Abstract
Background and Purpose:
Demonstration of lepra bacilli is essential for definite or unequivocal diagnosis of pure neuritic leprosy (PNL) on nerve biopsy. However, nerves always do not show bacilli owing to the changes of previous therapy or due to low bacillary load in tuberculoid forms. In absence of granuloma or lepra bacilli, other morphologic changes in endoneurium and perineurium can be of help in making a probable diagnosis of PNL and treating the patient with multidrug therapy.
Materials and Methods:
Forty-six biopsies of PNL were retrospectively reviewed and histologic findings were compared with 25 biopsies of non leprosy neuropathies (NLN) including vasculitic neuropathy and chronic inflammatory demyelinating polyneuropathy (CIDP). The distribution of endoneurial infiltrate and fibrosis, perineurial thickening, and myelin abnormalities were compared between PNL and NLN biopsies and analyzed by Chi-square test.
Results:
Out of 46 PNL casses, 24 (52.17 %) biopsies were negative for acid fast bacilli (AFB). In these cases, the features which favor a diagnosis of AFB-negative PNL were endoneurial infiltrate (51.1%), endoneurial fibrosis (54.2%), perineurial thickening (70.8%), and reduced number of myelinated nerve fibers (75%).
Interpretation and Conclusion:
Nerve biopsy is an efficient tool to diagnose PNL and differentiate it from other causes of NLN. In absence of AFB, the diagnosis of PNL is challenging. In this article, we have satisfactorily evaluated the various hisopthological features and found that endoneurial inflammation, dense fibrosis, and reduction in the number of myelinated nerve fibers are strong supportive indicators of PNL regardless of AFB positivity.
Keywords: Leprosy, nerve biopsy, pure neuritic leprosy
Introduction
Leprosy is an important public health problem responsible for causing severe physical deformities as well as social issues. Leprosy was eliminated as a public health problem at national level in December 2005; however, higher case detection was subsequently published due to lack of registration of cases.[1] Peripheral neuropathy can present as mono or polyneuropathies or mononeuropathy multiplex in absence of cutaneous lesions. These cases are referred to as pure neuritic leprosy (PNL) which accounts for 4-8% of all leprosy cases.[2,3,4] It can be effectively treated with multidrug therapy and nerve biopsy is the gold standard for diagnosis of PNL. The nerve biopsy is an inflammatory neuropathy with presence of endoneurial inflammation, granuloma, or foam cells with lepra bacilli.[5] Presence of lepra bacilli acid fast bacilli (AFB) is an unequivocal evidence for the diagnosis of PNL.[5] However, nerves do not always show AFB; and in such situations, other histopathological alterations in conjunction with clinical and electroneuromyographical (ENMG) findings helps in supporting the diagnosis of PNL. The ENMG can show axonal, demyelinating, or mixed patterns. In most of the instances, the pathologist happens to interpret the nerve biopsy with limited clinical details and the morphologic alterations remains the major basis of making a probable diagnosis of PNL. The similar histopathologic alterations can be seen in neuropathies other than leprosy particularly vasculitis and chronic inflammatory demyelinating polyneuropathy (CIDP) which are also inflammatory neuropathies. The present article reflects the histopathologic analysis of the nerve biopsy samples done on patients with a clinical diagnosis of PNL. The morphologic features have been compared with that of NLN group which includes vasculitic neuropathies and CIDP. The article particularly tries to highlight the morphologic differences between AFB-negative PNL and NLN.
Objectives
To characterize the morphologic alterations in AFB-negative PNL group from the biopsied nerve samples and to compare and differentiate, these features with NLN group to make a probable diagnosis of PNL.
Materials and Methods
Study design
The study was a retrospective histopathologic analysis of the 46 nerve biopsies of PNL done in the Department of Pathology, Nizam's Institute of Medical Sciences during January 2011 to March 2013. The nerve biopsies are routinely performed by the pathologists for the patients being referred with a clinical diagnosis of peripheral neuropathy. A written informed consent is taken from patient before the biopsy. During the study period, 60 biopsies were diagnosed as leprosy neuropathy; however, 14 cases were excluded from the study since these patients had skin lesions. The nerve biopsies were not always obtained prior to the initiation of treatment. The nerves selected for biopsy were sural (n = 52) and ulnar (n = 8). The NLN group included vasculitic neuropathy (n = 15) and CIDP (n = 10). The diagnosis of vasculitis and CIDP was made after analyzing the clinical, ENMG, and biopsy features and these were inconsistent with a diagnosis of PNL. All the biopsies for the NLN group were from sural nerve.
