Abstract
This data article contains complementary figures and results related to the research article entitled “Negative Fgf8-Bmp2 feed-back is controlled by miR-130 during early cardiac specification” [15], which reveals what specific role miR-130 plays during the cardiac induction process. This study evidenced miR-130 a putative microRNA that targets Erk1/2 (Mapk1) 3′UTR- as a necessary linkage in the control of Fgf8 signaling, mediated by Bmp2. Thus, miR-130 regulates a negative Fgf8-Bmp2 feed-back loop responsible to achieve early cardiac specification. A significant aspect supporting our conclusions is given by the expression pattern of miR-130 during early cardiac specification, as well as by those results obtained after the designed experimental procedures. The data presented here reveal that miR-133 is also expressed within the precardiac areas during early cardiogenesis, pattern which is comparable to that of FGFR1, receptor involved in the Fgf8/ERK signaling pathway. Interestingly, our miR-133 overexpression experiments resulted in a decrease of Fgf8 expression, whereas we observed an increase of Bmp2 and subsequently of cardiac specific markers Nkx-2.5 and Gata4. Additionally, our loss-of-function experiments -through Fgf8 siRNA electroporation- showed an increase of miR-133 expression. Finally, after our Bmp2 experiments, we observed that miR-133 is upstream-regulated by Bmp2. All those results suggest that miR-133 also constitutes a crucial linkage in the crosstalk between Fgf8 and Bmp2 signaling by regulating the Fgf8/ERK pathway during cardiac induction.
Graphical abstract
Specifications table
| Subject area | Biology |
|---|---|
| More specific subject area | Embryonic development |
| Type of data | Text file and figures |
| How data was acquired | TSSS20 Ovodyne Electroporator (Intracel), Nikon digital SIGHT DS-U1: bright and fluorescent light |
| Data format | Raw |
| Experimental factors | Electroporation, beads implantation, culture embryo |
| Experimental features | Whole-mount in situ hybridization, immunohistochemistry |
| Data source location | University of Extremadura, Badajoz, Spain |
| Data accessibility | The data are supplied with this article |
Value of the data
-
•
miR-133 modulates Fgf8 during cardiac induction.
-
•
miR-133 is regulated by Bmp2.
-
•
miR-133 exerts a regulatory role in Fgf8–Bmp2 signaling during early cardiac specification.
1. Data
Fgf8 constitutes a crucial factor involved in MAPK/ERK signaling pathway [8]. Also, FGF receptors have demonstrated to play a significant role during the early steps in the Fgf8/ERK signaling cascade [6,9]. Although there is no evidence about the role of FGF receptors during early cardiogenesis, there are previous studies where FGFR1 has been identified in the endoderm underlying the precardiac mesoderm [16,17]. However, its functional role has been preferentially involved in cell proliferation rather than in cell differentiation [19]. It has been reported that cardiomyocyte proliferation is suppressed after miR-133 overexpression [13], and that miR-133 is also involved in late stages of mouse cardiac development as well as in molecular mechanisms regulating adult cardiovascular diseases [1,2,7,12,18].
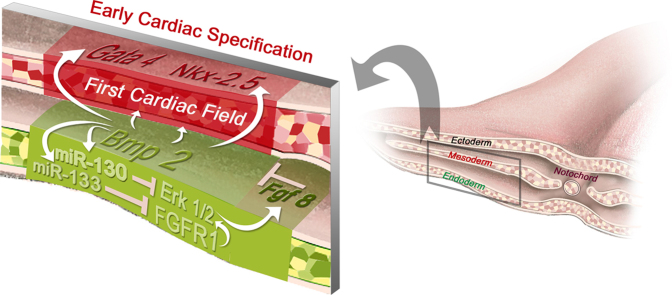
To further analyze what mechanism regulates these signaling pathways, we will explore herein miR-133 expression and function. Although previous studies have provided data about miR-133 expression in chick embryo [5], it has only been analyzed in late stages of cardiac development. Thus, we have analyzed miR-133 expression from the primitive streak stages to the primitive cardiac tube formation (Fig. 1), expression which is already detectable at gastrula stages throughout the entire primitive streak, including the precardiac cells. Subsequently, its expression spreads laterally and concentrates into the first cardiac field (FCF) and the underlying endoderm, to finally express restrictively at the splachnic mesoderm of the primitive endocardial tube. This topographical location of miR-133 shows a similar distribution to that of Fgfr1 [16,17]. Since FGFR1 has been proposed as a crucial factor involved in Fgf8/ERK signaling pathway in different experimental models [8,11], it is reasonable to consider a close relationship between miR-133 and Fgfr1 during cardiogenesis.
