Abstract
Imbalanced matrix metalloproteinase (MMP)-2 activity and transforming growth factor expression (TGF-β) are involved in vascular remodeling of hypertension. Atorvastatin and sildenafil exert antioxidant and pleiotropic effects that may result in cardiovascular protection. We hypothesized that atorvastatin and sildenafil alone or in association exert antiproliferative effects by down-regulating MMP-2 and TGF-β, thus reducing the vascular hypertrophy induced by two kidney, one clip (2K1C) hypertension.
Sham and 2K1C rats were treated with oral atorvastatin 50 mg/kg, sildenafil 45 mg/kg, or both, daily for 8 weeks. Blood pressure was monitored weekly. Morphologic changes in the aortas were studied. TGF-β levels were determined by immunofluorescence. MMP-2 activity and expression were determined by in situ zymography, gel zymography, Western blotting, and immunofluorescence. The effects of both drugs on proliferative responses of aortic smooth muscle cells to PDGF and on on MMP-2 activity in vitro were determined. Atorvastatin, sildenafil, or both drugs exerted antiproliferative effects in vitro. All treatments attenuated 2K1C-induced hypertension and prevented the increases in the aortic cross-sectional area and media/lumen ratio in 2K1C rats. Aortas from 2K1C rats showed higher collagen deposition, TGF-β levels and MMP-2 activity and expression when compared with Sham-operated animals. Treatment with atorvastatin and/or sildenafil was associated with attenuation of 2K1C hypertension-induced increases in these pro-fibrotic factors. However, these drugs had no in vitro effects on hr-MMP-2 activity.
Atorvastatin and sildenafil was associated with decreased vascular TGF-β levels and MMP-2 activity in renovascular hypertensive rats, thus ameliorating the vascular remodeling. These novel pleiotropic effects of both drugs may translate into protective effects in patients.
Keywords: Atorvastatin, Hypertension, Matrix metalloproteinase, Sildenafil
Graphical abstract
Highlights
-
•
Atorvastatin and sildenafil exert antioxidant and other pleotropic effects.
-
•
Imbalanced MMP-2 activity and TGF-β expression promote vascular remodeling in hypertension.
-
•
Atorvastatin and sildenafil exerted antiproliferative effects in vitro.
-
•
Both drugs prevented hypertension-induced increases pro-fibrotic factors.
-
•
These additional pleiotropic effects may translate into protective effects in patients.
1. Introduction
Vascular remodeling is critically involved in the pathogenesis of hypertension and is initiated by smooth muscle cell proliferation and migration that result in vascular hyperplasia and hypertrophy [1]. This alteration is a consequence of imbalanced vascular matrix metalloproteinase (MMP) activity, which promotes excessive degradation of extracellular matrix and induces structural modifications of the vasculature, particularly in hypertensive subjects [1,2]. Indeed, upregulated MMP activity, especially MMP-2, has been implicated in vascular remodeling in hypertensive patients [3,4] and in animal models of hypertension [5–7]. In addition to its effects on the components of the extracellular matrix, MMP-2 activates latent TGF-β [8] thus further contributing to vascular pathogenetic mechanisms that impair vascular function [9]. Indeed, TGF-β is a major profibrotic factor implicated in extracellular matrix reorganization and collagen deposition during vascular fibrosis [10]. Therefore, drugs that inhibit MMP activity may contribute to TGF-β downregulation and prevent vascular remodeling in hypertension.
Statins inhibit 3-hydroxy-3-methylglutaryl (HMG)-coenzyme A reductase activity, an enzyme that plays a major role in the biosynthesis of cholesterol [11]. However, in the last few decades, growing evidence has accumulated to support the notion that statins exert beneficial cardiovascular effects independent of their effects on cholesterol levels [12]. Similarly, phosphodiesterase-5 inhibitors, particularly sildenafil, which are usually prescribed to patients with erectile dysfunction or with pulmonary hypertension, have also been shown to exert protective effects that may justify their use in patients with other cardiovascular diseases [13]. In fact, there is evidence that both statins and phosphodiesterase-5 inhibitors may exert pleiotropic effects that counteract important mechanisms that promote vascular remodeling in hypertension. For example, statins inhibit MMP-9 secretion by vascular cells [14,15] and suppress TGF-β expression decreasing vascular fibrosis [16]. Moreover, sildenafil prevents cardiovascular remodeling [17] and inhibits MMP-9 and TGF-β expression [18,19]. Interestingly, some studies suggest that the combination of a statin with sildenafil further increases the protective effects of each drug alone [20–22]. However, although there is some evidence that both statins and phosphodiesterase-5 inhibitors may inhibit profibrotic mechanisms, it is not known whether atorvastatin or sildenafil attenuates the vascular remodeling associated with hypertension, or whether the combination of these drugs increases the possible effects associated with each drug alone.
In this study, we aimed at testing the hypothesis that both atorvastatin or sildenafil, inhibit proliferative mechanisms involving MMP-2 and TGF-β that result in vascular remodeling of renovascular (two kidney, one clip; 2K1C) hypertension. In addition, we examined whether the combination of both drugs improves the possible protective effects of each drug alone.
2. Materials and methods
The present study was carried out in accordance with National Institutes of Health (NIH; USA) guidelines. All experimental protocols with animals were approved by our Institutional Animal Care and Use Committee of the Ribeirao Preto Medical School, University of Sao Paulo.
2.1. Isolation of rat aortic smooth muscle cells
Rat aortic smooth muscle cells (RASMCs) were isolated from Sprague-Dawley rats (6–8 weeks of age) as previously described [23]. The rats were anesthetized with an intraperitoneal injection of pentobarbital. The aorta was isolated and immersed in 20% fetal bovine serum-Dulbecco's modified Eagle's medium (DMEM) containing 1000 U/mL heparin. Fat and connective tissue were removed and the aorta was incubated in DMEM (serum-free) with collagenase type II (2 mg/mL) for 45 min at 37 °C. After removal of the endothelium, the vessel was cut lengthwise, and the smooth muscle cells were removed mechanically.
2.2. Experiments with rat aortic smooth muscle cells to examine antiproliferative effects of atorvastatin or sildenafil in the presence of platelet-derived growth factor
RASMCs were examined by microscopy and used between passages 5 and 8. Approximately 20,000 cells/well were plated in 24-well plates with 1 mL DMEM and F-12 HAM'S medium with 10% fetal bovine serum for 24 h followed by 24 h in serum-free medium. The RASMCs were pre-treated with vehicle (Control, untreated cells), atorvastatin (0.1, 1, or 10 μM) and sildenafil (0.1, 1, or 10 μM) for 1 h and stimulated with platelet-derived growth factor-BB (PDGF-BB) 1 ng/mL for 24 h [24].
2.3. Assessment of cell proliferation
Growth-arrested RASMCs (serum starvation for 24 h) were subject to experimental conditions in the presence of 5 μCi/mL 3H-thymidine (NEN) for 24 h. 3H-thymidine incorporation into trichloroacetic acid-precipitated DNA was quantified by scintillation spectroscopy as described [24].
2.4. Renovascular (2K1C) hypertension animal model and treatments
Male Wistar rats weighing from 180 to 200 g were maintained under the animal house conditions (12 h light/dark cycle at 25 °C), and allowed free access to rat chow and water.