Inclusion criteria for PNL
Sensory loss along the distribution of nerve with or without motor deficit.
Mononeuropathy, mononeuropathy multiplex, overlapping neuritis (polyneuropathy).
No detectable cutaneous lesions.
Thickened nerves with preserved reflexes.
Exclusion criteria
Leprosy neuropathy with skin lesions.
Histopathology
The nerve biopsies were divided into four parts and processed routinely. Formalin-fixed paraffin-embedded tissues were cut longitudinally and transversely at 5 μ thickness and stained with hematoxylin and eosin stain. In addition special stains like Masson Trichome to assess fibrosis, 5% Ziehl Neelsen stain for detection of lepra bacilli and Kulchitsky Pal (Kpal) stain to estimate myelinated fiber loss and demyelination were done in all the cases. A careful search for lepra bacilli was done in all the cases. Care was taken not to confuse myelin debris for acid fast bacilli. The histopathological alterations analyzed in PNL and NLN group included:
Epineurial and endoneurial infiltrate along with the degree of inflammation.
Endoneurial fibrosis, perineurial thickening, endoneurial edema.
The presence of endoneurial granuloma and foamy macrophages.
Reduction in myelinated nerve fibers.
The histopathologic features were analyzed by three pathologists separately.
Statistical analysis
The frequency of each of the histopathologic variables has been mentioned as a percentage and compared between PNL and NLN group. The categorical values were analyzed using Chi-square test using Statistical Package of Social Sciences (SPSS) statistics software version 20 to derive a statistical significance of histopathologic variables between AFB-negative PNL and NLN group.
Results
The 46 patients of PNL were in the age range of 16-69 years with male to female ratio of 1.8:1. Peripheral nerves; particularly common peroneal and ulnar were thickened in 15 cases and muscle wasting was seen in 7 cases. There were deformities in the form of claw hand (n = 5) and foot drop (n = 4). Ulceration of toes and feet were seen in 10 cases. The neuropathy was in the form of mononeuropathy multiplex (n = 36), mononeuropathy (n = 3), and polyneuropathy (n = 7). The ENMG pattern in most cases was that of sensory axonal neuropathy and on occasion a mixed sensory motor neuropathy. After applying the Ridley-Jopling classification on nerve biopsies, it was classified as Tuberculoid (TT) (n = 6, 13%), borderline tuberculoid (BT) (n = 22, 47.8 %), and borderline lepromatous (BL) (n = 11, 23.9%), and Borderline (BB) (n = 5, 10.8%) forms. Two cases showed non-specific histology. The patients whose biopsies showed borderline (BB) features and non specific histology had received MDT for varied duration.
The inflammatory infiltrate varied according to the form of leprosy. The main histologic features of tuberculoid leprosy were presence of epithelioid granulomas, endoneurial inflammation with or without necrosis, and low bacillary load. Majority of the cases showed significant endoneurial fibrosis and marked or near total loss of myelinated fibers on Kpal stain. The inflammation was seen extending to perineurium and epineurial vessels. Caseous necrosis was seen in one of the cases of tuberculoid leprosy [Figure 1]. As against this, borderline lepromatous cases were characterized by presence of histiocytes with scant inflammation and high bacillary load. The myelin stains showed evidence of demyelination in addition to fiber loss [Figure 2]. The borderline form of leprosy showed only inflammatory infiltrate no foam cell, no giant cells, or any demonstrable organisms.[6,7] There were two cases which showed nonspecific histological changes in the form of markedly thickened perineurium and dense endoneurial fibrosis. These two cases were on treatment with multidrug therapy for leprosy and were thus considered as healed lesions. Two cases showed upgrading type of lepra reaction. Among these, one patient was on treatment for 4 years and presented with polyneuropathy and the other patient was on treatment for 2 years and presented with tingling numbness and paresthesia of the lower limbs for 2 months. Both the cases showed beaded bacilli. Among the 46 PNL cases, 22 (47.8%) were positive for acid fast bacilli.
