Abstract
Tumor cells generate extracellular superoxide anions and are protected against intercellular apoptosis-inducing HOCl- and NO/peroxynitrite signaling through the expression of membrane-associated catalase. This enzyme decomposes H2O2 and thus prevents HOCl synthesis. It efficiently interferes with NO/peroxynitrite signaling through oxidation of NO and decomposition of peroxynitrite. The regulatory potential of catalase at the crosspoint of ROS and RNS chemical biology, as well as its high local concentration on the outside of the cell membrane of tumor cells, establish tight control of intercellular signaling and thus prevent tumor cell apoptosis. Therefore, inhibition of catalase or its inactivation by singlet oxygen reactivate intercellular apoptosis-inducing signaling. Nitric oxide and peroxynitrite are connected with catalase in multiple and meaningful ways, as (i) NO can be oxidated by compound I of catalase, (ii) NO can reversibly inhibit catalase, (iii) peroxynitrite can be decomposed by catalase and (iv) the interaction between peroxynitrite and H2O2 leads to the generation of singlet oxygen that inactivates catalase. Therefore, modulation of the concentration of free NO through addition of arginine, inhibition of arginase, induction of NOS expression or inhibition of NO dioxygenase triggers an autoamplificatory biochemical cascade that is based on initial formation of singlet oxygen, amplification of superoxide anion/H2O2 and NO generation through singlet oxygen dependent stimulation of the FAS receptor and caspase-8. Finally, singlet oxygen is generated at sufficiently high concentration to inactivate protective catalase and to reactivate intercellular apoptosis-inducing ROS signaling. This regulatory network allows to establish several pathways for synergistic interactions, like the combination of modulators of NO metabolism with enhancers of superoxide anion generation, modulators of NO metabolism that act at different targets and between modulators of NO metabolism and direct catalase inhibitors. The latter aspect is explicitely studied for the interaction between catalase inhibiting acetylsalicylic acid and an NO donor. It is also shown that hybrid molecules like NO-aspirin utilize this synergistic potential. Our data open novel approaches for rational tumor therapy based on specific ROS signaling and its control in tumor cells.
Keywords: Nitric oxide, Peroxynitrite, Singlet oxygen, Catalase, Intercellular apoptosis-inducingsignaling, Synergistic effect
Graphical abstract
Highlights
-
•
Membrane-associated catalase protects tumor cells against ROS/RNS signaling.
-
•
NO can be oxidated by catalase, but can also reversibly inhibit the enzyme.
-
•
ONOO− is decomposed by catalase but also drives its inactivation through singlet oxygen.
-
•
Modulation of the NO level triggers singlet oxygen generation and catalase inactivation.
-
•
This signaling network allows to establish synergistic antitumor effects.
1. Introductory review
1.1. Extracellular superoxide anion production: a hallmark of malignant cells
Sustained extracellular superoxide anion production through membrane-associated NADPH oxidase (NOX) is controlled by activated oncogenes and represents a characteristic feature of malignant cells both in vitro and in vivo [1–18]. Extracellular superoxide anions and their dismutation product H2O2 are required for the stimulation of proliferation and for the maintenance of the transformed state [1,3,5,6,19–21]. Extracellular superoxide anion generation therefore seems to represent a crucial step in oncogenesis and a striking example of autocrine stimulation of malignant cells.
The procarcinogenic effects of transformed cell-derived superoxide anions are counteracted by versatile superoxide anion-controlled intercellular ROS signaling pathways that cause apoptosis induction selectively in transformed cells [22–39]. Intercellular apoptosis-inducing signaling can be established as an interplay between nontransformed neighbouring effector cells and transformed target cells, or in an autocrine/paracrine signaling by the transformed cells themselves [38,40–42]. Thereby, the efficiency and the selectivity of this elimination mechanism depend on extracellular superoxide anions that characterize the malignant cells. Intercellular apoptosis signaling of malignant cells is essentially mediated by the HOCl and the NO/peroxynitrite signaling pathway [31,32,34,35,37–39], whereas the nitrylchloride signaling pathway [43] and the metal catalyzed Haber Weiss reaction [44] are of minor importance.
Whereas cells transformed in vitro are regularly sensitive for intercellular ROS-mediated apoptosis induction, bona fide tumor cells derived from tumors are regularly resistant against intercellular apoptosis signaling, despite activated NOX [40–42]. More than 70 human tumor cell lines, established from the most frequent and the most aggressive tumors, have been uniformly found to be protected against NOX-dependent apoptosis signaling through expression of membrane-associated catalase (39; Bauer, unpublished).
Acquisition of resistance against ROS represents one characteristic and regularly occurring feature of experimental tumor progression in vivo [45–49]. The ‘H2O2-catabolizing phenotype’ of tumor cells, as defined by Deichman and coworkers, correlates perfectly with resistance against intercellular and autocrine ROS signalling. Resistance is based on the expression of membrane-associated catalase that inhibits both central signalling pathways [40–42].
1.2. Details of the intercellular apoptosis-inducing signaling pathways
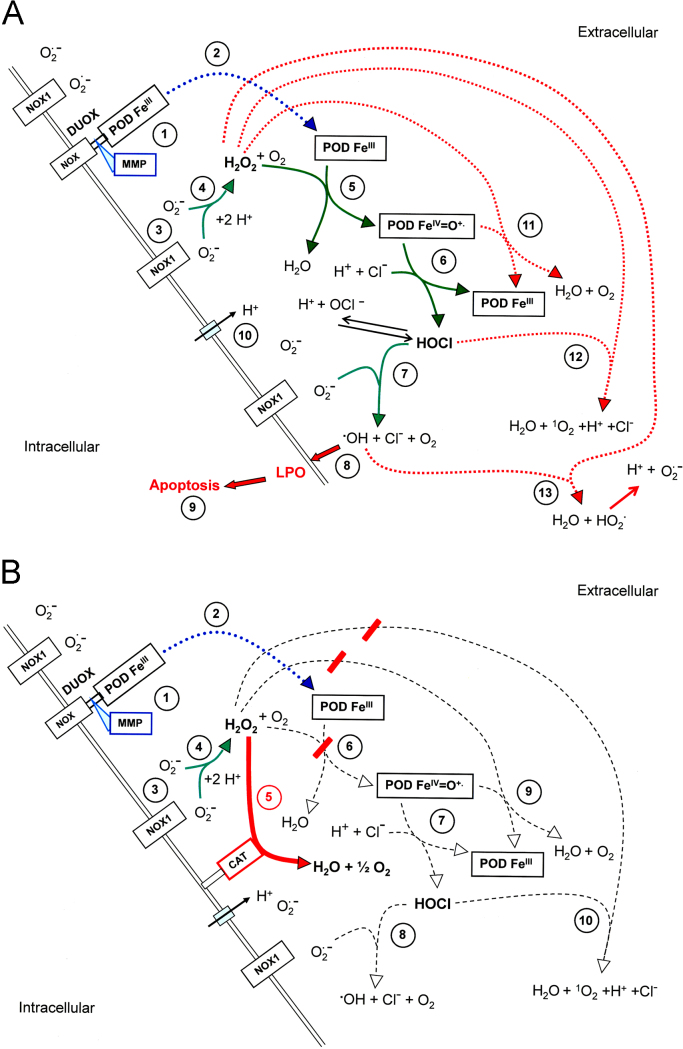
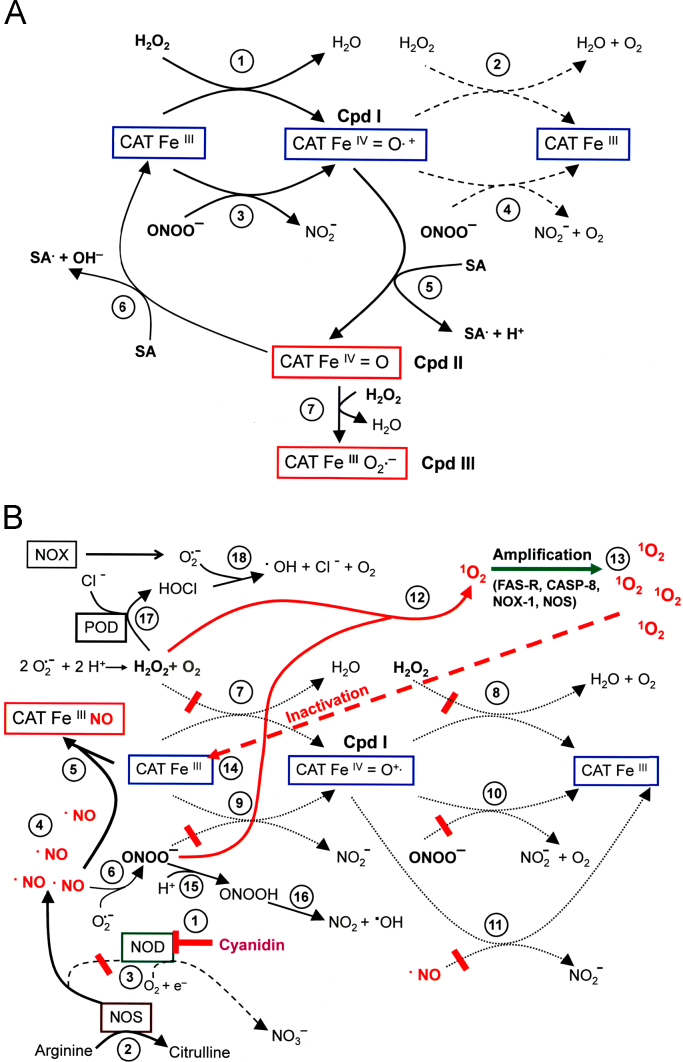
The HOCl and the NO/peroxynitrite signaling pathway have been elucidated through (i) inhibitor studies, (ii) establishment of models based on the results of the inhibitor experiments, (iii) verification or falsification by reconstitution experiments and (iv) siRNA-based analysis. The HOCl signaling pathway of transformed cells (Fig. 1A) depends on the extracellular generation of superoxide anions by NOX1, dismutation of superoxide anions to H2O2 (2O2·−+2H+→H2O2+O2), generation of HOCl by the peroxidase domain of DUOX1 which is released from DUOX1 through the action of matrix metalloprotease [50] (H2O2+PODFeIII→PODFeIV=O·++H2O; POD Fe IV=O·++Cl−+H+→PODFeIII+HOCl), and the interaction between HOCl and superoxide anions in the vicinity of the membrane of the target cells (HOCl+O2·−→·OH+O2+Cl−) [32,51,52]. The resultant hydroxyl radical therefore causes lipid peroxidation specifically in the membrane of the transformed cells and thus triggers the mitochondrial pathway of apoptosis, involving caspase-9- and caspase-3 activity [53]. In the case of excess H2O2 compared to peroxidase, HOCl signaling is strongly impaired [41,42]. The negative effect of H2O2 on HOCl signaling can be abrogated by the addition of (i) low concentrations of catalase or catalase mimetics that decompose excess inhibitory H2O2 to a degree that still allows H2O2-dependent HOCl synthesis, (ii) excess peroxidase or (iii) NO donors that counteract H2O2-dependent processes [41,42]. The negative effect of excess H2O2 on HOCl signaling might be explained (i) by a switch of peroxidase activity to catalase activity in analogy to MPO [54], (ii) the reaction between H2O2 and HOCl (H2O2+HOCl→1O2+H2O+H++Cl−) [55,56], or (iii) the reaction between H2O2 and hydroxyl radicals (H2O2+·OH→HO·2+H2O) [57]. The rate constant of the reaction between H2O2 and hydroxyl radicals (option iii; reaction #13 in Fig. 1A) is in the order of 107 M−1 s−1, whereas the reaction between unsaturated fatty acids in the membrane and hydroxyl radicals is two-three orders of magnitude higher. Therefore, directly at the surface of the membrane, interaction of hydroxyl radicals with H2O2 is unlikely, in contrast to lipid peroxidation by hydroxyl radicals. However, in the layer above the cell membrane, defined by the free diffusion path length of hydroxyl radicals, H2O2 may be speculated to interact with hydroxyl radicals, due to mobility of both molecules and immobility of the membrane. Further experimental work is required to define to which extend options (i–iii) from above contribute to the inhibition of the HOCl signaling pathway.
Fig. 1.
The HOCl signaling pathway A. Transformed cells. The peroxidase domain (PODFeIII) (#1) is released from DUOX through the activity of matrix metalloproteases (#2). (DUOX is expressed on transformed, nontransformed and tumor cells). The membrane of malignant cells, but not that of nontransformed cells contains active NADPH oxidase1 (NOX1) that generates extracellular superoxide anions (#3). These form H2O2 after dismutation (#4) and are used as substrate by PODFeIII for the synthesis of HOCl in a two step reaction (#5 and #6) that involves compound I (PODFeIV=O·+) as intermediate. HOCl reacts with NOX1-derived superoxide anions (#7), resulting in the formation of hydroxyl radicals (#8) that cause lipid peroxidation and subsequent activation of the mitochondrial pathway of apoptosis (#9). Proton pumps in the cell membrane (#10) favour the generation of HOCl from OCl−. In the presence of excess H2O2 compared to peroxidase, HOCl signaling is impaired through a catalase-like activity of peroxidase (#11), the reaction between HOCl and H2O2 (#12) and the reaction between H2O2 and hydroxyl radicals (#13). B. Tumor cells. Tumor cells carry catalase (CAT) covalently attached to the outside of their membrane. Catalase decomposes H2O (#5) and thus inhibits HOCl synthesis (#6,#7). This prevents HOCl/superoxide anion interaction and formation of apoptosis-inducing hydroxyl radicals (#8).
Nontransformed cells that lack activated NOX1 are neither able to synthesize HOCl nor can they generate hydroxyl radicals after addition of exogenously added HOCl. Tumor cells prevent HOCl signaling through expression of membrane-associated catalase that decomposes H2O2 and thus removes the substrate for POD-dependent HOCl synthesis (Fig. 1B). Inhibition of catalase, its inactivation by singlet oxygen or prevention of its expression through specific siRNA abrogate its protective effect and allow the reactivation of HOCl signaling in tumor cells [40–42,53].
The NO/peroxynitrite signaling pathway of transformed cells depends on cell-derived NO [58–60] and extracellular superoxide anions [34] (Fig. 2A). Thereby, the available concentration of NO is controlled by (i) the activity of arginase, (ii) the concentration of arginine, (iii) the activity of NO synthase (NOS) [58–61], (iv) the activity of NO dioxygenase (NOD) that oxidizes NO to nitrate [62–65], and (v) by the oxidation of NO by molecular oxygen [66]. The diffusion-driven reaction between target cell-derived superoxide anions and NO (·NO+O2·−→ ONOO−) [67–71] seems to represent the central step of the NO/peroxynitrite signaling pathway, as it can be completely prevented by inhibition of NO synthesis, inhibition of superoxide anion generation, scavenging of superoxide anions, as well as by decomposition of peroxynitrite through decomposition catalysts [34,42]. Protonation of peroxynitrite to peroxynitrous acid and homolysis of peroxynitrous acid into NO2 and apoptosis-inducing hydroxyl radicals (ONOO−+H+→ONOOH; ONOOH→·NO2+·OH) [68, 72–74] seems to be the final step of the NO/peroxynitrite pathway, as apoptosis induction by this pathway can be completely inhibited by the hydroxyl radical scavenger mannitol. On a first glance, this finding is unexpected, as the established chemistry of peroxynitrite indicates that the reaction of peroxynitrite with CO2, leading to the formation of nitrosoperoxycarboxylate (ONOOCOO−), should be more favourable than the formation of peroxynitrous acid under the experimental conditions used [75–78]. This specific aspect will be experimentally illuminated in the Section 4 (Figs. 5 and 6), thereby taking into consideration that malignant cells show a high activity of proton pumps in their membrane [79]. The resultant high local concentration of protons on the outside of the cell membrane should facilitate the formation of peroxynitrous acid form peroxynitrite in direct vicinity of the membrane. Fig. 2 A shows that NO/peroxynitrite-dependent apoptosis induction may not only be counteracted by the reaction between peroxynitrite and CO2 [75–78], but also through the interaction between H2O2 and peroxynitrite [80], the interaction between H2O2 and hydroxyl radicals [57], the oxidation of NO by oxygen [66] and the reaction between N2O3 and the anion of H2O2 [81]. These counteracting pathways are by no means complete and also require additional discussion. For example, the reaction between H2O2 and hydroxyl radicals (reaction #28 in Fig. 2A) with a reaction constant in the order of 107 M−1 s−1 should have no chance to occur directly at the surface of the cell membrane, as the rate constant of lipid peroxidation of unsaturated fatty acids by hydroxyl radicals is two-to-three orders of magnitude higher. However, experimental evidence presented in reference [53] (inhibition of peroxynitrite/peroxynitrous acid/hydroxyl radical-dependent apoptosis induction by excess H2O2 after strong inhibition of tumor cell protective catalase) shows that this reaction seems to be feasible distant from the membrane, but within the range of the free diffusion range of hydroxyl radicals, most likely as the membrane is immobile in contrast to the reaction partners H2O2 and hydroxyl radical.
Fig. 2.
The NO/peroxynitrite signaling pathway A. Transformed cells. Arginase controls the level of arginine (#1) which is the substrate for NO synthase (NOS) (#2). A substantial concentration of NOS-derived NO is converted into by NO dioxygenase (NOD) (#3) that is connected to the activity of cytochrome P 450 oxidoreductase (POR). The remaining NO passes the cell membrane (#4) and reacts with NOX1-derived superoxide anions (#5), resulting in the formation of peroxynitrite (ONOO−) (#6). Proton pumps in the cell membrane (#7) favour the protonation of peroxynitrite to peroxynitrous acid (ONOOH) (#8). Peroxynitrous acid readily decomposes into NO2 and hydroxyl radicals (#9), which cause lipid peroxidation (LPO) (#10) and apoptosis induction through the mitochondrial pathway of apoptosis (#11). The oxidation of NO by molecular oxygen (#12) leads to the formation of the peroxynitrite radical (ONOO.), which reacts with NO to form ONOONO (#13). ONOONO decomposes into 2 NO2 (#14). NO2 reacts with NO to form dinitrogentrioxide (N2O3) (#15). NOX1 generates extracellular superoxide anions (#16) that dismutate to H2O2 (#17). , the anion of H2O2 (#18) reacts with N2O3, resulting in the formation of peroxynitrite (#19). The reaction between peroxynitrite and CO2 (#20, 21) leads to the formation of nitrosoperoxycarboxylate () (#22). Nitrosoperoxycarboxylate decomposes into NO2 and the carbonate radical (CO3.–) (#23). CO3·− reacts with H2O2, resulting in the formation of HCO3−+HO2· (#24), followed by the formation of superoxide anions (#25). NO2 reacts with NO to form N2O3 (#26). The reaction between H2O2 and peroxynitrite leads to the formation of singlet oxygen (1O2) (#27), whereas the reaction between H2O2 and hydroxyl radicals (#28, 29) might impair apoptosis induction. B. Tumor cells. The concentration of extracellular NO is controlled through steps #1–#4, as discussed under A. Nox1-derived superoxide anions (#5) dismutate to form H2O2 (#6) that is decomposed by membrane-associated catalase (#7), involving compound I as intermediate (CATFeIV=O·+). Compound I of catalase is generated through interaction of native catalase with one molecule of H2O2 and oxidates NO to (#8). Oxidation of NO impairs the formation of ONOO− (#9, #10). Residual ONOO− is decomposed by catalase in a two step reaction that involves formation of compound I (#11). The oxidation of NO and the decomposition of peroxynitrite by membrane-associated catalase causes a tight control of NO/peroxynitrite signaling.
