Abstract
Introduction:
Microvascular anastomosis is a crucial procedure in replantation surgeries. Venous insufficiency is one of the most consistent cause of failure or re-exploration in these surgeries necessitating the use of venous grafts.
Materials and Methods:
We discuss our study of 9 such replantation surgeries executed in calendar year 2013-2014, including a double finger replantation done in the same patient having total amputation of 4 fingers of the same (right) hand, in which an arterial segment was used as a microvascular interposition graft for venous anastomosis. Out of these 9 surgeries, 3 were re-exploration procedures for venous compromise and 6 were successful primary replantations.
Results:
In all, 8 replants were successful and one failed due to arterial compromise.
Discussion:
In our experience and extensive review of the previously available literature, we would like to portray the advantages of arterial segments as microvascular grafts in replant surgeries. Specifically, in a crush amputation injury for which the use of a vascular interposition graft is being contemplated. If any other digit is also amputated and is unsuitable for replantation, it can act as a potential donor site to harvest the arterial segment. However, when dealing with single finger amputation, the surgeon must be confident about the single digital arterial anastomosis, before harvesting the second digital artery as a microvascular graft.
Conclusion:
In our study, we found the use of arterial grafts in microvascular anastomosis of veins advantageous, as arterial segments have better ability to resist spasm due to environmental changes, better pressure tolerance as compared to venous segments, and provide an appropriate calibre match and ease of harvest in the same operative field.
KEY WORDS: Arterial, grafts, microvascular, replantation
INTRODUCTION
Replantation surgery, especially involving fingers and even toes as a matter of fact, has become one of the emerging areas of expertise and a sub speciality in microvascular surgery. This is due to some very fundamental facts since such injuries are found in all spectra of population, be it factory workers, children, housewives and even in people engaging in modern sedentary lifestyles, and in both rural and urban set ups. Furthermore, losing a finger, apart from being a functional and aesthetic loss is a big social setback in childhood and even adulthood and more so amongst youngsters. No wonder the stakes to replace the lost finger (and even toes) have become very high.
Today in the age of microvascular surgery, these once mighty challenges have become a reality to a very large extent. Nonetheless, these surgeries are still vulnerable to complications, both intra and post operatively.
Replantation of the digit is a fairly straightforward yet intricate surgery which has to be meticulously planned. It is time bound and often demands the best of microvascular and innovative skills. The sequence of replantation, with of course some variations, involves — Bony fixation, tendon repair, nerve, venous and finally arterial repair. This sequence is often altered for surgical convenience and prioritised accordingly as per the injury.
Vascular grafts have been used in these surgeries quite frequently, both as primary procedures or in failed primary anastomoses on subsequent re-exploration.
Microvascular surgeons have traditionally believed in vein segments as both arterial and venous vascular grafts. Here, through this article, we portray the use of arterial segments as vascular interposition grafts for venous microvascular anastomosis.
MATERIALS AND METHODS
In our institute, we execute such surgeries on a routine basis. We discuss our study of 9 such replantation surgeries executed in the calendar year 2013-2014, including a double finger replantation done in the same patient who had total amputation of 4 fingers of the same (right) hand, in which an arterial segment was used as a microvascular interposition graft for venous anastomosis (Figures 1–8). Of the cases evaluated (fingers replanted), 6 fingers had a crush amputation injury, and 3 fingers had a clean cut amputation.
Figure 1.
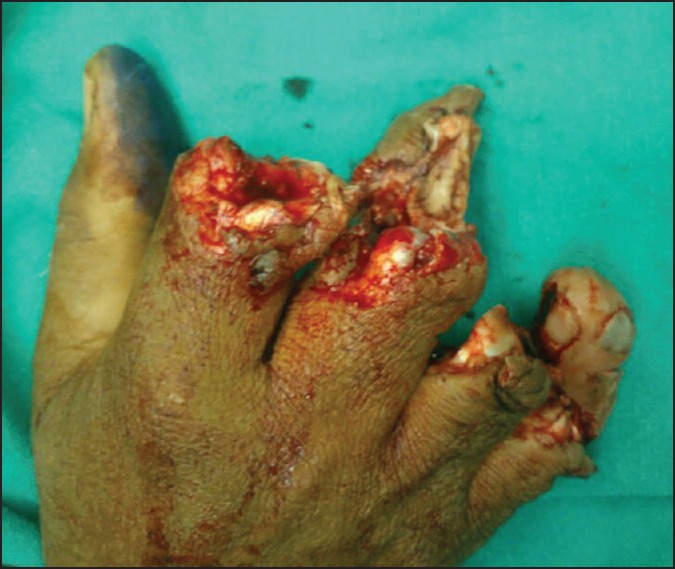
Crush amputation of four fingers of right hand-dorsal view
Figure 8.
