Abstract
Aims:
To assess sleep quality in patients with primary headaches before and after prophylactic treatment using a validated sleep-screening instrument.
Materials and Methods:
A total of 147 patients, including 63 tension type headache (TTH) and 84 migraine patients were included. Patients were examined in terms of frequency and severity of headaches and sleep quality before and 12 weeks after prophylactic treatment with either propranolol or amitriptyline.
Results:
Baseline Visual Analogue Score (VAS) in migraine patients was 7.99 ± 1.39 compared with 6.86 ± 1.50 in TTH group (P < 0.001). VAS score after the first month of treatment was 6.08 ± 1.88 in migraine patients and 5.40 ± 1.61 in TTH (P = 0.023). VAS scores decreased after the third month of treatment to 4.32 ± 2.29 in migraine patients and 4.11 ± 1.66 in TTH patients (P = 0.344). The decrease was significant for patients treated with amitriptyline but not for those with propranolol. Baseline Pittsburgh Sleep Quality (PSQI) scores were 5.93 ± 2.43 in migraine patients and 6.71 ± 2.39 in TTH patients. Poor quality of sleep (PSQI ≥ 6) prior to prophylactic treatment was observed in 61.4% of migraine patients and in 77.7% of TTH patients. Comparison of PSQI scores before and 3 months following treatment showed significantly improved quality of sleep in all treatment groups; the greatest significance was detected in migraine patients with initial PSQI scores of ≥6 and treated with amitriptyline (P < 0.001).
Conclusions:
Increased understanding of routine objective sleep measures in migraine patients is needed to clarify the nature of sleep disturbances associated with primary headaches. This may in turn lead to improvements in headache treatments.
Keywords: Migraine, prophylactic treatment, sleep quality
Introduction
Migraine is a recurrent neurovascular disorder involving several pathophysiological mechanisms[1] and characterized by moderate to severe unilateral throbbing headache associated with symptoms such as nausea, vomiting, and sensitivity to light and sound.[2] Migraine has several subtypes, multiple comorbidities, and variable prognoses. The pathophysiology of migraine is not fully understood, but it has long been known that various triggers including changes in lifestyle, sleep, diet, or hormones may precipitate migraine attacks. The current understanding is that migraine is due to a decreased threshold in central mechanisms and intracranial hypersensitivity, in combination with a wide range of external and internal precipitating factors.[1,3,4,5] Sleep disturbance is one of the most commonly cited causes of migraine headache.[6,7] Headache and sleep share a complex relationship since sleep disturbances may trigger headaches, and headaches in turn may cause sleep abnormalities in these patients. Sleep patterns were attributed to significant variations in headache over time in 46% of tension-type and 60% of migraine patients.[7] Vgontzas et al. (2008) suggested that sleep disturbances in patients with migraine are not explained solely by chance association or on the basis of co-morbid depression and anxiety.[8] Rather, a biological basis underlying a common pathway for regulation of sleep and pathogenesis of migraine may exist.
Since the mechanisms underlying migraine headache are still insufficiently understood, different of medications are used for migraine prophylaxis. Available guidelines commonly recommend beta-blockers or tricyclic antidepressants (TCA) as the first choice for migraine prophylaxis.[9] The efficacy of TCAs in migraine treatment has been shown to be independent of their antidepressant action.[10,11] Both amitriptyline and propranolol appear to have rapid eye movement (REM)-suppressant effects, and disruption of REM sleep has been shown to trigger migraine attacks.[12] Disturbed sleep is associated with a reduction in pain threshold, presumably reflecting central sensitization and advocating further investigation into sleep disturbance as a potential modifiable risk factor for the prevention of headaches. Identification of specific headache characteristics related to sleep disturbances may lead to improvements in headache treatments.
The aim of the present study was to assess the sleep quality and sleep patterns in patients with primary headaches before and after prophylactic headache treatment using a validated sleep-screening instrument, and to determine the relationship between headache characteristics and sleep disturbances.
