Abstract
Over the last 50 years, laboratories around the world analyzed the pharmacological effect of Bacopa monniera extract in different dimensions, especially as a nerve tonic and memory enhancer. Studies in animal model evidenced that Bacopa treatment can attenuate dementia and enhances memory. Further, they demonstrate that Bacopa primarily either acts via antioxidant mechanism (i.e., neuroprotection) or alters different neurotransmitters (serotonin (5-hydroxytryptamine, 5-HT), dopamine (DA), acetylcholine (ACh), γ-aminobutyric acid (GABA)) to execute the pharmacological effect. Among them, 5-HT has been shown to fine tune the neural plasticity, which is a substrate for memory formation. This review focuses on the studies which trace the effect of Bacopa treatment on serotonergic system and 5-HT mediated key molecular changes that are associated with memory formation.
1. Introduction
Bacopa monniera (L.) Wettst., which belongs to the family Scrophulariaceae, is an annual creeping plant found in wet, damp, and marshy areas. The leaves and stem of the plant are used for medicinal purposes traditionally [1]. In the ancient Indian system of medicine, namely, Ayurveda, B. monniera known as “Bhrami” has been classified under Medhya Rasayana and described in ancient ayurvedic medical encyclopedias, namely, Charaka Samhita, Sushrutha Samhita, and Astanga Hrdaya, as cure for mental disorders and loss of intellect and memory. It has been tested in different animal models to understand its effect on memory [2, 3] and antiamnesic activity [4–9]. These pharmacological properties lead to clinical trial of B. monniera extract in elderly persons to improve cognitive performance and memory [10–15]. In parallel, Bacopa is a main constituent in the preparation of ayurvedic medicine prescribed for cognitive dysfunction. In addition, several research groups and pharmaceutical companies formulated Bacopa for clinical use in different countries including India, New Zealand, Australia, and United States of America. Earlier, many reviews have discussed pharmacological property of B. monniera in a broad perspective; however, no comprehensive article has yet shown its effect on molecular level. In this review, we summarize the in vivo experiments that suggest that B. monniera treatment enhances cognitive function by altering the molecular targets through serotonergic system.
2. Bioactive Compounds in B. monniera Leaf Extract
Series of biochemical studies identified different pharmacological compounds from ethanolic extracts of Bacopa, which include alkaloids (brahmine, nicotine, and herpestine), saponins (monnierin, hersaponin), sterols (b-sitosterol, stigma-sterol), d-mannitol, acid A, and betulinic acid [16–18]. The principal constituents of B. monniera are triterpene saponins of the dammarane class, which have been named bacosides and bacopasaponins. There are two types of saponins, jujubogenin and pseudojujubogenin, which differ only in the nature of the sugar units in the glycosidic chain and the position of the olefinic side chain in the aglycone. These saponins are complex mixture of closely related structures, namely, bacosides A1 [19] and A3 [20] and bacopasaponins A–G [21–23]. Two new dammarane-type jujubogenin bisdesmosides, bacopasaponins E and F [24], pseudojujubogenin glycosides, bacopasides I and II [25], phenylethanoid glycosides, namely, monnierasides I–III with the known analogue plantainoside B [26], and bacopasides III, IV, and V [27] have also been identified. The major chemical entity shown responsible for neuropharmacological effects of B. monniera is bacoside A (64.28%) and bacoside B (27.11%); the latter differs only in optical rotation. The bacoside A (bacogenins A1, A2, A3, and A4) derives from two triterpenoid saponins: pseudojujubogenin and jujubogenin on acid hydrolysis [16–18, 28]. All these bacogenins (especially A4) are rich in the standardized extract of Bacopa which is termed as bacosides-enriched standardized extract of Bacopa (BESEB CDRI-08) that contains 55 ± 5% bacosides (Lumen Marketing Company, Chennai, India), and BESEB CDRI-08 is mentioned as BME in this paper.
3. Neuropharmacological Activity of BME
3.1. Learning and Memory
Bacopa treatment has been reported to improve behavior of different laboratory animal models under variety of experimental conditions. Oral administration of BME improved spatial learning of rats and mice in Morris water maze [4, 5, 29–31]. Interestingly, several other studies demonstrated that it also improved spatial working memory in different mazes like plus maze [32, 33], Y-maze [34, 35], radial arm maze [34, 36], Barnes maze [36], T-maze [37], Hole board [35], and modified Y maze [38]. In addition, it also improved negative reinforcement (foot-shock motivated brightness discrimination task, conditioned avoidance response) and positive reinforcement (conditioned taste aversion) based memory [2, 39]. Similarly, in passive avoidance task and fear conditioning task Bacopa treatment increased the transfer latency and freezing response [33, 35, 37, 38, 40–42], whereas, in contextual cues associated with odor, BME treated rats showed less latency to retrieve the reward [43] and exhibited improved discrimination of novel object [38, 44, 45]. In addition, it has been stated that Bacopa treatment induced dendritic arborization of neurons in hippocampal and basolateral amygdala [46, 47], which possibly enhanced neural plasticity.
4. B. monniera Extract Treatment Ameliorates Chemicals Induced Dementia
Interestingly, several studies investigated the pharmacological effect of BME against different chemical compounds that induce anterograde/retrograde amnesia by targeting different neuronal system. These studies reported that BME effectively attenuated anterograde/retrograde amnesia induced by chemical compounds such as scopolamine, an acetylcholine receptor antagonist [2, 6, 7, 22, 36, 40, 48, 49], diazepam, a positive allosteric modulators of γ-aminobutyric acid (GABA) type A receptor [4], Nω-nitro-l-arginine (L-NNA), a nitric oxide synthase inhibitor [8, 9], BN52021, a receptor antagonist for platelet activating factor [48], and sodium nitrite, a anticholinergic drug [48]. In addition, memory impairments caused by Okadaic acid, a selective inhibitor of protein phosphatase [31], aluminium-chloride which causes oxidative damage [50], autistic symptoms induced by sodium valproate, a weak blocker of sodium ion channels, and inhibitor of GABA transaminase [51] were also ameliorated by Bacopa treatment.
5. Uptake of Bacosides
We have learned from pioneering works about different active compounds in B. monniera extract [16–18]. As a first step to validate the effect of BME on the reported behavioral improvements, Charles et al. [35] confirmed that orally treated BME was uptaken into the system. HPLC analysis showed the presence of bioactive compound bacoside A in the serum of BME treated rats. The bioactive compounds in the BME could directly or indirectly interact with neurotransmitter systems to enhance learning and memory. Since the bacosides present in the BME are nonpolar glycosides [25–27], they can cross the blood-brain barrier (BBB) by simple lipid-mediated passive diffusion [52], and its bioavailability in brain has been confirmed by the biodistribution of radiopharmaceuticals [53] effectively activating the cascade which participates in the memory enhancing mechanism.
6. Activation of Neurotransmitter Systems by Bacoside
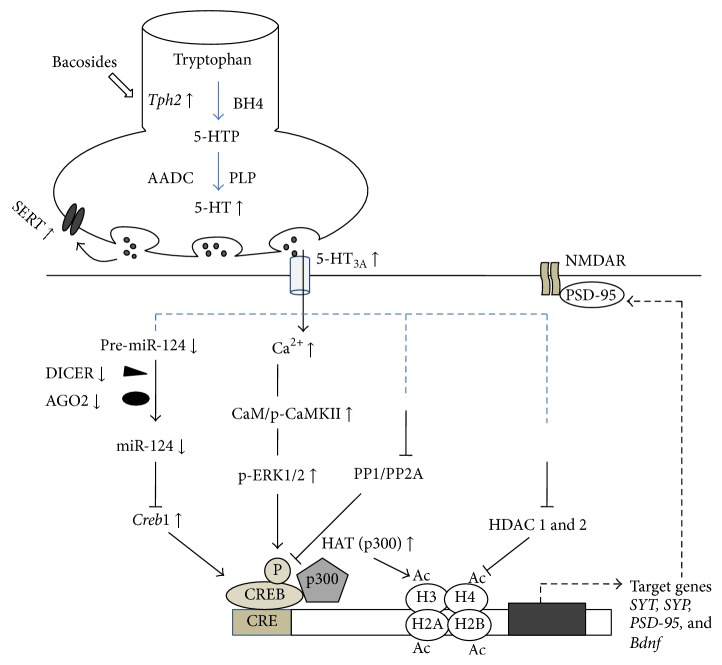
The balanced functions of various neurotransmitters such as acetylcholine (ACh) [2, 40], serotonin (5-hydroxytryptamine, 5-HT) [2, 54], catecholamine [55], γ-aminobutyric acid (GABA) [56], and glutamate (Glu) [8] were all altered by BME treatment. It has been reported that the BME treatment increased the 5-HT level in the hippocampus, hypothalamus, and cerebral cortex [54], and also modified the ACh concentration directly/indirectly through other neurotransmitter systems. As a first step, Rajan et al. [41] estimated the level of neurotransmitters to understand the effect of BME treatment. They found that BME treatment during postnatal period significantly upregulated the level of 5-HT, ACh, GABA, and Glu. In contrast, it reduced the level of dopamine (DA). Notably, the reported inhibitory effects of cholinesterase activity of BME may possibly increase the level of ACh and enhance memory [33, 40]. On the other hand, 5-HT receptors present in the GABAergic neuron [57] may activate the GABAergic neurons [58, 59], which enhances the release of GABA. In fact, increased GABA level in hippocampus could activate the inhibitory GABA receptors on cholinergic system that leads to inhibition of ACh release [60, 61], but 5-HT receptors may directly act on the cholinergic system and increase release of Ach [62]. These proceedings and the observed trend in the 5-HT level have drawn the attention to analyse the effect of BME on 5-HT system. Further, studies were designed to test the pathway associated with 5-HT system (Figure 1). Observed effect of BME on neurotransmitter systems and the molecules involved in the signaling pathway are shown in Table 1.