Fig. 1.
Whole-mount ISH analyses for miR-133 during early chick development. Note the expression from the primitive streak (ps), through FCF, to the primitive endocardial tube level (pet). The white lines indicate the transverse section level, showing miR-133 expression in the precardiac mesoderm (arrow) and the underlying endoderm (arrowhead). Hensen׳s node: Hn. Neural plate: np.
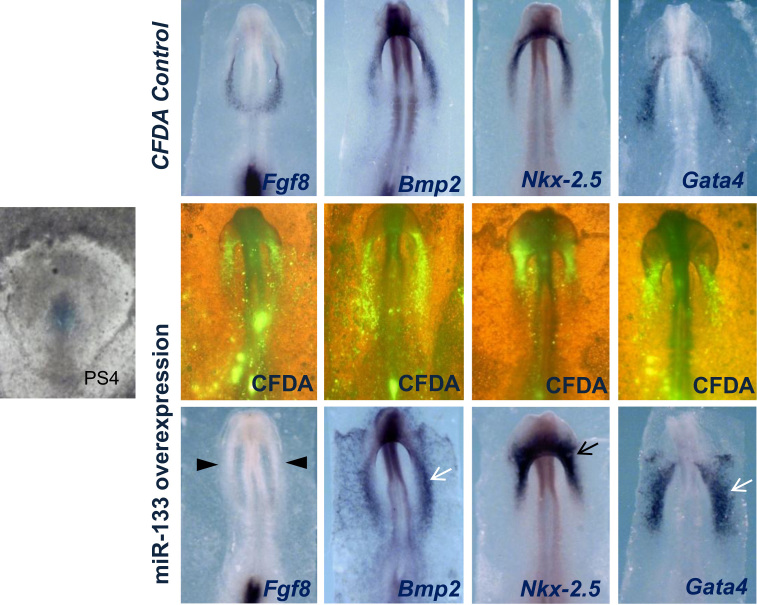
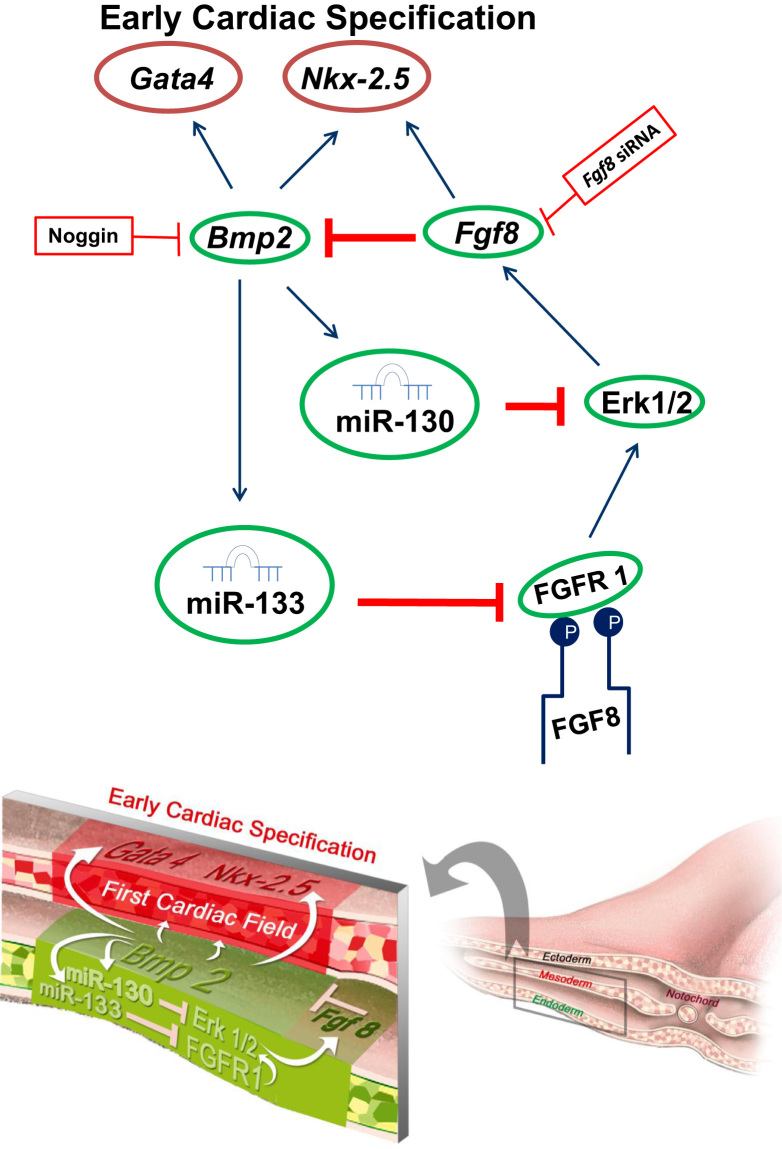
To assess the potential involvement of miR-133 in Fgf8-Bmp2 cooperation during cardiogenesis, we analyzed the effects of miR-133 overexpression on the precardiac primitive streak cells, resulting in Fgf8 inhibition and Bmp2 increase. Subsequently, Nkx-2.5 and Gata4 increase as well (Fig. 2). Interestingly, our loss-of-function experiments through Fgf8 siRNA electroporation showed an increased miR-133 expression (Fig. 3). It is also noteworthy that our Bmp2 overexpression experiments induced miR-133 expression, whereas noggin soaked beads administration -specifically into FCF, showed a decrease of miR-133 expression in the ipsilateral endocardial tube (Fig. 4), suggesting that miR-133 is upstream-regulated by Bmp2. All the above results clearly indicate that a reciprocal repression between miR-133 and Fgf8 regulates cardiac induction through Bmp2 signaling (Fig. 5), thus constituting complementary data to those obtained after our miR-130 analysis [15]. However, although miR-133 may be identified as a putative microRNA that targets FGFR1 3′UTR -site broadly conserved among vertebrates, its connection for Fgf8/ERK pathway in this process remains to be further established.
Fig. 2.
Effect of miR-133 gain-of-function on primitive endocardial tube specification. Embryos electroporated at the level of the primitive streak precardiac (left image) at stage PS4: Whole-mount ISH for Fgf8, Bmp2, Nkx-2.5 and Gata4. Note the dramatic reduced Fgf8 expression (arrowheads), whereas Bmp2, Nkx-2.5 and Gata4 are significantly increased (arrows), at the primitive endocardial tube level. Visualization of CFDA expression of embryos processed for ISH is shown in the immediate upper panel.
Fig. 3.

Effect of Fgf8 loss-of-function on primitive endocardial tube specification. Whole-mount ISH for miR-133. Embryos electroporated at the level of the primitive streak precardiac cells (left image), either with the control construct or Fgf8 siRNA expressing construct. Note that at the primitive endocardial tube level miR-133 is markedly increased (arrow). EGFP expression of experimental embryos is shown in the immediate left panel.
Fig. 4.