Figure 1.
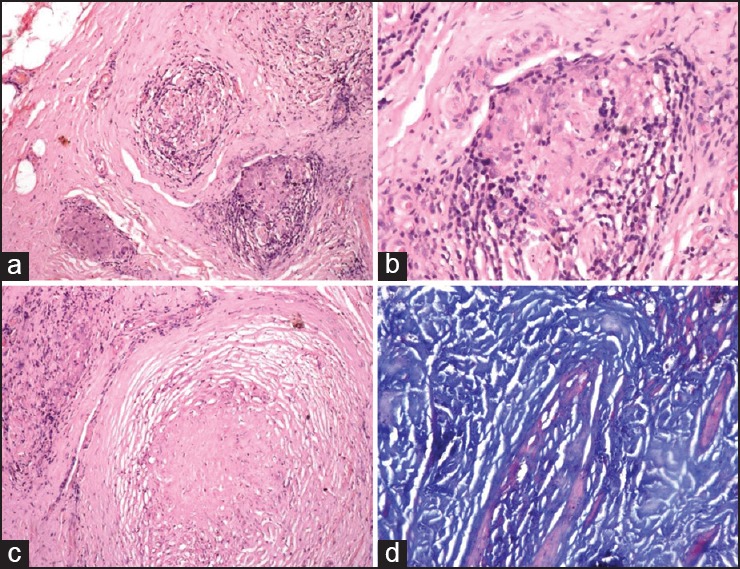
Biopsy features of tuberculoid leprosy PNL (a) Cross-section of the nerve biopsy showing endoneurial infiltrate and perineurial thickening. (H and E ×40). (b) Presence of well-formed epithelioid cell granuloma (arrow) in the endoneurium (H and E ×100). (c) Endoneurial necrosis, arrow (H and E ×40). (d) Ziehl Neelsen (5%) stain for lepra bacilli is negative (H and E1000)
Figure 2.
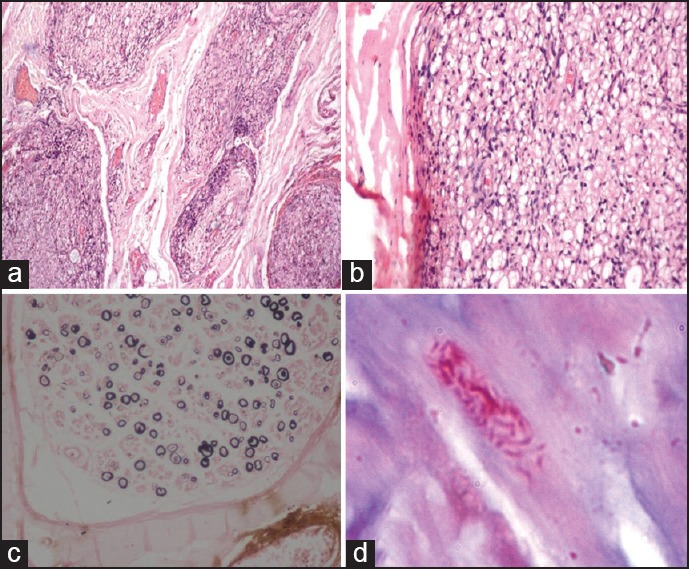
Biopsy features of borderline lepromatous leprosy PNL. (a) The cross-section of the nerve showing minimal endoneurial inflammation (H and E ×40). (b) The endoneurium showing foam cells (H and E ×100). (c) Kpal stain showing reduction in the number of myelinated fibers with few thinly myelinated fibers (arrowheads) (Kpal ×100). (d) Ziehl Neelsen (5%) stain showing lepra bacilli (arrow)
The hisopathological features evaluated in both AFB-positive and AFB-negative PNL group are summarized in Table 1. Both the groups showed significant moderate to dense epineurial and endoneurial inflammatory infiltrate chiefly comprising mononuclear cells [Figure 3a]. In addition, endoneurial fibrosis and perineurial thickening were also significant in both the groups [Figure 3b and c]. A demyelinating pattern as evidenced by the presence of thinly myelinated nerve fibers were seen in three cases of AFB-positive PNL group which belonged to the spectrum of borderline lepromatous form.
