Abstract
It is well known that plant photosynthesis and respiration are two fundamental and crucial physiological processes, while the critical role of the antioxidant system in response to abiotic factors is still a focus point for investigating physiological stress. Although one key metabolic process and its response to climatic change have already been reported and reviewed, an integrative review, including several biological processes at multiple scales, has not been well reported. The current review will present a synthesis focusing on the underlying mechanisms in the responses to elevated CO2 at multiple scales, including molecular, cellular, biochemical, physiological, and individual aspects, particularly, for these biological processes under elevated CO2 with other key abiotic stresses, such as heat, drought, and ozone pollution, as well as nitrogen limitation. The present comprehensive review may add timely and substantial information about the topic in recent studies, while it presents what has been well established in previous reviews. First, an outline of the critical biological processes, and an overview of their roles in environmental regulation, is presented. Second, the research advances with regard to the individual subtopics are reviewed, including the response and adaptation of the photosynthetic capacity, respiration, and antioxidant system to CO2 enrichment alone, and its combination with other climatic change factors. Finally, the potential applications for plant responses at various levels to climate change are discussed. The above issue is currently of crucial concern worldwide, and this review may help in a better understanding of how plants deal with elevated CO2 using other mainstream abiotic factors, including molecular, cellular, biochemical, physiological, and whole individual processes, and the better management of the ecological environment, climate change, and sustainable development.
Keywords: abiotic stress, antioxidant system, elevated CO2, drought, global warming, photosynthesis, respiration
Introduction
The major components of climate change include elevated atmospheric carbon dioxide concentrations (elevated CO2), warming, and altered precipitation patterns, as well as their interactions within and with other environmental factors (IPCC, 2013). Based on updated information, with increases in global atmospheric CO2 concentrations of 43% from the pre-industrial level of 280 μmol mol-1 in 1750 to the present level of 400 μmol mol-1 (an annual increase of 1.35%), the global CO2 concentration has increased by about 1.55 ppm CO2 per year over the past 55 years. It continues to be elevated at an unprecedented pace of ∼1.0 μmol mol-1 per year, as a result of the further increase in the cumulative emissions of CO2 to the atmosphere during the 21st century (400 μmol mol-1 in 2011 vs. 936 μmol mol-1 in 2100; IPCC, 2013; NASA, 2014). Meanwhile, the global mean surface temperature is expected to increase by 2.6–4.8°C by the end of the 21st century (2081–2100), relative to the 1986–2005 level under RCP8.5, based on a more undisciplined management scenario with higher greenhouse gas emissions (IPCC, 2013). The climate changes, such as elevated CO2, rising temperature, and altered precipitation, have resulted in drastic impacts on the natural ecosystems, such as in vegetation function, sustainable food production, and crop yields (Lobell et al., 2011; Peñuelas et al., 2013; Ruiz-Vera et al., 2013; Xu et al., 2013a, 2014; Lavania et al., 2015), leading to more profound impacts when the climate changes are combined with other environmental constraints, such as air pollution, nutrition limitation, and their interactions (Gillespie et al., 2012; Peñuelas et al., 2012; Xu et al., 2013a,b; Wang et al., 2015).
Herein, we focus on the critical biological processes of plants with regard to climate change, including (mainly) photosynthesis, respiration, the antioxidant system, and the related metabolic activities. Photosynthesis and respiration are two fundamental physiological processes of plants, because the former involves initial carbon fixation, light energy transfer, and oxygen release, and the latter works on carbon efflux, energy production, and the relevant substrate metabolisms, such as those providing the carbon skeleton. They play a critical role in balancing the carbon budget and maintaining the carbon sink in terrestrial ecosystems, as well as in the response and feedback to climate change (Melillo et al., 1993; Prentice et al., 2001; Sage, 2004; Long et al., 2006; Atkin et al., 2010; Atkin, 2015). The excessive accumulation of reactive oxygen species (ROS) often occurs in plants grown under abiotic stress, while an enzymatic and non-enzymatic antioxidant defense system may work to protect plants against oxidative stress-induced damage, which can be affected by climate change (such as elevated CO2, drought, and heat waves; Pérez-López et al., 2009; Gill and Tuteja, 2010; Xu et al., 2014; Zinta et al., 2014; Way et al., 2015).
Rising CO2 has affected almost all crucial biological processes, including photosynthesis, respiration, and antioxidant systems, as well as other key secondary metabolisms in plants (Poorter et al., 1997; Long et al., 2004; Matros et al., 2006; Peñuelas et al., 2013; Singh and Agrawal, 2015). All other effects of elevated CO2 on individual plants and ecosystems may be partly derived from these fundamental biological responses (Long et al., 2004; Ainsworth and Rogers, 2007; Peñuelas et al., 2013; Zinta et al., 2014). Genetic variations relative to the biological processes’ traits might also be impacted by elevated CO2, closely linking to these responses in various spatiotemporal aspects, from molecular, biochemical, and physiological, through individual levels and ecosystems, up to the entire Earth’s life system, interacting with multiple environmental factors (both biotic and abiotic) as well as human-driven disturbances at different temporal scales (Long et al., 2004; Teng et al., 2009; Peñuelas et al., 2012, 2013; Jagadish et al., 2014; Zinta et al., 2014).
As stated above, plant responses to climate change have become a hot topic in botanical research across various scales in the recent decades. Many reports have reviewed the biological responses to CO2 enrichment, and their interactions with environmental change, including photosynthesis and stomatal behavior (e.g., Long et al., 2004; Ainsworth and Long, 2005; Ainsworth and Rogers, 2007). Our earlier review by Xu et al. (2013a) examined plant growth, carbon and nitrogen (N) allocations, gas exchange responses to elevated CO2 with drought and high temperature. Although this review discussed the changes in growth and photosynthesis, and water use efficiency (WUE) in higher plants exposed to CO2 enrichment with abiotic variables, the various underlying mechanisms of the critical biological processes that are affected, modulated, and controlled by elevated CO2 with other abiotic environmental variables were not fully covered, particular at the molecular, organelle, cell, biochemical, physiological, organ, individual, and ecosystem scales. Actually, no systematic synthesis of these has been well reviewed, thus far. Therefore, in this review, based on correcting and synthesizing any new progress of the relevant research concerning plant biology and climatic change, we attempted to systematically summarize the considerable study results that have reported the responses of photosynthesis, respiration, and the antioxidant systems, as well as the key substrate metabolisms to elevated CO2 with other environmental variables. Particularly, we reviewed the underlying mechanisms and the response pathways, as well as their interrelationships. Finally, the future perspectives for this study related to the possible implications are briefly presented and discussed. Thus, the present review may be of current interest in terms of its interdisciplinary and systematic synthesis, providing comprehensive information on the important historical and new experimental results, relative theoretical analysis, underlying mechanisms, and potential applications to promote further research.
Responses of Critical Biological Processes to Elevated CO2
Photosynthetic Response to Elevated CO2 Concentrations
Response Magnitude
The responses of photosynthesis to elevated CO2 concentrations have been reviewed in many reports [e.g., Drake et al., 1997 most for enclosure results; Long et al., 2004; Nowak et al., 2004; Ainsworth and Long, 2005; Ainsworth and Rogers, 2007 for free-air CO2 enrichment (FACE)]. The stimulation of the light-saturated photosynthetic CO2 assimilation rate (Asat) is a general response to CO2 enrichment, with an average of 31% in the FACE experiments (Ainsworth and Rogers, 2007), and 23–58% in the potted plant experiments from earlier reports (Ryle et al., 1992; Drake et al., 1997). The magnitude of the stimulation by CO2 enrichment varies with the different plant functional types (PFTs), with a maximum for trees and C3 grasses; moderate for shrubs, C3 and C4 crops, and legumes; and minimum for C4 grass (even with a negative response; Drake et al., 1997; Ainsworth and Long, 2005; Ainsworth and Rogers, 2007). Therefore, there is greater variation in the stimulation by elevated CO2, depending on the plant species, PFTs, and their surroundings, specifically environmental conditions like nutrition and water resource availability. For instance, elevated CO2 leads to an increase in the Asat of Arabidopsis thaliana leaves by 82%, since the N availability is ample (Markelz et al., 2014). However, a recent study of soybean plants indicated that elevated CO2 did not produce significant effects on midday net photosynthetic rate (Anet), either in the FACE or open-top chamber (OTC) studies (Bunce, 2014), suggesting that the Anet at high photosynthetic photon flux density (PPFD) might be limited by a low ribulose 1, 5-bisphosphate carboxylase/oxygenase (Rubisco) carboxylation capacity (Bunce, 2014). Actually, other abiotic and biotic factors such as high temperature (e.g., Ruiz-Vera et al., 2013), drought (Xu et al., 2014), N deficit (Markelz et al., 2014), genetic variation (Ainsworth et al., 2004), and leaf senescence (Liu et al., 2014) may also diminish the photosynthetic response to elevated CO2.
The same results appeared in Lolium perenne and Medicago lupulina plants in controlled chambers (Farfan-Vignolo and Asard, 2012). Moreover, C4 plants may have no response to elevated CO2, because their CO2 concentrating mechanism (CCM) may concentrate the CO2 12–20 times at the site of Rubisco, which is relatively higher than in C3 species (von Caemmerer and Furbank, 2003; Ainsworth and Rogers, 2007). Case studies confirmed this theoretical conclusion under well-watered conditions in either enclosure (e.g., Xu et al., 2014) or FACE experiments (e.g., Leakey et al., 2006; Markelz et al., 2011). However, under a water deficit, the stimulation of the C4 Asat by elevated CO2 still appears, because the drought-induced impairment of C4 photosynthesis might be ameliorated by elevated CO2 (Markelz et al., 2011; Meng et al., 2014; Xu et al., 2014). Moreover, C4 plants can avoid photorespiration to promote CO2 fixation with higher light use efficiency (von Caemmerer and Furbank, 2003; Long et al., 2006). On the other hand, the down-regulation of the photosynthesis capacity is also more profound in C3 species than in C4 species (Morgan et al., 2001; Duarte et al., 2014), due in part to the N dilution, possibly because C3 plants need to invest more N from the leaf into Rubisco, relative to the C4 species, so that the former may easily undergo more severe N dilution under CO2 enrichment (Sage et al., 1987; Yin, 2002; Luo et al., 2004; Sage, 2004), with no CCM (von Caemmerer and Furbank, 2003).