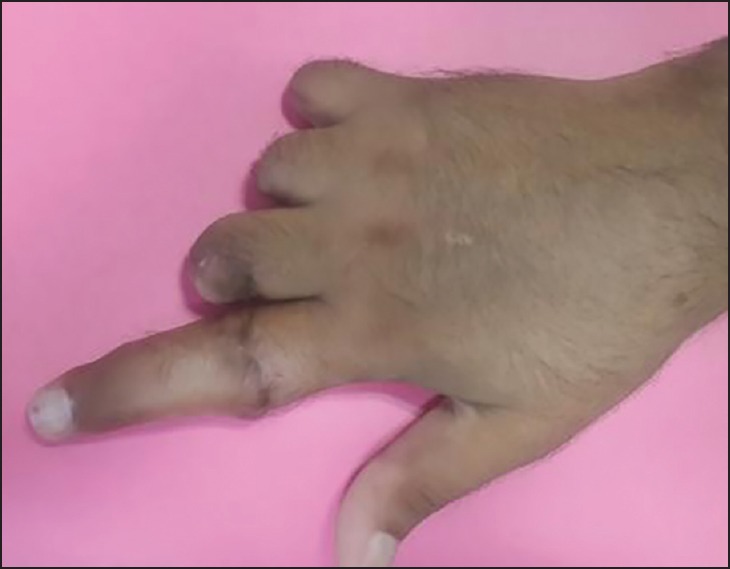
Dorsal view at 2 months
Figure 2.
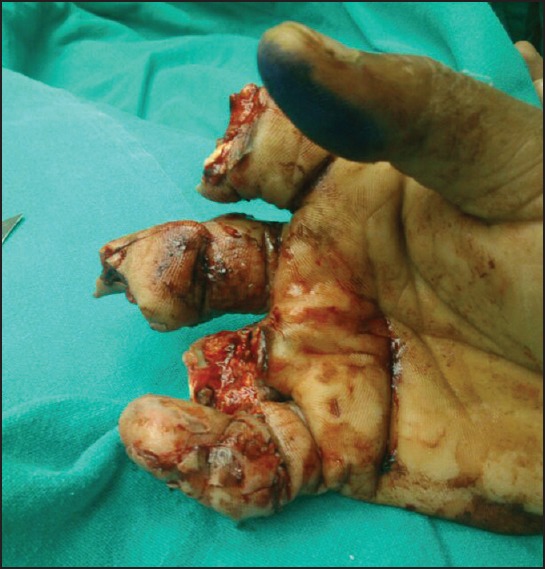
Ventral view of injured hand
Figure 3.
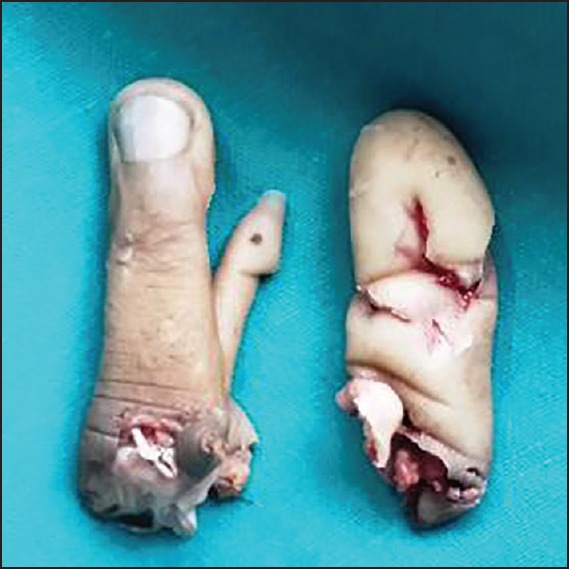
Amputated index and middle finger
Figure 4.