Table 1.
Histopathological features in PNL (AFB positive and AFB negative) and NLN group
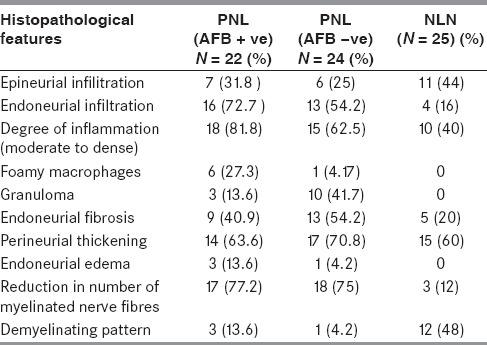
Figure 3.
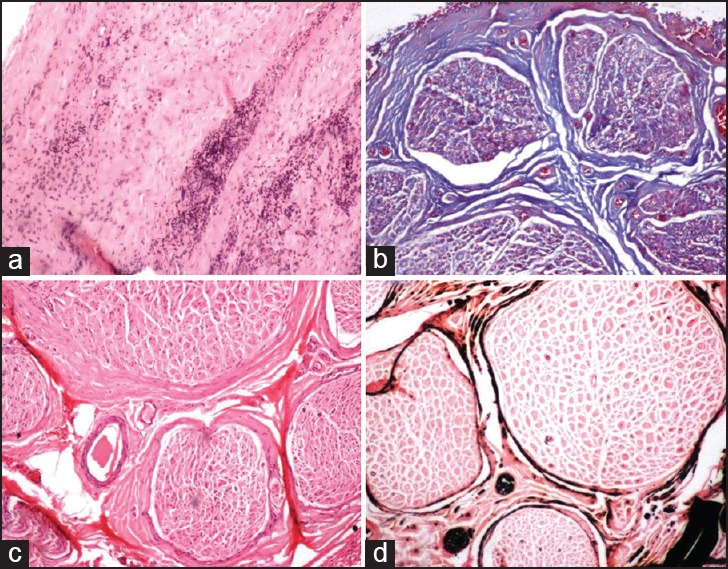
The features evaluated in PNL and NLN cases including (a) Epineurial inflammation, endoneurial inflammation, and perineurial thickening (H and E ×40). (b) Presence of endoneurial fibrosis (Masson trichrome ×40). (c) Perineurial thickening (Arrow) (H and E ×40) and (d) Reduction in the myelinated fiber density (Kpal ×40)
The presence of epithelioid granulomas and foamy macrophages loaded with acid fast bacilli indicates two extreme polar forms of the disease. The presence of mononuclear cell infiltrate in absence of granulomas mimics other inflammatory disorders of the peripheral nerves like vasculitic neuropathy or CIDP. To differentiate PNL from these entities, the PNL group (AFB-positive and AFB-negative) was compared with that of the NLN group. A comparison of the histopathological features between PNL and NLN group are summarized in Table 1. The endoneurial infiltrate was more frequent in PNL group as compared to NLN (72.7% in AFB-positive PNL, 51.1% in AFB-negative PNL, and 16% in NLN). The inflammatory infiltrate was chiefly composed of mononuclear cells, epithelioid cells, and foamy macrophages. The vasculitic neuropathies showed predominantly inflammatory infiltrate around the epineurial vessels with destruction of the vessel wall [Figure 4]. Fibrinoid necrosis was seen in five cases. There were four cases of CIDP which showed minimal inflammation [Figure 5]. Among the non-inflammatory alterations, endoneurial inflammation, and fibrosis were statistically significant when AFB-negative PNL group was compared with NLN group [P value - 0.005 and 0.013 respectively, Table 2]. Though the frequency of perineurial thickening was high, it was not statistically significant when both the groups were compared (P value – 0.426).