Under anesthesia (ketamine 100 mg/kg and xylazine 10 mg/kg i.p.), 2K1C hypertension was performed by placing 0.2 mm silver clip around the left renal artery. As normotensive controls, sham-operated rats underwent the same surgical procedure without placement of the renal artery clip. Body weight and systolic blood pressure (SBP) were assessed weekly. SBP were measured by tail-cuff plethysmography, and the rats were considered hypertensive when SBP>160 mm Hg two weeks after the surgery.
The animals were randomly assigned to one of eight groups: 2K1C and Sham groups that received ethanol 2% (vehicle used to dilute both drugs); 2K1C and Sham groups that received atorvastatin at 50 mg/kg per day [25]; 2K1C and Sham groups that received sildenafil at 45 mg/kg [17]; and 2K1C and Sham groups that received the combination of atorvastatin 45 mg/kg and sildenafil 50 mg/kg per day. The treatments were started two weeks after 2K1C surgery and were maintained for eight additional weeks. All treatments were given by oral gavage. The animals were killed by decapitation after 10 weeks of hypertension and their thoracic aortas were isolated and cleaned of connective tissue and fat. Arterial blood samples were centrifuged at 1000g for 10 min and plasma fractions were immediately stored at −70 °C until used for biochemical measurements.
2.5. Morphometric analysis of the aorta and assessment of aortic collagen content
The thoracic aortas were carefully removed and cleaned of connective tissue and fat. After that, the aortas were fixed in 4% phosphate-buffered paraformaldehyde (pH 7.4) for 24 h, followed by 70% ethanol (at least 24 h) and then embedded in paraffin. The blocks of paraffin were cut at four micrometer thick slices and stained with hematoxylin and eosin (H&E). The morphometric parameters including media cross-sectional area (CSA) and media to lumen diameter (M/L) were quantified as previously described using ImageJ Program (Rasband, W.S., ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2011) [5].
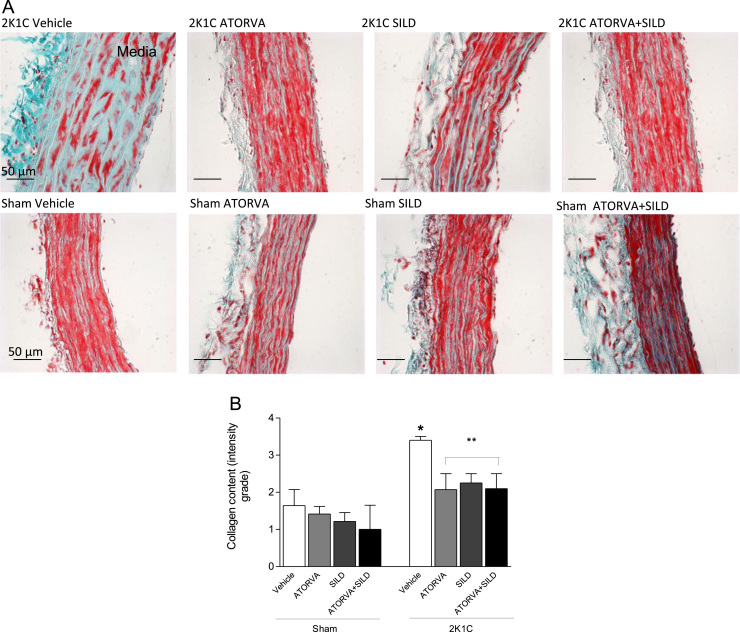
Trichrome staining (Gomori) was used to determine the collagen content in the aortic media layer with light microscopy (DMLB; Leica, Bensheim, Germany) and the image was captured at 400×. These structural analyses in the media were evaluated by two skilled blinded observers. The evaluation of collagen surface was scored quali-quantitatively as absent (0), low (1), moderate (2), or strong (3) in the study groups. Each score reflects changes in the intensity and extension of staining.
2.6. Assessment of TGF-β by immunofluorescence
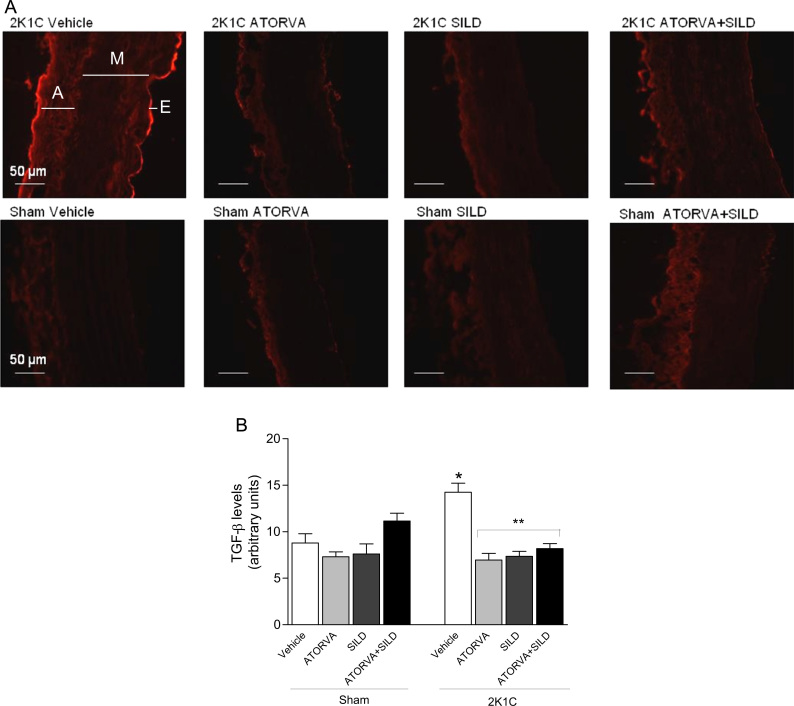
The aortas were frozen in Tissue-tek O.C.T. compound and 4-μm thick cryostat sections were incubated with antibody against TGF-β1 (polyclonal rabbit anti-TGF-β 1; 1:500, ab92486, Abcam, USA) at room temperature in dark humidified chambers for 1 h. Slices were washed 3 times with cold PBS and anti-rabbit rhodamine conjugated secondary antibody (1:200, AP187R, Millipore, USA) was added for 1 h. Immunofluorescence images were viewed with a fluorescent microscope (Leica Imaging Systems Ltd., Cambridge, England) and the images were captured at 400×. Red fluorescence intensity was evaluated by using ImageJ Program (National Institutes of Health) in 40 fields selected around the vessel circumference (interassay coefficient of variation less than 3%), and the arithmetic of 40 fields was calculated for each slide [26].
2.7. Measurement of aortic MMP-2 levels by gelatin zymography
Gelatin zymography was performed as previously described [27]. Frozen aortic tissue samples (approximately 30 mg) were homogenized with cold RIPA-buffer on ice. The protein concentration in the supernatant was performed with Bradford protein assay. Tissue extracts diluted 1:1 with 2× sample buffer were subjected to electrophoresis on 7% SDS-PAGE co-polymerized with gelatin (0.1%). After electrophoresis, the gels were soaked in a 2% Triton X-100 solution for 30 min twice at room temperature. Then, the gels were incubated in Tris–HCl buffer (10 mmol L−1 CaCl2, pH 7.4) overnight, at 37 °C. The staining was carried out for 3 h with Coomassie Brilliant Blue G-250 (0.05%) and destained with 25% methanol and 7% acetic acid for 2 h. Gelatinolytic activity was detected as unstained bands against the blue background of stained gelatin, and quantified by densitometry using a Kodak Electrophoresis Documentation and Analysis System (EDAS) 290 (Kodak, Rochester, NY). Intergel analysis was possible after normalization of the gelatinolytic activity with an internal standard (fetal bovine serum).
