Abstract
Eggs of the lung fluke genus Paragonimus were detected in red-capped mangabeys (Cercocebus torquatus) in Nigeria. We assess the role of these primates as potential sylvatic hosts and the clinical effects of the parasite on monkeys. DNA sequenced from eggs in feces were 100% identical in the ITS2 region to Paragonimus africanus sequences from humans in Cameroon. Paragonimus-positive monkeys coughed more than uninfected monkeys. Experimental de-worming led to reduction in parasite intensity and a corresponding reduction of coughing to baseline levels in infected monkeys. This report provides the first evidence of Paragonimus sp. in C. torquatus, of P. africanus in Nigerian wildlife, and the first molecular evidence of the parasite in African wildlife. Coughing, sometimes interpreted as a communication behavior in primates, can actually indicate infection with lung parasites. Observations of coughing in primates may, in turn, provide a useful mechanism for surveillance of Paragonimus spp, which are re-emerging human pathogens, in wildlife reservoirs.
Keywords: Paragonimus africanus, Nigeria, Primates, Cercocebus torquatus, Coughing, Respiratory disease
Graphical abstract

Highlights
-
•
We provide molecular evidence of Paragonimus sp. infection in African wildlife.
-
•
P. africanus in primates is 100% identical to humans at the ITS2 region.
-
•
Coughing in wild primates can indicate infection with lung flukes.
-
•
We offer a method for surveillance of wildlife for potentially zoonotic lung flukes.
1. Introduction
Paragonimiasis is a food-borne illness of the lung caused by trematodes of the genus Paragonimus. Humans can become infected with this lung fluke after consuming raw or undercooked freshwater crustaceans. Before infecting mammalian hosts, Paragonimus species require a snail as the first intermediate host, and a freshwater crab or crayfish as the second intermediate host. In mammalian definitive hosts, the infective metacercariae excyst in the duodenum and migrate to the lungs, causing pulmonary paragonimiasis, with respiratory symptoms (e.g. coughing) that mimic tuberculosis (Toscano et al., 1995). Ectopic paragonimiasis occurs when flukes migrate internally, causing damage to muscles and organs, including the brain (cerebral paragonimiasis) (Blair, 2014). Paragonimus spp. infect more people globally than any other foodborne trematode, and infections cause an estimated 196,710 disability adjusted life-years (Fürst et al., 2012). These estimates do not account for infections in Africa.
Paragonimus spp. is best known from Asia and Latin America (Fürst et al., 2012). However, two known species, Paragonimus africanus and P. uterobilaterlis, infect humans in Africa (Blair, 2014). Paragonimus uterobilateralis is considered the principal causative agent of paragonimiasis in Nigeria (Aka et al., 2008). The epidemiology of Paragonimus sp. infections in Nigeria is tightly bound to post-colonial history. Prior to the Biafran war in Nigeria (1967–1970), paragonimiasis was known only from a handful of cases (Nnochiri, 1968; Nwokolo, 1964). During the war, food shortages and limited access to cooking facilities led to increased consumption of inadequately cooked or raw crab, and cases of paragonimiasis increased dramatically (Nwokolo, 1972). Human infections nearly disappeared again after the war, until recent surveys revealed unexpected high prevalence (up to 13.2%) in communities in the Southeast part of Nigeria (Aka et al., 2008). The epidemiology of Paragonimus sp. in Nigeria differs from that in Cameroon, where the disease has a longer history of endemicity due to the cultural practice of eating raw crabs in some areas (World Health Organization, 1995).
