Abstract
Cardiopulmonary Resuscitation (CPR), defibrillation, and epinephrine administration are pillars of advanced cardiac life support (ACLS). Intraosseous (IO) access is an alternative route for epinephrine administration when intravenous (IV) access is unobtainable. Previous studies indicate the pharmacokinetics of epinephrine administration via IO and IV routes differ, but it is not known if the difference influences return of spontaneous circulation (ROSC). The purpose of this prospective, experimental study was to determine the effects of humeral IO (HIO) and IV epinephrine administration during cardiac arrest on pharmacokinetics, ROSC, and odds of survival. Swine (N = 21) were randomized into 3 groups: humeral IO (HIO), peripheral IV (IV) and CPR/defibrillation control. Cardiac arrest was induced under general anesthesia. The swine remained in arrest for 2 min without intervention. Chest compressions were initiated and continued for 2 min. Epinephrine was administered and serial blood samples collected for pharmacokinetic analysis over 4 min. Defibrillation and epinephrine administration proceeded according to ACLS guidelines continuing for 20 min or until ROSC.
Seven HIO swine, 4 IV swine, and no control swine had ROSC. There were no significant differences in ROSC, maximum concentration; except at 30 s, and time-to-concentration-maximum between the HIO and IV groups. Significant differences existed between the experimental groups and the control. The HIO delivers a higher concentration of epinephrine than the IV route at 30 s which may be a survival advantage. Clinicians may consider using the IO route to administer epinephrine during CA when there is no preexisting IV access or when IV access is unobtainable.
Keywords: Intraosseous, Return of spontaneous circulation, Epinephrine, Pharmacokinetics, Resuscitation
Highlights
-
•
No difference in concentration maximum (Cmax) and time to maximum concentration (Tmax) in epinephrine between humeral intraosseous and intravenous routes of administration over time.
-
•
Humeral intraosseous delivers higher concentration than intravenous at 30 s after administration of epinephrine.
-
•
Humeral intraosseous facilitates rapid delivery of epinephrine during cardiac arrest.
-
•
Use of humeral intraosseous had higher number of subjects survived.
1. Introduction
Incidence of death attributable to cardiovascular disease has declined over the past 15 years but still accounts for 1 of every 3 deaths in the United States. Cardiovascular disease continues to be the leading cause of death in the United States [1], [2]. The incidence of sudden cardiac arrest (CA) in 2013 was approximately 326,200 occurrences of out-of-hospital CA and 209,000 occurrences of in-hospital CA [1]. Research has shown that survival depends on a rapid sequence of therapeutic interventions termed the “chain of survival.” Vascular access is a vital step in this chain [3], [4],[5]. Several studies demonstrate establishing rapid vascular access is essential in enhancing outcomes during cardiac arrest [3], [6], [7], [8], [9], [10]. However, during CA, environmental conditions and cardiovascular compromise may make intravenous (IV) vascular access difficult or impossible and time consuming. The intraosseous (IO) route has been demonstrated to be a reliable and effective alternative when IV access cannot be obtained [3], [11], [12], [13]. Several organizations including the American Heart Association (AHA), the European Resuscitation Council (ERC), and several others recommend the use of IO access if IV access is not readily available [3], [14], [15], [16], [17], [18], [19], [20].
Few studies have investigated the effects of IO epinephrine pharmacokinetics specifically maximal plasma concentration (Cmax) and time to maximum plasma concentration (Tmax) during CA. Investigations conducted by coauthors of this study found that epinephrine administered via tibial IO route achieved a lower Cmax and a demonstrated a prolonged Tmax when compared to either the sternal IO and IV routes [5], [11]. Hoskins et al. reported a prolonged Tmax when administering epinephrine during CA using the tibial IO route compared to central venous route [21]. No study to date has examined the effects of IO administration of epinephrine on return of spontaneous circulation (ROSC) and pharmacokinetic measurements of plasma epinephrine during cardiac arrest with ongoing CPR.
The purpose of this study was to determine the effects of the humeral IO administration of epinephrine compared to IV on ROSC and pharmacokinetics in a swine model of cardiac arrest. The research questions that guided this study were:
-
1.
Are there statistically significant differences in the rate ROSC between the humeral IO, IV, and control groups?
-
2.
Are there statistically significant differences in Cmax and Tmax of epinephrine between the humeral IO, IV, and control groups?
