Abstract
Objectives:
Very few studies in India have addressed the role of vitamin D in cognitive function. The present study was conducted to assess the serum levels of 25-hydroxyvitamin D (25(OH)D) and its association with markers of cognitive impairment and homocysteine levels in the elderly Indian population.
Materials and Methods:
The study population consisted of patients with dementia (Group A, n = 32), mild cognitive impairment (MCI; Group B, n = 24), and elderly age-matched controls (Group C, n = 30). Measurement of serum levels of 25(OH)D and total homocysteine were done.
Results:
Significant decreased concentration of 25(OH)D and increased concentration of homocysteine was observed. Association of serum levels of vitamin D with markers of cognitive decline as well as serum homocysteine levels was observed in patients with dementia and MCI when compared to controls.
Conclusion:
Correlation of vitamin D with markers of cognitive decline and homocysteine opens a new door for early diagnosis of cognitive impairment.
Keywords: Cognitive decline, dementia, homocysteine, MMSE score, vitamin D
Introduction
Nutritional factors play a vital role in promoting health and the majority of evidence has linked nutritional deficiencies to aggravating cognitive deterioration.[1] Historically, antioxidative nutrients and B-vitamins have been evaluated for neuroprotective effects. In recent years, emerging evidence has linked vitamin D not only to its classical function in mineral homeostasis, but also to various chronic illnesses involving neurocognitive decline.[2] Some studies in older adults have either linked lower 25-hydroxyvitamin D (25(OH)D) levels, the clinical indicator of vitamin D status in the blood, with measures of poor cognitive performance or higher 25(OH) D levels with measures of better cognitive performance.[3] However, the association between 25(OH)D concentrations and cognitive performance is not yet clear.
Vitamin D is a secosteroid hormone involved with over 2,000 genes in the body. Worldwide low vitamin D concentrations are prevalent for all age groups; however, the older population is especially at high risk for vitamin D deficiency due to the decreased cutaneous synthesis and dietary intake of vitamin D. Several studies have revealed a high prevalence of vitamin D deficiency even in areas of the world that receive ample sunlight. In Australia, India, and Saudi Arabia, 30-50% of children and adults have 25(OH)D levels below 50 nmol/L.[4,5] Vitamin D deficiency has also been linked to vascular dysfunction and ischemic stroke risk[6] as well as brain atrophy.[7] Previous prospective studies have established that low vitamin D concentrations in elderly adults are associated with an increased risk of cognitive decline.[8]
Vitamin D obtained from sun exposure, food, and supplements is biologically inert and must undergo two hydroxylation reactions in the body for activation. The first occurs in the liver and converts vitamin D to 25(OH)D, also known as calcidiol. The second occurs primarily in the kidney and forms the physiologically active 1,25-dihydroxyvitamin D (1,25(OH)2 D), also known as calcitriol. Serum concentration of 25(OH)D is the best indicator of vitamin D status. It reflects vitamin D produced cutaneously and that obtained from food and supplements and has a fairly long circulating half-life of 15 days.[9] At the molecular level, the brain has the ability to synthesize the active form of vitamin D (1,25(OH)2 D) within many cell types and regions with predominance in the hypothalamus and the large neurons within the substantia nigra.[10] Functionally, vitamin D contributes to neuroprotection by modulating the production of nerve growth factor (NGF), neurotrophin, glial cell-derived neurotrophic factor (GDNF), nitric oxide synthase (NOS), and choline acetyltransferase.[11] Compelling evidence suggests a beneficial role of the active form of vitamin D in the development of brain as well as in adult brain function. The vitamin D receptor and catalytic enzymes are colocalized in the human cortex and hippocampus, which are key areas for cognition and are involved in complex planning, processing, and the formation of new memories. Therefore, vitamin D has come under investigation for its role in cognitive preservation.