The African civet (Viverra civetta) is considered the natural host of P. uterobilateralis in Nigeria (Voelker and Sachs, 1974), with the swamp mongoose (Atilax paludinosus) and domestic dog (Canis familiaris) harboring the parasite in Cameroon and Liberia, respectively (Voelker and Vogel, 1965). Paragonimus africanus has a broader host range, infecting the mongoose (Crossarchus obscurus), palm civet (Nandinia binotata), drill monkey (Mandrillus leucophaeus), potto (Perodicticus potto), and domestic dog (C. familiaris) in Cameroon (Voelker and Vogel, 1965; Sachs and Voelker, 1975). The intermediate and definitive hosts of P. africanus range throughout the contiguous forest of southeastern Nigeria bordering Cameroon (Kingdon, 2005; Abraham and Akpan, 2011), suggesting that P. africanus could be more widely distributed than is currently appreciated.
We report the discovery of Paragonimus sp. eggs in red-capped mangabeys (Cercocebus torquatus) in Nigeria. We sequenced Paragonimus sp. DNA directly from eggs in feces to identify it to species and compare it to parasites reported in human populations. We also made observations of primate hosts for clinical signs of infection. Finally, we examined clinical observational data prior to and following treatment of the study population with anthelminthic drugs. We use this information to assess the presence of Paragonimus sp. in red-capped mangabeys in Nigeria, the role of these primates as potential hosts, and the clinical effects of the parasite on monkeys.
2. Materials and methods
Between May and August 2012, we collected fecal samples and recorded coughing opportunistically from a group of 49 [23 adults/sub-adults (≥3 yo) and 12 juveniles (<3 yo)] individually identifiable red-capped mangabeys that lived in a 1-ha open topped forest enclosure within the natural home range of the species (Fig. 1). The population was provisioned daily, but also had access to wild foods within the enclosure. The animals had access to water ad libitum, from a natural stream that ran through the enclosure. They were exposed to natural predators (e.g. snakes and birds of prey) and parasites. All animals were rescued from the bushmeat and pet trades in Nigeria as young juveniles, or were captive-born. Thirteen individuals were moved from a sancutary to the open-topped enclosure in 2004 as part of the rehabilitation and release program of the Centre for Education, Research and Conservation of Primates and Nature (CERCOPAN), and the remaining 36 individuals were born in the enclosure.
Fig. 1.
Map of collection site. Map shows the location of the study population (black circle) relative to the Oban Division of Cross River National Park (CRNP), in Cross River State, Nigeria.
For 30 days in late May and June 2012, we collected triplicate fecal samples from each individual. Then, in late June 2012, the population was treated for Paragonimus sp. via orally administered praziquantel (approximately 20 mg/kg for three consecutive days). We collected subsequent triplicate fecal samples from each individual over 30 days post-treatment, for a total of 294 samples (147 pre-treatment and 147 post-treatment). Over the same time periods (30 days pre-treatment and 30 days post-treatment), we recorded all observed instances of coughing between 6:00 and 17:00 daily. The Institutional Animal Care and Use Committee at University of Wisconsin, Madison approved all research activities (protocol v1490).
Fecal samples were collected from known individuals immediately following defecation, stored temporarily in plastic bags, and fixed within 2 h of collection. We removed two aliquots from each sample for preservation of gastrointestinal parasite eggs and DNA separately. One aliquot was fixed in 10% formalin for microscopic analysis, and the other in RNAlater® nucleic acid stabilizing solution for genetic analysis. Samples were transported to the University of Wisconsin, Madison following all applicable import, export, and International Air Transport Association regulations. One gram of formalin-preserved feces was concentrated by sedimentation and examined microscopically at X10 and X40 magnification (Greiner and McIntosh, 2009). We calculated prevalence (percent of individuals infected) as number of individuals shedding eggs divided by the total number of individuals examined, and we approximated mean and median intensity of infection (number of eggs per gram (epg) of a particular parasite species in the feces of a single infected host) (Bush et al., 1997; Greiner and McIntosh, 2009). We calculated pre- and post-treatment intensity by taking the average epg of triplicate samples for each individual. We compared Paragonimus sp. prevalence and intensity to host characteristics and rates of coughing using Fisher's exact test, Mann–Whitney test, and Spearman rank correlation. We then compared parasite intensity and coughing rates pre- and post-treatment using paired Wilcoxon rank sum test. For all pre- and post-treatment comparisons, we used one-tailed tests under the directional hypotheses that coughing frequency would be positively associated with parasite infection.