In addition to N limitation, photosynthetic acclimation under higher CO2 levels may result from high stomatal and internal resistances, higher starch levels, and diluted chlorophyll concentrations (Delucia et al., 1985; Teng et al., 2009). Under elevated CO2, carbohydrate accumulations, such as starch size and number of chloroplasts (Teng et al., 2006, 2009), can be enhanced, partially due to the carbon substrate increase. However, the excessive carbohydrate accumulation may cause feedback inhibition or physical damage at the chloroplast level, reducing the photosynthetic capacity (Delucia et al., 1985; Aranjuelo et al., 2011). More importantly, the Rubisco response, excessive sugar feedback, and the related gene expression may, together; play crucial roles in plants’ photosynthetic acclimation under higher CO2 concentrations, particularly for long-term CO2 enrichment under a nitrogen availability deficit (see details below).
Molecular Mechanisms: Role of Rubisco
The stimulation of photosynthesis in C3 species by short-term elevated CO2 has been well established, and confirmed under almost all experimental conditions, particularly with FACE (e.g., Long et al., 2004; Ainsworth and Rogers, 2007; Duarte et al., 2014). However, with long-term exposure to elevated CO2 or other limitations, photosynthetic acclimation or the down-regulation of the photosynthetic capacity may occur, depending on the species, plant developmental stage, and environmental conditions (Moore et al., 1999; Urban et al., 2012; Sanz-Sáez et al., 2013).
Rubisco has been identified as a controlling rate enzyme for carbon fixation (Eichelmann et al., 2009). Here, we succinctly summarize the five major mechanisms that might explain the response to elevated CO2, involving Rubisco: (1) under current CO2 concentration levels, although the value of the Rubisco Michaelis–Menten constant (Km) for CO2 is close to the current intercellular CO2 concentration (Ci) (c. 190 μmol mol-1) at the site of carboxylation (von Caemmerer and Evans, 1991; Ainsworth and Rogers, 2007). CO2, as a substrate of photosynthesis, does not have to reach saturation; therefore, the rising CO2 can lead to an immediate increase in the Rubisco carboxylation velocity, due to an increase in the carbon substrate availability. (2) The Rubisco catalyzing function has two intrinsic side features: carboxylation and oxygenation. The carboxylation rate is ∼2.2 fold greater than the oxygenation rate at 25°C in C3 plants; that is, about one-third of the ribulose-1,5-bisphosphate (RuBP) may be consumed in the oxygenation reaction (Ainsworth and Rogers, 2007). Thus, elevated CO2, as a competing substrate, can competitively inhibit the oxygenation of RuBP (light-dependent photorespiration) through the down-regulation of Rubisco’s affinity for O2, while competitively promoting the carboxylation of RuBP via the up-regulation of Rubisco’s affinity for CO2 (Bowes, 1991; Long, 1991; Ainsworth and Rogers, 2007; Kane et al., 2013; Moroney et al., 2013). Consequently, this leads to the stimulation of photosynthesis, which may be compromised by heat and drought due to the enhancement of Rubisco’s affinity for O2 (Wingler et al., 1999; Tingey et al., 2003; Carmo-Silva et al., 2008; Moroney et al., 2013) (Figure 1). On the other hand, (3) with continually increasing CO2, the ATP products may not meet enough of the demand for RuBP regeneration, and a reduction in Rubisco’s activation state may occur, usually accompanied by a decrease in the capacity for RuBP regeneration, as well as in the RuBP pool, as indicated by a decline in the ATP:ADP ratio in the chloroplast (Eichelmann et al., 2009; Watanabe et al., 2014). (4) A reduction in the Rubisco content via N dilution, particularly under long-term elevated CO2, may finally contribute to the reduction of carboxylation at the Rubisco active site. In addition, the nitrogen use efficiency (NUE) might be increased due to the optimization of the resource use (Moore et al., 1999; Luo et al., 2004; Fukayama et al., 2012; Urban et al., 2012; Palmroth et al., 2013; Sanz-Sáez et al., 2013). Because the leaf N of C3 species can be more invested in Rubisco (more than 25% vs. 10–15% of the leaf N in C3 and C4 plants, respectively), the former may be affected more profoundly by N dilution.
FIGURE 1.
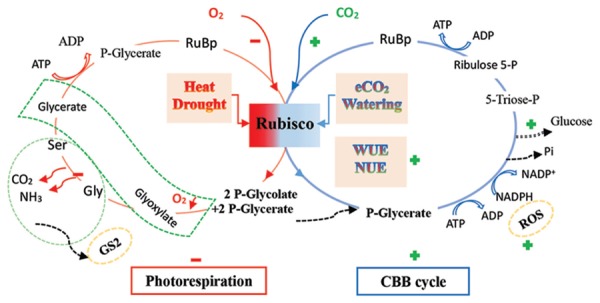
A diagrammatic outline of the Calvin–Benson–Basshan (CBB) cycle and photorespiration pathway in plants in response to elevated CO2 with abiotic factors. Rubisco has two sites of carboxylation and oxygenation. Elevated CO2 (eCO2) may promote carboxylation, but repress oxygenation under ample environmental conditions, such as well-watering, whereas extreme abiotic stress, such as heat and drought, may repress carboxylation but promote oxygenation (Wingler et al., 1999; Tingey et al., 2003). An energy consumption trade-off between the key cycles may occur, possibly modified by the CO2 level, in which photorespiration may be promoted to quench reactive oxygen species (ROS), related to glutamine synthetase (GS2) to recycle ammonia, diminishing photo-oxidation and photo-inhibition (dotted orange line ellipse; Kozaki and Takeba, 1996; Watanabe et al., 2014). A low Gly:Ser ratio provides evidence that photorespiration is repressed in eCO2 (Kebeish et al., 2007). Water use efficiency (WUE) and nitrogen use efficiency (NUE), despite the N dilution, should be enhanced by elevated CO2, by decreasing stomatal conductance and investing relatively more N into the Rubisco protein (Palmroth et al., 2013). The photorespiration process is compartmentalized into the chloroplast (red line ellipse), peroxisome (dotted green line bent rectangle), and mitochondrion (dotted green line ellipse). The green plus and red minus signs denote the stimulation and suppression via rising CO2, respectively (mainly referring to Kozaki and Takeba, 1996; Wingler et al., 1999; Tingey et al., 2003; Ainsworth and Rogers, 2007; Moroney et al., 2013; Xu et al., 2013a; Watanabe et al., 2014).
Finally, (5) Hexokinase (HXK), as a sensor of excessive photosynthate, may be involved in the downregulation of the Rubisco content (Ainsworth and Rogers, 2007; Kirschbaum, 2011; see below). In summary, with respect to the Rubisco response, parts (1) and (2) above may explain the stimulation of photosynthesis by elevated CO2, while the last three points may provide a mechanism for understanding the downregulation of the photosynthetic capacity under relatively long-term elevated CO2 or other resource deficit conditions, such as a scarcity of N.
Sugar Feedback Inhibition of Photosynthesis
Under higher CO2 concentrations, a prevailing explanation of the downregulation of photosynthesis may be ascribed to the sugar feedback inhibition hypothesis: certain reactive bioprocess activities within the Calvin–Benson–Basshan (CBB) cycle may be inhibited by elevated CO2, due to the overload of the chemical reaction substrates. The hypothesis of the sugar feedback mechanism suggests that excessive photosynthate in chloroplasts under elevated CO2 may trigger the sugar signal network (HXK acting as a flux sensor) to down-regulate the Rubisco contents through the gene expression processes, affecting the subunit of Rubisco (Drake et al., 1997; Stitt and Krapp, 1999; Long et al., 2004; Ainsworth and Rogers, 2007).
As noted above, HXK acting as a flux sensor in mesophyll cells may involve the down-regulation of the Rubisco content associated with genetic expressions under elevated CO2; however, plants may prefer to reduce the Rubisco activity relative to the RuBP regeneration capacity (Ainsworth and Rogers, 2007). For instance, Aranjuelo et al. (2011) found a decline in wheat Rubisco and its activase protein content accompanying a photosynthetic down-regulation. In addition, the effects of the source-sink balance in response to CO2 enrichment may play an important role in the regulation of the photosynthetic capacity.
Based on the “source-sink” hypothesis, some plants with a strong sink can encounter photosynthetic down-regulation, to some extent, under higher CO2, which can generally be repressed by other limitations, such as intrinsic genetic constraints or the specific plant developmental stage (such as the flowering stage; Lewis et al., 2002; Ainsworth et al., 2004). Moreover, when enhanced carbohydrate availability exceeds the plants’ ability to fully utilize carbohydrates, due to nutrient or inherent internal growth limitations, the feedback may lead to a lower level of photosynthesis (Kirschbaum, 2011), which may lead to an imbalance in the carbon sink:source ratio (Bryant et al., 1998; Aranjuelo et al., 2011). For instance, wheat plants exposed to high atmospheric CO2 are incapable of excessive accumulation of leaf photoassimilate, due to the lack of an increase in the carbon sink strength (Aranjuelo et al., 2011).
Furthermore, the respiratory ATP may be consumed more under elevated CO2 (Watanabe et al., 2014); for example, the rate of the carbohydrate/sugar export (i.e., the cost related to the carbohydrate export) is higher under elevated CO2 than under normal CO2 (Watanabe et al., 2014), which may cause a negative feedback effect on photosynthesis. This also highlights the close link between the photosynthetic and respiratory bioprocesses, between both the CBB and tricarboxylic acid (TCA) cycle, under climate change (Moroney et al., 2013; Watanabe et al., 2014).
Response of Respiration to Elevated CO2
Photorespiration
Photorespiration enables the photosynthetic process to recycle the phosphoglycolate produced by the oxygenase reaction of Rubisco, consequently avoiding more carbon loss, with some protective regulation functions for plants, such as in the oxidative defense mechanism (Bowes et al., 1971; Husic et al., 1987; Kozaki and Takeba, 1996; Wingler et al., 1999; Carmo-Silva et al., 2008; Moroney et al., 2013). However, as reported in the review by Ainsworth and Rogers (2007), at room temperature (25°C), photorespiration can lead to a loss of 23–30% of the carbon fixed by photosynthesis with the rising temperature, whereas the CO2 fixation may be increased by ∼53% if only the carboxylation reaction occurs, without the oxygenation reaction (Monteith, 1977; Long et al., 2006).