Fig. 5.
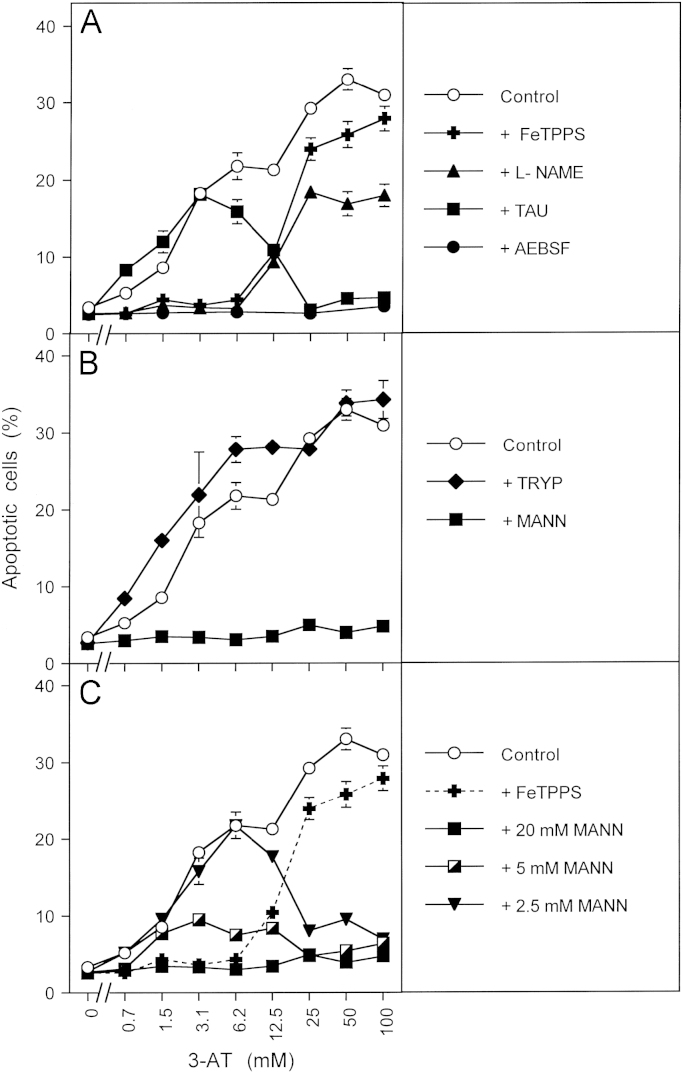
NO/peroxynitrite signaling is mediated by hydroxyl radicals and not by the carbonate radical 9000 MKN-45 human gastric carcinoma cells per assay received no inhibitor (“control”) or the following inhibitors or scavengers: (A) 25 µM of the peroxynitrite decomposition catalyst FeTPPS, 2.4 mM of the NOS inhibitor l-NAME, 50 mM of the HOCl scavenger taurine (TAU), 100 µM of the NOX inhibitor AEBSF; (B) 20 mM of the carbonate radical scavenger tryptophan (TRYP), 20 mM of the hydroxl radical scavenger mannitol (MANN); (C) 25 µM FeTPPS or the indicated concentrations of mannitol (MANN). 3-AT was added at the indicated concentrations and the percentages of apoptotic cells were determined after 5.5 h. Statistical analysis: Apoptosis induction by 3.1 – 100 mM 3-AT was highly significant (p<0.001). Inhibition of apoptosis by AEBSF and mannitol (between 3 and 100 mM 3-AT), by taurine (25–100 mM) and by l-NAME and FeTPPS (3.1 and 6.2 mM 3-AT) were highly significant (p<0.001), whereas there was no significant inhibitory effect by tryptophan.
Fig. 6.
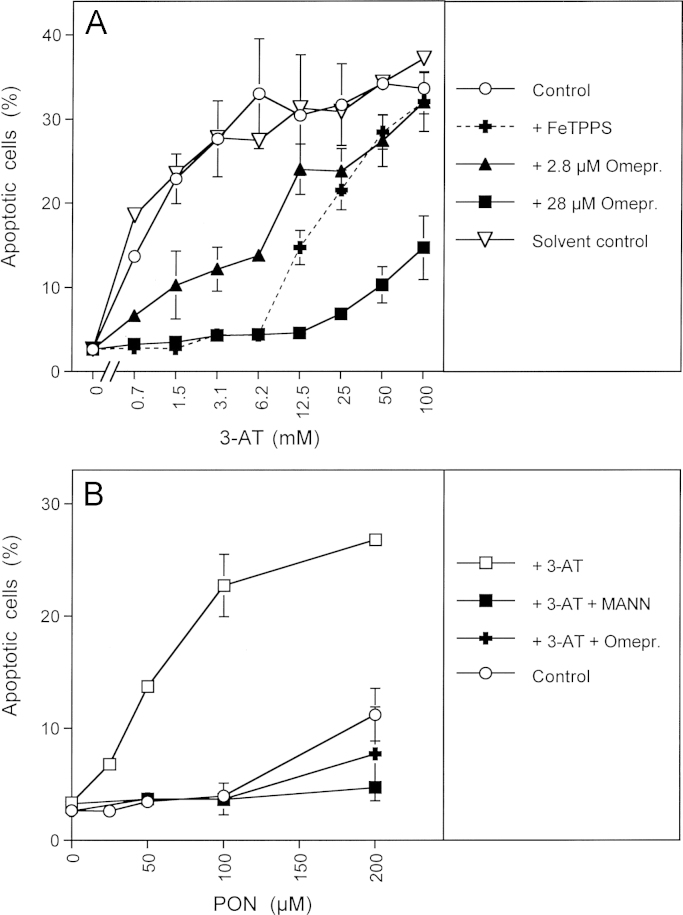
Proton pumps control the formation of peroxynitrous acid from peroxynitrite A. 9000 MKN-45 cells per assay received either no inhibitor (control) or 25 µM FeTPPS, 2.8 or 28 mM of the proton pump inhibitor omeprazol (Omepr.) or the corresponding solvent control (methanol). 3-AT was added at the indicated concentrations and the percentages of apoptotic cells were determined after 5.5 h. B. 12500 MKN-45 cells per assay received either no addition (control), or 50 mM 3-AT, 50 mM 3-AT plus 20 mM mannitol (MANN) or 50 mM 3-AT plus 28 µM omeproazol (omepr.). 100 µM AEBSF was added to all assays and peroxynitrite was added at the indicated concentrations 15 min later. The percentages of apoptotic cells were determined after 70 min. Statistical analysis: (A) Apoptosis induction by 3-AT and its inhibition by 28 µM omeprazole was highly significant at all concentrations of 3-AT, whereas there was no significant effect of the solvent control. The inhibitory effect of 2.8 µM omeprazole was highly significant between 0.7 and 6.2 mM 3-AT, the inhibitory effect of FeTPPS was highly significant between 0.7 and 12.5 mM 3-AT (p<0.001). (B) In the presence of 3-AT, apoptosis induction by 50–200 µM peroxynitrite and its inhibition by mannitol and omeprazole was highly significant (p<0.001).
Furthermore, reaction # 19 in Fig. 2A is not favoured at neutral pH, as the pKa of H2O2 is 11.75. In addition, the reaction between the carbonate radical (CO3·–) and H2O2 (reaction # 24 in Fig. 2A) may be competed out by the reaction between the carbonate radical and superoxide anions, which is three orders of magnitude higher (not shown in Fig. 2A for reduction of complexity).
Nontransformed cells cannot form substantial concentrations of extracellular peroxynitrite in the presence of NO, as they lack extracellular superoxide anion production [31–34].
Based on the recently described multifunctionality of catalase [42,82,83], membrane-associated catalase of tumor cells interferes with NO/peroxynitrite signaling through oxidation of NO [82,84], thus interfering with peroxynitrite formation, as well as through decomposition of peroxynitrite [42,83] (Fig. 2B).
Gradual inhibition of catalase in human tumor cells such as the gastric carcinoma cell line MKN-45, first allowed reactivation of NO/peroxynitrite signalling which was followed by reactivation of the HOCl signalling pathway at higher concentrations of the inhibitor. Interestingly, cell lines derived from mammary carcinoma, Ewing sarcoma, small cell lung carcinoma, neuroblastoma and ovarial carcinoma showed NO/peroxynitrite signaling exclusively after inhibition of their protective catalase at all concentrations of inhibitors ([42,85] Bauer, unpublished results).
1.3. Central role of membrane-associated catalase for the protection of tumor cells
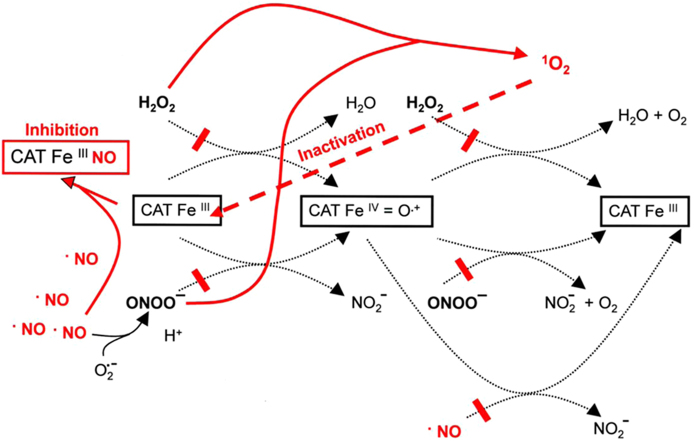
The combination of membrane-associated NOX1 and extracellular membrane-bound catalase seems to represent a unique and regular feature of tumor cells (Fig. 3A). NOX1 is the source for extracellular superoxide anions that (i) are required for the generation of proliferation-stimulating H2O2, but (ii) also drive the efficiency and selectivity of the HOCl and the NO/peroxynitrite signaling pathway that endanger the survival of the malignant cell. Catalase interferes with HOCl and NO/peroxynitrite signaling through decomposition of H2O2 and peroxynitrite. Both reactions use compound I (CATFeIV=O·+) as intermediate. The proof of compound I formation by both substrates has been established through the demonstration of compound I-dependent oxidation of methanol to formaldehyde after the interaction of either H2O2 or peroxynitrite with catalase [42]. As shown by Brunelli et al. 2001 [82], catalase has the potential to oxidate NO. Oxidation of NO by catalase requires first the formation of compound I which is followed by a two step mechanism, resulting in the overall reaction H2O2+2·NO→2H++2 [82,84], with an involvement of compound II (CATFeIV=O) and NO+ as intermediates (Fig. 3B). Thus, catalase interferes with peroxynitrite formation in addition to its potential to decompose peroxynitrite. However, as the formation of peroxynitrite through superoxide anion/NO interaction is extremely fast [68–71], whereas the interaction between NO and catalase seems to be lower [86], decomposition of peroxynitrite rather than oxidation of NO can be expected to be the major controlling effect of catalase on NO/peroxynitrite signaling. At sufficiently high concentrations, NO has the potential to reversibly inhibit catalase by forming an inactive CATFeIIINO complex [87]. Superoxide anions can reactivate NO-inhibited catalase [86], but also may inhibit catalase through reduction of compound I to the inactive compound II and through direct interaction with native ferricatalase, leading to the inactive compound III [88–93] (Fig. 3B). Thus, extracellular catalase controls the crosspoint between H2O2− and NO/peroxynitrite-dependent signaling processes and at the same time is subject to the control by NO and superoxide anions.
Fig. 3.
Mechanistic details of the action of membrane-associated catalase of tumor cells (A) Tight control of NO/peroxynitrite and HOCl signaling through membrane-associated catalase of tumor cells. Membrane-associated NOX1 generates extracellular superoxide anions (#1) that form H2O2 after dismutation (#2). H2O2 is decomposed by membrane-associated catalase in a two step reaction, involving compound I (CATFeIV=O·+) as intermediate (#3 and #4). Thus catalase efficiently inhibits the HOCl signaling pathway as it prevents the generation of HOCl by peroxidase (#5) and subsequent HOCl/superoxide anion interaction and formation of apoptosis-inducing hydroxyl radicals (#6). The activity of arginase (#7), the concentration of its substrate arginine, the activity of NO synthase (NOS) (#8) and the activity of NO dioxygenase (NOD) (#9) determine the concentration of free NO (#10). Compound I of catalase (CATFeIV=O·+) oxidates NO to (#11) and thus counteracts NO-mediated inhibition of catalase (#12), as well as peroxynitrite formation through NO/superoxide anion interaction (#13). Residual peroxynitrite is decomposed by catalase in a two step reaction that involves the formation of compound I (#14 and # 15). Catalase-mediated oxidation of NO and decomposition of peroxynitrite prevent the formation of peroxynitrous acid (ONOOH) (#16) and its subseqent decomposition into NO2 and apoptosis-inducing hydroxyl radicals (#17). Thus catalase establishes a tight control of the NO/peroxynitrite signaling pathway. (B) Details of the interactions between NO and catalase. NOX-derived superoxide anions (#1) dismutate to H2O2 (#2) which reacts with native ferricatalase (CATFeIII), resulting in the formation of compound I (CATFeIV=O·+). Compound I is reduced to the inactive compound II (CATFeIV=O) by NO in a one electron transfer reaction (#4). The resultant nitrosonium ion (NO+) readily reacts with water (NO++H2O→2H+) (#5). Compound II reacts with a second molecule of NO (#6), which leads to the formation of and to the restoration of native ferricatalase. Taken together, the oxidation of NO by catalase follows the equation H2O2+2·NO→2H++2. The reaction between NO and superoxide anions results in the formation of peroxynitrite (ONOO−) (#7), which reacts with ferricatalase and leads to the formation of compound I (#8). If the concentration of NO is sufficiently high, catalase can be reversibly inhibited by NO through formation of an inactive CATFeIIINO complex (#9). Superoxide anions reactivate NO-inhibited catalase (#10), but also can cause inhibition of the enzyme (#11) either through reduction of compound I to the inactive compound II or through formation of inactive compound III (CATFeIIIO2·−).
As catalase can be inhibited by superoxide anions [88–93], the protective activity of catalase on tumor cells requires the presence of additional membrane-associated SOD that scavenges superoxide anions beyond a level that inhibits catalase [39,94]. As recently shown, the activity of extracellular SOD is low enough to still allow superoxide anion-dependent interaction with HOCl and NO, but high enough to prevent superoxide anion-dependent inhibition of catalase [39,94].
Recent work shows that transglutaminase 2 is responsible for the covalent attachment of catalase to the membrane or extracellular matrix, after the enzyme has been released from the tumor cells [95]. The release of catalase from tumor cells per se has been shown long ago, but has not found much attention in the past [96,97]. According to our own findings, these authors have focussed on free catalase in the supernatants of the cells and may have missed the majority of extracellular catalase that is bound to the membrane. The association of catalase to the membranes ensures the high local concentration of the enzyme that is required for efficient interference with peroxynitrite-dependent effects on the membrane. As peroxynitrite is generated outside of the tumor cells, protection against peroxynitrite requires the presence of catalase at the same site and at appreciable local concentration. Intracellular catalase cannot interfere with extracellular peroxynitrite for the simple reason that peroxynitrite hits the membrane before it could be reached by the intracellular enzyme [42,95]. This allows a direct and specific test for extracellular catalase and its inhibition through application of exogenously added peroxynitrite [53]. The differential role of intra- and extracellular catalase in the protection of cells against exogenous peroxynitrite and H2O2 confirmed that protection against exogenous peroxynitrite can only be achieved by extracellular catalase, whereas protection against H2O2 is achieved by intra- and extracellular catalase [95]. Membrane-associated catalase and NOX are not only interacting as antagonists with respect to intercellular ROS-dependent apoptosis signaling, but they are also connected in a corresponding response. Inhibition of NOX1 or scavenging of H2O2, the dismutation product of NOX-derived superoxide anions, causes a rather rapid decrease in the concentration of protective catalase [95]. This indicates that high local expression of catalase on the membrane of tumor cells is not due to stable constitutive expression but rather reflects the result of a dynamic interaction between cellular superoxide anion/H2O2 production and catalase expression.
1.4. Inhibition and inactivation of membrane-associated catalase allows selective apoptosis induction in tumor cells
Inhibition of membrane-associated catalase by 3-aminotriazole (3-AT) or by neutralizing monoclonal antibodies, as well as siRNA-mediated prevention of catalase expression caused NOX-driven reactivation of intercellular apoptosis-inducing NO/peroxynitrite and HOCl signaling and selective apoptosis induction in tumor cells [40,42]. A recent study was focussed on apoptosis induction in tumor cells by secondary plant products, with catalase as hypothetical target structure. The study was based on the long established knowledge that salicylic acid [98–104] as well as anthocyanidins [105–111] induce apoptosis selectively in malignant cells in vitro and show antitumor effects in vivo [99,107,112,113]. In addition, the tumorpreventive effect of acetylsalicylic acid, the i. e. the prodrug for salicylic acid, has been demonstrated in multiple studies [114–117]. Salicylic acid-dependent apoptosis induction in tumor cells does not seem to be attributed to inhibition of cyclooxygenase (COX), as salicylic acid also affects COX1- and COX2-deficient tumor cells [118,119] and as certain other nonsteroidal antiinflammatory drugs that inhibit COX as efficiently as salicylic acid do not induce apoptosis [99]. One common feature of salicylic acid- and anthocyanidin-dependent apoptosis induction seems to be the induction of the mitochondrial pathway of apoptosis [102,103,106,109,111] and the provocation of ROS generation [102,105,109]. Though salicylic acid is known to inhibit plant and mammalian catalases through a one electron transfer that transforms compound I into the inactive compound II [120], the interaction of salicylic acid with tumor cell protective catalase had not yet been suggested as potential initial step during salicylic acid-dependent apoptosis induction in tumor cells. No information on an effect of anthocyanidins on catalase existed when we started our study. The paper by Scheit and Bauer 2015 [53] demonstrates that salicylic acid as well as anthocyanidins (like cyanidin, malvidin, pelargonin and others) cause a strong inhibition of tumor cell protective catalase and, as a consequence thereof, they reactivate intercellular apoptosis signaling through the NO/peroxynitrite and the HOCl signaling pathway. As shown in Fig. 4, the mechanism of catalase inhibition by these two groups of secondary plant compounds is completely different. Salicylic acid seemed to inhibit catalase directly and its action did not require the formation of singlet oxygen. Based on the data from the literature and our own findings, reduction of compound I to inactive compound II or inactive compound III seems to be the mechanism underlying the inhibitory effect of salicylic acid on catalase (Fig. 4A). It can easily be seen that the peroxidatic cycle of catalase through reduction of compound I to compound II and subsequent restoration of native catalase by a second molecule of salicylic acid interferes with the catalatic cycle of catalase, but also causes a gradual consumption of salicylic acid. This decrease was reflected in the kinetics of the salicylic acid dependent effect on tumor cells, which was indicative of abortive inhibition with time [53]. Inhibition of catalase by salicylic acid did not require ongoing superoxide anion synthesis, whereas the consquence of inhibition, i. e. the reactivated intercellular signaling pathways were strictly dependent on extracellular superoxide anions [53].