We extracted DNA from 150 mg of the fecal sample with the highest egg count (1,067 epg) using the Zymo ZR Fecal DNA MiniPrep Kit (Zymo Research Corporation, Irvine, CA, USA), following the manufacturer's protocols. PCR and nucleotide sequencing were performed on the internal transcribed spacer 2 region (ITS2) using primers 3S (5′-CGGTGGATCACTCGGCTCGT-3′) and A28 (5′-CCTGGTTAGTTTC TTTTCCTCCGC-3′), previously used to amplify P. africanus (Nkouawa et al., 2009). PCR was performed using Phusion High-Fidelity PCR mastermix (New England BioLabs, Ipswich, MA), and cycled in a BioRad CFX96 platform (Bio-Rad Laboratories, Hercules, CA, USA) with the following cycling parameters: 98 °C for 30 min; 40 cycles of 98 °C for 10 s, 55 °C for 30 s, 72 °C for 90 s; and a final extension at 72 °C for 10 min. Amplicons were electrophoresed on an agarose gel stained with ethidium bromide and then purified using the Zymoclean Gel DNA Recovery Kit (Zymo Research Corporation, Irvine, CA, USA). Amplicons were sequenced on ABI 3730xl DNA Analyzers (Applied Biosystems, Grand Island, NY, USA) at the University of Wisconsin–Madison Biotechnology Center DNA Sequencing Facility. Sequences were aligned to published Paragonimus sequences using CLUSTAL W (Thompson et al., 1994).
3. Results
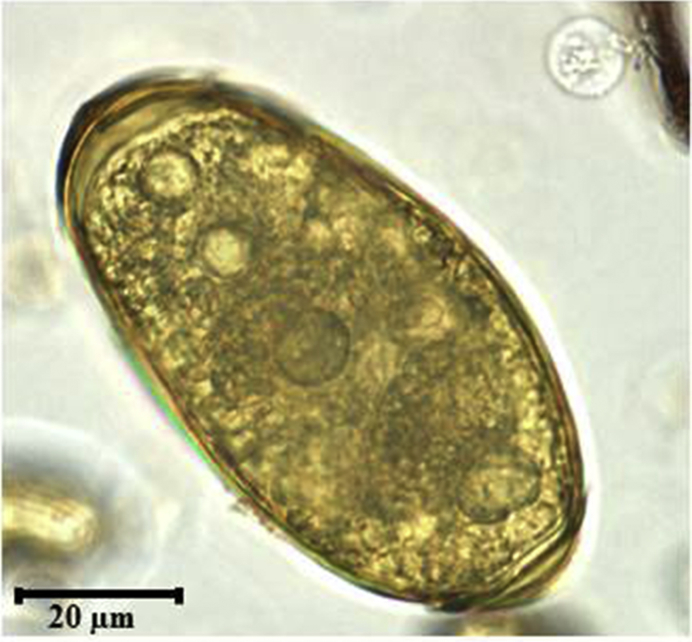
We recovered parasite eggs that were consistent with P. africanus (87.3 ± 7.9 X 45.4 ± 4.5) in 43% of the population. (Fig. 2). Our DNA sequence (Gen Bank accession number KR780065) was 100% identical to a P. africanus sequence from a human in Kumba, Cameroon and 99% identical to another from Bulutu, Cameroon (Nkouawa et al., 2009). Mean intensity prior to treatment was 96.70 epg (95% CI: 29.73–163.67; median = 32.67; range = 1–1067).
Fig. 2.
Microscopic image of Paragonimus africanus egg.
