Abstract
Inhibitory neurotransmission is primarily mediated by γ-aminobutyric acid (GABA) activating synaptic GABA type A receptors (GABAAR). In schizophrenia, presynaptic GABAergic signaling deficits are among the most replicated findings; however, postsynaptic GABAergic deficits are less well characterized. Our lab has previously demonstrated that although there is no difference in total protein expression of the α1–6, β1–3 or γ2 GABAAR subunits in the superior temporal gyrus (STG) in schizophrenia, the α1, β1 and β2 GABAAR subunits are abnormally N-glycosylated. N-glycosylation is a posttranslational modification that has important functional roles in protein folding, multimer assembly and forward trafficking. To investigate the impact that altered N-glycosylation has on the assembly and trafficking of GABAARs in schizophrenia, this study used western blot analysis to measure the expression of α1, α2, β1, β2 and γ2 GABAAR subunits in subcellular fractions enriched for endoplasmic reticulum (ER) and synapses (SYN) from STG of schizophrenia (N=16) and comparison (N=14) subjects and found evidence of abnormal localization of the β1 and β2 GABAAR subunits and subunit isoforms in schizophrenia. The β2 subunit is expressed as three isoforms at 52 kDa (β252 kDa), 50 kDa (β250 kDa) and 48 kDa (β248 kDa). In the ER, we found increased total β2 GABAAR subunit (β2ALL) expression driven by increased β250 kDa, a decreased ratio of β248 kDa:β2ALL and an increased ratio of β250 kDa:β248 kDa. Decreased ratios of β1:β2ALL and β1:β250 kDa in both the ER and SYN fractions and an increased ratio of β252 kDa:β248 kDa at the synapse were also identified in schizophrenia. Taken together, these findings provide evidence that alterations of N-glycosylation may contribute to GABAergic signaling deficits in schizophrenia by disrupting the assembly and trafficking of GABAARs.
Introduction
Schizophrenia is a chronic psychiatric disorder that affects multiple brain regions, neurotransmitter systems and cell types, and presents with variable combinations of symptoms. Negative and cognitive symptoms associated with this illness have a profound effect on patient outcome, and have been shown to correlate with dysfunctional GABAergic signaling.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 A consistent finding in schizophrenia research is the decreased expression of GAD67, an enzyme necessary for the synthesis of the neurotransmitter γ-aminobutyric acid (GABA).13, 14, 15, 16, 17, 18, 19, 20, 21 Altered inhibitory neurotransmission from GABAergic interneurons onto cortical pyramidal neurons has been shown to disrupt the excitatory:inhibitory balance in the cortex and contribute to disruptions of neural synchrony in schizophrenia1, 3, 5, 6, 9, 22, 23 and other neuropsychiatric disorders.5, 6 These presynaptic GABAergic deficits have been extensively studied in schizophrenia, whereas postsynaptic GABAA receptor (GABAAR) subunit abnormalities have been more difficult to characterize due to extensive homology between subunits and the variety of potential subunits that may be expressed and incorporated into intact receptors.6, 24, 25, 26, 27
Alterations of transcript and protein expression of several GABAAR subunits in a brain-region, cortical lamina and cell type-specific manner have been described in schizophrenia.13, 15, 17, 28, 29, 30, 31, 32, 33, 34, 35 We have previously reported that there is no change to the total protein expression of the α1–6, β1–3 and γ2 GABAAR subunits in the superior temporal gyrus (STG; Brodmann area 22),36 an area that we focused on given prior studies indicating decreased volume, increased GABAAR density and GABAergic signaling abnormalities in this cortical region in schizophrenia.37, 38, 39, 40 Although protein expression of these GABAAR subunits was unchanged in STG in schizophrenia, we identified significant alterations in the posttranslational processing of the α1, β1 and β2 GABAAR subunits; specifically, we observed abnormalities of immature N-linked glycosylation of the α1 and β1 GABAAR subunits, and altered total N-glycosylation of the β2 GABAAR subunit in schizophrenia.36
N-glycosylation has an essential role in proper protein folding and assembly, endoplasmic reticulum (ER) quality control mechanisms and forward trafficking from the ER to the plasma membrane.41, 42, 43, 44, 45, 46, 47, 48 Previous studies suggested that these functional processes are disrupted in schizophrenia, and we have identified alterations of N-linked glycosylation of multiple neurotransmitter-associated proteins that are consistent with abnormal ER function.36, 49, 50, 51 A smaller immature N-glycan has been observed attached to the α1 GABAAR subunit in schizophrenia, which suggests that this subunit undergoes early glycoprotein processing and may be retained in the calnexin–calreticulin protein folding cycle in the ER. More of the β149 kDa GABAAR subunit isoform is immaturely glycosylated in schizophrenia, which could result in increased incorporation of this subunit into synaptically targeted GABAARs. The abnormal total N-glycosylation of the β2 subunit that we have previously reported may alter receptor targeting after ER exit and result in decreased β2 expression at the synapse. As N-glycosylation abnormalities are evident on isoforms of both the β1 and β2 GABAAR subunits, this atypical pattern of posttranslational modifications may serve to ensure preferential incorporation of one β-subunit over the other to compensate for presynaptic GABAergic signaling deficits in the disorder. Accordingly, we predict that the ratio of β1-containing versus β2-containing GABAARs could be altered at the synapse. On the basis of the previously reported N-glycosylation deficits in schizophrenia, we hypothesize that deficits in initial protein processing, including abnormal posttranslational protein modifications, may alter neurotransmitter receptor assembly and trafficking and contribute to the pathophysiology of schizophrenia.
To ascertain whether N-glycosylation alterations contribute to aberrant forward trafficking of GABAARs in schizophrenia, in this study, we examined the subcellular localization of GABAAR subunits that we previously found to be abnormally N-glycosylated in STG in schizophrenia. We also examined the subcellular distribution of the γ2 GABAAR subunit given its role in synaptic targeting of intact receptors,52, 53, 54, 55, 56, 57, 58 and the α2 GABAAR subunit, which has been implicated in schizophrenia.32, 59, 60, 61 We determined the expression of the α1, α2, β1, β2 and γ2 GABAAR subunits in defined subcellular compartments from postmortem STG from schizophrenia and comparison subjects. By assessing the abundance of these GABAAR subunits in subcellular compartments at the proximal and distal ends of the forward trafficking pathway, we anticipated that we would identify alterations in subcellular localization or subunit composition of intact GABAARs in schizophrenia that may contribute to the pathophysiology of this disorder.
Materials and methods
Subjects and tissue acquisition
Samples of the full thickness of gray matter from the left STG (Brodmann area 22) of 16 schizophrenia subjects and 14 non-psychiatrically ill comparison subjects (Table 1 and Supplementary Table S1) were obtained from the Mount Sinai Medical Center brain collection, as previously described.36, 62, 63 Patients who were diagnosed with schizophrenia on the basis of DSM-III-R criteria and confirmed by at least two clinicians, had a documented history of the onset of psychotic symptoms before age 40 and at least 10 years of hospitalization, were prospectively recruited.64 Each subject was assessed for psychiatric illnesses, history of drug or alcohol abuse and tests of cognition. CERAD guidelines were used to evaluate the brain macro- and microscopically.65 Comparison subjects were similarly evaluated, and had no history of documented substance abuse or psychiatric illness. Subjects that had been in a coma for more than 6 h before death, had a history of substance abuse or death as a result of suicide were excluded from this study. Consent to perform an autopsy on the body and brain for diagnostic and research purposes was obtained from the next-of-kin for each subject.63, 65 Tissue samples were pulverized with small amounts of liquid nitrogen and stored at −80 °C until processed for study.
Table 1. Summary of subject demographics.
| Comparison | Schizophrenia | |
|---|---|---|
| n | 14 | 16 |
| Age | 79.4±9.3 | 75.8±11.9 |
| Sex | 4 M/10 F | 11 M/5 F |
| PMI (h) | 10.0±7.3 | 11.4±4.4 |
| Tissue pH | 6.3±0.2 | 6.4±0.3 |
| On/off Rx | 0/14 | 11/5 |
Abbreviations: F, female; M, male; PMI, postmortem interval; Rx, antipsychotic medication.
Values are expressed as means±s.d. Off Rx indicates patients that had not received antipsychotic medications for 6 weeks or more at the time of death.
Subcellular fractionation
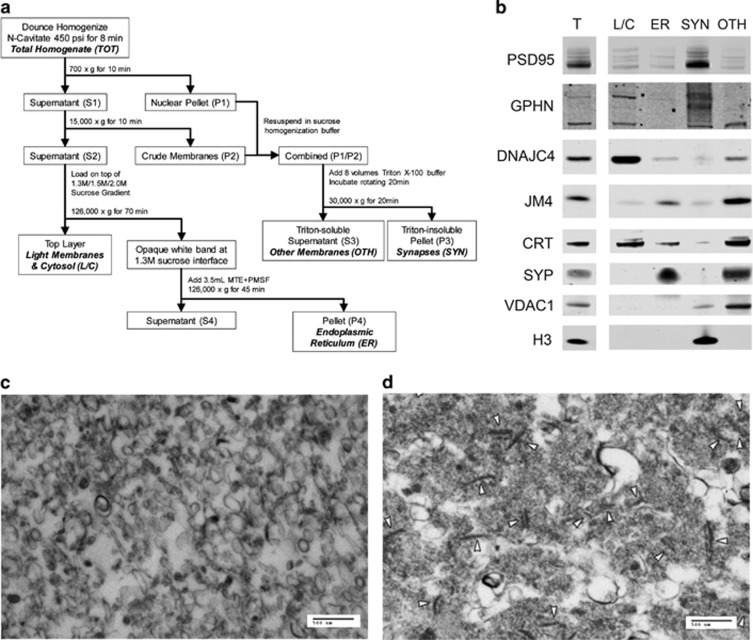
Subcellular fractionation was performed using nitrogen cavitation, differential sucrose gradient ultracentrifugation and Triton solubilization (Figure 1a). This protocol yields fractions enriched for light membrane/cytosol, ER, and synapses (SYN). It also yields a relatively nonspecific residual fraction containing markers for mitochondria, extrasynaptic membranes, ER lumen and other membrane and vesicle-associated proteins; this triton-soluble fraction, referred to as the ‘other intermediate membrane' fraction, does not contain nuclear or excitatory synaptic markers (Figure 1b).
