Abstract
The current unprecedented outbreak of Ebola virus (EBOV) disease in West Africa has demonstrated the urgent need for a vaccine. Here, we describe the evaluation of an EBOV vaccine candidate based on Kunjin replicon virus-like particles (KUN VLPs) encoding EBOV glycoprotein with a D637L mutation (GP/D637L) in nonhuman primates. Four African green monkeys (Cercopithecus aethiops) were injected subcutaneously with a dose of 109 KUN VLPs per animal twice with an interval of 4 weeks, and animals were challenged 3 weeks later intramuscularly with 600 plaque-forming units of Zaire EBOV. Three animals were completely protected against EBOV challenge, while one vaccinated animal and the control animal died from infection. We suggest that KUN VLPs encoding GP/D637L represent a viable EBOV vaccine candidate.
Keywords: Ebola virus, Kunjin replicon, vaccine, nonhuman primates
Ebola virus (EBOV) is responsible for a severe form of viral hemorrhagic fever in humans, with case-fatality rates of up to 90%. Outbreaks of Ebola disease were first documented in 1976 in the African countries Zaire (now Democratic Republic of the Congo) and Sudan [1]. Since then, a number of outbreaks have been reported in central Africa, including recent outbreaks in Uganda (31 cases and 21 deaths) in 2012 and the Democratic Republic of the Congo in 2012 (57 cases and 29 deaths [2]) and 2014 (66 cases and 49 deaths [3]). The largest outbreak of Ebola disease ever recorded started in 2014 in West Africa and has so far yielded 22 057 cases (13 675 of which are laboratory confirmed) and 8795 deaths [4]. The unprecedented scale of the current Ebola disease outbreak has demonstrated the urgent need for an effective vaccine, particularly for Ebola disease crisis responders (eg, healthcare, sanitation, and mortuary workers; laboratory technicians; and contact tracers), who are at significantly higher risk of becoming infected. Other groups at increased risk of exposure include those sharing accommodation with known or suspected EBOV-infected individuals, as well as biomedical research scientists working with EBOV. Vaccination may also provide postexposure protection for contacts; such a capability would be invaluable for Ebola disease outbreak management [5].
EBOV is an enveloped virus with single-strand, negative-sense, nonsegmented RNA molecule encoding the following genes: 3′-NP-VP35-VP40-GP-VP30-VP24-L-5′. No vaccine for EBOV is currently licensed [1]. The most advanced EBOV vaccine candidates to date are (1) a recombinant chimpanzee adenovirus 3 vaccine encoding the EBOV glycoprotein (cAd3-EBOV-GP), which is being developed by GlaxoSmithKline and the National Institute of Allergy and Infectious Diseases and is currently being fast tracked through phase 1 clinical trials; and (2) a recombinant vesicular stomatitis virus (VSV) vaccine, in which the VSV G protein has been replaced with EBOV glycoprotein (rVSVΔG-EBOV-GP), that is currently being developed by NewLink Genetics and Public Health Agency of Canada, with phase 1 clinical trials soon to commence [6]. Both vaccines have demonstrated 100% efficacy in nonhuman primate studies. The cAd3-EBOV-GP (1010 plaque-forming units [PFU]) vaccine given intramuscularly protected 4 of 4 cynomolgus macaques (Macaca fascicularis) against challenge with 1000 PFU of Zaire EBOV 5 weeks after vaccination [7, 8]. rVSV-based vaccines against Zaire and Sudan EBOV and Marburg virus have also shown effective protection in nonhuman primate models [9]. A number of other preclinical vaccines have also been described, including inactivated virus, virus-like particles (VLPs) produced upon expression of VP40 and GP proteins, DNA vaccines expressing glycoprotein (GP) or nucleocapsid protein, and various recombinant viral and replicon vectors primarily expressing surface GP [1, 6]. Overall, vaccine studies so far indicate a critical role of antibodies in protection, with GP being the main inducer of protective antibodies in nonhuman primates [1, 6].
We have previously developed vaccine vectors based on RNA replicons of a naturally attenuated Australian strain of West Nile virus, Kunjin [10]. Kunjin replicons were constructed by deletion of viral structural genes from the Kunjin genome [10]. The lack of structural genes makes Kunjin replicon RNA incapable of productive infection, but capable of replicating and thus amplifying vaccine antigen gene(s) inserted in place of deleted structural genes. Packaging of replicon RNA into secreted VLPs significantly enhances replicon RNA transduction in vivo and thus vaccine efficacy [11]. In our previous study, a Kunjin replicon VLP expressing a D637L mutant of GP (KUN-VLP-GP/D637L) at a dose of 5 × 106 VLPs was shown to be effective in protecting 80% of guinea pigs against lethal challenge with guinea pig–adapted EBOV [12]. The D637L mutation promotes shedding of GP due to improved cleavage by TACE [13], thereby reducing cytotoxicity, which allowed manufacture of higher titers of VLPs in vitro and presumably also extended replicon-based GP expression in vivo [12].