Figure 1.
Diagram showing the possible mechanism of serotonin mediated signaling pathway activated by BME during learning. (↑: increase; ↓: decrease).
Table 1.
Summary of Bacopa monniera treatment effects on serotonergic system and its associated pathway.
| Neurotransmitters | Effects | Genes (mRNA) |
Effects | Genes (Protein) |
Effects | References |
|---|---|---|---|---|---|---|
| Serotonin | ↑ | Tph2 | ↑ | [35] | ||
| SERT | ↑ | |||||
|
| ||||||
| Serotonin Dopamine Acetylcholine GABA Glutamate |
↑ ↓ ↑ ↑ ↑ |
5-HT1 A | — | |||
| 5-HT2 A | ↑ | |||||
| 5-HT3 A | ↑ | |||||
| 5-HT4 | — | [41] | ||||
| 5-HT5 | — | |||||
| 5-HT6 | — | |||||
| 5-HT7 | ↓ | |||||
|
| ||||||
| Nrf2 | ↑ | SYP SYT t-αCaMKII p-αCaMKII PSD-95 |
↑ ↑ ↑ ↑ ↑ |
[43] | ||
|
| ||||||
|
Dicer
Ago2 miR-124 Creb1 |
↓ ↓ ↓ ↑ |
DICER AGO2 t-CREB1/2 p-CREB1/2 |
↓ ↓ ↑ ↑ |
[39] | ||
|
| ||||||
|
Bdnf
PP1α |
↑ ↓ |
t-ERK1/2 | ↑ | |||
| p-ERK1/2 | ↑ | |||||
| t-CREB1/2 | ↑ | |||||
| p-CREB1/2 | ↑ | |||||
| Ac-H3 | ↑ | [42] | ||||
| Ac-H4 | ↑ | |||||
| HDAC1 | ↓ | |||||
| HDAC2 | ↓ | |||||
| p300 | ↑ | |||||
| PP2A | ↓ | |||||
↓: decrease; ↑: increase.
7. BME Treatment Regulates the Synthesis of Serotonin
Earlier studies demonstrated that increasing level of tryptophan hydroxylase (TPH) mRNA expression elevated TPH activity and 5-HT metabolism, which profoundly could influence the synaptic 5-HT activity [63, 64]. Further, serotonin transporter (SERT) is known to critically uptake the 5-HT by transport across presynaptic membrane [65]. The upregulated level of 5-HT by BME raises the question, does it alter the level of TPH2 and SERT? Interestingly, Charles et al. [35] showed that TPH2, SERT mRNA expression was upregulated and the level persisted even a week after the BME treatment [35]. The upregulated SERT expression could regulate the reuptake of released 5-HT and control the duration and intensity of serotonergic activity at the synapse. This could be one of the mechanisms that enhance the learning and memory processing and it fits well into established concept in different models [66, 67]. In addition to these studies, in silico analysis suggested that interaction of bacosides (A, A3) with TPH2 possibly alters the activity of TPH2 that could be one of the mechanisms for increased 5-HT synthesis [68].
8. Activation of 5-HT Receptor by BME Treatment
Previously, it has been found that synaptically released 5-HT exerts its function through their diverse receptors [69]. Activated receptors either positively or negatively regulate the downstream signaling cascade that is involved in regulation of synaptic plasticity [70–72]. In view of these reports, expression of 5-HT receptors (5-HT1A, 5-HT2A, 5-HT4, 5-HT5A, 5-HT6, and 5-HT7) after BME treatment was examined. Notably, 5-HT3A receptor expression was increased compared to all other receptors. It is the only metabotropic receptor, and its expression could be stimulated by endogenous 5-HT which may facilitate the hippocampal-dependent task [73, 74]. Hence, the role of 5-HT3A in hippocampal-dependent learning could be tested by using 5-HT3 antagonist 1-(m-chlorophenyl)-biguanide (mCPBG), which effectively impairs the retention of the conditioned response [75] in both short- and long-term memories [76]. The 5-HT3 antagonist mCPBG has facilitated gaining insight into the BME induced 5-HT3A receptor mediated role in hippocampal-dependent learning and its regulation of other neurotransmitters. Interestingly, treatment of BME ameliorated the antagonistic effect of mCPBG. The combination of mCPBG and BME treatment recorded improvement in behavioural task accompanying the upregulation of 5-HT3A receptor. Considering the interaction of multiple neurotransmitters involved in learning and memory network [77–80], it could be interesting to know the interaction of 5-HT3 receptor in activation/inhibition of other neurotransmitter systems.
The upregulated 5-HT3A receptor might regulate serotonergic system and may interact with other neurotransmitters that are involved in learning and memory [58, 67, 81]. It should be noted that 5-HT3A is a heteroreceptor; its stimulation by means of mCPBG has been reported to enhance GABA and DA levels and inhibit the release of ACh [74]. The activation of 5-HT3 receptors in dopaminergic neuron could facilitate the release of DA [82, 83], and mCPBG inhibits dopamine uptake by binding with dopamine transporter [84], thereby increasing the synaptic dopamine level. On the other hand, the anticholinesterase activity of BME [40] and other regulatory mechanisms of BME are also involved in the regulation of ACh level and memory enhancement [33, 85].
A noteworthy point is that it did not alter the level of Glu. This suggests that glutamate neurons in the hippocampus may not colocalize with 5-HT3A receptor [59]. The observed changes are indication of the facilitatory effect of BME on long-term and intermediate forms of memory through 5-HT3A receptor.
9. Activation of Protein Kinases-CREB Pathway
A pioneering study in 1976 described that serotonin stimulation increases the level of cyclic adenosine monophosphate (cAMP) by the adenyl cyclase in the neuronal cells [86]. Subsequent study by Castellucci et al. [87] established that activation of cAMP mediates downstream signaling process through phosphorylating proteins, namely, cAMP-dependent protein kinase or protein kinase A (PKA). Upon activation, cAMP-dependent PKA dissociates into regulatory and catalytic subunits. The catalytic subunit of PKA drives to activate mitogen activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK1/2) [88, 89]. It has been shown that activation of protein kinases (MAPK/ERK) can induce the phosphorylation of the key transcription factor CREB, which is a positive regulator of memory consolidation [90–93]. These proceedings triggered us to test whether the BME treatment induced activation of 5-HT3A receptor regulated synaptic plasticity through protein kinase and cAMP response element binding (CREB) protein signaling pathway. It is noteworthy to mention that treatment of BME increased the phosphorylation of ERK1/2 and provides a physiological and functional meaning for the observed different forms of memory [42]. If the p-ERK activity is decreased/increased, one would expect concomitant changes in the CREB and CREB targeted gene expression and functional consequences [94–97]. It should be noted that the induction of p-CREB1 is involved in the regulation of synaptic proteins synthesis, which are known to be involved in synaptic plasticity related events in hippocampus [98] and their synthesis is necessary for the consolidation of long-term memory (LTM) [99–102]. Preethi et al. [39] found that level of both total and phosphorylated CREB protein was increased in the BME treated individuals. When BME treated before m-CPBG treatment, the mCPBG mediated suppression of CREB phosphorylation was attenuated by BME, thus adding additional support to the effect of BME in regulation of PKA-CREB pathway.