Fig. 4.
Salicylic acid and anthocyanidins use different strategies to inhibit tumor cell protective catalase. (A) Direct inhibition of catalase by salicylic acid Fig. 4A combines recent results on the catalatic cycle of catalase for its classical substrate H2O2 and the alternative substrate peroxynitrite [42] with previously established central findings [120]. The catalatic cycle (reactions #1–4) involves the formation of compound I (CATFeIV=O·+) and is extremely fast. It starts and ends with active ferricatalase (CATFeIII). The interaction between salicylic acid (SA) and catalase requires the presence of compound I (reaction #5) and is characterized as an one electron reduction of compound I (CATFeIV=O+·) to compound II (CATFeIV=O). Compound II is inactive with respect to the catalatic cycle, but can interact with a second molecule of salicylic acid (reaction #6) and thus regenerate active ferricatalase (CATFeIII). Reaction steps #5 and #6 represent the peroxidatic cycle of catalase. As the peroxidatic cycle is much slower than the catalatic cycle [120], it results in (partial) inhibition of catalase and thus allows free H2O2 to get used as substrate for POD-dependent HOCl synthesis. In parallel, peroxynitrite has a chance to get protonated and then to generate hydroxyl radicals and NO2. This seems to restore intercellular ROS-dependent apoptosis signaling through the NO/peroxynitrite and the HOCl signaling pathways, which are otherwise controlled by catalase [53]. As further alternative, compound II can be converted to the inactive compound III (CATFeIIIO2·−) by H2O2, leading to a dead end of catalase activity and sustained restoration of intercellular ROS signaling. (B) Singlet oxygen-mediated inactivation of tumor cell protective catalase by anthocyanidins. Anthocyanidins inhibits NOD (#1) and thus NO generated by NOS (#2) is no longer dioxygenated by NOD (#3). This causes an increase in free NO (#4), a transient inhibition of catalase by NO (#5) (87), and an increase in the formation of peroxynitrite (#6). The NO-mediated inhibition also interferes with catalase-mediated decomposition of H2O2 (#7,8), decomposition of peroxynitrite (#9,10) and oxidation of NO (#11). Therefore, the concentrations of free H2O2 and peroxynitrite are now increased and a chance for the generation of singlet oxygen is established (#12). The ligand-independent activation of the FAS receptor by singlet oxygen [123] and the subsequent caspase-8-dependent stimulation of NOX activity [124,125] and NOS induction [156] cause a further increase of NO, superoxide anions, H2O2 and peroxynitrite. They thus amplify the generation of more singlet oxygen (#13). Singlet oxygen inactives catalase (#14) [121,122] and restores efficient signaling through the NO/peroxynitrite (#15, #16) and the HOCl signaling pathway (#17,#18) and subsequent activation of the mitochondrial pathway of apoptosis [53].
In contrast to the action of salicylic acid, the inhibitory effect of anthocyanidins on catalase was strictly dependent on ongoing extracellular superoxide anion generation and seemed to be mediated by singlet oxygen. Singlet oxygen is known for its potential to inactivate catalase through reaction with a histidine residue close to the active center [121,122]. Based on the results obtained with sequential addition of inhibitors, the complex picture shown in Fig. 4B emerged as the most likely mechanism underlying the inactivation of catalase by anthocyanidins. It is in line with our data and the known interactions of the molecular species defined as being involved in this scenario. It represents a two-step mechanism that utilizes the amplificatory role of the FAS receptor that can be activated by singlet oxygen in the absence of its ligand [123] and that activates NOX1 in a caspase-8-dependent reaction [124,125]. This caspase-8-dependent activity is different from the death receptor-mediated cell death process. The experimental dissection between caspase-8-dependent stimulation of NOX from death receptor-mediated, caspase-8-dependent apoptosis induction was possible through utilization of cell lines with relatively low FAS receptor expression, allowing the FAS-related effects on ROS signaling but not the direct induction of cell death through the classical FAS receptor-mediated pathway. As shown in Fig. 4B, the increase in available NO after inhibition of NOD by an anthocyanidin is suggested to cause transient inhibition of at least a few catalase molecules, in line with the finding by Brown, 1995 [87]. As a consequence, in the vicinity of the inhibited catalase (i) NO is no longer oxidated, (ii) therefore more peroxynitrite can be formed and (iii) peroxynitrite and H2O2 are not decomposed by the enzyme. The interaction between peroxynitrite and H2O2 allows for local formation of singlet oxygen (1O2), in line with the findings by Di Mascio et al. (1994) [80], who suggested a direct interaction of the two molecules as underlying reaction scheme (H2O2+ONOO−→1O2 +H2O+NO2–). However, this reaction seems to be more complex. Therefore, the details of the interaction are discussed in the Supplementary part of reference# [126]. Singlet oxygen generated after local inhibition of catalase may either inactivate neighbouring catalase molecules directly or activate the FAS receptor in a ligand-independent mode, in line with the findings of Zhuang et al., 2001 [123] that were confirmed by Riethmüller et al., 2015 [126]. As a consequence of FAS receptor activation, caspase-8-dependent activation of NOX and induction of NOS expression may lead to an increase of H2O2 and peroxynitrite. This amplification step allows for more singlet oxygen generation and subsequent inactivation of catalase molecules to a degree that allows reactivation of intercellular apoptosis-inducing signaling.
Fig. 4B shows that NO plays central and determining roles during anthocyanidin-mediated catalase inactivation through this amplificatory mechanism and also during subsequent intercellular apoptosis signaling. The cell lines used for this analysis, like many other tumor cell lines studied by us, do not carry sufficient FAS receptor to allow direct induction of FAS receptor-dependent cell death. Thus it was possible to determine the enhancing effect of the FAS receptor on the ROS-related signaling pathways leading to singlet oxygen generation. It is obvious and also was experimentally determined that tumor cell lines with high FAS receptor expression activate the direct FAS receptor-dependent cell death in the presence of singlet oxygen, in addtion to the pathways described here. However, these interactions reach a degree of complexity that hampers the analysis of discrete signaling pathways.
In the cell system studied here, the FAS receptor and caspase-8 are signaling partners that are not involved in direct induction of apoptosis, but rather trigger ROS-related signaling pathways that cause apoptosis indirectly through establishment of intercellular signaling. Therefore, the effect of caspase-8 is restricted here to the very early step of catalase inactivation and is not involved in execution of cell death through intercellular ROS signaling. The requirement for caspase-8-dependent process was dispensable when superoxide anion generation was enhanced by stimulation of NOX1 through TGF-beta1, thus showing that this step was the contribution of the caspase-8-dependent process required for catalase inactivation [53,85]
1.5. Synergistic effect between direct and indirect inhibitors/inactivators of catalase
Fig. 4B indicates that the initial formation of singlet oxygen from free H2O2 and peroxynitrite after transient NO-mediated inhibition of catalase is a critical step that requires further amplification before singlet oxygen-dependent inactivation of catalase can establish a significant effect. These initial events are especially critical, as NO-mediated inhibition of catalase and singlet oxygen formation are actively counteracted by oxidation of NO and decomposition of peroxynitrite and H2O2 by residual active catalase. The first round of activation of the FAS receptor therefore seems to be a “point of ignition” that triggers further ROS/RNS interactions that finally lead to optimal catalase inactivation and reactivation of intercellular signaling. Even suboptimal inactivation of catalase by direct catalase inhibitors such as salicylic acid might allow local protection of tumor cell-derived H2O2 and peroxynitrite and thus facilitate the critical initial singlet oxygen generation. This might enhance further singlet oxygen-dependent catalase inactivation. Recent results are in line with this concept, as the combination of salicylic acid with cyanidin caused an impressive synergistic effect [53,127]. In line with the model summarized in Fig. 4B, the synergistic effect between salicylic acid and cyanidin was dependent on the generation of singlet oxygen, but was independent of the FAS receptor and caspase-8 [127]. It seemed to follow the steps (1) catalase inactivation, (2) intercellular NOX-dependent signaling through subsequent NO/peroxynitrite and HOCl signaling and (3) execution through the mitochondrial pathway of apoptosis, involving caspase-9 and caspase-3. The synergistic effect characterized a rather general principle, as different catalase inhibitors (such as neutralizing antibodies against catalase, ascorbic acid, Methyldopa) and anti-SOD (that causes indirect inhibition of catalase through establishing a sufficiently high concentration of superoxide anion for inhibition of catalase) had the same effect as salicylic acid when they were combined with cyanidin or other modulators of the NO metabolism [127].
1.6. Establishment of a potential novel therapeutic approach based on intercellular ROS signaling after catalase inactivation
Several lines of evidence indicate that the apoptosis-inducing signaling system and its control by catalase as described here might be relevant for the situation in vivo and therefore might offer the chance for novel therapeutic approaches:
-
1.
The combination of active NOX1 with membrane-associated catalase was regularly found in human and animal tumor systems [38,39];
-
2.
Tumor progression experiments in vivo showed that progression required the induction and selection of cells that are protected against H2O2, which was shown to be due to their expression of membrane-associated catalase [45–49];
-
3.
Inactivation of tumor cell protective catalase reactivated intercellular apoptosis signaling [40–42,53,85],
-
4.
Compounds with selective apoptosis-inducing potential for tumor cells and antitumor activity in vivo, such as salicylate and anthocyanidins target tumor cell catalase and establish synergistic effects [53,127].
Therefore, the NO-driven signaling system that establishes singlet oxygen-dependent inactivation of catalase based on autoamplificatory loops might bear a great chance to establish a rational and novel approach. Consequently, the solidity of the model shown in Fig. 4B should be tested by verification or falsification of the central deductions based on this model. If the model is correct and potentially useful for further therapeutic application:
-
1.
modulation of the available NO concentration by (i) an increase of the NOS substrated arginine, (ii) through inhibition of arginase, (iii) by inducers of NOS expression (iv) by NOD inhibitors others than anthocyanidins,and (v) by direct application of NO donors should result in singlet oxygen-dependent catalase inactivation and subsequent intercellular apoptosis signaling selectively in tumor cells;
-
2.
stepwise experimental dissection of the autoamplificatory loop shown in Fig. 4B, like addition of exogenous singlet oxygen or activation of the FAS receptor should allow to reach the same biological effectas modulation of the NO concentration, i.e. inactivation of catalase and reactivation of intercellular ROS-dependent apoptosis signaling;
-
3.
the combination of modulators of NO metabolism with catalase inhibitors or with stimulators of NADPH oxidase; and the combination of effector molecules that target different regulatory points of NO metabolism should establish synergistic effects on singlet oxygen-dependent catalase inactivation;
-
4.
the combination of catalase inhibitors with exogenous NO, derived from an NO donor should cause a synergistic effect as well and be directed against tumor cell protective catalase.
The experimental part of this contribution presents the results of testing the validity of these predictions and also focuses on the specific aspect of apoptosis induction mediated by peroxynitrite.
2. Experimental complementation
2.1. Materials and methods
2.1.1. Materials
Acetylsalicylic acid, 4-(2-Aminoethyl)benzenesulfonyl fluoride (AEBSF), 3-aminotriazole (3-AT), arginine, catalase from bovine liver, cyanidin, cycloheximide (CHX), DEA NONOate, glucose oxidase (GOX), histidine, mannitol, neutralizing monoclonal antibodies against catalase (clone CAT-505, mouse, IgG1), monoclonal antibodies directed against laminin, monoclonal antibodies directed against the EGF receptor, monoclonal antibodies (clone DX2) directed against human FAS receptor (Apo-1/CD95), the NOS inhibitor N-omega-nitro-l-arginine methylester hydrochloride (l-NAME), NO-aspirin (NCX 4040), malvidin, omeprazole, salicylic acid, taurine, tryptophan, Mn-SOD from Escherichia coli, were obtained from Sigma-Aldrich (Schnelldorf, Germany). The peroxidase inhibitor 4-Aminobenzoyl hydrazide (ABH) was obtained from Acros Organics (Geel, Belgium). The catalase mimetic EUK 134 [chloro([2,2′-[1,2-ethanediylbis[(nitrilo-κN)methylidyne]]bis[6-methoxyphenolato-κO]]]-manganese was a product of Cayman and was obtained from Biomol (Hamburg, Germany). Inhibitors for caspase-8 (Z-IETD-FMK) and caspase-9 (Z-LEHD-FMK) were obtained from R&D Systems (Wiesbaden-Nordenstadt, Germany). The peroxynitrite decomposition catalyst 5-, 10-, 15-, 20-Tetrakis(4-sulfonatophenyl)porphyrinato iron(III) chloride (FeTPPS) was obtained from Calbiochem (Merck Biosciences GmbH, Schwalbach/Ts, Germany). Photofrin (a product of Axcan, Canada) was obtained from Meduna Arzneimittel GmbH (Aschaffenburg, Germany). TGF-beta1 was purified from human platelets as recently described [22].
Itraconazol (Sempera) was obtained from Janssen-Cilag, Neuss, Germany. The arginase inhibitors 2(S)-Amino-6-boronohexanoic acid.NH4 (A-6-BHA), (2S)-(+)-Amino-5-iodoacetamidopentanoic acid (A-5-IAAPA), S-(2-Boronoethyl)-l-cysteine.NH4 (BEC), Nω-Hyroxy-nor-l-arginine.acetate (NOR–NOHA) and NG-Hydroxy-l-arginine.monoacetate (NOHA) were obtained from Axxora/Enzo Life Sciences, Lörrach, Germany.
Detailed information on inhibitors has been previously published [34,40,85,128]. The site of action of inhibitors and scavengers is shown in the supplementary material of Bauer et al. (2014) [128]; Bauer and Zarkovic (2015) [85].
Preparation of photofrin dilutions and their addition to the assays was performed at dimmed out light. Singlet oxygen generation by photofrin was induced by illumination with visible light under the working bench for the indicated times.
2.1.2. Cells and media for cell culture
The human gastric adenocarcinoma cell line MKN-45 (ACC 409) (established from the poorly differentiated adenocarcinoma of the stomach (medullary type) of a 62 year-old woman) was purchased from DSMZ, Braunschweig, Germany. The human Ewing sarcoma cell lines CHP 100 and A 4573 have been obtained from Dr. U. Kontny, Department of Pediatrics and Adolescent Medicine, University Medical Centre Freiburg, Germany. A 4573 cells have deficient expression of caspase-8 [129]. Nontransformed diploid fibroblasts Alpha-1 have been established in our institute from the foreskin of a healthy proband. MKN-45 and GUMBUS were cultured in RPMI 1640 medium, containing 10% fetal bovine serum (FBS). Fetal bovine serum (Biochrom, Berlin, Germany) had been heated for 30 min at 56 °C prior to use. Medium was supplemented with penicillin (40 U/ml), streptomycin (50 µg/ml), neomycin (10 µg/ml), moronal (10 U/ml) and glutamine (280 µg/ml). Care was taken to avoid cell densities below 300,000/ml and above 106/ml. L929 and Alpha-1 cells were cultivated as adherent cultures in Eagle's Minimum Essential Medium (EMEM), supplemented with 5% heat-treated FBS, penicillin (40 U/ml), streptomycin (50 µg/ml), neomycin (10 µg/ml), moronal (10 U/ml) and glutamine (280 µg/ml).
3. Methods
3.1. Apoptosis induction
3.1.1. Autocrine apoptosis induction by intercellular ROS signaling
Cells in complete medium were seeded in 96-well tissue culture clusters at a density of 12,500 cells/100µl. Reactivation of intercellular apoptosis-inducing ROS signaling required the inhibition or inactivation of membrane-associated catalase of tumor cells. This was either achieved by direct catalase inhibitors such as 3-aminotriazole (3-AT) (Figs. 5 and 6A), salicylic acid (Fig. 10) and acetylsalicylic acid (Fig. 14), or through modulation of the endogenous NO concentration that triggered cell-derived catalase-inactivating singlet oxygen generation ( Figs. 7–13, 15). Modulation of the NO concentration was achieved through addition of arginine, arginase inhibitors, inhibitors of NO dioxygenase (NOD) (cyanidin, malvidin), stimulation of the FAS receptor and addition of interferon gamma. Details of the treatments are summarized in the respective. In all experiments, assays were performed in duplicate. After the indicated time of incubation at 37 °C and 5% CO2 that allowed intercellular ROS-mediated apoptosis induction, the percentage of apoptotic cells was determined by inverted phase contrast microscopy based on the classical criteria for apoptosis, i.e., nuclear condensation/fragmentation or membrane blebbing [42,130,131]. The characteristic morphological features of intact and apoptotic cells, as determined by inverted phase contrast microscopy have been published [29,42,128]. At least 200 neighbouring cells from randomly selected areals were scored for the percentage of apoptotic cells at each point of measurement. Control assays ensured that the morphological features ‘nuclear condensation/fragmentation’ as determined by inverse phase contrast microscopy were correlated to intense staining with bisbenzimide and to DNA strand breaks, detectable by the TUNEL reaction [22,29,34]. A recent systematic comparison of methods for the quantitation of apoptotic cells has shown that there is a perfect coherence between the pattern of cells with condensed/fragmented nuclei (stained with bisbenzimide) and TUNEL-positive cells in assays with substantial apoptosis induction, whereas there was no significant nuclear condensation/fragmentation in control assays [52,128].
Fig. 10.