In the current study, we extended our vaccine studies of KUN-VLP-GP/D637L to nonhuman primates, specifically African green monkeys (Cercopithecus aethiops) [14], a species that is much less aggressive than macaques and therefore presents a lower risk to investigators.
VLPs were prepared as described previously [12] and concentrated by centrifugation through Amicon Ultra centrifugal filter devices, with a 100 000 molecular weight cut off (Millipore, Billerica, Massachusetts), followed by partial purification through 15% sucrose cushion. Partially purified VLPs were resuspended in Dulbecco's modified Eagle's medium (DMEM), and VLP titers were determined by immunofluorescence staining assays, using anti-EBOV GP monoclonal antibody (FE37, Abcam) and Vero cells fixed and stained at 2 days after infection with 10-fold serial dilutions of VLPs [12]. Four 4–5-kg C. aethiops were vaccinated subcutaneously with 109 VLPs per animal in 2 mL of DMEM into 4 different sites. One control animal was injected with 2 mL of DMEM. Four weeks later, animals were vaccinated for a second time with the same dose of VLPs (or DMEM).
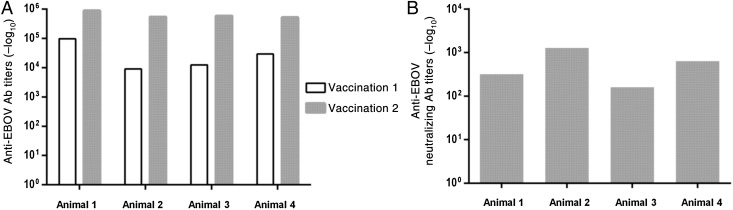
Four weeks after the first vaccination and 3 weeks after the second vaccination, serum specimens were collected and analyzed for anti-GP antibody responses, using an enzyme-linked immunosorbent assay with inactivated EBOV as antigen and horseradish peroxidase–conjugated mouse anti-human immunoglobulin G. End point titers were determined as the highest dilution showing an OD450 that was statistically different (mean ± 3 SD) from that of samples from the control animal [15]. All 4 animals vaccinated with KUN-VLP-GP/D637L developed a robust anti-GP antibody response, with titers ranging from 1:9100 (animal 2) to 1:98 300 (animal 1) after the first immunization and from 1:520 900 (animal 4) to 1:893 600 (animal 1) after the second immunization (Figure 1A).
Figure 1.
Induction of anti-Ebola virus (EBOV) antibodies (Abs) in immunized animals. A, Titers of anti-EBOV Abs determined by an enzyme-linked immunosorbent assay, using inactivated EBOV as antigen. B, Titers of EBOV-neutralizing Abs determined by a virus-neutralization assay.
Sera were also analyzed for EBOV-neutralizing antibodies, using microneutralization assays on Vero cells. Briefly, serum specimens collected from animals 3 weeks after the second immunization was were treated at 56°C for 30 minutes, serially diluted in DMEM with 2% fetal bovine serum, and incubated with 200 PFU of EBOV for 60 minutes at 37°C. The reaction mix was then used to infect Vero cells. Forty-eight hours after infection, cells were fixed and incubated with anti-GP mouse monoclonal antibodies, followed by incubation with horseradish peroxidase–conjugated rabbit anti-mouse antibodies and staining with AEC substrate (Sigma). Positively stained foci were counted, and neutralization titers were determined as the dilution reducing the amount of stained cells by 50%, compared with the amount in a serum specimen from the unimmunized animal. Neutralizing titers after the second vaccination ranged from 1:160 to 1:1280 (Figure 1B).
Three weeks after the second vaccination, animals were challenged intramuscularly with 600 PFU of Zaire EBOV strain Mayinga passaged once in an African green monkey, amplified, and titrated on Vero cells. The temperature of infected animals was measured every second day, and serum specimens were collected on days 4 and 6 after infection to analyze EBOV titers, using plaque assays on Vero cells.