10. Activation of CREB Regulation through MicroRNA-124 by BME
Long-term memory formation requires synthesis of new proteins [103, 104], which is regulated by mRNA transport and translation [105]. At this point, several studies proposed that microRNAs (miRNAs) are one of the factors that regulate expression of gene [106, 107] which could be regulated by level of miRNA/biosynthesis of miRNA. There are two molecules, Dicer and Ago2, involved in the regulation of miRNA biosynthesis [108]. It is noteworthy to mention that there is an interaction between miR-124 and 5-HT, because the stimulation of the latter has been shown to downregulate the expression of miR-124 during 5-HT-induced synaptic facilitation [109]. Thus, we thought that BME treatment might alter the level of miR-124 expression and the molecules involved in its biosynthesis pathway. Subsequently, we found that BME treatment reduced the level of Dicer, Ago2 mRNA, and protein [39]. Reduction in Dicer has been known to enhance synaptic plasticity [110]; the formation of miRNA-induced silencing complex (miRISC) requires the activation of Ago2 [111]. Further, this study revealed that reduction of Dicer and Ago2 directly downregulated miR-124 level in BME treated individuals. Conversely, inhibition of 5-HT activity by treating with mCPBG showed upregulated Dicer, Ago2, and miR-124 [39]. It has been postulated that the downregulation of miR-124 would lead to the upregulation of CREB [109]. Though it is well established that 5-HT can upregulate Creb1 mRNA level [112], recent studies claimed that miR-124 might directly bind to Creb1 3′UTR and regulates the expression of CREB [109, 113]. Indeed, upregulated CREB reciprocally regulates the miRNA [109, 114]. This in turn regulates the activation of immediate early genes that ultimately facilitates synaptic plasticity [115–118]. These cellular events demonstrate that BME possibly regulates the transcriptional regulators to fine tune transcription factors.
11. Phosphorylation of CREB Regulated by BME Treatment
Contrary to the protein kinases, protein phosphatases (PPs) act as dephosphorylating enzymes that dephosphorylate the molecules like CREB [119]. PPs critically regulate the phosphorylation events that favor forgetting [120], cognitive decline in ageing [121, 122], and suppress learning and memory. In brain, several PPs are known to be expressed. Among them, Ser/Thr phosphatases (PP1, PP2) are the most likely candidates that negatively act on the phosphorylation of CREB [123–125] and thereby downregulate the transcription of CREB targeted genes [120, 126, 127]. BME treatment significantly reduced the PP1α and PP2A level in hippocampus, which appears to be responsible for observed BME mediated enhanced memory [42]. This study revealed the contribution of BME in regulation of CREB phosphorylation that favors the transcription of CREB targeted genes to memory formation. Moreover, it supported the earlier reports which showed inhibition of PPs to enhance memory formation [120, 124, 125, 128–130], but the exact mechanism that inhibits PPs is not yet studied.
12. Chromatin Modifications Differentially Regulated by BME Treatment
Studies in memory highlighted chromatin alteration and epigenetic changes that are associated with CREB activation. Contribution of histone tail acetylation and deacetylation in chromatin are widely known to be involved in the formation of long-term memory and synaptic changes [131–133]. Histone deacetylase (HDAC) inhibitors are known to induce acetylation of histones (H3, H4). It has been reported that HDAC inhibitors repress the HDAC-PP1 complex and thus block dephosphorylation of CREB [134, 135]. On the other hand, in vitro and in vivo studies claimed that transcriptional induction of CREB occurred by pSer133, which requires histone acetylase (HAT)—CREB binding protein (CBP/p300) [136, 137]. P300 contains intrinsic HAT activity and it has been shown to interact with CREB [138–140]. Manipulation in p300 leads to reduction in the histone acetylation and impairs hippocampus dependent memory [141–143]. These reports prompted us to examine the potential role of BME in chromatin modifications especially with histone acetylation and deacetylation.
An earlier study reported significant enhancement of p300 level in hippocampus of BME treated groups, but not in control groups after training [42]. These reports suggest that BME plays an agonistic role for p300 in hippocampus; further it may acetylate H3 and H4 histones [144–146]. Accordingly, we found that BME treatment induced marked increment in the level of Ac-H3 and Ac-H4 in hippocampus [42]. These results agree with the earlier studies, in which HDAC inhibitors have been found to induce acetylation of histones (H3, H4) and improve memory [147–150]. In addition, the level of HDAC 1 and HDAC 2 in the hippocampus of BME treated group was decreased compared to control group. The reduction in HDAC 1 and HDAC 2 levels together with increased acetylation of histones in BME groups added additional evidence to the mechanism of BME [42].
13. BME Treatment Activates the Synaptic Proteins to Induce Synaptic Plasticity
Behavioural response to the stimuli is basic functional circuit formation between the neuronal cells. The molecular mechanism underlying the circuit (synaptic plasticity) is likely to provide insight to role of molecules/molecular complexes. The communications between the neuronal cells are initiated by the recruitment of adhesion molecules in pre-post synaptic neurons [151, 152]. Synaptic plasticity depends on activity strength, which leads to release of neurotransmitters to the synaptic cleft. However, the release of neurotransmitters is critically regulated by synaptic proteins synaptotagmin-I (SYT-1) and synaptophysin (SYP). SYT-1 is sensitive to Ca2+ and conserved at least in vertebrates [153]. This synaptic vesicle protein is exclusively involved in synaptic vesicle docking and regulating release of neurotransmitter [154]. Another key synaptic protein SYP is playing important role in regulation of synaptic vesicle association by protein-protein interactions [153]. It is a vesicle-associated regulatory protein which is involved in plasticity related changes in the hippocampus [155, 156]. The levels of SYT-1 and SYP were upregulated after BME treatment which possibly established the synaptic communication and synaptic function [43]. BME treatment upregulated the synaptic proteins (SYT-1, SYP), which is possibly by the elevated level of 5-HT. The level of signaling components is essential for neurotransmission and synaptic plasticity. The upregulated synaptic proteins could enhance neurotransmission and synaptic plasticity. However, this should be transferred to postsynaptic neurons. There are two key postsynaptic proteins (post synaptic density protein 95 (PSD-95) and Ca2+/calmodulin dependent protein kinase II (CaMKII)) distributed densely. Acute phosphorylation and localization of PSD-95 and CaMKII is fundamental to synaptic function [157]. They are critical for long-term potentiation (LTP) and information storage [158, 159]. Translocation of CaMKII to postsynaptic region by autophosphorylation is necessary for early phase of memory formation, where it controls the phosphorylation of different postsynaptic proteins [160]. The induction and phosphorylation of CaMKII depends on the release of 5-HT [161]. Genetic manipulation and pharmacological studies pointed out the critical role of CaMKII in synaptic plasticity and memory formation [161, 162]. BME treatment upregulated the induction and phosphorylation of CaMKII; it could be by the level of 5-HT, thus the improved memory recorded. PSD-95 is a core component in the architecture of synapses [163, 164] involved in localization of receptors, clustering of synaptic signaling proteins, and synapse stabilisation [164–166]. The level of PSD-95 increases at synapses during learning/learning-induced plasticity [167, 168]. Earlier, we demonstrated that PSD-95 was upregulated after BME treatment [43]; upregulated PSD-95 may increase the interaction between PSD proteins and enhances synaptic transmission [169–172]. These results suggest that BME treatment activates the synaptic proteins; thus neurotransmission and synaptic plasticity are enhanced between the neurons.
14. Conclusion
Taken together, bacosides present in the Bacopa extract has been known to improve cognitive function by modulating different neurotransmitters. However, this review focused on the studies which provide much attention to the serotonergic system, in which, starting from in silico approach to alternation in 5-HT levels, their receptors and associated signaling cascades known to be involved in synaptic plasticity and memory enhancement were discussed. These studies provide molecular evidence to possible mechanism of BME on serotonergic system and its associated pathway.
Acknowledgments
The authors would like to thank the Editors and three anonymous reviewers for their comments to improve the paper. Koilmani Emmanuvel Rajan is thankful to his students Dr. Ganesh Ambigapathy and Dr. Prisila Dulcy Charles for their contribution in developing Bacopa project and Lumen Marketing Company (Chennai, India) for providing the standardised B. monniera extract (CDRI-08). Department of Animal Science is supported by UGC-SAP-DRS-II.
Abbreviations
- 5-HT:
5-Hydroxytryptamine
- ACh:
Acetylcholine
- BESEB CDRI-08:
Bacosides-enriched standardized extract of Bacopa
- CaMKII:
Ca2+/calmodulin dependent protein kinase II
- cAMP:
Cyclic adenosine monophosphate
- CBP:
CREB binding protein
- CREB:
Cyclic adenosine monophosphate (cAMP) response element binding
- DA:
Dopamine
- ERK1/2:
Extracellular signal-regulated kinase
- GABA:
γ-Amino butyric acid
- Glu:
Glutamate
- HAT:
Histone acetylase
- HDAC:
Histone deacetylase
- L-NNA:
Nω-nitro-l-arginine
- LTM:
Long-term memory
- LTP:
Long-term potentiation
- MAPK:
Mitogen activated protein kinase
- mCPBG:
1-(m-Chlorophenyl)-biguanide
- miRISC:
miRNA-induced silencing complex
- miRNAs:
MicroRNAs
- PKA:
Protein kinase A
- PPs:
Protein phosphatases
- PSD-95:
Postsynaptic density protein 95
- SERT:
Serotonin transporter
- SYP:
Synaptophysin
- SYT1:
Synaptotagmin I
- TPH:
Tryptophan hydroxylase.