During the past decade, homocysteine has received increasing attention, as elevated levels of homocysteine have been implicated as an independent risk factor for cardiovascular disease and have also been associated with various other diseases and/or clinical conditions, including Alzheimer's disease (AD), neural tube defects, schizophrenia, etc.[12,13] Earlier studies have revealed homocysteine as a neurotoxin, which is capable of directly damaging the medial temporal lobe, the area of the brain that rapidly degenerates in AD.[14] Homocysteine is easily lowered with intake of inexpensive B-vitamins. Many factors are thought to raise levels of homocysteine; among them are poor diet; poor lifestyle, especially smoking and high coffee and alcohol intake; some prescription drugs; diabetes; rheumatoid arthritis; and poor thyroid function. Also, Kriebitzsch et al., have showed that 1,25(OH)2 D influences cellular homocysteine levels in murine preosteoblastic MC3T3-E1 cells by direct regulation of cystathionine β-synthase.[15]
Homocysteine is a sulfur-containing amino acid. It is a pivotal intermediate in methionine cycle, an important metabolic pathway that generates S-adenosylmethionine (SAM), a methyl group donor that regulates protein, lipid, biogene amine, and DNA methylation, as well as many other methylation reactions. Thus, changes in homocysteine levels lead to alterations in methylation cycle, thereby, causing defective methylation reactions in the body. As a result, defect in this complex mechanism of homocysteine leads to the condition called hyperhomocysteinemia, which is considered as a risk factor for dementia.[16]
A preponderance of evidence has laid the groundwork for the hypothesis that vitamin D and homocysteine can reduce the risk of dementia. Hence, the present study was conducted to evaluate the role of vitamin D in cognitive impairment by assessing the serum levels of 25(OH)D in patients with dementia and mild cognitive impairment (MCI) and compare the same with elderly age-matched controls. We also evaluated the potential association between vitamin D inadequacy and markers of cognitive impairment, that is, Mini-Mental State Examination (MMSE) and Addenbrook's Cognitive Examination (ACE) score and homocysteine levels.
Materials and Methods
Study design and participants
In this prospective study, total of 86 study participants aged >50 years were enrolled. The study population was divided into three groups; viz., patients suffering from AD and vascular dementia as well as patients with vascular cognitive impairment (VCI) not amounting to dementia (Group A, n = 32), patients with MCI (Group B, n = 24), and elderly age-matched controls (Group C, n = 30). The standard Diagnostic and Statistical Manual of Mental Disorders IV (DSM IV) diagnostic criterion was used for the above diagnoses. Dementia and MCI cases were referred from two hospitals located in Mumbai; Sir H. N. Reliance Foundation Hospital and Research Centre, and TN Medical College and BYL Nair Hospital. Elderly age-matched controls were healthy individuals from the nearby vicinity of Sir H. N. Reliance Foundation Hospital and Research Centre falling in the same age category.
Ethics consideration
Due approval was taken from the Scientific Advisory Committee and the Institutional Ethics Committee for this study. The study was carried out in accordance with the “Ethical Guidelines for Biomedical Research on Human Participants, 2006” by the Indian Council of Medical Research and the Declaration of Helsinki, 2008.
Written informed consent was obtained from all the study participants.
At the time of recruitment, all the participants were subjected to a baseline clinical and neurological examination.
Psychological assessment
Cognitive assessment of participants enrolled for this study was conducted by a clinical psychologist. All the participants underwent a comprehensive geriatric assessment, including history, medication history, and physical and neurological examination. They were put through questions for assessment of the MMSE and ACE. In MMSE, functions such as registration, attention, calculation, recall, language (comprehension, reading, writing, and naming), ability to follow simple commands, and orientation were examined. However, in ACE along with MMSE, other additional questions for remote memory, verbal fluency, naming, language, and visuospatial were assessed to test various specific domains of cognition. For MMSE, scores 27 or above are considered normal. However, scores below 24 indicates impairment in cognition. Similarly, in ACE, scores 90 or above are considered normal and scores below 90 indicates cognitive impairment.
Laboratory analysis
Six milliliter of blood was collected by venipuncture after overnight fasting. After centrifugation at 3,000rpm for 10min; serum was separated and stored at -80°C for enzyme-linked immunosorbent assay (ELISA). Analysis of blood glucose levels, lipid profile, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), VDRL, and thyroid profile (triiodothyronine (T3), thyroxine (T4), and thyroid-stimulating hormone (TSH)) were performed on fresh serum on the same day. Biochemical estimations were performed using a clinical chemistry autoanalyzer Konelab i20 (Thermo Scientific, Waltham, USA). In hematology, erythrocyte sedimentation rate (ESR) and complete blood count (CBC) were performed using VES-MATIC 20 and Sysmex 2000I hematology analyzer, respectively. Serum levels of 25(OH)D using kits from Immunodiagnostic Systems Ltd, USA, and total Homocysteine from MyBioSource, USA, were determined by ELISA kit.