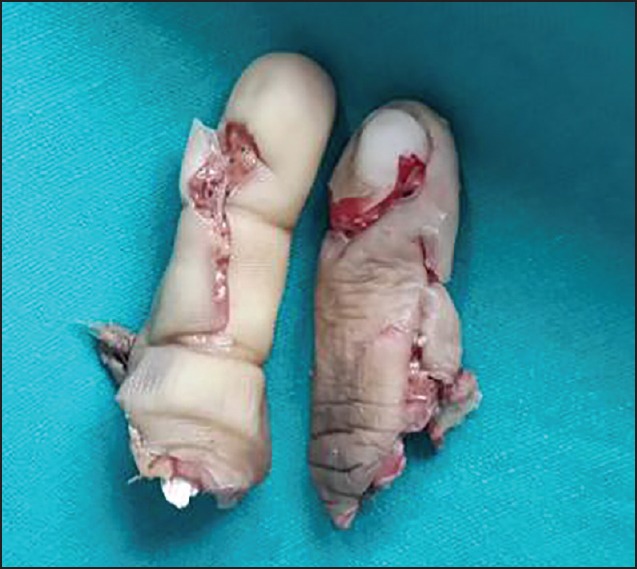
Amputated index finger having multiple fractures
Figure 5.
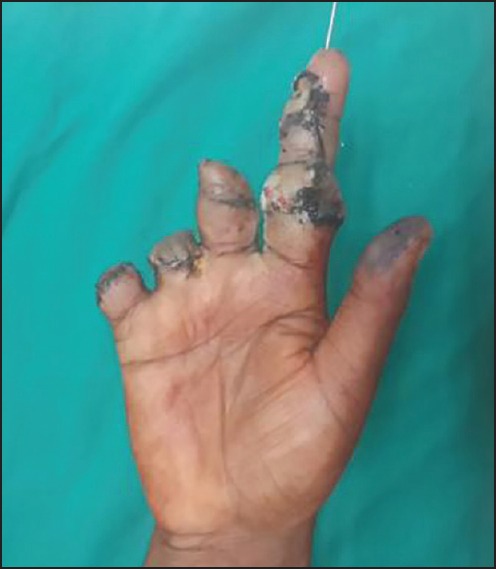
Replanted index finger at 1-week — ventral view, amputated part of middle finger replanted over stump of index finger
Figure 6.
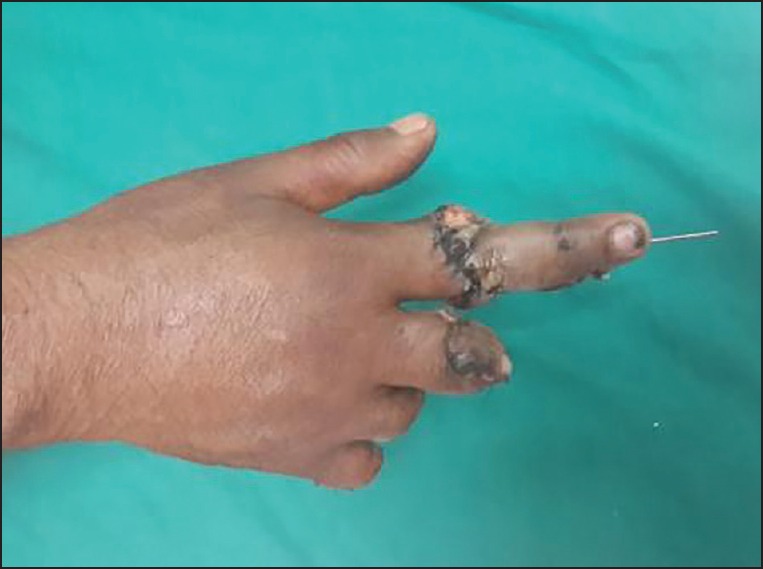
Replanted finger dorsal view
Figure 7.
