Abstract
Purpose. To explore the potential of diffusion-weighted (DW) magnetic resonance imaging (MRI) using apparent diffusion coefficient (ADC) for predicting the response to neoadjuvant chemotherapy in nasopharyngeal carcinoma (NPC). Methods and Materials. Ninety-two consecutive patients with NPC who underwent three cycles of neoadjuvant chemotherapy were retrospectively analyzed. DW and anatomical MRI were performed before and after neoadjuvant chemotherapy prior to radiotherapy. Pretreatment ADCs and percentage increases in ADC after chemotherapy were calculated for the primary lesions and metastatic adenopathies. Receiver operating characteristic curve analysis was used to select optimal pretreatment ADCs. Results. Pretreatment mean ADCs were significantly lower for responders than for nonresponders (primary lesions, P = 0.012; metastatic adenopathies, P = 0.013). Mean percentage increases in ADC were higher for responders than for nonresponders (primary lesions, P = 0.008; metastatic adenopathies, P < 0.001). The optimal pretreatment primary lesion and metastatic adenopathy ADCs for differentiating responders from nonresponders were 0.897 × 10−3 mm2/sec and 1.031 × 10−3 mm2/sec, respectively. Conclusions. NPC patients with low pretreatment ADCs tend to respond better to neoadjuvant chemotherapy. Pretreatment ADCs could be used as a new pretreatment imaging biomarker of response to neoadjuvant chemotherapy.
1. Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common malignancies in Southeast Asia, especially in the southern provinces of China [1]. In patients with advanced NPC, neoadjuvant chemotherapy has been established as an alternative treatment for reducing tumor size, thereby facilitating local control and improving the disease-free survival rate, but not the overall survival rate, after administration of subsequent concurrent chemoradiotherapy [2–7]. However, not all patients respond to neoadjuvant chemotherapy, so identification of nonresponders at the time of pretreatment staging would allow the treatment regimens of individual patients to be modified or altered to concurrent chemoradiotherapy.
Currently, anatomical magnetic resonance imaging (MRI) is normally used to assess the tumor response after neoadjuvant chemotherapy in NPC [8, 9], though changes in the morphologically based measures of tumor diameter and volume occur relatively late during the treatment course. Furthermore, anatomical MRI cannot be used to predict the tumor response to neoadjuvant chemotherapy prior to treatment. The value of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) with respect to predicting tumor response is often hampered by its false-positive and expensive check cost [10, 11]. Therefore, it would be advantageous to identify novel imaging biomarkers that could predict the response to neoadjuvant chemotherapy prior to treatment in patients with NPC.
Diffusion-weighted (DW) MRI measures the diffusivity of water molecules within tissue extracellular spaces, which is quantified using apparent diffusion coefficients (ADCs). In general, intratumoral cell death induced by treatment increases water diffusion and leads to an increase in the ADC. Some early studies showed that DW MRI is helpful for predicting or detecting the response to chemoradiotherapy or radiosensitivity in head and neck carcinoma [12–19]. However, the efficacy of pretreatment ADCs obtained by DW MRI for predicting the response to neoadjuvant chemotherapy in NPC, or other head and neck carcinomas, has not been reported. The purpose of this study was to evaluate the potential of DW MRI using ADCs for predicting the response to neoadjuvant chemotherapy in patients with NPC.
2. Materials and Methods
2.1. Patients
This retrospective study was approved by the institutional review board and written informed consent was obtained from all participating patients or their next of kin. This study comprised 98 ethnic Chinese patients who were newly diagnosed with untreated and nonmetastatic NPC and who underwent both DW MRI and anatomical MRI before (baseline MRI) and after neoadjuvant chemotherapy but before radiotherapy (follow-up MRI) between March 2010 and April 2014. Median time between baseline MRI and the start of chemotherapy was 8 days (range, 2–21 days). Median time between the completion of chemotherapy and follow-up MRI was 16 days (range, 6–21 days). All 98 patients underwent the same neoadjuvant chemotherapeutic regimen and subsequently received intensity-modulated radiotherapy (IMRT). Six patients were later excluded from the study for the following reasons: four failed to complete three cycles of neoadjuvant chemotherapy, and two had inadequate DW MRI image quality due to excessive susceptibility or motion artifacts. The remaining 92 patients included 70 males (mean age, 46.5 years; range, 21–73 years) and 22 females (mean age, 44.3 years; range, 20–71 years). The World Health Organization (WHO) histologic type distribution was as follows: type I (n = 1), type II (n = 9), and type III (n = 82). According to the 2010 American Joint Committee on Cancer (AJCC) tumor-node-metastases (TNM) staging system [20], the prechemotherapy clinical stage distribution was as follows: stage III, 52 patients; IVA, 29; and IVB, 11.