Figure 1.
Fractions enriched for light membranes and cytosol (L/C), endoplasmic reticulum (ER), excitatory and inhibitory synapses (SYN) and other intermediate membranes (OTH) generated from postmortem human cortex. (a) Schematic depiction of the centrifugation, differential sucrose gradient and Triton solubilization steps to produce L/C, ER, SYN and OTH-enriched fractions from nitrogen-cavitated samples. Briefly, after nitrogen-cavitated cortical homogenate samples undergo sequential centrifugations, S2 is loaded on top of a differential sucrose gradient and ultracentrifuged to separate the ER from other light membranes and cytosolic components based on membrane density. The ER membranes appear as a semi-opaque white band, and the L/C remains suspended in the translucent top layer. P1 and P2 from the first centrifugation steps are resuspended, combined and solubilized by a brief incubation with Triton X-100 buffer. Following centrifugation, the Triton-insoluble synaptic membranes are concentrated in the resulting P3, while S3 contains the remaining heavy and intermediate membrane components. (b) Representative images from western blots of total homogenate, L/C, ER, SYN and OTH fractions probed for various subcellular markers to validate the efficacy of the fractionation method in postmortem human cortex. Target marker proteins include postsynaptic density protein 95 (PSD95), for excitatory synapses; gephyrin (GPHN), for inhibitory synapses and extrasynaptic membrane; DnaJ/hsp40 homolog subfamily C member 4 (DNAJC4), for cytosol; PRA1 family protein 2 (JM4), for ER and Golgi membranes; calreticulin (CRT), for ER lumen; synaptophysin (SYP), for extrasynaptic membranes; voltage dependent anion-selective channel protein 1 (VDAC), for mitochondria; and histone 3 (H3), for nuclei. (c and d) Representative electron microscopy (EM) image of the ER and SYN fractions (scale bars, 500 nm). (c) ER membrane is enriched and no other identifiable structures or organelles are evident in the ER fraction. (d) Synapses, indicated by white arrowheads, are enriched and no other intact structures or organelles are visualized in the SYN fraction. MTE, D-mannitol, Tris-base, and EDTA; PMSF, phenylmethylsulfonyl fluoride.
For each subject, 50 mg of pulverized tissue was homogenized on ice by 10 strokes in a glass–teflon homogenizer in 1.25 ml of 1 × Isotonic Extraction Buffer (Sigma-Aldrich, St. Louis, MO, USA) diluted with sterile water, then transferred into a nitrogen cavitation vessel (Parr Instrument Company, Moline, IL, USA) and pressurized at 450 psi for 8 min for further disruption of cell membranes.66, 67 The homogenates were collected through the outlet port of the vessel by nitrogen decompression; 950 μl was used for subcellular fractionation and the remainder reserved as total homogenate.
The homogenate from each subject was centrifuged at 700 g for 10 min at 4 °C. The supernatant (S1) was subsequently centrifuged at 15 000 g for 10 min at 4 °C and the pellet (P1) was resuspended in 75 μl of sucrose homogenization buffer (5 mM Tris-HCl, pH 7.4, 320 mM sucrose and a protease inhibitor tablet (Complete Mini; Roche Diagnostics, Mannheim, Germany)). After the second centrifugation, the supernatant (S2) was loaded on top of a differential sucrose gradient (prepared with 1 ml each of 2.0 M sucrose, 1.5 M sucrose, then 1.3 M sucrose in a 14 × 89 mm polyallomer ultracentrifuge tube (Beckman Coulter, Indianapolis, IN, USA)), and the pellet (P2) was resuspended in 75 μl sucrose homogenization buffer and combined with the resuspended P1.66, 67
To the combined P1/P2 resuspension, 1.2 ml of Triton X-100 buffer (10 mM Tris-HCl, pH 7.4, 1 mM Na3VO4, 5 mM NaF, 1 mM EDTA, 1 mM EGTA, 5%v/v Triton X-100) was added and samples were incubated for 20 min at 4 °C on a rotator before being centrifuged for 20 min at 30 000 g at 4 °C. The triton-insoluble pellet was resuspended in 125–150 μl of 1 × phosphate-buffered saline (PBS) with a protease inhibitor tablet (Roche Diagnostics) and sonicated 5 × for 1 s at level 4 (Sonic Dismembrator Model 100, Fisher Scientific, Pittsburgh, PA, USA) to produce the final SYN fraction.68 The supernatant (S3) was reserved to produce the final other intermediate membrane fraction.68
The sucrose gradient was ultracentrifuged at 126 000 g (35 000 r.p.m. in a SW60Ti rotor (Beckman Coulter)) at 4 °C for 70 min. The upper layer was reserved to produce the final light membrane/cytosol fraction.66, 67 A dense, semi-opaque white band at the interface of the upper layer and the 1.3 M sucrose layer was aspirated and combined with 3.0–3.5 ml of ice-cold 1 × MTE+PMSF buffer (270 mM D-mannitol, 10 mM Tris-base and 0.1 mM EDTA adjusted to pH 7.4, with 1 mM phenylmethylsulfonyl fluoride) and ultracentrifuged in a new polyallomer ultracentrifuge tube at 126 000 g at 4 °C for 45 min. The supernatant was decanted and pellet dried for 2–3 min before being resuspended in 50 μl of ice-cold 1 × PBS with 0.5% v/v Triton X-100, pH 7.4, to produce the final ER fraction.66, 67
Electron microscopy
To validate enrichment of ER membranes in the ER fraction (Figure 1c) and symmetrical and asymmetrical synapses in the SYN fraction (Figure 1d) by electron microscopy (EM), fraction samples from two non-psychiatrically ill subjects were prepared as previously described.66, 67 Briefly, fractions were fixed in 4% gluteraldehyde in 0.1 M cacodylate buffer (pH 7.4) at 4 °C for at least 24 h. The University of Alabama at Birmingham HRIF Electron Microscopy Core then processed the samples and post-stained with uranyl acetate and lead citrate for EM imaging on a Tecnai F20 FEG transmission electron microscope (FEI, Hillsboro, OR, USA).
Western blot sample preparation
Protein concentration of the homogenate and fraction samples was determined with BCA assays (Thermo Fisher Scientific, Pittsburgh, PA, USA). Western blot samples were prepared by dilution with sucrose homogenization buffer and the addition of 6 × loading buffer (0.5 M Tris-HCl, 36% glycerol, 4.5% sodium dodecyl sulfate and 2% β-mercaptoethanol) to a final protein concentration of 0.556 μg μl−1 (10 μg in 18 μl).
Deglycosylation
Peptide N-glycosidase F (PNGase F; New England Biolabs, Ipswich, MA, USA) was used to cleave total N-glycans in samples of total homogenate, ER and SYN fractions. For each fraction, 25 μg of protein was denatured with Denaturation Solution (New England Biolabs) and 10 × PNGase F Reaction Buffer (New England Biolabs) by incubation at 70 °C for 10 min. The deglycosylating enzyme PNGase F and 10% NP40 were added and samples incubated overnight at 40 °C. To each sample, 6 × loading buffer was added and heated at 70 °C for 10 min. Non-enzyme-treated negative control samples with or without NP40 were prepared identically to the enzyme-treated samples with the same buffers, replacing the enzyme and NP40 with water.36, 49, 50, 51
Western blot analysis
Fraction samples were run on three 12-well 4–12% Bis-Tris polyacrylamide gels (Life Technologies, Grand Island, NY, USA). For each subject, 10 μg of total homogenate, light membrane/cytosol, ER, SYN and other intermediate membrane fractions were loaded. Novex Sharp Pre-stained Protein Standard (Life Technologies) was run on each gel. Gels were suspended in a bath of 1 × NuPAGE MES sodium dodecyl sulfate running buffer (Life Technologies) and run on a Novex Mini Cell nuPAGE system (Life Technologies) at 55 V for 20 min, followed by 150 V for 80 min.