-
3.
Are there statistically significant differences in the odds of survival between the humeral IO, IV, and control groups?
-
4.
Are there statistically significant differences in the plasma concentration of epinephrine between the humeral IO and IV groups?
2. Methods
This study was a prospective, between groups, experimental design approved by the TriService Research Laboratory Institutional Animal Care and Use Committee. The animals received care in compliance with the Animal Welfare Act and the Guide to the Care and Use of Laboratory Animals. The investigators used a swine model because of ease of care, relatively inexpensive, and, more importantly, because the cardiovascular system and bone marrow of swine are comparable to humans and accepted as analogs in research [22], [23].
We used a computer generated random number to assign twenty-one Yorkshire-cross swine (Sus scrofa) to three groups: humeral IO (n = 7), peripheral IV (n = 7), and CPR/defibrillation control (n = 7). Food was withheld after midnight before the experiment. Water was allowed ad libitum up to the time of the experiment.
Thirty minutes prior to instrumentation, the swine were sedated, anesthetized, and placed on mechanical ventilation. Anesthesia was induced with an intramuscular injection of Telazol (4–8 mg/kg) and inhaled isoflurane (4%–5%). An 18-gauge peripheral IV was started in an auricular (ear) vein in all subjects. The auricular peripheral IV was used as the site of epinephrine administration for the IV experimental group. The auricular IV was maintained at a keep-vein-open (KVO) rate to ensure patency. After placement of an endotracheal tube, the isoflurane concentration was decreased to a maintenance dose (1%–2%) until CA was induced.
The animals were ventilated at 8–10 mL/kg tidal volume with a Narkomed 3A anesthesia machine (Dräger, Telford, PA). Respiratory rate was set at 10–14 breaths per minute. In all groups a 20 gauge catheter was placed in the left carotid artery using a cut-down technique. The arterial catheter was connected to a Phillips MP 50 system (Phillips Healthcare, Andover, MA) for continuous monitoring of systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP). The arterial line was also connected to a Vigileo Hemodynamic Monitor (Edwards Lifesciences, Irvine, CA) for continuous monitoring of cardiac output (CO) and stroke volume (SV). The Phillips MP 50 system was also used to monitor heart rate and rhythm, pulse oximetry (SpO2), end-tidal carbon dioxide (ETCO2), and rectal temperature. Normothermia was maintained using a forced air-warming blanket to maintain body temperature ≥37.0 °C.
Swine in the HIO group had a 15 gauge × 45 mm EZ-IO device (Teleflex Medical, San Antonio, TX) inserted in the humerus following surgical exposure. Surgical exposure was necessary to ensure correct placement of the HIO device because of the thick overlying soft tissue present in swine not found in humans. Placement of the HIO needle was verified by aspiration of bone marrow and ease of irrigation with 10 mL of normal saline.
Swine were stabilized for 5 min prior to beginning the experiment. Cardiac arrest was induced in all swine using the transcutaneous electrical induction technique. Specifically, a needle was inserted at the left sternal border between the second and third intercostal space at a depth of 3.25 cm. A second needle was inserted immediately caudal to the xiphoid process at a depth of 6 cm. Lead wires were attached to both needles. One lead wire was connected to the negative pole of three 9-V batteries connected in series. The other lead wire was rapidly tapped on the positive pole placing the swine into ventricular fibrillation. Cardiac arrest was operationally defined as any nonperfusing arrhythmia resulting in a SBP ≤60 mm/Hg. The subjects remained in arrest for 2 min without intervention. Most of the swine had ventricular fibrillation within 10 s, and all achieved arrest within 30 s. CPR was initiated at 2 min post-arrest using the “Thumper” Mechanical Compression Device, Model 1008 (Michigan Instruments, Grand Rapids, MI). The device was used to reproducibly compress the sternum to a predetermined depth of two inches and a rate of hundred compressions per minute. Ventilations were administered at 12 breaths per minute [24].
At 4 min post-arrest, the investigators administered epinephrine 1 mg to the IV and humeral IO groups according to group assignment followed by 10 mL of NS flush. The control group did not receive epinephrine. CPR continued for an additional 4 min. During this 4 min, the researchers collected aerial arterial blood samples pharmacokinetic analysis from the carotid arterial line. Seven samples were collected at 30, 60, 90, 120, 150,180 and 240 s. At 8 min post-arrest, the swine were defibrillated with 360 J (J). Defibrillation was repeated at 2 min intervals and epinephrine administration repeated every 4 min for 20 min (24 min post-arrest) or until ROSC was achieved. Animals achieving ROSC received standard AHA post-cardiac arrest care and were monitored for 30 min. Anesthesia, as tolerated by the animal, was immediately resumed.