Statistical analysis
Statistical evaluation of the results was performed using the Statistical Package for Social Sciences (SPSS) software, version 21.0 (SPSS, Chicago, IL, USA). The Kolmogorov-Smirnov test was used to confirm assumptions of normal distributions. Comparison between groups was done using either one-way analysis of variance (ANOVA; if normally distributed) or Kruskal-Wallis test (if not normally distributed) with post-hoc tests. Correlation between two numerical variables was assessed using Spearman's rho correlation coefficient. Student's t-test was used to compare the patient group with the control group. Baseline differences were analyzed using Mann-Whitney U-test and independent samples t-tests (continuous data). A p-value < 0.05 was accepted as statistically significant.
Results
Baseline characteristics
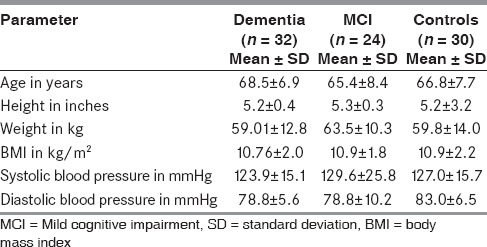
As shown from Table 1, all the groups were comparable with respect to the demographic details and anthropometric measurements.
Table 1.
Baseline characteristics of study population
Markers of cognitive decline
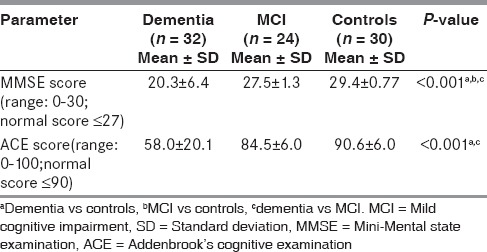
Table 2 shows significantly lower MMSE score in patients with dementia and MCI group when compared to age-matched controls. ACE score was found to be significantly lower in dementia group as compared to age-matched controls. Also significant difference was observed between MMSE and ACE score of dementia and MCI group (P < 0.001).
Table 2.
Comparison of markers of cognitive decline by MMSE and ACE
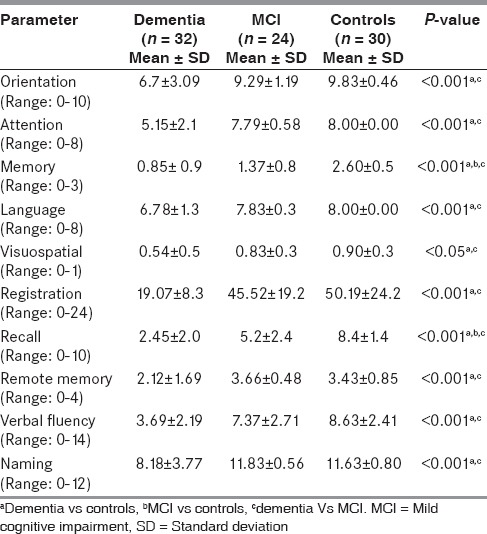
As seen in Table 3, a comprehensive analysis of MMSE and ACE indicated differential significance for each cognitive domain across the study groups. Scores of cognitive domains such as orientation, attention, memory, language, registration, remote memory, recall, verbal fluency, and naming were reduced significantly in patients with dementia when compared to MCI cases and age-matched controls (P < 0.001). Also, significantly decreased scores of visuospatial domain were observed in patients with dementia when compared to MCI cases and age-matched controls (P < 0.05). However, significantly decreased scores in domains like memory and recall were seen in patients with MCI when compared to age-matched controls.
Table 3.
Detailed comparison of cognitive domains across the study groups
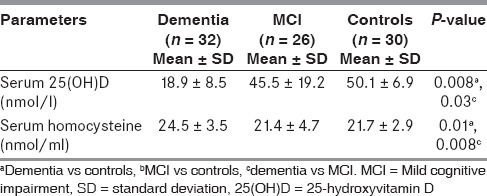
Serum levels of 25(OH)D and homocysteine across the study groups
As shown in Table 4, we observed significantly decreased concentration of 25(OH)D and increased concentration of homocysteine in serum of patients with dementia when compared to age-matched controls (P < 0.01) and patients with MCI (P < 0.05).