Neoadjuvant chemotherapy consisted of docetaxel (60 mg/m2 or 65 mg/m2 d1), cisplatin (60 mg/m2 or 65 mg/m2 d1), and fluorouracil (600 mg/m2 or 650 mg/m2 d1–5) via intravenous infusion, repeated every 21 days for 3 cycles. After neoadjuvant chemotherapy, all patients were treated with definitive IMRT followed by concomitant chemotherapy (30–40 mg/m2 cisplatin or nedaplatin weekly).
2.2. Imaging Protocol
All patients underwent MRI using a 1.5 Tesla system (Signa CV/i, GE Healthcare, Milwaukee, WI, USA). The region from the suprasellar cistern to the inferior margin of the sternal end of the clavicle was examined using a head and neck combined coil. A T2-weighted fast spin-echo (FSE) sequence in the axial plane with a matrix of 512 × 512 and repetition time (TR)/echo time (TE) = 2889 ms/70.8 ms, a T1-weighted FSE sequence in the axial, coronal, and sagittal planes with a matrix of 560 × 560 and TR/TE = 627 ms/8.6 ms, and an echo-planar DWI sequence with a matrix of 224 × 224, TR/TE = 1360 ms/89.8 ms, and b-values of 0, 100, 500, and 1,000 s/mm2 were obtained before injection of contrast material. After intravenous injection of Gd-DTPA at a dose of 0.1 mmol/kg body weight, T1-weighted axial and sagittal sequences and T1-weighted fat-suppressed coronal sequences were performed sequentially, with parameters similar to those applied before the Gd-DTPA injection. The section thickness and interslice gap were 5 mm and 0 mm, respectively.
2.3. Image Assessment
The MRI scans were evaluated independently on a work station (Medi-PACS, Vancouver, Canada) by two radiologists, each with over 10 years of experience interpreting NPC MR images; any differences were resolved by consensus. Both radiologists were blinded to the therapeutic responses to neoadjuvant chemotherapy and other clinical findings.
Regions of interest (ROIs) were placed on the primary lesions and metastatic adenopathies on the images acquired using a b-value of 0 s/mm2 (excluding any necrotic regions identified with the aid of the T2-weighted and T1-weighted postcontrast MR images), and then the ROIs were automatically copied to the other b-value images by the software. Subsequently, all ROIs were merged per lesion for each b-value, and the average SI was calculated for the entire lesion. ADCs were derived using the following equation: ADC = −ln[SI(b)/SI(0)]/b, where b is the diffusion weighting factor and SI(b) and SI(0) are the signal intensities with and without diffusion-sensitizing gradients, respectively [19]. Percentage increases in ADC (ADC%) were calculated as follows: ADC% = (ADCpost − ADCpre)/ADCpre × 100, where ADCpre and ADCpost are the pre- and posttreatment ADCs, respectively.
For each lesion, contours were drawn around the lesion border at each slice position based on anatomical MRI. Subsequently, the volume of each lesion was calculated using the following equation: (Σsurface at each slice position) ∗ (slice thickness + interslice gap). Percentage increases in volume (V%) were calculated as follows: V% = (Vpost − Vpre)/Vpre × 100, where Vpre and Vpost are the pre- and posttreatment tumor volume, respectively.
The Response Evaluation Criteria in Solid Tumors (RECIST) guidelines were used to classify patients as responders or nonresponders on the basis of anatomical MRI [21]. A patient was considered to be a responder if all assessable lesions (both primary lesion and metastatic adenopathies) completely disappeared or partially reduced (≥30% in the sum of the maximal diameters) on the follow-up MRI. A patient was considered to be a nonresponder if measurable lesions were stable (<30% reduction or <20% increase in the sum of the maximal diameters) or progressed (≥20% increase in the area(s) of the original lesion(s) or the appearance of new lesions) on the follow-up MRI.
2.4. Statistical Analysis
Interreader agreement was evaluated with Cohen K coefficient for the image assessment. A K value of 0.4–0.6 indicated moderate agreement; 0.6–0.8, good agreement; and above 0.8, very good agreement [22]. The independent-samples t-test was used to compare the responders and nonresponders with respect to tumor volume (mean tumor volume of primary lesions and metastatic adenopathies before and after chemotherapy), pre- and posttreatment mean ADCs, and percentage increases in ADCs after chemotherapy. Fisher's exact test was used to compare age, sex, tumor pathology, and clinical stage between responders and nonresponders. Spearman's rank correlation was performed to evaluate the correlation between (a) the changes in ADCs and change in tumor volume on follow-up MRI and (b) pretreatment tumor ADCs and percentage change in tumor volume after chemotherapy.