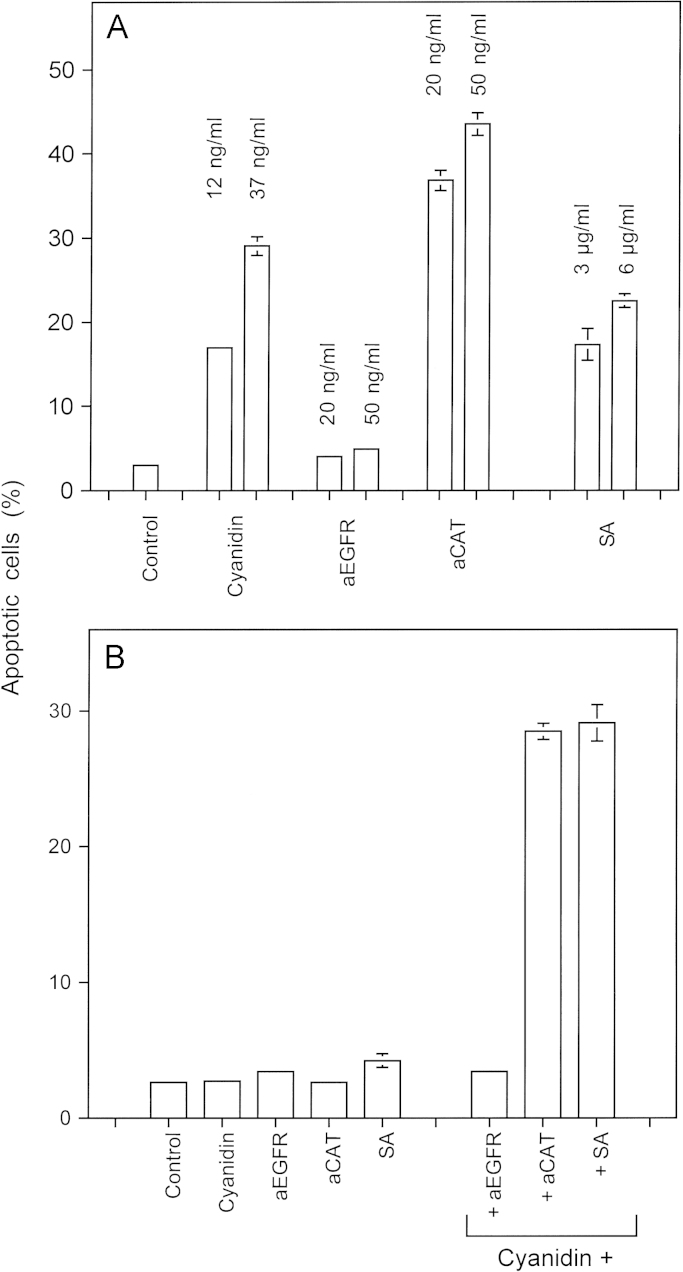
Synergistic interaction between the NOD inhibitor cyanidin and catalase inhibitors. (A) 12,500 MKN-45 cells received no addition (control) or the indicated concentrations of cyanidin, antibody directed towards the EGF receptor (aEGFR), neutralizing antibodies directed against human catalase (aCAT) or salicylic acid (SA). The percentages of apoptotic cells obtained by sole application of these compounds was determined after 6.5 h. (B) 12,500 MKN-45 cells received no addition (control) or 0.15 ng/ml cyanidin, 1 ng/ml aEGFR, 1 ng/ml aCAT, 0.1 µg/ml SA applied alone or 0.15 ng/ml cyanidin combined with either 1 ng/ml aEGFR, 1 ng/ml aCAT, 0.1 µg/ml SA. The percentages of apoptotic cells were determined after 6 h. Statistical analysis: (A) Apoptosis induction by cyaniding, aCAT and salicylic acid was highly significant (p<0.001). (B) The synergistic effect between cyaniding and aCAT and cyaniding and salicylic acid was highly significant (p<0.001).
Fig. 14.
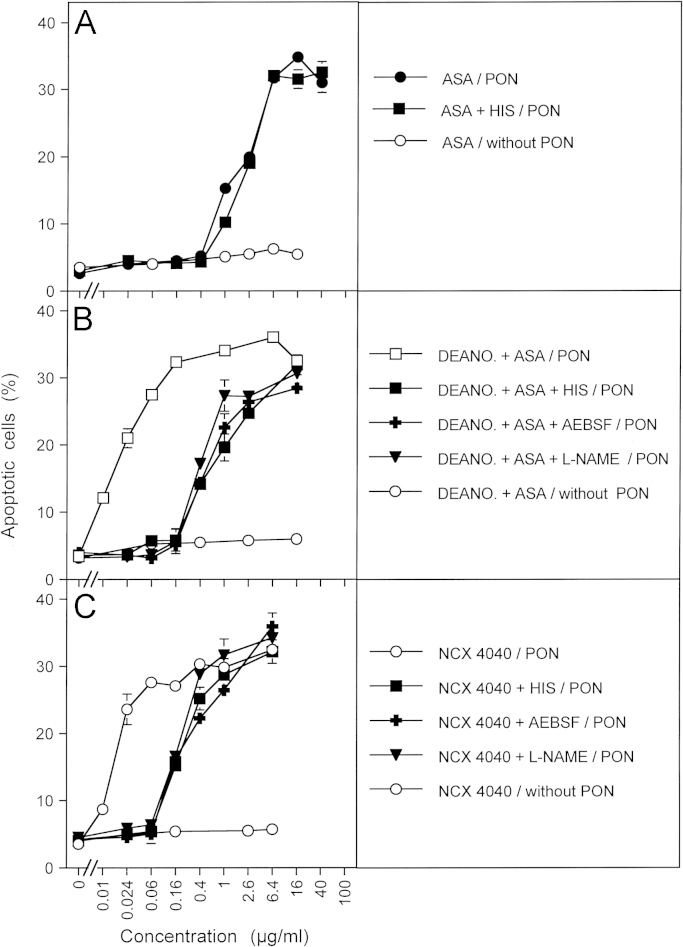
Synergistic effect between the catalase inhibitor acetyl salicylic acid and the NO donor DEA NONOate 12,500 MKN-45 cells per assay received the indicated concentrations of acetyl salicylic acid (ASA) alone or in combination with 2 mM histidine (HIS) (A), or the indicated concentrations of ASA in combination with 0.2 mM DEA NONOate (DEANO.), or the indicated concentrations of ASA in combination with 0.2 mM DEANONOate plus 2 mM histidine (HIS), 100 µM AEBSF, 2.4 mM l-NAME where indicated (B) or the indicated concentrations of the NO-acetyl salicylic acid hybrid molecule NCX 4040 alone or in combination with 2 mM histidine (HIS), 100 µM AEBSF, 2.4 mM l-NAME (C). The assays were incubated for 30 min at 37 °C. AEBSF was then added at a concentration of 100 µM (except for the assays that already contained AEBSF) and 200 µM peroxynitrite (PON) were added as indicated in the legends. The percentages of apoptotic cells were determined after 90 min. The figure shows that ASA sensitizes tumor cells for apoptosis induction by peroxynitrite, i.e. inhibits membrane-associated catalase, independent of singlet oxygen (A). The presence of the NO donor allows for a synergistic effect that is dependent on the action of cell-derived singlet oxygen (B). NO-ASA triggers an effect that is analogous to the synergistic effect between NO and ASA. Statistical analysis: A: Apoptosis induction by peroxynitrite in the presence of 1–100 µg/ml acetylsalicylic acid was highly significant (p<0.001). Histidine did not inhibit this reaction significantly. The difference between assays with and without peroxynitrite was highly significant (p<0.001). (B) The leftward shift of the curve in the presence of the NO donor DEA NONOate was highly significant (p<0.001). The shift-back effect of histidine, AEBSF and l-NAME was highly significant (p<0.001). (C) Apoptosis induction by NCX 4040/peroxynitrite in the concentration range between 0.024 and 6.4 µg/ml NCX 4040 was highly significant. The rightward shift of the curves after addition of histidine, AEBSF and l-NAME was highly significant (p<0.001).
Fig. 7.
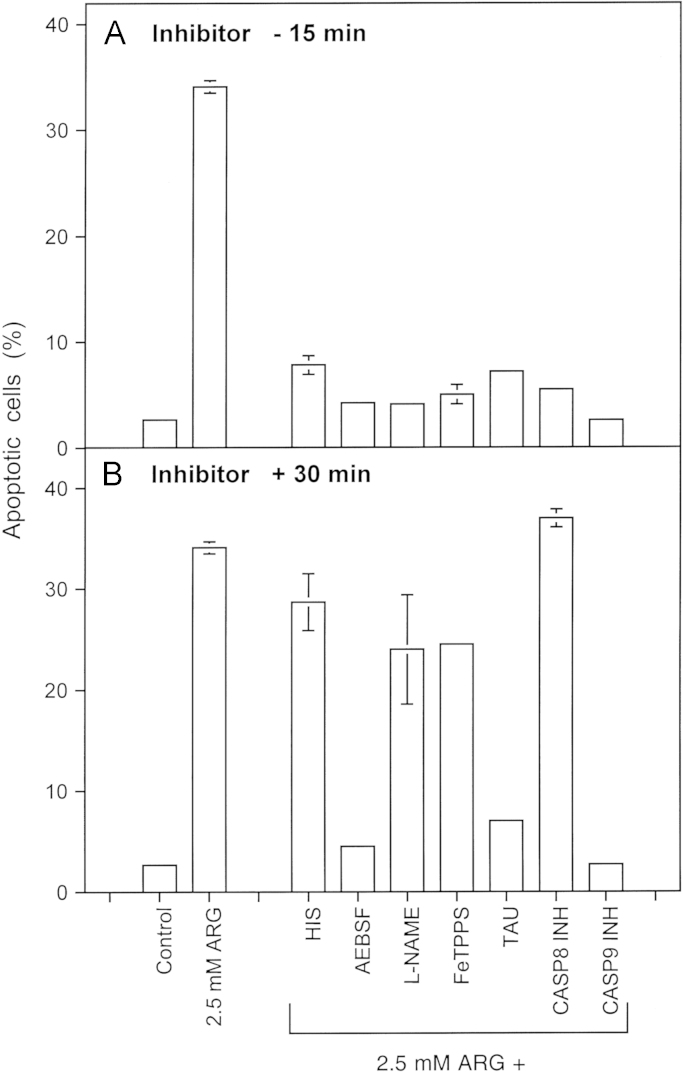
Reactivation of intercellular ROS-dependent signaling through addition of the NOS substrate arginine 12,500 MKN-45 cells per assay received either no addition (control), 2.5 mM arginine (ARG) or 2.5 mM arginine plus either 2 mM histidine (HIS), 100 µM AEBSF, 2.4 mM l-NAME, 25 µM FeTPPS, 50 mM taurine (TAU), 25 µM caspase-8 inhibitor (CASP8 INH) or 25 µM caspase-9 inhibitor (CASP9 INH). The inhibitors were either added 15 min before the addition of arginine (A) or 30 min after addition of arginine (B). The percentages of apoptotic cells were determined after 3.5 h. Statistical analysis: Apoptosis induction mediated by arginine and its inhibition by all inhibitors applied before arginine addition was highly significant (p<0.001). When the inhibitors had been applied 30 min after arginine, only the inhibition by AEBSF, taurine and caspase-9 inhibitor was highly significant (p<0.001), whereas the other inhibitors caused no significant effects.
Fig. 8.
Reactivation of intercellular ROS-dependent signaling through addition of arginase inhibitors 12,500 MKN-45 tumor cells per assay received the indicated concentrations of five chemically different arginase inhibitors. Where indicated, assays contained 2 mM of the singlet oxygen scavenger histidine (HIS) in addition to the arginase inhibitors. The percentages of apoptotic cells were determined after 5 h. Abbreviations of arginase inhibitors: A-6-BHA: 2(S)-Amino-6-boronohexanoic acid.NH4 A-5-IAAPA: (2S)-(+)-Amino-5-iodoacetamidopentanoic acid BEC: S-(2-Boronoethyl)-l-cysteine.NH4 NOR-NOHA: Nω-Hyroxy-nor-l-arginine.acetate NOHA: NG-Hydroxy-l-arginine.monoacetate This experiment demonstrates that five different arginase inhibitors cause apoptosis induction in the tumor cells in a concentration-dependent mode. In each case, apoptosis induction is abrogated when the singlet oxygen scavenger histidine had been present during treatment. Statistical analysis: Apoptosis induction by all arginase inhibitors at higher concentrations, as well as inhibition by histidine were highly significant (p<0.001).
Fig. 9.
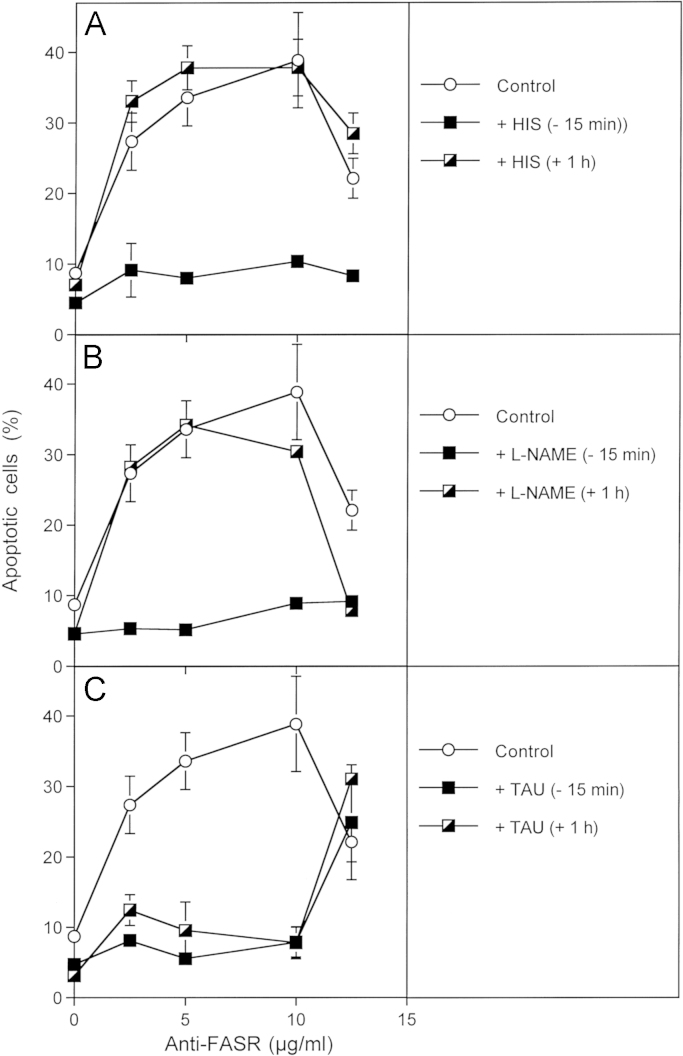
Activation of the FAS receptror causes the reactivation of intercellular ROS-mediated apoptosis signalling 12,500 MKN-45 cells per assay received no inhibitors (control) or 2 mM histidine (HIS), 2.4 mM l-NAME or 50 mM taurine (TAU) either 15 min before or 1 h after addition of the indicated concentrations of antibodies directed against the FAS receptor. The percentages of apoptotic cells were determined after 12 h. Statistical analysis: Apoptosis induction by anti-FAS receptor as well as its inhibition by histidine and l-NAME added prior to the antibody were highly significant (p<0.001), whereas later addition of these inhibitors did not cause significant inhibition. In contrast, there was no significant difference between the effects of early and late addition of taurine (p<0.001) that inhibited apoptosis induction by anti-FAS receptor up to 10 µg/µl with high significance (p<0.001).
Fig. 11.
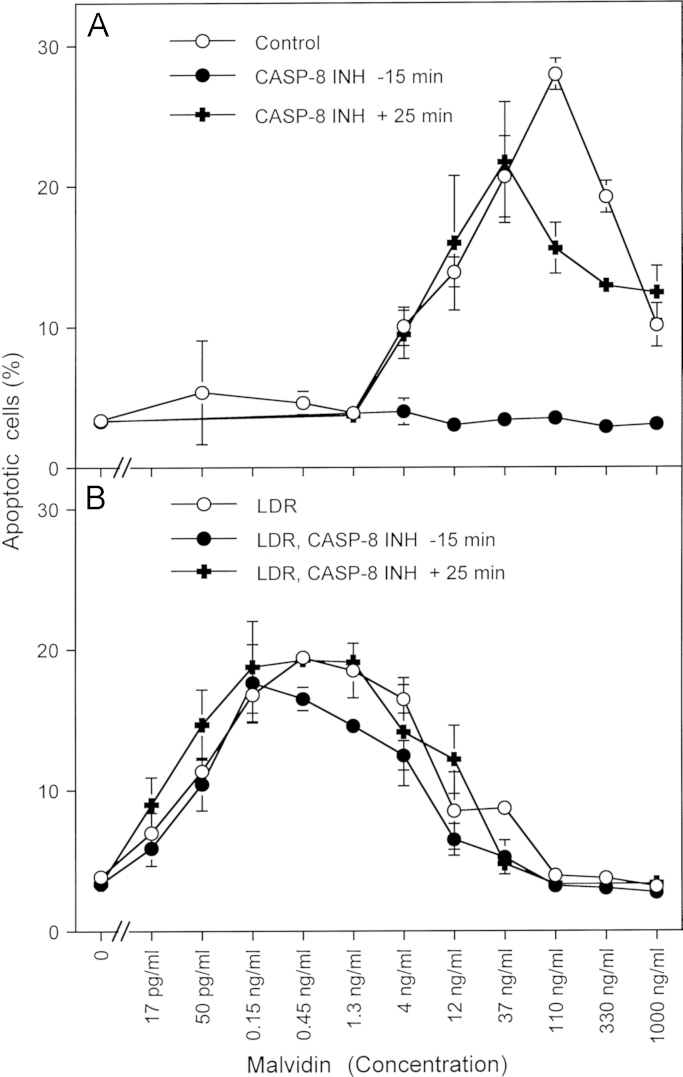
Synergistic effect between the NOD inhibitor malvidin and low dose gamma radiation-enhanced superoxide anion generation (A) 12,500 MKN-45 cells per assay received no inhibitor (control) or 25 µM caspase-8 inhibitor (CASP-8 INH) either 15 min before or 20 min after the addition of the indicated concentrations of malvidin. The percentages of apoptotic cells were determined after 12 h. B. MKN-45 cells were irradiated at a density of 200,000 cells/ml with 75 mGy gamma radiation and incubated for 1 h. The cells were seeded at a density of 12,500 cells per assay and received no inhibitor (control) or 25 µM caspase-8 inhibitor (CASP-8 INH) either 15 before or 20 min after the addition of the indicated concentrations of malvidin. The percentages of apoptotic cells were determined after 12 h. Statistical analysis:A: Apoptosis induction by 4 ng/ml and 1000 ng/ml was significant (p<0.01), apoptosis induction by 12–330 ng/ml was highly significant (p<0.001). Inhibition by caspase-8 inhibitor added 15 min before cyanidin was highly significant (p<0.001), whereas caspase-8 inhibitor added 25 min after cyanidin only caused highly significant inhibition (p<0.001) at 110 ng/ml cyanidin. B: The low dose irradiation-dependent shift of the curve compared to A was highly significant (p<0.001). There was no significant inhibition by caspase-8 inhibitor.
Fig. 12.
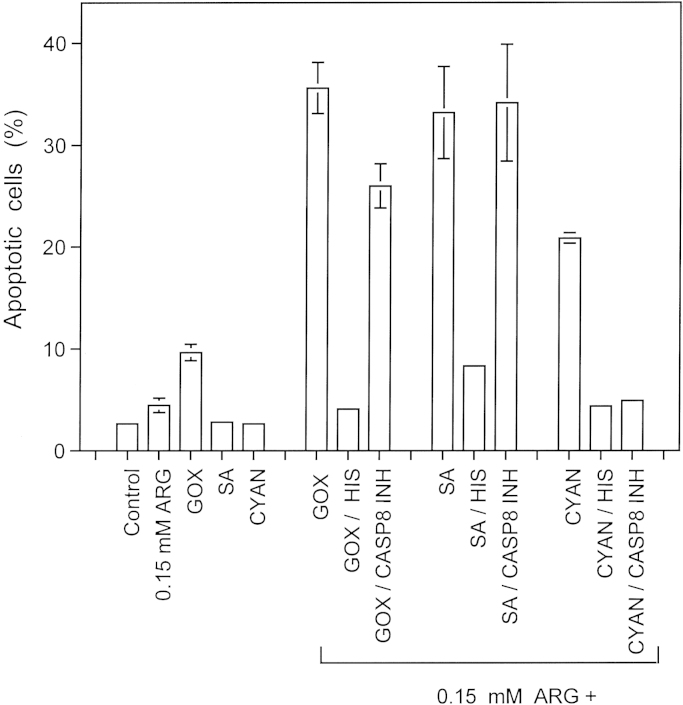
Synergistic effects between the NOS substrate arginine and H2O2-generating glucose oxidase, the catalase inhibitor salicylic acid and the NOD inhibitor cyaniding 12,500 MKN-45 cells per assay received no addition (control) or 0.15 mM arginine (ARG), 10 mU/ml glucose oxidase (GOX), 0.1 µg/ml salicylic acid (SA) or 10 ng/ml cyanidin (CYAN) as individual addition or 0.15 mM arginine combined with 10 mU/ml glucose oxidase (GOX), 0.1 µg/ml salicylic acid (SA) or 10 ng/ml cyanidin (CYAN). Where indicated, assays had received 2 mM histidine (HIS) or 25 µM caspase-8 inhibitor (CASP8 INH) 15 min before the other compounds. The percentages of apoptotic cells were determined after 3.5 h. Statistical analysis: Apoptosis induction by individual compounds was not significant, whereas apoptosis induction through synergistic effects between 0.15 mM arginine and GOX, salicylic acid and cyanidin, as well as inhibition of apoptosis by histidine were highly significant (p<0.001). Caspase-8 inhibitor only cause highly significant inhibition of the synergistic effect between cyanidin and arginine (p<0.001).