Conflict of Interests
The authors have declared that no conflict of interests exists.
References
- 1.Mathew K. M. The Flora of Tamil Nadu and Carna. Tiruchirappalli, India: Rapinat Herbarium St. Joseph's College; 1984. [Google Scholar]
- 2.Singh H. K., Dhawan B. N. Neuropsychopharmacological effects of the ayurvedic nootropic Bacopa monniera Linn. (Brahmi) Indian Journal of Pharmacology. 1997;29(5):S359–S365. [Google Scholar]
- 3.Kongkeaw C., Dilokthornsakul P., Thanarangsarit P., Limpeanchob N., Scholfield C. N. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. Journal of Ethnopharmacology. 2014;151(1):528–535. doi: 10.1016/j.jep.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakar S., Saraf M. K., Pandhi P., Anand A. Bacopa monniera exerts antiamnesic effect on diazepam-induced anterograde amnesia in mice. Psychopharmacology. 2008;200(1):27–37. doi: 10.1007/s00213-007-1049-8. [DOI] [PubMed] [Google Scholar]
- 5.Saraf M. K., Anand A., Prabhakar S. Scopolamine induced amnesia is reversed by Bacopa monniera through participation of kinase-CREB pathway. Neurochemical Research. 2010;35(2):279–287. doi: 10.1007/s11064-009-0051-4. [DOI] [PubMed] [Google Scholar]
- 6.Saraf M. K., Prabhakar S., Pandhi P., Anand A. Bacopa monniera ameliorates amnesic effects of diazepam qualifying behavioral-molecular partitioning. Neuroscience. 2008;155(2):476–484. doi: 10.1016/j.neuroscience.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 7.Saraf M. K., Prabhakar S., Khanduja K. L., Anand A. Bacopa monniera attenuates scopolamine-induced impairment of spatial memory in mice. Evidence-Based Complementary and Alternative Medicine. 2011;2011:10. doi: 10.1093/ecam/neq038.236186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraf M. K., Prabhakar S., Anand A. Bacopa monniera alleviates Nω-nitro-l-arginine-induced but not MK-801-induced amnesia: A mouse Morris water maze study. Neuroscience. 2009;160(1):149–155. doi: 10.1016/j.neuroscience.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Anand A., Saraf M. K., Prabhakar S. Antiamnesic effect of B. monniera on L-NNA induced amnesia involves calmodulin. Neurochemical Research. 2010;35(8):1172–1181. doi: 10.1007/s11064-010-0171-x. [DOI] [PubMed] [Google Scholar]
- 10.Calabrese C., Gregory W. L., Leo M., Kraemer D., Bone K., Oken B. Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: a randomized, double-blind, placebo-controlled trial. Journal of Alternative and Complementary Medicine. 2008;14(6):707–713. doi: 10.1089/acm.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan A., Stevens J. Does bacopa monnieri improve memory performance in older persons? Results of a randomized, placebo-controlled, double-blind trial. Journal of Alternative and Complementary Medicine. 2010;16(7):753–759. doi: 10.1089/acm.2009.0342. [DOI] [PubMed] [Google Scholar]
- 12.Stough C., Scholey A., Cropley V., et al. Examining the cognitive effects of a special extract of Bacopa monniera (CDRI 08: Keenmind): a review of ten years of research at Swinburne University. The Journal of Pharmacy and Pharmaceutical Sciences. 2013;16(2):254–258. doi: 10.18433/j35g6m. [DOI] [PubMed] [Google Scholar]
- 13.Dave U. P., Dingankar S. R., Saxena V. S., et al. An open-label study to elucidate the effects of standardized Bacopa monniera extract in the management of symptoms of attention-deficit hyperactivity disorder in children. Advances in Mind-Body Medicine. 2014;28:10–15. [PubMed] [Google Scholar]
- 14.Barbhaiya H. C., Desai R. P., Saxena V. S., et al. Efficacy and tolerability of BacoMind on memory improvement in elderly participants—a double blind placebo controlled study. Journal of Pharmacology and Toxicology. 2008;3(6):425–434. doi: 10.3923/jpt.2008.425.434. [DOI] [Google Scholar]
- 15.Morgan A., Stevens J. Does Bacopa monnieri improve memory performance in older persons? Results of a randomized, placebo-controlled, double-blind trial. Journal of Alternative and Complementary Medicine. 2010;16(7):753–759. doi: 10.1089/acm.2009.0342. [DOI] [PubMed] [Google Scholar]
- 16.Chatterji N., Rastogi R. P., Dhar M. L. Chemical examination of Bacopa monniera Wettst: isolation of chemical constituent. Indian Journal of Chemistry. 1963;1:212–215. [Google Scholar]
- 17.Chatterji N., Rastogi R. P., Dhar M. L. Chemical examination of Bacopa monniera Wettst: the constitution of bacoside A. Indian Journal of Chemistry. 1965;3:24–29. [Google Scholar]
- 18.Basu N., Rastogi P. R., Dhar M. L. Chemical examination of Bacopa monniera Wettst Part III: the constitution of Bacoside-B. Indian Journal of Chemistry. 1967;5:p. 84. [Google Scholar]
- 19.Jain P., Kulshreshtha D. K. Bacoside A1, A minor saponin from Bacopa monniera . Phytochemistry. 1993;33(2):449–451. doi: 10.1016/0031-9422(93)85537-2. [DOI] [Google Scholar]
- 20.Rastogi S., Pal R., Kulshreshtha D. K. Bacoside A3—a triterpenoid saponin from Bacopa monniera . Phytochemistry. 1994;36(1):133–137. doi: 10.1016/s0031-9422(00)97026-2. [DOI] [PubMed] [Google Scholar]
- 21.Garai S., Mahato S. B., Ohtani K., Yamasaki K. Dammarane-type triterpenoid saponins from Bacopa monniera . Phytochemistry. 1996;42(3):815–820. doi: 10.1016/0031-9422(95)00936-1. [DOI] [PubMed] [Google Scholar]
- 22.Garai S., Mahato S. B., Ohtani K., Yamasaki K. Bacopasaponin D—a pseudojujubogenin glycoside from Bacopa monniera . Phytochemistry. 1996;43(2):447–449. doi: 10.1016/0031-9422(96)00250-6. [DOI] [PubMed] [Google Scholar]
- 23.Hou C.-C., Lin S.-J., Cheng J.-T., Hsu F.-L. Bacopaside III, bacopasaponin G, and bacopasides A, B, and C from Bacopa monniera . Journal of Natural Products. 2002;65(12):1759–1763. doi: 10.1021/np020238w. [DOI] [PubMed] [Google Scholar]
- 24.Mahato S. B., Garai S., Chakravarty A. K. Bacopasaponins E and F: two jujubogenin bisdesmosides from Bacopa monniera . Phytochemistry. 2000;53(6):711–714. doi: 10.1016/s0031-9422(99)00384-2. [DOI] [PubMed] [Google Scholar]
- 25.Chakravarty A. K., Sarkar T., Masuda K., Shiojima K., Nakane T., Kawahara N. Bacopaside I and II: two pseudojujubogenin glycosides from Bacopa monniera . Phytochemistry. 2001;58(4):553–556. doi: 10.1016/s0031-9422(01)00275-8. [DOI] [PubMed] [Google Scholar]
- 26.Chakravarty A. K., Sarkar T., Nakane T., Kawahara N., Masuda K. New phenylethanoid glycosides from Bacopa monniera . Chemical and Pharmaceutical Bulletin. 2002;50(12):1616–1618. doi: 10.1248/cpb.50.1616. [DOI] [PubMed] [Google Scholar]
- 27.Chakravarty A. K., Garai S., Masuda K., Nakane T., Kawahara N. Bacopasides III-V: three new triterpenoid glycosides from Bacopa monniera . Chemical and Pharmaceutical Bulletin. 2003;51(2):215–217. doi: 10.1248/cpb.51.215. [DOI] [PubMed] [Google Scholar]
- 28.Rastogi R. P. Compendium of Indian Medicinal Plants. Vol. 1. New Delhi, India: CSIR; 1990. [Google Scholar]
- 29.Uabundit N., Wattanathorn J., Mucimapura S., Ingkaninan K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer's disease model. Journal of Ethnopharmacology. 2010;127(1):26–31. doi: 10.1016/j.jep.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 30.Anand T., Kumar G. P., Pandareesh M. D., Swamy M. S. L., Khanum F., Bawa A. S. Effect of bacoside extract from Bacopa monniera on physical fatigue induced by forced swimming. Phytotherapy Research. 2012;26(4):587–593. doi: 10.1002/ptr.3611. [DOI] [PubMed] [Google Scholar]
- 31.Dwivedi S., Nagarajan R., Hanif K., Siddiqui H. H., Nath C., Shukla R. Standardized extract of Bacopa monniera attenuates okadaic acid induced memory dysfunction in rats: effect on Nrf2 pathway. Evidence-Based Complementary and Alternative Medicine. 2013;2013:18. doi: 10.1155/2013/294501.294501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Achliya G. S., Barabde U., Wadodkar S., Dorle A. Effect of Bramhi Ghrita, an polyherbal formulation on learning and memory paradigms in experimental animals. Indian Journal of Pharmacology. 2004;36(3):159–162. [Google Scholar]
- 33.Joshi H., Parle M. Brahmi rasayana improves learning and memory in mice. Evidence-Based Complementary and Alternative Medicine. 2006;3(1):79–85. doi: 10.1093/ecam/nek014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathew J., Gangadharan G., Kuruvilla K. P., Paulose C. S. Behavioral deficit and decreased GABA receptor functional regulation in the hippocampus of epileptic rats: effect of Bacopa monnieri . Neurochemical Research. 2011;36(1):7–16. doi: 10.1007/s11064-010-0253-9. [DOI] [PubMed] [Google Scholar]