To determine the optimal pretreatment ADC cutoff values with which to differentiate responders from nonresponders, receiver operating characteristic (ROC) curves and the areas under the curve (AUCs) were to evaluate the effectiveness of different criteria. The optimal cutoff value was defined as the value corresponding to the highest average sensitivity and specificity. The overall accuracy was represented by the AUC: the larger the area, the better the test. Sensitivity, specificity, and accuracy were calculated using the standard definitions [23]. SPSS version 16.0 (IBM, Armonk, NY, USA) was used for all data analyses, except for Fisher's exact test and ROC curve analysis that were performed using MedCalc software version 10.3.0.0 (MedCalc software, Mariakerke, Belgium). P-values < 0.05 were considered significant.
3. Results
3.1. Interobserver Agreement
In the per-lesion analysis, there was excellent agreement for the image assessment between observers 1 and 2, with K coefficients of 0.930 and 0.932 for pre- and posttreatment volume of primary lesions, 0.937 and 0.934 for pre- and posttreatment volume of metastatic adenopathies, 0.927 and 0.924 for pre- and posttreatment ADCs of primary lesions, and 0.931 and 0.928 for pre- and posttreatment ADCs of metastatic adenopathies. Any differences between observers 1 and 2 were resolved by consensus.
3.2. Treatment Outcomes
After completion of neoadjuvant chemotherapy, the primary tumor treatment responses were distributed as follows: complete resolution, 24 (26.1%) patients; partial resolution, 55 (59.8%) patients; and stability, 13 (14.1%) patients. The treatment responses of the metastatic cervical lymph nodes were distributed as follows: complete resolution, 44 (55.0%) patients; partial resolution, 26 (32.5%) patients; and stability, 10 (12.5%) patients. When the treatment responses of the primary tumor and metastatic cervical lymph nodes were considered together, 76 (82.6%) of the 92 patients were categorized as responders, and 16 (17.4%) were categorized as nonresponders (Table 1). No significant differences were observed between responders and nonresponders with respect to age, sex, tumor histology, or clinical stage (Table 2).
Table 1.
Response to neoadjuvant chemotherapy in 92 patients with NPC.
| Response | Number of patients | ||
|---|---|---|---|
| NP (n = 92) |
LN (n = 80)∗ |
Combination (NP + LN) |
|
| Responders | |||
| Complete response | 24 (26.1%) | 44 (55.0%) | 20 (21.7%) |
| Partial response | 55 (59.8%) | 26 (32.5%) | 56 (60.9%) |
| Nonresponders | |||
| Stable disease | 13 (14.1%) | 10 (12.5%) | 16 (17.4%) |
| Progressive disease | 0 (0%) | 0 (0%) | 0 (0%) |
NP = nasopharynx; LN = regional neck lymph nodes.
∗12 patients with N0 disease were not included in the analysis.
Table 2.
Clinicopathologic features of the responders and nonresponders.
| Characteristic | Number of patients | P-value | |
|---|---|---|---|
| Responders (n = 76) | Nonresponders (n = 16) | ||
| Sex | |||
| Male | 58 | 12 | 1.000 |
| Female | 18 | 4 | |
| Age | |||
| ≥50 years | 26 | 6 | 0.802 |
| <50 years | 50 | 10 | |
| WHO pathologic type | |||
| Type 1 | 1 | 0 | 0.834 |
| Type 2 | 7 | 2 | |
| Type 3 | 68 | 14 | |
| Clinical stage (2010) | |||
| III | 43 | 9 | 0.997 |
| IVA | 24 | 5 | |
| IVB | 9 | 2 | |
WHO = World Health Organization.
3.3. DW MRI and Tumor Volume
The mean tumor volumes of the primary lesions and metastatic adenopathies at prechemotherapy MRI were 37.2 cm3 (median, 36.6 cm3; range, 3.8–96.5 cm3) and 18.4 cm3 (median, 16.2 cm3; range, 1.3–49 cm3), respectively. No significant difference was observed between responders and nonresponders in terms of the mean pretreatment tumor volume (primary lesions, 36.6 cm3 ± 2.7 (standard error) versus 40.4 cm3 ± 6.7, P = 0.770; metastatic adenopathies, 18.6 cm3 ± 1.7 versus 19.5 cm3 ± 4.5, P = 0.906). However, after completion of neoadjuvant chemotherapy, responders had a smaller mean tumor volume than nonresponders (primary lesions, 9.2 cm3 ± 1.2 versus 28.0 cm3 ± 4.7, P = 0.014; metastatic adenopathies, 2.6 cm3 ± 0.5 versus 12.6 cm3 ± 2.9, P = 0.003) (Table 3).
Table 3.