Vaccinated animals 2 and 3 showed no significant increase in temperature throughout the experiment, and vaccinated animal 1 showed a slight increase in temperature on day 12 after challenge, which returned to normal the next day (Figure 2A). These 3 animals also showed no detectable EBOV in serum specimens obtained after challenge (Figure 2B) and were protected against EBOV challenge (Figure 2A). Vaccinated animal 4 unexpectedly developed an elevated temperature on day 6 after challenge and died on day 7 (Figure 2A), despite high antibody titers that were similar in magnitude to those seen in immunized animals 1–3 (Figure 1). The control animal showed elevated serum EBOV titers on days 4 and 6 (Figure 2B), had an increased temperature on day 10, and died on day 13 (Figure 2A), somewhat later than might be expected. Conceivably, the outbred nature of primates used in the experiment may account for observed variations in the response to immunization and to infection, with variable innate responses potentially involved [16].
Figure 2.
Monitoring of animals after challenge with Ebola virus (EBOV). A, Body temperature measurements of EBOV-infected animals. The dagger indicates time of death. B, Titers of EBOV detected in serum on days 4 and 6 after infection. Abbreviations: PFU, plaque-forming units; SD, standard deviation.
This first nonhuman primate study using the KUN-VLP-GP/D637L vaccine thus illustrated that 2 immunizations with 109 VLPs can protect 75% of nonhuman primates against challenge with 600 PFU of the Zaire strain of EBOV. Our previous studies in guinea pigs showed a significant improvement in protection when the dose of VLPs was increased 5-fold [12]. Studies using Venezuelan equine encephalitis virus replicon VLPs in nonhuman primates also showed substantial improvement in protection when the VLP dose was increased, with 1010 VLPs required to induce 100% protection [17]. These latter studies might suggest that increasing the dose of the KUN-VLP-GP/D637L vaccine would also improve protection and, perhaps, also eliminate the need for a second vaccination.
In summary, the study presented herein suggests the KUN-VLP-GP/D637L vaccine represents a viable EBOV vaccine candidate, with further studies in nonhuman primates with higher doses of VLPs and larger number of animals warranted.
Notes
Financial support. This work was supported by the National Institutes of Health (exploratory/development grant R21 AI053579).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Marzi A, Feldmann H. Ebola virus vaccines: an overview of current approaches. Expert Rev Vaccines 2014; 13:521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. http://www.who.int/mediacentre/factsheets/fs103/en/ .
- 3. http://www.cdc.gov/vhf/ebola/outbreaks/drc/2014-august.html .
- 4.http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Accessed 28 January 2015.
- 5.Geisbert TW, Daddario-DiCaprio KM, Williams KJ, et al. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J Virol 2008; 82:5664–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanapathipillai R, Restrepo AM, Fast P, et al. Ebola Vaccine - An Urgent International Priority. N Engl J Med 2014; 371:2249–51. [DOI] [PubMed] [Google Scholar]
- 7.Geisbert TW, Geisbert JB, Leung A, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J Virol 2009; 83:7296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–9. [DOI] [PubMed] [Google Scholar]
- 9.Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis 2011; 204(suppl 3):S1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pijlman GP, Suhrbier A, Khromykh AA. Kunjin virus replicons: an RNA-based, non-cytopathic viral vector system for protein production, vaccine and gene therapy applications. Expert Opin Biol Ther 2006; 6:135–45. [DOI] [PubMed] [Google Scholar]
- 11.Harvey TJ, Liu WJ, Wang XJ, et al. Tetracycline-inducible packaging cell line for production of flavivirus replicon particles. J Virol 2004; 78:531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynard O, Mokhonov V, Mokhonova E, et al. Kunjin virus replicon-based vaccines expressing Ebola virus glycoprotein GP protect the guinea pig against lethal Ebola virus infection. J Infect Dis 2011; 204(suppl 3):S1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolnik O, Volchkova V, Garten W, et al. Ectodomain shedding of the glycoprotein GP of Ebola virus. EMBO J 2004; 23:2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis KJ, Anderson AO, Geisbert TW, et al. Pathology of experimental Ebola virus infection in African green monkeys. Involvement of fibroblastic reticular cells. Arch Pathol Lab Med 1997; 121:805–19. [PubMed] [Google Scholar]
- 15.Ksiazek TG, West CP, Rollin PE, Jahrling PB, Peters CJ. ELISA for the detection of antibodies to Ebola viruses. J Infect Dis 1999; 179(suppl 1):S192–8. [DOI] [PubMed] [Google Scholar]
- 16.Smith LM, Hensley LE, Geisbert TW, et al. Interferon-beta therapy prolongs survival in rhesus macaque models of Ebola and Marburg hemorrhagic fever. J Infect Dis 2013; 208:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbert AS, Kuehne AI, Barth JF, et al. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. J Virol 2013; 87:4952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]