Widely accepted results show that photorespiration can be restricted when C3 plants are grown under high CO2 concentrations (Bowes, 1991; Tingey et al., 2003; Long et al., 2004), because in C3 plants, the carboxylation capacity of Rubisco, with a low catalytic activity (operating below its Km for CO2), is easily promoted by high CO2. Meanwhile, an increase in the CO2 concentration, leading to a high CO2:O2 ratio, may reduce its oxygenation reaction capacity, inhibiting photorespiration (Bowes, 1991; Tingey et al., 2003; see above). For example, based on the earlier report by Sharkey (1988), the photorespiration rate should fall by ∼50% when the CO2 level is doubled. In A. thaliana plants grown under elevated CO2, although the accumulations of several major amino acids (including glutamate, aspartate, asparagine, and alanine) were enhanced, a lower level of glycine (Gly), an intermediate of photorespiration, was observed in the plants, leading to a decline in the Gly:Ser ratio, indicating a lower photorespiration activity (Kebeish et al., 2007; Figure 1).
Enhanced photoperoxidation in chloroplasts can induce a destruction of the chlorophyll and a disassembly of the chloroplast membranes, leading to a decline in photosynthesis (Heath and Packer, 1968). Conversely, the constraints of photorespiration by elevated CO2 may also reduce the H2O2 products, weakening oxidation stress, possibly protecting the photosynthetic apparatus (Watanabe et al., 2014; Zinta et al., 2014). Based on the fact that photorespiration has a protective function against photo-oxidation (Kozaki and Takeba, 1996; Zinta et al., 2014), possibly via the up-regulation of glutamine synthetase (GS2) to recycle ammonia, diminishing photo-oxidation and photo-inhibition (Kozaki and Takeba, 1996). This brings with it another dilemma: a decline in photorespiration under rising CO2 levels may cancel the protective role, leading to a higher level of photo-oxidation than the higher rate of carboxylation stimulated by elevated CO2 can maintain. In order to solve this dilemma, further research is required to cope with climate change, possibly by manipulating the modulated photorespiration bioprocess (Moroney et al., 2013).
Mitochondrial Respiration
Mitochondrial respiration involves the carbon balance in the whole plant, with 20–80% of the carbon fixed in photosynthesis being released again through the respiration process. The respiration of the leaves in both the light and dark can account for ∼50% of the whole-plant respiratory CO2 (Ayub et al., 2014). The response of dark leaf respiration (Rd) to elevated CO2 remains debatable, with a decrease in the major reports, while increasing or remaining stable in a number of experiments (e.g., Ryle et al., 1992; Curtis and Wang, 1998; Drake et al., 1999; Amthor, 2000; Gonzalez-Meler et al., 2004; Loreto et al., 2007; Ayub et al., 2014). For instance, there is a 15–18% range in the reduction of foliar respiration when plants are grown under a doubled CO2 concentration, relative to the ambient CO2 level from one review (Drake et al., 1999; references). However, no significant response to the leaf Rd was observed in L. perenne plants exposed to high CO2; although, the leaves grown in elevated CO2 had a relative lower Rd (Ryle et al., 1992). A small response in the leaf respiration rate to a short-term CO2 elevation (a 1.5% decrease) was obtained from the deciduous tree species used in an earlier experiment by Amthor (2000), with similar evidence found in soybean plants from a recent report by Ayub et al. (2014). Thus, for the plants grown under elevated CO2, the Rd decrease response is general, not universal.
Correspondingly, the underlying mechanism has also been proposed in two contrasting hypotheses: elevated CO2 may enhance the Rd due to the great increase in the respiratory substrates, such as sugar; whereas the N dilution induced by elevated CO2 might reduce the demand on dark respiration to support the protein turnover, leading to a decline in the Rd (Thomas et al., 1993; Gonzalez-Meler et al., 2004; Fukayama et al., 2011; Markelz et al., 2014). A recent report showed that CO2 enrichment can accelerate the accumulation of the relevant carbohydrates, such as sugar, starch, and respiratory glycolysis intermediates like hexose-P, phosphoglycerate (PGA), and phosphoenolpyruvate (PEP) in A. thaliana plants, which may enhance the respiration potential (Watanabe et al., 2014). Recent evidence has also indicated that the promotion to greater photo-assimilation availability at elevated CO2 leads to a great transcriptional up-regulation of the genes, in association with the respiratory pathway (Leakey et al., 2009a; Fukayama et al., 2011; Markelz et al., 2014), supporting the first hypothesis. However, this may depend on the availability of the nutritional components, including nitrogen in the plants and/or the soil. For instance, based on a recent report by Markelz et al. (2014), widely and greatly adaptive responses of the expression of the respiratory genes were obtained when the plants were exposed to elevated CO2. However, the transcriptional reprogramming with the stimulation of leaf respiration by elevated CO2 can be suppressed by limited nitrogen availability (Markelz et al., 2014).
Response of Antioxidant System to Elevated CO2
The ROS in plants, including superoxide radicals (), hydrogen peroxide (H2O2), the hydroxyl radical (OH⋅), and the perhydroxy radical (), often accumulate when plants are subjected to abiotic stress, while the antioxidant defense system with enzymatic and non-enzymatic machinery may work to protect the plants against damage due to oxidative stress. This occurs particularly in the face of stressful environmental changes, such as adverse climatic changes like droughts and heat waves (Figure 2) (Schwanz and Polle, 1998; Pérez-López et al., 2009; Gill and Tuteja, 2010; Sekmen et al., 2014; Zinta et al., 2014). Generally, when plants become senesced, with some antioxidants increasing and others decreasing, the ROS may accumulate in a large amount, and the antioxidant system does not work well. This is often indicated by enhanced lipid peroxidation and decreased levels of antioxidant enzymes, such as superoxide dismutase (SOD) and catalase (CAT), leading to programmed cell death (PCD), particularly under severe abiotic stress (Dhindsa et al., 1981; Hodges and Forney, 2000; Gill and Tuteja, 2010; Duarte et al., 2013).
FIGURE 2.
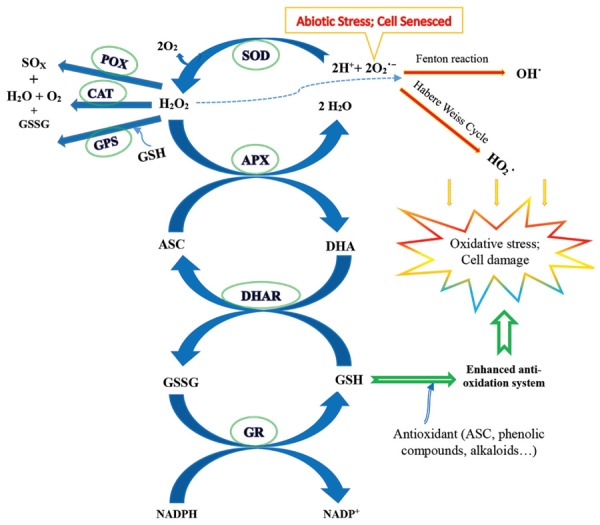
A diagrammatic outline of the antioxidant defense systems and the responses to elevated CO2 with abiotic stress. Elevated CO2 may alleviate the damage of oxidative stress from abiotic stress factors, such as heat, drought, and ozone, by ameliorating the antioxidant defense systems of non-enzymatic compounds, potentially including ascorbate (ASC), glutathione (GSH), phenolic compounds, and alkaloids, and the relevant enzymes, possibly including superoxide dismutase (SOD), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), glutathione reductase (GR), peroxidase (POX), catalase (CAT), and glutathione peroxidase (GPX). ROSs, including superoxide radicals (), hydrogen peroxide (H2O2), hydroxyl radicals (OH⋅), and perhydroxy radicals (), accumulate when plants undergo abiotic stress or are senesced by the Fenton reaction and/or the Habere Weiss mechanism (Hodges and Forney, 2000; Gill and Tuteja, 2010). Whether the rising CO2 mitigates oxidative damage and the response magnitude, and which parts play major roles, depends on the plant species, crop varieties, developmental stage, abiotic factors, and their combinations (e.g., Hodges and Forney, 2000; Gill and Tuteja, 2010; Abd Elgawad and Asard, 2013; Kumari et al., 2013; Zinta et al., 2014). GSSG, oxidized glutathione; DHA, dehydroascorbate. This diagram is based mainly on the studies by Gill and Tuteja (2010) and Zinta et al. (2014).
Elevated CO2 may increase the levels of antioxidants, including polyphenols, ascorbate (ASC), alkaloids, and some antioxidant enzyme activities (such as CAT and SOD), with a significant enhancement in the antioxidant capacity, leading to declines in the ROS levels (Mishra and Agrawal, 2014; Zinta et al., 2014). For example, when the plants were exposed to elevated CO2, increases in the ASC and phenol levels were obtained in Beta vulgaris (Kumari et al., 2013), and increases in the ASC, glutathione (GSH), and ASC/GSH, as well as in their redox status, were found in L. perenne and M. lupulina (Farfan-Vignolo and Asard, 2012). Ascorbate synthesis can be triggered and enhanced by excessive carbohydrate production due to elevated CO2 (Smirnoff and Wheeler, 2000; Zinta et al., 2014), which is closely linked to carbon metabolism (Smirnoff and Wheeler, 2000), and together improve the plant-antioxidant defense system. Moreover, with a delay in the onset of senescence and/or severe stress under elevated CO2 conditions, it is suggested that the antioxidant profiles, such as the accumulation of antioxidant compounds and antioxidant enzyme activity, may show better performance in dealing with the biological process of senescence (Hodges and Forney, 2000). For instance, a reduction in the oxidative stress under elevated CO2 was found in Zingiber officinale (Ghasemzadeh et al., 2010), Catharanthus roseus (Singh and Agrawal, 2015), a temperate grassland shrub, Caragana microphylla (Xu et al., 2014), a bean, Vigna radiate (Mishra and Agrawal, 2014), and A. thaliana plants (Zinta et al., 2014).