Figure 4.
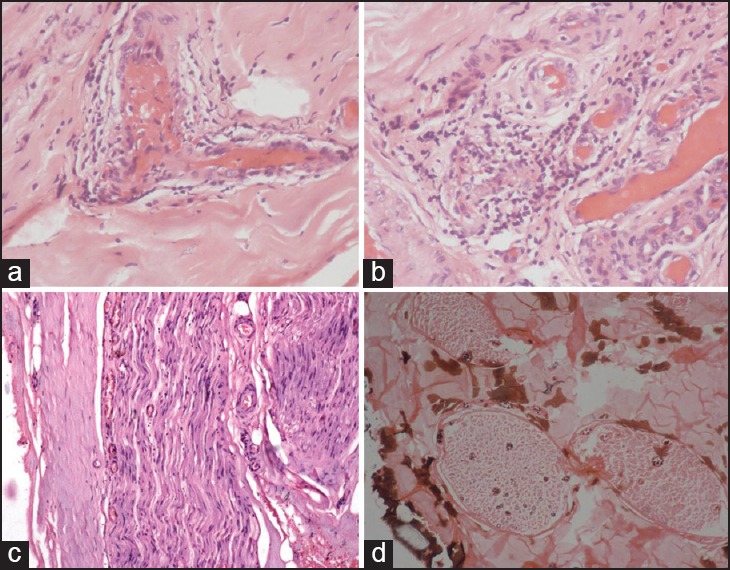
Nerve biopsy features of vasculitic neuropathy. (a and b) Presence of epineurial inflammation with vascular wall destruction (Circle) (H and E ×100). (c) Absence of endoneurial inflammation or granulomas (H and E ×40). (d) Non-uniform involvement of Kpal stain with sectorial fiber loss (Kpal ×40)
Figure 5.
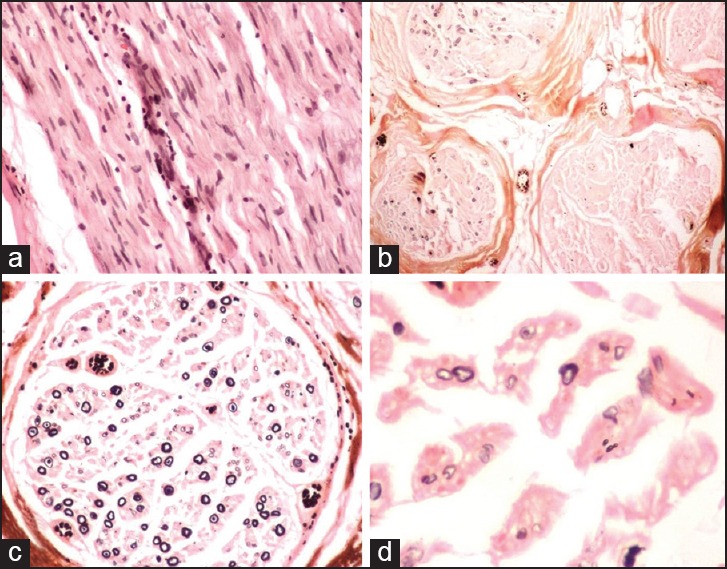
Nerve biopsy features of CIDP (a) The longitudinal section of the nerve showing endoneurial infiltrate (H and E ×100). (b) Non-uniform involvement of the fascicles (Kpal ×40). (c and d) The endoneurium showing loss if myelinated fibers as well as thinly myelinated fibers on Kapl stain
Table 2.
Commparison of the histopathological features in PNL and NLN group
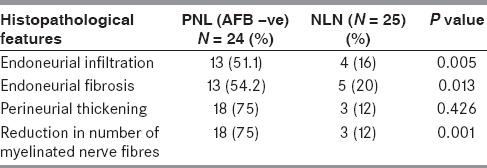
There was marked reduction in the number of small and large myelinated fibers in all the fascicles in PNL group [Figure 3d], whereas a non-uniform loss of myelinated nerve fibers with a demyelinating pattern was noted in the NLN group; particularly CIDP. (P value – 0.001) The reduction in the number of myelinated fibers was minimal in four cases of NLN. So, the findings which were statistically significant in AFB-negative PNL in comparison to NLN were endoneurial infiltrate, endoneurial fibrosis, and reduction in the number of myelinated nerve fibers [Table 2].