After electrophoresis, proteins were transferred onto 0.45 μm nitrocellulose membranes (Bio-Rad, Hercules, CA, USA) at 16 V for 30 min using a Bio-Rad semi-dry transfer apparatus. Membranes were cut just above 160 kDa, just below 60 kDa, and just above 40 kDa, followed by a brief PBS rinse. For each set of three gels, membranes of the same molecular weight range were incubated using the appropriate primary antibody in the same box, except for the 60–40 kDa range membranes, which were probed separately for GABAAR subunits. Membranes were incubated with primary antibodies against VCP, gephryin, JM4, DNAJC4 and the α1, α2, β1, β2, γ2 GABAAR subunits (Supplementary Table S2). Conditions for primary antibodies were optimized to be within the linear range of detection for the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA) at a resolution of 169 μm and intensity level of 3. After washing three times with 1 × PBS+0.1% Tween, each membrane was incubated with the appropriate IRDye-labeled secondary antibody (LI-COR Biosciences) for 1 h, then washed twice with PBS+0.1% Tween and once with MilliQ water before being scanned. After scanning, the membranes were stored in MilliQ water at 4 °C.
Antibody specificity for the GABAAR subunits was determined by comparison of the predicted molecular mass of the target protein with the antibody manufacturers observed molecular mass and observed molecular mass of immunoreactive bands from western blots of postmortem cortical homogenate from a non-psychiatrically ill comparison subject. The α1, α2 and γ2 GABAAR antibodies strongly associated with protein bands at the expected molecular mass of the respective subunit. Specificity of the GABAAR β1 subunit was determined by incubating the primary antibody with the antigenic peptide (sc-31426P, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 10 min before probing a western blot of total cortical homogenate. Protein bands, which were not evident after this incubation but were apparent when probed with primary antibody alone were determined to represent the GABAAR β1 subunit. There was no peptide antigen available for the GABAAR β2 subunit; however, similar to the GABAAR β1 subunit, we were able to verify which bands represented GABAAR β3 subunits using the antigenic peptide (sc-31430P, Santa Cruz Biotechnology) incubated with the GABAAR β3 antibody (sc-31430, Santa Cruz Biotechnology) and comparing western blots to identify which bands were specific to β3 subunit expression. We then compared these with a western blot probed with an antibody that recognizes all the three GABAAR β-subunits (sc-28794, Santa Cruz Biotechnology) and identified the immunoreactive bands that were recognized by the GABAAR β2 antibody, but not the β1 or β3 subunit antibodies, as representing the GABAAR β2 subunit.
Data analysis
Image Studio software (LI-COR Biosciences) was used to collect the near-infrared fluorescence value, expressed as signal with left–right median intralane background subtracted, for each protein band under investigation in the total homogenate and subcellular fractions. The number of subjects per group was determined using the previously reported mean and standard deviation of GABAAR α1, β1 and β2 protein expression in STG to detect a 20% difference with statistical power=0.80. The protein expression of α1, α2, β1, β2 and γ2 GABAAR subunits, as well as the protein expression of gephyrin and JM4, was determined by measuring the signal intensity of each band and normalizing to a loading control as well as a marker for each specific fraction. We used VCP as the loading control due to its ubiquitous expression in brain, immunoreactivity in each subcellular fraction, and unchanged expression in multiple brain regions in schizophrenia.36, 69, 70 We used gephyrin as the normalizing factor for the SYN fraction because of its role as a cytoskeletal scaffold for GABAAR-containing synapses.56, 58, 71, 72 JM4 is a marker of ER and Golgi membranes expressed in cortical neurons used as the normalizing factor for the ER fraction due to its consistent and uniform expression in that fraction. Before GABAAR subunit normalization, we verified that VCP, gephyrin and JM4 were not different between groups in the total homogenate or subcellular fractions. For the α2 and β2 GABAAR subunits, which are expressed as multiple isoforms, individual protein bands for each isoform and all isoform bands together, were measured. Although the β1 GABAAR subunit is also expressed as a doublet in our western blot conditions, the individual isoforms did not have enough separation between bands to be measured individually.
VCP-normalized signal intensity was used to assess protein expression for each target in the total homogenate lanes to validate our prior finding of unchanged GABAAR subunit expression in schizophrenia STG from a different subject cohort36 and for use as a within-subject normalizing factor for protein expression in subcellular fractions. For the ER fraction lane, VCP-normalized GABAAR subunit signal intensity was normalized to VCP-normalized JM4 signal intensity and divided by the VCP-normalized GABAAR subunit signal intensity in the total homogenate lane. Similarly, for the SYN fraction lane, VCP-normalized GABAAR subunit signal intensity was normalized to VCP-normalized gephyrin signal intensity and expressed relative to VCP-normalized GABAAR subunit signal intensity in the total homogenate lane. We evaluated the expression of the target GABAAR subunits and, where applicable, calculated the ratio of individual isoforms to total subunit expression in each fraction lane. In addition, the ratios of α1:α2, β1:β2 and β2:β2 (total subunit and ratios to individual subunit isoforms) GABAAR subunit isoforms were assessed in each fraction between schizophrenia and comparison subjects.
Statistica (StatSoft, Tulsa, OK, USA) and Prism 6.0 (GraphPad Software, La Jolla, CA, USA) software were used for all the statistical analyses. Individual data points were excluded from statistical analysis if the protein expression of the target or normalizing factor was below the threshold for detection or greater than 4 s.d. from the mean. We first assessed all the dependent variables for normal distributions using the D'Agostino and Pearson omnibus normality test. For dependent variables that were not normally distributed, we used the Mann–Whitney U-test, and, for normally distributed dependent variables, we determined differences between groups using two-tailed Student's t-tests. To determine whether there were any significant associations between the dependent variables and potential covariates, we performed correlation analysis between the dependent measures and age, pH and postmortem interval, and found no significant correlations for any measures. There were no differences between diagnostic groups for age, pH or postmortem interval. Direct measures of GABAAR subunits in the total homogenates were compared to confirm our prior report of unchanged expression in the total STG homogenates in schizophrenia,36 as well as to validate their utility as normalizing factors for subunit expression by subject in each fraction. Direct measures of GABAAR subunits in the ER and SYN fractions were assessed between diagnostic groups to test specific hypotheses. Calculated measures of subunit ratios in the ER and SYN fraction were not based on independent hypotheses and were corrected for multiple testing using the Benjamini–Hochberg q-value,73 which controls for the false discovery rate. For multiple comparison tests, q*=0.05.
Although the male:female ratio differs between groups, post hoc two-way analysis of variance was performed for all the significant dependent measures and no sex effect was identified. In addition, post hoc Mann–Whitney U-tests between males and females within diagnostic groups were performed and no difference in expression within groups was identified (Supplementary Figure 1). Post hoc Mann–Whitney U-tests were also performed to assess differences between schizophrenia subjects ‘on' antipsychotic medication versus ‘off' medication for all the significant dependent measures. ‘Off' medication in this study is defined as >6 weeks of abstinence from antipsychotic medication before death. A medication effect was identified for the ratios of β1:β2ALL and β1:β250 kDa in the SYN fraction (Supplementary Figure 2); no effect of neuroleptic treatment was discernible for any of the other significant variables. The majority of subjects in this study are Caucasian, with two Asian and one Hispanic subjects in the comparison group and three Black subjects and one subject of unknown race in the schizophrenia group; due to small group sizes, no meaningful post hoc statistical analyses of any effect of race on dependent measures were possible. For all the statistical analyses, α=0.05.
Results
The β2 GABAAR subunit, specifically the β250 kDa isoform, is increased in the ER fraction in schizophrenia
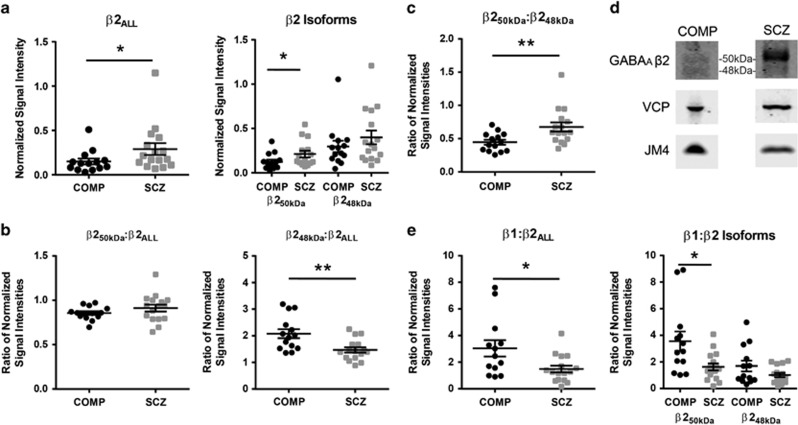
The β2 GABAAR subunit is visualized as multiple isoforms in a fraction-specific manner, with bands at ~52 and 50 kDa in the total homogenate; 50 and 48 kDa in the ER fraction (Figure 2d); and 52, 50 and 48 kDa in the SYN fraction. ER expression of all β2 isoforms (β2ALL) was 93% higher in schizophrenia (U(14,16)=61, P=0.03; Figure 2a, Table 2), and ER expression of the primary 50 kDa β2 isoform (β250 kDa), which is seen in all the subcellular fractions, was 70% higher in the ER in schizophrenia (U(14,15)=59, P<0.05; Figure 2a, Table 2). There was no difference in the relative expression of the 48 kDa β2 isoform (β248 kDa) in the ER (Figure 2a, Table 2) and no difference in the relative expression of β2ALL, β250 kDa, β248 kDa or 52 kDa β2 (β252 kDa) GABAAR subunit isoforms in the total homogenate or SYN fractions between schizophrenia and comparison subjects (Table 2).
Figure 2.