However, with the elevated CO2-alleviated oxidation stress evidence coming from a number of reports (e.g., Pérez-López et al., 2009; Xu et al., 2014), these results have not been confirmed in some species, such as in Spinacia oleracea leaves (Hodges and Forney, 2000). Farfan-Vignolo and Asard (2012) reported that CO2 enrichment could exacerbate lipid peroxidation in M. lupulina, but not in L. perenne plants, with no rising-CO2 responses in the ascorbate peroxidase (APX) and peroxidase (POX) in M. lupulina. No significant responses in the antioxidant enzyme activity, including APX, glutathione reductase (GR), POX, CAT, and SOD, were found when B. vulgaris plants were exposed to high levels of O3 with elevated CO2, except in the inhibition of APX (Kumari et al., 2013).
Instead, in Quercus pubescens and Q. ilex plants grown under elevated CO2, down-regulation of the protective systems was observed (Schwanz and Polle, 1998). According to the findings of Schwanz and Polle (1998), although the GR in oak leaves remains stable, the activities of SOD, CAT, POX, and APX, as well as the sum of dehydroascorbate and ASC, were reduced in CO2-elevated environments. Base on a recent report (Singh and Agrawal, 2015), the activities of the SOD, CAT, and APX declined, but the GR and POX were stimulated, finally leading to a significant reduction in the , H2O2, and malondialdehyde (MDA) contents in C. roseus plants grown under elevated CO2. Recently, marked decreases in the ROS levels (, H2O2) and reductions in some antioxidant enzymes, such as CAT and SOD, were observed simultaneously in mung bean plants exposed to elevated CO2, suggesting that a lower level of ROS might match the lower activity of antioxidant enzymes (Mishra and Agrawal, 2014).
Based on a report by AbdElgawad et al. (2015), who used C3 grasses (L. perenne, Poa pratensis) and C3 legumes (M. lupulina, Lotus corniculatus) as experimental materials, elevated CO2 can reduce the H2O2 level, lipid peroxidation, and lipoxygenase (LOX) activities, while it decreased the SOD, CAT, glutathione peroxidase (GPX), and GR levels, but did not affect the ASC-GSH cycle (AbdElgawad et al., 2015). Thus, the predominant form of the enzymatic antioxidant defense may strongly depend on the species and the abiotic stress (Duarte et al., 2013; Singh and Agrawal, 2015).
The activities and gene transcription expression levels of ROS scavenging enzymes in A. thaliana at elevated CO2 remained unchanged, particularly under well-watered conditions (Zinta et al., 2014). However, the excessive gene transcriptional response related to antioxidant metabolism due to O3 pollution was partly repressed by elevated CO2 in soybean (Glycine max) plants under field conditions, again arguing the protective role of elevated CO2 (Gillespie et al., 2012). Lipid peroxidation, indicated by MDA accumulation, would be lessened by CO2 enrichment, especially under other severe abiotic stress conditions such as drought (Salazar-Parra et al., 2012; Xu et al., 2014; AbdElgawad et al., 2015), heat wave stress (Xu et al., 2014; Zinta et al., 2014; AbdElgawad et al., 2015), O3 pollution (Yan et al., 2010; Kumari et al., 2013), and salinity (Pérez-López et al., 2009), implying that oxidative stress induced by severe environmental constraints may be mitigated, generally or at least partly, by CO2 fertilization. It is again highlighted that the positive vs. negative roles of elevated CO2 concentrations in antioxidant enzyme regulation under severe stressful abiotic environments may depend considerably on different species (Schwanz and Polle, 1998, 2001; Guo et al., 2006; Kumari et al., 2013; Xu et al., 2014; Zinta et al., 2014).
Moreover, based on a recent report using wheat plants, with increasing sugar levels via CO2 enrichment, sugar-derived reactive carbonyls (RCs; aggressive by-products of oxidative stress), including methylglyoxal (MG), were provoked by elevated CO2, which can negate the functions of multiple proteins, and impair the biological membrane, suggesting that plant diabetes may be inducible (Takagi et al., 2014), supporting an earlier study by Schwanz and Polle (1998). Thus, whether and how much elevated CO2 affects antioxidant systems in plant tissues depends on the plant species, crop variety, developmental stage, abiotic factors, and the combination of these (e.g., Hodges and Forney, 2000; Gill and Tuteja, 2010; Mishra and Agrawal, 2014; Xu et al., 2014; Zinta et al., 2014). This is a debatable issue, requiring further research.
Response of Crucial Metabolites to Elevated CO2
The metabolism changes of certain important metabolites, and the related genetic variations induced by elevated CO2 have been found in a number of research reports. For instance, an accumulation of carbon compounds under elevated CO2 occurs in wheat leaves accompanied by an up-regulation of phosphoglycerate mutase (PGAM) involving carbohydrate transport, but a down-regulation of the adenosine diphosphate glucose pyrophosphatase protein for synthesizing starch; thus affecting the carbon flux within the plants’ tissues, and the balance between the carbon sink and source (Aranjuelo et al., 2011). Changes in the major chemical components induced by elevated CO2 have also been reported in many studies. Generally, under elevated CO2, there may be a decrease in the total N and organic N compounds, which define the elevated CO2-induced dilution effectiveness. However, there is an increase in the total non-structural carbohydrates (TNC), including starch and sugar (e.g., glucose, fructose, sucrose; Lavola and Julkunen-Tiitto, 1994; Poorter et al., 1997; Luo et al., 2004; Markelz et al., 2014), with a mostly stable level in the total structural carbohydrates (cellulose plus hemicellulose), lignin, and lipids (review by Poorter et al., 1997; Markelz et al., 2014). Nitrogen assimilation may be enhanced by elevated CO2 (Ribeiro et al., 2012), and a recent report indicated that elevated CO2 may promote N assimilation and transamination-related enzyme activities, such as glutamate oxoglutarate aminotransferase (GOGAT) and glutamate oxalate transaminase (GOT), and lead to an increase in the phloem amino acid content in M. truncatula (Guo et al., 2013).
Elevated CO2 can change not only the primary metabolic processes, but also the secondary metabolic composition in plant tissues (Lavola and Julkunen-Tiitto, 1994; Poorter et al., 1997; Matros et al., 2006; Lavola et al., 2013). Here, we mainly address the secondary metabolite responses, because there are fewer studies related to the key metabolites. Plant secondary metabolites often indicate that these compounds have no primary functions in the maintenance of life processes in plants; however, they are involved in the biological processes of plants dealing with environmental stress, with regard to adaptation and defense (Lavola and Julkunen-Tiitto, 1994; Ramakrishna and Ravishankar, 2011). Changes in the secondary metabolites with rising CO2 have been reported in several relevant studies. For example, an alteration in the carbon allocation under elevated CO2 has revised the carbon-nutrient balance (CNB) hypothesis (Bryant et al., 1983), increasing the C:N ratio in plant tissues (e.g., Poorter et al., 1997; Cotrufo et al., 1998; Xu et al., 2007), while increasing the levels of the C-based secondary compounds due to easier synthesis, in plants with excess carbon relative to the other nutrients (such as N; Lavola and Julkunen-Tiitto, 1994; Matros et al., 2006). However, a contradiction may arise when elevated CO2-induced N dilution limits the carbohydrate reserves, leading instead to a reduction in some secondary substances (Lavola and Julkunen-Tiitto, 1994). However, with rising CO2, a large accumulation of some secondary metabolites, including phenylpropanoids, tannins, triterpenoids, phenolic acids, and alkaloids, was observed, despite the effectiveness of the dilution (Lavola and Julkunen-Tiitto, 1994; Matros et al., 2006; Ghasemzadeh et al., 2010; Lavola et al., 2013). For example, in tobacco leaves there was a large accumulation of phenylpropanoids, including the major carbon-rich compound chlorogenic acid (CGA), and the scopolin and scopoletin coumarins (Matros et al., 2006). In the flavonoid response, although both the kaempferol and fisetin were increased by elevated CO2 in ginger (Zingiber officinale Roscoe; Ghasemzadeh et al., 2010), whether there was a decrease or increase in birch plants grown in elevated CO2 depended on the genetic type or environmental conditions (Lavola and Julkunen-Tiitto, 1994; Lavola et al., 2013). The glucosinolate accumulation was enhanced in Brassica plants exposed to elevated CO2, possibly changing the feeding behavior of specialized herbivores (Klaiber et al., 2013). In other metabolites, including lignin, cell wall polysaccharides, and terpenes, no obvious response was found, depending on the compound composition, species, genotype, nutrient status (such as N availability), and other environmental factors (Poorter et al., 1997; Lindroth et al., 2001; Matros et al., 2006; Lavola et al., 2013; AbdElgawad et al., 2014; Singh and Agrawal, 2015). For example, the levels of the condensed tannins, most flavonols, and phenolic acids in birch plants can be stimulated by elevated CO2 and elevated UVB, but this effect disappeared at high temperatures (Lavola et al., 2013).
Isoprene is a volatile hydrocarbon molecule, generally emitted by certain vegetation types, particularly tree species, protecting plants against damage from abiotic stress, and playing an important role in tropospheric chemistry and climate change due to its highly reactive molecular properties, especially in the formation processes of ozone and secondary organic aerosols (Sharkey and Singsaas, 1995; Claeys et al., 2004; Sun et al., 2012). However, it has been confirmed that isoprene may have an active function in protecting the photosynthetic apparatus against oxidative stress from abiotic stress (such as heat), by quenching the ROS via the promotion of oxidative defense machinery (Gill and Tuteja, 2010; Morfopoulos et al., 2014). In a number of related reports, elevated CO2 has produced various effects on plant-derived isoprene emissions, including increases (Sharkey et al., 1991; Tognetti et al., 1998), remaining unchanged (Rosenstiel et al., 2003; Sun et al., 2012), and, most often, showing decreases (e.g., Wilkinson et al., 2009; Possell and Hewitt, 2011; Morfopoulos et al., 2014). The reason for the decreasing isoprene emission may be that the available reducing power captured by light may cause a large consumption, due to carbon fixation rather than isoprene synthesis, in CO2 enrichment conditions, resulting in a reduction in isoprene emissions (Morfopoulos et al., 2014). A reduction in the isoprene emission capacity may be attributable to a decrease in both the isoprene synthase activity and pool size of dimethylallyldiphosphate (DMADP), an immediate isoprene precursor (Sun et al., 2012). Actually, DMADP synthesis is involved in the primary photosynthetic product of glyceraldehyde-3-phosphate (GAP), linked to a leaf’s photosynthetic carbon metabolism (Loreto and Sharkey, 1993; Lichtenthaler, 1999; Sun et al., 2012; Trowbridge et al., 2012). Reduced ATP induced by elevated CO2 may also diminish DMADP synthesis (Sun et al., 2012). Thus, the isoprene emission capacity may be determined by the status of the balance between the primary metabolites, such as sugar, and the secondary metabolites, such as isoprene (Loreto and Sharkey, 1993; Sun et al., 2012), again highlighting the importance of the primary-secondary metabolite balance (abbreviated by PSMB) with CO2 enrichment. Based on the response model suggested by Morfopoulos et al. (2014), Figure 3 succinctly describes a pathway involved in the downregulation of isoprene biosynthesis in response to elevated CO2. Under elevated CO2, more electron flux may be used in the CBB cycle for photosynthesis, whereas less electrons may flow into the photorespiration cycle, xanthophyll cycle, and the methylerythritol 4-phosphate (MEP, 5) pathway to synthesize isoprene, as well as other redox reactions, such as quenching ROS (e.g., GR reaction demands of NADPH; Gill and Tuteja, 2010). It is worth noting that the isoprene biosynthesis and emission, in and from plants, may be tightly associated with photosynthesis, photorespiration, the xanthophyll cycle, and oxidative defense systems in response to CO2 enrichment, with abiotic environmental changes (Gill and Tuteja, 2010; Moroney et al., 2013; Morfopoulos et al., 2014; Figure 3).
