatomy or function in neurodegeneration. Initial studies of the facial nerve injury model demonstrated that microglia remove synaptic input to motor neurons and the process is influenced by cytokine release,50 suggesting that microglia might contribute to synapse loss in neurodegenerative disease. The hypothesis is supported by studies demonstrating that synapse loss develops concomitantly with neuroinflammation, in multiple sclerosis, AD, and HD.51,36 It has also been found that the complement components C1q and C3 are upregulated and co-localize with retinal synapses in a mouse model of glaucoma.42 Interestingly, mice with mutations in complement component 1a (C1qa) are protected from glaucoma,52 and mice deficient for complement component 5 (C5) display a less severe glaucoma than C5-sufficient mice.53 Taken together, these studies suggest that the complement system is central to synaptic and/or neuronal integrity during neuroinflammation. Nevertheless, the evidence supporting a role for microglia in synaptic stripping or synapse degeneration in these diseases is lacking, and the idea remains steeped in controversy.54,5 Recently, however, it was demonstrated that hypoxia and the inflammatory stimulus lipopolysaccharide (LPS) trigger long-term synaptic depression (LTD) in rat hippocampal slices via microglial CR3 and NADPH oxidase.55 Additionally, systemic administration of LPS and resulting microglia activation leads to specific loss of inhibitory synapses, increased excitatory synaptic transmission, and activation of downstream neuroprotective signal transduction.56 Taken together, these findings demonstrate that in the adult brain, when microglia respond to a variety of signals from both central and peripheral inflammatory processes, the result may strongly influence synaptic maintenance and function.
Several studies have suggested that microglia actively participate in the pathogenesis of neurodegenerative diseases. With aging and neurodegeneration, microglia become dystrophic and senescent, resulting in a loss of neuroprotective capacity.57 This may be compounded by markedly reduced microglial ramification, resulting in increased areas of brain parenchyma lacking coverage by microglial processes, thus compromising the ability of microglia to scan their environment adequately.58 Progressive, aging-related microglial degeneration and loss of microglial neuroprotection may contribute to aging and the onset of sporadic AD.59 Interestingly, the complement protein C1q, which mediates synapse elimination during development, is increased 300-fold in the normal aging mouse and human brain.60 This increase was seen primarily adjacent to synapses, and aged C1q-deficient mice displayed reduced cognitive and memory deficits compared with their wild-type littermates.60 One could hypothesize from such data that early developmental synapse removal mechanisms mediated by microglia are somehow reactivated in a pathological manner in the aging and/or AD brain. Age and neurodegenerative-associated microglial dysfunction may also result in dysfunctional phagocytosis and clearance of amyloid beta peptides (Aβ) and other aberrant proteins, resulting in their toxic accumulation directly affecting synapses.61 Recent evidence indicates that variants in the microglial gene encoding triggering receptor expressed on myeloid cells-2 (TREM2) are linked with an increased risk of developing AD and may result in enhanced Aβ plaque deposition, an inflammatory microglial phenotype, and failure to clear phagocytic debris.62 Polymorphism in cluster of differentiation 33 (CD33) is an AD risk allele associated with diminished microglial internalization of Aβ1-42 and increased microglial activation in humans.63 Microglial cytokines, interleukin-12 (IL-12), and IL-23, also have potential roles in AD pathogenesis. Inhibition of the p40 subunit common to both IL-12 and IL-23 in the amyloid precursor protein/presenilin 1 (APP/PS1) mouse model resulted in reduced cerebral amyloid load, decreased soluble Aβ species, and reversed cognitive deficits.64 It is reasonable to surmise that these kinds of microglial behaviors may also produce direct or indirect changes in synaptic properties. Thus, the microglial regulation of synapses in health and disease will be a promising area of future research (Fig. 2).
Figure 2.
