Abstract
In addition to its surface glycoprotein (GP), Ebola virus directs the production of large quantities of a truncated glycoprotein isoform (sGP) that is secreted into the extracellular space. We recently reported that sGP actively diverts host antibody responses against the epitopes that it shares with GP and thereby allows itself to absorb anti-GP antibodies, a phenomenon we termed “antigenic subversion.” To investigate the effect of antigenic subversion by sGP on protection against virus infection, we compared immune responses induced by different prime-boost immunization regimens with GP and sGP DNA vaccines in mice and their efficacy against lethal Ebola virus challenge. Similar levels of anti-GP antibodies were induced by 2 immunizations with sGP and GP DNA vaccines. However, 2 immunizations with GP but not sGP DNA vaccine fully protected mice from lethal challenge. Boosting with sGP or GP DNA vaccine in mice that had been primed by GP or sGP DNA vaccine augmented the levels of anti-GP antibody responses and further improved protective efficacy against Ebola virus infection. These results show that both the quality and the levels of anti-GP antibody responses affect the efficacy of protection against Ebola virus infection.
Keywords: Ebola, vaccine, soluble GP, antigenic subversion
Since their first identification during the Ebola virus (EBOV) outbreak in 1976 in Zaire, 5 different EBOV species, including Zaire (ZEBOV), Sudan (SEBOV), Bundibugyo (BEBOV), Tai Forest, and Reston, have been isolated from outbreaks in humans and nonhuman primates (NHPs), and their amino acid sequences differ by as much as 40% [1]. Among them, ZEBOV, SEBOV, and BEBOV have caused large human outbreaks with high fatality rates, ranging from 20% to 90% [1–4]. Of particular concern, human outbreaks of EBOV infection have become increasingly frequent in recent years [5, 6], and the current ZEBOV outbreak, which has caused >24 000 human infections and close to 10 000 deaths as of 11 March 2015, once again demonstrates that its serious threat to public health is real and imminent. A number of vaccine strategies are under development, and at least 6 vaccine approaches, including recombinant adenovirus replicons [7], recombinant vesicular stomatitis virus (VSV) [8], recombinant parainfluenza virus [9], recombinant virus-like replicon particles [10], recombinant rabies virus [11], and protein-based virus-like particles (VLPs) [12, 13], have been demonstrated to protect against EBOV infection in both small-animal models, such as mice and guinea pigs, and NHPs.
The ability to develop a vaccine is critically dependent on our understanding of the mechanisms by which EBOV suppresses, distracts, or otherwise evades the host immune response [14]. The studies using different vaccine platforms in NHPs have shown that protection is invariably correlated with serum antibody levels against the viral membrane glycoprotein (GP) [15, 16]. Further, recent studies showed that passive transfer of purified immunoglobulin G (IgG) from convalescent NHP sera or a combination of 3 mouse monoclonal antibodies protected recipient NHPs against EBOV infection [17, 18]. It has also been shown that, after vaccination of NHPs with a recombinant VSV GP vaccine, depletion of CD8+ T cells prior to vaccination did not affect protective efficacy against EBOV challenge, whereas depletion of B cells or CD4+ T cells prior to vaccination impaired induction of antibody responses to GP and abrogated protection against EBOV infection [19]. These results indicate that antibody responses against GP may play an important role in mediating protection against EBOV infection. EBOV GP forms trimeric spikes on virion surfaces, similar to the influenza virus hemagglutinin and human immunodeficiency virus envelope (Env) proteins [20]. An unusual feature of EBOV GP biosynthesis is its separation into 2 disjointed reading frames, which are joined together by slippage of the viral polymerase at an editing site to generate a messenger RNA (mRNA) transcript that directs synthesis of GP [21–23]. However, only about 20% of the mRNA transcripts are edited. The remaining unedited transcripts (80%) have a premature stop codon, resulting in synthesis of a truncated GP product (sGP) that forms homodimers and is secreted in large quantities into the extracellular space [23]. In addition, it has also been reported that passage of ZEBOV in Vero cells or Thp1 cells leads to mutant viruses with mutated viral genomic RNA, which directs transcription of mRNA for GP as a primary product [24, 25]. However, it was further demonstrated that, during infection of guinea pigs, the mutant viruses quickly revert back to the wild-type viral genomic RNA that directs transcription of mRNA for sGP as a primary product [25]. Thus, while synthesis of GP may be achieved through different mechanisms, it is clear that the sGP is the major glycoprotein product during in vivo ZEBOV infection. We recently reported a mechanism of ZEBOV immune invasion in which production of sGP by ZEBOV could potentially subvert induction of antibody responses against GP by preferentially stimulating expansion of B cells that produce antibodies more reactive to sGP, thereby enabling sGP to absorb such antibodies [26]. We termed our observation “antigenic subversion,” which is distinct from a simple mechanism of passive absorption by sGP for anti-GP antibodies. In extension of our previous findings, we investigated immune responses induced by immunization with sGP and GP DNA vaccines and determined their efficacy for protection against ZEBOV infection in the present study.
METHODS
Virus and Biosafety
Mouse-adapted ZEBOV stock was propagated in Vero E6 cells. All experiments involving infectious ZEBOV were performed at the biosafety level 4 (BSL-4) facility at the Texas Biomedical Research Institute (TxBiomed; San Antonio, Texas).
Cell Lines and Plasmids
293 T cells and JC53 cells were maintained in Dulbecco's modified Eagle's medium (Mediatech) supplemented with 10% fetal bovine serum (Hyclone, ThermoFisher) and penicillin/streptomycin. The DNA constructs expressing ZEBOV GP and sGP proteins have been described in previous studies [26]. For large-scale preparation, the plasmids were amplified in Escherichia coli DH5α and purified with a Qiagen Endo-Free Megaprep kit. The plasmids were then resuspended at 1 µg/µL in sterile phosphate-buffered saline (PBS) and stored at −80°C until used for immunization.
Immunization of Mice, Sample Collection, and Challenge
Female BALB/c mice (6–8 weeks old) were purchased from Charles River Laboratory and housed at the Emory University animal facility (Atlanta, Georgia) or the TxBiomed animal BSL-4 facility. All animal studies were performed in accordance with approved institutional animal care and use committee protocols at Emory University and the TxBiomed. Immunization of mice was performed by intramuscular injection of 50 µg of DNA vaccine dissolved in 100 µL of PBS into 1 quadriceps muscle, followed by injection of the same formulation in the opposite quadriceps muscle 4 weeks later. Blood samples were collected from the retro-orbital sinus under anesthesia at 2 weeks after each immunization and were stored at −80°C until analysis.
Lethal ZEBOV challenge studies were performed in the animal BSL-4 facility at TxBiomed. After the final immunization, mice were challenged by intraperitoneal injection with 1000 plaque-forming units (approximately 30 000 50% lethal doses) of mouse-adapted ZEBOV diluted in PBS. After challenge, mice were monitored daily for weight changes and signs of disease.
Enzyme-Linked Immunosorbent Assay (ELISA)
ZEBOV GP–specific antibodies were measured in individual mouse serum samples by ELISA, using established protocols [26–28]. Briefly, the assays were performed in a 96-well plate coated overnight at 4°C with purified histidine-tagged GP at a concentration of 2 µg/mL. Serial dilutions of serum samples were incubated at room temperature for 2 hours on coated and blocked ELISA plates, and the bound immunoglobulins were detected with horseradish peroxidase–conjugated goat anti-mouse IgG secondary antibodies (Southern Biotechnology Associates). The wells were developed with tetramethylbenzidine (Sigma). The color reaction was stopped with hydrochloric acid (0.2 N), and the absorbance at 450 nm was determined by an ELISA reader. A standard curve was constructed by coating each ELISA plate with serial 2-fold dilutions of purified mouse IgG with known concentrations, and the concentrations of GP-specific antibodies in serum samples were calculated using obtained standard curves and expressed as the weight of antigen-specific antibody per volume of serum sample.
Pseudovirion Neutralization Assay
Neutralizing antibodies against ZEBOV GP were analyzed by means of a single-round infectivity assay that we used in our previous studies [28]. Briefly, 293T-cells were cotransfected with Env-defective HIV backbone and ZEBOV GP in pCAGGS vector, using Fugene HD (Roche). Supernatants were harvested 48 hours after transfection, clarified, and filtered using a 0.45-μm-pore filter. Pseudoviruses were titered by infecting JC53 cells [29], which express β-galactosidase and luciferase under a tat-activated promoter, causing infected cells turning blue with X-Gal staining. Neutralization assays were performed as described elsewhere [28], with minor modifications. Briefly, pseudoviruses were preincubated with dilutions of heat-inactivated antisera and supplemented with heat-inactivated naive mouse sera (Innovative Research) so that 5% of the total volume was mouse serum. Pseudovirus-antiserum mixtures were then added to 30% confluent JC53 cells and incubated for 48 hours. Virus infection and neutralization was measured by a luciferase reporter assay, and neutralization was measured as the decrease in luciferase expression versus that for virus-only controls [29]. Neutralizing activity is expressed as the percentage reduction of virus titers in sample wells, compared with titers in control wells without mouse sera: [(virus titer in control well-virus titer in sample well)/(virus titer in control well)] × 100%.
RESULTS
Immunization Study Design
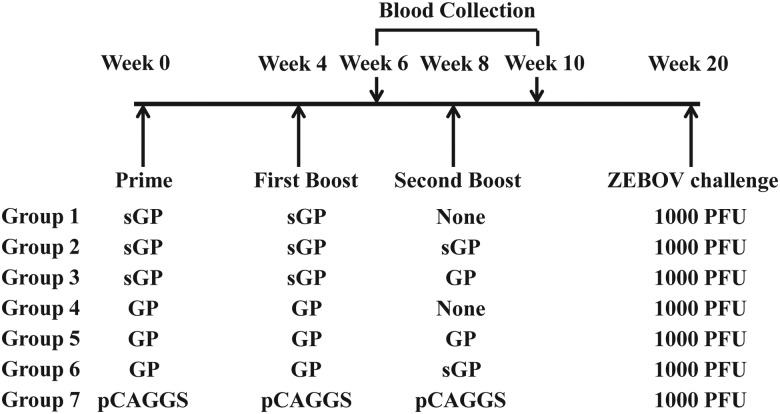
The results from our recent studies indicate that anti-GP antibody responses induced by ZEBOV GP and sGP DNA vaccines are qualitatively different with respect to their cross-reactivity to sGP [26]. In the present study, we sought to investigate whether immune responses induced by GP or sGP DNA immunizations might exhibit different efficacies for protection against ZEBOV infection. As outlined in Figure 1, 7 groups of mice (5 per group) were used in this study. Group 1 received 2 immunizations with sGP DNA vaccine, group 2 received 3 immunizations with sGP DNA vaccine, and group 3 received 2 immunizations with sGP DNA vaccine and then a boosting immunization with GP DNA vaccine. On the other hand, group 4 received 2 immunizations with GP DNA vaccine, group 5 received 3 immunizations with GP DNA vaccine, and group 6 received 2 immunizations with GP DNA vaccine and then a boosting immunization with sGP DNA vaccine, for comparing with groups 1, 2, and 3, respectively. The control group (group 7) received 3 immunizations with the empty plasmid DNA vector pCAGGS. Immunizations were performed by intramuscular injection of 50 µg of DNA vaccines at 4-week intervals, and blood samples were collected 2 weeks after the second and third immunizations for analysis of antibody responses.
Figure 1.
Schematic diagram of immunization and challenge study design. Female Balb/c mice (groups of 5) were vaccinated at 4-week intervals with different immunization regimens. Blood samples were collected 2 weeks after the second and third immunizations, and mice were challenged 12 weeks after the final immunization by 1000 plaque-forming units (PFU) of mouse-adapted Ebola virus (EBOV). Abbreviations: GP, glycoprotein; sGP, truncated Ebola virus glycoprotein isoform.
Characterization of Antibody Responses Induced by Different Prime-Boost Immunization Regimens With GP and sGP DNA Vaccines
Serum samples collected after the second and third immunizations were analyzed for the levels of anti-GP and sGP antibody responses by ELISA. As shown in Figure 2A, similar levels of anti-GP antibodies were induced in mice after 2 immunizations with GP or sGP DNA vaccine. Further, a third immunization with sGP or GP DNA vaccine augmented the levels of anti-GP antibodies in mice that had been primed by sGP or GP DNA vaccine. The highest levels of anti-GP antibody response were detected in group 5 mice, which received 3 immunizations with GP DNA vaccine. In contrast, while sGP DNA vaccine also induced high levels of anti-sGP antibodies, GP DNA vaccine only induced low levels of anti-sGP antibodies, even after 3 immunizations (Figure 2B). Nonetheless, levels of anti-sGP antibodies were effectively boosted by sGP DNA vaccine in mice that had been primed by GP DNA vaccine (group 6), and boosting with GP DNA vaccine in mice that had been primed by sGP DNA vaccine also augmented the levels of anti-sGP antibodies to higher levels (group 3).
Figure 2.
Antibody response induced by different regimens of Ebola virus (EBOV) glycoprotein (GP) and truncated EBOV GP isoform (sGP) DNA vaccines. Groups of mice were vaccinated by intramuscular injection with 50 µg of DNA (25 µg/leg) according to the schedule shown in Figure 1. A, Antibody response against GP. B, Antibody response against sGP. The levels of antibody response induced by EBOV GP DNA constructs in mice were measured by an enzyme-linked immunosorbent assay, using purified histidine-tagged GP or sGP proteins as coating antigen. The antibody concentration was determined from a standard curve. Results are mean values and standard deviations for samples from individual animals of each group. Abbreviation: IgG, immunoglobulin G.
Serum samples collected after the third immunization were also analyzed for their neutralizing activity against ZEBOV GP–mediated pseudovirion infection, to determine whether homologous or heterologous boosting with sGP or GP DNA vaccines may further augment neutralizing antibody responses. As shown in Figure 3, similar levels of neutralizing activities were detected in sera from mice that had received 2 immunizations with sGP or GP DNA vaccine (groups 1 and 4, respectively). However, additional boosting by the same sGP or GP DNA vaccine only increased sera neutralizing activity slightly (groups 2 and 5, respectively). On the other hand, additional boosting by the different DNA vaccine did not further enhance serum neutralizing activities (group 3 and group 6). Nevertheless, the differences in the neutralizing activity of sera from these different groups were not statistically significant.
Figure 3.
Neutralization of Ebola virus (EBOV) glycoprotein (GP)–mediated pseudovirion infection. Neutralizing activity of immune sera collected after the third immunization was determined by incubating 500 plaque-forming units (PFU) of GP-pseudotyped virus with a 1:900 dilution of serum samples from mice that received different immunization regimens, shown in Figure 1. Neutralization was measured as the decrease in luciferase expression, compared with virus-only controls, after 48 hours, as described in “Materials and Methods” section. Results reported are means and standard deviations for samples from individual animals of each group.
Protective Efficacy of Different Immunization Regimens Against Lethal ZEBOV Challenge
After the final immunization and blood sample collection, mice were sent to the TxBiomed and challenged by intraperitoneal injection of 1000 plaque-forming units of mouse-adapted ZEBOV 12 weeks after the third immunization in BSL-4 animal facilities. Mice were monitored daily for 21 days after challenge to record weight changes, disease symptoms, and survival rates. As presented in Table 1, all control group mice (group 7) died by day 5 after challenge. Only 1 of 5 mice in group 1 that received 2 immunizations with sGP DNA survived the challenge, whereas 3 mice died on day 5 after challenge and 1 mouse died on day 8 after challenge, with a slightly delayed mean time to death (5.3 days), compared with that for the control group. All mice in other vaccinated groups survived the challenge. However, weight loss and illness were observed in groups 2, 3, and 4. In comparison, mice in groups 5 and 6 remained healthy throughout the challenge period.
Table 1.
Protective Efficacy Against Lethal Challenge by Mouse-Adapted Ebola Virus
| Group | Immunization Regimen | Animals With Weight Loss, No.a (n = 5) | Animal With Illness, No.b (n = 5) | Animals That Survived, No.c (n = 5) |
|---|---|---|---|---|
| 1 | sGP DNA (2 doses) | 5 | 5 | 1 |
| 2 | sGP DNA (3 doses) | 1 | 5 | 5 |
| 3 | sGP DNA (2 doses) + GP DNA (1 dose) | 2 | 5 | 5 |
| 4 | GP DNA (2 doses) | 2 | 4 | 5 |
| 5 | GP DNA (3 doses) | 0 | 0 | 5 |
| 6 | GP DNA (2 doses) + sGP DNA (1 dose) | 0 | 0 | 5 |
| 7 | pCAGGS (3 doses) | 5 | 5 | 0 |
Abbreviations: GP, Ebola virus glycoprotein; sGP, truncated Ebola virus glycoprotein isoform.
a Weight changes were monitored daily after challenge. Weight loss was defined a decrease of >5% in body weight from day 0.
b Mice were monitored after challenge for disease signs, such as ruffled fur or hunched back, or lack of activity. They were considered to be ill if any of these symptoms were observed.
c Mice that exhibited severe disease symptoms or a body weight loss of >25% from day 0 were euthanized in accordance with institutional animal care and use committee guidelines.
DISCUSSION
The mechanism for immune protection against EBOV has not been clearly defined. Nonetheless, evidence suggests that induction of a strong antibody response against GP likely plays an important role on protection against EBOV infection. A number of studies have shown that successful protection against EBOV infection in NHPs can be achieved by vaccines that target the EBOV GP antigen alone [8, 10, 11, 30, 31]. Moreover, analysis of EBOV vaccine efficacy in NHPs indicates that effective protection correlates with the levels of anti-GP antibodies induced by vaccination [15, 16]. Consequently, most vaccine strategies focus on inducing immune responses against GP for protection against EBOV infection. We showed in recent studies that the ZEBOV sGP, which is the main viral GP product and is efficiently secreted during virus infection, could profoundly modulate the profiles of anti-GP antibody responses induced by vaccination [26]. In this study, we further investigated the effect of modulation of anti-GP antibody responses by sGP on protection against lethal challenge by ZEBOV, and our results indicate that both the quality and levels of anti-GP antibody responses may affect the efficacy of protection.
Comparison of immune response induced by immunization with sGP or GP DNA vaccines showed that similar levels of anti-GP antibody responses, as well as neutralizing activity against GP-mediated pseudovirion infection, were induced after 2 immunizations. However, only 1 of 5 mice that received 2 immunizations with sGP DNA survived lethal ZEBOV challenge, whereas all 5 mice that received 2 immunizations with GP DNA survived the challenge. These results indicate that, despite induction of similar levels of antibody responses to GP, the immune responses induced by sGP or GP DNA vaccines are not equally protective against ZEBOV infection. On the other hand, it was also observed that an additional boosting immunization with either sGP or GP DNA vaccine effectively augmented anti-GP antibody responses to higher levels in mice that had received 2 immunizations with the sGP DNA vaccine and protected these mice against lethal ZEBOV challenge. This observation is in agreement with a previous study that showed protection of guinea pigs against ZEBOV infection by immunization with an sGP DNA vaccine [32], indicating that protection against ZEBOV infection can be obtained by immunization with the sGP DNA vaccine in small-animal models. Moreover, an additional boosting immunization by the sGP DNA vaccine in mice that had received 2 immunizations with the GP DNA vaccine was shown to augment anti-GP antibody responses but not neutralizing activities to higher levels, and the vaccinated mice were effectively protected against ZEBOV infection, with no disease symptoms or weight loss. Taken together, these results show that the qualitative limitation of sGP-induced immune responses may be overcome by induction of higher levels of anti-GP antibody responses and that induction of higher levels of anti-GP antibodies by sGP or GP DNA vaccine correlates with improved protection against lethal EBOV challenge. Nonetheless, protection against ZEBOV infection requires the induction of a higher level of anti-GP antibody response by sGP DNA vaccine than by GP DNA vaccine. We have shown in previous studies that antibody responses induced by sGP and GP DNA vaccines are qualitatively different, with antibodies induced by sGP DNA exhibiting preferential reactivity to sGP [26]. It is possible that sGP–cross-reactive antibodies induced by sGP DNA vaccines are more readily absorbed by sGP proteins produced during ZEBOV infection, and as a result it will require the presence of a larger amount of such antibodies to control virus infection.
In summary, the present results provide further support for the correlation between protection against ZEBOV infection and levels of anti-GP antibody responses induced by each vaccine. We have also shown in previous studies that immunization of mice with 2 different doses of ZEBOV VLPs induced different levels of antibodies against GP, and vaccination of mice with the high dose ZEBOV VLP vaccine induced higher levels of antibodies against GP and protected mice against lethal ZEBOV challenge [27]. Of note, immunization with the low-dose ZEBOV VLP in mice induced levels of anti-GP antibodies comparable to those induced by 2 immunizations with the GP DNA vaccine in the present study but failed to protect mice from lethal ZEBOV challenge. Similar observations have also been made in other studies comparing protective efficacies of different vaccine approaches. In an early study, it was observed that immunization with ZEBOV VLPs but not inactivated ZEBOV virions conferred complete protection against lethal EBOV challenge in mice, despite induction of a similar level of antibody responses against GP by both vaccines [33]. Further, studies with recombinant adenovirus replicon–based vaccines showed that more-effective protection against ZEBOV infection was achieved in NHPs by immunization with a replicon expressing membrane-bound GP than by immunization with a replicon expressing a transmembrane domain–truncated GP [31]. A more recent study with a recombinant rabies virus vaccine also showed that, while similar levels of antibody responses were induced by 2 immunizations with the live virus vaccine or 2 immunizations with the inactivated recombinant rabies virus vaccine, complete protection of NHPs was achieved only by immunization with the live virus vaccine [11]. Thus, the mechanism of immune protection against EBOV infection is likely to be complex, and the correlates for protection may vary for different vaccine approaches. Together, the results from these studies indicate that a certain threshold of immune response, with contributions from both antibody and T-cell responses, will be required for achieving an effective protection against EBOV infection. However, as each vaccine approach will be different with respect to its ability to induce antibody or T-cell responses, such a threshold will be different for different vaccine approaches. On the other hand, the levels of antibody responses against GP by each vaccine approach represent an important indicator for the overall immune responses induced by vaccination and therefore may serve as a useful correlate for immune protection against EBOV infection. Furthermore, future studies to define the targets of protective antibody responses induced by sGP and GP DNA vaccines, as well as their required dosages for protection against EBOV infection, will also advance our understanding of the critical role of antibody response for protection against EBOV infection and provide valuable information for development of more effective EBOV vaccines.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant AI093406).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Towner JS, Sealy TK, Khristova ML, et al. Newly discovered Ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog 2008; 4:e1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldmann H, Volchkov VE, Volchkova VA, Klenk HD. The glycoproteins of Marburg and Ebola virus and their potential roles in pathogenesis. Arch Virol Suppl 1999; 15:159–69. [DOI] [PubMed] [Google Scholar]
- 3.Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis 2004; 4:487–98. [DOI] [PubMed] [Google Scholar]
- 4.Reed DS, Mohamadzadeh M. Status and challenges of filovirus vaccines. Vaccine 2007; 25:1923–34. [DOI] [PubMed] [Google Scholar]
- 5.Groseth A, Feldmann H, Strong JE. The ecology of Ebola virus. Trends Microbiol 2007; 15:408–16. [DOI] [PubMed] [Google Scholar]
- 6.Weingartl HM, Embury-Hyatt C, Nfon C, Leung A, Smith G, Kobinger G. Transmission of Ebola virus from pigs to non-human primates. Sci Rep 2012; 2:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin JE, Sullivan NJ, Enama ME, et al. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccine Immunol 2006; 13:1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones SM, Feldmann H, Stroher U, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med 2005; 11:786–90. [DOI] [PubMed] [Google Scholar]
- 9.Bukreyev A, Rollin PE, Tate MK, et al. Successful topical respiratory tract immunization of primates against Ebola virus. J Virol 2007; 81:6379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbert AS, Kuehne AI, Barth JF, et al. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. J Virol 2013; 87:4952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaney JE, Marzi A, Willet M, et al. Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLoS Pathog 2013; 9:e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis 2007; 196(suppl 2):S430–7. [DOI] [PubMed] [Google Scholar]
- 13.Swenson DL, Wang D, Luo M, et al. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin Vaccine Immunol 2008; 15:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamadzadeh M, Chen L, Schmaljohn AL. How Ebola and Marburg viruses battle the immune system. Nat Rev Immunol 2007; 7:556–67. [DOI] [PubMed] [Google Scholar]
- 15.Wong G, Richardson JS, Pillet S, et al. Immune parameters correlate with protection against ebola virus infection in rodents and nonhuman primates. Sci Transl Med 2012; 4:158ra146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol 2009; 7:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dye JM, Herbert AS, Kuehne AI, et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci U S A 2012; 109:5034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu X, Audet J, Wong G, et al. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med 2012; 4:138ra181. [DOI] [PubMed] [Google Scholar]
- 19.Marzi A, Engelmann F, Feldmann F, et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci U S A 2013; 110:1893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, Saphire EO. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 2008; 454:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez A, Trappier SG, Mahy BW, Peters CJ, Nichol ST. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc Natl Acad Sci U S A 1996; 93:3602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volchkov VE, Becker S, Volchkova VA, et al. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology 1995; 214:421–30. [DOI] [PubMed] [Google Scholar]
- 23.Volchkov V, Volchkova V, Slenczka W, Klenk H, Feldmann H. Release of viral glycoproteins during Ebola virus infection. Virology 1998; 245:110–9. [DOI] [PubMed] [Google Scholar]
- 24.Shabman RS, Jabado OJ, Mire CE, et al. Deep sequencing identifies noncanonical editing of Ebola and Marburg virus RNAs in infected cells. mBio 2014; 5:e02011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volchkova VA, Dolnik O, Martinez MJ, Reynard O, Volchkov VE. Genomic RNA editing and its impact on Ebola virus adaptation during serial passages in cell culture and infection of guinea pigs. J Infect Dis 2011; 204(suppl 3):S941–6. [DOI] [PubMed] [Google Scholar]
- 26.Mohan G, Li W, Ye L, Compans R, Yang C. Antigenic subversion: a novel mechanism of host immune evasion by Ebola virus. PLoS Pathog 2012; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Carrion R, Jr, Ye L, et al. Protection against lethal challenge by Ebola virus-like particles produced in insect cells. Virology 2009; 383:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye L, Lin J, Sun Y, et al. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology 2006; 351:260–70. [DOI] [PubMed] [Google Scholar]
- 29.Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature 2003; 422:307–12. [DOI] [PubMed] [Google Scholar]
- 30.Bukreyev A, Marzi A, Feldmann F, et al. Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge. Virology 2009; 383:348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan NJ, Geisbert TW, Geisbert JB, et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med 2006; 3:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Sanchez A, Yang Z, et al. Immunization for Ebola virus infection. Nat Med 1998; 4:37–42. [DOI] [PubMed] [Google Scholar]
- 33.Warfield KL, Bosio CM, Welcher BC, et al. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci U S A 2003; 100:15889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]