Fig. 13.
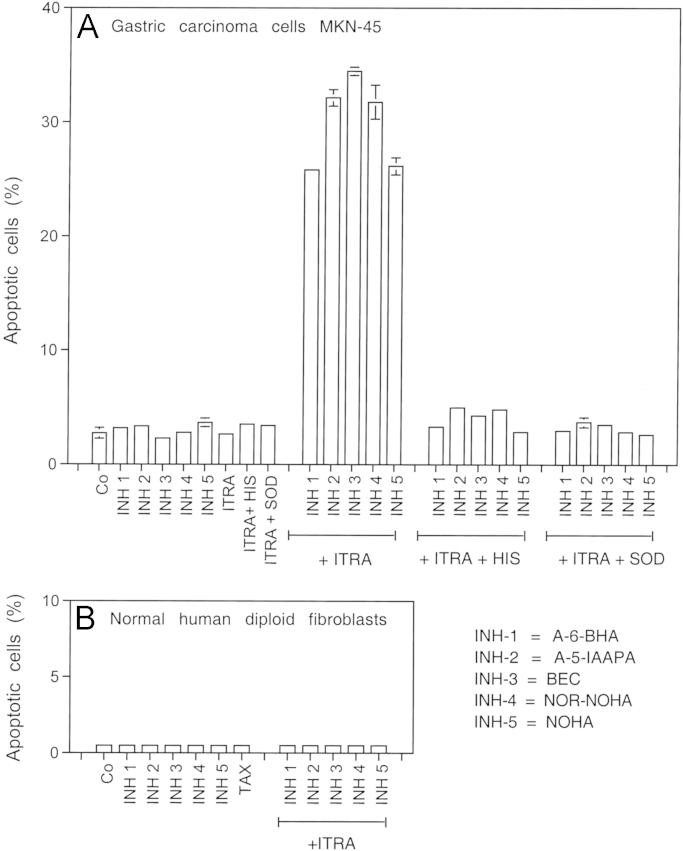
Synergistic action between five different arginase inhibitors and suboptimal concentrations of the NOD inhibitor itraconazole. 12,500 MKN-45 tumor cells or 10,000 human diploid fibroblasts Alpha-1 per assay were treated without further additions (“co”), 2 µM of inhibitors 1–4, 4 µM of inhibitor 5 in the absence or presence of 0.1 µg/ml itraconazol. Where indicated, assays received 2 mM histidine or 100 U/ml Mn-SOD before addition of the other compounds. The percentages of apoptotic cells were determined after five hours in duplicate experiments. The data shown in Fig. 13 demonstrate that all arginase inhibitors act synergistically with itraconzole in the specific apoptosis induction in tumor cells. The synergistic action depends on singlet oxygen and superoxide anions, as it was blocked by histidine as well as SOD. The combination of arginase inhibitors and itraconazol used in this experiment had no apoptosis inducing effect on normal diploid fibroblasts. The lack of reaction is due to the lack of extracellular superoxide anion generation in nontransformed cells. Statistical analysis: A: None of the individually applied compounds caused significant apoptosis induction, whereas the synergistic effects between itraconazole and the arginase inhibitors were highly significant (p<0.001). Inhibition of these synergistic effects by histidine and SOD were highly significant (p<0.001). B: There was no significant apoptosis induction in normal human diploid fibroblasts.
Fig. 15.
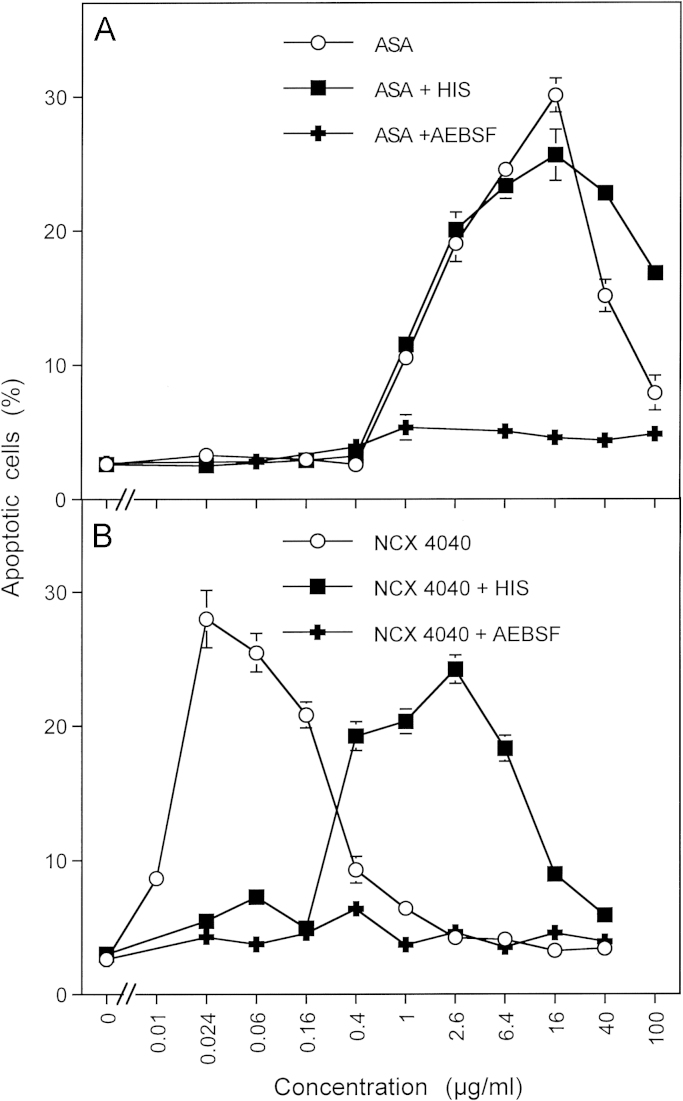
Singlet oxygen-dependent and –independent activity of NO-acetyl salicylic acid. 12,500 MKN-45 cells were treated with increasing concentrations of acetyl salicylic acid (ASA) (A) or NO-acetyl salicylic acid (NCX 4040) (B) in the absence and presence of 2 mM histidine (HIS) or 100 µM AEBSF. The percentages of apoptotic cells were determined after 3.5 h. Statistical analysis: (A) Apoptosis induction by acetylsalicylic acid (1-40 µg/ml) as well as its inhibition by histidine were highly significant (p<0.001). AEBSF caused no significant inhibitory effect. (B) The shift of the concentration-response curve of NCX 4040 compared to acetylsalicylic acid (A) was highly significant, as were the effects of histidine and AEBSF (p<0.001).
Apoptosis induction by exogenous peroxynitrite is described in the legend to Figs. 6B and 14 (Fig. 15).
3.2. Irradiation
Cells (200,000 cells/ml in suspension) were irradiated using a Compagnie Oris (Gif-sur-Yvette, France) IBL 437 Cs137 gamma source. The dose rate was 37.5 m Gy/s for the experiments in this study. Immediately after irradiation, the cells were centrifuged and resuspended in fresh medium. The cells were then used either directly in the experiments or incubated in 5% CO2 at 37 °C in a humidified incubator for the time indicated in the and then centrifuged and resuspended in fresh medium.
3.3. Statistics
Statistical analysis. In all experiments, assays were performed in duplicate and empirical standard deviation were calculated. Absence of standard deviation bars indicates that the standard deviation was too small to be reported by the graphic program. Empirical standard deviations merely demonstrate reproducibility in parallel assays but do not allow statistical analysis of variance. The experiments have been repeated at least twice (with duplicate assays). The Yates continuity corrected chi-square test was used for the statistical determination of significances (p<0.01=significant; p<0.001=highly significant).
4. Results
4.1. The role of peroxynitritous acid-derived hydroxyl radicals for apoptosis signaling through the NO/peroxynitrite pathway
In the presence of CO2, the formation of nitrosoperoxycarboxylate (ONOOCOO−) from peroxynitrite and subsequent decomposition into NO2 and carbonate radicals is known as the dominating reaction [75–78]. Therefore, protonation of peroxynitrite to peroxynitrous acid and subsequent decomposition into NO2 and hydroxyl radicals are generally regarded as being unfavourable. However, our previous inhibitor experiments with mannitol pointed to a central role of hydroxyl radicals as final element of the NO/peroxynitrite signaling pathway [42]. In order to specifically address this question, MKN-45 cells were treated with the catalase inhibitor 3-AT in the presence of selected inhibitors (Fig. 5). The inhibitor profile (Fig. 5A) showed that apoptosis induction was dependent on superoxide anions at all concentrations of 3-AT applied, as it was inhibited by the NOX inhibitor AEBSF. Up to 12.5 mM 3-AT, the NO/peroxynitrite pathway was dominating, as seen by the inhibitory effect of the peroxynitrite decomposition catalyst FeTPPS and the NOS inhibitor l-NAME. At higher concentrations of 3-AT, HOCl signaling was responsible for apoptosis induction as shown by the effect of the HOCl scavenger taurine. As shown in Fig. 5B, scavenging of hydroxyl radicals with the specific scavenger mannitol completely inhibited apoptosis induction over the whole concentration range of 3-AT, whereas the carbonate radical scavenger tryptophan had no inhibitory effect. This finding confirms the apoptosis-inducing role of hydroxyl radicals for the NO/peroxynitrite and the HOCl signaling pathway (in line with the schemes shown in Figs. 1 and 2). It also shows that there is no indication for a functional role of carbonate radicals for apoptosis induction in this specific context. When the concentration of mannitol was gradually decreased, the inhibitory effect on NO/peroxynitrite signaling was gradually abolished for NO/peroxynitrite signaling, whereas the effect on HOCl signaling remained much stronger (Fig. 5C).
In order to unravel the paradoxical finding of biologically significant peroxynitrous acid-derived hydroxyl radical generation despite expected dominating generation of nitrosoperoxycarboxylate (ONOOCOO−), we took into consideration that the membrane of tumor cells is known for its high activity of proton pumps [79]. Therefore, in close vicinity to the membrane, peroxynitrite might have a higher chance to form peroxynitrous acid rather than to react with CO2. If this assumption was correct, proton pump inhibitors should have a negative effect on NO/peroxynitrite signaling. As shown in Fig. 6A, the proton pump inhibitor omeprazol had a strong inhibitory effect on NO/peroxynitrite signaling and a weaker effect on HOCl signaling. 28 µM of omeprazol completely inhibited NO/peroxynitrite signaling and caused more than 50% inhibition of HOCl signaling. The strong inhibitory effect of omeprazol was also seen when exogenous peroxynitrite was applied to the cells in the presence of 3-AT (Fig. 6B). Thus, interference with peroxynitrous acid formation had a similar effect as scavenging peroxynitrous acid-derived hydroxyl radicals by mannitol. The negative effect of the proton pump inhibitor on HOCl signaling is explained by interference with HOCl formation from OCl–.
4.2. Triggering of singlet oxygen-dependent reactivation of intercellular apoptosis inducing signaling through the addition of arginine, arginase inhibitor or exogenous NO
Addition of the NOS substrate arginine at a concentration of 2.5 mM (Fig. 7) caused an analogous apoptosis-inducing effect as recently described for the NOD inhibitor cyanidin [53]. In analogy to the mechanism described in Fig. 4B, apoptosis induction triggered by arginine was dependent on an early step that involved singlet oxygen, NO, peroxynitrite and caspase-8. This was followed by subsequent HOCl signaling and caspase-9 action. Based on the reaction schemes and the inhibitor data, superoxide anions (and their dismutation product H2O2) seem to be required for both steps.
When the available concentration of arginine was increased by application of five chemically different arginase inhibitors (Fig. 8), in each case a concentration-dependent and singlet oxygen-mediated process of apoptosis induction was initiated. This result is in line with the result found for the direct application of arginine and therefore strenghtens the underlying biochemical concept.
The application of 0.2 or 2 mM of the NO donor DEANONOate did not induce apoptosis in MKN-45 cells, but sensitized the cells for apoptosis induction by exogenous peroxynitrite, indicating an inhibitory effect on catalase (Supplementary Fig. 1). When the NO donor had been added in the presence of the singlet oxygen scavenger histidine and the NOX inhibitor AEBSF for prevention of peroxynitrite formation through NO/superoxide anion interaction, the catalase inhibiting effect was partially reduced. This result allowed to differentiate between direct inhibition of catalase by NO (in the presence of histidine and AEBSF) and the additional inactivation through singlet oxygen generation after peroxynitrite/H2O2 interaction, according to findings by Di Mascio et al., 1994 [80] and extended by Riethmüller et al., 2015 [126].
4.3. Activation of the FAS receptor reactivates intercellular ROS-mediated apoptosis through a singlet oxygen-dependent mechanism
Activation of the FAS receptor by antibodies caused apoptosis induction dependent on the concentration of the antibodies (Fig. 9). Apoptosis induction was determined by an early step that seemed to be mediated by singlet oxygen and NO, as it was inhibited by histidine and l-NAME, provided the inhibitors had been added before the antibodies. Addition of the inhibitors one hour after the antibodies did not lead to any inhibition. This early step was followed by subsequent HOCl-dependent signaling that caused apoptosis induction and that was independent of NO. Addition of the HOCl scavenger taurine one hour after the antibodies caused essentially the same inhibitory effect as its early addition. There was no detectable direct FAS receptor-dependent apoptosis induction in the cell line used, as this would be expected to not being inhibited by scavengers like histidine, l-NAME or taurine.
In line with these and previous findings, direct application of exogenous singlet oxygen through illumination of photofrin also caused reactivation of intercellular apoptosis inducing signaling. The efficiency, selectivity and complexity of this process that is in line with the model shown in Fig. 4B is presented in [126].
4.4. Inactivation of catalase as common consequence of modulation of the NO concentration, FAS receptor activation and singlet oxygen generation
As tumor cells are efficiently protected against exogenous H2O2 and peroxynitrite by their membrane-associated catalase, apoptosis induction by direct application of these compounds requires inhibition of the enzyme [40,42,95]. This finding is illustrated for H2O2 in Supplementary Fig. 2A. Pretreatment of the tumor cells with either 5 mM arginine, illuminated photofrin, NOS-inducing interferon gamma or FAS receptor activating antibodies caused an analogous sensitization of the cells for apoptosis induction by H2O2, indicative for the inactivation of catalase (Supplementary Figs. 2B and 3A–C). This sensitization was completely prevented by histidine, confirming that it was mediated by singlet oxygen. Inhibition of the process by the NOS inhibitor l-NAME points to the expected involvement of NO. Sensitization by arginine occured despite the presence of the protein synthesis inhibitor cycloheximide, whereas the effects of interferon gamma and the FAS receptor required ongoing protein synthesis. This may indicate that the latter effects required induction of NOS expression.
Control experiments with the NO donor DEANONOate confirmed that arginine- and illuminated photofrin-treated cells were also sensitive for peroxynitrite and that the inactivation of catalase that sensitized the cells for NO/peroxynitrite-dependent apoptosis induction had been mediated by singlet oxygen (Supplementary Fig. 4).
4.5. The establishment of synergistic effects: a chance for efficient antitumor effects based on the modulation of NO metabolism
The establishment of synergistic effects is a frequently used approach for therapeutic applications. Its major benefit is a drastic reduction of the necessary concentrations of compounds involved, compared to the sole application of individual compounds. Unwanted side effects of individual compounds may thus be avoided and in addition, the approach may become more economic. For analytical purposes, the unravelling of the mechanisms of synergistic effects open new chances to understand novel cross connections in signaling processes. Our previous study [53] has demonstrated that the direct catalase inhibitor salicylic acid and the NOD inhibitor cyanidin caused a remarkable synergistic effect. This finding is summarized in Fig. 10. Fig. 10A illustrates that high concentrations of salicylic acid, neutralizing antibody directed against catalase (aCAT) and cyanidin induce apoptosis in tumor cells after sole application. Fig. 10B demonstrates that much lower concentrations of these compounds had no apoptosis-inducing effect when applied alone, but that the combination of a low concentration of cyanidin with low concentrations of either aCAT or salicylic acid established a remarkable synergistic effect. The combination of cyanidin with control antibody that did not target catalase had no effect.
The reaction scheme shown in Fig. 4B allowed to predict that the combination of compounds that affect different targets within the biochemical network described might establish useful synergistic effects. As shown in Fig. 11A, the NOD inhibitor malvidin caused apoptosis induction in tumor cells in a concentration-dependent mode. The reaction required an early caspase-8-dependent step. When the cells had been pretreated with low dose gamma irradiation (75 mGy) and allowed to establish bystander-mediated enhancement of NOX1-dependent superoxide anion production [132] before malividin was added, a remarkable synergistic effect was seen (Fig. 11B). Apoptosis induction by the synergistically acting effectors was independent of caspase-8. This confirms our previous finding that the caspase-8-dependent reaction during singlet oxygen-mediated catalase inactivation was dispensable when the superoxide anion concentration was increased [52,85]. This finding also confirmed that in the context of these experiments caspase-8, was not involved in the execution of cell death, but that its action was rather restricted to the modulation of singlet oxygen generation. If this conclusion was correct, caspase-8-deficient tumor cells should not show apoptosis induction by malvidin applied alone, but should die by apoptosis after stimulation of NOX1 activity by low dose radiation, which compensated for the specific modulatory function of caspase-8.
Supplementary Fig. 5A shows that apoptosis was induced in the caspase-8-positive Ewing sarcoma cell line CHP100 after malividin treatment, in a reaction dependent on caspase-8. After establishing a synergistic effect between low dose radiation-mediated stimulation of NOX irradiation and malividin treatment, apoptosis induction was independent of caspase-8 activity. The caspase-8-deficient Ewing sarcoma cell line A573 was not affected by treatment with malvidin alone, but showed very efficient apoptosis induction when low dose radiation and malvidin treatment had been combined (Supplementary Fig. 5B). This finding confirms that caspase-8 plays a modulatory role and that it is dispensable when NOX is stimulated. Also, caspase-8 seems not to be required for the execution of apoptosis after intercellular ROS signaling in this cell system.