2.8. Assessment of aortic gelatinolytic activity by in situ zymography and aortic MMP-2 levels by immunofluorescence
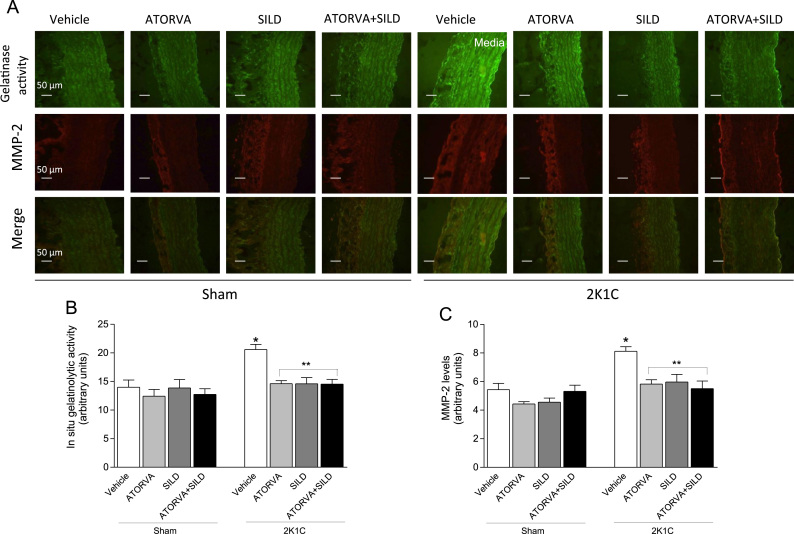
In situ gelatinolytic activity in the media of frozen thoracic aorta was performed as previously described [28]. Frozen 4 μm sections were incubated with dye-quenched (DQ) Gelatin (E12055, Molecular Probes, Oregon 411, USA) diluted 1:20 for 30 min in dark humidified chambers. The images were examined with fluorescent microscopy (Leica Imaging Systems Ltd., Cambridge, England) and captured at 400×. The intensity of the green fluorescent signal was evaluated by using ImageJ Program (NIH – National Institute of Health).
To co-localized aortic gelatinolytic activity with MMP-2 expression immunofluorescence for MMP-2 was performed. After DQ gelatin, the sections were rinsed 3× with cold PBS and incubated with mouse monoclonal MMP-2 antibody (1:500; MAB3308, Millipore, USA) for 1 h. Slices were then incubated with anti-mouse rhodamine conjugated secondary antibody (1:200, AP181R, Millipore, USA) Sections were examined with fluorescent microscopy (Leica Imaging Systems Ltd., Cambridge, England) and the image was captured at 400×. The intensity of the red fluorescent signal was evaluated by using ImageJ Program [28].
2.9. Assessment of aortic MMP-2 expression by Western blot analysis
Aortic samples of about 30 mg were lysed with cold RIPA-buffer. The protein concentration of tissue homogenate was determined with Bradford protein assay. 40 μg of protein extracts were separated by 12% polyacrylamide gels. Then, the proteins were transferred to nitrocellulose membranes (GE Healthcare, Madison, WI, USA). The membranes were blocked for 1 h at room temperature with TBS-T (NaCl 100 mM; Tris–Cl 100 mM; Tween 0.1%) containing 5% BSA (bovine serum albumin) and incubated overnight at 4 °C with primary antibody against MMP-2 (1:1000; MAB3308, Millipore, USA). The membranes were then incubated with 1:1000 horseradish peroxidase (HRP)-secondary goat anti-rabbit antibody (AP132P, Millipore, USA) for 1 h. The protein bands were revealed with ECL chemiluminescence kit (GE Healthcare). MMP-2 expression was normalized with respect to β-actin expression (1:1000; MAB1501, Millipore, USA).
2.10. Assessment of direct in vitro effects of atorvastatin or sildenafil on MMP-2 activity
To examine whether atorvastatin, sildenafil or both drugs (at concentrations varying from 0.1 to 10 µM) inhibit MMP-2 activity in vitro, we measured the direct effects of these drugs on recombinant human MMP-2 (rhMMP-2) activity obtained as previously described [29]. Proteolytic activity of 300 ng of rhMMP-2 was measured in absence or presence of these drugs by using the Gelatinolytic Activity Kit (E12055, Molecular Probes, OR, USA) in a microplate spectrofluorometer (at λexcitation 495, λemission 515 nm; Gemini EM, Molecular Devices, Sunnyvale, CA, USA) after 30 min of incubation at 37 °C, as previously described [28]. Phenanthroline (0.1 mM) was used as a positive control for MMP-2 activity inhibition.
2.11. Statistical analysis
Results are expressed as means±S.E.M. Comparisons between groups were assessed by two-way analysis of variance (ANOVA) followed by the Bonferroni correction and by the Kruskal–Wallis test or t test as appropriate using GraphPad Prism software. A probability value <0.05 was considered significant.
3. Results
3.1. Both atorvastatin and sildenafil inhibit PDGF-BB-stimulated RASMCs proliferation
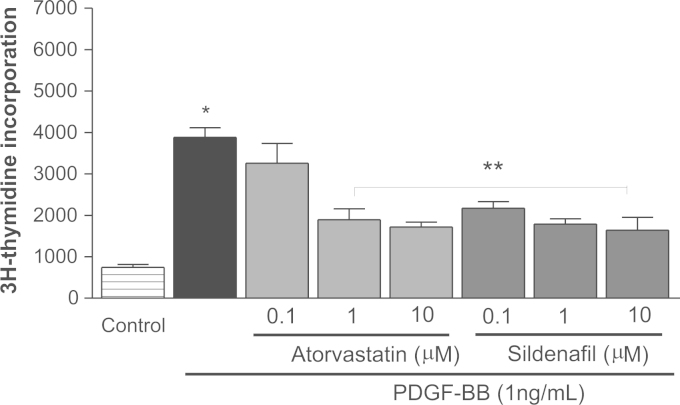
PDGF-BB is a major inducer of smooth muscle cell proliferation and migration and plays an important role in the pathogenesis of vascular diseases including hypertension [30]. The RASMCs were stimulated with PDGF-BB for 24 h and both atorvastatin (1 and 10 μM) and sildenafil (0.1, 1 and 10 μM) significantly inhibited PDGF-BB-induced cell proliferation (all P<0.05; Fig. 1).
Fig. 1.
Effects of atorvastatin and sildenafil on cell proliferation. Rat aortic smooth muscle cells (RASMCs) were treated with atorvastatin or sildenafil at different concentrations and PDGF-BB (1 ng/mL) was added 1 h later for 24 h. 3H-thymidine incorporation was measured. Control cells are untreated RASMCs without fetal bovine serum in the medium. Data are expressed as mean±S.E.M. (n=5 in the control group and n=6 in the other groups). *P<0.05 versus Control. **P<0.05 versus PDGF-BB alone.