FIGURE 3.

A diagrammatic representation of isoprene biosynthesis downregulation in response to elevated CO2. Light energy from the sun (1) is transferred into the plant metabolic bioprocesses using an electron transport chain (ETC). Under elevated CO2, more electron flux may be used for the CBB cycle for photosynthesis (2), while less electrons may flow into the photorespiration cycle (3), xanthophyll cycle (4), and the methylerythritol 4-phosphate pathway (MEP), (5) to synthesize isoprene (6), as well as other redox reactions, including the quenchers of ROS (7) (based mainly on Morfopoulos et al., 2014).
The role of hormone pathways in regulating the growth and metabolic responses to elevated CO2 is not well known, despite there being a few reports (e.g., Li et al., 2011; Ribeiro et al., 2012; Zavala et al., 2013). Elevated CO2 can promote an accumulation in the salicylic acid (SA, Zavala et al., 2013) and brassinosteroids (BR; Jiang et al., 2012), while reducing Jasmonates (JA) and ethylene concentrations (Zavala et al., 2013; Vaughan et al., 2014). Elevated CO2-induced genes were associated with the metabolic processes of the BR regulator in plant tissues (Li et al., 2006), which can alleviate the heat-induced inhibition of photosynthesis, by increasing the carboxylation efficiency and enhancing the antioxidant systems in Lycopersicon esculentum (Ogweno et al., 2008). In one recent report, BRs were found to enhance the stimulation of plant growth and photosynthetic potential under elevated CO2 (Jiang et al., 2012). An increase in the indole-3-acetic acid (IAA), isopentenyl-adenosine (iPA), and dihydrozeatin riboside (DHZR) was found, while a decrease in the ABA and unchangeable zeatin riboside (ZR) occurred in Pinus tabulaeformis plants exposed to elevated CO2, which can encounter O3 exposure effects to alleviate damage (Li et al., 2011). The iPA, DHZR, and ZR are recognized as the most commonly active cytokinins (CTKs) in plants. The results of the experiment by Ribeiro et al. (2012) indicated that elevated CO2 may play a role similar to gibberellin (GA) in the integration of carbohydrate and nitrogen metabolisms underlying the optimal biomass determination. When the Arabidopsis plants exhibited the inhibition of growth via the GA biosynthesis inhibitor (low-GA regime), the activities of the enzymes involved in photosynthesis, including the CBB cycle enzymes [phosphoglycerate kinase (PGK) and transketolase (TK)], were enhanced by elevated CO2, whereas the activities of the enzymes related to organic acid metabolism, such as the NAD-dependent malate dehydrogenase (MDH), were inhibited (Ribeiro et al., 2012). Moreover, nitrate reductase (NR) can be stimulated by elevated CO2 (by 31%) in plants with a low-GA content, indicating that rising CO2 may mediate inorganic N metabolism in association with GA (Ribeiro et al., 2012). This clearly indicates that elevated CO2 can substitute for the relevant metabolic bioprocesses in the low-GA species, which may have a marked potential application for plants, particularly staple crops, to cope with future climate change in a high CO2 concentration environment.
General Gene Expression Profile Under Elevated CO2
The genes expressed differently between ambient and elevated CO2 might encode great changes in their metabolic functions (Li et al., 2006), including increases in the expression of a subset of genes encoding stress-related functions, and decreases in the expression of genes encoding chloroplast functions and other processes of photosynthesis (Moore et al., 1999; Miyazaki et al., 2004; Li et al., 2006). The decline in the gene expression may partly lead to so-called photosynthetic acclimation to long-term elevated CO2, particularly under limited environmental conditions or in carbon sink limited species (Jifon and Wolfe, 2002; Ainsworth et al., 2004; Long et al., 2004; Fukayama et al., 2012; Xu et al., 2013a). For example, Fukayama et al. (2012) found the overexpression of Rubisco activase in rice leaves grown under elevated CO2, possibly leading to a decrease in the photosynthetic capacity. However, the gene expression in response to CO2 fumigation may depend on different developmental stages at the time of sampling, and different physiological conditions of the ecotypes of A. thaliana (Li et al., 2006). Moreover, limited N, a typical example of a nutrition resource deficit, may lower the stimulation of photosynthesis by elevated CO2, due to excess photoassimilate availability, triggering sugar-signaling feedback. This reduces the expression of the photosynthetic genes, especially in Rubisco, leading to the allocation of photosynthetic N into sinks that are more necessary for relative biosynthesis (Moore et al., 1999; Leakey et al., 2009b; Markelz et al., 2014). Based on a report by Duanmu et al. (2009), the enhanced expression of the limited CO2-induced gene HLA3 may increase the HCO3- transport and photosynthetic Ci affinity, which may counter the down-regulation of the photosynthetic capacity under CO2 enrichment, if the gene can be transported to higher plants (Price et al., 2011). This demonstrates the potential modified gene applications in the improvement of photosynthetic regulation traits in high CO2 climates.
In a rice cultivar, gene expression for D1 protein (a protein of PSII gene) was down-regulated by 20% at heat stress under elevated CO2, but this change did not occur in another cultivar, indicated that elevated CO2 may enhance the damage of D1 protein, depending on genotypic variation (Gesch et al., 2003). Based on a recent study in poplar plants (Liu et al., 2014), only eight significantly changed key genes involved in crucial metabolisms in response to elevated CO2 were identified by a qRT-PCR test. During wheat plant senescence, up-regulation of genes related to nitrogen remobilization, and down-regulation of genes related to carbon remobilization were observed under elevated CO2, reflecting greater grain N-sink strength of developing grains (Buchner et al., 2015). Based on a microarray analysis, the A. thaliana photosynthetic gene expression can be most adversely affected by abiotic stress, such as heat and drought, where almost all of genes were down-regulated. However, the greatest down-regulation in gene expression can be diminished by elevated CO2 (Zinta et al., 2014). From the genome-wide expression profiling of the mRNA in A. thaliana leaves (Zinta et al., 2014), 3643 differentially expressed genes appeared between plants exposed to climate extremes and ambient CO2, whereas only 2841 genes were obtained when grown under elevated CO2. Specifically, both the up-regulated and down-regulated genes were remarkably lower in plants exposed to elevated CO2, than in ambient CO2. For example, under stressful conditions such as heat and drought, the down-regulations of the genes involved in the light reactions (photosystem I and II, light-harvesting complex II), pigment synthesis, and the Calvin cycle can be dampened by elevated CO2, being consistent with changes in photosynthetic rates. It is indicated that elevated CO2 may repress the impact of climate extremes on gene expression in rosette leaves (Zinta et al., 2014). It is worth noting that we only presented a general description here, and a detailed list of the gene expression differences may be found in the report by Zinta et al. (2014).
Elevated CO2 often can induce a marked decline in photorespiration (see above), suggesting that there may be an involvement of the expression of the genes related to photorespiration pathway including both transcripts and metabolite levels (Sharkey, 1988; Novitskaya et al., 2002; Foyer et al., 2009; Florian et al., 2014; Wang et al., 2014). A BOUT DE SOUFFLE (BOU) gene encoding a mitochondrial carrier may be involved in photorespiration in Arabidopsis because of the knockout mutant bou-2 can arrest growth at ambient CO2, but not at high CO2 concentration, implying BOU gene linking glycine decarboxylase (GDC) activity, may regulate the response to CO2 concentration changes (Eisenhut et al., 2013). Plants defective (glyk1 mutants), the gene encoding glycerate kinase (GLYK), cannot grow in normal CO2 level but fully recover at elevated CO2, which the reasonable reason why the mutant requires a high CO2 concentration is unknown (Timm and Bauwe, 2013). The transcript levels of some photorespiratory genes up-regulated such as plastid chaperonin proteins (CPN60B), and those down-regulated such as GDC under heat and drought stresses, were largely repressed under elevated CO2, but that is not universal for all genes (Zinta et al., 2014). Furthermore, according to a study by Florian et al. (2014), the transcript levels of photorespiratory genes in Arabidopsis were almost unchanged at high CO2 concentration except a decline in transcript levels of glycine decarboxylase H-protein (GDCH1) that functions in photorespiratory carbon recovery. Thus, whether and how the photorespiratory gene expression play a major role in responses to atmospheric CO2 concentration changes are mostly unknown (Foyer et al., 2009; Timm and Bauwe, 2013; Florian et al., 2014), which needs to be tested further.