After analyzing and comparing the histopathologic features of PNL and NLN, the nerve biopsies of PNL were satisfactorily categorized in the following subtypes as:
Confirmed diagnosis [22 cases (47.8%): Inflammatory infiltrate composed of AFB-loaded macrophages.
Highly probable diagnosis [10 cases (21.7%): The presence of epithelioid granulomas in the absence of AFB].
Probable diagnosis [12 cases (26.1%): The presence of inflammatory infiltrate composed exclusively of mononuclear cells without transformation into either epithelioid cells or AFB-loaded macrophages in the nerve compartments, particularly in the endoneurium and in the absence of AFB].
Possible diagnosis [2 cases (4.3%): Non-inflammatory histological alterations like perineurial thickening and endoneurial fibrosis].
Discussion
The present study highlights the morphologic changes occurring in nerve biopsies of PNL. It reflects our experience of systematic evaluation of the biopsied nerve samples in arriving at a diagnosis of leprosy even in absence of demonstration of lepra bacilli. An attempt to make a diagnosis of PNL on morphologic grounds is of utmost importance since leprosy is a common public health problem in India and a correct diagnosis can benefit the patient with MDT. Various classification systems have been proposed which take into account the clinical, histologic, and immunologic characteristic including the pure neuritic and indeterminate forms.[8,9] The clinical presentation can vary from mononeuropathy, mononeuropathy multiplex, and polyneuropathy. Though mononeuropathy is the most common presentation of neuritic form of leprosy,[10] Garbino et al., observed that 42.85% cases presented as mononeuropathy multiplex and 31.25% as polyneuropathy.[11] The present study showed mononeuropathy multiplex in 78.26% cases. Though polyneuropathy is uncommon, various studies on PNL have mentioned it to be a definite presentation in PNL.[11,12] In fact in one study from India, symmetrical neuropathy outnumbered mononeuropathy multiplex in a series of 19 cases of leprous neuropathy.[13] Polyneuropathy was observed in 15.2% of our patients. Deformities like claw hand and foot drop were present in 19.57% cases as compared to 50 % cases noted by Mendiratta et al.,[14] Mc Dougall et al., found a preponderance of borderline tuberculoid and borderline lepromatous forms similar to the present study.[15] However Pedley et al., in their study of 119 patients in Nepal found a preponderance of borderline lepromatous form.[16]
The selection of nerve for biopsy depends on its clinical involvement on the basis of thickening or alterations in ENMG. However, clinically significant nerve thickening may be delayed in PNL even though it is pathologically affected.[17] Secondly, the major peripheral nerves cannot be easily biopsied and the smaller cutaneous branches may not yield diagnosis due to less number of fascicles.[15] Sural nerve was the choice of biopsy in our study because of technical advantage; it being a superficial and pure sensory nerve and also has more number of fascicles to avoid a false negative diagnosis. The other studies on PNL have also mentioned sural as the common nerve to be biopsied.[11,18,19]
The alterations seen in nerve biopsies in PNL have been studied in depth by Chimelli (1997) and Garbino (2004).[11,20] Chimelli et al., also evaluated the value of nerve biopsy in 53 leprosy patients and found inflammation in 75% cases, granulomas in 13.2% cases, and fibrosis in 66% cases.[20] Antunes et al., have earlier tried to elaborate the inflammatory and non-inflammatory changes in the nerve biopsies and highlighted the utility of these changes is AFB-negative samples similar to that of our study.[18]
The presence of AFB in either schwann cells or macrophages associated with or without inflammatory infiltrate is an unequivocal finding in leprosy neuritis. In absence of AFB, epithelioid granulomas especially in endoneurial compartment suggest high probability of leprosy.[18] However, in presence of endoneurial infiltrate and absence of a well-defined granuloma, vasculitis and CIDP needs to be considered in differential diagnosis.[20] This was the main basis of comparing the PNL biopsies with that of vasculitis and CIDP.