3.2. Effects of treatments on systolic blood pressure (SBP) and body weight
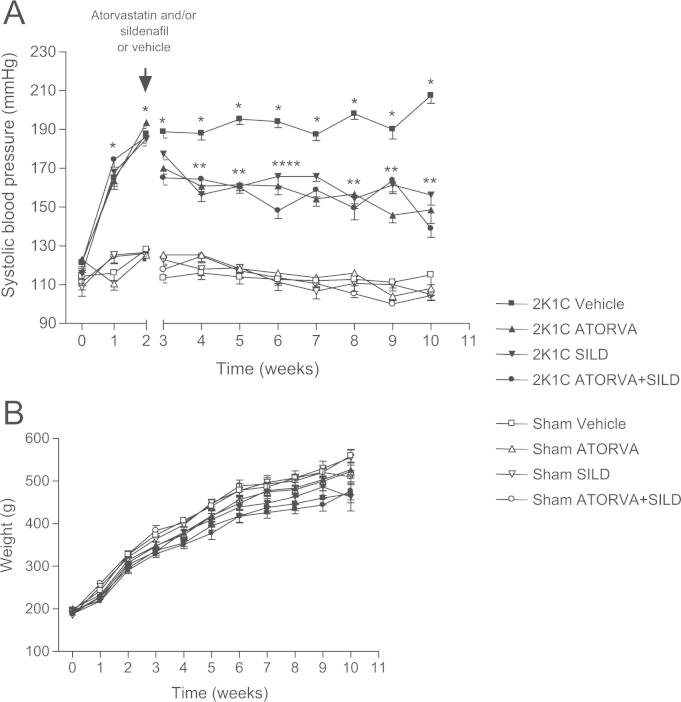
We found similar baseline SBP in all groups and no significant changes in SBP were seen in the groups of Sham-operated animals treated with Vehicle or with the drugs (Fig. 2A; P>0.05). While SBP increased progressively in 2K1C rats treated with vehicle (207±4 mm Hg after 10 weeks), treatment with atorvastatin, sildenafil, or with both drugs exerted similar antihypertensive effects (SBP=148±7, 156±5, and 138±4 mm Hg, respectively; P<0.05, Fig. 2A).
Fig. 2.
Systolic blood pressure (mmHg) measured by tail-cuff method (a) and body weight (b) in the eight experimental groups along 10 weeks of study. Data are shown as mean±S.E.M. *P<0.01 versus Sham Vehicle group; #P<0.01 versus 2K1C Vehicle group. (2K1C+Vehicle: n=8; 2K1C+ATORVA: n=7; 2K1C+SILD: n=8; 2K1C+ATORVA+SILD: n=9; Sham+Vehicle: n=8; Sham+ATORVA: n=10; Sham+SILD: n=8; Sham+ATORVA+SILD: n=10).
No significant differences were found with respect to body weight gain (P>0.05; Fig. 2B).
3.3. Treatment with atorvastatin or sildenafil, or the combination of drugs, ameliorated the vascular remodeling in 2K1C rats
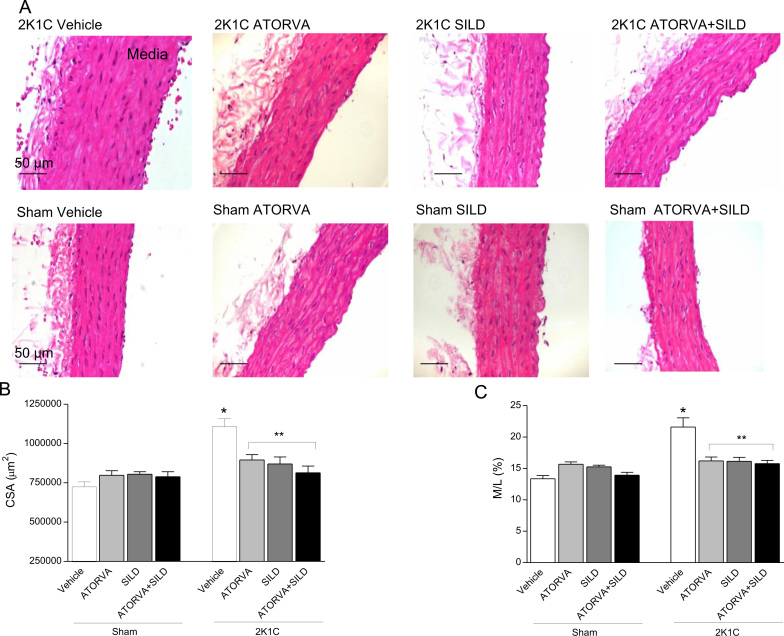
2K1C hypertension was associated with vascular hypertrophy as revealed by increased aortic cross-sectional area (CSA) and increased media to lumen (M/L) ratio in hypertensive rats compared to Sham-operated animals (both P<0.05; Fig. 3A–C). Interestingly, while no effects were found in Sham-operated animals treated with drugs, both atorvastatin or sildenafil, or the combination of drugs blunted the morphological alterations induced by 2K1C hypertension (all P<0.05; Fig. 3A–C).
Fig. 3.
Panel (a) shows aortic structural alterations induced by 2K1C hypertension and the effects of treatment with atorvastatin (ATORVA), sildenafil (SILD), or both drugs. Panels (b) and (c) show the cross sectional area (CSA) and media to lumen ratio (M/L), respectively, in each study group. Data are expressed as mean±S.E.M. *P<0.05 versus Sham Vehicle group; **P<0.05 versus 2K1C Vehicle group. (Sham+Vehicle: n=9; Sham+ATORVA: n=9; Sham+SILD: n=14; Sham+ATORVA+SILD: n=11; 2K1C+vehicle: n=9; 2K1C+ATORVA: n=10; 2K1C+SILD: n=11; 2K1C+ATORVA+SILD: n=14).
In agreement with the morphological results, increased collagen surface area was found in 2K1C hypertensive rats treated with vehicle as compared with Sham-operated rats (P<0.05; Fig. 4A and B). Again, while no effects were found in Sham-operated animals treated with drugs, both atorvastatin or sildenafil, or the combination of drugs blunted the increases in collagen surface area induced by 2K1C hypertension (all P<0.05; Fig. 4A and B).
Fig. 4.
Collagen surface area in the media layer of aortas from rats and effects of treatments. Panel (a) shows representative photomicrographs of aortic samples stained by Trichrome (Gomori) (400×). Panel (b) shows quantitative evaluation of the collagen surface area stained in blue. Data are expressed as mean±S.E.M. *P<0.05 versus Sham Vehicle group; **P<0.05 versus 2K1C Vehicle group. (Sham+Vehicle: n=7; Sham+ATORVA: n=6; Sham+SILD: n=7; Sham+ATORVA+SILD: n=5; 2K1C+vehicle: n=5; 2K1C+ATORVA: n=7; 2K1C+SILD: n=7; 2K1C+ATORVA+SILD: n=5).
3.4. Both atorvastatin or sildenafil, or the combination of drugs, blunted the increases in aortic expression of TGF-β induced by hypertension
Hypertension increased the aortic expression of TGF-β in 2K1C rats treated with vehicle compared with Sham-operated animals (P<0.05; Fig. 5A and B). While no significant differences were found in Sham-operated animals treated with drugs, both atorvastatin or sildenafil, or the combination of drugs blunted the increases in aortic TGF-β content induced by 2K1C hypertension (all P<0.05; Fig. 5A and B).
Fig. 5.
TGF-β expression in the media layer of aortas from rats. Panel (a) shows representative photomicrographs (400×) of TGF-β expression by immunofluorescence (in red; A=adventicia; M=media; E=endothelium). Panel (b) shows the quantification of red staining of TGF-β. Data are expressed as mean±S.E.M. *P<0.05 versus Sham Vehicle group; **P<0.05 versus 2K1C Vehicle group. (Sham+Vehicle: n=3; Sham+ATORVA: n=5; Sham+SILD: n=5; Sham+ATORVA+SILD: n=6; 2K1C+vehicle: n=4; 2K1C+ATORVA: n=4; 2K1C+SILD: n=3; 2K1C+ATORVA+SILD: n=4).