In order to clarify whether the contribution by anthocyanidins to the synergistic effects represented a general aspect of NO rather than a specific feature of NOD manipulation, arginine was used as one of the synergy-establishing partner in the following experiment. The combination of a low concentration of arginine with either H2O2-generating glucose oxidase, with catalase inhibiting salicylic acid or with the NOD inhibitor cyanidin caused a significant singlet oxygen-dependent synergistic effect in each case (Fig. 12). Whereas the combination of NO-modulating arginine with GOX and salicylic acid rendered apoptosis induction independent of the activity of caspase-8, establishment of a synergy effect between arginine and cyanidin, i.e. two compounds that both affected NO concentration, though at different steps, remained dependent on caspase-8 activity. When NO was increased by the addition of arginase inhibitors, a strong synergistic effect with the NOD inhibitor itraconazole were observed (Fig. 13), as to be predicted from the reaction scheme shown in Fig. 4B. Control experiments thereby ensured that these synergistic effects were dependent on the action of singlet oxygen and of superoxide anions, and were specific for tumor cells (Fig. 13B).
4.6. Utilizing NO donors for the establishment of synergistic effects with a catalase inhibitor
If the conclusions derived from our data were correct, the combination of a singlet oxygen-independent direct catalase inhibitor with an NO donor should also result in a singlet oxygen-dependent synergistic effect. And if this could be verified, compounds like NO-aspirin (NO-acetylsalicylic acid) [133,134] might be discussed as being hybrid molecules that comprise two compounds necessary for the establishment of a synergistic effect that targets tumor cell protective catalase. Fig. 14 shows that these predictions can be confirmed by robust data. Acetylsalicylic acid sensitized tumor cells for the apoptosis-inducing effect of exogenous peroxynitrite, indicating that membrane-associated catalase that decomposes peroxynitrite must have been inhibited or inactivated (Fig. 14A). In line with the findings established for salicylic acid [52], sensitization of tumor cells by acetylsalicylic acid was independent of singlet oxygen, as it was not prevented by the singlet oxygen scavenger histidine. DEANONOate applied alone did not induce apoptosis, as expected by the NO-oxidating and peroxynitrite-decomposing potential of membrane-associated catalase. However, the combination of the NO donor with low concentrations of acetylsalicylic acid caused a remarkable synergistic effect that was dependent on singlet oxygen, superoxide anions and NO, as deduced from the inhibitor profile. The NO-acetylsalicylic acid NCX 4040-induced apoptosis with a similar efficiency as the combination of acetylsalicylic acid and the NO donor and more than 100 fold more efficient than acetylsalicylic acid. (Fig. 14C). This efficiency was drastically lowered when superoxide anions or singlet oxygen were scavenged or when NOS was inhibited. The residual singlet oxygen-independent activity of NCX 4040 in the presence of these inhibitors was indistinguishable of that of a direct catalase inhibitor. If these conclusions were correct and sensitization for the apoptosis-inducing effect of peroxynitrite did indicate inactivation or inhibition of catalase, then (i) NCX4040 should induce apoptosis in tumor cells (in the absence of exogenous peroxynitrite and after longer incubation time to allow intercellular signaling) with higher efficiency than acetylsalicylic and (ii) apoptosis induction by both compounds should be inhibited by the NOX inhibitor AEBSF, (iii) apoptosis induction by acetylsalicylic acid should be independent of singlet oxygen and (iv) apoptosis induction should be dependent on singlet oxygen in the lower concentration range of NCX 4040 and independent on singlet oxygen at higher concentrations of NCX 4040. Fig. 15 shows that all of these predictions were verified by the performed experiment, demonstrating that NCX 4040 acts as a hybrid composed of two synergistically compounds. A detailed kinetic analysis of the apoptosis-mediating effect of NCX 4040 illuminated that NCX 4040 first induces a fast singlet oxygen- and NO-dependent process that can be deduced to represent catalase inactivation, as it allows a longer lasting secondary effect of HOCl signaling-dependent apoptosis induction that is mediated by superoxide anions, H2O2, peroxidase, HOCl and hydroxyl radicals (Supplementary Fig. 6).
5. Discussion
Reactive oxygen species (ROS) such as superoxide anions, H2O2 and their multiple reaction products, as well as reactive nitrogen species (RNS) such as NO, ONOO− and their complex metabolites are involved in multistep oncogenesis at many distinct and partially adverse steps [38]. The procarcinogenic effects of ROS comprise (i) mutagenic effects related to tumor initiation and tumor progression [135–137], (ii) ROS-related mechanisms during tumor promotion [136,138], (iii) establishment and maintenance of the transformed state through extracellular superoxide anion generation by membrane-associated NADPH oxidase (NOX1) [1–21], (iv) changes in the cytoskeleton of transformed cells [139], (v) induction of cell motility [140], (vi) stimulation of tumor angiogenesis [141,142] and (vii) enhancement of metastasis [143,144]. Likewise, NO and its multiple derivatives, either derived from the tumor cells themselves or from cells within the tumor microenvironment have been shown to contribute to distinct steps of multistep oncogenesis such as tumor initiation, progression, angiogenesis and metastasis [145–152].
These procarcinogenic effects of ROS and RNS are counteracted by intercellular apoptosis induction selectively in transformed cells through the HOCl and the NO/peroxynitrite signaling pathway (Figs. 1 and 2). These pathways have in common (i) that extracellular NOX-derived superoxide anions generated specifically by transformed cells drive the efficiency and the selectivity of apoptosis induction with respect to the malignant phenotype of the cells; (ii) that hydroxyl radicals are the final apoptosis inducer through their potential for lipid peroxidation and (iii) that apoptosis is mediated by the mitochondrial pathway of apoptosis involving caspase-9 and caspase-3 [53]. As nontransformed cells lack sustained extracellular superoxide anion generation, they can neither establish intercellular apoptosis signaling nor are they affected by it. Intercellular apoptosis signaling through the HOCl and the NO/peroxynitrite signaling pathway has been discussed as potential mechanism for the elimination of transformed cells [32,37–39]. Mathematical modelling supports this view [153].
The efficiency of the elimination mechanism that is based on the HOCl and/or NO/peroxynitrite signaling pathway indicates that tumor formation in vivo should depend on a resistance mechanism to overcome this specific restraint. Independent and earlier findings by Deichman’s group showed that experimental tumor progression in vivo leads to the selection of a tumor cell phenotype that is characterized by its “H2O2-catabolizing potential” [45–49]. This is in perfect agreement with our recent findings that bona vida tumor cells that had been established from tumors were regularly resistant against intercellular apoptosis induction through HOCl and NO/peroxynitrite signaling [38–42].Their resistance is based on the expression of membrane-associated catalase that utilizes its location at the right site and its multifunctionality for the control of both signaling pathways [40,42] (Fig. 3A). As membrane-associated catalase prevents HOCl signaling through decomposition of H2O2, the substrate required for peroxidase-mediated HOCl synthesis, and as it interferes with NO/peroxynitrite signaling through oxidation of NO and decomposition of peroxynitrite [42], it establishes a tight control of apoptosis-inducing signaling.
Inhibition of membrane-associated catalase therefore reactivates intercellular signaling and thus causes selective apoptosis induction in tumor cells. After gradual inhibition, tumor cell lines like MKN-45, Gumbus and many others establish first NO/peroxynitrite signaling, which is followed by HOCl signaling at higher concentrations of the catalase inhibitor. In contrast, cell lines derived from neuroblastoma, Ewing sarcoma, small lung cell carcinoma, mammary carcinoma and ovarial carcinoma establish NO/peroxynitrite signaling at all concentrations of inhibitor ([39,42] ; Bauer, unpublished observation).
Due to the efficient reaction between peroxynitrite and CO2, that leads to the formation of nitrosoperoxycarboxylate [75–78], the pathway of peroxynitrite to peroxynitrous acid formation and homolysis into NO2 and hydroxyl radicals [68,72–74] seemed to be unfavourable. However, the experiments shown in Figs. 5 and 6 strengthen the role of hydroxyl radicals for apoptosis induction, as the hydroxyl radical scavenger mannitol completely inhibited apoptosis induction. They also exclude the carbonate radical as apoptosis inducer, as the carbonate radical scavenger tryptophan had no inhibitory effect. Our results indicate that the high concentration of proton pump-derived H+ controls peroxynitrous acid formation in close vicinity to the target membrane and thus leads to the generation and action of hydroxyl radicals at the required site. It is reasonable to assume that distant of the cell membrane, peroxynitrite/CO2 interaction will be the dominant reaction that most likely hampers the NO/peroxynitrite signaling pathway.
A recent study showed that two secondary plant products with antitumor activity in vitro and in vivo, i. e. salicylic acid and cyanidin, caused inactivation of tumor cell protective catalase and thus reactivated intercellular apoptosis-inducing signaling through the HOCl and the NO/peroxynitrite pathway [53]. However, these compounds used different strategies for the inactivation of catalase, as summarized in Fig. 4A,B. Whereas salicylic acid caused singlet oxygen-independent direct inactivation of catalase through formation of inactive compound II and compound III, cyanidin triggered a complex singlet oxygen-dependent autoamplificatory mechanism that resulted in singlet oxygen-dependent inactivation of catalase. The understanding of the initial and crucial step in this mechanism requires to take into consideration (i) the central role of catalase for the protection against NO/peroxynitrite signaling (Fig. 3A), (ii) the multiple modes of NO/catalase interaction, i. e. oxidation of NO by catalase versus inhibition of catalase by NO (Fig. 3 B); (iii) the increase in the concentration of free H2O2 and peroxynitrite after NO-mediated inhibition of catalase (Fig. 4B), (iv) the generation of singlet oxygen through the reaction between H2O2 and peroxynitrite [80] and (v) the potential of singlet oxygen to inactivate catalase [120,121]. Even if the initial concentration of singlet oxygen generated by this mechanism was too low to allow substantial inactivation of catalase for reactivation of intercellular signaling, the potential of singlet oxygen to trigger the FAS receptor in a ligand independent mode [122] and the FAS receptor/caspase-8-dependent enhancement of NOX activity and NOS expression [124,125] finally seem to establish the generation of sufficiently high concentrations of H2O2, peroxynitrite and their reaction product singlet oxygen.
Alternatively or in addition to the reaction proposed by Di Mascio et al., 1994 [80], to explain the generation of singlet oxygen through the direct interaction between peroxynitrite and H2O2 (H2O2+ONOO−→1O2 +H2O+NO2−), the reaction between hydroxyl radicals derived from peroxynitrous acid and H2O2 might lead to the generation of ·HO2 radicals (H2O2+·OH→·HO2+H2O) [154]. Subsequent spontaneous dismutation of ·HO2/O2·− might generate singlet oxygen in addition to H2O2 [155]. The significance of these reactions was verified by Riethmüller et al. (2015) [126].
The validity of this model, derived from the original analysis of the effect of cyanidin [53] was confirmed through the complementary results presented here, as modulation of the concentration of free NO through alternative ways [58–61] such as addition of arginine, inhibition of arginase, induction of NOS by interferon gamma also caused singlet oxygen-dependent inactivation of catalase and subsequent intercellular apoptosis signaling. The amplificatory role of caspase-8 in the context of this mechanism was confirmed, as it was shown that caspase-8 was dispensable when either the generation of superoxide anions was enhanced by low dose radiation, the concentration of H2O2 was enhanced through glucose oxidase, or when suboptimal catalase inhibition was combined with a treatment that increased the concentration of NO. These findings and the limitation of the functional role of caspase-8 to the initial reactions exclude that caspase-8 was involved in the direct execution of cell death through activation of a cell death receptor-dependent mechanism.
The restriction of caspase-8 activity to the enhancement of ROS signaling was experimentally warranted through the use of cell lines that do not express sufficient FAS receptor for induction of cell death-dependent apoptosis. When cell lines were used that had a high expression of the FAS receptor, singlet oxygen generation after modulation of endogenous NO not only caused catalase inactivation and intercellular apoptosis inducing ROS signaling, but triggered the FAS receptor-dependent pathway in parallel. This caused an enhancement of apoptosis but was an obstacle for clear experimental dissection of the individual reactions (Bauer, unpublished observation).
The validity of the model presented in Fig. 4 B is also ensured by the finding that starting the amplificatory reaction cycle at later steps, like activation of the FAS rececptor or through addition of exogenous singlet oxygen, led to the same biochemical endpoints as starting the cycle with an increase in the concentration of free NO. In all cases, singlet oxygen-dependent inactivation of catalase and reactivation of intercellular signaling were the final and biologically significant consequences. A detailed analysis of the complex reactions following the addition of exogenous singlet oxygen is presented by Riethmüller et al. (2015) [126].
The establishment of synergistic effects (i) through the combination of modulators of the NO metabolism (like the NOS substrate arginine or the NOD inhibitors cyanidin and malvidin) with enhancers of superoxide anion production (like low dose gamma irradiation) or with H2O2 producing glucose oxidase; (ii) through the combination of arginase inhibitors with the NOD inhibitor itraconazole; (iii) through the combination of the NOS substrate arginine with the NOD inhibitor cyanidin; or (iv) through the combination of the NOS substrate arginine or the NOD inhibitor cyanidin with the direct catalase inhibitor salicylic acid is a further rigid test of the validity of the model presented in Fig. 4B, based on our recent publication [53]. The establishment of synergistic effects is therefore highly interesting from an analytical point of view, but also might open novel approaches for the development of new antitumor strategies that are (i) specific with respect to the malignant phenotype of the targeted cells and (ii) require only relatively low concentrations of compounds due to the effectiveness of synergistic effects. This might be one conceivable way to minimize unwanted side effects.
The synergistic effect between modulators of NO metabolism and suboptimal concentrations of a catalase inhibitor is especially intriguing, as it mimicks a situation where a small population of protective catalase molecules is already inactive and the NO-driven process is continuously proceeding, leading to further increase of catalase inactivation through singlet oxygen action. The data presented here show that the generation of NO by an exogenous NO donor in combination with the direct catalase inhibitors acetyl salicylic acid (the prodrug of salicylic acid) is sufficient for the establishment of the synergistic effect. This confirms the central role of NO in this process. It also showed that singlet oxygen-independent inhibition of catalase shifted to a much more effective, but singlet oxygen-dependent process when acetyl salicylic acid and the NO donor were combined. In line with this finding, the nitric oxide releasing aspirin NCX 4040 [133,134] caused a singlet oxygen-dependent highly efficient inactivation of catalase which was shifted to a less efficient direct catalase inhibitory effect related to the acetyl salicylic acid moiety, when either singlet oxygen was scavenged or when its generation was prevented. This finding indicates that hybrid molecules like NCX 4040 seem to bundle synergistic effects attributed to the interaction of their subcompounds. As NCX 4040 was shown to target catalase and as it effect was completely abolished when intercellular ROS signaling was prevented, this compound seems to represent a striking example for an therapeutic that utilizes the specific ROS signaling chemistry as presented in this manuscript.
Work in progress is focussed on the development of hybrid molecules that target the NO metabolism and enhance superoxide anion production, thus ensuring a sustained production of endogenous singlet oxygen at low concentrations of the effector molecules and a specific effect on tumor cells with protective catalase and active NOX. Parallel to the completion of this article, we have succeeded in the synthesis and analysis of a highly promising prototype of such a compound.
Acknowledgements
I thank Dr. U. Kontny for the gift of Ewing sarcoma cell lines and J. Brandel for valuable help during preparation of the graphs. I am grateful for intellectual support by the COST consortium ‘ChemBioRadical’ (COST Action CM0603). I thank my family for continuous support. This work was supported by grants from EuroTransBio (ETB1 0315012B), RiscRad (F16-CT-2003-508842), the Clotten Stiftung Freiburg (Apoptoseinduktion) and the Hans Sauer Stiftung München (2012-040).
Glossary
- 3-AT
3-Aminotriazole (catalase inhibitor)
- ABH
4-Aminobenzoyl hydrazide (POD inhibitor)
- aCAT
Antibody directed towards catalase
- AEBSF
4-(2-Aminoethyl)benzenesulfonyl fluoride (NOX inhibitor)
- ASA
Acetylsalicylic acid
- Casp8
Caspase8
- CAT
Catalase
- CATFeIII
Catalase (native enzyme)
- CATFeIV=O+
Compound I of catalase
- CATFeIV=O
Compound II of catalase
- CATFeIIIO2·–
Compound III of catalase
- CATFeIIINO
Catalase/NO complex
- Cpd I, II, III
Compound I, II, III
- DEANO.
DiethylaminoNONOate (NO donor)
- DUOX
Dual oxidase
- EUK-134
[chloro([2,2′-[1,2-ethanediylbis[(nitrilo-κN)methylidyne]]bis[6-methoxyphenolato-κO]]]-manganese (catalase mimetic)
- FeTPPS
5-, 10-, 15-, 20-Tetrakis(4-sulfonatophenyl)porphyrinato iron(III) chloride (peroxynitrite decomposition catalyst)
- GOX
Glucose oxidase
- HIS
Histidine (singlet oxygen scavenger)
- ITRA
Itraconazole
- LDR
Low dose gamma radiation
- l-NAME
N-omega-nitro-l-arginine methylester hydrochloride
(NOS inhibitor)
- LPO
Lipid peroxidation
- MANN
Mannitol (hydroxyl radical scavenger)
- MMP
Matrix metalloprotease
- MPO
Myeloperoxidase
- NCX 4040
NO-aspirin, NO-acetylsalicylic acid
- NO
Nitric oxide
- NOD
NO dioxygenase
- NOS
NO synthase
- NOX
NADPH oxidase
- Omepr.
Omeprazole (Proton pump inhibitor)
- POD
peroxidase
- PODFeIII
peroxidase (native enzyme)
- PODFeIV=O·+
Compound I of peroxidase
- PODFeIV=O
Compound II of peroxidase
- PON
Peroxynitrite
- POR
Cytochrome P 450 oxidoreductase
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- SA
Salicylic acid
- siRNA
small interfering RNA
- SOD
Superoxide dismutase
- TAU
Taurine (HOCl scavenger)
- TGF-beta1
Transforming growth factor-beta1
- TRYP
Tryptophane (carbonate radical scavenger)
- O2·−
Superoxide anion
- H2O2
Hydrogen peroxide
- HO2−
Anion of hydrogen peroxide
- HO·2
Hydroperoxyl radical
- ·OH
Hydroxyl radical
- OH−
Hydroxyl anion
- 1O2
singlet oxygen
- HOCl
Hypochlorous acid
- ·NO
Nitric oxide
- ·NO2
Nitrogen dioxide
- NO2−
Nitrite
- NO3−
Nitrate
- ONOO−
Peroxynitrite
- ONOOH
Peroxynitrous acid
- ONOO·
Peroxynitrite radical
- NO+
Nitrosonium ion
- N2O3
Dinitrogentrioxide
- N2O4
Dinitrogentretraoxide
- ONOONO
Nitrosoperoxy-nitric oxide; isomer of N2O4
- ONOOCOO−
Nitrosoperoxycarboxylate
- CO3·−
Carbonate radical
- CO32−
Carbonate
Footnotes
Supplementary material associated with this article can be found in the online version at: doi:10.1016/j.redox.2015.07.017.