Because antioxidant defense systems would be enhanced by elevated CO2, the gene expression levels of antioxidant enzymes may be also promoted accordingly (Gillespie et al., 2012; Mishra and Agrawal, 2014; Zinta et al., 2014). In A. thaliana plants, CO2 enrichment can up-regulate the gene transcriptional expression of an antioxidant enzyme, dehydroascorbate reductase (DHAR), but down-regulate that of CAT, particularly under stressful environments. However, the gene expression changes in others such as APX, GR, GPX, POX, and SOD to elevated CO2 were not significant (Zinta et al., 2014). Additionally, a high transcript abundance for the majority of the genes coding antioxidant recycling enzymes enhanced by high O3 concentration was also not affected by elevated CO2 (Gillespie et al., 2012). Elevated CO2 did not modify the up-regulation of transcripts of oxidative-stress-related genes induced by herbivory or elevated O3 in soybean plants (Casteel et al., 2008). Kontunen-Soppela et al. (2010) indicating that CO2 enrichment cannot alleviate harmful effects from O3 pollution based on a gene expression test in birch plants. Thus, the authors could not conclude that CO2 enrichment can up-regulate the gene transcriptional expression levels of the antioxidant enzymes under stressful environment. Further studies are needed urgently to elucidate the molecular responses in the diverse antioxidant systems in responses to elevated CO2 with the key environmental factors including drought, heat, and ozone (Gillespie et al., 2012; Zinta et al., 2014).
One recent research study described the results of a gene bioinformatics analysis of hardy winter wheat (Triticum aestivum), with different low temperature adaptive capacities in response to elevated CO2 (Kane et al., 2013). The genes induced by elevated CO2 was three times higher in the non-acclimated (NA) relative to cold-acclimated (CA) conditions (1,022 vs. 372). The greatest down-regulation of genes appeared in the plant defense responses in the NA plants. On the other hand, CA can reverse this down-regulation, due to the cold-induced genes involved in the plant’s resistance to pathogenesis, and cellular and chloroplast protection (Kane et al., 2013), suggesting that cold-adapted hardy winter plants may be less affected by elevated CO2. Conversely, the plants that are more sensitive to cold weather may be regulated both easily and drastically via CO2 enrichment. Of note is the down-regulative interaction of high CO2 levels with low temperature adaptations, which requires further investigation.
Another recent microarray study describes the expression of the respiratory genes in A. thaliana plants exposed to elevated CO2, with both limited and sufficient N availabilities (Markelz et al., 2014). This analysis showed that 4439 transcripts were significantly different between the ambient and elevated CO2. Particularly, the transcriptional response of the genes related to protein synthesis was greatest during the day, due to elevated CO2 induction. These genes included those related to the components of glycolysis, the TCA cycle, the mitochondrial electron transport chain (ETC), and the mitochondrial protein import complexes. The evidence of the up-regulation of the transcription of the genes, with relation to respiration under elevated CO2 levels, has also been obtained from rice (Fukayama et al., 2011) and soybean plants (Leakey et al., 2009b). Furthermore, 1,708 transcripts differed significantly in abundance between the limited N and ample N availabilities, while 258 transcripts differed significantly due to the interactions of the CO2 level and N availability, again indicating that the expression of the genes related to the key physiological bioprocesses in response to elevated CO2 may be markedly affected by other environmental factors, such as N limitation (Markelz et al., 2014) and day length (Queval et al., 2012). It is worth pointing out that the systematicness and complexity of the underlying molecular mechanisms may coexist in the plant response to elevated CO2, and its interaction with other multiple abiotic factors including nutrition condition.
In addition to the relevant studies concerning the specific gene manipulation and genome-wide transcriptional analysis, with strong selective pressure due to the novel CO2 level, the evolutionary adaption to an atmospheric CO2 concentration change has been found in many reports of the stomatal developmental response (Gray et al., 2000; Ward and Kelly, 2004). Moreover, because the previous studies concerning the response to CO2 enrichment are often limited to one generation of the plant life-cycle (Ward and Kelly, 2004), to further understand the genetic variations in the plants exposed to long-term elevated CO2, Teng et al. (2009) found that the maternal genetic effects of elevated CO2 cannot be retrieved in their offspring after undergoing 15 generations of A. thaliana grown in a long-term elevated CO2 atmosphere, indicating the lack of genetic variation and specific adaptations for CO2-enriched responsiveness (Teng et al., 2009). It is suggested that selective pressure from elevated CO2 may be not enough to produce a genetic modification to adapt to new environmental changes. This issue should be investigated further, with long-term exposure to elevated CO2.
Elevated CO2 Interactions with Multiple Abiotic Stresses
There have been several review reports concerning the interactions between elevated CO2 and other abiotic factors, such as temperature or drought, on plant growth and physiological processes (e.g., Morison and Lawlor, 1999; Ainsworth and Rogers, 2007; Peñuelas et al., 2013; Ruiz-Vera et al., 2013). However, the underlying mechanism concerning the responses to CO2 enrichment with multiple factors has rarely been systematically reviewed (Xu et al., 2013a; Jagadish et al., 2014; Way et al., 2015). Although the related descriptions have been presented in the appropriate places above, here, we present a succinct statement, particularly for the underlying mechanisms in physiological responses to elevated CO2, in combination with several abiotic factors, such as drought and heat waves.
Water deficits and heat waves are considered to be the most critical stress factors with markedly potential effects on plant growth, crop yield, vegetation productivity, photosynthetic capacity, promotion of ROS accumulation (such as H2O2), and the oxidative enhancement of functional molecules, such as active proteins and DNA (e.g., Mittler, 2006; Xu and Zhou, 2006; Barnabás et al., 2008; Xu et al., 2009b, 2013a, 2014; Zinta et al., 2014). With the exception of a few reports (e.g., Coleman et al., 1991; Roden and Ball, 1996), most of the relevant studies have concluded that CO2 fertilization may mitigate the adverse impacts of environmental stresses, such as heat, drought, O3 pollution, and their combinations (Biswas et al., 2013; Xu et al., 2013a, 2014; Zinta et al., 2014). These aspects of the mitigation of CO2 enrichment include relatively increased individual growth (e.g., Xu et al., 2014) and community production (Naudts et al., 2014), enhanced photosynthesis (Biswas et al., 2013; Xu et al., 2014; Zinta et al., 2014), elevated WUE and NUE (Palmroth et al., 2013), optimized chlorophyll fluorescence (Biswas et al., 2013; Xu et al., 2014; Zinta et al., 2014), up-regulated antioxidant defense metabolism via increased lipophilic antioxidants and membrane-protecting enzymes (Naudts et al., 2014; Xu et al., 2014), and decreased photorespiration with low H2O2 production (Foyer and Noctor, 2009; Munne-Bosch et al., 2013; Zinta et al., 2014).
Elevated CO2 may help the leaf tissues of a dominant grass in Northern China to partly escape the negative effects of heat and drought stresses on plant growth, canopy structure, leaf development, photosynthetic potential, and antioxidant systems (Xu et al., 2014). For A. thaliana plants, the combination of the heat and drought-induced inhibition of photosynthesis was 62% under ambient CO2, but the reduction in photosynthesis was only 40% with elevated CO2. Furthermore, the protein carbonyl content, a marker of protein oxidation, increased significantly during a heat wave and drought, in which the effects were repressed by increased CO2 (Zinta et al., 2014). The dramatic differences between the altered transcriptional expression of A. thaliana plants subjected to a combination of heat and drought stresses were demonstrated in the presence and absence of elevated CO2, with less down-regulation of the genes involved in the light reactions (photosystem I and II, light-harvesting complex II), pigment synthesis, and the CBB under elevated CO2. Additionally, there was less limitation to the photosynthetic parameters, such as Anet, maximum photochemical efficiency (Fv/Fm), gs, and chlorophyll content (Zinta et al., 2014), possibly due to the effectiveness of the mitigation of the CO2 enrichment. Moreover, following cancelation of the extreme heat and drought stresses, the plant growth and physiological activities related to the positive responses to growth may partly resume at high CO2 concentrations, and the oxidative stress can be greatly alleviated, although they cannot reach the control levels (Xu et al., 2009a, 2010; Xu and Zhou, 2011; Zinta et al., 2014). Again, this implies that CO2 fertilization may alleviate the damage of extreme climatic events, such as snap heat waves and droughts, compromising part of the loss and accelerating recovery in case of the elimination of severe abiotic stress. However, a recent report indicated that high temperature, with no elevated CO2, provokes the drought sensitivity of the leaf to gas exchange, while the latter did not affect the Eucalyptus radiata seedling response to drought, and cannot alleviate the negative effects of rising temperature on drought stress (Duan et al., 2014). From another point of view, drought, warming, air pollution, and, particularly, their combination may substantially negate the elevated-CO2 stimulation in photosynthesis, plant growth, and productivity (Biswas et al., 2013; Ruiz-Vera et al., 2013; Xu et al., 2013a; Duan et al., 2014), which is worth noting.
A hot issue has arisen, in which the photosynthetic responses to elevated CO2 and its combination with climatic change may differ completely between plant species within their photosynthetic pathways. Because of the C4 specific photosynthetic pathway with a CO2-concentrating pump (von Caemmerer and Furbank, 2003), they cannot benefit from elevated CO2 relative to C3 plants (Leakey et al., 2006; Morgan et al., 2011; Bütof et al., 2012; Xu et al., 2014). However, there is a practical and explicable positive response of growth and photosynthesis to elevated CO2, with drought and heat stress in C4 plants. (1) Although no obvious response to CO2 enrichment occurs under ample water availability, great stimulation in the growth and photosynthetic capacity may be obtained under water deficits, due to the marked alleviations of drought stress via gs reduction and WUE elevation, and oxidative stress mitigation under elevated CO2 (e.g., Long et al., 2004; Ghasemzadeh et al., 2010). (2) The higher temperature might benefit the C4 species that originate from, and currently grow under warming conditions, and because the photorespiration of C3 plants increases with rising temperature, leading to a reduction in the Anet. The C4 plants lack photorespiration pathways, with no effect on photosynthesis (Long et al., 2006; Long and Ort, 2010; Morgan et al., 2011). Actually, both hypotheses have been well tested in several reports (Morgan et al., 2001, 2011; Leakey et al., 2006; Xu et al., 2014). This positive response to a combination of CO2 enrichment and warming, as well as water deficits, highlights the fact that C4 plants may have a great potential advantage in future climatic change. A higher CO2 concentration with warming and drought suggests that C4 plants may prosper in these vulnerable ecosystems in arid and semiarid regions in the future (Morgan et al., 2011; Lobell et al., 2013; Xu et al., 2014). However, elevated CO2 induced an electron transfer rate (ETR) enhancement in one C3 species, Halimione portulacoides, and one C4 species, Spartina maritime, but with lower photosynthetic efficiency in the C4 plants due to an increase in the dissipated energy flux, indicated by higher non-photochemical quenching (NPQ), suggesting that the abundance of C3 species may increase in Mediterranean halophyte vegetation (Duarte et al., 2014). Thus, the future climatic change may induce a rapid shift in some terrestrial vegetation, because of the different responses between the species with the specific photosynthetic pathways, such as C3 and C4 plants, depending on the combination of multiple climatic factors.