- 35.Charles P. D., Ambigapathy G., Geraldine P., Akbarsha M. A., Rajan K. E. Bacopa monniera leaf extract up-regulates tryptophan hydroxylase (TPH2) and serotonin transporter (SERT) expression: implications in memory formation. Journal of Ethnopharmacology. 2011;134(1):55–61. doi: 10.1016/j.jep.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 36.Gupta A., Karchuli M. S., Upmanyu N. Comparative evaluation of ethanolic extracts of Bacopa monnieri, Evolvulus alsinoides, Tinospora cordifolia and their combinations on cognitive functions in rats. Current Aging Science. 2013;6(3):239–243. doi: 10.2174/18746098112059990036. [DOI] [PubMed] [Google Scholar]
- 37.Vollala V. R., Upadhya S., Nayak S. Learning and memory-enhancing effect of Bacopa monniera in neonatal rats. Bratislava Medical Journal. 2011;112(12):663–669. [PubMed] [Google Scholar]
- 38.Le X. T., Pham H. T. N., Do P. T., et al. Bacopa monnieri ameliorates memory deficits in olfactory bulbectomized mice: possible involvement of glutamatergic and cholinergic systems. Neurochemical Research. 2013;38(10):2201–2215. doi: 10.1007/s11064-013-1129-6. [DOI] [PubMed] [Google Scholar]
- 39.Preethi J., Singh H. K., Charles P. D., Rajan K. E. Participation of microRNA 124-CREB pathway: a parallel memory enhancing mechanism of standardised extract of Bacopa monniera (BESEB CDRI-08) Neurochemical Research. 2012;37(10):2167–2177. doi: 10.1007/s11064-012-0840-z. [DOI] [PubMed] [Google Scholar]
- 40.Das A., Shanker G., Nath C., Pal R., Singh S., Singh H. K. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba—anticholinesterase and cognitive enhancing activities. Pharmacology Biochemistry and Behavior. 2002;73(4):893–900. doi: 10.1016/s0091-3057(02)00940-1. [DOI] [PubMed] [Google Scholar]
- 41.Rajan K. E., Singh H. K., Parkavi A., Charles P. D. Attenuation of 1-(m-chlorophenyl)-biguanide induced hippocampus-dependent memory impairment by a standardised extract of Bacopa monniera (BESEB CDRI-08) Neurochemical Research. 2011;36(11):2136–2144. doi: 10.1007/s11064-011-0538-7. [DOI] [PubMed] [Google Scholar]
- 42.Preethi J., Singh H. K., Venkataraman J. S., Rajan K. E. Standardised extract of Bacopa monniera (CDRI-08) improves contextual fear memory by differentially regulating the activity of histone acetylation and protein phosphatases (PP1α, PP2A) in hippocampus. Cellular and Molecular Neurobiology. 2014;34(4):577–589. doi: 10.1007/s10571-014-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dulcy C. P., Singh H. K., Preethi J., Rajan K. E. Standardized extract of Bacopa monniera (BESEB CDRI-08) attenuates contextual associative learning deficits in the aging rat's brain induced by D-galactose. Journal of Neuroscience Research. 2012;90(10):2053–2064. doi: 10.1002/jnr.23080. [DOI] [PubMed] [Google Scholar]
- 44.Piyabhan P., Wetchateng T. Cognitive enhancement effects of Bacopa monnieri (Brahmi) on novel object recognition and VGLUT1 density in the prefrontal cortex, striatum, and hippocampus of sub-chronic phencyclidine rat model of schizophrenia. Journal of the Medical Association of Thailand. 2013;96(5):625–632. [PubMed] [Google Scholar]
- 45.Piyabhan P., Wetchateng T., Sirseeratawong S. Cognitive enhancement effects of Bacopa monnieri (Brahmi) on novel object recognition and NMDA receptor immunodensity in the prefrontal cortex and hippocampus of sub-chronic phencyclidine rat model of schizophrenia. Journal of the Medical Association of Thailand. 2013;96(2):231–238. [PubMed] [Google Scholar]
- 46.Vollala V. R., Upadhya S., Nayak S. Enhanced dendritic arborization of amygdala neurons during growth spurt periods in rats orally intubated with Bacopa monniera extract. Anatomical science international. 2011;86(4):179–188. doi: 10.1007/s12565-011-0104-z. [DOI] [PubMed] [Google Scholar]
- 47.Vollala V. R., Upadhya S., Nayak S. Enhanced dendritic arborization of hippocampal CA3 neurons by Bacopa monniera extract treatment in adult rats. Romanian Journal of Morphology and Embryology. 2011;52(3):879–886. [PubMed] [Google Scholar]
- 48.Kishore K., Singh M. Effect of bacosides, alcoholic extract of Bacopa monniera Linn. (brahmi), on experimental amnesia in mice. Indian Journal of Experimental Biology. 2005;43(7):640–645. [PubMed] [Google Scholar]
- 49.Sumathi T., Shobana C., Christinal J., Anusha C. Protective effect of Bacopa monniera on methyl mercury-induced oxidative stress in cerebellum of rats. Cellular and Molecular Neurobiology. 2012;32(6):979–987. doi: 10.1007/s10571-012-9813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thippeswamy A. H., Rafiq M., Viswantha G. L. S., Kavya K. J., Anturlikar S. D., Patki P. S. Evaluation of Bacopa monniera for its synergistic activity with rivastigmine in reversing aluminum-induced memory loss and learning deficit in rats. Journal of Acupuncture and Meridian Studies. 2013;6(4):208–213. doi: 10.1016/j.jams.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Sandhya T., Sowjanya J., Veeresh B. Bacopa monniera (L.) Wettst ameliorates behavioral alterations and oxidative markers in sodium valproate induced autism in rats. Neurochemical Research. 2012;37(5):1121–1131. doi: 10.1007/s11064-012-0717-1. [DOI] [PubMed] [Google Scholar]
- 52.Pardridge W. M. Blood-brain barrier biology and methodology. Journal of NeuroVirology. 1999;5(6):556–569. doi: 10.3109/13550289909021285. [DOI] [PubMed] [Google Scholar]
- 53.De K., Chandra S., Misra M. Evaluation of the biological effect of brahmi (Bacopa monnieri Linn) extract on the biodistribution of technetium-99m radiopharmaceuticals. Life Science Journal. 2008;5(2):45–49. [Google Scholar]
- 54.Sheikh N., Ahmad A., Siripurapu K. B., Kuchibhotla V. K., Singh S., Palit G. Effect of Bacopa monniera on stress induced changes in plasma corticosterone and brain monoamines in rats. Journal of Ethnopharmacology. 2007;111(3):671–676. doi: 10.1016/j.jep.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 55.Reis H. J., Guatimosim C., Paquet M., et al. Neuro-transmitters in the central nervous system and their implication in learning and memory processes. Current Medicinal Chemistry. 2009;16(7):796–840. doi: 10.2174/092986709787549271. [DOI] [PubMed] [Google Scholar]
- 56.Kant G. J., Wylie R. M., Vasilakls A. A., Ghosh S. Effects of triazolam and diazepam on learning and memory as assessed using a water maze. Pharmacology Biochemistry and Behavior. 1996;53(2):317–322. doi: 10.1016/0091-3057(95)02028-4. [DOI] [PubMed] [Google Scholar]
- 57.Morales M., Battenberg E., De Lecea L., Bloom F. E. The type 3 serotonin receptor is expressed in a subpopulation of GABAergic neurons in the rat neocortex and hippocampus. Brain Research. 1996;731(1-2):199–202. doi: 10.1016/S0006-8993(96)00557-4. [DOI] [PubMed] [Google Scholar]
- 58.Turner T. J., Mokler D. J., Luebke J. I. Calcium influx through presynaptic 5-HT 3 receptors facilitates GABA release in the hippocampus: in vitro slice and synaptosome studies. Neuroscience. 2004;129(3):703–718. doi: 10.1016/j.neuroscience.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 59.Dorostkar M. M., Boehm S. Opposite effects of presynaptic 5-HT3 receptor activation on spontaneous and action potential-evoked GABA release at hippocampal synapses. Journal of Neurochemistry. 2007;100(2):395–405. doi: 10.1111/j.1471-4159.2006.04218.x. [DOI] [PubMed] [Google Scholar]
- 60.Ramírez M. J., Cenarruzabeitia E., Lasheras B., del Río J. Involvement of GABA systems in acetylcholine release induced by 5-HT3 receptor blockade in slices from rat entorhinal cortex. Brain Research. 1996;712(2):274–280. doi: 10.1016/0006-8993(95)01471-3. [DOI] [PubMed] [Google Scholar]
- 61.Díez-Ariza M., Ramírez M. J., Lasheras B., Del Río J. Differential interaction between 5-HT3 receptors and GABAergic neurons inhibiting acetylcholine release in rat entorhinal cortex slices. Brain Research. 1998;801(1-2):228–232. doi: 10.1016/S0006-8993(98)00562-9. [DOI] [PubMed] [Google Scholar]
- 62.Consolo S., Bertorelli R., Russi G., Zambelli M., Ladinsky H. Serotonergic facilitation of acetylcholine release in vivo from rat dorsal hippocampus via serotonin 5-HT3 receptors. Journal of Neurochemistry. 1994;62(6):2254–2261. doi: 10.1046/j.1471-4159.1994.62062254.x. [DOI] [PubMed] [Google Scholar]
- 63.Chamas F., Serova L., Sabban E. L. Tryptophan hydroxylase mRNA levels are elevated by repeated immobilization stress in rat raphe nuclei but not in pineal gland. Neuroscience Letters. 1999;267(3):157–160. doi: 10.1016/s0304-3940(99)00340-7. [DOI] [PubMed] [Google Scholar]