3. Results
We examined the random assignment of animals to groups by running descriptive statistics by group. The “average” animal weighed 69.14 kg, had a heart rate of 72.5 BPM, and a temperature of 36.9 °C. We also evaluated heterogeneity by running Kruskal–Wallis tests (non-parametric ANOVA) for each of the measures by group. There were no significant differences indicating that the groups were equivalent on these parameters (See Table 1 provides the baseline measures). Only swine that were between 126 and 128 days old were used in all groups.
Table 1.
Descriptive statistics by group.
| Base measures | IV |
Humerus |
CPR defib |
Total |
p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | ||
| Weight in kg | 71.84 | 2.35 | 68.83 | 6.31 | 67.91 | 5.74 | 69.53 | 5.14 | 0.46 |
| Systolic (in.) | 99.00 | 6.53 | 94.86 | 10.37 | 104.57 | 16.13 | 99.48 | 11.82 | 0.33 |
| Diastolic (in.) | 65.57 | 6.37 | 61.43 | 13.00 | 61.29 | 10.13 | 62.76 | 9.89 | 0.60 |
| Heart rate (BPM) | 77.86 | 9.92 | 66.57 | 9.50 | 74.86 | 11.25 | 73.10 | 10.89 | 0.22 |
| Mean arterial pressure | 79.57 | 6.70 | 75.00 | 13.63 | 74.71 | 12.11 | 76.43 | 10.88 | 0.64 |
| Temperature in C | 37.03 | 0.92 | 37.00 | 0.40 | 37.07 | 0.73 | 37.03 | 0.68 | 0.85 |
| Cardiac output | 5.10 | 1.13 | 4.13 | 0.88 | 6.27 | 1.65 | 5.17 | 1.50 | 0.05 |
| Stroke volume | 69.57 | 15.22 | 64.86 | 7.36 | 86.43 | 29.18 | 73.62 | 20.76 | 0.26 |
| End-tidal CO2 | 44.86 | 6.23 | 40.86 | 6.28 | 40.17 | 4.62 | 42.05 | 5.91 | 0.40 |
| Oxygen | 94.86 | 5.27 | 94.57 | 5.00 | 94.57 | 3.82 | 94.67 | 4.50 | 0.92 |
Next, we evaluated pairwise outcomes of survivability using Fisher's Exact Test (FET) and odds ratio. There was evidence that the HIO group was significantly different from the control group (FET, p < .001) and that the IV group was significantly different from the control (FET, p = .035). There was insufficient evidence to demonstrate a significant difference in effect between the IO humerus group and the IV group (FET, p = .096) (See Table 2 for a summary of subjects that survived and those that did not).
Table 2.
Cross-tabulation of group members by status.
| Groups |
Total | ||||
|---|---|---|---|---|---|
| CPR with defib | HIO | IV | |||
| Survive? | Yes | 0 | 7 | 4 | 11 |
| No | 7 | 0 | 3 | 10 | |
| Total | 7 | 7 | 7 | 21 | |
The odds of survival for the HIO group in comparison with the control group (adjusting cells upwards .5 to account for zeros) was 225.00, 95% CI (3.926, 12895.826), and the odds of survival for the IV group in comparison to the control was 19.286, 95% CI (.798, 466.265). The survival odds ratio for the HIO group versus the IV group was 11.667, 95% CI (.483, 282.061).
The concentration is reported in ng per mL. To evaluate potential differences in Cmax by group (HIO vs. IV), we ran Wilcoxon signed ranks. The results were not statistically significant (W = 31.00, p = .4557) (See Fig. 1 for a side-by-side boxplot of Cmax by group).
Fig. 1.
Boxplots of Cmax by treatment group.
We performed this same analysis for Tmax. The Wilcoxon signed ranks test approaches significance (W = 10.50, p = .062) (See Fig. 2 is the side-by-side boxplots in maximum concentration in seconds).
Fig. 2.
Boxplots of Tmax by treatment group.