3.5. Treatment with atorvastatin or sildenafil, or the combination of both, decreased aortic MMP-2 levels and activity and reduced in situ aortic gelatinolytic activity
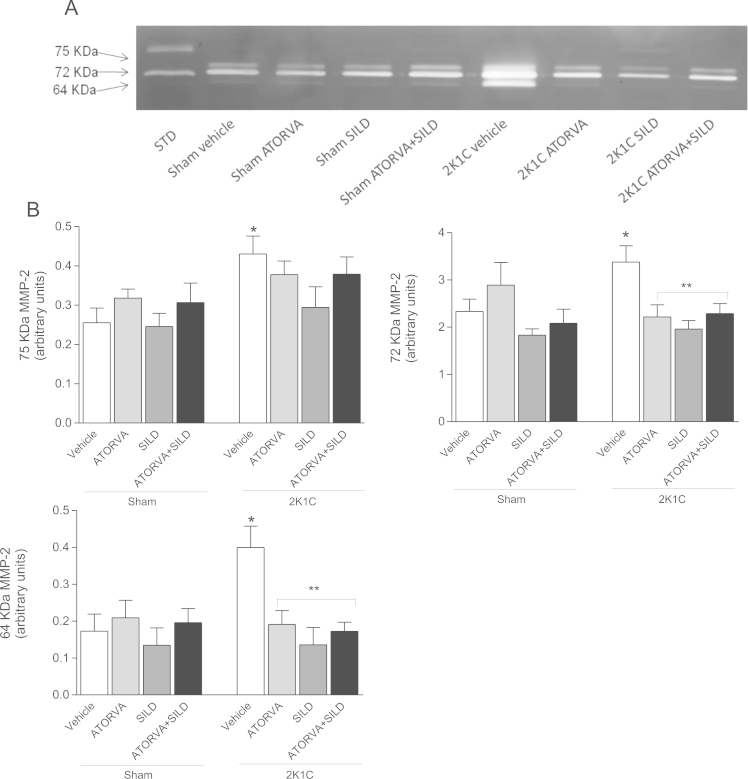
A representative zymogram of aortic extracts displaying bands corresponding to the three usual molecular weights of MMP-2 (75 kDa, 72 kDa, and 64 kDa) is shown in Fig. 6A. As expected, 2K1C hypertension increased the aortic levels of the 3 isoforms of MMP-2 when compared with Sham-operated animals treated with vehicle (all P<0.05; Fig. 6A and B). While the drugs exerted no effects in Sham-operated animals, both atorvastatin and sildenafil, alone or in combination, attenuated 2K1C hypertension-induced increases in aortic 72 kDa and 64 kDa MMP-2 levels (all P<0.05; Fig. 6A and B). No significant effects were found on the aortic levels of the 75 kDa MMP-2 levels.
Fig. 6.
Representative sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) gelatin zymogram of aortic samples (Panel (a)). Molecular weights of MMP-2 bands (75, 72 and 64 kDa MMP-2) were identified after electrophoresis on 7% SDS–PAGE. STD: internal standard. Panel (b) shows the quantification of each molecular weight form (75, 72 and 64 kDa) of MMP-2 in the aortic extracts. Data are expressed as mean±S.E.M. *P<0.05 versus Sham Vehicle group; **P<0.05 versus 2K1C Vehicle group. (Sham+Vehicle: n=18; Sham+ATORVA: n=9; Sham+SILD: n=9; Sham+ATORVA+SILD: n=9; 2K1C+vehicle: n=18; 2K1C+ATORVA: n=10; 2K1C+SILD: n=9; 2K1C+ATORVA+SILD: n=10).
To confirm aortic zymogram findings, we assessed aortic MMP-2 levels by immunofluorescence and in situ gelatinolytic aortic activity. Hypertension increased aortic in situ gelatinolytic activity, as revealed by increased green fluorescence in the media layer in the 2K1C+Vehicle group compared with Sham-operated animals (P<0.05; Fig. 7A and B), and the increased in situ gelatinolytic activity co-localized with increased aortic MMP-2 expression assessed by immunofluorescence (P<0.05; Fig. 7A and C). Interestingly, both atorvastatin or sildenafil, or the combination, blunted both increases in aortic in situ gelatinolytic activity and MMP-2 levels indeed by hypertension (all P<0.05; Fig. 7A–C).
Fig. 7.
Effects of treatments on in situ gelatinolytic activity and MMP-2 levels detected by immunofluorescence in the aortas. Panel (a) shows representative photographs of in situ gelatinolytic activity (400×), MMP-2 detected by immunofluorescence, and their co-localization (merge) in the aortas. Panel (b) shows the quantification of aortic in situ gelatinolytic activity detected as bright green fluorescence. Panel (c) shows the quantification of aortic MMP-2 levels detected by immunofluorescence as bright red fluorescence. Data are expressed as mean±S.E.M. *P<0.05 versus Sham Vehicle group; **P<0.05 versus 2K1C Vehicle group. (Sham+Vehicle: n=6; Sham+ATORVA: n=7; Sham+SILD: n=8; Sham+ATORVA+SILD: n=7; 2K1C+vehicle: n=5; 2K1C+ATORVA: n=7; 2K1C+SILD: n=8; 2K1C+ATORVA+SILD: n=8).
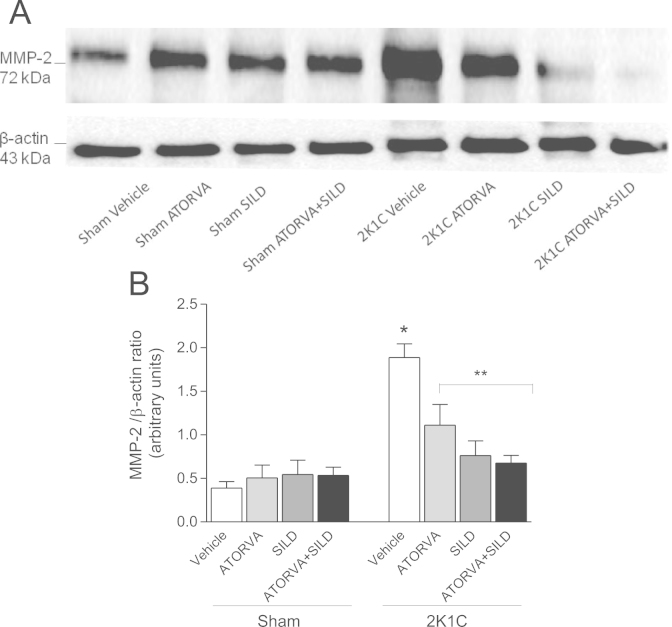
To further confirm these findings, we assessed aortic MMP-2 expression by Western blot analysis. In agreement with the results reported above, 2K1C hypertension increased aortic MMP-2 expression more than three-fold (P<0.05; Fig. 8A and B), and treatment with atorvastatin or silfenafil, or both, significantly attenuated hypertension-induced increases in aortic MMP-2 expression (all P<0.05; Fig. 8A and B).
Fig. 8.
Expression of MMP-2 in aortas from Sham and 2K1C rats treated with atorvastatin, sildenafil or the combination of both drugs normalized by β-actin expression. Panel (a) shows representative Western blotting results. Panel (b) shows the quantification in the different study groups. Data are expressed as mean±S.E.M. *P<0.05 versus Sham Vehicle group; **P<0.05 versus 2K1C Vehicle group. (Sham+Vehicle: n=5; Sham+ATORVA: n=6; Sham+SILD: n=6; Sham+ATORVA+SILD: n=7; 2K1C+vehicle: n=7; 2K1C+ATORVA: n=5; 2K1C+SILD: n=6; 2K1C+ATORVA+SILD: n=5).