Materials and Methods
This uncontrolled, cross-sectional, prospective study enrolled 230 consecutive outpatients attending our tertiary headache center for the first time between January and June of 2014. Of these, 118 suffered from “migraine without aura” (henceforth referred to as “migraine”) and 112 from tension type headache (TTH), according to the diagnostic criteria of the International Classification for Headache disorders, 2nd edition.[13] The study was approved by the Ethics in Research Committee of the local University Review Board. All patients enrolled in this study provided individual written informed consent.
The patients underwent extensive physical, neurological, and psychological examination performed by a neurologist specializing in headache disorders and a clinical psychologist. Exclusion criteria included any of the following: Symptoms of depression, anxiety, or other comorbid psychiatric disorders; other types of headache disorders or more than one type of headache (e.g., migraine and TTH attacks in the same patient); other clinical conditions characterized by sleep disturbance (e.g., obstructive sleep apnea, restless les syndrome); chronic use of drugs known to affect sleep (e.g., benzodiazepines, antidepressants, anticonvulsants, antipsychotics, antihistamines); morbid obesity (body mass index > 35 kg/m2); epilepsy; and pregnancy or breast feeding. Of the initial 230 patients, 108 with migraine and 87 with TTH who were not on prophylactic treatment for headache in the 8 weeks prior to randomization were included in the study.
Depression was screened using Hamilton Depression Scale and anxiety was screened with Hamilton Anxiety Scale. Patients having high scores were referred to Psychiatry department for re-evaluation and patients diagnosed with comorbid depression and/or anxiety were excluded from the study. Patients who had relatively high scores in tests but were not diagnosed as having psychiatric disorder in examinations were included in the study.
During the initial 4-week baseline period, patients received no prophylactic treatment for headache. The number of headache attacks (NOA), the duration in hours, and the severity of the attacks were recorded in a headache diary by each patient. The severity of pain was expressed using a visual analog scale (VAS). Acute medications were used if needed. After 4 weeks, patients underwent 12 weeks of prophylactic treatment with either propranolol or amitriptyline. As a result of patients’ insufficient compliance, irregular visits during baseline, use of other drugs affecting sleep patterns, or other reasons, only 147 patients completed the study.
Detailed information on the clinical characteristics of migraine and TTH was again collected, employing face-to-face interviews prior to and 1 and 3 months after prophylactic treatment. The quality of sleep was measured using the Pittsburgh Sleep Quality Index (PSQI).[14] PSQI is a self-rated questionnaire validated in Turkish[15] and scored on a 0-3 scale assessing various factors related to sleep quality, including estimates of sleep duration and latency and the frequency and severity of specific sleep-related difficulties. A PSQI score of ≥6 was considered abnormal.[14] The headache frequency (NOA), severity (VAS), and PSQI scores were compared between TTH and migraine patients, grouped by prophylactic treatment administered with gender taken into account.
Statistical analysis
Statistical analysis was performed using Statistical Package of Social Sciences (SPSS) v.19 (IBM, Chicago, IL, USA). We tested the normality of the continuous variable datasets. Comparisons between groups were made using the student t-test for normally distributed data and the Mann-Whitney U test for data that failed normality. Time-dependent data were analyzed using the Friedman test. The Wilcoxon rank sum test was used to compare data within groups with baseline values. Comparison of categorical variables between groups was performed using the Chi square, Cochran Q, or McNemar test. General linear model (GLM) multivariate analyses were used to determine independent risk factors related to PSQI. Results are presented as means ± SD. P values < 0.05 were considered statistically significant.
Results
Demographic and clinical data
A total of 147 patients (130 females; mean age 34.3 ± 8.7 years), including 63 TTH (53 females; mean age 34.5 ± 7.7 years) and 84 migraine patients (77 female; mean age 34.2 ± 9.3 years) completed the study. Patients had been experiencing headaches for a mean of 5.9 ± 3.9 years (range 1-20 years; median 4 years). Patients were treated for 12 weeks: Migraine patients were administered either amitriptyline (40 patients; MA group) or propranolol (44 patients; MP group); all TTH patients were administered amitriptyline (TA group).