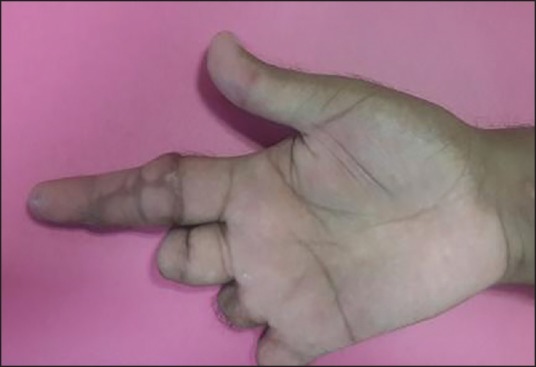
Replanted finger at 2 months — ventral view
Of the 8 patients, 5 were adults and 3 were children. All the cases were taken for surgery within 12 hours of the injury. Level of amputation was variable with the most distal most being as far as the distal interphalangeal joint and proximal up to the metacarpophalangeal joint.
The operative sequence followed was — Bony fixation with prior adequate shortening, flexor/extensor tendon repair, 2 dorsal digital veins and single digital artery repair followed by both digital nerve repairs.
We used an arterial segment as a microvascular graft varying from 5 mm to 12 mm in length. The microvascular arterial graft was harvested from the same (injured) finger in all these cases except the one with 4 finger amputation.
In the patient with 4 finger amputation, the arterial segment was harvested from one of the amputated digits which was not suitable for replantation as it had multiple fractures. However, under the microscope, we found a non-contused healthy appearing segment of the digital artery of about 8-10 mm length.
All replantation procedures were given post-operative dextran infusion (100 ml stat, followed by 40 microdrops/min) for 5 days and injection Fragmin 2500 IU after 6 hours, repeated 12 hourly for 3 days. Tablet aspirin 75 mg O.D. was started on 3rd post-operative day and continued for 4 weeks. The affected hand was kept elevated, warmed (under a blanket at room temperature) and was monitored clinically every 2 hours for the first 48 hours and 6 hourly thereafter for 5 days.
RESULTS
In our series of 9 replantation surgeries involving the use of arterial segments as microvascular grafts we made the following observations.
In six cases, primary arterial graft was used for venous anastomosis and successful repair was achieved, and in three cases, all having a crush component, microvascular interposition grafting was done at the time of re-exploration (all being due to venous insufficiency). Out of the 3 re-exploration procedures, 2 fingers were successfully salvaged while one could not be salvaged due to concomitant arterial compromise.
DISCUSSION
In our experience of replantation and revascularisation at our setup, venous insufficiency, both immediately and in the early postoperative period, has been one of the most consistent factors causing failure of these extensive procedures.
Venous compromise in such scenarios can be due to a number of reasons. Usually, the veins involved in such injuries, especially in distal amputations, amputations involving the little finger and those in young children are of very small calibre, which:
Are friable and unable to withstand anastomotic surgical insult.
Have a considerable segment of intimal injury due to the trauma per se due to their relatively superficial location.
Are unlike digital arteries, have no or minimal tunica media to maintain their patency.
Are superficial in location and undergo spasm easily due to increased temperature sensitivity.
Though replantation involves a lot of planning, no one can underrate the fact that the vascular anastomosis is the most critical and outcome defining part of these surgeries.
For obtaining a good vascular anastomosis, both the cut vascular ends should be revised till an uninjured healthy segment is obtained. This attempt often complicates the surgery as, adequate length of the vascular pedicle cannot be obtained, and further shortening of the finger is undesirable as it either defeats the functional or aesthetic outcome. Here comes the role of vascular grafts.
Artery versus vein graft
There is a large body of literature, which has demonstrated differences between venous and arterial grafts.
Veins are more susceptible to vasoactive substances than arteries.[1]
The venous wall is supplied by the vasa vasorum, whereas the arterial wall may be supplied through the lumen in addition to the vasa vasorum.[2].