3.6. Lack of direct inhibitory effects of atorvastatin or sildenafil on in vitro MMP-2 activity
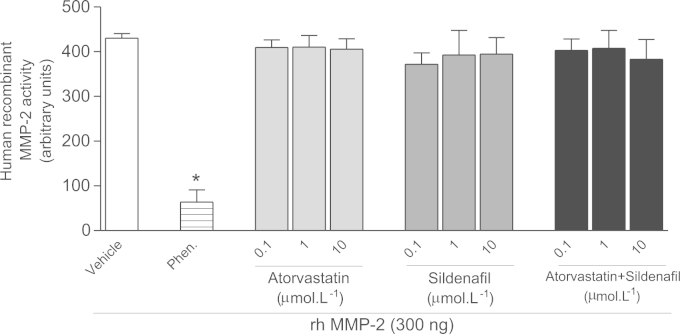
Given the positive results shown above, we examined the possibility that atorvastatin or sildenafil could directly inhibit MMP-2. While phenanthroline (non-selective Zn+2 chelating MMP inhibitor) inhibited rhMMP-2 activity by more than 80%, neither atorvastatin, nor sildenafil, either alone of combined, affected MMP-2 activity (P>0.05; Fig. 9).
Fig. 9.
Effects of atorvastatin, sildenafil, or the combination of both drugs at different concentrations (from 0.1 to 10 μM) on the activity of human recombinant MMP-2. Phenanthroline (Phen) 0.1 mM was used as a positive control for MMP-2 inhibition. Data are shown as mean±S.E.M. (n=5 per group). *P<0.05 versus Vehicle.
4. Discussion
The present study shows that both atorvastatin or sildenafil inhibit proliferative mechanisms and therefore attenuate the vascular remodeling associated with hypertension. To our knowledge, this is the first study to show that atorvastatin or sildenafil prevent aortic hypertrophy caused by 2K1C hypertension, and that this effect possibly involves lowered expression and activity of TGF-β and MMP-2. Our findings may suggest a mechanism by which these drugs exert beneficial, pleiotropic effects in hypertension.
Our findings showed clear antiproliferative effects of both atorvastatin and sildenafil, which attenuated PDGF-BB-induced RASMCs proliferation in vitro and blunted hypertension-induced media hypertrophy. Supporting the antiproliferative effects reported here, both atorvastatin and sildenafil inhibited vascular smooth muscle cells proliferation stimulated under other conditions, possibly as a result of interference with regulation of cell cycle [31,32]. Importantly, while previous studies have already shown that both atorvastatin [33] or sildenafil [17,34] may revert cardiac remodeling, this is the first study to show that both drugs blunt the vascular remodeling induced by renovascular hypertension.
The 2K1C hypertension model critically depends on the activation of the renin–angiotensin system, with increased angiotensin-converting enzyme activity and angiotensin II levels, which promotes oxidative stress and proliferative mechanisms leading to arterial remodeling [35]. In agreement with the present results, previous studies have notably implicated enhanced vascular MMP-2 activity and TGF-β levels as major players in the collagen deposition and other proliferative and profibrotic alterations associated with vascular remodeling of 2K1C hypertension [6,7,26]. In fact, MMP-2 activates TGF-β, which interacts with its receptors to promote classic profibrotic pathways that lead to increased synthesis of collagen and other components of the extracellular matrix [8,10]. Moreover, angiotensin II, which is implicated in the pathogenesis of 2K1C hypertension, stimulates TGF-β expression and activity in vascular smooth muscle cells [36]. Then, angiotensin II-induced vascular remodeling depends partly of TGF-β stimulation. Although unproven, the improvement of the vascular alterations after treatments with atorvastatin or sildenafil shown in the present study probably results of inhibition of profibrotic pathways, as suggested by the lower TGF-β levels that we found in the aortas from hypertensive rats treated with atorvastatin or sildenafil. The lower aortic TGF-β levels, in turn, may reflect decreased MMP-2 expression and activity with both drugs [8,10]. This suggestion is supported by previous studies showing that atorvastatin blunts the increases in TGF-β expression in response to angiotensin II infusion in rats [16]. Moreover, atorvastatin prevents collagen secretion by smooth muscle cells [37,38]. Similarly, sildenafil reduced TGF-β expression in the DOCA-salt hypertension model, thus allowing regression of renal injury [19].
Activated MMPs proteolyze a variety of extracellular matrix components including collagen, and therefore favor pathological vascular remodeling by mechanisms that add to those activated by TGF-β [1,39]. The increased MMP-2 levels and gelatinolytic activity that we found in 2K1C hypertension confirms previous studies and may reflect increased oxidative stress resulting from enhanced angiotensin II signaling [5,7,40,41]. Because we found in a previous study that both atorvastatin or sildenafil completely blunted 2K1C hypertension-induced oxidative stress under the same conditions of the present study [42], it is possible that antioxidant effects exerted by both drugs may have inhibited hypertension-induced increases in vascular MMP-2 expression and activity, as shown with other antioxidant compounds [28]. Similar antioxidant effects of sildenafil leading to lower MMP activity has been shown under other conditions [18,20], and the same has also been reported with atorvastatin [14,15,43]. Moreover, the inhibition of vascular remodeling associated with lower MMP-2 expression and activity that we found are not explained by direct inhibition of MMP-2 by either atorvastatin or sildenafil, as demonstrated by lack of significant effects of both drugs on rhMMP-2 activity that we found in the present study.
Both atorvastatin and sildenafil upregulate intracellular signaling pathways involved in the vascular nitric oxide (NO) signaling [44]. Atorvastatin increases endothelial NO synthase expression and activity, thus increasing NO production [42,44], whereas sildenafil inhibits phosphodiesterase-5, increasing cyclic GMP tissue levels [45]. Interestingly, while the combination of both drugs resulted in improved effects in previous studies [20–22], the same was not true in our 2K1C hypertension model and vascular remodeling, and therefore pathways activated by cGMP may not be critically involved in the effects reported here. Alternatively, since atorvastatin and sildenafil activate the same pathway, the effects on intracellular cGMP levels were not amplified when both drugs were associated. Consistent with our findings, both drugs produced similar antihypertensive effects in other animal models of hypertension [46–48], and in hypertensive patients [49,50].
Some limitations of the present study should be taken into consideration. The aortas used in the histological analysis in the present study were fixed by simple immersion into the fixative solution, and not after fixative solution perfusion via vascular system, which could avoid arterial retraction upon excision of the aortas [51]. While arterial retraction tends to increase vascular cross-sectional area as compared to in vivo conditions, this effect is probably not relevant when comparing aortas from treated and untreated animals. Another limitation is that the doses of sildenafil or atorvastatin used in the present study are more than one order of magnitude above those used to treat patients. However, experimental studies in rats usually require such doses given significant differences in many pharmacologic parameters when rats are compared to humans.
In conclusion, treatment with atorvastatin and sildenafil was associated with decreased vascular TGF-β levels and MMP-2 activity in renovascular hypertensive rats and prevention of vascular remodeling. These findings show novel beneficial pleiotropic effects of atorvastatin and sildenafil in a relevant hypertension model and that may translate into clinical protective effects in hypertensive patients. The similarity of biochemical effects associated with both drugs may underlie the lack of sinergism between them.