Table 1 shows the distribution of VAS, NOA, and PSQI scores according to headache type and prophylactic medication during the initial period and following 1 and 3 months of treatment. Overall, mean values of VAS, NOA, and PSQI decreased significantly during the treatment period (P = 0.013, 0.006, and 0.007, respectively). The mean baseline VAS score in migraine patients was 7.99 ± 1.39 (MA group: 8.00 ± 1.23; MP group: 7.98 ± 1.54) compared with 6.86 ± 1.50 in the TTH group (P < 0.001). The mean VAS score after the first month of treatment was 6.08 ± 1.88 in migraine patients (MA group: 6.11 ± 2.05; MP group: 6.05 ± 1.73) and 5.40 ± 1.61 in TTH patients (P = 0.023). VAS scores decreased after the third month of treatment to 4.32 ± 2.29 in migraine patients (MA group: 4.51 ± 2.06; MP group: 4.36 ± 2.50) and 4.11 ± 1.66 in TTH patients (P = 0.344). The decrease was significant for patients treated with amitriptyline (MA group: P = 0.003; TA group: P = 0.002) but not for those treated with propranolol (P = 0.666). VAS scores were similar between genders initially (P = 0.849) but decreased significantly in females over the treatment period (P = 0.021).
Table 1.
The distribution of VAS, NOA and PSQI scores
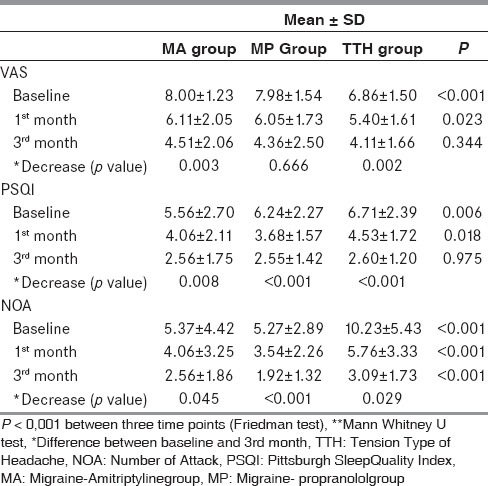
The initial NOA was 5.29 ± 3.2 in migraine patients (MA group: 5.37 ± 4.42; MP group: 5.27 ± 2.89) and 10.23 ± 5.43 in the TA group. At the time of the final visit, the NOA was significantly decreased in all three groups, most notably in MP patients (MP group: P < 0.001; MA group: P = 0.045; TA group: P = 0.029).
Hamilton Depression Scale results were 5.70 ± 3.53 (range: 0-14; median: 5.0) in migraine patients and 6.84 ± 3.44 (range: 1-15, median: 7.0) in TTH group. Hamilton Anxiety Scale results were 4.19 ± 0.41 (range: 0-15, median: 3.0) in migraine group and 7.31 ± 0.48 (range: 3-20, median: 7.0) in TTH group.