Appendix A. Supplementary material
Supplementary material
References
- 1.Irani K., Xia Y., Zweier J.L., Sollott S.J., Der C.J., Fearon E.R., Sundaresan M., Finkel T., Goldschmidt-Clermont P.J. Mitogenic signalling by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 2.Irani K., Goldschmidt-Clermont P.J. Biochem. Pharmacol. 1998;55:1339–1346. doi: 10.1016/s0006-2952(97)00616-3. [DOI] [PubMed] [Google Scholar]
- 3.Suh Y.-A., Arnold R.S., Lassegue B., Shi J., Xu X., Sorescu D., Chung A.B., Griendling K.K., Lambeth J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 4.Bittinger F., Gonzalez-Garcia J.L., Lein C.L., Brochhausen C. Offner F und Kirkpatrick CJ: Production of superoxide by human malignant melanoma cells. Melanoma Res. 1998;8:381–38387. doi: 10.1097/00008390-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Yang J.Q., Li S., Domann F.E., Buettner G., Oberley L.W. Superoxide generation in v-Ha-ras-transduced human keratinocyte HaCaT cells. Mol. Carcinog. 1999;26:180–18188. [PubMed] [Google Scholar]
- 6.Mitsushita J., Lambeth J.D., Kamata T. The superoxide-generating oxidase nox1 is functionally required for ras oncogenic transformation. Cancer Res. 2004;64:3580–3585. doi: 10.1158/0008-5472.CAN-03-3909. [DOI] [PubMed] [Google Scholar]
- 7.Laurent E., McCoy J.W., Maccina R.A., Liu W., Cheng G.J., Robine S., Papkoff J., Lambeth J.D. Nox1 is overexpressed in human colon cancers and correlates with activating mutations in K-Ras. Int. J. Cancer. 2008;123:100–10107. doi: 10.1002/ijc.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Q., Cavallin L.E., Yan B., Zhu S., Duran E.M., Wang H., Hala L.P., Dong C., Cesarman E., Mesri E.A., Goldschmidt-Clermont P.J. Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi's sarcoma. Proc. Natl. Acad. Sci. USA. 2009;106:8683–868688. doi: 10.1073/pnas.0812688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim E.-Y., Seo J.-M., Kim C., Lee J.-E., Lee K.-M., Kim J.-H. BLT2 promotes the invasion and metastasis of aggressive bladder cancer through a reactive oxygen species-linked pathway. Free Rad. Biol. Med. 2010;49:1072–1081. doi: 10.1016/j.freeradbiomed.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Du J., Liu J., Smith B.J., Tsao M.S., Cullen J. Role of RAC-1-dependent NADPH oxidase in the growth of pancreatic cancer. Cancer Gene Ther. 2011;18:135–143. doi: 10.1038/cgt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrend L., Henderson G., Zwacka R.M. Reactive oxygen species in oncogenic transformation. Biochem. Soc. Trans. 2003;31:1441–1444. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- 12.Geiszt M., Leto L. The NOX family of NAD(P)H oxidases: host defense and beyond. J. Biol. Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- 13.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 14.Tominaga K., Kawahara T., Sano t, Toida K., Kuwano Y., Sasaki H., Kawai T. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-base oxidase system in the human stomach. Free Radic. Biol. Med. 2007;43:1627–1638. doi: 10.1016/j.freeradbiomed.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Lambeth J.D. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007;43:332–33347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Lazaro M. Excessive superoxide anion generation plays a key role in carcinogenesis. Int. J. Cancer. 2007;120:1378–1380. doi: 10.1002/ijc.22493. [DOI] [PubMed] [Google Scholar]
- 17.Kamata T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci. 2009;100:1382–1388. doi: 10.1111/j.1349-7006.2009.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg F., Chandel N.S. Reactive oxygen species-dependent signaling regulates cancer. Cell. Mol. Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold R.S., Shi J., Murad E., Whalen A.M., Sun C.Q., Palavarapu R., Parthasarathy S., Petros J.A., Lambeth J.D. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc. Natl. Acad. Sci. USA. 2001;98:5550–555555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamulitrat W., Schmidt R., Tomakidi P., Stremmel W., Chunglok W., Kawahara T., Rokutan K. Association of gp91phox homolog NOX1 with anchorage-independent growth and MAP kinase activation of transformed human keratinocyte. Oncogene. 2003;22:6045–606053. doi: 10.1038/sj.onc.1206654. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Lazaro M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007;252:1–8. doi: 10.1016/j.canlet.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Jürgensmeier J., Schmitt C.P., Viesel E., Höfler P., Bauer G. TGF-ß-treated normal fibroblasts eliminate transformed fibroblasts by induction of apoptosis. Cancer Res. 1994;54:393–39398. [PubMed] [Google Scholar]
- 23.Jürgensmeier J., Höfler P., Bauer G. TGF-ß-induced elimination of transformed fibroblasts by normal cells: independence of cell-to-cell contact and dependence on reactive oxygen species. Int. J. Oncol. 1994;5:525–52531. doi: 10.3892/ijo.5.3.525. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer D., Jürgensmeier J., Bauer G. Catechol interferes with TGF-ß-induced elimination of transformed cells by normal cells: implications for the survival of transformed cells during carcinogenesis. Int. J. Cancer. 1995;60:520–52526. doi: 10.1002/ijc.2910600416. [DOI] [PubMed] [Google Scholar]
- 25.Langer C., Jürgensmeier J.M., Bauer G. Reactive oxygen species act both at TGF-ß-dependent and -independent steps during induction of apoptosis of transformed cells by normal cells. Exp. Cell Res. 1996;222:117–124. doi: 10.1006/excr.1996.0015. [DOI] [PubMed] [Google Scholar]
- 26.Hipp M.-L., Bauer G. Intercellular induction of apoptosis in transformed cells does not depend on p53. Oncogene. 1997;15:791–79797. doi: 10.1038/sj.onc.1201247. [DOI] [PubMed] [Google Scholar]
- 27.Jürgensmeier J., Bauer G. Interference of Bcl-2 with intercellular control of carcinogenesis. Int. J. Cancer. 1997;71:698–704. doi: 10.1002/(sici)1097-0215(19970516)71:4<698::aid-ijc30>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Panse J., Hipp M.-L., Bauer G. Fibroblasts transformed by chemical carcinogens are sensitive for intercellular induction of apoptosis: Implications for the control of oncogenesis. Carcinogenesis. 1997;18:259–264. doi: 10.1093/carcin/18.2.259. [DOI] [PubMed] [Google Scholar]
- 29.Beck E., Schäfer R., Bauer G. Sensitivity of transformed fibroblasts for intercellular induction of apoptosis is determined by their transformed phenotype. Exp. Cell Res. 1997;234:47–56. doi: 10.1006/excr.1997.3587. [DOI] [PubMed] [Google Scholar]
- 30.Zucker B., Bauer G. Intercellular induction of apoptosis of transformed cells is modulated by their intracellular glutathione concentration. Int. J. Oncol. 1997;10:141–14146. doi: 10.3892/ijo.10.1.141. [DOI] [PubMed] [Google Scholar]
- 31.Engelmann I., Dormann S., Saran M., Bauer G. Transformed target cell-derived superoxide anions drive apoptosis induction by myeloperoxidase. Redox Rep. 2000;5:207–214. doi: 10.1179/135100000101535762. [DOI] [PubMed] [Google Scholar]
- 32.Herdener M., Heigold S., Saran M., Bauer G. Target cell-derived superoxide anions cause efficiency and selectivity of intercellular induction of apoptosis. Free Radic. Biol. Med. 2000;29:1260–1271. doi: 10.1016/s0891-5849(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 33.Schwieger A., Bauer L., Hanusch J., Sers C., Schäfer R., Bauer G. Ras oncogene expression determines sensitivity for intercellular induction of apoptosis. Carcinogenesis. 2001;22:1385–1392. doi: 10.1093/carcin/22.9.1385. [DOI] [PubMed] [Google Scholar]
- 34.Heigold S., Sers C., Bechtel W., Ivanovas B., Schäfer R., Bauer G. Nitric oxide mediates apoptosis induction selectively in transformed fibroblasts compared to nontransformed fibroblasts. Carcinogenesis. 2002;23:929–92941. doi: 10.1093/carcin/23.6.929. [DOI] [PubMed] [Google Scholar]
- 35.Ivanovas B., Bauer G. Selective and nonselective apoptosis induction in transformed and nontransformed fibroblasts by exogenous reactive oxygen and nitrogen species. Anticancer Res. 2002;22:841–84856. [PubMed] [Google Scholar]
- 36.Bauer G. Reactive oxygen and nitrogen species: efficient, selective and interactive signals during intercellular induction of apoptosis. Anticancer Res. 2000;20:4115–414140. [PubMed] [Google Scholar]
- 37.Bauer G., Chatgilialoglu C., Gebicki J.L., Gebicka L., Gescheidt G., Golding B.T., Goldstein S., Kaizer J., Merenyi G., Speier G., Wardman P. Biologically relevant small radicals. Chimia. 2008;62:1–9. [Google Scholar]
- 38.Bauer G. Tumor cell protective catalase as a novel target for rational therapeutic approaches based on specific intercellular ROS signaling. Anticancer Res. 2012;32:2599–2624. [PubMed] [Google Scholar]
- 39.Bauer G. Targeting extracellular ROS signaling of tumor cells. Anticancer Res. 2014;34:1467–1482. [PubMed] [Google Scholar]
- 40.Bechtel W., Bauer G. Catalase protects tumor cells against apoptosis induction by intercellular ROS signaling. Anticancer Res. 2009;29:4541–454557. [PubMed] [Google Scholar]
- 41.Bechtel W., Bauer G. Modulation of intercellular ROS signaling of human tumor cells. Anticancer Res. 2009;29:4559–4570. [PubMed] [Google Scholar]
- 42.Heinzelmann S., Bauer G. Multiple protective functions of catalase against intercellular apoptosis-inducing ROS signaling of human tumor cells. Biol. Chem. 2010;391:675–67693. doi: 10.1515/BC.2010.068. [DOI] [PubMed] [Google Scholar]
- 43.Steinebach C., Bauer G. An alternative signalling pathway based on nitryl chloride during intercellular induction of apoptosis. In vitro Appl. Mol. Toxicol. 2001;14:107–120. doi: 10.1089/10979330152560504. [DOI] [PubMed] [Google Scholar]
- 44.Schimmel M., Bauer G. Proapoptotic and redox state-related signalling of reactive oxygen species generated by transformed fibroblasts. Oncogene. 2002;21:5886–5896. doi: 10.1038/sj.onc.1205740. [DOI] [PubMed] [Google Scholar]
- 45.Deichman G.I., Vendrov E.L. Characteristics of in vitro transformed cells essential for their in vivo survival, selection and metastatic activity. Int. J. Caner. 1986;37:401–40409. doi: 10.1002/ijc.2910370312. [DOI] [PubMed] [Google Scholar]
- 46.Deichman G.I., Kluchareva T.E., Matveeva V.A., Kushlinsky N.E., Bassalyk L.S., Vendrov E.L. Clustering of discrete cell properties essential for tumorigenicity and metastasis. I. Studies of syrian hamster embryo fibroblasts spontaneously transformed in vitro. Int. J. Cancer. 1989;44:904–90907. doi: 10.1002/ijc.2910440526. [DOI] [PubMed] [Google Scholar]
- 47.Deichman G., Matveeva V.A., Kashkina L.M., Dyakova N.A., Uvarova E.N., Nikiforov M.A., Gudkov A.V. Cell transforming genes and tumor progression in vivo unified secondary phenotypic cell changes. Int. J. Cancer. 1998;75:277–283. doi: 10.1002/(sici)1097-0215(19980119)75:2<277::aid-ijc17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 48.Deichman G. Natural selection and early changes of phenotype of tumor cells in vivo: Acquisition of new defense mechanisms. Biochemistry. 2000;65:78–94. [PubMed] [Google Scholar]
- 49.Deichman G. Early phenotypic changes of in vitro transformed cells during in vivo progression: possible role of the host innate immunity. Sem. Cancer Biol. 2002;12:317–326. doi: 10.1016/s1044-579x(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 50.Pottgießer S.J., Heinzelmann S., Bauer G. Intercellular HOCl-mediated apoptosis induction in malignant cells: interplay between NOX1-dependent superoxide anion generation and DUOX-related HOCl-generating peroxidase activity. Anticancer Res. 2015 in press. [PubMed] [Google Scholar]
- 51.Candeias L.P., Patel K.B., Stratford M.R.L., Wardmann P. Free hydroxyl radicals are formed on reaction between the neutrophil-derived species superoxide anion and hypochlorous acid. FEBS. 1993;333:151–15153. doi: 10.1016/0014-5793(93)80394-a. [DOI] [PubMed] [Google Scholar]
- 52.Folkes L.K., Candeias L.P., Wardman P. Kinetics and mechanisms of hypochlorous acid reactions. Arch. Biochem. Biophys. 1995;323:120–12126. doi: 10.1006/abbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- 53.Scheit K., Bauer G. Direct and indirect inactivation of tumor cell protective catalase by salicylic acid and anthocyanidins reactivates intercellular ROS signaling and allows for synergistic effects. Carcinogenesis. 2015;36:400–40411. doi: 10.1093/carcin/bgv010. [DOI] [PubMed] [Google Scholar]
- 54.Kettle A.J., Winterbourn C.C. A kinetic analysis of the catalase activity of myeloperoxidase. Biochemistry. 2001;40:10204–10212. doi: 10.1021/bi010940b. [DOI] [PubMed] [Google Scholar]
- 55.Connick R.E. The interaction of hydrogen peroxide and hypochlorous acid in acidic solutions containing chloride ion. J. Am. Chem. Soc. 1947;69:1509–151514. [Google Scholar]
- 56.Held A.M., Halko D.J., Hurst J.K. Mechanisms of chlorine oxidation of hydrogen peroxide. J. Am. Chem. Soc. 1978;100:5732–575740. [Google Scholar]
- 57.Christensen H., Sehested K., Corfitzen H. Reactions of hydroxyl radicals with hydrogen peroxide at ambient and elevated temperature. J. Phys. Chem. 1982;86:1588–1590. [Google Scholar]
- 58.Moncada S., Higgs E.A. The l-arginine-nitric oxide pathway. New Engl. J. Med. 1993;329:2002–202012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 59.Moncada S., Palmer R.M.J., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 60.Moncada S., Higgs E.A., Hodson H.F., Knowles R.G., Lopez-Jaramillo P., McCall T., Palmer R.M.J., Radomski W., Rees D.D., Schulz R. The l-arginine: nitric oxide pathway. J Cardiovasc Pharmacol. 1991;17(Suppl. 3):S1–S9. [Google Scholar]
- 61.Moncada S., Higgs E.A. Molecular mechanisms and therapeutic strategies related to nitric oxide. The FASEB J. 1995;9:1319–1330. [PubMed] [Google Scholar]
- 62.Gardner P.R., Martin L.A., Hall D., Gardner A.M. Dioxygen-dependent metabolism of nitric oxide in mammalian cells. Free Radic. Biol. Med. 2001;31:191–19204. doi: 10.1016/s0891-5849(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 63.Hallstrom C.K., Gardner A.M., Gardner P.R. Nitric oxide metabolism in mammalian cells: substrate and inhibitor profiles of a NADPH-cytochrome P450 oxidoreductase-coupled microsomal nitric oxide dioxygenase. Free Radic. Biol. Med. 2004;37:216–228. doi: 10.1016/j.freeradbiomed.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 64.Gardner P.R. Vol. 436. 2008. Assay and characterization of the NO dioxygenase activity of flavohemoglobins. Globins and other nitric oxide-reactive proteins; pp. 217–237. (Methods Enzymol.). [DOI] [PubMed] [Google Scholar]
- 65.Schmidt K., Mayer B. Consumption of nitric oxide by endothelial cells: Evidence for the involvement of a NAD(P)H-, flavin and heme-dependent dioxygenase reaction. FEBS Lett. 2004;577:199–204. doi: 10.1016/j.febslet.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 66.Goldstein S., Czapski G. Kinetics of nitric oxide autooxidation in aqueous solution in the absence and presence of various reductants. The nature of the oxidizing intermediates. J. Am. Chem. Soc. 1995;117:12078–1212084. [Google Scholar]
- 67.Saran M., Michel C., Bors W. Reaction of NO with O2−. Implication for the action of endothelium-derived relaxing factor (EDRF) Free Radic. Res. Commun. 1990;10:221–22226. doi: 10.3109/10715769009149890. [DOI] [PubMed] [Google Scholar]
- 68.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., Freeman B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury form nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koppenol W.H., Moreno J.J., Pryor W.A., Ischiropoulos H., Beckman J.S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol. 1992;5:834–83842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 70.Huie R.E., Padmaja S. The reaction of NO with superoxide. Free Radic. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 71.Goldstein S., Czapski G. The reaction of.NO with O2·− and HO2·−: a pulse radiolysis study. Free Rad. Biol. Med. 1995;19:505–50510. doi: 10.1016/0891-5849(95)00034-u. [DOI] [PubMed] [Google Scholar]
- 72.Gatti R.M., Alvarez B., Vasquez-Vivar J., Radi R., Augusto O. Formation of spin trap adducts during the decomposition of peroxynitrate. Arch. Biochem. Biophys. 1998;349:36–46. doi: 10.1006/abbi.1997.0451. [DOI] [PubMed] [Google Scholar]
- 73.Merényi G., Lind J., Goldstein S., Czapski G. Peroxynitrous acid homolyzes into ·OH and ·NO2 radicals. Chem. Res. Toxicol. 1998;11:712–71713. doi: 10.1021/tx980043h. [DOI] [PubMed] [Google Scholar]
- 74.Goldstein S., Meyerstein D., van Eldik R., Czapski G. Peroxynitrous acid decomposes via homolysis: evidence from high-pressure pulse radiolysis. J. Phys. Chem. A. 1999;103:6587–6590. [Google Scholar]
- 75.Goldstein S., Czapski G. Formation of peroxynitrate from the reaction of peroxynitrite with CO2: evidence for carbonate radical production. J. Am. Chem. Soc. 1998;120:3458–3463. [Google Scholar]
- 76.Squadrito G.L., Pryor W.A. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic. Biol. Med. 1998;25:392–39403. doi: 10.1016/s0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 77.Espey M.G., Miranda K.M., Thomas D.D., Xavier S.A., Citrin D., Vitek M.P., Wink D.A. A chemical perspective on the interplay between NO, reactive oxygen species, and reactive nitrogen species. Ann. N.Y. Acad. Sci. 2002;962:195–206. doi: 10.1111/j.1749-6632.2002.tb04068.x. [DOI] [PubMed] [Google Scholar]
- 78.Augusto O., Bonini M.G., Amanso A.M., Linares E., Santos C.X., De Menzes S.L. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic. Biol. Med. 2002;32:841–84859. doi: 10.1016/s0891-5849(02)00786-4. [DOI] [PubMed] [Google Scholar]
- 79.De Milito A., Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 2005;1:779–786. doi: 10.2217/14796694.1.6.779. [DOI] [PubMed] [Google Scholar]