Effect of Bmp2 gain- and loss-of-function on primitive endocardial tube specification: whole-mount ISH for miR-133 . (A) Embryo electroporated at the level of the primitive streak precardiac at stage PS4 (left image), either with the control construct or with Bmp2 expressing construct. Note the significant increase of miR-133 expression (arrow) at the primitive endocardial tube level. Visualization of EGFP expression of embryos processed for ISH is shown in the immediate left panel. (B) After noggin soaked bead application just inside FCF, at PS11 stage (right drawing), miR-133 is diminished at the level of the ipsilateral endocardial tube (arrowhead).
Fig. 5.
Proposed model for early cardiac specification. Our model indicates that the earliest cardiac markers -Nkx-2.5 and Gata4- are induced by Bmp2, which is repressed by Fgf8. Moreover, Bmp2 induces miR-130 and miR-133. Both repress Erk1/2 and FGFR1, respectively. Since Fgf8 is controlled by these two factors -Erk1/2 and FGFR1, Bmp2 modulates Fgf8 expression. Thus, miR-130 and miR-133 act as necessary linkages in the control of Fgf8 signaling, mediated by Bmp2, establishing a negative feed-back loop responsible to achieve the initial cardiac specification.
2. Experimental design, materials and methods
Fertilized eggs (Granja Santa Isabel, Córdoba, Spain) were incubated at 38 °C in forced-draft humidified incubators. Embryos were staged (PS stages: [14]; HH stages: [10]) and subjected to early chick (EC) embryo culture [3]. Two groups of embryos were selected for experiments:
2.1. Group 1. Embryo electroporation of precardiac primitive streak cells
Cultured embryos were injected and electroporated in precardiac primitive streak cells. For gain-of-function experiments, two different groups of embryos were electroporated with Bmp2 expressing construct (pIRES-Bmp2-EGFP) and pre-miR-133, respectively. For loss-of-function experiments, a group of embryos was electroporated with Fgf8 siRNA expressing construct (pSilencer-Fgf8). For control embryos, EGFP expressing construct (pCAGGs-EGFP), or CFDA, was electroporated. Embryos were additionally incubated for 14 to 16 h.
2.2. Group 2. Bead implantation
For loss-of-function experiments, beads were soaked in noggin (an antagonist of BMP signals) solution and implanted at the desired site. The embryos were additionally incubated for 6–8 h.
After the experimental procedures, the selected embryos were fixed overnight in 4% PFA, dehydrated in methanol and stored at −20 °C. Subsequently, they were processed for whole mount in situ hybridization: embryos were hydrated by incubation in graded methanol/PBT steps to a pure sterile PBT solution and processed for ISH following standard procedure [4] using antisense-Nkx2.5, -Bmp2, -Fgf8, and -Gata4 labeled probes, respectively. A group of embryos was processed [5] for ISH with LNA-labelled microRNA probes (Exiqon) against miR-133.
Acknowledgments
We thank María Pérez for her invaluable technical support. This work has been partially financed with Grants to research groups CTS005 (to VGM) from the Junta de Extremadura, with FEDER co-financing, and CVI-6556 (to DF) from the Junta de Andalucía Regional Council.