Sleep profile in migraine and TTH patients
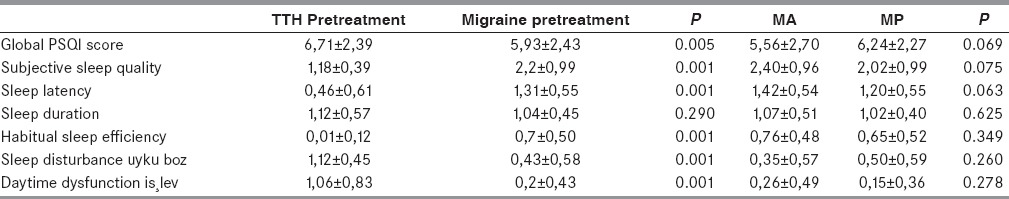
Baseline PSQI scores were 5.93 ± 2.43 in migraine patients and 6.71 ± 2.39 in TTH patients. Sleep quality was worse among patients with TTH compared with migraine for each sleep item analyzed (P = 0.05) [Table 2]. Poor quality of sleep (PSQI ≥ 6) prior to prophylactic treatment was observed in 61.4% of migraine patients and in 77.7% of TTH patients. Sleep quality did not change after the first month of treatment in the MA group (P = 0.068), but a significant improvement was observed after 3 months (P = 0.008). Sleep quality improved significantly after the first month of treatment, and improvement persisted after the third month in the MP (P = 0.006 first month, P < 0.001 third month) and TA groups (P = 0.019 first month; P < 0.001 third month). Baseline VAS scores were higher in patients with PSQI scores ≥ 6, although this difference was not significant (VAS = 8.13 ± 1.45 in patients with PSQI score ≥ 6; VAS = 7.78 ± 1.29 in patients with PSQI score <6; P = 0.260). The mean NOA was 5.3 ± 2.6 in patients with good sleep quality, significantly lower than that in patients with poor sleep quality (7.9 ± 4.2; P = 0.001). Older patients had higher initial PSQI scores (P = 0.019) in the migraine but not the TTH group. There was no correlation between PSQI score and gender or headache duration in either group. Comparison of PSQI scores before and 3 months following treatment showed significantly improved quality of sleep in all treatment groups (MA: P = 0.008; MP: P = 0.004; TA: P = 0.017); the greatest significance was detected in migraine patients with initial PSQI scores of ≥6 and treated with amitriptyline (P < 0.001).
Table 2.
PSQI subscores in migraine and TTH patients
The number of patients experiencing poor quality of sleep decreased from 101 patients (68.7%) prior to treatment to 43 patients (29.3%) at the end of the first month and to 8 patients (5.4%) at the end of the third month. There was a significant increase in sleep quality over the study period (P = 0.0001).
PSQI differed significantly among time periods according to the GLM analyses; VAS (P = 0.004) and NOA (P = 0.0001) were found to be significant independent risk factors in relation to PSQI, whereas gender (P = 0.419), age (P = 0.293), drug group (P = 0,176), type of migraine (P = 0.840), and duration of headache (P = 0.732) were not.
Discussion
Migraine and sleep maintain a complex relationship. The comorbidity of migraine and sleep disorders is estimated to be between 30 and 55%.[16] In a study evaluating patients with migraine or TTH, 60% of subjects were determined to have sleep disorders.[17] In our study, sleep disturbances were found in 61.4% of patients with migraine and 77.7% patients with TTH, according to PSQI scores, and this was in agreement with other studies. A potentially causal relationship appears to be reciprocal: Sleep disorders can be the cause and/or result of headaches, and vice versa. VAS scores and NOA were higher in patients with PSQI scores ≥ 6. Chronically abnormal sleep patterns have been associated with more frequent and more severe migraine attacks.[18] Since the present study was cross-sectional, we cannot determine whether frequent headaches were the cause of sleep disturbance, or vice versa, or whether another common susceptibility was responsible for this association. The discovery that headache and sleep processes share physiologic, neurochemical, and pathophysiologic characteristics has been an important milestone in our understanding of the relationship between the two. One possibility is that pain arouses the system, preventing initiation of sleep or altering sleep architecture.[19] Another possibility is that poor sleep patterns can alter pain processing and thereby cause pain.[20] The circadian rhythm is controlled by the hypothalamic suprachiasmatic nucleus; the fact that the posterior hypothalamus and the suprachiasmatic nucleus are also implicated in the pathology of headaches may be another key factor in our understanding.[21] Further, receptors for cytokines, which are involved in the production of pain, are expressed in the suprachiasmatic nucleus.[22] Sleep has also been shown to relieve migraines. Although migraine patients frequently cite sleep problems as a trigger of attacks, a large majority of migraineurs report sleep as an effective acute treatment.[12] Although potential relationships between migraine and sleep have been postulated in several studies, alteration in the quality of sleep following prophylactic treatment has not been assessed in detail.