- 80.Di Mascio P., Bechara E.J.H., Medeiros M.H.G., Briviba K., Sies H. Singlet molecular oxygen production in the reaction of peroxynitrite with hydrogen peroxide. FEBS Lett. 1994;355:287–289. doi: 10.1016/0014-5793(94)01224-5. [DOI] [PubMed] [Google Scholar]
- 81.Goldstein S., Czapski G. Formation of peroxynitrite from the nitrosation of hydrogen peroxide by an oxygenated nitric oxide solution. Inorg. Chem. 1996;35:5935–595940. [Google Scholar]
- 82.Brunelli L., Yermilov V., Beckman J.S. Modulation of catalase peroxidatic and catalatic activity by nitric oxide. Free Radic. Biol. Med. 2001;30:709–714. doi: 10.1016/s0891-5849(00)00512-8. [DOI] [PubMed] [Google Scholar]
- 83.Gebicka L., Didil J. Catalytic scavenging of peroxynitrite by catalase. Int. J. Inorg. Biochem. 2009;103:1375–1379. doi: 10.1016/j.jinorgbio.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 84.Wink D.A., Mitchell J.B. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998;25:434–43456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 85.Bauer G., Zarkovic N. Revealing mechanisms of selective, concentration-dependent potentials of 4-hydroxy-2-nonenal to induce apoptosis in cancer cells through inactivation of membrane-associated catalase. Free Radic. Biol. Med. 2015;81:128–144. doi: 10.1016/j.freeradbiomed.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 86.Kim Y.S., Han S. Superoxide reactivates nitric oxide-inhibited catalase. Biol. Chem. 2000;381:1269–1271. doi: 10.1515/BC.2000.156. [DOI] [PubMed] [Google Scholar]
- 87.Brown G.C. Reversible binding and inhibition of catalase by nitric oxide. Eur. J. Biochem. 1995;232:188–191. doi: 10.1111/j.1432-1033.1995.tb20798.x. [DOI] [PubMed] [Google Scholar]
- 88.Kono Y., Fridovich I. Superoxide radical inhibits catalase. J. Biol. Chem. 1982;257:5751–575754. [PubMed] [Google Scholar]
- 89.Shimizu N., Kobayashi K., Hayashi K. The reaction of superoxide radical with catalase. Mechanism of the inhibition of catalase by superoxide radical. J. Biol. Chem. 1984;259:4414–444418. [PubMed] [Google Scholar]
- 90.Fridovich I. Biological effects of the superoxide radical. Arch. Biochem. Biophys. 1986;247:1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- 91.Gebicka L., Metodiewa D., Gebicki J.L. Pulse radiolysis of catalase in solution. I. Reactions of O2.− with catalase and its compound I. Int. J. Radiat. Biol. 1989;55:45–50. doi: 10.1080/09553008914550051. [DOI] [PubMed] [Google Scholar]
- 92.Pigeolet E., Corbisier P., Houbion A., Lambert D., Michiels C., Raes M., Zachary M.-D., Remacle J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing Dev. 1990;51:283–297. doi: 10.1016/0047-6374(90)90078-t. [DOI] [PubMed] [Google Scholar]
- 93.Lardinois O.M. Reactions of bovine liver catalase with superoxide radicals and hydrogen peroxide. Free Radic. Res. 1995;22:251–25274. doi: 10.3109/10715769509147544. [DOI] [PubMed] [Google Scholar]
- 94.Bauer G. HOCl-dependent singlet oxygen and hydroxyl radical generation modulate and induce apoptosis of malignant cells. Anticancer Res. 2013;33:3589–3602. [PubMed] [Google Scholar]
- 95.Böhm B., Heinzelmann S., Motz M., Bauer G. Extracellular localization of catalase is associated with the transformed state of malignant cells. Biol. Chem. 2015 doi: 10.1515/hsz-2014-0234. in press. [DOI] [PubMed] [Google Scholar]
- 96.Sandstrom P.A., Buttke T.M. Autocrine production of extracellular catalase prevents apoptosis of the human CEM T-cell line in serum-free medium. Proc. Natl. Acad. Sci. USA. 1993;90:4708–474712. doi: 10.1073/pnas.90.10.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moran E.C., Kamiguti A.S., Cawley J.C., Pettitt A.R. Cytoprotective antioxidant activity of serum albumin and autocrine catalase in chronic lymphatic leukaemia. Br. J. Haematol. 2002;116:316–328. [PubMed] [Google Scholar]
- 98.Elwood P.C., Gallagher A.M., Duthie G.G., Mur L.A.J., Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–131309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 99.Aas A.T., Tønnessen T.I., Brun A., Salford L.G. Growth inhibition of rat glioma cells in vitro and in vivo by aspirin. J. Neuro-Oncol. 1995;24:171–17180. doi: 10.1007/BF01078487. [DOI] [PubMed] [Google Scholar]
- 100.Bellosillo B., Pique M., Barragan M., Dastano E., Villamor N., Colomer D., Montserrat E., Pons G., Gil J. Aspirin and salicylate induce apoptosis and activation of caspases in B-cell chronic lymphocytic leukemia cells. Blood. 1998;4:1406–141414. [PubMed] [Google Scholar]
- 101.Klampfer L., Cammenga J., Wisniewski H.-G., Nimer S.D. Sodium salicylate activates caspases and induces apoptosis of myeloid leukemia cell lines. Blood. 1999;93:2386–2394. [PubMed] [Google Scholar]
- 102.Chung Y.M., Bae Y.S., Lee S.Y. Molecular ordering of ROS production, mitochondrial changes, and caspase activation during sodium salicylate-induced apoptosis. Free Radic. Biol. Med. 2003;34:434–43442. doi: 10.1016/s0891-5849(02)01301-1. [DOI] [PubMed] [Google Scholar]
- 103.Battaglia V., Salvi M., Toninello A. Oxidative stress is responsible for mitochondrial permeability transition induction by salicylate in liver mitochondria. J. Biol. Chem. 2005;280:33864–33872. doi: 10.1074/jbc.M502391200. [DOI] [PubMed] [Google Scholar]
- 104.Elder D.J.E., Hague A., Hicks D.J., Paraskeva C. Differential growth inhibition by the aspirin metabolite salicylate in human colorectal tumor cell lines: enhanced apoptosis in carcinoma and in vitro-transformed adenoma relative to adenoma cell lines. Cancer Res. 1996;56:2273–2276. [PubMed] [Google Scholar]
- 105.Hou D.-X., Fujii M., Terahara N., Yoshimote M. Molecular mechanisms behind the chemopreventive effects of anthocyanidins. J. Biomed. Biotechnol. 2004;2004(5):321–325. doi: 10.1155/S1110724304403040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang Y.-C., Huang H.-P., Hsu J.-D., Yang S.-F., Wang C.-J. Hibiscus anthocyanins rich extract-induced apoptotic cell death in human promyelocytic leukemia cells. Toxicol. Appl. Pharmacol. 2005;205:201–20212. doi: 10.1016/j.taap.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 107.Chen P.-N., Chu S.-C., Chiou H.-L., Chiang C.-L., Yang S.-F., Hsieh Y.-S. Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutr. Cancer. 2005;53:232–23243. doi: 10.1207/s15327914nc5302_12. [DOI] [PubMed] [Google Scholar]
- 108.Shih P.-H., Yeh C.-T., Yen G.-C. Effects of anthocyanidin on the inhibition of proliferation and induction of apoptosis in human gastric adenocarcinoma cells. Food Chem. Toxicol. 2005;43:1557–1566. doi: 10.1016/j.fct.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 109.Feng R., Ni H.-M., Wang S.Y., Tourkova I.L., Shurin M.R., Harada H. Cyanidin-3-rutinoside, a natural polyphenol antioxidant, selectively kills leukemic cells by induction of oxidative stress. J. Biol. Chem. 2007;282:13468–13476. doi: 10.1074/jbc.M610616200. [DOI] [PubMed] [Google Scholar]
- 110.Reddivari L., Vanamala J., Chintharlapalli S., Safe S.H., Creighton J.C. Anthocyan fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways. Carcinogenesis. 2007;28:2227–2235. doi: 10.1093/carcin/bgm117. [DOI] [PubMed] [Google Scholar]
- 111.Wang l-S., Stoner G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–28290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rao C.V., Rivenson A., Simi B., Zang E., Van Kelloff G. Steele, Reddy B.S. Chemoprevention of colon carcinogenesis by sulindac, a non-steroidal anti-inflammatory agent. Cancer Res. 1995;55:1464–1472. [PubMed] [Google Scholar]
- 113.Giardiello F.M., Offerhaus G.J.A., DjuBois R.N. The role of non-steroidal anti-inflammatory drugs in colorectal cancer prevention. Eur. J. Cancer. 1995;31A:1071–1076. doi: 10.1016/0959-8049(95)00137-8. [DOI] [PubMed] [Google Scholar]
- 114.Thun M.J., Namboodiri M.M., Heath C.W., Jr Aspirin use and reduced risk of fatal colon cancer. New Engl. J. Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 115.Harris R.E., Namboodiri K.K., Farrar W.B. Nonsteroidal anti.inflammatory drugs and breast cancer. Epidemiology. 1996;7:203–205. doi: 10.1097/00001648-199603000-00017. [DOI] [PubMed] [Google Scholar]
- 116.Rothwell P.M., Fowkes F.G.R., Belch J.F.F., Ogawa H., Warlow C.P., Meade T.W. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 117.Rothwell P.M., Price J.F., Fowkes F.G.R., Zanchetti A., Roncaglioni M.C., Tognoni G., Lee R., Belch J.F.F., Wilson M., Mehta Z., Meade T.W. Short-term effects of daily aspirin on cancer incidence mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–161612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 118.Hanif R., Pittas A., Feng Y., Koutsos M.I., Qiao L., Staiano-Coico L., Shiff S.I., Rigas B. Effects of nonsteriodal antiinflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem. Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 119.Rigas B., Shiff S.I. Is inhibition of cyclooxygenase required for the chemopreventive effect of NSAIDs in colon cancer? A model reconciling the current contradiction. Med. Hypotheses. 2000;54:210–215. doi: 10.1054/mehy.1999.0023. [DOI] [PubMed] [Google Scholar]
- 120.Durner J., Klessig D.F. Salicylic acid is a modulator of tobacco and mammalian catalases. J. Biol. Chem. 1996;271:28492–28501. doi: 10.1074/jbc.271.45.28492. [DOI] [PubMed] [Google Scholar]
- 121.Escobar J.A., Rubio A., Lissi E.A. SOD and catalase inactivation by singlet oxygen and peroxyl radicals. Free Radic. Biol. Med. 1996;20:285–290. doi: 10.1016/0891-5849(95)02037-3. [DOI] [PubMed] [Google Scholar]
- 122.Kim Y.K., Kwon O.J., Park J.-W. Inactivation of catalase and superoxide dismutase by singlet oxygen derived from photoactivated dye. Biochimie. 2001;83:437–444. doi: 10.1016/s0300-9084(01)01258-5. [DOI] [PubMed] [Google Scholar]
- 123.Zhuang S., Demir J.T., Kochevar I.E. Protein kinase C inhibits singlet oxygen-induced apoptosis by decreasing caspase-8 activation. Oncogene. 2001;20:6764–676776. doi: 10.1038/sj.onc.1204867. [DOI] [PubMed] [Google Scholar]
- 124.Suzuki Y., Ono Y., Hirabayashi Y. Rapid and specific reactive oxygen species generation via NADPH oxidase activation during FAS-mediated apoptosis. FEBS Lett. 1998;425:209–212. doi: 10.1016/s0014-5793(98)00228-2. [DOI] [PubMed] [Google Scholar]
- 125.Reinehr R., Becker S., Eberle A., Grether-Beck S., Häussinger D. Involvement of NADPH oxidase isoforms and src family kinases in CD95-dependent hepatocyte apoptosis. J. Biol. Chem. 2005;280:27179–2727194. doi: 10.1074/jbc.M414361200. [DOI] [PubMed] [Google Scholar]
- 126.Riethmüller M., Burger N., Bauer G. Singlet oxygen treatment of tumor cells triggers extracellular singlet oxygen generation, catalase inactivation and reactivation of intercellular apoptosis-inducing signaling. Redox Biol. 2015:157–168. doi: 10.1016/j.redox.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scheit K., Bauer G. Synergistic effects between catalase inhibitors and modulators of nitric oxide metabolism on tumor cell apoptosis. Anticancer Res. 2014;34:5337–535350. [PubMed] [Google Scholar]
- 128.Bauer G., Bereswill S., Aichele P., Glocker E. Helicobacter pylori protects protects oncogenically transformed cells from reactive oxygen species-mediated intercellular induction of apoptosis. Carcinogenesis. 2014;35:1582–1591. doi: 10.1093/carcin/bgu074. [DOI] [PubMed] [Google Scholar]
- 129.Lissat A., Vraetz T., Tsokos M., Klein R., Braun M., Koutelia N., Fisch P., Romero M.E., Long L., Noelke P., Mackall C.L., Niemeyer C.M., Kontny U. Interferon-γ sensitizes resistant Ewing’s sarcoma cells to tumor necrosis factor apoptosis-inducing ligand-induced apoptosis by up-regulation of caspase-8 without altering chemosensitivity. Am. J. Pathol. 2007;176:1917–191930. doi: 10.2353/ajpath.2007.060993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kerr J.F.R., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–49515. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Temme J., Bauer G. Low-dose gamma irradiation enhances superoxide anion production by nonirradiated cells through TGF-β1-dependent bystander signaling. Radiat. Res. 2013;179:422–42432. doi: 10.1667/RR3161.2. [DOI] [PubMed] [Google Scholar]
- 133.Rigas B., Kashfi K. Nitric oxide-donating NSAIDs as agents for cancer prevention. Trends Mol. Med. 2004;10:324–330. doi: 10.1016/j.molmed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 134.Tesei A., Rosetti M., Ulivi P., Fabbri F., Medri L., Vannini I., Bolla M., Amadori D., Zoli W. Study of the molecular mechanism of pro-apoptotic activity of NCX 4040, a novel nitric oxide-releasing aspirin, in colon cancer cell lines. J. Transl. Med. 2007;5:52–62. doi: 10.1186/1479-5876-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Feig D.I., Reid T.M., Loeb L.A. Reactive oxygen species in tumorigenesis. Cancer Res. 1994;54:1890–1894. [PubMed] [Google Scholar]
- 136.Storz P. Reactive oxygen species in tumor progression. Front. Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 137.Chiera F., Meccia E., Degan P., Aquilina G., Pietraforte D., Minetti M., Lambeth D., Bignami M. Overexpression of human NOX1 complex induces genome instability in mammalian cells. Free Radic. Biol. Med. 2008;44:332–33342. doi: 10.1016/j.freeradbiomed.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 138.Cerutti P.A. Prooxidant states and tumor promotion. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 139.Shinohara M., Shang W.-H., Kubodera M., Hanada S., Mitsushita J., Kato M., Miyazaki H., Suminoto H., Kamata T. Nox1 redox signaling mediates oncogenic Ras-induced disruption of stress fibers and focal adhesions by down-regulating Rho. J. Biol. Chem. 2007;282:17640–17648. doi: 10.1074/jbc.M609450200. [DOI] [PubMed] [Google Scholar]
- 140.Sadok A., Bourgarel-Rey V., Gattacceca F., Penel C., Lehmann M., Kovacic H. Nox1-dependent superoxide production controls colon adenocarcinoma cell migration. Biochem. Biophys. Acta. 2008;1783:23–33. doi: 10.1016/j.bbamcr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 141.Arbiser J.L., Petros J., Klafter R., Govindajaran B., McLaughlin E.R., Brown L.F., Cohen C., Moses M., Kilroy S., Arnold R.S., Lambeth J.D. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc. Acad. Natl. Acad. Sci. USA. 2002;99:715–71720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blanchetot C., Boonstra J. The ROS-NOX connection in cancer and angiogenesis. Crit. Rev. Euk. Gene Expr. 2008;18:35–45. doi: 10.1615/critreveukargeneexpr.v18.i1.30. [DOI] [PubMed] [Google Scholar]
- 143.Ferraro D., Corso S., Fasano E., Panieri E., Santangelo R., Borrelo S., Giordano S., Pani G., Galeotti T. Pro-metastatic signaling by c-Met through Rac-1 and reactive oxygen species. Oncogene. 2006;25:3689–3698. doi: 10.1038/sj.onc.1209409. [DOI] [PubMed] [Google Scholar]
- 144.Lopez-Lazaro M. Why do tumors metastasize? Cancer Biol. Ther. 2007;6:141–14144. doi: 10.4161/cbt.6.2.3950. [DOI] [PubMed] [Google Scholar]
- 145.Thomsen L.L., Lawton F.G., Knowles R.G., Beesley J.E., Riveros-Moreno V., Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994;54:1352–1354. [PubMed] [Google Scholar]
- 146.Thomsen L.L., Miles D.W., Happerfiel L., Bobrow L.G., Knowles R.G., Moncada S. Nitric oxide synthase in human breast cancer. Br. J. Cancer. 1995;72:41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thomsen L.L., Scott J.M.J., Topley P., Knowles R.G., Keerie A.-J., Frend A. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: Studies with 1400W, a novel inhibitor. Cancer Res. 1997;57:3300–333304. [PubMed] [Google Scholar]
- 148.Thomsen L.L., Miles D.W. Role of nitric oxide in tumor progression: lessons form human tumors. Cancer Metastasis Rev. 1998;17:107–118. doi: 10.1023/a:1005912906436. [DOI] [PubMed] [Google Scholar]
- 149.Jenkins D.C., Charles I.G., Baylis S.A., Lelchuk R., Radomski M.W., Moncada S. Human colon cancer cell lines show a diverse pattern of nitric oxide synthase gene expression and nitric oxide generation. Br. J. Cancer. 1994;70:847–84849. doi: 10.1038/bjc.1994.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jenkins D.C., Charles I.G., Thomsen L.L., Moss D.W., Holmes L.S., Baylis S.A., Rhodes P., Westmore K., Emson P.C., Moncada S. Roles of nitric oxide in tumor growth. Proc Natl. Acad. Sci. USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wink D.A., Vodovotz Y., Laval J., Laval F., Dewhirst M.W., Mitchell J.B. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–71721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 152.Wink D.A., Mitchell J.B. Nitric oxide and cancer: an introduction. Free Radic. Biol. Med. 2003;34:951–95954. doi: 10.1016/s0891-5849(02)01362-x. [DOI] [PubMed] [Google Scholar]
- 153.Kundrát P., Bauer G., Jacob P., Friedland W. Mechanistic modelling suggests that the size of preneoplastic lesions is limited by intercellular induction of apoptosis in oncogenically transformed cells. Carcinogenesis. 2012;33:253–259. doi: 10.1093/carcin/bgr227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alvarez B., Denicola A., Radi R. Reaction between peroxynitrite and hydrogen peroxide: Formation of oxygen and slowing of peroxynitrite decomposition. Chem. Res. Toxicol. 1995;8:859–864. doi: 10.1021/tx00048a006. [DOI] [PubMed] [Google Scholar]
- 155.Badway J.A., Karnovsky M.L. Active oxygen species and the functions of phagocytic leukocytes. Annu. Rev. Biochem. 1980;49:695–69726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- 156.Selleri C., Sato T., Raiola A.M., Rotoli B., Young N.S., Maciejewski J.P. Induction of nitric oxide synthase is involved in the mechanism of FAS-mediated apoptosis in hematopoietic cells. Br. J. Hematol. 1997;99:481–48489. doi: 10.1046/j.1365-2141.1996.4323240.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material