Microglia–synapse interactions. (A) In the developing CNS, microglia actively phagocytose and remove synapses to sculpt neuronal circuits. This is activity-dependent and is hypothesized to involve the classical complement cascade whereby weak synapses are “tagged” by the complement factors C1q and complement receptor 3 (C3) in neurons and then phagocytosed by microglia via expression of receptors for C1q and C3. (B) In the normal adult CNS, microglia briefly contact synapses, once per hour for approximately 5 min, in an activity-dependent manner, and microglial brain-derived neurotrophic factor (BDNF) is thought to promote learning-related glutamatergic synapse formation. (C) In CNS disease models, hypoxia combined with lipopolysaccharide (LPS) can lead to long-term synaptic depression (LTD), and glaucoma can reactivate complement-mediated synapse loss. In addition, microglial activation and cytokine release is hypothesized to strip synapses during chronic neurodegenerative disease.
Synapses and myelin-forming glia
The primary function of myelin is to insulate axons and enhance the fast saltatory conduction of neuronal action potentials. In the CNS, myelin is formed by oligodendrocytes and in the peripheral nervous system (PNS) this function is performed by myelinating Schwann cells.65 Myelin has been implicated in regulating neuronal network behavior, thereby contributing to synaptic plasticity.66 Indeed, evidence now suggests that myelin contributes to cognition and learning, and studies have demonstrated that changes in white-matter structure are associated with learning fine motor tasks and even foreign language acquisition.67,68 This is thought to occur via signaling of myelin proteins that can suppress axon sprouting and synaptogenesis.69 Recent research has also suggested a role for the transcription factor myelin regulatory factor (MyRF) in mediating the production of adult-born oligodendrocytes that were shown to be crucial for rodents to learn a new motor skill.70
In the PNS, Schwann cells have been shown to play key roles in synaptic function, maintenance, and development at the neuromuscular junction (NMJ). Electron microscopy studies have revealed that Schwann cells are closely positioned alongside presynaptic and postsynaptic components of the NMJ, allowing them to detect synaptic activity and regulate neurotransmission.71 Experiments using Drosophila have found that bone morphogenetic protein (BMP) acts as a retrograde signal from muscle to regulate NMJ synapse assembly, 72,73 and the Schwann cell–secreted morphogen wingless (Wg) was recently shown to regulate glutamate receptor clustering and synaptic function at the NMJ. 74 Interestingly, work by Bishop and colleagues using time-lapse imaging of fluorescently labeled axons and serial electron microscopy has demonstrated that retracting axons at the NMJ shed numerous membrane-bound synaptic organelles, called axosomes, which are subsequently engulfed by neighboring Schwann cells.75 It is thought that this cellular mechanism exists to regulate synaptic pruning in the PNS during development.
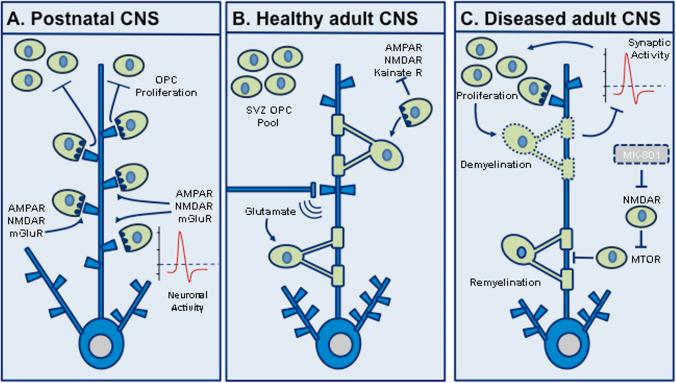
Recently, an intriguing relationship between neurons and oligodendrocyte precursor cells (OPCs; also known as NG2 cells) has emerged. OPCs are a large population of glial cells that exist during development and adulthood in all regions of the CNS and comprise approximately 5% of all cells in the brain.76 Remarkably, OPCs form their own glutamatergic synapses with axons of neighboring neurons77,78,79 and express AMPA, kainate, NMDA, and metabotropic glutamate receptors.80 The purpose of these OPC–neuronal synapses is hypothesized to provide a means for neuronal activity to modulate oligodendrocyte development. Animal models have shown that synaptic activity may influence OPC proliferation. Sensory deprivation during the formation of the mouse barrel cortex reduces glutamatergic synaptic input onto OPCs, which in turn increases their proliferation.81 Interestingly, OPCs continue to receive synaptic inputs during mitosis and transfer their synapses to new daughter cells.82 This behavior persists until OPCs differentiate into fully mature oligodendrocytes, when the synaptic structures are then dismantled altogether.83