The endothelium of arteries may secrete more endothelium-derived relaxing factor[3] and may release more nitric oxide (NO) and endothelium-derived hyperpolarising factor.[4,5]
The structure of the vein is subject to low pressure whereas that of the artery is subject to high pressure. After grafting, the venous grafts have to adapt to the higher pressure.
To better understand the biological behaviour of the grafts, their common features and their differences, a clinical classification may be useful for a practising surgeon. Based on experimental studies of their vaso reactivity combined with anatomical, physiological and embryological considerations, the following classification for arterial grafts has been described:
Type I: Somatic arteries;
Type II: Splanchnic arteries and
Type III: Limb arteries.
Type I arteries have enhanced endothelial function and release more NO and other relaxing factors. Type II arteries such as the gastroepiploic artery and Type III arteries such as the radial artery have higher pharmacological reactivity to vasoconstrictors [Figure 9].[6,7]
Figure 9.
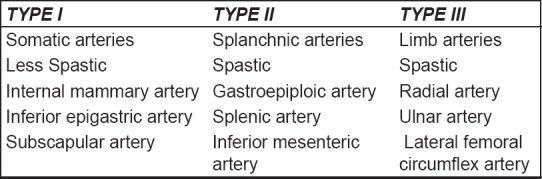
Classification of arterial vascular grafts
So Type II and III arteries are prone to spasms due to higher contractility.
Contractility and incidence of spasm
While the true cause of vasospasm remains unclear, vasospasm is presumed to be the extreme form of vasoconstriction response to stimuli (spasmogens). These stimuli may be physical (such as mechanical stimulation or temperature changes) or pharmacological (such as nerve stimulation or vasoconstrictor substances).[8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]
It has been suggested[8] that there are two types of vasoconstrictors that are important spasmogens for arterial grafts. Type I (endothelin, prostanoids [TxA2 and PGF2α] and α1-adrenoceptor agonists) are the most potent vasoconstrictors which strongly contract arterial grafts even when the endothelium is intact, while Type II vasoconstrictors (such as 5-hydroxytryptamine) only induce a weak vasoconstriction when the endothelium is intact. However, these vasoconstrictors probably play an important role in the spasm of arterial grafts if the endothelium is lost due to surgical handling.
Sources of microvascular arterial graft
The subscapular arterial tree may be used as a source of microvascular grafts to replace damaged or diseased portions of arteries, particularly in the hand and forearm. The probability of finding at least one usable terminal branch (1 mm diameter) that is at least 12.0 cm in length was found to be 98%.[25] Several other arteries have been used as donors of arterial grafts, popular ones being the dorsalis pedis and posterior interosseous arteries.[26]
The spasmogenic influences in the proximal and distal venous segments after an arterial interposition graft cannot be excluded, but it does not preclude the use and potential advantages of arterial microvascular grafts over venous segments.
CONCLUSION
In our experience, an anastomosis of 2 veins and single digital artery gives an adequate vasculature in digital replantation, so the second digital artery repair in replantation surgeries may be avoided whenever possible.[27] It is this artery that could well be used as vascular graft in failed and/or difficult venous anastomosis as it closely matches the recipient vessel calibre and can be harvested very conveniently in the same position without surgical exploration in any other virgin territory. Grafts for digital vessel repair taken from elsewhere fail to match the calibre of the recipient ends and confer another separate surgical exploration. Furthermore, an arterial segment used for interposition graft has better ability to resist spasm than venous segments, in response to environmental changes and better pressure tolerance as compared to venous segments.
To sum up, through this article, we highlight the potential advantages of using an arterial graft in microvascular anastomosis of veins in replant surgeries, specifically when there is associated crush injury to digits for which a small length of vascular interposition graft has to be used.
However, the condition of the arterial microvascular segment being considered as a vascular interposition graft harvested from a digit considered unsuitable for replantation has to be assessed intra-operatively and if found injured has to be done away with and one has to look for conventional virgin sites for vascular graft harvest.
Nonetheless, harvesting the digital artery from the digit not being replanted leaves us an option to repair both digital arteries in the replanted digit if need arises; this can facilitate simultaneous dissection which saves time which is all the more important in replantation procedures.