Migraine attacks have been well-documented to occur during specific stages of sleep. In particular, attacks are more likely to occur during periods of REM sleep, with morning arousal associated with longer durations of stage IV and REM sleep. Most antidepressants alter sleep, as evidenced by modifications in patterns of electroencephalography recorded during sleep. These effects are most consistent in REM sleep and tend to be in the direction opposite of those found in sleep abnormalities. Amitriptyline is effective in reducing migraine attacks, and its effect is unrelated to migraine severity.[23] Amitriptyline also has sleep-promoting effects. It reduces the time spent in REM sleep and increases the metabolism of catecholamines, especially norepinephrine.[24] Amitriptyline also prevents reuptake and increases turnover of central serotonin and increases availability of biogenic amines at central synapses.[25,26] Although lipophilic drugs such as propranolol also reduce REM frequency, their effect on sleep is not well-understood.[27]
Both amitriptyline and propranolol were used for migraine prophylaxis in our study, which showed a reduction in the duration, frequency and severity of attacks in all groups after the 3-month treatment period. Differences within the MP group were more prominent following the first month. VAS scores and NOA were found to be significant independent risk factors related to PSQI scores. Our study showed that in patients with migraine and TTH, sleep quality and headaches improved in parallel. These findings led us to conclude that effective prophylaxis for headaches provides important benefits in addition to decreasing pain attacks, and that this is especially important with regard to sleep quality. Both headaches and sleep quality improved in all patients upon prophylactic treatment.
The limitation of this study is that this was a non-controlled study and we did not perform a crossover analysis of these patients on propranolol going to amitriptyline group. Controlled studies with a larger group of patients investigating the more robust medication in terms of improving sleep and quality of life is needed.
Conclusion
There was a high prevalence of sleep abnormalities, including poor quality of sleep, in patients with “migraine without aura.” Increased understanding of routine objective sleep measures in such patients is needed to clarify the nature and etiology of sleep disturbances associated with primary headaches. This may in turn lead to improvements in headache treatments.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
References
- 1.Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neurol. 2012;15:S15–22. doi: 10.4103/0972-2327.99993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 3.Pearce J. Insulin induced hypoglycaemia in migraine. J Neurol Neurosurg Psychiatry. 1971;34:154–6. doi: 10.1136/jnnp.34.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao NS, Pearce J. Hypothalamic-pituitary-adrenal axis studies in migraine with special reference to insulin sensitivity. Brain. 1971;94:289–98. doi: 10.1093/brain/94.2.289. [DOI] [PubMed] [Google Scholar]
- 5.Gasparini CF, Sutherland HG, Griffiths LR. Studies on the pathophysiology and genetic basis of migraine. Curr Genomics. 2013;14:300–15. doi: 10.2174/13892029113149990007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sookoian S, Gemma C, Gianotti TF, Burgueño A, Alvarez A, González CD, et al. Serotonin and serotonin transporter gene variant in rotating shift workers. Sleep. 2007;30:1049–53. doi: 10.1093/sleep/30.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelman L, Rains JC. Headache and sleep: Examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45:904–10. doi: 10.1111/j.1526-4610.2005.05159.x. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas A, Cui L, Merikangas KR. Are sleep difficulties associated with migraine attributable to anxiety and depression? Headache. 2008;48:1451–9. doi: 10.1111/j.1526-4610.2008.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pryse-Phillips WE, Dodick DW, Edmeads JG, Gawel MJ, Nelson RF, Purdy RA, et al. Guidelines for the diagnosis and management of migraine in clinical practice. Canadian Headache Society. CMAJ. 1997;156:1273–87. [PMC free article] [PubMed] [Google Scholar]