Acknowledgments
This study was funded by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors thank Sandra de Oliveira Conde for excellent technical assistance.
References
- 1.Sluijter J.P., de Kleijn D.P., Pasterkamp G. Vascular remodeling and protease inhibition – bench to bedside. Cardiovasc. Res. 2006;69:595–603. doi: 10.1016/j.cardiores.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Chow A.K., Cena J., Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br. J. Pharmacol. 2007;152:189–205. doi: 10.1038/sj.bjp.0707344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontana V., Silva P.S., Belo V.A., Antonio R.C., Ceron C.S., Biagi C., Gerlach R.F., Tanus-Santos J.E. Consistent alterations of circulating matrix metalloproteinases levels in untreated hypertensives and in spontaneously hypertensive rats: a relevant pharmacological target. Basic Clin. Pharmacol. Toxicol. 2011;109:130–137. doi: 10.1111/j.1742-7843.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 4.Yasmin, McEniery C.M., Wallace S., Dakham Z., Pulsalkar P., Maki-Petaja K., Ashby M.J., Cockcroft J.R., Wilkinson I.B. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005;25:372. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 5.Castro M.M., Rizzi E., Figueiredo-Lopes L., Fernandes K., Bendhack L.M., Pitol D.L., Gerlach R.F., Tanus-Santos J.E. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis. 2008;198:320–331. doi: 10.1016/j.atherosclerosis.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Ceron C.S., Castro M.M., Rizzi E., Montenegro M.F., Fontana V., Salgado M.C., Gerlach R.F., Tanus-Santos J.E. Spironolactone and hydrochlorothiazide exert antioxidant effects and reduce vascular matrix metalloproteinase-2 activity and expression in a model of renovascular hypertension. Br. J. Pharmacol. 2010;160:77–87. doi: 10.1111/j.1476-5381.2010.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins-Oliveira A., Castro M.M., Oliveira D.M., Rizzi E., Ceron C.S., Guimaraes D., Reis R.I., Costa-Neto C.M., Casarini D.E., Ribeiro A.A., Gerlach R.F., Tanus-Santos J.E. Contrasting effects of aliskiren versus losartan on hypertensive vascular remodeling. Int. J. Cardiol. 2013;167:1199–1205. doi: 10.1016/j.ijcard.2012.03.137. [DOI] [PubMed] [Google Scholar]
- 8.Wang M., Zhao D., Spinetti G., Zhang J., Jiang L.Q., Pintus G., Monticone R., Lakatta E.G. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler. Thromb. Vasc. Biol. 2006;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 9.Liu R.M., Gaston Pravia K.A. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic. Biol. Med. 2010;48:1–15. doi: 10.1016/j.freeradbiomed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leask A., Abraham D.J. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 11.Endres M. Statins and stroke. J. Cereb. Blood Flow Metab. 2005;25:1093–1110. doi: 10.1038/sj.jcbfm.9600116. [DOI] [PubMed] [Google Scholar]
- 12.Wang C.Y., Liu P.Y., Liao J.K. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol. Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai W., Kloner R.A. Is inhibition of phosphodiesterase type 5 by sildenafil a promising therapy for volume-overload heart failure? Circulation. 2012;125:1341–1343. doi: 10.1161/CIRCULATIONAHA.112.094912. [DOI] [PubMed] [Google Scholar]
- 14.Izidoro-Toledo T.C., Guimaraes D.A., Belo V.A., Gerlach R.F., Tanus-Santos J.E. Effects of statins on matrix metalloproteinases and their endogenous inhibitors in human endothelial cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 2011;383:547–554. doi: 10.1007/s00210-011-0623-0. [DOI] [PubMed] [Google Scholar]
- 15.Luan Z., Chase A.J., Newby A.C. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler. Thromb. Vasc. Biol. 2003;23:769–775. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 16.Ruperez M., Rodrigues-Diez R., Blanco-Colio L.M., Sanchez-Lopez E., Rodriguez-Vita J., Esteban V., Carvajal G., Plaza J.J., Egido J., Ruiz-Ortega M. HMG-CoA reductase inhibitors decrease angiotensin II-induced vascular fibrosis: role of RhoA/ROCK and MAPK pathways. Hypertension. 2007;50:377–383. doi: 10.1161/HYPERTENSIONAHA.107.091264. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira-Melo S.E., Yugar-Toledo J.C., Coelho O.R., De Luca I.M., Tanus-Santos J.E., Hyslop S., Irigoyen M.C., Moreno H., Jr. Sildenafil reduces cardiovascular remodeling associated with hypertensive cardiomyopathy in NOS inhibitor-treated rats. Eur. J. Pharmacol. 2006;542:141–147. doi: 10.1016/j.ejphar.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 18.Dias-Junior C.A., Cau S.B., Oliveira A.M., Castro M.M., Montenegro M.F., Gerlach R.F., Tanus-Santos J.E. Nitrite or sildenafil, but not BAY 41-2272, blunt acute pulmonary embolism-induced increases in circulating matrix metalloproteinase-9 and oxidative stress. Thromb. Res. 2009;124:349–355. doi: 10.1016/j.thromres.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Bae E.H., Kim I.J., Joo S.Y., Kim E.Y., Kim C.S., Choi J.S., Ma S.K., Kim S.H., Lee J.U., Kim S.W. Renoprotective effects of sildenafil in DOCA-salt hypertensive rats. Kidney Blood Press Res. 2012;36:248–257. doi: 10.1159/000343414. [DOI] [PubMed] [Google Scholar]
- 20.Neto-Neves E.M., Dias-Junior C.A., Uzuelli J.A., Pereira R.P., Spiller F., Czaikoski P.G., Tanus-Santos J.E. Sildenafil improves the beneficial hemodynamic effects exerted by atorvastatin during acute pulmonary thromboembolism. Eur. J. Pharmacol. 2011;670:554–560. doi: 10.1016/j.ejphar.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q.M., Wei Y., Zheng Y., Waeber C. Efficacy of combined atorvastatin and sildenafil in promoting recovery after ischemic stroke in mice. Am. J. Phys. Med. Rehabil. 2013;92:143–150. doi: 10.1097/PHM.0b013e3182643f1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro M.M., Rizzi E., Rascado R.R., Nagassaki S., Bendhack L.M., Tanus-Santos J.E. Atorvastatin enhances sildenafil-induced vasodilation through nitric oxide-mediated mechanisms. Eur. J. Pharmacol. 2004;498:189–194. doi: 10.1016/j.ejphar.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 23.Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J. Cell Biol. 1971;50:172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuckerbraun B.S., Shiva S., Ifedigbo E., Mathier M.A., Mollen K.P., Rao J., Bauer P.M., Choi J.J., Curtis E., Choi A.M., Gladwin M.T. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121:98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 25.Wassmann S., Laufs U., Baumer A.T., Muller K., Ahlbory K., Linz W., Itter G., Rosen R., Bohm M., Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 26.Ceron C.S., Rizzi E., Guimaraes D.A., Martins-Oliveira A., Gerlach R.F., Tanus-Santos J.E. Nebivolol attenuates prooxidant and profibrotic mechanisms involving TGF-beta and MMPs, and decreases vascular remodeling in renovascular hypertension. Free Radic. Biol. Med. 2013;65:47–56. doi: 10.1016/j.freeradbiomed.