Of course, the surgeon has to be very sure of the single arterial anastomosis when dealing with single finger amputation before harvesting the contralateral digital artery for venous interposition graft.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.He GW, Angus JA, Rosenfeldt FL. Reactivity of the canine isolated internal mammary artery, saphenous vein, and coronary artery to constrictor and dilator substances: Relevance to coronary bypass graft surgery. J Cardiovasc Pharmacol. 1988;12:12–22. doi: 10.1097/00005344-198807000-00003. [DOI] [PubMed] [Google Scholar]
- 2.van Son JA, Smedts F, Vincent JG, van Lier HJ, Kubat K. Comparative anatomic studies of various arterial conduits for myocardial revascularization. J Thorac Cardiovasc Surg. 1990;99:703–7. [PubMed] [Google Scholar]
- 3.Lüscher TF, Diederich D, Siebenmann R, Lehmann K, Stulz P, von Segesser L, et al. Difference between endothelium-dependent relaxation in arterial and in venous coronary bypass grafts. N Engl J Med. 1988;319:462–7. doi: 10.1056/NEJM198808253190802. [DOI] [PubMed] [Google Scholar]
- 4.Liu ZG, Ge ZD, He GW. Difference in endothelium-derived hyperpolarizing factor-mediated hyperpolarization and nitric oxide release between human internal mammary artery and saphenous vein. Circulation. 2000;102:III296–301. doi: 10.1161/01.cir.102.suppl_3.iii-296. [DOI] [PubMed] [Google Scholar]
- 5.Zhang RZ, Yang Q, Yim AP, Huang Y, He GW. Different role of nitric oxide and endothelium-derived hyperpolarizing factor in endothelium-dependent hyperpolarization and relaxation in porcine coronary arterial and venous system. J Cardiovasc Pharmacol. 2004;43:839–50. doi: 10.1097/00005344-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 6.He GW, Yang CQ. Comparison among arterial grafts and coronary artery. An attempt at functional classification. J Thorac Cardiovasc Surg. 1995;109:707–15. doi: 10.1016/S0022-5223(95)70352-7. [DOI] [PubMed] [Google Scholar]
- 7.He GW. Arterial grafts for coronary artery bypass grafting: Biological characteristics, functional classification, and clinical choice. Ann Thorac Surg. 1999;67:277–84. doi: 10.1016/s0003-4975(98)01207-7. [DOI] [PubMed] [Google Scholar]
- 8.He GW, Yang CQ, Starr A. Overview of the nature of vasoconstriction in arterial grafts for coronary operations. Ann Thorac Surg. 1995;59:676–83. doi: 10.1016/0003-4975(94)01011-0. [DOI] [PubMed] [Google Scholar]
- 9.He GW, Rosenfeldt FL, Buxton BF, Angus JA. Reactivity of human isolated internal mammary artery to constrictor and dilator agents. Implications for treatment of internal mammary artery spasm. Circulation. 1989;80:I141–50. [PubMed] [Google Scholar]
- 10.Liu MH, Floten HS, Furnary AP, Yim AP, He GW. Inhibition of vasoconstriction by angiotensin receptor antagonist GR117289C in arterial grafts. Ann Thorac Surg. 2000;70:2064–9. doi: 10.1016/s0003-4975(00)01935-4. [DOI] [PubMed] [Google Scholar]
- 11.Wei W, Floten HS, He GW. Interaction between vasodilators and vasopressin in internal mammary artery and clinical significance. Ann Thorac Surg. 2002;73:516–22. doi: 10.1016/s0003-4975(01)03322-7. [DOI] [PubMed] [Google Scholar]
- 12.Luu TN, Dashwood MR, Chester AH, Tadjkarimi S, Yacoub MH. Action of vasoactive intestinal peptide and distribution of its binding sites in vessels used for coronary artery bypass grafts. Am J Cardiol. 1993;71:1278–82. doi: 10.1016/0002-9149(93)90540-s. [DOI] [PubMed] [Google Scholar]