The β2 GABAAR subunit is abnormally expressed in an isoform-specific manner and the ratios of β1 and β2 subunit isoforms are altered in the ER in schizophrenia. Western blot analysis of total β2 GABAAR subunit (β2ALL) and individual β2 GABAAR subunit 50 kDa and 48 kDa isoforms (β250 kDa and β248 kDa, respectively), the ratios of β2 GABAAR subunit isoforms to each other, and the ratio of β1:β2 GABAAR subunit and subunit isoform expression in the ER fraction in schizophrenia and comparison subjects. (a) ER fraction-normalized expression of β2ALL, and specifically the β250 kDa GABAAR subunit isoform, is increased in schizophrenia. (b) The ratio of β248 kDa:β2ALL GABAAR subunit fraction-normalized expression is decreased in the ER in schizophrenia. (c) The ratio of β250 kDa:β248 kDa GABAAR subunit fraction-normalized signal intensities is increased in schizophrenia. (d) Representative images of western blots of the β2 GABAAR subunit, VCP and JM4 from the ER fraction from comparison and schizophrenia subjects with the β250 kDa and β248 kDa protein bands indicated. (e) The ratio of β1:β2ALL, and specifically the ratio of β1:β250 kDa GABAAR subunit expression is significantly less in the ER fraction in schizophrenia. Data are expressed as either the signal intensity of protein targets in the ER fraction normalized to VCP as a loading control and JM4 as an ER marker relative to the VCP-normalized signal intensity of the same target in the total homogenate, or expressed as a ratio of normalized signal intensities, for each data point with means±s.e.m. for each group indicated in a, b, c and e. *P<0.05, **P<0.01. COMP, comparison subject; ER, endoplasmic reticulum; GABAAR, γ-aminobutyric acid type A receptor; SCZ, schizophrenia; VCP, valosin-containing protein.
Table 2. α1, α2, β1 and γ2 GABAA receptor subunit protein expression is unchanged in the total homogenate, ER and synapse-enriched fractions of the STG in schizophrenia.
| GABAAR subunit | Comparison | Schizophrenia | Test statistic (d.f.) | P-value |
|---|---|---|---|---|
| Total | ||||
| α1 | 9.42±13.88 | 15.91±26.47 | U (13,15)=95 | |
| α2ALL | 0.29±0.23 | 0.31±0.25 | U (14,16)=105 | |
| α251 kDa | 0.13±0.12 | 0.12±0.08 | U (13,15)=107 | |
| α249 kDa | 0.16±0.13 | 0.19±0.19 | U (14,16)=104 | |
| β1 | 3.37±1.86 | 3.15±1.23 | t (27)=0.39 | |
| β2ALL | 0.43±0.29 | 0.36±0.25 | U (14,16)=95 | |
| β252 kDa | 0.15±0.10 | 0.11±0.05 | U (14,16)=90 | |
| β250 kDa | 0.25±0.20 | 0.20±0.12 | U (14,16)=96 | |
| β248 kDa | 0.11±0.07 | 0.11±0.07 | t (28)<0.01 | |
| γ2 | 13.64±12.19 | 20.42±20.43 | U (13,16)=67 | |
| ER | ||||
| α1 | 0.23±0.19 | 0.26±0.18 | U (12,15)=80 | |
| α2ALL | 0.21±0.24 | 0.17±0.14 | U (13,16)=99 | |
| α251 | 0.29±0.32 | 0.25±0.16 | U (13,16)=91 | |
| β1 | 0.40±0.36 | 0.34±0.27 | U (13,16)=99 | |
| β2ALL | 0.15±0.12 | 0.27±0.27 | U (14,16)=61 | 0.03 |
| β250 kDa | 0.12±0.09 | 0.21±0.15 | U (14,15)=59 | <0.05 |
| β248 kDa | 0.30±0.24 | 0.40±0.31 | U (14,16)=93 | |
| γ2 | 1.91±1.49 | 2.20±0.77 | t (25)=1.03 | |
| Synaptic | ||||
| α1 | 21.83±19.70 | 25.19±18.32 | U (13,14)=75 | |
| α2ALL | 23.65±23.55 | 19.71±13.29 | t (27)=0.54 | |
| α251 | 50.41±31.80 | 49.88±35.37 | U (13,15)=82 | |
| α249 | 28.57±31.81 | 23.06±19.54 | U (13,14)=80 | |
| β1 | 33.31±33.48 | 29.93±23.39 | U (13, 15)=94 | |
| β2ALL | 31.00±23.01 | 39.87±25.53 | t (27)=0.98 | |
| β252 kDa | 22.13±11.82 | 39.55±25.90 | U (13,15)=64 | |
| β250 kDa | 28.09±21.46 | 37.39±21.84 | U (14,15)=74 | |
| β248 kDa | 39.34±31.59 | 45.62±35.16 | U (14,15)=96 | |
| γ2 | 199.80±96.41 | 232.70±120.50 | t (22)=0.74 | |
Abbreviations: ER, endoplasmic reticulum; GABAAR, γ-aminobutyric acid type A receptor; STG, superior temporal gyrus.
Values are reported as means±s.d. For normally distributed dependent measures, data were analyzed using Student's t-tests; for dependent measures that were not normally distributed, data were analyzed using the Mann–Whitney U-test. Test statistics which met the threshold for significance (α=0.05) are listed in bold.
The ratios of β2 GABAAR subunit isoforms are altered in the ER and SYN fractions in schizophrenia
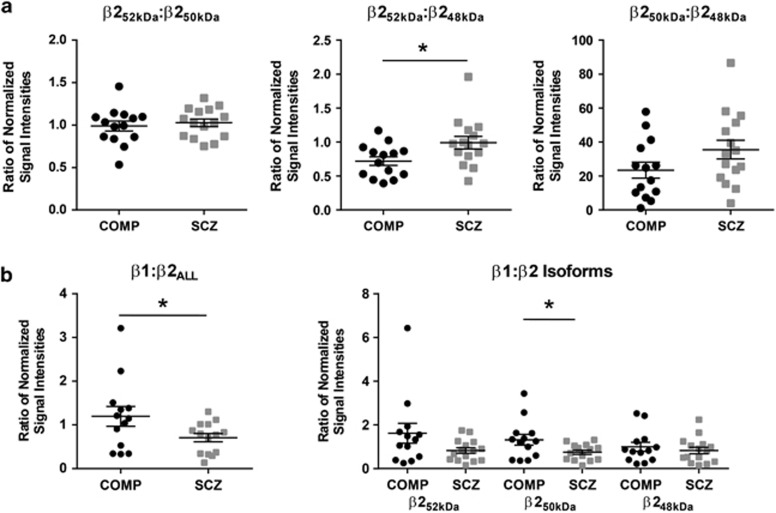
In the ER fraction, the ratio of β248 kDa:β2ALL GABAAR subunit expression is decreased in schizophrenia (t(28)=3.2, P<0.01, q<0.01; Figure 2b, Table 3) and is not different in the total homogenate or the SYN fraction (Table 3). The ratio of β250 kDa:β248 kDa GABAAR subunit expression is increased in the ER in schizophrenia (U(14,16)=45, P<0.01, q=0.01; Figure 2c, Table 3) but unchanged in the SYN fraction (Figure 3a, Table 3). The ratio of β252 kDa:β248 kDa GABAAR subunit expression is not different in total homogenate (Table 3), but is increased in the SYN fraction (U(14,15)=54, P=0.03, q<0.01; Figure 3a, Table 3). There is no difference in the ratio of β250 kDa:β2ALL GABAAR subunit expression between diagnostic groups in the ER (Figure 2b, Table 3), nor is there a difference in the total homogenate or SYN fractions for the ratios of β250 kDa:β2ALL or β252 kDa:β250 kDa GABAAR subunit expression (Figure 3a, Table 3).
Table 3. Ratios of GABAAR subunit isoforms are altered in schizophrenia in the ER and synaptic fractions.
| GABAAR subunit isoform ratios | Comparison | Schizophrenia | Test statistic (d.f.) | P-value | q-value |
|---|---|---|---|---|---|
| Total | |||||
| α1:α2 | 50.41±31.80 | 49.88±35.37 | U (12,12)=71 | ||
| β1:β2ALL | 9.33±4.89 | 9.20±4.83 | U (14,14)=96 | ||
| β1:β252 kDa | 24.67±14.42 | 27.46±10.69 | U (13,14)=63 | ||
| β1:β250 kDa | 15.16±5.97 | 17.36±9.04 | U (14,14)=91 | ||
| β1:β248 kDa | 31.94±14.35 | 33.11±16.75 | t (25)=0.19 | ||
| β252 kDa:β2ALL | 0.36±0.08 | 0.35±0.12 | t (28)=0.26 | ||
| β250 kDa:β2ALL | 0.60±0.12 | 0.60±0.19 | U (14,16)=99 | ||
| β248 kDa:β2ALL | 0.26±0.04 | 0.30±0.12 | U (14,16)=93 | ||
| β252 kDa:β250 kDa | 0.62±0.21 | 0.60±0.21 | t (28)=0.25 | ||
| β252 kDa:β248 kDa | 1.39±0.38 | 1.20±0.40 | t (28)=1.35 | ||
| β250 kDa:β248 kDa | 2.39±0.73 | 2.12±0.86 | U (14,16)=81 | ||
| ER | |||||
| α1:α2 | 1.40±0.97 | 1.15±0.65 | t (26)=0.81 | ||
| β1:β2ALL | 3.04±2.21 | 1.48±1.02 | U (13,16)=53 | 0.03 | 0.03 |
| β1:β250 kDa | 3.56±2.60 | 1.63±1.04 | t (27)=2.72 | 0.01 | 0.02 |
| β1:β248 kDa | 1.69±1.45 | 1.01±0.64 | t (27)=1.70 | ||
| β250 kDa:β2ALL | 0.86±0.08 | 0.91±0.16 | t (28)=1.23 | ||
| β248 kDa:β2ALL | 2.08±0.63 | 1.47±0.40 | t (28)=3.21 | <0.01 | <0.01 |
| β250 kDa:β248 kDa | 0.45±0.14 | 0.68±0.27 | U (14,16)=45 | <0.01 | 0.01 |
| Synaptic | |||||
| α1:α2 | 1.07±0.88 | 1.01±0.72 | U (13,13)=84 | ||
| β1:β2ALL | 1.19±0.82 | 0.71±0.36 | U (13,14)=49 | 0.04 | 0.01 |
| β1:β252 kDa | 3.04±2.21 | 1.48±1.02 | U (13,15)=66 | ||
| β1:β250 kDa | 3.04±2.21 | 1.48±1.02 | t (25)=2.20 | 0.04 | 0.01 |
| β1:β248 kDa | 3.04±2.21 | 1.48±1.02 | t (26)=0.67 | ||
| β252 kDa:β2ALL | 0.88±0.19 | 0.95±0.18 | t (26)=0.99 | ||
| β250 kDa:β2ALL | 0.90±0.06 | 0.94±0.13 | U (14,14)=80 | ||
| β248 kDa:β2ALL | 1.31±0.38 | 1.19±0.59 | U (14,15)=69 | ||
| β252 kDa:β250 kDa | 0.99±0.22 | 1.03±0.17 | t (27)=0.52 | ||
| β252 kDa:β248 kDa | 0.72±0.24 | 0.99±0.36 | U (14, 15)=54 | 0.03 | <0.01 |
| β250 kDa:β248 kDa | 23.46±17.42 | 35.56±21.43 | t (27)=1.66 | ||
Abbreviations: ER, endoplasmic reticulum; GABAAR, γ-aminobutyric acid type A receptor.
Values are reported as means±s.d. For the normally distributed dependent measures, data were analyzed using Student's t-tests; for the dependent measures that were not normally distributed, data were analyzed using the Mann–Whitney U-test. Test statistics which met the threshold for significance (α=0.05, q*=0.05) are listed in bold.
Figure 3.
The ratio of β1:β2ALL, β1:β250 kDa and β252 kDa:β248 kDa GABAAR subunit expression is increased in the SYN fraction in schizophrenia. Western blot analysis of the ratios of β1 and β2 GABAAR subunit isoform expression in the SYN fraction in schizophrenia and comparison subjects. (a) The ratio of β252 kDa:β248 kDa GABAAR subunit expression is increased in schizophrenia, with no difference between groups for the ratio of β252 kDa:β250 kDa or β250 kDa:β248 kDa GABAAR subunit expression in the SYN fraction. (b) The ratio of β1:β2ALL and β1:β250 kDa GABAAR subunit expression is decreased in the SYN fraction in schizophrenia. Data are expressed as a ratio of the signal intensity of protein targets in the SYN fraction normalized to VCP as a loading control and gephyrin as an inhibitory synaptic marker relative to the VCP-normalized signal intensity of the same targets in total homogenate for each subject; data are means±s.e.m. *P<0.05. COMP, comparison subject; GABAAR, γ-aminobutyric acid type A receptor; SCZ, schizophrenia; SYN, synapse; VCP, valosin-containing protein.
The ratio of β1 to β2 GABAAR subunit expression is decreased in the ER and SYN fractions in schizophrenia
There was no difference in the relative amount of the β1 GABAAR subunit expressed in the total, ER or SYN fractions between schizophrenia and comparison subjects (Figure 4, Table 2). However, the ratio of β1:β2 ALL GABAAR subunit expression, while not different in total homogenate (Table 3), was significantly reduced in the ER (U (13,16)=53, P=0.03, q=0.03; Figure 2e, Table 3) and SYN fractions (U (13,14)=49, P=0.04; Figure 3b, Table 3) in schizophrenia. The ratio of β1:β250 kDa expression was also less in both the ER (U (13,16)=49, P=0.02, q=0.02; Figure 2e, Table 3) and SYN (t (25)=2.2, P=0.04; Figure 3b, Table 3) fractions in schizophrenia, with no difference between groups in total homogenate (Table 3). The ratios of β1:β252 kDa and β1:β248 kDa were unchanged in the total homogenate (Table 3) and SYN fractions (Figure 3b, Table 3) in schizophrenia and the ratio of β1:β248 kDa was also unchanged in ER (Figure 2a, Table 3). Post hoc analysis of medication status found the ratio of β1:β2ALL in the SYN fraction decreased in schizophrenia subjects ‘off' medication relative to comparison subjects (U (3,13)=5, P=0.05) as well as a decrease of the β1:β2ALL ratio in schizophrenia subjects ‘off' medication versus ‘on' medication (U (3,11)=3, P=0.04) in the SYN fraction (Supplementary Figure 2). Similarly, the β1:β250 kDa ratio was greater in comparison subjects than schizophrenia subjects ‘off' medication (U (3, 13)=2, P=0.01) and was greater in schizophrenia subjects ‘on' medication versus ‘off' medication (U (3,14)=0, P<0.01; Supplementary Figure 2).
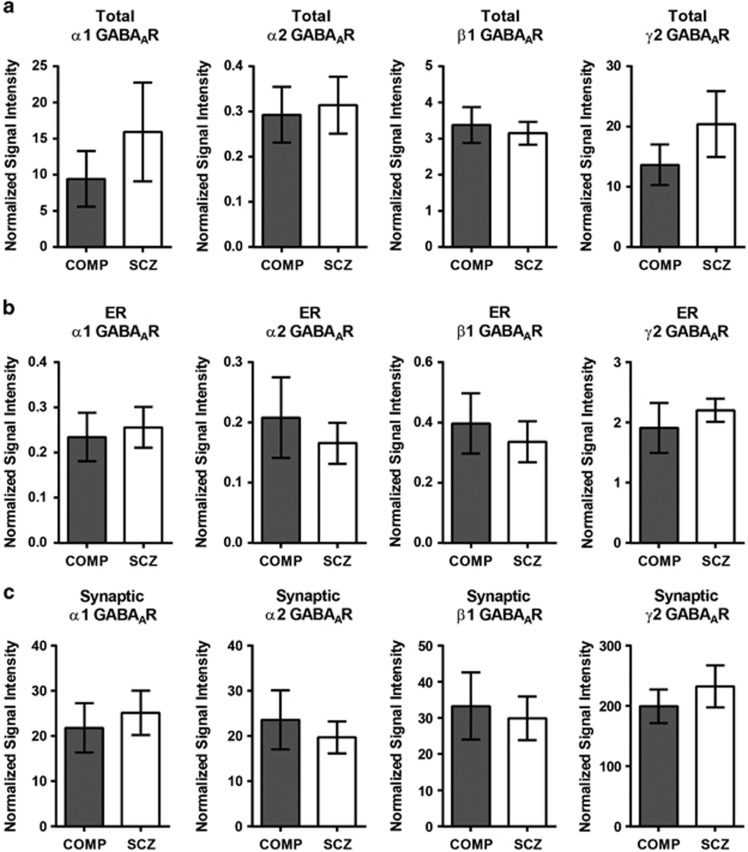
Figure 4.
α1, α2, β1 and γ2 GABAAR subunit expression are not different between diagnostic groups in the total homogenate, ER or SYN fractions. Western blot analysis of α1, α2ALL, β1 and γ2 GABAAR subunit expression in schizophrenia and comparison subjects. There are no differences between diagnostic groups in the protein expression of α1, α2ALL, β1 or γ2 GABAAR subunits in (a) the total homogenates, (b) ER fractions or (c) the SYN fractions. Data are expressed as the mean signal intensity (±s.e.m.) of protein targets in the ER fraction normalized to VCP as a loading control, and JM4 as an ER marker or gephyrin as an inhibitory synapse marker, relative to the VCP-normalized signal intensity of the same target in the total homogenate. COMP, comparison subject; ER, endoplasmic reticulum; GABAAR, γ-aminobutyric acid type A receptor; SCZ, schizophrenia; SYN, synapse; VCP, valosin-containing protein.
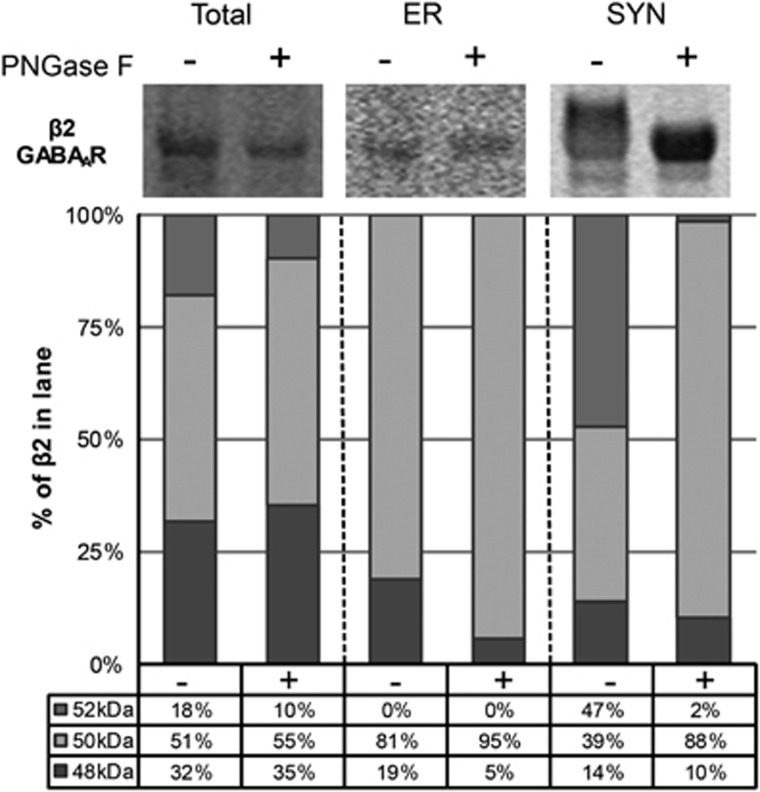
The β252 kDa GABAAR subunit isoform represents an N-glycosylated form of β2
After cleavage of immature and mature N-glycans with PNGase F from subcellular fractions, the relative contribution of each β2 GABAAR subunit isoform to the total β2 GABAAR subunit protein expression was assessed in each fraction. The signal intensity of the β252 kDa GABAAR subunit isoform is greatly reduced and the signal intensity of the β250 kDa GABAAR subunit isoform exhibits a corresponding increase in signal intensity in the SYN fraction after deglycosylation (Figure 5). Although the calculated percentage of β248 kDa GABAAR in the ER fraction is also modestly reduced after PNGase F treatment, this is likely an artifact due to the low signal intensity values for protein bands measured in this assay from those lanes.
Figure 5.
The β252 kDa GABAAR subunit isoform is N-glycosylated in postmortem human cortex. Representative images of western blots probed for the β2 GABAAR subunit in total homogenate, ER and SYN fractions with and without N-glycans cleaved by treatment with the deglycosylating enzyme PNGase F and corresponding graphs of β2 GABAAR subunit isoform protein expression as a percentage of total β2 GABAAR subunit in each lane. In brief, subcellular fractions generated from cortical homogenates were denatured and deglycosylated with PNGase F. Image Studio software was used to measure the signal intensity of protein bands at 52, 50 and 48 kDa in each lane. The signal intensity of each isoform was then divided by the sum of signal intensities for all the three isoforms to determine the percentage of total β2 GABAAR subunit expressed in the lane. After deglycosylation with PNGase F, the percentage of β252 kDa GABAAR is greatly reduced with a corresponding increase of β250 kDa GABAAR expressed in the SYN fraction. The calculated percentage of β248 kDa GABAAR in the ER fraction is also reduced after PNGase F treatment; however, this is likely an artifact due to the low signal intensity values for protein bands measured in those lanes. ER, endoplasmic reticulum; GABAAR, γ-aminobutyric acid type A receptor; PNGase F, peptide N-glycosidase F; SCZ, schizophrenia; SYN, synapse.
Protein expression of α1, α2, β1 and γ2 GABAAR subunits is unchanged in the STG in schizophrenia
The α1 subunit appears as a single band at 52 kDa in the total homogenate, ER and SYN fractions. In the total homogenate and the SYN fraction, the α2 subunit appears as a doublet (α2ALL). The higher molecular mass band at ~51 kDa (α251 kDa) is expressed discretely in the ER and the lower molecular mass band at 49 kDa (α249 kDa) is expressed in the total homogenate and SYN fractions. The β1 GABAAR subunit appears as a doublet at ~50–52 kDa in the total homogenate, ER and SYN fractions. The γ2 GABAAR subunit is present in all the fractions and appears as a single band at ~51 kDa. There is no difference in the protein expression of the α1, β1 and γ2 GABAAR subunits between schizophrenia and comparison subjects in total homogenate, ER or SYN fractions (Figure 4, Table 2). There is also no significant difference between diagnostic groups in the expression of α2ALL (Figure 4, Table 2), nor the α251 kDa or α249 kDa isoforms when assessed individually in any fraction (Table 2).
Discussion
These data indicate abnormal subcellular expression of the β1 and β2 GABAAR subunits in the schizophrenia brain. We have previously reported that the 49 kDa β1 GABAAR subunit is more immaturely N-glycosylated and the total N-glycosylation of the β2 subunit is altered in schizophrenia, suggesting a mechanism underlying abnormal GABAAR subunit assembly, altered cell surface expression and trafficking disruptions in this illness.36 Although we did not find any abnormalities in β1 GABAAR subunit expression in subcellular compartments, we identified increased expression of β2ALL driven by increased β250 kDa in the ER in schizophrenia.
To assess the relative abundance and localization of the β1 and β2 GABAAR subunits and β2 GABAAR subunit isoforms, we calculated the ratios of β1 and β2 isoforms in the ER and SYN fractions to assess potential differences in GABAAR subunit composition between schizophrenia and comparison subjects. In the ER fraction, we identified a significant decrease in the ratios of β1:β2ALL, β1:β250 kDa and β248 kDa:β2ALL and a significant increase in the ratio of β250 kDa:β248 kDa. Together, these data suggest a relative reduction in the expression of β1 GABAAR subunits, as well as a reduction of β248 kDa isoform expression in the ER in schizophrenia. In addition, these data suggest that the β250 kDa isoform is expressed more abundantly in the ER, which may reflect that this isoform is more likely to be incorporated into intact receptors trafficked to the cell membrane for expression at the synapse.
Consistent with our findings in the ER, the ratios of β1:β2ALL and β1:β250 kDa GABAAR subunits were decreased in the SYN fraction, suggesting a relative increase of β250 kDa isoform expression and a relative decrease in β1 GABAAR subunit expression at the synapse in schizophrenia. We also determined that the ratio of β252 kDa:β248 kDa GABAAR subunit isoforms is increased in the SYN fraction in schizophrenia, which we consider to be indicative of increased synaptic β252 kDa and decreased synaptic β248 kDa. Because we could not measure the β252 kDa GABAAR isoform in the ER fraction, and the difference associated with β252 kDa is only apparent relative to β248 kDa isoform expression in the SYN fraction, it is unclear whether the β252 kDa isoform is specifically increased at the synapse; however, despite this confound, the altered ratios in the SYN fraction appear to be consistent with a relative increase of the β252 kDa and β250 kDa GABAAR subunit isoforms, and a relative decrease of β1 and β248 kDa GABAAR subunits incorporated into synaptic GABAARs. We have also shown that the β252 kDa GABAAR subunit in the SYN fraction likely represents an N-glycosylated form of β2 which, consistent with previous in vitro studies, suggests that N-glycosylated forms of the β2 GABAAR subunit may be preferentially incorporated into intact, synaptically expressed receptors. Interestingly, the decreased ratios of β1:β2ALL and β1:β250 kDa in the SYN fraction in schizophrenia appear to be ameliorated by the effects of antipsychotic medications, with schizophrenia subjects ‘on' medication more closely resembling comparison subjects. This suggests that treatment with antipsychotic medication may result in increased expression of β1-containing GABAARs expressed at the synapse relative to those containing β2 subunits, possibly by inhibiting incorporation of the β250 kDa subunit into synaptically targeted receptors.
In humans, the β2 GABAAR subunit protein is expressed as four isoforms (β2L, β2S, β2S1 and β2S2) that are the result of mRNA splice variants which can be regulated by epigenetic modifications at key neurodevelopmental time points.74, 75, 76, 77 The β2L and β2S isoforms are distinguished by the inclusion of exon 10 in the β2L isoform, to produce subunits with predicted molecular masses of 60 kDa and 54 kDa, respectively.75, 76 The β2S1 and β2S2 isoforms were subsequently identified with predicted molecular masses of 36 kDa and 42 kDa, respectively, and are differentiated from the β2L and β2S isoforms by the exclusion of the fourth transmembrane domain of the subunit.75 On the basis of the predicted molecular masses of β2 GABAAR subunit isoforms, we propose that the antibody used in this study labeled posttranslationally modified and unmodified forms of the β2S and/or β2S2 isoforms.
In genetic studies, chromosome 5q34, where the GABRB2 gene is located, has been identified as a region of interest for schizophrenia-related risk alleles.34, 78, 79 In addition, schizophrenia has been associated with a recombination hotspot80 and there is evidence for multiple schizophrenia-associated single-nucleotide polymorphisms in both coding and non-coding regions of the GABRB2 gene.74, 76, 80, 81, 82 Some GABRB2 single-nucleotide polymorphism haplotypes appear to be subject to regulation by parental imprinting or other epigenetic modifications, which may explain inconsistencies in previous reports examining the role of chromosome 5 in schizophrenia susceptibility risk in different patient populations.74, 79, 83 The variability of GABRB2 mRNA splice variant expression and corresponding alterations in β2 GABAAR subunit protein isoforms expressed as a result of epigenetic or neurodevelopmental changes supports the two-hit model of schizophrenia,84 which posits that genetic predisposition to the disorder in combination with some environmental factor(s) contributes to the onset of psychosis and the conversion from prodromal to symptomatic patient phenotypes.
It has been established that the subunit composition of heteropentameric GABAARs affects the signaling properties of the receptor,25, 26, 52, 85, 86, 87, 88, 89, 90, 91, 92 and it has more recently been shown that the specific β2 GABAAR subunit isoform incorporated into the intact receptor has a significant role in the functional and electrophysiological properties of GABAARs.75, 82, 89 In addition to isoform-specific differences in GABAergic signaling, posttranslational modifications of the β2 GABAAR subunit are known to affect heteropentamer assembly, receptor trafficking, cell surface expression, membrane stability, ligand-binding affinity, channel gating properties and receptor kinetics.91, 93 N-glycosylation-deficient β2 GABAAR subunits expressed in binary α1β2 GABAARs in the plasma membrane in vitro display reduced current amplitude and decreased long single-channel openings; N-glycosylation of β2 GABAAR at N104 has been shown to affect heteropentamer assembly; and proper immature N-glycosylation at N173 affects the stability of individual β2 GABAAR subunits in the ER,93 which together illustrate that early protein processing can substantially affect not only the composition and surface expression of specific β2 GABAAR subunit isoforms, but also the signaling properties of intact GABAARs.
On the basis of prior findings that the α2 GABAAR subunit is expressed more highly in intact GABAARs in axosomatic synapses of pyramidal neurons in the dorsolateral prefrontal cortex in schizophrenia,60 in conjunction with our prior report demonstrating a smaller immature N-glycan attached to the α1 GABAAR subunit in schizophrenia,36 we anticipated finding increased abundance of α1 GABAAR subunits in the ER fraction, consistent with retention of this subunit in the calnexin–calreticulin protein-folding cycle, and a decreased ratio of α1:α2 GABAAR subunits in both the ER and SYN fractions. Although we found no difference in the abundance of the α1 or α2 GABAAR subunits, nor a change in the ratio of α1:α2 GABAAR subunits in the ER or SYN fractions in schizophrenia versus comparison subjects, this can be reconciled with the earlier findings. It has been shown in vitro that only 25% of translated subunits are assembled into intact GABAARs, which are then trafficked to the cell membrane;53, 94 and although our previous N-glycosylation findings indicate possible α1 GABAAR subunit retention in the ER, it is also possible that aberrantly N-glycosylated α1 GABAAR subunits may be rapidly expelled from the ER and undergo ER-associated degradation via the ubiquitin–proteasome system instead of remaining sequestered in the ER.42, 53, 54, 55, 95
Because α1 and β2 GABAAR subunits preferentially co-assemble in intact receptors, and our data suggest relatively more β2 GABAAR expression in the ER and SYN fractions, another possible explanation may be that the increased expression of β2 versus β1 GABAAR subunits in schizophrenia facilitates α1 versus α2 assembly into intact, synaptically targeted GABAARs. In addition, because we used specific biochemical methods to isolate a synapse-enriched fraction from cortical homogenate, the SYN fraction is enriched for a combination of excitatory and inhibitory synapses, as well as a combination of axosomatic and dendritic synapses and, as such, we may be unable to identify alterations that are specific to inhibitory axosomatic synapses on pyramidal neurons. The possibility that altered ratios of α1:α2 GABAAR subunits may be masked when measured in our assays, or that α-subunit-specific alterations may be more readily evident in the other cortical areas, such as the dorsolateral prefrontal cortex, cannot be ruled out.
We measured the expression of γ2 GABAAR subunits in the fractions as an indirect measure of intact GABAAR localization, owing to the role of the γ2 subunit in synaptic targeting via its interaction with GABAAR-associated protein, GABARAP, an essential component of the GABAAR trafficking machinery.52, 53, 55, 56, 57 We found no difference in γ2 GABAAR subunit expression in the ER or SYN fractions between schizophrenia and comparison subjects. This suggests that intact synaptically targeted heteropentameric GABAARs are being assembled in the ER and localized to the synapse, but does not exclude the possibility that other GABAAR subunits or specific subunit isoforms incorporated into γ2-containing GABAAR s may be altered in schizophrenia.
As with all the postmortem studies, there are several limitations to this work. As mentioned previously, the diagnostic groups were not equally matched for sex. Although we did not identify any sex effects for any significant dependent measure (Supplementary Figure 1), the relatively small sample size may not be sufficient to reliably identify sex-specific abnormalities of GABAAR subunit expression and localization in schizophrenia. In addition, the age range of subjects in this study was 53–97 years at the time of death; thus, these findings may not be generalizable to younger patients in the earlier stages of the disorder. Post hoc statistical analyses were performed in an effort to control for these limitations.
Given our previous report of increased immature N-glycosylation of the β149 kDa GABAAR subunit and altered total N-glycosylation of the β2 GABAAR subunit, our current data indicating increased β250 kDa and decreased β1 and β248 kDa GABAAR subunits in both the ER and SYN fractions and increased β252 kDa in the SYN fraction in schizophrenia provide evidence that proper ER processing and synaptic targeting of β1- and β2-containing GABAARs are affected by N-glycosylation abnormalities in schizophrenia. Our current data suggest that there is an increase of N-glycosylated β252 kDa GABAAR subunits expressed synaptically in the STG in schizophrenia. The disparate expression of β2 subunit isoforms at the synapse suggests a GABAAR subunit-mediated postsynaptic abnormality in GABAergic signaling in schizophrenia and, as such, could potentially be a target for pharmacological intervention. The subunit composition of GABAARs is disrupted in multiple brain regions in schizophrenia, and although prior studies have highlighted alterations in membrane expression of the α1 and α2 GABAAR subunits, further investigation of the functional consequences of aberrant β1 and β2 GABAAR subunit isoform membrane expression may provide additional insight into the etiology of GABAergic signaling deficits in schizophrenia.
Acknowledgments
We thank Jana B Drummond for her assistance in the development of the subcellular fractionation protocol. This work is supported by National Institutes of Health Grant MH53327 (JHM-W), MH064673 (VH) and MH066392 (VH).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Liu F, Moss SJ, Brandon NJ. GABAA receptors and their associated proteins: Implications in the etiology and treatment of schizophrenia and related disorders. Neuropharmacology. 2009;57:481–495. doi: 10.1016/j.neuropharm.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo T-UW. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry. 1999;46:616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Early developmental disturbances of cortical inhibitory neurons: contribution to cognitive deficits in schizophrenia. Schizophr Bull. 2014;40:952–957. doi: 10.1093/schbul/sbu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. J Physiol. 2012;590 (Pt 4:715–724. doi: 10.1113/jphysiol.2011.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Rev. 2000;31:251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the ‘right stuff'. Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia. Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Guidotti AR, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase 67 (GAD 67 ) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Avilés JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Wang X, Adler AL, Mozhui K, Lu L, Williams RW. Complex control of GABA(A) receptor subunit mRNA expression: variation, covariation, and genetic regulation. PLoS One. 2012;7:e34586. doi: 10.1371/journal.pone.0034586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Whiting P, McKernan RM, Iversen LL. Another mechanism for creating diversity in gamma-aminobutyrate type A receptors: RNA splicing directs expression of two forms of gamma 2 phosphorylation site. Proc Natl Acad Sci USA. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21:999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor α1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (hGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Weickert CS. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Rooney RJ, Thuras PD. Expression of GABAA α2-, β1- and ɛ-receptors are altered significantly in the lateral cerebellum of subjects with schizophrenia, major depression and bipolar disorder. Transl Psychiatry. 2013;3:e303. doi: 10.1038/tp.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Wang X, Beveridge NJ, Tooney PA, Scott RJ, Carr VJ, et al. Transcriptome sequencing revealed significant alteration of cortical promoter usage and splicing in schizophrenia. PLoS One. 2012;7:e36351. doi: 10.1371/journal.pone.0036351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshen TL, Middleton FA, Tahl AR, Rockwell GN, Purcell SM, Aldinger KA, et al. Genetic investigation of chromosome 5q GABAA receptor subunit genes in schizophrenia Mol Psychiatry 2005101074–1088.1057. [DOI] [PubMed] [Google Scholar]
- Bullock WM, Cardon K, Bustillo J, Roberts RC, Perrone-Bizzozero NI. Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry. 2008;165:1594–1603. doi: 10.1176/appi.ajp.2008.07121845. [DOI] [PubMed] [Google Scholar]
- Mueller TM, Haroutunian V, Meador-Woodruff JH. N-Glycosylation of GABAA receptor subunits is altered in Schizophrenia. Neuropsychopharmacology. 2014;39:528–537. doi: 10.1038/npp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Huang X-F. Increased density of GABAA receptors in the superior temporal gyrus in schizophrenia. Exp Brain Res. 2006;168:587–590. doi: 10.1007/s00221-005-0290-9. [DOI] [PubMed] [Google Scholar]
- Chen J, Xu Y, Zhang K, Liu Z, Xu C, Shen Y, et al. Comparative study of regional homogeneity in schizophrenia and major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:36–43. doi: 10.1002/ajmg.b.32116. [DOI] [PubMed] [Google Scholar]
- Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, et al. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;63:465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF.Auditory cortex anatomy and asymmetry in schizophreniaIn: Lajtha A, Javitt D, Kantrowitz J (eds). Handbook of Neurochemistry and Molecular Neurobiology Springer: Boston, MA USA; 2009353–381. [Google Scholar]
- Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Cai G, Salonikidis PS, Fei J, Schwarz W, Schülein R, Reutter W, et al. The role of N-glycosylation in the stability, trafficking and GABA-uptake of GABA-transporter 1. Terminal N-glycans facilitate efficient GABA-uptake activity of the GABA transporter. FEBS J. 2005;272:1625–1638. doi: 10.1111/j.1742-4658.2005.04595.x. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Lau KS, Demetriou M, Nabi IR. Adaptive regulation at the cell surface by N-glycosylation. Traffic. 2009;10:1569–1578. doi: 10.1111/j.1600-0854.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- Roth J, Zuber C, Park S, Jang I, Lee Y, Kysela KG, et al. Protein N-glycosylation, protein folding, and protein quality control. Mol Cells. 2010;30:497–506. doi: 10.1007/s10059-010-0159-z. [DOI] [PubMed] [Google Scholar]
- Parodi AJ. Protein glucosylation and its role in protein folding. Annu Rev Biochem. 2000;69:69–93. doi: 10.1146/annurev.biochem.69.1.69. [DOI] [PubMed] [Google Scholar]
- Ito Y, Takeda Y. Analysis of glycoprotein processing in the endoplasmic reticulum using synthetic oligosaccharides. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:31–40. doi: 10.2183/pjab.88.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivari S, Molinari M. Glycoprotein folding and the role of EDEM1, EDEM2 and EDEM3 in degradation of folding-defective glycoproteins. FEBS Lett. 2007;581:3658–3664. doi: 10.1016/j.febslet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophr Res. 2013;146:177–183. doi: 10.1016/j.schres.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, McMillan LD, Haroutunian V, Meador-Woodruff JH. N-linked glycosylation of cortical N-methyl-D-aspartate and kainate receptor subunits in schizophrenia. Neuroreport. 2013;24:688–691. doi: 10.1097/WNR.0b013e328363bd8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res. 2010;117:92–98. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91:1009–1022. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking. Mol Neurobiol. 2002;26:251–268. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Lüscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Lüscher B. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cho R, Carter CS. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Keefe RSE, Lieberman JA, Barch DM, Csernansky JG, Goff DC, et al. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biol Psychiatry. 2011;69:442–449. doi: 10.1016/j.biopsych.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio MD, Wood K, Haroutunian V, Meador-Woodruff JH. Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology. 2013;38:1910–1920. doi: 10.1038/npp.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powchik P, Davidson M, Nemeroff CB, Haroutunian V, Purohit DP, Losonczy M, et al. Alzheimer's-disease-related protein in geriatric schizophrenic patients with cognitive impairment. Am J Psychiatry. 1993;150:1726–1727. doi: 10.1176/ajp.150.11.1726. [DOI] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Gorman JM, Haroutunian V. Variations in differential gene expression patterns across multiple brain regions in schizophrenia. Schizophr Res. 2005;77:241–252. doi: 10.1016/j.schres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Purohit DP, Perl DP, Haroutunian V, Powchik P, Davidson M, Davis KL. Alzheimer disease and related neurodegenerative diseases in elderly patients with schizophrenia: a postmortem neuropathologic study of 100 cases. Arch Gen Psychiatry. 1998;55:205–211. doi: 10.1001/archpsyc.55.3.205. [DOI] [PubMed] [Google Scholar]
- Hammond JC, Meador-Woodruff JH, Haroutunian V, McCullumsmith RE. AMPA receptor subunit expression in the endoplasmic reticulum in frontal cortex of elderly patients with schizophrenia. PLoS One. 2012;7:e39190. doi: 10.1371/journal.pone.0039190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan D, Mount D, Moore S, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal partitioning of hexokinase 1 suggests disruption of a glutamate transport protein complex in schizophrenia. Schizophr Res. 2014;154:1–13. doi: 10.1016/j.schres.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, et al. Human postmortem tissue: what quality markers matter. Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Expression of four housekeeping proteins in elderly patients with schizophrenia. J Neural Transm. 2009;116:487–491. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Mukherjee J, Maric H-M, Schindelin H, Sieghart W, Moss SJ. Gephyrin, the enigmatic organizer at GABAergic synapses. Front Cell Neurosci. 2012;6:23. doi: 10.3389/fncel.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, et al. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- Zhao C, Wang F, Pun FW, Mei L, Ren L, Yu Z, et al. Epigenetic regulation on GABRB2 isoforms expression: developmental variations and disruptions in psychotic disorders. Schizophr Res. 2012;134:260–266. doi: 10.1016/j.schres.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Zhao C, Xu Z, Wang F, Chen J, Ng S-K, Wong P-W, et al. Alternative-splicing in the exon-10 region of GABA(A) receptor beta(2) subunit gene: relationships between novel isoforms and psychotic disorders. PLoS One. 2009;4:e6977. doi: 10.1371/journal.pone.0006977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley D, Lennon D, Carter D. Cloning, sequence analysis and expression of two forms of mRNA coding for the human β 2 subunit of the GABA A receptor. Mol Brain Res. 1995;28:175–179. doi: 10.1016/0169-328x(94)00228-7. [DOI] [PubMed] [Google Scholar]
- Magrane M, Consortium U UniProt Knowledgebase: a hub of integrated protein data. Database (Oxford) 2011;2011:bar009. doi: 10.1093/database/bar009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Brynjolfsson J, Petursson H, Potter M, Dudleston K, Barraclough B, et al. Localization of a susceptibility locus for schizophrenia on chromosome 5. Nature. 1988;336:164–167. doi: 10.1038/336164a0. [DOI] [PubMed] [Google Scholar]
- Pato CN, Lander ES, Schulz SC. Prospects for the genetic analysis of schizophrenia. Schizophr Bull. 1989;15:365–372. doi: 10.1093/schbul/15.3.365. [DOI] [PubMed] [Google Scholar]
- Ng S-K, Lo W-S, Pun FW, Zhao C, Yu Z, Chen J, et al. A recombination hotspot in a schizophrenia-associated region of GABRB2. PLoS One. 2010;5:e9547. doi: 10.1371/journal.pone.0009547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tsang S-Y, Zhao C, Pun FW, Yu Z, Mei L, et al. GABRB2 in schizophrenia and bipolar disorder: disease association, gene expression and clinical correlations. Biochem Soc Trans. 2009;37 (Pt 6:1415–1418. doi: 10.1042/BST0371415. [DOI] [PubMed] [Google Scholar]
- Zhao C, Xu Z, Chen J, Yu Z, Tong K-L, Lo W-S, et al. Two isoforms of GABA(A) receptor beta2 subunit with different electrophysiological properties: differential expression and genotypical correlations in schizophrenia. Mol Psychiatry. 2006;11:1092–1105. doi: 10.1038/sj.mp.4001899. [DOI] [PubMed] [Google Scholar]
- Pun FW, Zhao C, Lo W-S, Ng S-K, Tsang S-Y, Nimgaonkar V, et al. Imprinting in the schizophrenia candidate gene GABRB2 encoding GABA(A) receptor β(2) subunit. Mol Psychiatry. 2011;16:557–568. doi: 10.1038/mp.2010.47. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the ‘Two hit hypothesis.'. J Psychiatr Res. 1999;33:543–548. doi: 10.1016/s0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Zhou C, Huang Z, Ding L, Deel ME, Arain FM, Murray CR, et al. Altered cortical GABAA receptor composition, physiology, and endocytosis in a mouse model of a human genetic absence epilepsy syndrome. J Biol Chem. 2013;288:21458–21472. doi: 10.1074/jbc.M112.444372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wu Z, Ning G, Guo Y, Ali R, MacDonald RL, et al. γ-Aminobutyric acid type A (GABAA) receptor α subunits play a direct role in synaptic versus extrasynaptic targeting. J Biol Chem. 2012;287:27417–27430. doi: 10.1074/jbc.M112.360461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Roberts JDB, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadingham KL, Wingrove PB, Wafford KA, Bain C, Kemp JA, Palmer KJ, et al. Role of the beta subunit in determining the pharmacology of human gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1993;44:1211–1218. [PubMed] [Google Scholar]
- Kang SU, Heo S, Lubec G. Mass spectrometric analysis of GABAA receptor subtypes and phosphorylations from mouse hippocampus. Proteomics. 2011;11:2171–2181. doi: 10.1002/pmic.201000374. [DOI] [PubMed] [Google Scholar]
- Laha KT, Tran PN. Multiple tyrosine residues at the GABA binding pocket influence surface expression and mediate kinetics of the GABAA receptor. J Neurochem. 2013;124:200–209. doi: 10.1111/jnc.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Regulation of GABA(A) receptor subunit expression by pharmacological agents. Pharmacol Rev. 2010;62:97–135. doi: 10.1124/pr.109.002063. [DOI] [PubMed] [Google Scholar]
- Lo WY, Lagrange AH, Hernandez CC, Harrison R, Dell A, Haslam SM, et al. Glycosylation of β2 subunits regulates GABAA receptor biogenesis and channel gating. J Biol Chem. 2010;285:31348–31361. doi: 10.1074/jbc.M110.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrie GH, Vallis Y, Stephenson A, Whitfield J, Browning B, Smart TG, et al. Assembly of GABAA receptors composed of alpha1 and beta2 subunits in both cultured neurons and fibroblasts. J Neurosci. 1997;17:6587–6596. doi: 10.1523/JNEUROSCI.17-17-06587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-W, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem. 2007;100:279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.