Tumor volume and ADCs of the primary tumor and metastatic adenopathies in 92 patients with NPC.
| Characteristic | Number of patients | P-value | |
|---|---|---|---|
| Responders (n = 76) | Nonresponders (n = 16) | ||
| Tumor volume (cm3) | |||
| NP pretreatment | 37.3 ± 2.7 | 36.2 ± 7.0 | 0.884 |
| NP posttreatment | 9.1 ± 1.2 | 23.7 ± 4.7 | 0.010 |
| LN pretreatment | 18.2 ± 1.7 | 20.0 ± 4.6 | 0.708 |
| LN posttreatment | 2.4 ± 0.5 | 14.3 ± 3.1 | 0.004 |
| ADC (×10−3 mm2/sec) | |||
| NP pretreatment | 0.809 ± 0.009 | 0.953 ± 0.038 | 0.003 |
| NP posttreatment | 1.276 ± 0.007 | 1.306 ± 0.033 | 0.182 |
| LN pretreatment | 0.966 ± 0.010 | 1.121 ± 0.045 | 0.007 |
| LN posttreatment | 1.354 ± 0.009 | 1.355 ± 0.045 | 0.983 |
| Increase in ADC (%) | |||
| NP | 58.5 ± 1.0 | 38.2 ± 3.1 | <0.001 |
| LN | 40.4 ± 0.5 | 21.2 ± 1.3 | <0.001 |
NP = nasopharynx; LN = regional neck lymph nodes.
Before neoadjuvant chemotherapy, the mean ADCs of responders were significantly lower than that of nonresponders (primary lesions: [0.798 ± 0.007] × 10−3 mm2/sec versus [1.019 ± 0.028] × 10−3 mm2/sec, P = 0.012; metastatic adenopathies: [0.964 ± 0.010] × 10−3 mm2/sec versus [1.135 ± 0.042] × 10−3 mm2/sec, P = 0.013). After completion of neoadjuvant chemotherapy, no significant difference in the mean ADCs was observed between responders and nonresponders (primary lesions: [1.274 ± 0.011] × 10−3 mm2/sec versus [1.366 ± 0.020] × 10−3 mm2/sec, P = 0.526; metastatic adenopathies: [1.354 ± 0.013] × 10−3 mm2/sec versus [1.427 ± 0.031] × 10−3 mm2/sec, P = 0.217). However, the mean percentage increases in the ADCs were significantly greater in responders than in nonresponders (primary lesions, 60.0% ± 2.4 versus 34.8% ± 3.2, P = 0.008; metastatic adenopathies, 40.7% ± 2.7 versus 26.5% ± 3.3, P < 0.001) (Figures 1 and 2) (Table 3). Additionally, the changes in the ADCs correlated with the change in tumor volume at follow-up MRI (primary lesions: r = 0.611, P < 0.001; metastatic adenopathies: r = 0.676, P < 0.001). Furthermore, a strong negative correlation was observed between the mean pretreatment tumor ADC and percentage change in tumor volume after chemotherapy (primary lesions: r = −0.570, P < 0.001; metastatic adenopathies: r = −0.423, P < 0.001).
Figure 1.
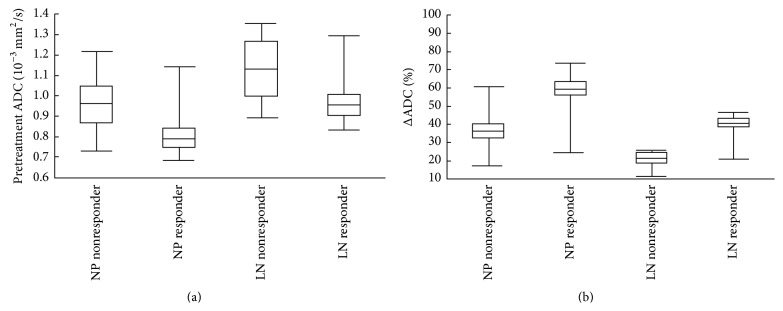
(a) Comparison of pretreatment ADCs for responders and nonresponders in patients with NPC. Responders had significantly lower pretreatment ADCs (primary lesions, P = 0.012; metastatic adenopathies, P = 0.013). (b) Comparison of ΔADCs for responders and nonresponders. Responders had significantly higher ΔADCs (primary lesions, P = 0.008; metastatic adenopathies, P < 0.001). Box-whisker plots are presented with median (-), interquartile range (box), and minima/maxima (-). NP = nasopharynx; LN = regional neck lymph nodes.
Figure 2.