Though still in its infancy, the field of neuronal–OPC synapse interactions is beginning to expose important ramifications for neurodegenerative and demyelinating diseases, such as multiple sclerosis (MS) and the leukodystrophies. Following lysolecithin (LPC)-induced demyelination in the adult mouse corpus callosum, a significant correlation between decreased synaptic activity and increased OPC proliferation has been observed.84 In this model, axons of the corpus callosum are found to innervate a pool of OPCs that are derived from the subventricular zone (SVZ), leading some researchers to hypothesize that neuron–OPC synapses participate in oligodendrocyte regeneration.80 In the mutant superoxide dismutase-1 (SOD1) transgenic mouse model of amyotrophic lateral sclerosis (ALS), oligodendrocyte loss was followed by a compensatory increase in OPC proliferation and differentiation. These OPCs, as well as their mature oligodendrocyte counterparts, were found to be dysfunctional.85 A similar study in the motor cortex of human ALS patients also found increased OPC proliferation as well as demyelination,86 highlighting OPCs as therapeutically targetable agents in motor neuron disease. Furthermore, following ischemia in the adult neocortex, GABAergic stimulation of OPCs correlated with increased BDNF expression—thought to be released by OPCs to aid recovery following ischemic insult.87 One study demonstrated that glutamate release in co-cultures of mouse dorsal root ganglion neurons and OPCs promoted myelination by stimulating the formation of cholesterol-rich signaling domains between the two cell types.88 The formation of these domains was associated with increased myelin basic protein expression via an NMDAR activation, Fyn kinase–dependent signaling pathway. Recent research blocking NMDARs using the specific antagonist MK-801 in a cuprizone model of demyelination significantly inhibited remyelination in the corpus callosum, via a mammalian target of rapamycin (mTOR)-dependent mechanism.89 Altogether, these studies highlight diverse and unexpected new roles for myelinating glia at neuronal synapses, carving new avenues of research into developmental neurobiology and advancing our understanding of demyelinating neurological disorders (Fig. 3).
Figure 3.
Oligodendrocyte–synapse interactions. (A) In the developing CNS, oligodendrocyte precursor cells (OPCs) form glutamatergic synapses with the axons of neighboring neurons in an activity-dependent fashion, which is hypothesized to inhibit and/or modulate subsequent OPC proliferation. (B) In the normal adult CNS, OPCs mature into oligodendrocytes and enwrap axons with myelin sheaths to ensure the fast saltatory conduction of neuronal action potentials in the CNS. Evidence suggests a pool of OPCs persists in the adult subventricular zone (SVZ). Oligodendrocytes also “listen” to neurotransmission via expression of neurotransmitter receptors. (C) In CNS disease models, neuron–OPC synapses are hypothesized to participate in oligodendrocyte regeneration following demyelinating insults. In the cuprizone model, the NMDA receptor (NMDAR) antagonist MK-801 blocks NMDAR activity and subsequent remyelination via a mammalian target of rapamycin (mTOR)-dependent mechanism. Furthermore, demyelination is thought to inhibit neuronal synaptic activity and in turn OPC proliferation, possibly preventing OPC differentiation. Metabotropic glutamate receptor, mGluR.