We also evaluated mean epinephrine concentrations for 30, 60, 90, 120, 150,180 and 240 s for the HIO and IV groups by Wilcoxon signed rank tests. Only the 30 s time period had statistically different concentrations (W = 34.00, p = .017) with the HIO group having more than four times greater concentration (620.91 versus 150.06). We also analyzed the concentrations of epinephrine over time by group (See Fig. 3 results of the epinephrine concentrations).
Fig. 3.
Epinephrine concentration over time.
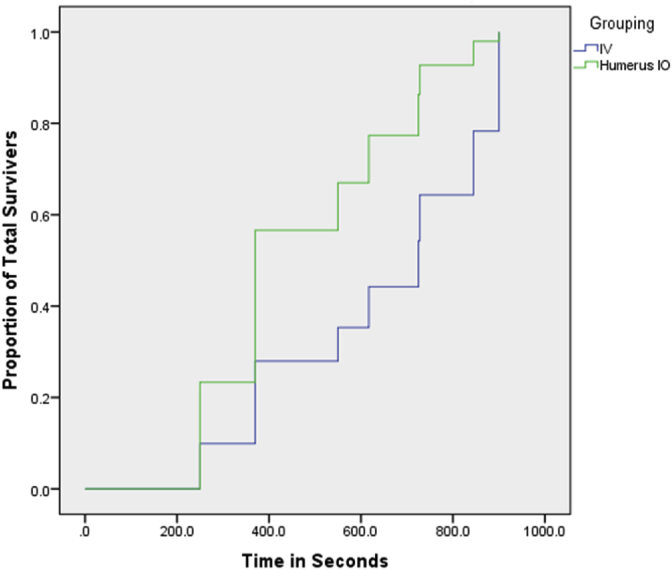
We also evaluated a Cox regression model for time to ROSC for surviving animals based on group assignment and found no statistically significant differences between the HIO and the IV groups (-2LL = 34.048, p = .176) (See Fig. 4 of the cumulative survival by group and by time).
Fig. 4.
Plot of proportion surviving by time by group.
4. Discussion
The combination of CPR, defibrillation, and epinephrine administration are pillars of advanced cardiac life support. The 2015 draft ECC guidelines emphasize the importance of epinephrine as the first-line agent to be used in treating CA. Additionally, the ECC draft guidelines support establishing early vascular access including the use of the IO route. The results of this study provided evidence supporting the early use of epinephrine and the use of the humeral IO route as an alternative when IV access is unobtainable.
Compared to CPR and defibrillation only, the administration of epinephrine via HIO or IV significantly improved the likelihood of achieving ROSC and increased the odds of survival. Unexpectedly, all animals in HIO group had ROSC compared to the four of seven animals in the IV group that had ROSC. The investigators surmised epinephrine administered via the HIO route might enter the circulation more directly than when administered in a peripheral ear vein. Perhaps because the HIO injection site is analogous to central venous access as the humeral circumflex vein empties directly into the axillary vein to the subclavian vein and to the superior vena cava. Another consideration is the HIO site is more proximal to the site of chest compressions compared to the peripheral ear IV site. Both of these explanations may explain the higher Cmax of epinephrine in the HIO group compared to the IV group at the 30 s time point. The clinical implication of these results may be the HIO route may facilitate more rapid delivery of epinephrine to the effect site during CA with ongoing CPR resulting in all subjects achieving ROSC in that group.
Another result of importance in this study was the lack of statistical difference in the overall Cmax and Tmax of epinephrine between the HIO and IV routes. Previous studies have indicated that the Cmax and Tmax of epinephrine administered via the IO and IV routes differ [5], [21], [25]. The investigators hypothesized the IV group would have a higher Cmax and shorter Tmax. However, this study demonstrated there was no statistically significant difference in the measured pharmacokinetic variables between the HIO and IV groups except for the HIO group having a higher Cmax than the IV group at the 30 s time point. Whether the higher Cmax of epinephrine given via the HIO route at the 30 s time point would be clinically significant in humans remains unknown. However, the results of this investigation indicate there may be a survival advantage when epinephrine is given by the HIO route. These findings provide supportive evidence and is consistent with Zuercher's finding that early IO epinephrine improved resuscitative outcome compared with delayed IV epinephrine [26].