- 64.Kim S. W., Park S. Y., Hwang O. Up-regulation of tryptophan hydroxylase expression and serotonin synthesis by sertraline. Molecular Pharmacology. 2002;61(4):778–785. doi: 10.1124/mol.61.4.778. [DOI] [PubMed] [Google Scholar]
- 65.Gainetdinov R. R., Caron M. G. Monoamine transporters: from genes to behavior. Annual Review of Pharmacology and Toxicology. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 66.Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- 67.Meneses A. 5-HT system and cognition. Neuroscience and Biobehavioral Reviews. 1999;23(8):1111–1125. doi: 10.1016/S0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 68.Rajathei D. M., Preethi J., Singh H. K., Rajan K. E. Molecular docking of bacosides with tryptophan hydroxylase: a model to understand the bacosides mechanism. Natural Products and Bioprospecting. 2014;4(4):251–255. doi: 10.1007/s13659-014-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meneses A. A pharmacological analysis of an associative learning task: 5-HT1 to 5-HT7 receptor subtypes function on a Pavlovian/instrumental autoshaped memory. Learning and Memory. 2003;10(5):363–372. doi: 10.1101/lm.60503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cassaday H. J., Hodges H., Gray J. A. The effects of ritanserin, RU 24969 and 8-OH-DPAT on latent inhibition in the rat. Journal of Psychopharmacology. 1993;7(supplement 1):63–71. doi: 10.1177/026988119300700110. [DOI] [PubMed] [Google Scholar]
- 71.Buhot M.-C. Serotonin receptors in cognitive behaviors. Current Opinion in Neurobiology. 1997;7(2):243–254. doi: 10.1016/S0959-4388(97)80013-X. [DOI] [PubMed] [Google Scholar]
- 72.Huang Y.-Y., Kandel E. R. 5-Hydroxytryptamine induces a protein kinase A/mitogen-activated protein kinase-mediated and macromolecular synthesis-dependent late phase of long-term potentiation in the amygdala. The Journal of Neuroscience. 2007;27(12):3111–3119. doi: 10.1523/jneurosci.3908-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harrell A. V., Allan A. M. Improvements in hippocampal-dependent learning and decremental attention in 5-HT3 receptor overexpressing mice. Learning and Memory. 2003;10(5):410–419. doi: 10.1101/lm.56103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fink K. B., Göthert M. 5-HT receptor regulation of neurotransmitter release. Pharmacological Reviews. 2007;59(4):360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- 75.Kilpatrick G. J., Butler A., Burridge J., Oxford A. W. 1-(m-Chlorophenyl)-biguanide, a potent high affinity 5-HT3 receptor agonist. European Journal of Pharmacology. 1990;182(1):193–197. doi: 10.1016/0014-2999(90)90513-6. [DOI] [PubMed] [Google Scholar]
- 76.Meneses A. Stimulation of 5-HT1A, 5-HT1B, 5-HT1A/2C, 5-HT3 and 5-HT4 receptors or 5-HT uptake inhibition: short- and long-term memory. Behavioural Brain Research. 2007;184(1):81–90. doi: 10.1016/j.bbr.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 77.Decker M. W., McGaugh J. L. The role of interactions between the cholinergic system and other neuromodulatory systems in learning and memory. Synapse. 1991;7(2):151–168. doi: 10.1002/syn.890070209. [DOI] [PubMed] [Google Scholar]
- 78.Matsukawa M., Ogawa M., Nakadate K., et al. Serotonin and acetylcholine are crucial to maintain hippocampal synapses and memory acquisition in rats. Neuroscience Letters. 1997;230(1):13–16. doi: 10.1016/s0304-3940(97)00460-6. [DOI] [PubMed] [Google Scholar]
- 79.Stancampiano R., Cocco S., Cugusi C., Sarais L., Fadda F. Serotonin and acetylcholine release response in the rat hippocampus during a spatial memory task. Neuroscience. 1999;89(4):1135–1143. doi: 10.1016/S0306-4522(98)00397-2. [DOI] [PubMed] [Google Scholar]
- 80.Nail-Boucherie K., Dourmap N., Jaffard R., Costentin J. Contextual fear conditioning is associated with an increase of acetylcholine release in the hippocampus of rat. Cognitive Brain Research. 2000;9(2):193–197. doi: 10.1016/S0926-6410(99)00058-0. [DOI] [PubMed] [Google Scholar]
- 81.Van Hooft J. A., Vijverberg H. P. M. 5-HT3 receptors and neurotransmitter release in the CNS: a nerve ending story? Trends in Neurosciences. 2000;23(12):605–610. doi: 10.1016/s0166-2236(00)01662-3. [DOI] [PubMed] [Google Scholar]
- 82.Blandina P., Goldfarb J., Craddock-Royal B., Green J. P. Release of endogenous dopamine by stimulation of 5-hydroxytryptamine3 receptors in rat striatum. Journal of Pharmacology and Experimental Therapeutics. 1989;251(3):803–809. [PubMed] [Google Scholar]
- 83.Alex K. D., Pehek E. A. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacology and Therapeutics. 2007;113(2):296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell A. D., Womer D. E., Simon J. R. The 5-HT3 receptor agonist 1-(m-chlorophenyl)-biguanide interacts with the dopamine transporter in rat brain synaptosomes. European Journal of Pharmacology: Molecular Pharmacology. 1995;290(2):157–162. doi: 10.1016/0922-4106(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 85.Siddiqui M. F., Levey A. I. Cholinergic therapies in Alzheimer's disease. Drugs of the Future. 1999;24(4):417–424. doi: 10.1358/dof.1999.024.04.668318. [DOI] [Google Scholar]
- 86.Brunelli M., Castellucci V., Kandel E. R. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- 87.Castellucci V. F., Kandel E. R., Schwartz J. H. Intracellular injection of the catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia . Proceedings of the National Academy of Sciences of the United States of America. 1980;77(12):7492–7496. doi: 10.1073/pnas.77.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bacskai B. J., Hochner B., Mahaut-Smith M., et al. Spatially resolved dynamics of cAMP and protein kinase a subunits in Aplysia sensory neurons. Science. 1993;260(5105):222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- 89.Martin K. C., Michael D., Rose J. C., et al. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia . Neuron. 1997;18(6):899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 90.Villarreal J. S., Barea-Rodriguez E. J. ERK phosphorylation is required for retention of trace fear memory. Neurobiology of Learning and Memory. 2006;85(1):44–57. doi: 10.1016/j.nlm.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 91.Sindreu C. B., Scheiner Z. S., Storm D. R. Ca2+–stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53(1):79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Restivo L., Tafi E., Ammassari-Teule M., Marie H. Viral-mediated expression of a constitutively active form of CREB in hippocampal neurons increases memory. Hippocampus. 2009;19(3):228–234. doi: 10.1002/hipo.20527. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki A., Fukushima H., Mukawa T., et al. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. The Journal of Neuroscience. 2011;31(24):8786–8802. doi: 10.1523/jneurosci.3257-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davis S., Vanhoutte P., Pagès C., Caboche J., Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo . The Journal of Neuroscience. 2000;20(12):4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhai H., Li Y., Wang X., Lu L. Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement. Cellular and Molecular Neurobiology. 2008;28(2):157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartsch D., Casadio A., Karl K. A., Serodio P., Kandel E. R. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95(2):211–223. doi: 10.1016/S0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- 97.Yin J. C. P., del Vecchio M., Zhou H., Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in drosophila. Cell. 1995;81(1):107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- 98.Zhao H., Li Q., Zhang Z., Pei X., Wang J., Li Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Research. 2009;1256:111–122. doi: 10.1016/j.brainres.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 99.Kandel E. R. The molecular biology of memory storage: a dialog between genes and synapses. Bioscience Reports. 2001;21(5):565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- 100.Müller U., Carew T. J. Serotonin induces temporally and mechanistically distinct phases of persistent PKA activity in Aplysia sensory neurons. Neuron. 1998;21(6):1423–1434. doi: 10.1016/s0896-6273(00)80660-1. [DOI] [PubMed] [Google Scholar]