Conclusions and future implications
Over the recent past, multiple studies have demonstrated the importance of non-cell-autonomous processes in the pathogenesis of neurodegenerative diseases. Many of the most convincing experiments have involved the cell type–specific expression or deletion of mutant genes that initiate disease, and several of these studies have implicated glia as active participants in the development of neurodegenerative pathology. There are a variety of mechanisms by which glia influence disease pathogenesis. However, recent studies suggest that glial modulation of synapse function and number is emerging as a critical component of the role glia play in the process of neurodegeneration. Synapse dysfunction has been demonstrated to precede the onset of detectable behavioral symptoms in a variety of animal models of neurodegenerative disease,90,91 and synapse loss is well documented to correlate with symptoms in AD. Thus, the role of glia in the process of developing synapse dysfunction and/or synaptic degeneration is clearly a key and potentially targetable component of pathogenesis in these disorders.
The studies reviewed above have revealed several potential ways by which glia participate in the development of synapse dysfunction and/or elimination. Astrocytes have a well-understood role in modulating the degree of excitatory glutamatergic input through active transport of glutamate away from the synaptic cleft, and several early studies suggested that astrocyte dysfunction lead to excitotoxic neuronal injury in amyotrophic lateral sclerosis (ALS).92,93 We have also reported that in a model of polyglutamine-induced neurodegeneration, reduced glia glutamate transport function influences synaptic transmission and subsequent neuronal survival.94 Subsequently, several of the studies reviewed above have demonstrated that disease mechanisms in AD and HD also involve modulation of astrocyte-mediated glutamate transport. However, the studies reviewed here have provided evidence that several glial cell types are involved in modulating synaptic structure and function through mechanisms unrelated to altered glutamate transport, including the direct engulfment of synaptic structures by both astrocytes and microglia. These findings suggest that glia are likely to actively participate in the pathogenic process that leads to synapse dysfunction and structural synapse loss during early-phase disease. Thus, the mechanisms that control how and when glia modulate neuronal synaptic structure and function are potential therapeutic targets for preventing eventual neurodegeneration that could develop downstream to synaptic change.
Key questions for future studies include: (1) What are the molecular signals that regulate glial behaviors early in neurodegenerative disease?; (2) Is there a functional or molecular signature for synapses targeted for removal by astrocytes and/or microglia?; (3) Are normal synapses removed by dysfunctional glia or are glia performing their normal function and removing dysfunctional synapses?; and (4) Are the signals that target synapses for removal initiated by injured neurons and/or reactive glia? Experiments aimed at addressing these questions are critical for determining whether interventions aimed at modulating glia in these disorders will have potential therapeutic benefit.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Andreae LC, Burrone J. The role of neuronal activity and transmitter release on synapse formation. Curr. Opin. Neurobiol. 2014;12:47–52. doi: 10.1016/j.conb.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison JH, Baxter MG. The aging cortical synapse: hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 2012;13:240–50. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petralia RS, Mattson MP, Yao PJ. Communication breakdown: the impact of aging on synapse structure. Aging Res. Rev. 2014;14:31–42. doi: 10.1016/j.arr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 2013;14:311–21. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Garden GA, La Spada AR. Intercellular (mis)communication in neurodegenerative disease. Neuron. 2012;73:886–901. doi: 10.1016/j.neuron.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synpase number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 8.Ullian EM, Barkis WB, Chen S, et al. Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Mol. Cell Neurosci. 2004;26:544–57. doi: 10.1016/j.mcn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Buard I, Steinmetz CC, Claudepierre T, Pfrieger FW. Glial cells promote dendrite formation and the reception of synaptic input in Purkinje cells from postnatal mice. Glia. 2010;58:538–545. doi: 10.1002/glia.20943. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat. Neurosci. 2010;13:22–24. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