Table 4.
Comparison of serum levels of 25(OH)D and homocysteine across the study groups
Relationship between markers of cognitive decline, serum homocysteine, and serum 25(OH)D concentrations in patient groups
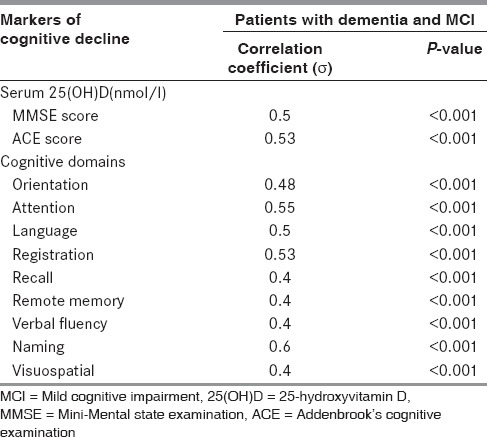
In the patient groups, serum 25(OH)D levels showed a positive correlation with markers of cognitive decline, that is, MMSE score (σ = 0.5) and ACE score (σ = 0.53). Similarly, serum 25(OH)D levels showed a strong positive correlation with cognitive domains like attention, language, registration, and naming; whereas, moderately positive correlation with domains like orientation, recall, remote memory, visuospatial, and verbal fluency with P-value less than 0.001 as shown in Table 5. We did not find significant correlation between serum homocysteine levels and cognitive domains of MMSE and ACE.
Table 5.
Spearman's correlation coefficient between markers of cognitive decline and serum 25(OH)D
Association between serum levels of homocysteine and 25(OH)D
Figure 1 shows an inverse correlation between serum levels of homocysteine and 25(OH) D (σ = −0.4 and P ≤ 0.001) in patients with dementia and MCI.
Figure 1.
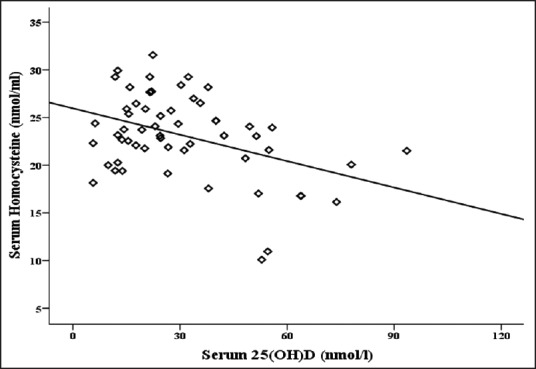
Correlation between serum levels of homocysteine and 25(OH)D in patients with Dementia and MCI
Discussion
In the cross-sectional design of our study, we observed significantly lower MMSE and ACE scores in patients with dementia and MCI group when compared to age-matched controls. Also significantly decreased concentration of 25(OH)D and increased concentration of homocysteine were found in serum of patients with dementia when compared to age-matched controls and patients with MCI. Furthermore, we found an association of serum levels of vitamin D with markers of cognitive decline and serum homocysteine levels.
The MMSE and ACE scores aid in understanding cognitive status of individual, and thus in the diagnosis of cognitive decline.[17] Though MMSE is a widely used screening tool across the world to assess cognitive dysfunction, the ACE is also a screening battery that examines the same domains as MMSE, but additionally examines memory in more detail, has a more detailed language component and visual-spatial functioning and has items for assessing executive functioning as well. In the present study, significantly decreased MMSE and ACE scores were seen in patients with dementia and MCI than elderly age-matched controls. These results are in accordance with findings of Bryant et al.[18] Similarly, individual cognitive domain assessed in MMSE and ACE showed significant difference between the study groups.