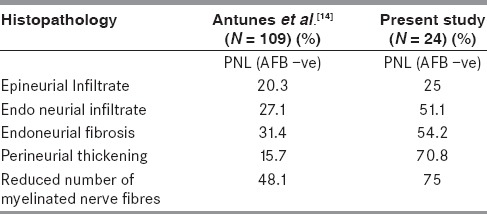
Antunes et al., in their study compared 144 cases of PNL with 196 cases of NLN and found that endoneurial infiltrate, fibrosis, and reduced number of myelinated nerve fibers were more common in PNL than NLN group similar to the present study.[18] It was observed that epineurial infiltrate, endoneurial infiltrate, endoneurial fibrosis, and reduction in number of myelinated nerve fibers were significant histological features in AFB-negative PNL group similar to our study [Table 3][18]. They also noted that perineurial thickening, fibrosis, mononuclear infiltrate, and endoneurial infiltrate were independently more frequent in PNL group and when assessed together could diagnose 100% AFB-negative PNL.[18]
Table 3.
Comparison of the histopathological features of AFB negative PNL group in the present study and Antunes et al.[14]
The presence of endoneurial fibrosis is often emphasized as a significant feature of leprosy neuropathy. This results due to progressive axonal degeneration and collagen deposition in the endoneurium. Axonal damage is the primary process in tuberculoid leprosy resulting as a bystander effect of destructive inflammatory process.[6] Special attention needs to be paid to presence of endoneurial inflammation and endoneurial fibrosis which can be easily picked by special stain with Masson trichrome. As against this, the lepromatous forms can show segmental myelin changes and relative preservation of axons in the initial stages irrespective of high bacillary load. The myelin changes have been attributed to Schwann cell damage caused by bacilli.[18] The similar features were observed in our study in three cases of borderline lepromatous form.
Similar to that described by Antunes et al., we tried to summarize the results of histopathologic analysis in deriving a definite, highly probable, probable, and possible diagnosis of PNL on nerve biopsies.[18] The results of both the studies were much comparable except confirmed diagnosis could be given in majority of our biopsies due to demonstration of AFB; India being one of the endemic regions for leprosy.
Irrespective of these morphologic alterations, it is also important to mention that an attempt should be made to demonstrate presence of lepra bacilli by specialized techniques like PCR [M. leprae deoxyribonucleic acid (DNA)] or immunohistochemistry (anti-PGL-1 antibody).[5] These were not performed in our study and is a limitation of the present study.
Conclusion
Nerve biopsy is an efficient tool to diagnose PNL and differentiate it from other causes of non-leprosy neuropathies presenting as mononeuropathy multiplex. In absence of AFB, the diagnosis of PNL is challenging. In this article, we have satisfactorily evaluated the various hisopthological features and found that endoneurial inflammation, endoneurial fibrosis, and reduction in the number of myelinated nerve fibers are supportive indicators to make a probable diagnosis of PNL regardless of AFB-positivity.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
References
- 1.Dogra S, Narang T, Kumar B. Leprosy — evolution of the path to eradication. Indian J Med Res. 2013;137:15–35. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Girdhar BK. Neuritic leprosy. Indian J Lepr. 1996;68:35–42. [PubMed] [Google Scholar]
- 3.Noordeen SK. Epidemiology of (Poly) neuritic type of leprosy. Lepr India. 1972;44:90–6. [Google Scholar]
- 4.Sehgal VN, Sardana KS. “Intriguing” repercussions of primary neuritic leprosy during the evolution of leprosy across the leprosy spectrum. Int J Dermatol. 2006;45:1121–3. doi: 10.1111/j.1365-4632.2006.02882.x. [DOI] [PubMed] [Google Scholar]