- 13.Chen ZW, Yang Q, Huang Y, Fan L, Li XW, He GW. Human urotensin II in internal mammary and radial arteries of patients undergoing coronary surgery. Vascul Pharmacol. 2010;52:70–6. doi: 10.1016/j.vph.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Bai XY, Liu XC, Yang Q, Tang XD, He GW. The interaction between human urotensin II and vasodilator agents in human internal mammary artery with possible clinical implications. Ann Thorac Surg. 2011;92:610–6. doi: 10.1016/j.athoracsur.2011.03.094. [DOI] [PubMed] [Google Scholar]
- 15.He GW, Yang CQ. Radial artery has higher receptor-mediated contractility but similar endothelial function compared with mammary artery. Ann Thorac Surg. 1997;63:1346–52. doi: 10.1016/s0003-4975(97)00106-9. [DOI] [PubMed] [Google Scholar]
- 16.He GW, Shaw J, Hughes CF, Yang CQ, Thomson DS, McCaughan B, et al. Predominant alpha 1-adrenoceptor-mediated contraction in the human internal mammary artery. J Cardiovasc Pharmacol. 1993;21:256–63. [PubMed] [Google Scholar]
- 17.He GW, Yang CQ. Characteristics of adrenoceptors in the human radial artery: Clinical implications. J Thorac Cardiovasc Surg. 1998;115:1136–41. doi: 10.1016/S0022-5223(98)70414-3. [DOI] [PubMed] [Google Scholar]
- 18.Seo B, Oemar BS, Siebenmann R, von Segesser L, Lüscher TF. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation. 1994;89:1203–8. doi: 10.1161/01.cir.89.3.1203. [DOI] [PubMed] [Google Scholar]
- 19.Yildiz O, Ciçek S, Ay I, Tatar H, Tuncer M. 5-HT1-like receptor-mediated contraction in the human internal mammary artery. J Cardiovasc Pharmacol. 1996;28:6–10. doi: 10.1097/00005344-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 20.He GW, Yang CQ. Comparison of nitroprusside and nitroglycerin in inhibition of angiotensin II and other vasoconstrictor-mediated contraction in human coronary bypass conduits. Br J Clin Pharmacol. 1997;44:361–7. doi: 10.1046/j.1365-2125.1997.t01-2-00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He GW, Yang CQ. Effect of thromboxane A2 antagonist GR32191B on prostanoid and nonprostanoid receptors in the human internal mammary artery. J Cardiovasc Pharmacol. 1995;26:13–9. doi: 10.1097/00005344-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Liu JJ, Phillips PA, Burrell LM, Buxton BB, Johnston CI. Human internal mammary artery responses to non-peptide vasopressin antagonists. Clin Exp Pharmacol Physiol. 1994;21:121–4. doi: 10.1111/j.1440-1681.1994.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 23.Ochiai M, Ohno M, Taguchi J, Hara K, Suma H, Isshiki T, et al. Responses of human gastroepiploic arteries to vasoactive substances: Comparison with responses of internal mammary arteries and saphenous veins. J Thorac Cardiovasc Surg. 1992;104:453–8. [PubMed] [Google Scholar]
- 24.Mügge A, Barton MR, Cremer J, Frombach R, Lichtlen PR. Different vascular reactivity of human internal mammary and inferior epigastric arteries in vitro . Ann Thorac Surg. 1993;56:1085–9. doi: 10.1016/0003-4975(95)90020-9. [DOI] [PubMed] [Google Scholar]
- 25.Valnicek SM, Mosher M, Hopkins JK, Rockwell WB. The subscapular arterial tree as a source of microvascular arterial grafts. Plast Reconstr Surg. 2004;113:2001–5. doi: 10.1097/01.prs.0000122235.09892.da. [DOI] [PubMed] [Google Scholar]
- 26.Arnez ZM, Lister GD. The posterior interosseous arterial graft. Plast Reconstr Surg. 1994;94:202–6. doi: 10.1097/00006534-199407000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Lee BI, Chung HY, Kim WK, Kim SW, Dhong ES. The effects of the number and ratio of repaired arteries and veins on the survival rate in digital replantation. Ann Plast Surg. 2000;44:288–94. doi: 10.1097/00000637-200044030-00007. [DOI] [PubMed] [Google Scholar]