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 27.Souza-Tarla C.D., Uzuelli J.A., Machado A.A., Gerlach R.F., Tanus-Santos J.E. Methodological issues affecting the determination of plasma matrix metalloproteinase (MMP)-2 and MMP-9 activities. Clin. Biochem. 2005;38:410–414. doi: 10.1016/j.clinbiochem.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Castro M.M., Rizzi E., Rodrigues G.J., Ceron C.S., Bendhack L.M., Gerlach R.F., Tanus-Santos J.E. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic. Biol. Med. 2009;46:1298–1307. doi: 10.1016/j.freeradbiomed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Goncalves A.N., Meschiari C.A., Stetler-Stevenson W.G., Nonato M.C., Alves C.P., Espreafico E.M., Gerlach R.F. Expression of soluble and functional full-length human matrix metalloproteinase-2 in Escherichia coli. J. Biotechnol. 2012;157:20–24. doi: 10.1016/j.jbiotec.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi E., Casali B., Regolisti G., Davoli S., Perazzoli F., Negro A., Sani C., Tumiati B., Nicoli D. Increased plasma levels of platelet-derived growth factor (PDGF-BB+PDGF-AB) in patients with never-treated mild essential hypertension. Am. J. Hypertens. 1998;11:1239–1243. doi: 10.1016/s0895-7061(98)00124-1. [DOI] [PubMed] [Google Scholar]
- 31.Laufs U., Marra D., Node K., Liao J.K. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1) J. Biol. Chem. 1999;274:21926–21931. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
- 32.Tantini B., Manes A., Fiumana E., Pignatti C., Guarnieri C., Zannoli R., Branzi A., Galie N. Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic Res. Cardiol. 2005;100:131–138. doi: 10.1007/s00395-004-0504-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhao X.Y., Li L., Zhang J.Y., Liu G.Q., Chen Y.L., Yang P.L., Liu R.Y. Atorvastatin prevents left ventricular remodeling in spontaneously hypertensive rats. Int. Heart J. 2010;51:426–431. doi: 10.1536/ihj.51.426. [DOI] [PubMed] [Google Scholar]
- 34.Takimoto E., Champion H.C., Li M., Belardi D., Ren S., Rodriguez E.R., Bedja D., Gabrielson K.L., Wang Y., Kass D.A. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat. Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 35.Ceron C.S., Rizzi E., Guimaraes D.A., Martins-Oliveira A., Cau S.B., Ramos J., Gerlach R.F., Tanus-Santos J.E. Time course involvement of matrix metalloproteinases in the vascular alterations of renovascular hypertension. Matrix Biol. 2012;31:261–270. doi: 10.1016/j.matbio.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Gao P., Xu T.T., Lu J., Li L., Xu J., Hao D.L., Chen H.Z., Liu D.P. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. J. Mol. Med. (Berl.) 2014;92:347–357. doi: 10.1007/s00109-013-1111-4. [DOI] [PubMed] [Google Scholar]
- 37.Schaafsma D., Dueck G., Ghavami S., Kroeker A., Mutawe M.M., Hauff K., Xu F.Y., McNeill K.D., Unruh H., Hatch G.M., Halayko A.J. The mevalonate cascade as a target to suppress extracellular matrix synthesis by human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 2011;44:394–403. doi: 10.1165/rcmb.2010-0052OC. [DOI] [PubMed] [Google Scholar]
- 38.Briones A.M., Rodriguez-Criado N., Hernanz R., Garcia-Redondo A.B., Rodrigues-Diez R.R., Alonso M.J., Egido J., Ruiz-Ortega M., Salaices M. Atorvastatin prevents angiotensin II-induced vascular remodeling and oxidative stress. Hypertension. 2009;54:142–149. doi: 10.1161/HYPERTENSIONAHA.109.133710. [DOI] [PubMed] [Google Scholar]
- 39.Castro M.M., Tanus-Santos J.E., Gerlach R.F. Matrix metalloproteinases: targets for doxycycline to prevent the vascular alterations of hypertension. Pharmacol. Res. 2011;64:567–572. doi: 10.1016/j.phrs.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Cau S.B., Guimaraes D.A., Rizzi E., Ceron C.S., Souza L.L., Tirapelli C.R., Gerlach R.F., Tanus-Santos J.E. Pyrrolidine dithiocarbamate down-regulates vascular matrix metalloproteinases and ameliorates vascular dysfunction and remodelling in renovascular hypertension. Br. J. Pharmacol. 2011;164:372–381. doi: 10.1111/j.1476-5381.2011.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guimaraes D.A., Rizzi E., Ceron C.S., Oliveira A.M., Oliveira D.M., Castro M.M., Tirapelli C.R., Gerlach R.F., Tanus-Santos J.E. Doxycycline dose-dependently inhibits MMP-2-mediated vascular changes in 2K1C hypertension. Basic Clin. Pharmacol. Toxicol. 2011;108:318–325. doi: 10.1111/j.1742-7843.2010.00656.x. [DOI] [PubMed] [Google Scholar]
- 42.Guimaraes D.A., Rizzi E., Ceron C.S., Pinheiro L.C., Gerlach R.F., Tanus-Santos J.E. Atorvastatin and sildenafil lower blood pressure and improve endothelial dysfunction, but only atorvastatin increases vascular stores of nitric oxide in hypertension. Redox Biol. 2013;1:578–585. doi: 10.1016/j.redox.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(III):39–43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 44.Laufs U., La Fata V., Plutzky J., Liao J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz B.G., Jackson G., Stecher V.J., Campoli-Richards D.M., Kloner R.A. Phosphodiesterase type 5 inhibitors improve endothelial function and may benefit cardiovascular conditions. Am. J. Med. 2013;126:192–199. doi: 10.1016/j.amjmed.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Wassmann S., Laufs U., Baumer A.T., Muller K., Konkol C., Sauer H., Bohm M., Nickenig G. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol. Pharmacol. 2001;59:646–654. doi: 10.1124/mol.59.3.646. [DOI] [PubMed] [Google Scholar]
- 47.Ge C.J., Hu S.J., Wu Y.S., Chen N.Y. Effects of atorvastatin on vascular remodeling in spontaneously hypertensive rats. J. Zhejiang Univ. Sci. 2003;4:612–615. doi: 10.1631/jzus.2003.0612. [DOI] [PubMed] [Google Scholar]
- 48.Rossoni G., Manfredi B., De Gennaro Colonna V., Berti M., Guazzi M., Berti F. Sildenafil reduces l-NAME-induced severe hypertension and worsening of myocardial ischaemia–reperfusion damage in the rat. Br. J. Pharmacol. 2007;150:567–576. doi: 10.1038/sj.bjp.0707131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanaki A.I., Sarafidis P.A., Georgianos P.I., Stafylas P.C., Kanavos K., Tziolas I.M., Lasaridis A.N. Low-dose atorvastatin reduces ambulatory blood pressure in patients with mild hypertension and hypercholesterolaemia: a double-blind, randomized, placebo-controlled study. J. Hum. Hypertens. 2012;26:577–584. doi: 10.1038/jhh.2011.80. [DOI] [PubMed] [Google Scholar]
- 50.Oliver J.J., Melville V.P., Webb D.J. Effect of regular phosphodiesterase type 5 inhibition in hypertension. Hypertension. 2006;48:622–627. doi: 10.1161/01.HYP.0000239816.13007.c9. [DOI] [PubMed] [Google Scholar]
- 51.Dobrin P.B. Effect of histologic preparation on the cross-sectional area of arterial rings. J. Surg. Res. 1996;61:413–415. doi: 10.1006/jsre.1996.0138. [DOI] [PubMed] [Google Scholar]