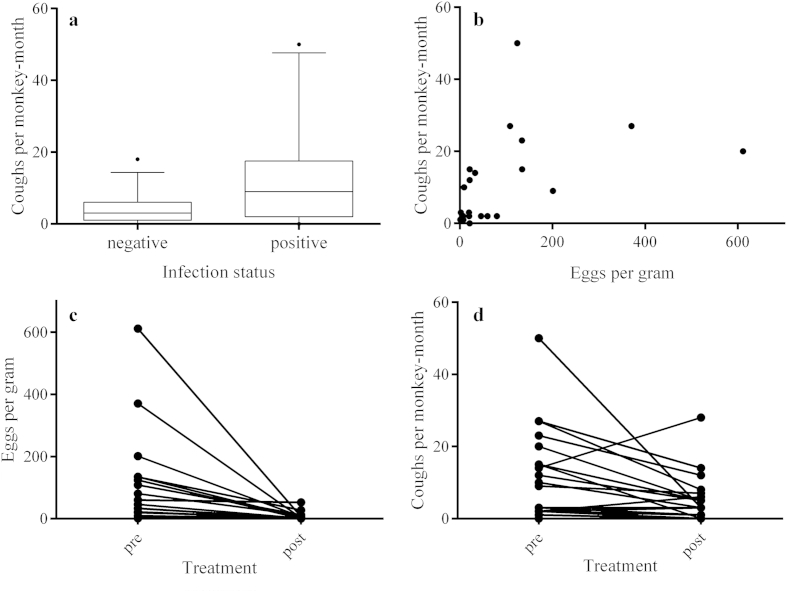
We found no association between parasitism (prevalence or intensity) and host sex or age. We recovered Paragonimus sp. from animals transferred to the enclosure and those born in the enclosure. We observed 516 coughs in 88% (n = 43) of individuals. Frequency of coughing was over two times higher in Paragonimus-positive individuals (μ = 11.42) than in Paragonimus-negative individuals (μ = 4.03; W = 192; p < .02; Fig. 3a). Coughing frequency also increased with the number of Paragonimus sp. eggs shed in feces (rs = .64, p < .001; Fig. 3b). De-worming led to a significant reduction in epg (μ = 6.13; p < .0001; Fig. 3c) and a corresponding reduction of coughing to baseline levels (μ = 4.95; p < .01; Fig. 3d) in infected monkeys.
Fig. 3.
Coughing and Paragonimus infection. a) Paragonimus infection status and coughs per monkey-month (CPMM) (W = 192; p < .02), b) intensity of Paragonimus infection [eggs per gram of feces (epg)] and CPMM (rs = .64, p < .001), c) de-worming and epg (μ = 6.13; W = 0; p < .0001), and d) de-worming and CPMM (μ = 4.95; W = 26.5; p < .01).
4. Discussion
We provide the first molecular evidence of Paragonimus sp. in African wildlife, and the first report of Paragonimus sp. infection in C. torquatus and P. africanus in Nigerian wildlife. Recent surveys revealing re-emergence of Paragonimus sp. in human populations in southeast Nigeria suggest a sylvatic cycle in which the parasite is maintained in crab-eating wildlife reservoirs (Blair, 2014). Indeed, C. torquatus in Gabon eat crabs as a normal part of the diet (Cooke, 2014). Our results demonstrate that C. torquatus can be a host for P. africanus, and infection is associated with respiratory illness. However, given limited sampling and DNA sequencing, we cannot exclude the possibility that this population hosts other species within the genus Paragonimus. Together with observations from Voelker and Sachs (1977) and Sachs and Voelker (1980), these results suggest that wild primates in Nigeria may help maintain Paragonimus sp. perhaps contributing to human disease.