Based on animal and human observational studies, vitamin D deficiency has long been suspected as a risk factor for various chronic illnesses involving neurocognitive decline.[19] There is growing evidence indicating the role of vitamin D in regulating the development and function of nerve cells and the potential ramifications of vitamin D deficiency in this respect. Measurement of circulating 25(OH)D concentration is recognized as the best functional measure of vitamin D status.[20] We found significantly reduced serum levels of 25(OH)D in patients of dementia group than in MCI and elderly age-matched controls, which corroborates the findings by Oudshoorn et al., 2008.[21] When we tried to do detailed group-wise analysis of this data, due to limited sample size we could not find significant correlation between the parameters. Thus, we have combined the data of Group A (patients with Dementia) and Group B (patients with MCI). To the best of our knowledge, our study examines the association between serum 25(OH)D levels and cognition in dementia and MCI cases for the first time in elderly Indian population. A significant positive association was seen between serum levels of vitamin D and markers of cognitive decline, that is, low MMSE and ACE scores in patients with dementia and MCI. These results are in line with a previous study on the association between serum 25(OH)D levels and cognitive functioning.[22] Also detailed analysis of association of serum 25(OH)D levels with individual cognitive domains showed a significant positive correlation. Cognitive domains like attention, registration, and naming showed more significant association with serum 25(OH)D than other domains, suggesting importance of assessment of these domains with more consideration.
A positive correlation of vitamin D status with MMSE and ACE scores can be interpreted in several ways. First, vitamin D deficiency may lead to a gradual decline in cognitive functions. Data, mostly from ex vivo and animal studies, suggest that vitamin D metabolites may have protective effects on neurons or neurotransmitter pathways which could benefit cognition. Recently, two studies discussed the role of vitamin D in maintaining brain function. One examined evidence linking vitamin D deficiency to brain function/dysfunction;[23] the other explored the role of vitamin D in preventing neurocognitive dysfunction.[24] Collectively, many of these reports build the foundation for the hypothesis that vitamin D can reduce the risk of dementia. Furthermore, there is strong evidence that 1,25(OH)2 D contributes to neuroprotection by modulating the production of nerve growth, decreasing L-type calcium channel expression, regulating the toxicity of reactive oxygen species, and neurotrophic factors such as NGF, GDNF, and NOS.[25] Furthermore, vitamin D and its metabolites are involved in other neuroprotective mechanisms including amyloid phagocytosis and clearance[26] and vasoprotection.[25] Therefore the positive, significant correlation between vitamin D status and markers of cognitive decline suggests the possibility that vitamin D insufficiency may play role in cognitive dysfunction in elderly adults.
A number of potential mechanisms linking low vitamin D levels with the risk of dementia have been identified. Vitamin D receptors are expressed throughout the brain, including areas involved in memory such as the hippocampus and dentate gyrus.[10] Similarly, the enzyme that synthesizes the active form of vitamin D, 1α-hydroxylase, is produced in several cerebral regions. In vitro, vitamin D stimulates macrophages, which increases the clearance of amyloid plaques, a hallmark of AD.[26] A recent study found that amyloid-β induction of iNOS, part of the inflammatory process of AD, is dependent on the disruption of the vitamin D-vitamin D receptor pathway.[27] Vitamin D supplementation ameliorates age-related decline in learning and memory in aged rats.[28] Therefore, role of vitamin D deficiency in cognitive dysfunction has come into limelight.
Homocysteine is a key intermediate in methionine metabolism, an important metabolic pathway that generates SAM. SAM is a methyl group donor that regulates protein, lipid, biogene amine, and DNA methylation, as well as many other methylation reactions. Thus, changes in homocysteine levels lead to alterations in methylation cycle, thereby, causing defective methylation reactions in the body. An elevated homocysteine concentration can impact SAM, the only methyl donor in the CNS, required for the synthesis of neurotransmitters. Impaired SAM synthesis may cause impaired myelin synthesis/repair and impaired neurotransmitter turnover, and thereby, interfere with cognitive function.[29] The first evidence that elevated homocysteine levels might be involved in mental disorder was recorded in a group of mentally retarded children. A large number of reports have since followed, in which elevated homocysteine levels have been found in cognitively impaired patients or patients with dementia.[30]
We observed that there is a significant increase in serum levels of homocysteine in dementia patients than MCI patients and elderly age-matched controls. These outcomes are in line with previously published studies, In the Framingham Offspring Study, a relationship between total plasma homocysteine and cognitive function was found in older, but not younger, adults. Whereas, epidemiological studies have confirmed that elevations in plasma total homocysteine temporally precede the development of dementia and that there is a continuous, inverse linear relation between plasma total homocysteine concentrations and cognitive performance in older persons.[31] Therefore, disordered homocysteine metabolism might negatively influence some biological pathways in the brain and enhance the risk of dementia. In the Hordaland study, plasma homocysteine concentrations were shown to predict memory decline in elderly people within 6 years of follow-up.[32]
In the present study, an inverse relationship was observed between serum levels of vitamin D and homocysteine in dementia and MCI patients, which may imply role of vitamin D deficiency in the elevation of serum homocysteine concentrations. Various studies have depicted increased serum concentrations of homocysteine as a well-established marker for cognitive impairment, and hence as a diagnostic marker for dementia. Hence, this inverse correlation may be helpful in designing treatment to prevent or reverse cognitive decline in elderly population.