- 5.Antunes SL, Chimelli LM, Rabello ET, Valentim VC, Corte-Real S, Sarno EN, et al. An immunohistochemical, clinical and electroneuromyographic correlative study of the neural markers in the neuritic form of leprosy. Braz J Med Biol Res. 2006;39:1071–81. doi: 10.1590/s0100-879x2006000800010. [DOI] [PubMed] [Google Scholar]
- 6.Midroni G. Leprous neuropathy. In: Midroni G, Bilbao JM, editors. Biopsy diagnosis of peripheral neuropathy. 1st ed. Newton: Butterworth Heinemann; 1995. pp. 223–39. [Google Scholar]
- 7.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–73. [PubMed] [Google Scholar]
- 8.Sehgal VN, Rege VL, Reys M. Correlation between clinical and histopathologic classification in leprosy. Int J Lepr Other Mycobact Dis. 1977;45:278–80. [PubMed] [Google Scholar]
- 9.Sehgal VN. A 7-group classification of leprosy for institutional and field work. Lepr Rev. 1989;60:75. [PubMed] [Google Scholar]
- 10.Mahajan PM, Jogaikar DG, Mehta JM. A study of pure neuritic leprosy: Clinical experience. Indian J Lepr. 1996;68:137–41. [PubMed] [Google Scholar]
- 11.Garbino JA, Ura S, Belone AF, Marciano LH, Fleury RN. Clinical and diagnostic aspects of the primarily neural leprosy. Hansen Int. 2004;29:124–9. [Google Scholar]
- 12.Jardim MR, Antunes SL, Santos AR, Nascimento OJ, Nery JA, Sales AM, et al. Criteria for diagnosis of pure neural leprosy. J Neurol. 2003;250:806–9. doi: 10.1007/s00415-003-1081-5. [DOI] [PubMed] [Google Scholar]
- 13.Khadilkar SV, Benny R, Kasegaonkar PS. Proprioceptive loss in leprous neuropathy: A study of 19 patients. Neurol India. 2008;56:450–5. doi: 10.4103/0028-3886.44824. [DOI] [PubMed] [Google Scholar]
- 14.Mendiratta V, Khan A, Jain A. Primary neuritic leprosy: A reappraisal at a tertiary care hospital. Indian J Lepr. 2006;78:261–7. [PubMed] [Google Scholar]
- 15.McDougall AC, Harman DJ, Waudby H, Hargrave JC. Peripheral nerve biopsies in the diagnosis of leprosy in Aboriginal patients from the Northern Teritory of Australia. J Neurol Neurosurg Psychiatry. 1978;41:874–81. doi: 10.1136/jnnp.41.10.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedley JC, Harman DJ, Waudby H, McDougall AC. Leprosy in peripheral nerves: Histopathological findings in 119 untreated patients in Nepal. J Neurol Neurosurg Psychiatry. 1980;43:198–204. doi: 10.1136/jnnp.43.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shetty VP, Ghate SD, Wakade AV, Thakar UH, Thakur DV, D’souza E, et al. Clinical, bacteriological, and histopathological characteristics of newly detected children with leprosy: A population based study in a defined rural and urban area of Maharashtra, Western India. Indian J Dermatol Venereol Leprol. 2013;79:512–7. doi: 10.4103/0378-6323.113081. [DOI] [PubMed] [Google Scholar]
- 18.Antunes SL, Chimelli L, Jardim MR, Vital RT, Nery JA, Corte-Real S, et al. Histopathological examination of nerve samples from pure neural leprosy patients: Obtaining maximum information to improve diagnostic efficiency. Mem Inst Oswaldo Cruz. 2012;107:246–53. doi: 10.1590/s0074-02762012000200015. [DOI] [PubMed] [Google Scholar]
- 19.Reddy R, Singh G, Sacchidanand S, Okade R, Shivakumar V, Uday A, et al. A comparative evaluation of skin and nerve histopathology in single skin lesion leprosy. Indian J Dermatol Venereol Leprol. 2005;71:401–5. doi: 10.4103/0378-6323.18944. [DOI] [PubMed] [Google Scholar]
- 20.Chimelli L, Freitas M, Nascimento O. Value of nerve biopsy in the diagnosis and follow-up of leprosy: The role of vascular lesions and usefulness of nerve studies in the detection of persistent bacilli. J Neurol. 1997;244:318–23. doi: 10.1007/s004150050094. [DOI] [PubMed] [Google Scholar]