Conclusion
We briefly summarize several key points. (1) Elevated CO2 generally increases the Anet, in which the positive responses strongly depend on the plant functional groups and species, with the expected stimulation from rising CO2, for almost all of the C3 species, but only for C4 plants under water deficit conditions (due to the CCM). The performance of Rubisco in fixing carbon is promoted by CO2 enrichment, because of its dual character. However, a downregulation in the photosynthetic capacity may occur because of the decreased ATP:ADP ratio, diluted N, and excessive photosynthate accumulation under continually rising CO2, particularly under N and/or carbon sink limitations. (2) An elevated CO2-induced suppression of photorespiration has been tested using a lower Gly:Ser ratio as an indicator, while a general negative response in mitochondrial respiration varies, depending on the species. The balance between the increased respiratory substrate and diluted N may play a key role in the rising CO2-induced response, with evidence from the expression up-regulation of the genes related to the respiratory pathway. (3) Plants may run an antioxidant defense system with both the enzymatic and non-enzymatic machinery protected from the damage of oxidative stress due to the generation of ROS under abiotic stresses (such as drought and heat), while elevated CO2 may partly promote the accumulation of antioxidants like polyphenols and ascorbate, and enhance some antioxidant enzyme activities to diminish the oxidative stress from abiotic factors, alone or combination, depending on the genetic variations and plant developmental stage. (4) Elevated CO2 leads to a lower N level and higher content of the total non-structural carbohydrates (TNC), including starch and sugars, while remaining mostly stable in the totals of the structural carbohydrates, lignin, and lipids. However, some secondary metabolites, such as phenylpropanoids, tannins, and phenolic acids, are enhanced by CO2 enrichment. Isoprene emissions may be weakened by elevated CO2, because biosynthesis may need to balance the ATP and NADPH with photosynthetic metabolism. (5) Elevated CO2 might mitigate the adverse effects of abiotic stresses via relatively increased individual growth, enhanced photosynthesis, increased resource use efficiency, promoted antioxidant defense metabolism, and decreased photorespiration under multiple environmental stresses. In terms of the photosynthetic pathway, CO2 enrichment did not affect C4 plants under ample environmental conditions, but promoted it when exposed to drought, warming and their combination, predicting a great potential advantage in future climatic change scenarios for the C4 species, particularly in arid and semiarid areas.
Future Perspectives
Promotion of the Relevant Research
In the future, we may focus on several crucial research aspects: (1) to further elucidate the underlying mechanisms of the response to CO2 enrichment in key biological processes, including photosynthesis, antioxidant machinery, and other related critical metabolic bioprocesses, such as hormone-involved regulation, as well as the relevant biochemical signal cascades; (2) to disentangle and compare the diverse responses from different species and PFTs to elevated CO2 or its combination with other abiotic factors; (3) to integrate various spatial-temporal scales from molecular, cellular, biochemical, physiological, individual, ecosystem, and global vegetation levels, and from instantaneous to annual or longer time-scales to elucidate the underlying genetic mechanisms in association with key biological processes under the effects of global environmental factors, including elevated CO2, warming, drought, and air pollution; (4) to strengthen the linkages to other relevant research subjects, including ecological, biogeoscience, environmental, climatic, and social-economic aspects, to find appropriate synthetic solutions to urgent practical issues like environmental contamination, ecosystem damage, and global warming impacts.
Potential Applications under Future Climate Change
Future climate change may impact key biological metabolic processes and their feedback. For example, environmental stresses may provoke the generation of ROS in chloroplasts, the site of photosynthesis, while future high CO2 levels may alleviate the limitations of these stresses. We might also use biotechnological tools such as the protection function against ROS to deal with future climatic change. In addition, Rubisco properties may be improved by regulating the transgenic expression of Rubisco activase in crops such as rice, possibly enhancing the photosynthetic capacity under rising CO2 (Fukayama et al., 2012), while the high CO2-induced downregulation of the photosynthetic capacity might induce the modification of the photosynthetic pathway (Price et al., 2011). Furthermore, the modified genetic capacity for the high utilization of photosynthate to strengthen sink storage may make plants capable of sustaining increased photosynthesis when the plants are grown in elevated atmospheric CO2, while additional thermo-tolerant transgenic crops may be required to cope simultaneously with climatic warming (Lavania et al., 2015). Finally, research should be conducted to strengthen the feasible applications from the relevant research results in response to CO2 enrichment, and its combination with multiple environmental factors for ecological management, climate change mitigation, sustainable development, and related policy decisions, but not at the expense of environment.
Author Contributions
YJ is co-first author, ZX and YJ collected and analyzed the data, ZX, YJ, and GZ wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The study was funded by the National Natural Science Foundation of China (41330531, 31170456), and China Special Fund for Meteorological Research in the Public Interest (Major projects) (GYHY201506001-3).
References
- Abd Elgawad H., Asard H. (2013). Elevated CO2 attenuates oxidative stress caused by drought and elevated temperature in four C3 plant species. Biotechnologia 94 156–202. [Google Scholar]
- AbdElgawad H., Farfan-ignolo E. R., de Vos D., Asard H. (2015). Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci. 231 1–10. 10.1016/j.plantsci.2014.11.001 [DOI] [PubMed] [Google Scholar]
- AbdElgawad H., Peshev D., Zinta G., Van den Ende W., Janssens I. A., Asard H. (2014). Climate extreme effects on the chemical composition of temperate grassland species under ambient and elevated CO2: a comparison of fructan and non-fructan accumulators. PLoS ONE 9:e92044 10.1371/journal.pone.0092044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth E. A., Long S. P. (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165 351–372. 10.1111/j.1469-8137.2004.01224.x [DOI] [PubMed] [Google Scholar]
- Ainsworth E. A., Rogers A. (2007). The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ. 30 258–270. 10.1111/j.1365-3040.2007.01641.x [DOI] [PubMed] [Google Scholar]
- Ainsworth E. A., Rogers A., Nelson R., Long S. P. (2004). Testing the ‘source-sink’ hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agr. Forest Meteorol. 122 85–94. 10.1016/j.agrformet.2003.09.002 [DOI] [Google Scholar]
- Amthor J. S. (2000). Direct effect of elevated CO2 on nocturnal in situ leaf respiration in nine temperate deciduous tree species is small. Tree Physiol. 20 139–144. 10.1093/treephys/20.2.139 [DOI] [PubMed] [Google Scholar]
- Aranjuelo I., Cabrera-Bosquet L., Morcuende R., Avice J. C., Nogués S., Araus J. L., et al. (2011). Does ear C sink strength contribute to overcoming photosynthetic acclimation of wheat plants exposed to elevated CO2? J. Exp. Bot. 62 3957–3969. 10.1093/jxb/err095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin O. (2015). New Phytologist and the ‘fate’ of carbon in terrestrial ecosystems. New Phytol. 205 1–3. 10.1111/nph.13185 [DOI] [PubMed] [Google Scholar]
- Atkin O., Millar H. A., Turnbull M. H. (2010). Plant respiration in a changing world. New Phytol. 187 268–272. 10.1111/j.1469-8137.2010.03343.x [DOI] [PubMed] [Google Scholar]
- Ayub G., Zaragoza-Castells J., Griffin K. L., Atkin O. K. (2014). Leaf respiration in darkness and in the light under pre-industrial, current and elevated atmospheric CO2 concentrations. Plant Sci. 226 120–130. 10.1016/j.plantsci.2014.05.001 [DOI] [PubMed] [Google Scholar]
- Barnabás B., Jäger K., Fehér A. (2008). The effect of drought and heat stress on reproductive processes. Plant Cell Environ. 31 11–38. 10.1111/j.1365-3040.2007.01727.x [DOI] [PubMed] [Google Scholar]
- Biswas D. K., Xu H., Li Y. G., Ma B. L., Jiang G. M. (2013). Modification of photosynthesis and growth responses to elevated CO2 by ozone in two cultivars of winter wheat with different years of release. J. Exp. Bot. 64 1485–1496. 10.1093/jxb/ert005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G. (1991). Growth at elevated CO2: photosynthetic responses mediated through Rubisco. Plant Cell Environ. 14 795–806. 10.1111/j.1365-3040.1991.tb01443.x [DOI] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. (1971). Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem. Biophys. Res. Commun. 45 716–722. 10.1016/0006-291X(71)90475-X [DOI] [PubMed] [Google Scholar]
- Bryant J. P., Chapin F. S., III, Reichardt P., Clausen T. (1983). Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40 357–368. 10.2307/3544308 [DOI] [Google Scholar]
- Bryant J., Taylor G., Frehner M. (1998). Photosynthetic acclimation to elevated CO2 is modified by source: sink balance in three component species of chalk grassland swards grown in a free air carbon dioxide enrichment (FACE) experiment. Plant Cell Environ. 21 159–168. 10.1046/j.1365-3040.1998.00265.x [DOI] [Google Scholar]