However, several limitation of this study must also be considered. First, the relatively small sample size in this study has limited the significance of statistical inference of the available data. The study could benefit from a larger sample size. Second, the study had not been able to exclude all possible confounding factors that may affect the participant's cognitive functioning, such as social activities, alcohol intake, smoking habit, mental activities, and many other factors that may had influenced participant's cognitive function. Furthermore, due to limited duration and budget of the project, we could not do the follow-up studies to assess difference between levels of vitamin D and homocysteine with relation to cognitive functioning at regular intervals for 2-3 years, which could have given more connotation to the study in the form of better understanding of the effect of vitamin D and homocysteine levels on cognitive decline.
Prediction and prevention of neurodegenerative processes are important for future prevention and management of other risk factors. In conclusion, the present study reveals that vitamin D deficiency and elevated homocysteine levels may be considered as early diagnostic markers of cognitive decline. A positive correlation between markers of cognitive decline and vitamin D levels depicts important role of vitamin D in maintaining normal cognition, whereas an inverse association between vitamin D and homocysteine levels suggests that vitamin D may aid in lowering elevated homocysteine levels, which is an established marker of cognitive impairment. Therefore, these findings potentially implicate role of vitamin D in neurocognitive function and may serve as a new and early marker for assessment of hyperhomocysteinemia which is a pivotal risk factor for dementia.
Acknowledgment
The authors wish to thank the Director and the Management of Sir H. N. Reliance Foundation Hospital and Research Centre for the necessary funds to carry out the project and the Scientific Committee for sanctioning this project.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
References
- 1.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A., 3rd High homocysteine and low B vitamins predict cognitive decline in aging men: The Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82:627–35. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 2.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–5. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 3.Drake VJ. Micronutrients and Cognitive Function. Research Newsletter-Spring/Summer 2011; Linus Pauling Institute, Oregon State University February. 2011 [Google Scholar]
- 4.McGrath J, Feron F, Eyles D, Mackay-Sim A. Vitamin D: The neglected neurosteroid? Trends Neurosci. 2001;24:570–2. doi: 10.1016/s0166-2236(00)01949-4. [DOI] [PubMed] [Google Scholar]
- 5.Marwaha RK, Tandon N, Reddy DR, Aggarwal R, Singh R, Sawhney RC, et al. Vitamin D and bone mineral density status of healthy school children in northern India. Am J Clin Nutr. 2005;82:477–82. doi: 10.1093/ajcn.82.2.477. [DOI] [PubMed] [Google Scholar]
- 6.Brøndum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M. 25-hydroxyvitamin D and symptomatic ischemic stroke: An original study and meta-analysis. Ann Neurol. 2013;73:38–47. doi: 10.1002/ana.23738. [DOI] [PubMed] [Google Scholar]
- 7.Annweiler C, Montero-Odasso M, Hachinski V, Seshadri S, Bartha R, Beauchet O. Vitamin D concentration and lateral cerebral ventricle volume in older adults. Mol Nutr Food Res. 2013;57:267–76. doi: 10.1002/mnfr.201200418. [DOI] [PubMed] [Google Scholar]
- 8.Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–41. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582–6S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 10.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alphahydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, et al. Vitamin D, cognition, and dementia: A systematic review and meta-analysis. Neurology. 2012;79:1397–405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Calle M, Usandizaga R, Sancha M, Magdaleno F, Herranz A, Cabrillo E. Homocysteine, folic acid and B-group vitamins in obstetrics and gynaecology. Eur J Obstet Gynecol Reprod Biol. 2003;107:125–34. doi: 10.1016/s0301-2115(02)00305-6. [DOI] [PubMed] [Google Scholar]