Currently, limited genetic data exist for African Paragonimus spp. For example, there was no sequence information available for P. uterobilateralis on GenBank as of August 7, 2015, and only two sequences from P. africanus were available (Nkouawa et al., 2009). Our P. africanus sequences were between 99% and 100% identical to sequences from humans in Cameroon, demonstrating only limited intraspecific variation in the ITS2 region of the parasite. Paragonimus africanus therefore appears to be genetically homogeneous across Nigeria and Cameroon, albeit based on a limited number of samples and only one genetic locus. Paragonimus mexicanus, previously considered the sole etiological agent of paragonimiasis in the Americas, is now believed to include cryptic species (López-Caballero et al., 2013). Further sampling may indicate similar genetic diversity in P. africanus.
Significantly, we found that coughing was more frequent in infected individuals, and that animals with more intense infections coughed more frequently. Furthermore, treatment with praziquantel in our study population led to reduced parasite burden and a corresponding reduction of coughing to baseline levels in infected monkeys. Determining whether coughing is communicative or physiological is problematic in studies of primate behavior (Hauser, 2000). Our results suggest that coughing may actually indicate respiratory disease, such as infection with lung flukes. This observation not only complicates interpretations of primate behavior, but it also suggests a method for clinical assessment of wild primates for paragonimiasis and similar respiratory pathogens. Interestingly, hunters in this area report using primate skulls and feces to treat cough (Friant et al., 2015). In interviews, hunters made reference to seeing monkeys in the area cough as justification for these traditional remedies (S.Friant, unpub.data), indicating an intriguing link between parasitism, clinical disease, local beliefs, and primate conservation.
Knowledge of sylvatic reservoirs will be critical for improved understanding and control of re-emerging paragonimiasis in Nigeria. Given the difficulties associated with eradicating multi-host pathogens, as well as their high potential for emergence and re-emergence, control of paragonimiasis will require not only sustained behavior change away from raw or undercooked crab consumption and improved education, but also surveillance of potential wildlife hosts. In the case of primates, observations of coughing should be considered suspicious for paragonimiasis.
Conflict of interest
None.
Acknowledgments
We are grateful to the Centre for Education, Research and Conservation of Primates and Nature (CERCOPAN) and the University of Calabar, Nigeria for their support in the field. We would also like to acknowledge T. Yoshino, S. Sibley, L. Teixeira, S. Paige, and B. Rohde for their technical contributions to this manuscript, and F. Onajde and R. Gbegbaje for their assistance in the field. This work was supported by the Fulbright U.S. Student Program, National Science Foundation Doctoral Dissertation Improvement Grant (DDIG: 1403861), National Institutes of Health Parasitology and Vector Biology Training Program (T32AI007414); PI: T. Yoshino, Robert Wood Johnson Health Foundation Dissertation Grant, Graduate Women in Science, and John Ball Zoological Society Conservation Grant.
References
- Abraham J.T., Akpan P.A. Vectors of Paragonimus uterobilateralis a causative fluke for paragonimiasis in cross river state. Niger. Afr. Res. Rev. 2011:5. [Google Scholar]
- Aka N.A., Adoubryn K., Rondelaud D., Dreyfuss G. Human paragonimiasis in africa. Ann. Afr. Med. 7. Ann. Afr. Med. 7. 2008 doi: 10.4103/1596-3519.55660. [DOI] [PubMed] [Google Scholar]