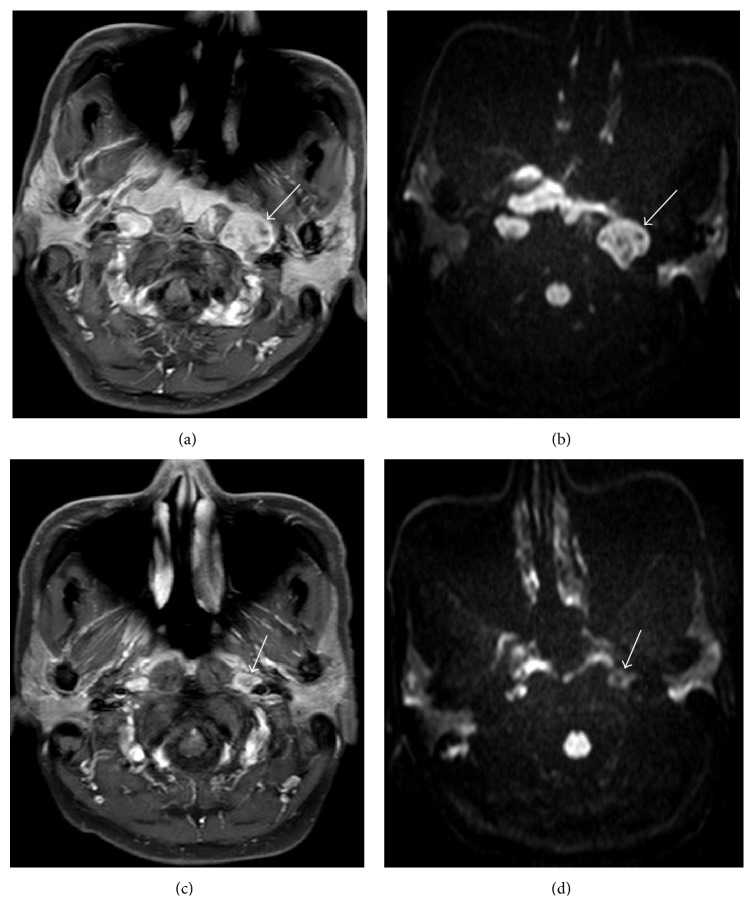
DW MRI findings in a 48-year-old female patient with NPC who responded to neoadjuvant chemotherapy. Pretreatment (a) axial contrast-enhanced T1-weighted image and (b) ADC maps showing an enlarged lymph node in the left retropharyngeal space (arrow). The mean pretreatment ADC of this lesion was 0.993 × 10−3 mm2/sec. Posttreatment (c) axial contrast-enhanced T1-weighted image and (d) ADC maps showing that the formerly enlarged right lymph node partially resolved; the volume of this lesion reduced by 83%. Neoadjuvant chemotherapy increased the mean ADC of this lesion to 1.383 × 10−3 mm2/sec.
3.4. ROC Curve Analysis
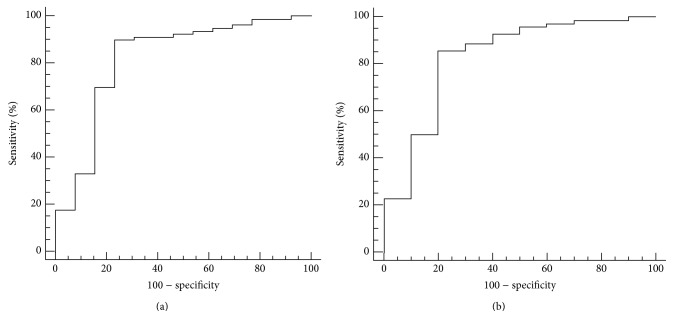
The optimal pretreatment primary tumor ADC for differentiating responders from nonresponders using ROC curve analysis was 0.897 × 10−3 mm2/sec; this cutoff value had a sensitivity of 89.9% (71/79; 95% confidence interval: 81.0–95.5%), specificity of 76.9% (10/13; 95% confidence interval: 46.2–95.0%), and area under the empirical ROC curve of 0.821 (95% confidence interval: 0.727–0.893). The optimal pretreatment metastatic adenopathy ADC cutoff value for differentiating responders from nonresponders was 1.031 × 10−3 mm2/sec, which yielded a sensitivity of 85.7% (60/70; 95% confidence interval: 75.3–92.9%), specificity of 80.0% (8/10; 95% confidence interval: 44.4–97.5%), and area under the empirical ROC curve of 0.830 (95% confidence interval: 0.730–0.905) (Figure 3).
Figure 3.
ROC curves of the ability of pretreatment primary tumor ADC (a) or metastatic adenopathy ADC (b) to predict the response to neoadjuvant chemotherapy in patients with nasopharyngeal carcinoma. The optimal pretreatment ADC cutoff values for the primary tumor and metastatic adenopathy were 0.897 × 10−3 mm2/sec and 1.031 × 10−3 mm2/sec, with areas under the ROC curves of 0.830 and 0.821, respectively.
4. Discussion
This study demonstrated that, in patients with NPC, responders to neoadjuvant chemotherapy have significantly lower pretreatment ADCs than nonresponders, and a strong negative correlation exists between the mean pretreatment ADCs and percentage change in tumor volume on follow-up MRI. The patients with lower pretreatment ADCs had a better response to neoadjuvant chemotherapy compared to those with higher pretreatment ADCs, in accordance with other clinical studies [24–27]. In rectal cancer [24, 25] and breast cancer [26, 27], responders had lower ADCs before neoadjuvant chemotherapy than nonresponders. Therefore, we suggest that pretreatment ADCs could be used as a novel imaging biomarker to predict the response before neoadjuvant chemotherapy in patients with NPC. This could facilitate tailored therapeutic approaches in NPC, with some patients spared from ineffective and unnecessary treatment toxicities.
After completion of neoadjuvant chemotherapy, the percentage increases in the ADCs of responders were significantly greater than that of nonresponders, and the changes in the ADCs correlated with the change in tumor volume at follow-up MRI. Previous studies have shown that low pretreatment ADCs indicate viable tumor tissue with a high cellularity, whereas high ADCs reflect less metabolic tumor tissue with a low cellularity [28, 29]. Tumor tissue with a high rate of cellular proliferation is more sensitive to chemotherapy or radiotherapy, which acts by inducing cellular damage and lysis in proliferating cells followed by a reduction in cellular volume, thereby enhancing the diffusion of water molecules and increasing the ADCs values on DW MRI. However, tumors with a low cellularity are likely to be in a situation of hypoxia and ischemia, which reduces the delivery of chemotherapeutic agents to the tumor. Furthermore, cancer cells that have a slow rate of metabolism are less sensitive to cytotoxic chemotherapy or radiotherapy [28]. Therefore the changes in the ADCs of tumors with a low cellularity after completion of chemotherapy or radiotherapy will always be lower than the changes in the ADCs of tumors with a high cellularity. Interestingly, some studies have shown that large changes in ADCs during the early stages of treatment (after the first or second cycle of neoadjuvant chemotherapy or 1-2 weeks after the start of radiotherapy), which occur prior to changes in tumor diameter or volume, indicate a better response to treatment [12, 18, 30]. Therefore, in most malignant tumors, an obvious increase in the ADC is regarded as an important imaging biomarker of successful treatment [12–19, 24–27].
Over the last 10 years or so, DW MRI has been successfully employed in head and neck cancer to distinguish between residual disease or tumor recurrence and inflammation or necrosis after completion of (chemo)radiotherapy [31]. Additionally, pretreatment ADCs or changes in ADCs during (chemo)radiotherapy have been reported as useful markers for predicting locoregional failure or progression-free survival in head and neck carcinoma [17, 32]. Some early studies indicated the potential of ADCs for evaluating treatment response in head and neck carcinoma [12, 14, 18]. King et al. [14] reported that a large change in ADCs within two weeks of treatment was predictive of a better response to (chemo)radiotherapy. Kim et al. [18] observed that low pretreatment ADCs or a significant increase in ADCs within one week of treatment was indicative of a higher rate of locoregional remission after concurrent chemoradiation. Recently, a study of 31 patients with NPC showed that high ADCs and early large increases in ADCs after initiation of neoadjuvant chemotherapy were indicative of a better response to subsequent concurrent chemoradiotherapy [12]. However, to our knowledge, the use of pretreatment ADCs for predicting the response to neoadjuvant chemotherapy in patients with NPC or other head and neck carcinomas has not yet been reported. This study showed that patients with NPC and low pretreatment ADCs were more likely to respond to neoadjuvant chemotherapy, and large increases in ADCs after completion of neoadjuvant chemotherapy correlated with a better response to neoadjuvant chemotherapy. Therefore, assessment of ADCs may help identify patients who will fail to respond to neoadjuvant chemotherapy, thereby enabling individualized treatment planning and allowing some patients to avoid unnecessary chemotherapy and the associated toxicities.
It should be stressed that DW MRI was not performed after each cycle of neoadjuvant chemotherapy in this study. Other studies reported that changes in ADCs after the first cycle of neoadjuvant chemotherapy could provide more detailed information on tumor response [30]. Furthermore, the sample size in this study was relatively small, and most of the patients with NPC responded to neoadjuvant chemotherapy, which could result in statistical bias. Thus, one should be wary of applying the ADC cutoff values defined in this population for defining responders and nonresponders. In addition, pathologic confirmation of imaging findings is not possible in patients with NPC, who are typically treated with radiotherapy rather than surgery. Thus, determining the treatment response to chemotherapy based on anatomical MRI may be inaccurate. Therefore, we acknowledge that prospective, large cohort, and multicentre studies are necessary to confirm our findings and recommendations.
5. Conclusions
In NPC, patients with low pretreatment ADCs tended to respond better to neoadjuvant chemotherapy. Pretreatment ADCs have potential as a novel imaging marker to predict the response to neoadjuvant chemotherapy, which could facilitate individual therapeutic approaches and allow some patients with NPC to avoid ineffective chemotherapy and unnecessary treatment toxicities.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China (no. 81402244), the Natural Science Foundation of Guangdong Province (Grant S2011040004296), the Science and Technology Planning Program of Guangdong Province (Grant 20120318050), and the Science and Technology Key Project of Foshan City (Grant 201008051).
Disclaimer
The authors alone are responsible for the content and writing of the paper.
Conflict of Interests
The authors report no conflict of interests.
Authors' Contribution
Guo-Yi Zhang and Yue-Jian Wang contributed equally to this work.
References
- 1.Chang E. T., Adami H.-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiology Biomarkers and Prevention. 2006;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 2.Hui E. P., Ma B. B., Leung S. F., et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. Journal of Clinical Oncology. 2009;27(2):242–249. doi: 10.1200/jco.2008.18.1545. [DOI] [PubMed] [Google Scholar]
- 3.Cvitkovic E., Eschwege F., Raha M., et al. Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV (≥N2, M0) undifferentiated nasopharyngeal carcinoma: a positive effect on progression-free survival. International Journal of Radiation Oncology, Biology, Physics. 1996;35(3):463–469. doi: 10.1016/s0360-3016(96)80007-1. [DOI] [PubMed] [Google Scholar]
- 4.Chua D. T. T., Sham J. S. T., Choy D., et al. Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 1998;83(11):2270–2283. doi: 10.1002/(sici)1097-0142(19981201)83:11lt;2270::aid-cncr6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Ma J., Mai H.-Q., Hong M.-H., et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Journal of Clinical Oncology. 2001;19(5):1350–1357. doi: 10.1200/JCO.2001.19.5.1350. [DOI] [PubMed] [Google Scholar]
- 6.Chua D. T. T., Ma J., Sham J. S. T., et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. Journal of Clinical Oncology. 2005;23(6):1118–1124. doi: 10.1200/jco.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 7.Xu L., Pan J., Wu J., et al. Factors associated with overall survival in 1706 patients with nasopharyngeal carcinoma: significance of intensive neoadjuvant chemotherapy and radiation break. Radiotherapy & Oncology. 2010;96(1):94–99. doi: 10.1016/j.radonc.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G.-Y., Liu L.-Z., Wei W.-H., Deng Y.-M., Li Y.-Z., Liu X. W. Radiologic criteria of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma treated with radiation therapy. Radiology. 2010;255(2):605–612. doi: 10.1148/radiol.10090289. [DOI] [PubMed] [Google Scholar]
- 9.Liao X.-B., Mao Y.-P., Liu L.-Z., et al. How does magnetic resonance imaging influence staging according to AJCC staging system for nasopharyngeal carcinoma compared with computed tomography? International Journal of Radiation Oncology Biology Physics. 2008;72(5):1368–1377. doi: 10.1016/j.ijrobp.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Lai V., Khong P. L. Updates on MR imaging and 18F-FDG PET/CT imaging in nasopharyngeal carcinoma. Oral Oncology. 2014;50(6):539–548. doi: 10.1016/j.oraloncology.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Chan S.-C., Kuo W.-H., Wang H.-M., et al. Prognostic implications of post-therapy 18F-FDG PET in patients with locoregionally advanced nasopharyngeal carcinoma treated with chemoradiotherapy. Annals of Nuclear Medicine. 2013;27(8):710–719. doi: 10.1007/s12149-013-0736-2. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Liu X., Zheng D., et al. Diffusion-weighted magnetic resonance imaging for early response assessment of chemoradiotherapy in patients with nasopharyngeal carcinoma. Magnetic Resonance Imaging. 2014;32(6):630–637. doi: 10.1016/j.mri.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Chawla S., Kim S., Dougherty L., et al. Pretreatment diffusion-weighted and dynamic contrast-enhanced MRI for prediction of local treatment response in squamous cell carcinomas of the head and neck. American Journal of Roentgenology. 2013;200(1):35–43. doi: 10.2214/ajr.12.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King A. D., Chow K.-K., Yu K.-H., et al. Head and neck squamous cell carcinoma: diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology. 2013;266(2):531–538. doi: 10.1148/radiol.12120167. [DOI] [PubMed] [Google Scholar]
- 15.Vandecaveye V., Dirix P., de Keyzer F., et al. Diffusion-weighted magnetic resonance imaging early after chemoradiotherapy to monitor treatment response in head-and-neck squamous cell carcinoma. International Journal of Radiation Oncology Biology Physics. 2012;82(3):1098–1107. doi: 10.1016/j.ijrobp.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 16.Lambrecht M., Dirix P., Vandecaveye V., de Keyzer F., Hermans R., Nuyts S. Role and value of diffusion-weighted MRI in the radiotherapeutic management of head and neck cancer. Expert Review of Anticancer Therapy. 2010;10(9):1451–1459. doi: 10.1586/era.10.121. [DOI] [PubMed] [Google Scholar]
- 17.Vandecaveye V., Dirix P., de Keyzer F., et al. Predictive value of diffusion-weighted magnetic resonance imaging during chemoradiotherapy for head and neck squamous cell carcinoma. European Radiology. 2010;20(7):1703–1714. doi: 10.1007/s00330-010-1734-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim S., Loevner L., Quon H., et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clinical Cancer Research. 2009;15(3):986–994. doi: 10.1158/1078-0432.CCR-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King A. D., Mo F. K. F., Yu K.-H., et al. Squamous cell carcinoma of the head and neck: diffusion-weighted MR imaging for prediction and monitoring of treatment response. European Radiology. 2010;20(9):2213–2220. doi: 10.1007/s00330-010-1769-8. [DOI] [PubMed] [Google Scholar]
- 20.Edge S. B., Byrd D. R., Compton C. C., Fritz A. G., Greene F. L., Trotti A., editors. AJCC Cancer Staging Handbook From the AJCC Cancer Staging Manual. 7th. New York, NY, USA: Springer; 2010. [Google Scholar]
- 21.Therasse P., Arbuck S. G., Eisenhauer E. A., et al. New guidelines to evaluate the response to treatment in solid tumors. Journal of the National Cancer Institute. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- 23.Galen R. S. Predictive value of laboratory tests. The American Journal of Cardiology. 1975;36(4):536–538. doi: 10.1016/0002-9149(75)90916-9. [DOI] [PubMed] [Google Scholar]
- 24.Dzik-Jurasz A., Domenig C., George M., et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. The Lancet. 2002;360(9329):307–308. doi: 10.1016/s0140-6736(02)09520-x. [DOI] [PubMed] [Google Scholar]
- 25.DeVries A. F., Kremser C., Hein P. A., et al. Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. International Journal of Radiation Oncology Biology Physics. 2003;56(4):958–965. doi: 10.1016/S0360-3016(03)00208-6. [DOI] [PubMed] [Google Scholar]
- 26.Park S. H., Moon W. K., Cho N., et al. Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology. 2010;257(1):56–63. doi: 10.1148/radiol.10092021. [DOI] [PubMed] [Google Scholar]
- 27.Nilsen L., Fangberget A., Geier O., Olsen D. R., Seierstad T. Diffusion-weighted magnetic resonance imaging for pretreatment prediction and monitoring of treatment response of patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. Acta Oncologica. 2010;49(3):354–360. doi: 10.3109/02841861003610184. [DOI] [PubMed] [Google Scholar]
- 28.Humphries P. D., Sebire N. J., Siegel M. J., Olsen O. E. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology. 2007;245(3):848–854. doi: 10.1148/radiol.2452061535. [DOI] [PubMed] [Google Scholar]
- 29.Guo A. C., Cummings T. J., Dash R. C., Provenzale J. M. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224(1):177–183. doi: 10.1148/radiol.2241010637. [DOI] [PubMed] [Google Scholar]
- 30.Sharma U., Danishad K. K. A., Seenu V., Jagannathan N. R. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR in Biomedicine. 2009;22(1):104–113. doi: 10.1002/nbm.1245. [DOI] [PubMed] [Google Scholar]
- 31.Tshering Vogel D. W., Zbaeren P., Geretschlaeger A., Vermathen P., De Keyzer F., Thoeny H. C. Diffusion-weighted MR imaging including bi-exponential fitting for the detection of recurrent or residual tumour after (chemo)radiotherapy for laryngeal and hypopharyngeal cancers. European Radiology. 2013;23(2):562–569. doi: 10.1007/s00330-012-2596-x. [DOI] [PubMed] [Google Scholar]
- 32.Berrak S., Chawla S., Kim S., et al. Diffusion weighted imaging in predicting progression free survival in patients with squamous cell carcinomas of the head and neck treated with induction chemotherapy. Academic Radiology. 2011;18(10):1225–1232. doi: 10.1016/j.acra.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]