- 101.Li B., Zhang S., Li M., Hertz L., Peng L. Chronic treatment of astrocytes with therapeutically relevant fluoxetine concentrations enhances cPLA2 expression secondary to 5-HT 2B-induced, transactivation-mediated ERK1/2 phosphorylation. Psychopharmacology. 2009;207(1):1–12. doi: 10.1007/s00213-009-1631-3. [DOI] [PubMed] [Google Scholar]
- 102.Peng S., Zhang Y., Zhang J., Wang H., Ren B. ERK in learning and memory: a review of recent research. International Journal of Molecular Sciences. 2010;11(1):222–232. doi: 10.3390/ijms11010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bailey C. H., Kandel E. R., Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron. 2004;44(1):49–57. doi: 10.1016/j.neuron.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 104.Kelleher R. J., III, Govindarajan A., Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44(1):59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 105.Ashraf S. I., McLoon A. L., Sclarsic S. M., Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila . Cell. 2006;124(1):191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 106.Wayman G. A., Davare M., Ando H., et al. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(26):9093–9098. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smalheiser N. R., Lugli G. MicroRNA regulation of synaptic plasticity. Neuromolecular Medicine. 2009;11(3):133–140. doi: 10.1007/s12017-009-8065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lugli G., Larson J., Martone M. E., Jones Y., Smalheiser N. R. Dicer and eIF2c are enriched at postsynaptic densities in adult mouse brain and are modified by neuronal activity in a calpain-dependent manner. Journal of Neurochemistry. 2005;94(4):896–905. doi: 10.1111/j.1471-4159.2005.03224.x. [DOI] [PubMed] [Google Scholar]
- 109.Rajasethupathy P., Fiumara F., Sheridan R., et al. Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron. 2009;63(6):803–817. doi: 10.1016/j.neuron.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Konopka W., Kiryk A., Novak M., et al. MicroRNA loss enhances learning and memory in mice. The Journal of Neuroscience. 2010;30(44):14835–14842. doi: 10.1523/jneurosci.3030-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu I. Y. C., Lyons W. E., Mamounas L. A., Thompson R. F. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. The Journal of Neuroscience. 2004;24(36):7958–7963. doi: 10.1523/jneurosci.1948-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu R.-Y., Fioravante D., Shah S., Byrne J. H. cAMP response element-binding protein 1 feedback loop is necessary for consolidation of long-term synaptic facilitation in Aplysia . Journal of Neuroscience. 2008;28(8):1970–1976. doi: 10.1523/jneurosci.3848-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lewis B. P., Shih I.-H., Jones-Rhoades M. W., Bartel D. P., Burge C. B. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 114.Winter J., Jung S., Keller S., Gregory R. I., Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nature Cell Biology. 2009;11(3):228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 115.Siegel G., Saba R., Schratt G. MicroRNAs in neurons: manifold regulatory roles at the synapse. Current Opinion in Genetics and Development. 2011;21(4):491–497. doi: 10.1016/j.gde.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 116.Millan M. J. MicroRNA in the regulation and expression of serotonergic transmission in the brain and other tissues. Current Opinion in Pharmacology. 2011;11(1):11–22. doi: 10.1016/j.coph.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 117.Alberini C. M., Ghirardi M., Metz R., Kandel E. R. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia . Cell. 1994;76(6):1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 118.Lonze B. E., Ginty D. D. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–623. doi: 10.1016/S0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 119.Lee Y.-S., Silva A. J. The molecular and cellular biology of enhanced cognition. Nature Reviews Neuroscience. 2009;10(2):126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Genoux D., Haditsch U., Knobloch M., Michalon A., Storm D., Mansuy I. M. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418(6901):970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- 121.Mansuy I. M., Shenolikar S. Protein serine/threonine phosphatases in neuronal plasticity and disorders of learning and memory. Trends in Neurosciences. 2006;29(12):679–686. doi: 10.1016/j.tins.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 122.Knobloch M., Farinelli M., Konietzko U., Nitsch R. M., Mansuy I. M. Aβ oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. Journal of Neuroscience. 2007;27(29):7648–7653. doi: 10.1523/jneurosci.0395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koshibu K., Gräff J., Beullens M., et al. Protein phosphatase 1 regulates the histone code for long-term memory. The Journal of Neuroscience. 2009;29(41):13079–13089. doi: 10.1523/jneurosci.3610-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koshibu K., Gräff J., Mansuy I. M. Nuclear protein phosphatase-1: an epigenetic regulator of fear memory and amygdala long-term potentiation. Neuroscience. 2011;173:30–36. doi: 10.1016/j.neuroscience.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 125.Mauna J. C., Miyamae T., Pulli B., Thiels E. Protein phosphatases 1 and 2A are both required for long-term depression and associated dephosphorylation of cAMP response element binding protein in hippocampal area CA1 in vivo. Hippocampus. 2011;21(10):1093–1104. doi: 10.1002/hipo.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Genoux D., Bezerra P., Montgomery J. M. Intra-spaced stimulation and protein phosphatase 1 dictate the direction of synaptic plasticity. The European Journal of Neuroscience. 2011;33(10):1761–1770. doi: 10.1111/j.1460-9568.2011.07669.x. [DOI] [PubMed] [Google Scholar]
- 127.Oberbeck D. L., McCormack S., Houpt T. A. Intra-amygdalar okadaic acid enhances conditioned taste aversion learning and CREB phosphorylation in rats. Brain Research. 2010;1348:84–94. doi: 10.1016/j.brainres.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peters M., Bletsch M., Catapano R., Zhang X., Tully T., Bourtchouladze R. RNA interference in hippocampus demonstrates opposing roles for CREB and PP1α in contextual and temporal long-term memory. Genes, Brain and Behavior. 2009;8(3):320–329. doi: 10.1111/j.1601-183x.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 129.Yin Y.-Y., Liu H., Cong X.-B., et al. Acetyl-L-carnitine attenuates okadaic acid induced tau hyperphosphorylation and spatial memory impairment in rats. Journal of Alzheimer's Disease. 2010;19(2):735–746. doi: 10.3233/JAD-2010-1272. [DOI] [PubMed] [Google Scholar]
- 130.Wang X., Takata T., Bai X., Ou F., Yokono K., Sakurai T. Pyruvate prevents the inhibition of the long-term potentiation induced by amyloid-β through protein phosphatase 2A inactivation. Journal of Alzheimer's Disease. 2012;30(3):665–673. doi: 10.3233/JAD-2012-101869. [DOI] [PubMed] [Google Scholar]
- 131.Guan Z., Giustetto M., Lomvardas S., et al. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111(4):483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- 132.Levenson J. M., Sweatt J. D. Epigenetic mechanisms in memory formation. Nature Reviews Neuroscience. 2005;6(2):108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- 133.Hsieh J., Gage F. H. Chromatin remodeling in neural development and plasticity. Current Opinion in Cell Biology. 2005;17(6):664–671. doi: 10.1016/j.ceb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 134.Canettieri G., Morantte I., Guzmán E., et al. Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nature Structural Biology. 2003;10(3):175–181. doi: 10.1038/nsb895. [DOI] [PubMed] [Google Scholar]
- 135.Levenson J. M., O'Riordan K. J., Brown K. D., Trinh M. A., Molfese D. L., Sweatt J. D. Regulation of histone acetylation during memory formation in the hippocampus. The Journal of Biological Chemistry. 2004;279(39):40545–40559. doi: 10.1074/jbc.m402229200. [DOI] [PubMed] [Google Scholar]
- 136.Korzus E., Rosenfeld M. G., Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42(6):961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Asahara H., Santoso B., Guzman E., et al. Chromatin-dependent cooperativity between constitutive and inducible activation domains in CREB. Molecular and Cellular Biology. 2001;21(23):7892–7900. doi: 10.1128/mcb.21.23.7892-7900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lundblad J. R., Kwok R. P. S., Laurance M. E., Harter M. L., Goodman R. H. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature. 1995;374(6517):85–88. doi: 10.1038/374085a0. [DOI] [PubMed] [Google Scholar]