References
- 1.Carè A., Catalucci D., Felicetti F., Bonci D., Addario A., Gallo P., Bang M.L., Segnalini P., Gu Y., Dalton N.D., Elia L., Latronico M.V., Høydal M., Autore C., Russo M.A., Dorn G.W., Ellingsen O., Ruiz-Lozano P., Peterson K.L., Croce C.M., Peschle C., Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 2.Cordes K.R., Srivastava D., Ivey K.N. MicroRNAs in cardiac development. Pediatr. Cardiol. 2010;31:349–356. doi: 10.1007/s00246-010-9639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman S.C., Collignon J., Schoenwolf G.C., Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev. Dyn. 2001;220:284–289. doi: 10.1002/1097-0177(20010301)220:3<284::AID-DVDY1102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Chapman S.C., Schubert F.R., Schoenwolf G.C., Lumsden A. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev. Biol. 2002;245:187–199. doi: 10.1006/dbio.2002.0641. [DOI] [PubMed] [Google Scholar]
- 5.Darnell D.K., Kaur S., Stanislaw S., Konieczka J.H., Yatskievych T.A., Antin P.B. MicroRNA expression during chick embryo development. Dev. Dyn. 2006;235:3156–3165. doi: 10.1002/dvdy.20956. [DOI] [PubMed] [Google Scholar]
- 6.Dell’Era P., Ronca R., Coco L., Nicoli S., Metra M., Presta M. Fibroblast growth factor receptor-1 is essential for in vitro cardiomyocyte development. Circ. Res. 2003;5:414–420. doi: 10.1161/01.RES.0000089460.12061.E1. [DOI] [PubMed] [Google Scholar]
- 7.Dong D.L., Chen C., Huo R., Wang N., Li Z., Tu Y.J., Hu J.T., Chu X., Huang W., Yang B.F. Reciprocal repression between microRNA-133 and calcineurin regulates cardiac hypertrophy: a novel mechanism for progressive cardiac hypertrophy. Hypertension. 2010;55:946–952. doi: 10.1161/HYPERTENSIONAHA.109.139519. [DOI] [PubMed] [Google Scholar]
- 8.Echevarria D., Belo J.A., Martinez S. Modulation of Fgf8 activity during vertebrate brain development. Brain Res. Rev. 2005;49:150–157. doi: 10.1016/j.brainresrev.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Hadari Y.R., Kouhara H., Lax I., Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamburger V., Hamilton H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 11.Hardy K.M., Yatskievych T.A., Konieczka J., Bobbs A.S., Antin P.B. FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev. Biol. 2011;11:20. doi: 10.1186/1471-213X-11-20. 〈http://www.biomedcentral.com/1471–213X/11/2〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horie T., Ono K., Nishi H., Iwanaga Y., Nagao K., Kinoshita M., Kuwabara Y., Takanabe R., Hasegawa K., Kita T., Kimura T. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem. Biophys. Res. Commun. 2009;389:315–320. doi: 10.1016/j.bbrc.2009.08.136. [DOI] [PubMed] [Google Scholar]
- 13.Liu N., Bezprozvannaya S., Williams A.H., Qi X., Richardson J.A., Bassel-Duby R., Olson E,N. MicroRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Sanchez C., Puelles L., Garcia-Martinez V., Rodriguez-Gallardo L. Morphological and molecular analysis of the early developing chick requires an expanded series of primitive streak stages. J. Morphol. 2005;264:105–116. doi: 10.1002/jmor.10323. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Sanchez C., Franco D., Bonet F., Garcia-Lopez V., Aranega A., Garcia-Martinez V. Negative Fgf8-Bmp2 feed-back is regulated by miR-130 during early cardiac specification. Dev. Biol. 2015;406:63–73. doi: 10.1016/j.ydbio.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Lunn J.S., Fishwick K.J., Halley P.A., Storey K.G. A spatial and temporal map of FGF/Erk1/2 activity and response repertoires in the early chick embryo. Dev. Biol. 2007;302:536–552. doi: 10.1016/j.ydbio.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Sugi Y., Sasse J., Barron M., Lough J. Developmental expression of fibroblast growth factor receptor-1 (cek-1; flg) during heart development. Dev. Dyn. 1995;202:115–125. doi: 10.1002/aja.1002020203. [DOI] [PubMed] [Google Scholar]
- 18.Villar A.V., Merino D., Wenner M., Llano M., Cobo M., Montalvo C., García R., Martín-Durán R., Hurlé J.M., Hurlé M.A., Nistal J.F. Myocardial gene expression of microRNA-133a and myosin heavy and light chains, in conjunction with clinical parameters, predict regression of left ventricular hypertrophy after valve replacement in patients with aortic stenosis. Heart. 2011;97:1132–1137. doi: 10.1136/hrt.2010.220418. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X., Sasse J., Lough J. Evidence that FGF receptor signaling is necessary for endoderm-regulated development of precardiac mesoderm. Mech. Ageing Dev. 1999;108:77–85. doi: 10.1016/s0047-6374(99)00003-2. [DOI] [PubMed] [Google Scholar]