This study was performed using a swine model which may not be generalizable to humans. However, the cardiovascular system and bone marrow of swine are comparable to humans and accepted as analogs in research [22], [23]. Additionally, second-line medications including vasopressors and antiarrhythmics were not employed during resuscitative efforts to eliminate the possibility of intervening variables affecting pharmacokinetic and resuscitative outcome measurements. The data from this study strongly suggest that if there is no preexisting IV access or immediate IV access is unobtainable during CA, placement of an IO device for the administration of epinephrine should implemented.
Future investigators might consider large, multicenter, retrospective studies in humans experiencing in or out-of-hospital cardiac arrests, comparing the IO and IV routes of epinephrine administration relative to the occurrence of ROSC, time to ROSC, and neurologic outcome 24 h post-arrest. The results of this study provides evidence indicating there may be a survival advantage when the HIO route is used to administer epinephrine compared to the peripheral IV route in a swine model of CA with ongoing CPR. Further, the evidence suggests the Cmax of epinephrine is higher when given by the HIO route compared to the IV route at the 30 s post-injection time point which may allow epinephrine to reach the effect site more quickly when the HIO route is used. The clinical significance of these results, applied to humans, remains unknown and should be investigated. Based on these results, clinicians may consider using the IO route for the rapid administration of epinephrine during CA when there is no preexisting IV access or when IV access is unobtainable.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to disclose.
Funding sources
This study was funded by a grant from the TriService Nursing Research Program (TSNRP) (N13-P10). The TSNRP had no role in the study design, collection, analysis and interpretation of data; writing of the manuscript; and the decision to submit the manuscript for publication.
Acknowledgments
The investigators thank the Naval Medical Research Unit-San Antonio for their support in completing this study.
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Kochanek K.D., Murphy S.L., Xu J., Arias E. Mortality in the United States. NCHS Data Brief. 2013;2014(178):1–8. [PubMed] [Google Scholar]
- 3.Neumar R.W., Otto C.W., Link M.S., Kronick S.L., Shuster M., Callaway C.W. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl. 3):S729–S767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 4.Anson J.A. Vascular access in resuscitation: is there a role for the intraosseous route? Anesthesiology. 2014;120(4):1015–1031. doi: 10.1097/ALN.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 5.Burgert J., Gegel B., Loughren M., Ceremuga T., Desai M., Schlicher M. Comparison of tibial intraosseous, sternal intraosseous, and intravenous routes of administration on pharmacokinetics of epinephrine during cardiac arrest: a pilot study. AANA J. 2012;80(4 Suppl):S6–S10. [PubMed] [Google Scholar]
- 6.Harris M., Balog R., Devries G. What is the evidence of utility for intraosseous blood transfusion in damage-control resuscitation? J. Trauma Acute Care Surg. 2013;75(5):904–906. doi: 10.1097/TA.0b013e3182a85f71. [DOI] [PubMed] [Google Scholar]
- 7.Lewis P., Wright C. Saving the critically injured trauma patient: a retrospective analysis of 1000 uses of intraosseous access. Emerg. Med. J. EMJ. 2014;32(6):463–467. doi: 10.1136/emermed-2014-203588. [DOI] [PubMed] [Google Scholar]
- 8.Reades R., Studnek J.R., Vandeventer S., Garrett J. Intraosseous versus intravenous vascular access during out-of-hospital cardiac arrest: a randomized controlled trial. Ann. Emerg. Med. 2011;58(6):509–516. doi: 10.1016/j.annemergmed.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Kudenchuk P.J., Cobb L.A., Copass M.K., Cummins R.O., Doherty A.M., Fahrenbruch C.E. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N. Engl. J. Med. 1999;341(12):871–878. doi: 10.1056/NEJM199909163411203. [DOI] [PubMed] [Google Scholar]