- 11.Dinz LP, Almeida JC, Tortelli V, et al. Astrocyte-induced synaptogenesis is mediated by transforming growth factor β signalling through modulation of D-serine levels in cerebral cortex neurons. J. Biol. Chem. 2012;287:41432–45. doi: 10.1074/jbc.M112.380824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krencik R, Weik JP, Liu Y, et al. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotech. 2011;29:528–45. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholersterol-induced synaptogensis in a CNS neuron. Mol. Cell Neurosci. 2005;29:190–201. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Christopherson KS, Ullian EM, Stokes CC, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–33. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Kucukdereli H, Allen NJ, Lee AT, et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Pro. Natl. Acad. Sci. U. S. A. 2011;108:440–9. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen NJ, Bennett ML, Foo LC, et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–4. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung WS, Clarke LE, Wang GX, et al. Astrocytes mediate synapse elimination through MGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang J, Jiang LI, Goldman SA, et al. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat. Neurosci. 1998;1:683–92. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 19.Araque A, Sanzgiri RP, Parpura V, et al. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J. Neurosci. 1998;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman EA. New roles for astrocytes: regulation of synaptic transmission . TINS. 2003;26:536–42. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Alvarez A, Araque A. Astrocyte-neuron interaction at tripartite synapses. Curr. Drug Targets. 2013;14:1220–4. doi: 10.2174/13894501113149990203. [DOI] [PubMed] [Google Scholar]
- 22.Panatier A, Vallee J, Haber M, et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–98. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Haber M, Zhou L, Murai KK. Cooperative astrocye and dendritic spine dynamics at hippocampal excitatory synapses. J. neurosci. 2006;26:8881–91. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao Z, Watanabe S, Christensen R, et al. Synapse location during growth depends on glia location. Cell. 2013;154:337–50. doi: 10.1016/j.cell.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tasdemir-Yilmaz OE, Freeman M. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 2014;28:20–33. doi: 10.1101/gad.229518.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mairet-Coello G, Polleux F. Involvement of ‘stress-response’ kinase pathways in Alzheimer's disease progression. Curr. Opin. Neurobiol. 2014;27C:110–117. doi: 10.1016/j.conb.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talantova M, Sanz-Blasco S, Zhang X, et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synapse loss. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E2518–27. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dal Pra I, Chiarini A, Pacchiana R, et al. Emerging concepts of how β-amyloid proteins and pro-inflammatory cytokines might collaborate to produce an ‘Alzheimer brain’. Mol. Med. Rep. 2008;1:173–8. [PubMed] [Google Scholar]
- 29.Mitew S, Kirkcaldie MT, Dickson TC, Vickers JC. Altered synapses and gliotransmission in Alzheimer's disease and AD model mice. Neurobiol. Aging. 2013;34:2341–51. doi: 10.1016/j.neurobiolaging.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Papa M, De Luca C, Petta F, et al. Astrocyte-neuron interplay in maladaptive plasticity. Neurosci. Biobehav. Rev. 2014;42:35–54. doi: 10.1016/j.neubiorev.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Faideau M, Kim J, Cormier K, et al. In vivo expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington's disease subjects. Hum. Mol. Genet. 2010;19:3053–3067. doi: 10.1093/hmg/ddq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradford J, Shin JY, Roberts M, et al. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc. Natl. Acad. Sci. USA. 2009;106:22480–85. doi: 10.1073/pnas.0911503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong X, Ao Y, Faas GC, et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat. Neurosci. 2014;17:694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiological reviews. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 35.Streit WJ. Microglia and neuroprotection: implications for Alzheimer's disease. Brain Res. Rev. 2005;48:234–239. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 37.Glass CK, Saijo K, Winner B, et al. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q, Rowan MJ, Anwyl R. β-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J. Neurosci. 2004;24:6049–56. doi: 10.1523/JNEUROSCI.0233-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–59. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 40.Tremblay M, Majewska AK. A role for microglia in synaptic plasticity? Commun. Integr. Biol. 2011;2:220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greter M, Merad M. Regulation of microglia development and homeostasis. Glia. 2013;61:121–7. doi: 10.1002/glia.22408. [DOI] [PubMed] [Google Scholar]
- 42.Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 43.Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 44.Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wake H, Moorhouse AJ, Jinno S, et al. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Achour B, Pascual O. Glia: the many ways to modulate synaptic plasticity. Neurochem. Int. 2010;57:440–5. doi: 10.1016/j.neuint.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moran LB, Graeber MB. The facial nerve axotomy model. Brain Res. Rev. 2004;44:154–178. doi: 10.1016/j.brainresrev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- 52.Howell GR, Macalinao DG, Sousa GL, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Invest. 2011;121:1429–44. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howell GR, Soto I, Ryan M, et al. Deficiency of complement componet 5 ameliorates glaucoma in DBA/2J mice. J Neuroinflammation. 2013;10:76. doi: 10.1186/1742-2094-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perry VH, O'Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN. Neuro. 2010;2:e00047. doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Malik A, Cho HB, et al. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron. 2014;82:195–207. doi: 10.1016/j.neuron.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z, Jalabi W, Hu W, et al. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat. Commun. 2014;225:4486. doi: 10.1038/ncomms5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Streit WJ, Miller KR, Lopes KO, Njie E. Microglial degeneration in the aging brain--bad news for neurons? Front Biosci. 2008;13:3423–38. doi: 10.2741/2937. [DOI] [PubMed] [Google Scholar]
- 58.Baron R, Babcock AA, Nemirovsky A A, et al. Accelerated microglial pathology is associated with Aβ plaques in mouse models of Alzheimer's disease. Aging Cell. 2014;13:584–95. doi: 10.1111/acel.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta. Neuropathol. 2009;118:475–85. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stephan AH, Madison DV, Mateos JM, et al. A dramatic increase in C1q protein in the CNS during normal aging. J Neurosci. 2014;33:13460–74. doi: 10.1523/JNEUROSCI.1333-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harry GJ. Microglia during development and aging. Pharmacol. Ther. 2013;139:313–26. doi: 10.1016/j.pharmthera.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guerreiro R, Wojtas A, Bras J, et al. Alzheimer Genetic Analysis Group. TREM2 variants in Alzheimer's disease. N. Engl. J. Med. 2013;368:117–27. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradshaw EM, Chibnik LB, Keenan BT, et al. CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nat. Neurosci. 2013;16:848–50. doi: 10.1038/nn.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.vom Berg J, Prokop S, Miller KR, et al. Inhibition of IL-12/IL-23 signalling reduces Alzheimer's disease-like pathology and cognitive decline. Nat. Medicine. 2012;18:1812–19. doi: 10.1038/nm.2965. [DOI] [PubMed] [Google Scholar]
- 65.Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoz LD, Simons M. The emerging functions of oligodendrocytes in regulating neuronal network behaviour. Bioessays. 2014;37:60–9. doi: 10.1002/bies.201400127. [DOI] [PubMed] [Google Scholar]
- 67.Scholz J, Klein MC, Behrens TE, et al. Training induces changes in white matter architecture. Nat. Neurosci. 2009;12:1370–1. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schlegel AA, Rudelson JJ, Tse PU. White matter structure changes as adults learn a second language. J Cogn Neurosci. 2012;24:1664–70. doi: 10.1162/jocn_a_00240. [DOI] [PubMed] [Google Scholar]
- 69.Fields RD. White matter in learning, cognition and psychiatric disorders. TINS. 2008;31:361–70. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKenzie IA, Ohayon D, Li H, et al. Motor skill learning requires active central myelination. Science. 2014;17:318–22. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng Z, Koirala S, Ko C. Synapse-glial interactions at the vertebrate neuromuscular junction. The Neuroscientist. 2005;11:503–13. doi: 10.1177/1073858405277409. [DOI] [PubMed] [Google Scholar]