- 13.Applebaum J, Shimon H, Sela BA, Belmaker RH, Levine J. Plasma homocysteine levels in newly admitted schizophrenic patients. J Psychiatr Res. 2004;4:413–6. doi: 10.1016/j.jpsychires.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–55. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 15.Kriebitzsch C, Verlinden L, Eelen G, van Schoor NM, Swart K, Lips P, et al. 1,25-dihydroxyvitamin D3 influences cellular homocysteine levels in murineprosteoblastic MC3T3-E1 cells by direct regulation of cystathionine β-synthase. J Bone Miner Res. 2011;26:2991–3000. doi: 10.1002/jbmr.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werder SF. Cobalamin deficiency, hyperhomocysteinemia, and dementia. Neuropsychiatr Dis Treat. 2010;6:159–95. doi: 10.2147/ndt.s6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spering CC, Hobson V, Lucas JA, Menon CV, Hall JR, O’Bryant SE. Diagnostic accuracy of the MMSE in detecting probable and possible Alzheimer's disease in ethnically diverse highly educated individuals: An analysis of the NACC database. J Gerontol A Biol Sci Med Sci. 2012;67:890–6. doi: 10.1093/gerona/gls006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff-Radford NR, Petersen RC, et al. Detecting Dementia with the Mini-Mental State Examination (MMSE) in highly educated individuals. Arch Neurol. 2008;65:963–7. doi: 10.1001/archneur.65.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soni M, Kos K, Lang IA, Jones K, Melzer D, Llewellyn DJ. Vitamin D and cognitive function. Scand J Clin Lab Invest Suppl. 2012;243:79–82. doi: 10.3109/00365513.2012.681969. [DOI] [PubMed] [Google Scholar]
- 20.Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: A systematic review. Am J Clin Nutr. 2009;89:S1997–2008. doi: 10.3945/ajcn.2009.27230D. [DOI] [PubMed] [Google Scholar]
- 21.Oudshoorn C, Mattace-Raso FU, van der Velde N, Colin EM, van der Cammen TJ. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2008;25:539–43. doi: 10.1159/000134382. [DOI] [PubMed] [Google Scholar]
- 22.Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460:202–5. doi: 10.1016/j.abb.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 23.McCann JC, Ames BN. Is there convincing biological or behavioural evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 24.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: Preventing “D”ecline? Mol Aspects Med. 2008;29:415–22. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlögl M, Holick MF. Vitamin D and neurocognitive function. Clin Interv Aging. 2014;9:559–68. doi: 10.2147/CIA.S51785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizwicki MT, Menegaz D, Zhang J, Barrientos-Duran A, Tse S, Cashman JR, et al. Genomic andnon-genomic signalling induced by 1a, 25(OH)2-vitamin D3 promotes the recovery of amyloid-b phagocytosis by Alzheimer's disease macrophages. J Alzheimers Dis. 2012;29:51–62. doi: 10.3233/JAD-2012-110560. [DOI] [PubMed] [Google Scholar]
- 27.Dursun E, Gezen-Ak D, Yilmazer S. A new mechanism for amyloid-b induction of iNOS: Vitamin D-VDR pathway disruption. J Alzheimers Dis. 2013;36:459–74. doi: 10.3233/JAD-130416. [DOI] [PubMed] [Google Scholar]
- 28.Briones TL, Darwish H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J Neuroinflammation. 2012;9:244. doi: 10.1186/1742-2094-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolander-Gouaille C. The role of homocysteine in dementia. CLI April. 2004 [Google Scholar]
- 30.Selhub J, Bagley LC, Miller J, Rosenberg IH. B vitamins, homocysteine, and neurocognitive function in the elderly. Am J Clin Nutr. 2000;71:614S–20. doi: 10.1093/ajcn/71.2.614s. [DOI] [PubMed] [Google Scholar]
- 31.Seshadri S. Elevated plasma homocysteine levels: Risk factor or risk marker for the development of dementia and Alzheimer's disease? J Alzheimer's Dis. 2006;9:393–8. doi: 10.3233/jad-2006-9404. [DOI] [PubMed] [Google Scholar]
- 32.Nurk E, Refsum H, Tell GS, Engedal K, Vollset SE, Ueland PM, et al. Plasma total homocysteine and memory in the elderly: The Hordaland homocysteine study. Ann Neurol. 2005;58:847–57. doi: 10.1002/ana.20645. [DOI] [PubMed] [Google Scholar]