- Buchner P., Tausz M., Ford R., Leo A., Fitzgerald G. J., Hawkesford M. J., et al. (2015). Expression patterns of C- and N-metabolism related genes in wheat are changed during senescence under elevated CO2 in dry-land agriculture. Plant Sci. 236 239–249. 10.1016/j.plantsci.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Bunce J. A. (2014). Limitations to soybean photosynthesis at elevated carbon dioxide in free-air enrichment and open top chamber systems. Plant Sci. 226 131–135. 10.1016/j.plantsci.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Bütof A., von Riedmatten L. R., Dormann C. F., Scherer-Lorenzen M., Welk E., Bruelheide H. (2012). The responses of grassland plants to experimentally simulated climate change depend on land use and region. Globe Change Biol. 18 127–137. 10.1111/j.1365-2486.2011.02539.x [DOI] [Google Scholar]
- Carmo-Silva A. E., Powers S. J., Keys A. J., Arrabaca M. C., Parry M. A. J. (2008). Photorespiration in C4 grasses remains slow under drought conditions. Plant Cell Environ. 31 925–940. 10.1111/j.1365-3040.2008.01805.x [DOI] [PubMed] [Google Scholar]
- Casteel C. L., O’Neill B. F., Zavala J. A., Bilgin D. D., Berenbaum M. R., Delucia E. H. (2008). Transcriptional profiling reveals elevated CO2 and elevated O3 alter resistance of soybean (Glycine max) to Japanese beetles (Popillia japonica). Plant Cell Environ. 31 419–434. 10.1111/j.1365-3040.2008.01782.x [DOI] [PubMed] [Google Scholar]
- Claeys M., Graham B., Vas G., Wang W., Vermeylen R., Pashynska V., et al. (2004). Formation of secondary organic aerosols through photooxidation of isoprene. Science 303 1173–1176. 10.1126/science.1092805 [DOI] [PubMed] [Google Scholar]
- Coleman J. S., Rochefort L., Bazzaz F. A., Woodward F. I. (1991). Atmospheric CO2, plant nitrogen status and the susceptibility of plants to an acute increase in temperature. Plant Cell Environ. 14 667–674. 10.1111/j.1365-3040.1991.tb01539.x [DOI] [Google Scholar]
- Cotrufo M. F., Ineson P., Scott A. (1998). Elevated CO2 reduces the nitrogen concentration of plant tissues. Global Change Biol. 4 43–54. 10.1046/j.1365-2486.1998.00101.x [DOI] [Google Scholar]
- Curtis P. S., Wang X. (1998). A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113 299–313. 10.1007/s004420050381 [DOI] [PubMed] [Google Scholar]
- Delucia E. H., Sasek T. W., Strain B. R. (1985). Photosynthetic inhibition after long-term exposure to elevated levels of atmospheric carbon dioxide. Photosyn. Res. 7 175–184. 10.1007/BF00037008 [DOI] [PubMed] [Google Scholar]
- Dhindsa R. S., Plumb-Dhindsa P. A., Thorpe T. A. (1981). Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 32 93–101. 10.1093/jxb/32.1.93 [DOI] [Google Scholar]
- Drake B. G., Berry J., Bunce J., Dijkstra P., Farrar J., Gifford R. M. (1999). Does elevated atmospheric CO2 concentration inhibit mitochondrial respiration in green plants? Plant Cell Environ. 22 649–657. 10.1046/j.1365-3040.1999.00438.x [DOI] [Google Scholar]
- Drake B. G., Gonzalez-Meler M. A., Long S. P. (1997). More efficient plants: a consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 609–639. 10.1146/annurev.arplant.48.1.609 [DOI] [PubMed] [Google Scholar]
- Duan H. L., Duursma R. A., Huang G. M., Smith R. A., Choat B., O’Grady A. P., et al. (2014). Elevated [CO2] does not ameliorate the negative effects of elevated temperature on drought-induced mortality in Eucalyptus radiata seedlings. Plant Cell Environ. 37 1598–1613. 10.1111/pce.12260 [DOI] [PubMed] [Google Scholar]
- Duanmu D., Miller A. R., Horken K. M., Weeks D. P., Spalding M. H. (2009). Knockdown of limiting-CO2–induced gene HLA3 decreases HCO3- transport and photosynthetic Ci affinity in Chlamydomonas reinhardtii. Proc. Nati. Acad. Sci. U.S.A. 106 5990–5995. 10.1073/pnas.0812885106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte B., Santos D., Marques J. C., Caçador I. (2013). Ecophysiological adaptations of two halophytes to salt stress: photosynthesis, PS II photochemistry and anti-oxidant feedback – Implications for resilience in climate change. Plant Physiol. Biochem. 67 178–188. 10.1016/j.plaphy.2013.03.004 [DOI] [PubMed] [Google Scholar]
- Duarte B., Santos D., Silva H., Marques J. C., Caçador I. (2014). Photochemical and biophysical feedbacks of C3 and C4 Mediterranean halophytes to atmospheric CO2 enrichment confirmed by their stable isotope signatures. Plant Physiol. Biochem. 80 10–22. 10.1016/j.plaphy.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Eichelmann H., Talts E., Oja V., Padu E., Laisk A. (2009). Rubisco in planta kcat is regulated in balance with photosynthetic electron transport. J. Exp. Bot. 60 4077–4088. 10.1093/jxb/erp242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M., Planchais S., Cabassa C., Guivarćh A., Justin A.-M., Taconnat L., et al. (2013). Arabidopsis a bout de souffle is a putative mitochondrial transporter involved in photorespiratory metabolism and is required for meristem growth at ambient CO2 levels. Plant J. 73 836–849. 10.1111/tpj.12082 [DOI] [PubMed] [Google Scholar]
- Farfan-Vignolo E. R., Asard H. (2012). Effect of elevated CO2 and temperature on the oxidative stress response to drought in Lolium perenne L. and Medicago sativa L. Plant Physiol. Biochem. 59 55–62. 10.1016/j.plaphy.2012.06.014 [DOI] [PubMed] [Google Scholar]
- Florian A., Timm S., Nikoloski Z., Tohge T., Bauwe H., Araújo W. L., et al. (2014). Analysis of metabolic alterations in Arabidopsis following changes in the carbon dioxide and oxygen partial pressures. J. Integr. Plant Biol. 56 941–959. 10.1111/jipb.12237 [DOI] [PubMed] [Google Scholar]
- Foyer C. H., Bloom A. J., Queval G., Noctor G. (2009). Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu. Rev. Plant Biol. 60 455–484. 10.1146/annurev.arplant.043008.091948 [DOI] [PubMed] [Google Scholar]
- Foyer C. H., Noctor G. (2009). Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid. Redox Signal. 11 861–905. 10.1089/ars.2008.2177 [DOI] [PubMed] [Google Scholar]
- Fukayama H., Sugino M., Fukuda T., Masumoto C., Taniguchi Y., Okada M, et al. (2011). Gene expression profiling of rice grown in free air CO2 enrichment (FACE) and elevated soil temperature. Field Crops Res. 121 195–199. 10.1016/j.fcr.2010.11.018 [DOI] [Google Scholar]
- Fukayama H., Ueguchi C., Nishikawa K., Katoh N., Ishikawa C., Masumoto C., et al. (2012). Overexpression of Rubisco activase decreases the photosynthetic CO2 assimilation rate by reducing Rubisco content in rice leaves. Plant Cell Physiol. 53 976–986. 10.1093/pcp/pcs042 [DOI] [PubMed] [Google Scholar]
- Gesch R. W., Kang I.-H., Gallo-Meagher M., Vu J. C. V., Boote K. J., Allen L. H., et al. (2003). Rubisco expression in rice leaves is related to genotypic variation of photosynthesis under elevated growth CO2 and temperature. Plant Cell Environ. 26 1941–1950. 10.1046/j.1365-3040.2003.01110.x [DOI] [Google Scholar]
- Ghasemzadeh A., Jaafar H. Z. E., Rahmat A. (2010). Elevated carbon dioxide increases contents of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiber officinale roscoe.) varieties. Molecules 15 7907–7922. 10.3390/molecules15117907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. S., Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48 909–930. 10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Gillespie K. M., Xu F., Richter K. T., McGrath J. M., Markelz R. C., Ort D. R., et al. (2012). Greater antioxidant and respiratory metabolism in field – grown soybean exposed to elevated O3 under both ambient and elevated CO2. Plant Cell Environ. 35 169–184. 10.1111/j.1365-3040.2011.02427.x [DOI] [PubMed] [Google Scholar]
- Gonzalez-Meler M. A., Taneva L., Trueman R. J. (2004). Plant respiration and elevated atmospheric CO2 concentration: cellular responses and global significance. Ann. Bot. 94 647–656. 10.1093/aob/mch189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. E., Holroyd G. H., van der Lee F. M., Bahrami A. R., Sijmons P. C., Woodward F. I., et al. (2000). The HIC signalling pathway links CO2 perception to stomatal development. Nature 408 713–716. 10.1038/35047071 [DOI] [PubMed] [Google Scholar]
- Guo H., Sun Y., Li Y., Tong B., Herris M., Zhu-Salzman K., et al. (2013). Pea aphid promotes amino acid metabolism both in Medicago truncatula and bacteriocytes to favor aphid population growth under elevated CO2. Global Change Biol. 19 3210–3223. 10.1111/gcb.12260 [DOI] [PubMed] [Google Scholar]
- Guo Z., Ou W., Lu S., Zhong Q. (2006). Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol. Biochem. 44 828–836. 10.1016/j.plaphy.2006.10.024 [DOI] [PubMed] [Google Scholar]
- Heath R. L., Packer L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125 189–198. 10.1016/0003-9861(68)90654-1 [DOI] [PubMed] [Google Scholar]
- Hodges D. M., Forney C. F. (2000). The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. J. Exp. Bot. 51 645–655. 10.1093/jexbot/51.344.645 [DOI] [PubMed] [Google Scholar]
- Husic D. W., Husic H. D., Tolbert N. E., Black Jr. C. C. (1987). The oxidative photosynthetic carbon cycle or C2 cycle. Crit. Rev. Plant Sci. 5 45–100. 10.1080/07352688709382234 [DOI] [Google Scholar]
- IPCC. (2013). “Summary for Policymakers,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds Stocker T. F., Qin D., Plattner G. K., Tignor M., Allen S. K., Boschung J.et al. (New York, NY: Cambridge University Press; ). [Google Scholar]
- Jagadish K. S. V., Kadam N. N., Xiao G., Melgar R. J., Bahuguna R. N., Quinones C., et al. (2014). Agronomic and physiological responses to high temperature, drought, and elevated CO2 interactions in cereals. Adv. Agron. 127 111–156. 10.1016/B978-0-12-800131-8.00003-0 [DOI] [Google Scholar]
- Jiang Y. P., Cheng F., Zhou Y. H., Xia X. J., Shi K., Yu J. Q. (2012). Interactive effects of CO2 enrichment and brassinosteroid on CO2 assimilation and photosynthetic electron transport in Cucumis sativus. Environ. Exp. Bot. 75 98–106. 10.1016/j.envexpbot.2011.09.002 [DOI] [Google Scholar]
- Jifon J., Wolfe D. (2002). Photosynthetic acclimation to elevated CO2 in Phaseolus vulgaris L. is altered by growth response to nitrogen supply. Global Change Biol. 8 101–1027. 10.1046/j.1365-2486.2002.00531.x [DOI] [Google Scholar]
- