Abstract
Most ebolaviruses can cause severe disease in humans and other primates, with high case fatality rates during human outbreaks. Although these viruses have been studied for almost 4 decades, little is know regarding the mechanisms by which they cause disease and what is important for protection or treatment after infection. Because of the sporadic nature of the outbreaks and difficulties accessing the populations affected by ebolaviruses, little is also known about what constitutes an appropriate immune response to infection in humans that survive infection. Such knowledge would allow a targeted approach to therapies. In contrast to humans, rodents are protected from disease on infection with ebolaviruses, although adapted versions of some of the viruses are lethal in mice, hamsters and guinea pigs. Using the recently described hamster model, along with T-cell depletion strategies, we show that CD4+ T cells are required for natural immunity to Ebola virus infection and that CD4-dependent antibody responses are required for immunity in this model.
Keywords: Ebola virus, immune response, antibody, T cell
Ebola virus (EBOV) is the prototypic etiologic agent of Ebola hemorrhagic fever (EHF) and is responsible for the current outbreak in West Africa, which has infected thousands of people, with a case-fatality rate of about 50% [1]. Ebolaviruses were first identified in 1976 in former Zaire (now the Democratic Republic of the Congo) and southern Sudan [2]. Despite the almost 4 decades of research on the virus, little is known about how the virus mechanistically causes disease and what parameters allows for some individuals to recover from infection. Much of the research efforts is hampered by the sporadic nature of the outbreaks and the fact that they often occur in remote villages in Central Africa, where sample collection is difficult and follow-up studies nearly impossible.
Several animal models are used to study pathogenesis and immune responses, including nonhuman primates (NHPs). Cynomolgus and rhesus macaques and African green monkeys succumb to infection with several species of ebolaviruses, and disease in these animals is markedly similar to what is observed in humans [3]. Conversely, immunocompetent laboratory mice do not develop disease on inoculation with wild-type ebolaviruses, although they are susceptible to infection and virus replicates in several tissues [4]. However, on serial passaging in mice, the virus accumulates mutations that allow it to become pathogenic. This adapted virus is lethal in mice, with mice succumbing to infection by 4–5 days after an intraperitoneal inoculation.
Recently, a hamster model of EHF has been developed that is superior to existing rodent models in that is displays most of the clinical hallmarks of EHF, including the coagulopathy associated with disease, which the mouse model largely lacks [4]. Similar to the mouse model, wild-type EBOV replicates in these animals without causing disease, whereas adapted EBOV is lethal. The aim of this study was to determine how hamsters are able to protect themselves from disease caused by wild-type EBOV and what role the components of the immune response play in this protection.
METHODS
Ethics and Biosafety Statement
Work with EBOV-infected hamsters and any potentially infectious material was conducted in Rocky Mountain Laboratory's biosafety level 4 facility, Division of Intramural Research, National Institutes of Allergy and Infectious Diseases, National Institutes of Health. Removal of samples from that facility was performed after inactivation according to standard operating protocols approved by the Institutional Biosafety Committee. The Institutional Animal Care and Use Committee of Rock Mountain Laboratories approved all animal experiments, which were performed by certified staff following Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
T-Cell Depletion and Inoculation in Syrian Hamsters
Female Syrian hamsters, 6–7 weeks of age (Harlan Laboratories), were used in this study. To deplete T-cell subsets, hamsters were injected intraperitoneally with 500 μL of a solution containing 175 μg of antibody while anesthetized with inhalational isoflurane, as described elsewhere [5]. The antibodies used were isotype control (immunoglobulin [Ig] G1 κ), anti-CD4 (clone GK1.5), and anti–rat CD8b (clone 341), all functional grade (eBioscience) [5]. Two days after depletion, hamsters were inoculated with 103 plaque-forming units of wild-type EBOV, strain Mayinga (EBOV-May) (Zaire 1976) grown on Vero E6 cells. For adoptive transfer studies, hamsters that were depleted of CD4+ cells or given an isotype control antibody and then inoculated with EBOV-May were euthanized 12 days after inoculation, and serum samples were obtained by cardiac puncture. The serum was gamma-irradiated (10 mrad) to inactivate any virus, and 1 mL of serum was administered intraperitoneally to CD4-depleted hamsters on day 4 and again on day 7 after inoculation.
Flow Cytometry
To determine the efficiency of T-cell depletion, 12 days after inoculation we excised spleens from hamsters that received either isotype control antibodies or depleting antibodies and then been inoculated with EBOV-May. Single-cell suspensions were prepared by gently rubbing the spleens through 70-μm mesh filters, followed by red blood cell lysing using ACK lysis buffer (Gibco) and washing in cold phosphate buffered saline (PBS)–ethylenediaminetetraacetic acid (EDTA). Cells (106) were then blocked with PBS containing 2 mmol/L EDTA, 2% fetal bovine serum, 2% mouse serum, and 2% rat serum for 10 minutes on ice. The cells were then stained with a mixture of anti–mouse CD4-allophycocyanin (1:250) and anti–rat CD8b-phycoerythrin (1:150) in blocking buffer for 15 minutes on ice in the dark. Cells were then washed in PBS-EDTA and fixed overnight with 4% paraformaldehyde. After the fixing step, cells were washed in PBS-EDTA containing 2% fetal bovine serum, flow cytometric analysis was performed using an LSR II flow cytometer (BD Biosciences), and data were acquired using FACSDiva software (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Enzyme-Linked Immosorbent Assays
Serum was obtained from cardiac puncture of hamsters 12 days after inoculation. Direct enzyme-linked immunosorbent assays were performed by coating plates with recombinant EBOV glycoprotein, as described elsewhere [6].
Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction
RNA from tissues was isolated using the RNeasy Mini kit (Qiagen), following the recommended protocol. RNA from whole-blood samples was isolated using the QIAamp Viral RNA Mini Kit (Qiagen), following the manufacturer's recommended protocol. Quantitative real-time reverse-transcription polymerase chain reaction (RT-PCR) was performed using a 1-step Rotorgene Fast RT-PCR kit (Qiagen) with a Rotorgene 6000 thermocycler (Qiagen). An EBOV primer and probe set specific for the nucleoprotein gene were used to amplify the RNA, and template abundance was calculated by comparing cycle threshold values with a standard curve generated from RNA standards derived from an EBOV stock of a known titer [7].
RESULTS
Role of CD4+ Cells in Protection From EBOV Disease in Hamsters
To determine whether the adaptive immune response of hamsters is required for their immunity to EBOV, groups of 3 hamsters were administered either control antibodies or antibodies that deplete either CD4+ or CD8+ cells 2 days before intraperitoneally inoculation with wild-type EBOV-May. We performed a subsequent experiment with 6 additional hamsters in the control and CD4-depleted groups after obtaining the results from the initial experiment, using 3 hamsters from each group. Prescott et al [5] have previously assessed the effectiveness of CD4+ and CD8+ T-cell depletion in Syrian hamsters.
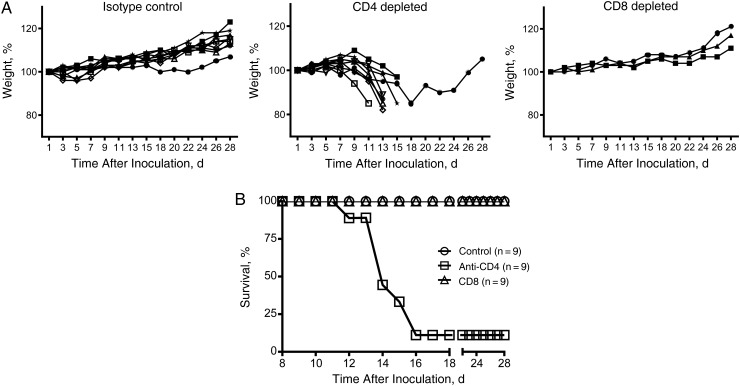
After inoculation, the hamsters were weighed regularly, and their health status was accessed for 28 days after inoculation (Figure 1). Whereas the controls and those depleted of CD8+ cells consistently gained weight and showed no signs of disease, hamsters in the CD4+ cell–depleted group began to lose weight and show signs of disease starting on day 7 after inoculation (Figure 1A). Disease signs included hunched posture, ruffled fur, and inability to ambulate. Starting on day 12 after inoculation, disease signs were severe enough to require euthanasia according to approved humane end point scoring, and 8 of 9 hamsters (89%) were euthanized 12–16 days after inoculation (Figure 1B). The single remaining hamster showed signs of disease and lost weight, but weight loss was delayed and this hamster recovered and regained weight throughout the course of the study.
Figure 1.
Disease and survival in T-cell–depleted hamsters infected with wild-type Ebola virus, strain Mayinga (EBOV-May). A, B, Syrian hamsters, after being given either isotype control antibodies or antibodies to deplete CD4+ or CD8+ cells, were weighed (A) and monitored for survival (B) for 4 weeks after challenge with EBOV-May. Hamsters given control antibodies or antibodies to deplete CD8+ cells gained weight throughout this period and showed no signs of disease. Those depleted of CD4+ cells began to lose weight by day 9 after inoculation; beginning on day 12 after inoculation, 8 of 9 hamsters were euthanized because of clinical disease.
Increased EBOV Replication After CD4 Depletion
Because CD4-depleted hamsters started showing signs of severe disease on day 12 after inoculation, we repeated the above experiment and euthanized groups of 9 hamsters that were given either isotype control antibodies or CD4-depleting antibodies (or CD8-depleting antibodies in 3 hamsters) 12 days after inoculation with EBOV-May. We prepared single-cell suspensions from the spleens of these animals to determine the extent of T-cell depletion at this time point. Cells were stained with anti-CD4 and anti-CD8 antibodies, and the percentages of cells expressing these markers were determined using flow cytometry (Figure 2A and 2B). Specific depletion resulted in reductions of approximately 73% for CD4-expressing and 90% for CD8-expressing cells at this time point (Figure 2B).
Figure 2.
Depletion efficacy and viral replication in T-cell–depleted hamsters. A, B, Splenocytes were isolated 12 days after inoculation and stained with anti-CD4 and anti-CD8 antibodies for flow cytometry. Depletion efficiency was calculated by determining the decrease in the percentage of CD4+ or CD8+ cells, and data represent mean and standard deviations for 3 hamsters. CD4+ cells were decreased by 73%, and CD8+ cells by 90%. C, The abundance of EBOV RNA was measured from the blood, livers, and spleens of depleted hamsters 12 days after inoculation by quantitative reverse-transcription polymerase chain reaction. In each case, there was a 3–4 log10 increase in viral RNA in the CD4-depleted hamsters. Results are shown as geometric means with 95% confidence intervals. *P < .001 (Mann–Whitney test). Abbreviations: NS, not significant; TCID50, median tissue culture infective dose.
We then examined whether this deficiency in T cells resulted in differences in the ability of EBOV-May to replicate in the blood, livers, or spleens of these hamsters. Viral RNA amounts in the CD4-depleted hamsters were higher in all cases, with a 3–4 log10 increase in viral RNA abundance for all tissues, which was highly statistically significant (Figure 2C).
Reduced EBOV-Specific Antibody Responses in CD4-Depleted Hamsters
CD4+ cells are necessary for the development of efficient antibody responses. To test whether CD4 depletion functionally inhibited T-cell responses in these experiments, we measured EBOV-specific antibodies in hamsters euthanized 12 days after inoculation with EBOV-May in animals depleted of either CD4+ or CD8+ cells (or control animals). Depletion of CD4 resulted in a reduction of anti-glycoprotein antibodies measured by enzyme-linked immunosorbent assay, compared with both control and CD8-depleted animals (Figure 3).
Figure 3.
Antibody titers in depleted hamsters inoculated with Ebola virus, strain Mayinga. Serum samples were obtained from hamsters 12 days after inoculation and used in a glycoprotein-specific enzyme-linked immunosorbent assay. Depletion of CD4+ cells resulted in a decrease in anti-glycoprotein antibodies. Data are given as means and standard deviations for 6 hamsters per group. Negative represents normal hamster control sera.
Passive Transfer of Antibodies and Protection From EBOV in CD4-Depleted Hamsters
After establishing that CD4+ cells are required for protection from EBOV disease, and that deficient CD4 responses reduces antibody response, we sought to investigate whether the mechanism by which hamsters are protected from disease is dependent on the antibody response or on other antibody-independent, CD4-dependent responses. To do this, we collected serum samples from 2 groups of hamsters as donors. The first group included hamsters given an isotype antibody 2 days before inoculation with EBOV, and the other group was depleted of CD4+ cells before inoculation. Serum samples from these donor hamsters were collected 12 days after inoculation and subjected to irradiation to inactivate any EBOV present.
Recipients were divided into 3 groups of 6 hamsters each. All animals were depleted of CD4 and then inoculated with EBOV 2 days later. One group received PBS (1 mL intraperitoneally) on days 4 and 7 after inoculation, a second received 1 mL of serum from hamsters given isotype control on the same schedule, and a third received serum from the CD4-depleted donor group on the same schedule. The recipient hamsters were monitored for signs of disease and survival (Figure 4).
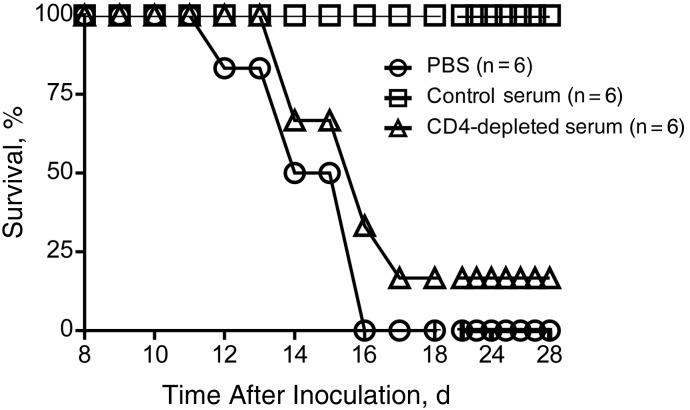
Figure 4.
Passive transfer of serum from infected hamsters protects CD4-depleted hamsters from disease. Twelve days after inoculation serum from either nondepleted or CD4-depleted hamsters that were inoculated with Ebola virus, strain Mayinga (EBOV-May) were transferred to CD4-depleted hamsters on days 4 and 7 after inoculation with EBOV-May. As a control, phosphate buffered saline (PBS) was administered to a group of hamsters at these same time points. Hamsters that received antibodies from nondepleted donors showed no clinical disease. Hamsters given PBS or serum from CD4-depleted donors became clinically ill, and all but 1 required euthanasia.
Beginning on day 12 after inoculation, hamsters given PBS showed signs of severe disease, and by day 16, all animals had to be euthanized owing to disease severity. Hamsters that were given serum from CD4-depleted hamsters also developed disease, although there was a 1-day delay in the time to euthanasia. One hamster in the group of 6 given CD4-depleted serum recovered after showing signs of disease (17% survival). All of the CD4-depleted hamsters given serum from non–CD4-depleted donors (control serum samples) survived without signs of disease (Figure 4).
DISCUSSION
Rodents are naturally protected from disease on infection with wild-type ebolaviruses. Despite their resistance to disease, inoculation results in virus replication and dissemination, suggesting that the host's immune response plays a role in controlling the virus and preventing disease [8]. This is evidenced by the observations that perturbations of the immune response in mice render them susceptible to disease. For instance, Signal transducer and activator of transcription (STAT)-1 knockout mice succumb to wild-type ebolavirus infection [9]. Similarly, mice lacking the receptor for type I interferon, which signals via the STAT pathway, also succumb to infection [10]. These observations suggest that an intact innate immune system is required for protection from lethal disease. This is not surprising, given observations that these pathogenic filoviruses have evolved several strategies to evade the host's immune response by encoding proteins with interferon antagonistic activity (reviewed in [11]).
Our present results demonstrate that, along with an efficient innate response, an appropriate adaptive immune response is required for immunity, and this adaptive response requires the generation of an efficient antibody response. Although the specific mechanistic events that lead to human disease are not known, circumvention and/or antagonism of the innate response, allowing the virus to replicate to high titers and then disarming the adaptive response, probably plays a key role. By examining species that are naturally immune but support replication, we can elucidate what is required for an appropriate response to infection and what might be maladaptive in humans.
The observation that antibody responses are required for efficient protection enables the rational development of interventions. Recent evidence suggests that efficient antibody responses are protective during EHF. Vaccination with a recombinant vesicular stomatitis virus encoding the EBOV glycoprotein is efficacious [12]. This vaccine generates EBOV-specific antibodies, and T-cell depletion experiments demonstrated that protection is dependent on the antibody response this vaccine elicits [13, 14]. Similarly, antibody therapy has been shown to be highly effective at preventing EHF in NHPs after exposure, suggesting that antibodies alone can protect against disease [15, 16].
Studies using rodent models along with rodent-adapted EBOV also demonstrate that antibody responses are important for protection. Vaccination using liposome-encapsulated EBOV antigens required an intact CD4 response to be efficacious against mouse-adapted EBOV, but CD8+ cells were dispensable. After immunization, depletion of either subset was dispensable, suggesting that antibodies already present may be required and sufficient for protection in the mouse model [17]. The relationship between wild-type EBOV in humans or NHPs and adapted viruses in rodents probably involves changes in the virus that allow it to antagonize the immune response. In the mouse and hamster models, suppression of the innate immune response by the rodent-adapted EBOV is critical for its ability to cause disease [4, 18].
Antibody responses are known to be important for effective clearance of ebolaviruses in humans [19]. Survivors of EBOV infection in the 1996 Gabon outbreaks mounted a strong humoral response, with measurable titers of both immunoglobulin M (IgM) and immunoglobulin G (IgG) during the symptomatic phase. In contrast, IgG was not detectable and IgM was detectable in only approximately one-third of fatal cases, suggesting that early and robust B-cell responses are important for eventual virus clearance. In these cases, CD4+ T-cell responses would also be important, because they are necessary for the development of affinity-matured antibodies, class switching from IgM to IgG, and a memory response. The requirement of cytotoxic T lymphocytes for recovery from infection in this context is not known. In the current study, antibody responses were critical for protection from disease, and both CD4+ and CD8+ T cell responses, other than the help required from CD4+ T cells for antibody production were dispensable, as evidenced by the observation that transfer of serum from hamsters infected with EBOV into CD4-depleted hamsters was completely protective, while CD8 depletion had no effect on disease outcome.
The results presented herein emphasize that efficient antibody responses are required for immunity to ebolaviruses in rodents. Innate immune responses are crucial in the early stages of infection to limit replication and allow the host enough time to generate robust adaptive immune responses. These findings demonstrate that a strong antibody response can prevent EHF in a biological system. Comparing rodent and human (or NHP) B-cell responses to ebolavirus infection might provide insight into how rodents are able to circumvent disease while primates develop EHF. These data suggest that approaches that promote antibody responses, by vaccination strategies, by transfer or administration of antibodies, or by promoting the de novo production of specific antibodies in the host, should be a focus for protection from disease caused by ebolaviruses.
Notes
Acknowledgments. We thank the Rocky Mountain Veterinary Branch for their care and handling of the animals. We also thank Blair DeBuysscher and Friederike Feldmann for assistance in performing animal procedures and Anita Mora for graphics.
Financial support. This work was supported by the Division of Intramural Research, the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and prevention. Ebola (Ebola virus disease). http://www.cdc.gov/vhf/ebola/. Accessed 3 January 2015.
- 2.Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ 1978; 56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 3.Nakayama E, Saijo M. Animal models for Ebola and Marburg virus infections. Front Microbiol 2013; 4:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebihara H, Zivcec M, Gardner D, et al. A Syrian golden hamster model recapitulating Ebola hemorrhagic fever. J Infect Dis 2013. 207:306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott J, Safronetz D, Haddock E, Robertson S, Scott D, Feldmann H. The adaptive immune response does not influence hantavirus disease or persistence in the Syrian hamster. Immunology 2013; 140:168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakayama E, Yokoyama A, Miyamoto H, et al. Enzyme-linked immunosorbent assay for detection of filovirus species-specific antibodies. Clin Vaccine Immunol 2010; 17:1723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marzi A, Yoshida R, Miyamoto H, et al. Protective efficacy of neutralizing monoclonal antibodies in a nonhuman primate model of Ebola hemorrhagic fever. PLoS One 2012; 7:e36192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strong JE, Wong G, Jones SE, et al. Stimulation of Ebola virus production from persistent infection through activation of the Ras/MAPK pathway. Proc Natl Acad Sci U S A 2008; 105:17982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raymond J, Bradfute S, Bray M. Filovirus infection of STAT-1 knockout mice. J Infect Dis 2011; 204(suppl):S986–90. [DOI] [PubMed] [Google Scholar]
- 10.Bray M. The role of the type I interferon response in the resistance of mice to filovirus infection. J Gen Virol 2001; 82(pt 6):1365–73. [DOI] [PubMed] [Google Scholar]
- 11.Ramanan P, Shabman RS, Brown CS, Amarasinghe GK, Basler CF, Leung DW. Filoviral immune evasion mechanisms. Viruses 2011; 3:1634–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldmann H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones SM, Feldmann H, Ströher U, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med 2005; 11:786–90. [DOI] [PubMed] [Google Scholar]
- 14.Marzi A, Engelmann F, Feldmann F, et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci U S A 2013; 110:1893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu X, Audet J, Wong G, et al. Sustained protection against Ebola virus infection following treatment of infected nonhuman primates with ZMAb. Sci Rep 2013; 3:3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettitt J, Zeitlin L, Kim DH, et al. Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci Transl Med 2013; 5:199ra113. [DOI] [PubMed] [Google Scholar]
- 17.Rao M, Bray M, Alving CR, Jahrling P, Matyas GR. Induction of immune responses in mice and monkeys to Ebola virus after immunization with liposome-encapsulated irradiated Ebola virus: protection in mice requires CD4+ T cells. J Virol 2002; 76:9176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahanty S, Gupta M, Paragas J, Bray M, Ahmed R, Rollin PE. Protection from lethal infection is determined by innate immune responses in a mouse model of Ebola virus infection. Virology 2003; 312:415–24. [DOI] [PubMed] [Google Scholar]
- 19.Baize S, Leroy EM, Georges-Courbot MC, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med 1999; 5:423–6. [DOI] [PubMed] [Google Scholar]