- Blair D. Paragonimiasis. In: Toledo R., Fried B., editors. Digenetic Trematodes, Advances in Experimental Medicine and Biology. Springer; New York: 2014. pp. 115–152. [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: Margolis et al. Revisit. J. Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Cooke C.A. Crab predation by red-capped mangabeys (Cercocebus torquatus) in Sette Cama, Gabon. Afr. J. Ecol. n/a–n/a. 2014 [Google Scholar]
- Friant S., Paige S.B., Goldberg T.L. Drivers of bushmeat hunting and perceptions of Zoonoses in nigerian hunting communities. PLoS Negl. Trop. Dis. 2015:9. doi: 10.1371/journal.pntd.0003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst T., Keiser J., Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:210–221. doi: 10.1016/S1473-3099(11)70294-8. [DOI] [PubMed] [Google Scholar]
- Greiner E.C., McIntosh A. Collection methods and diagnostic procedures for primate parasitology. In: Huffman M.A., Chapman C.A., editors. Primate Parasite Ecology;: the Dynamics and Study of Host-parasite Relationships. Cambridge University Press; 2009. pp. 3–27. [Google Scholar]
- Hauser M.D. A primate dictionary? Decoding the function and meaning of another species' vocalizations. Cogn. Sci. 2000;24:445–475. [Google Scholar]
- Kingdon J. Princeton University Press; Princeton: 2005. The Kingdon Pocket Guide to African Mammals. [Google Scholar]
- López-Caballero J., Oceguera-Figueroa A., León-Règagnon V. Detection of multiple species of human Paragonimus from Mexico using morphological data and molecular barcodes. Mol. Ecol. Resour. 2013;13:1125–1136. doi: 10.1111/1755-0998.12093. [DOI] [PubMed] [Google Scholar]
- Nkouawa A., Okamoto M., Mabou A.K., Edinga E., Yamasaki H., Sako Y., Nakao M., Nakaya K., Blair D., Agatsuma T., Enyong P., Shibahara T., Moyou-Somo R., Ito A. Paragonimiasis in Cameroon: molecular identification, serodiagnosis and clinical manifestations. Trans. R. Soc. Trop. Med. Hyg. 2009;103:255–261. doi: 10.1016/j.trstmh.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Nnochiri E. Oxford University Press; London: 1968. Parasitic Disease and Urbanization in a Developing Community. [Google Scholar]
- Nwokolo C. Paragonimiasis in Eastern Nigeria. J. Trop. Med. Hyg. 1964;67:1. [PubMed] [Google Scholar]
- Nwokolo C. Outbreak of paragonimiasis in Eastern Nigeria. Lancet. 1972;299:32–33. doi: 10.1016/s0140-6736(72)90017-7. [DOI] [PubMed] [Google Scholar]
- Sachs R., Voelker J. A primate, Mandrillus leucophaeus, as natural host of the African lung fluke Paragonimus africanus in West Cameroon. Tropenmed. Parasitol. 1975;26:205–206. [PubMed] [Google Scholar]
- Sachs, R., Voelker, J., 1980. Paragonimus lung fluke infection in wild primates, with special reference to food habits of African drill (Mandrillus leucophaeus). Presented at the Erkrankungen der Zootiere, Verhandlungsbericht des XXII. Internationalen Symposiums uber die Erkrankungen der Zootiere, 28 Mai–1 Juni 1980, Arnhem (Netherlands), Akademie Verlag., pp. 147–152.
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano C., Yu H.S., Nunn P. World Health Organization; 1995. Paragonimiasis and tuberculosis, diagnostic confusion: a literature review. [Google Scholar]
- Voelker J., Vogel H. 2 new paragonimus species from West africa: Paragonimus africanus and Paragonimus uterobilateralis (Troglotrematidae; Trematoda) Z. Für Tropenmedizin Parasitol. 1965;16:125–148. [PubMed] [Google Scholar]
- Voelker J., Sachs R. 3rd International Congress of Parasitology. 1974. Observations on the life history of Paragonimus uterobilateralis: the African civet (Viverra civetta) as natural reservoir in Nigeria. (München) [Google Scholar]
- Voelker J., Sachs R. Monkeys and lower primates as natural and experimental hosts of the African lung flukes, Paragonimus africanus and P. uterobilateralis (author's transl) Tropenmed. Parasitol. 1977;28:137–144. [PubMed] [Google Scholar]
- World Health Organization . 1995. Control of Foodborne Trematode Infections: Report of a WHO Study Group (WHO Technical Report Series) (Manila, Philippines) [PubMed] [Google Scholar]