- 139.Chan H. M., La Thangue N. B. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. Journal of Cell Science. 2001;114(13):2363–2373. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- 140.Vo N., Goodman R. H. CREB-binding protein and p300 in transcriptional regulation. Journal of Biological Chemistry. 2001;276(17):13505–13508. doi: 10.1074/jbc.r000025200. [DOI] [PubMed] [Google Scholar]
- 141.Oliveira A. M. M., Wood M. A., McDonough C. B., Abel T. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learning and Memory. 2007;14(9):564–572. doi: 10.1101/lm.656907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oliveira A. M. M., Estévez M. A., Hawk J. D., Grimes S., Brindle P. K., Abel T. Subregion-specific p300 conditional knock-out mice exhibit long-term memory impairments. Learning and Memory. 2011;18(3):161–169. doi: 10.1101/lm.1939811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marek R., Coelho C. M., Sullivan R. K. P., et al. Paradoxical enhancement of fear extinction memory and synaptic plasticity by inhibition of the histone acetyltransferase p300. The Journal of Neuroscience. 2011;31(20):7486–7491. doi: 10.1523/jneurosci.0133-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ogryzko V. V., Schiltz R. L., Russanova V., Howard B. H., Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87(5):953–959. doi: 10.1016/S0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 145.Schiltz R. L., Mizzen C. A., Vassilev A., Cook R. G., Allis C. D., Nakatani Y. Overlapping but distinct patterns of histone acetylation by the human coactivators p300 and PCAF within nucleosomal substrates. The Journal of Biological Chemistry. 1999;274(3):1189–1192. doi: 10.1074/jbc.274.3.1189. [DOI] [PubMed] [Google Scholar]
- 146.McManus K. J., Hendzel M. J. Quantitative analysis of CBP- and P300-induced histone acetylations in vivo using native chromatin. Molecular and Cellular Biology. 2003;23(21):7611–7627. doi: 10.1128/mcb.23.21.7611-7627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vecsey C. G., Hawk J. D., Lattal K. M., et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB: CBP-dependent transcriptional activation. The Journal of Neuroscience. 2007;27(23):6128–6140. doi: 10.1523/jneurosci.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanchis-Segura C., Lopez-Atalaya J. P., Barco A. Selective boosting of transcriptional and behavioral responses to drugs of abuse by histone deacetylase inhibition. Neuropsychopharmacology. 2009;34(13):2642–2654. doi: 10.1038/npp.2009.125. [DOI] [PubMed] [Google Scholar]
- 149.Kilgore M., Miller C. A., Fass D. M., et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of alzheimer's disease. Neuropsychopharmacology. 2010;35(4):870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Itzhak Y., Anderson K. L., Kelley J. B., Petkov M. Histone acetylation rescues contextual fear conditioning in nNOS KO mice and accelerates extinction of cued fear conditioning in wild type mice. Neurobiology of Learning and Memory. 2012;97(4):409–417. doi: 10.1016/j.nlm.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Washbourne P., Dityatev A., Scheiffele P., et al. Cell adhesion molecules in synapse formation. The Journal of Neuroscience. 2004;24(42):9244–9249. doi: 10.1523/jneurosci.3339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Craig A. M., Graf E. R., Linhoff M. W. How to build a central synapse: clues from cell culture. Trends in Neurosciences. 2006;29(1):8–20. doi: 10.1016/j.tins.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nakhost A., Houeland G., Castellucci V. F., Sossin W. S. Differential regulation of transmitter release by alternatively spliced forms of synaptotagmin I. The Journal of Neuroscience. 2003;23(15):6238–6244. doi: 10.1523/JNEUROSCI.23-15-06238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schwarz T. L. Synaptotagmin promotes both vesicle fusion and recycling. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(47):16401–16402. doi: 10.1073/pnas.0407390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Holahan M. R., Rekart J. L., Sandoval J., Routtenberg A. Spatial learning induces presynaptic structural remodeling in the hippocampal mossy fiber system of two rat strains. Hippocampus. 2006;16(6):560–570. doi: 10.1002/hipo.20185. [DOI] [PubMed] [Google Scholar]
- 156.Sun D., McGinn M. J., Zhou Z., Harvey H. B., Bullock M. R., Colello R. J. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Experimental Neurology. 2007;204(1):264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 157.Petersen D. J., Chen X., Vinade L., et al. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. The Journal of Neuroscience. 2003;23(35):11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barria A., Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48(2):289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 159.Vaynman S., Ying Z., Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;144(3):825–833. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barria A., Muller D., Derkach V., Griffith L. C., Soderling T. R. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276(5321):2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 161.Moyano S., Del Río J., Frechilla D. Role of hippocampal-CaMKII in serotonin 5-HTA1 receptor-mediated learning deficit in rats. Neuropsychopharmacology. 2004;29(12):2216–2224. doi: 10.1038/sj.npp.1300504. [DOI] [PubMed] [Google Scholar]
- 162.Achterberg K. G., Buitendijk G. H., Kool M. J., et al. Temporal and region-specific requirements of αCaMKII in spatial and contextual learning. The Journal of Neuroscience. 2014;34(34):11180–11187. doi: 10.1523/jneurosci.0640-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hata Y., Takai Y. Roles of postsynaptic density-95/synapse-associated protein 90 and its interacting proteins in the organization of synapses. Cellular and Molecular Life Sciences. 1999;56(5-6):461–472. doi: 10.1007/s000180050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nikonenko I., Boda B., Steen S., Knott G., Welker E., Muller D. PSD-95 promotes synaptogenesis and multiinnervated spine formation through nitric oxide signaling. The Journal of Cell Biology. 2008;183(6):1115–1127. doi: 10.1083/jcb.200805132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Garner C. C., Nash J., Huganir R. L. PDZ domains in synapse assembly and signalling. Trends in Cell Biology. 2000;10(7):274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- 166.Charych E. I., Akum B. F., Goldberg J. S., et al. Activity-independent regulation of dendrite patterning by postsynaptic density protein PSD-95. The Journal of Neuroscience. 2006;26(40):10164–10176. doi: 10.1523/jneurosci.2379-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Skibinska A., Lech M., Kossut M. PSD95 protein level rises in murine somatosensory cortex after sensory training. NeuroReport. 2001;12(13):2907–2910. doi: 10.1097/00001756-200109170-00030. [DOI] [PubMed] [Google Scholar]
- 168.Yoshii A., Sheng M. H., Constantine-Paton M. Eye opening induces a rapid dendritic localization of PSD-95 in central visual neurons. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):1334–1339. doi: 10.1073/pnas.0335785100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sturgill J. F., Steiner P., Czervionke B. L., Sabatini B. L. Distinct domains within PSD-95 mediate synaptic incorporation, stabilization, and activity-dependent trafficking. The Journal of Neuroscience. 2009;29(41):12845–12854. doi: 10.1523/jneurosci.1841-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.El-Husseini A. E.-D., Schnell E., Chetkovich D. M., Nicoll R. A., Bredt D. S. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290(5495):1364–1368. [PubMed] [Google Scholar]
- 171.Tomita S., Nicoll R. A., Bredt D. S. PDZ protein interactions regulating glutamate receptor function and plasticity. The Journal of Cell Biology. 2001;153(5):F19–F23. doi: 10.1083/jcb.153.5.f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sun Q. J., Duan R. S., Wang A. H., et al. Alterations of NR2B and PSD-95 expression in hippocampus of kainic acid-exposed rats with behavioural deficits. Behavioural Brain Research. 2009;201(2):292–299. doi: 10.1016/j.bbr.2009.02.027. [DOI] [PubMed] [Google Scholar]