- 72.Marques G, Bao H, Haerry TE, et al. The drosophila BMP type II receptor wishful thinking regulates neuromuscular synapse morphology and function. Neuron. 2002;33:529–43. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- 73.McCabe BD, Marques G, Haghighi AP, et al. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the drosophila neuromuscular junction. Neuron. 2003;39:241–54. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 74.Kerr KS, Fuentes-Medel Y, Brewer C, et al. Glial Wingless/Wnt regulates glutamate receptor clustering and synaptic physiology at the drosophila neuromuscular junction. J. Neurosci. 2014;34:2910–20. doi: 10.1523/JNEUROSCI.3714-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bishop DL, Misgeld T, Walsh MK, et al. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–61. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 76.Sun W, Dietrich D. Synaptic integration by NG2 cells. Front. Cell. Neurosci. 20. 2013;7:255. doi: 10.3389/fncel.2013.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocytes precursor cells in the hippocampus. Nature. 2000;405:187–91. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 78.Lin SC, Huck JH, Roberst JD JD, et al. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773–85. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 79.Maldonado PP, Angulo MC. Multiple Modes of Communication between Neurons and Oligodendrocyte Precursor Cells. Neuroscientist. 2014 Apr 10; doi: 10.1177/1073858414530784. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 80.Maldonado PP, Velez-Fort M, Angulo MC. Is neuronal communication with NG2 cells synaptic or extrasynaptic? J. Anat. 2011;219:8–17. doi: 10.1111/j.1469-7580.2011.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mangin JM, Li P, Scafidi J, Gallo V. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat. Neurosci. 2012;15:1192–4. doi: 10.1038/nn.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ge WP, Zhou W, Luo Q, et al. Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc. Natl. Acad. Sci. U. S. A. 2009;106:328–33. doi: 10.1073/pnas.0811353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kukley M, Nishiyama A, Dietrich D. The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J. Neurosci. 2010;30:8320–31. doi: 10.1523/JNEUROSCI.0854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ortiz F, Sahel A, Kerninon C, et al. Alterations of NG2 cell synaptic connectivity following demyelination of corpus callosum.. XI European Meeting on Glial Cells in Health and Disease Abstract T06-18B.2013. [Google Scholar]
- 85.Philips T, Bento-Abreu A, Nonneman A, et al. Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain. 2013;136:471–82. doi: 10.1093/brain/aws339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang SH, Li Y, Fukaya M, Lorenzini I, et al. Degeneration and impaired regeneration of grey matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 2013;16:571–9. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanaka Y, Tozuka Y, Takata T, et al. Excitatory GABAergic activation of cortical dividing glial cells. Cereb. Cortex. 2009;19:2181–95. doi: 10.1093/cercor/bhn238. [DOI] [PubMed] [Google Scholar]
- 88.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–51. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li C, Xiao L, Liu X, et al. A functional role of NMDA receptor in regulating the differentiation of oligodendrocytes precursor cells and remyelination. Glia. 2013;61:732–49. doi: 10.1002/glia.22469. [DOI] [PubMed] [Google Scholar]
- 90.Overk CR, Masliah E. Pathogenesis of synaptic degeneration in Alzheimer's disease and Lewy body disease. Biochem. Pharmacol. 2014;88:508–16. doi: 10.1016/j.bcp.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sepers MD, Raymond LA. Mechanisms of syaptic dysfunction and excitotoxicity in Huntington's disease. Drug Discov. Today. 2014;19:990–996. doi: 10.1016/j.drudis.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 92.Di Giorgio FP, Carrasco MA, Siao MC, et al. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat. Neurosci. 2007;10:608–14. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nagai M, Re DB, Nagata T, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 2007;10:615–22. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Custer SK, Garden GA, Gill N, et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat. Neurosci. 2006;10:1302–11. doi: 10.1038/nn1750. [DOI] [PubMed] [Google Scholar]