- 10.Dorian P., Cass D., Schwartz B., Cooper R., Gelaznikas R., Barr A. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N. Engl. J. Med. 2002;346(12):884–890. doi: 10.1056/NEJMoa013029. (Erratum appears in N Engl J Med 2002 Sep 19;347(12):955) [DOI] [PubMed] [Google Scholar]
- 11.Burgert J.M., Austin P.N., Johnson A. An evidence-based review of epinephrine administered via the intraosseous route in animal models of cardiac arrest. Mil. Med. 2014;179(1):99–104. doi: 10.7205/MILMED-D-13-00231. [DOI] [PubMed] [Google Scholar]
- 12.Rea T.D., Eisenberg M.S., Sinibaldi G., White R.D. Incidence of EMS-treated out-of-hospital cardiac arrest in the United States. Resuscitation. 2004;63(1):17–24. doi: 10.1016/j.resuscitation.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Rea T.D., Pearce R.M., Raghunathan T.E., Lemaitre R.N., Sotoodehnia N., Jouven X. Incidence of out-of-hospital cardiac arrest. Am. J. Cardiol. 2004;93(12):1455–1460. doi: 10.1016/j.amjcard.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Weiser G., Hoffmann Y., Galbraith R., Shavit I. Current advances in intraosseous infusion - a systematic review. Resuscitation. 2012;83(1):20–26. doi: 10.1016/j.resuscitation.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Kleinman M.E., de Caen A.R., Chameides L., Atkins D.L., Berg R.A., Berg M.D. Part 10: pediatric basic and advanced life support: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2010;122(16 Suppl. 2):S466–S515. doi: 10.1161/CIRCULATIONAHA.110.971093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinman M.E., Chameides L., Schexnayder S.M., Samson R.A., Hazinski M.F., Atkins D.L. Pediatric advanced life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics. 2010;126(5) doi: 10.1542/peds.2010-2972D. e1361–99. [DOI] [PubMed] [Google Scholar]
- 17.Physicians ACoE . 2011. Clinical Policy Statement: Alternative Methods to Vascular Access in the Emergency Department.http://www.acep.org/Clinical–-Practice-Management/Alternative-Methods-to-Vascular-Access-in-the-Emergency-Department (cited 2015 2/28/21015). Available from: [DOI] [PubMed] [Google Scholar]
- 18.American College of Surgeons CoT . Advanced Trauma Life Support for Doctors, Student Course Manual. ninth ed. American College of Surgeons; Chicago, IL: 2012. [Google Scholar]
- 19.Fowler R., Gallagher J.V., Isaacs S.M., Ossman E., Pepe P., Wayne M. The role of intraosseous vascular access in the out-of-hospital environment (resource document to NAEMSP position statement) Prehosp. Emerg. Care. 2007;11(1):63–66. doi: 10.1080/10903120601021036. [DOI] [PubMed] [Google Scholar]
- 20.Gerhardt RTM R.L., De Lorenzo R.A., Butler F.K. Office of the Surgeon General, Department of the Army; Falls Church, VA: 2012. Fundamentals of Combat Casualty Care. Combat Casualty Care: Lessons Learned from OEF and OIF. [Google Scholar]
- 21.Hoskins S.L., do Nascimento P., Jr., Lima R.M., Espana-Tenorio J.M., Kramer G.C. Pharmacokinetics of intraosseous and central venous drug delivery during cardiopulmonary resuscitation. Resuscitation. 2012;83(1):107–112. doi: 10.1016/j.resuscitation.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 22.Hannon J.P., Bossone C.A., Wade C.E. Normal physiological values for conscious pigs used in biomedical research. Lab. Anim. Sci. 1990;40(3):293–298. [PubMed] [Google Scholar]
- 23.Swindle M.M., Makin A., Herron A.J., Clubb F.J., Jr., Frazier K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012;49(2):344–356. doi: 10.1177/0300985811402846. [DOI] [PubMed] [Google Scholar]
- 24.Berg R.A., Hemphill R., Abella B.S., Aufderheide T.P., Cave D.M., Hazinski M.F. Part 5: adult basic life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl. 3):S685–S705. doi: 10.1161/CIRCULATIONAHA.110.970939. [DOI] [PubMed] [Google Scholar]
- 25.Burgert J., Gegel B., Johnson D., Loughren M. The Pharmacokinetics of intravenous, tibial intraosseous, and sternal intraosseous epinephrine during CPR in a swine model of cardiac arrest. Minerva Medica. 2014;105(2 Suppl. 1):35. [Google Scholar]
- 26.Zuercher M., Kern K.B., Indik J.H., Loedl M., Hilwig R.W., Ummenhofer W. Epinephrine improves 24-hour survival in a swine model of prolonged ventricular fibrillation demonstrating that early intraosseous is superior to delayed intravenous administration. Anesth. Analg. 2011;112(4):884–890. doi: 10.1213/ANE.0b013e31820dc9ec. [DOI] [PubMed] [Google Scholar]