- 10.Couch JR. Amitriptyline Versus Placebo Study Group. Amitriptyline in the prophylactic treatment of migraine and chronic daily headache. Headache. 2011;51:33–51. doi: 10.1111/j.1526-4610.2010.01800.x. [DOI] [PubMed] [Google Scholar]
- 11.Couch JR, Ziegler DK, Hassanein R. Amitriptyline in the prophylaxis of migraine. Effectiveness and relationship of antimigraine and antidepressant effects. Neurology. 1976;26:121–7. doi: 10.1212/wnl.26.2.121. [DOI] [PubMed] [Google Scholar]
- 12.Alberti A. Headache and sleep. Sleep Med Rev. 2006;10:431–7. doi: 10.1016/j.smrv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. (2nd edition) 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 14.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 15.Senol V, Soyuer F, Senol PN. Pitssburg, Epworth tests evaluation in patients living in nursing home. Turk J Geriatr. 2013;16:60–8. [Google Scholar]
- 16.Montagna P, Chokroverty S. Sleep. Handb Clin Neurol. 2011;98:9–10. doi: 10.1016/B978-0-444-52006-7.00048-4. [DOI] [PubMed] [Google Scholar]
- 17.Ong JC, Stepanski EJ, Gramling SE. Pain coping strategies for tension-type headache: Possible implications for insomnia? J Clin Sleep Med. 2009;5:52–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Seidel S, Zeitlhofer J, Wöber C. First Austrian case of hypnic headache: Serial polysomnography and blood pressure monitoring in treatment within domethacin. Cephalalgia. 2008;28:1086–90. doi: 10.1111/j.1468-2982.2008.01611.x. [DOI] [PubMed] [Google Scholar]
- 19.Karthik N, Kulkarni GB, Taly AB, Rao S, Sinha S. Sleep disturbances in ‘migraine without aura’ — a questionnaire based study. J Neurol Sci. 2012;321:73–6. doi: 10.1016/j.jns.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 20.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Peres MF, Sanchez del Rio M, Seabra ML, Tufik S, Abucham J, Cipolla-Neto J, et al. Hypothalamic involvement in chronic migraine. J Neurol Neurosurg Psychiatry. 2001;71:747–51. doi: 10.1136/jnnp.71.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovati C, D’Amico D, Bertora P, Raimondi E, Rosa S, Zardoni M, et al. Correlation between presence of allodynia and sleep quality in migraineurs. Neurol Sci. 2010;31:155–8. doi: 10.1007/s10072-010-0317-2. [DOI] [PubMed] [Google Scholar]
- 23.Gomersall JD, Stuart A. Amitriptyline in migraine prophylaxis. Changes in pattern of attacks during a controlled clinical trial. J Neurol Neurosurg Psychiatry. 1973;36:684–90. doi: 10.1136/jnnp.36.4.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillin JC, Wyatt RJ, Fram D, Snyder F. The relationship between changes in REM sleep and clinical improvement indepressed patients treated with amitriptyline. Psychopharmacology (Berl) 1978;59:267–72. doi: 10.1007/BF00426633. [DOI] [PubMed] [Google Scholar]
- 25.Himwich HE, Alpers HS. Psychopharmacology. Annu Rev Pharmacol. 1970;10:313–34. doi: 10.1146/annurev.pa.10.040170.001525. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann E, Cravens J. The effects of long term administration of psychotropic drugs on human sleep. 3. The effects of amitriptyline. Psychopharmacologia. 1973;33:185–202. doi: 10.1007/BF00429087. [DOI] [PubMed] [Google Scholar]
- 27.Betts TA, Alford C. Beta-blockers and sleep: A controlled trial. Eur J Clin Pharmacol. 1985;28:65–8. doi: 10.1007/BF00543712. [DOI] [PubMed] [Google Scholar]


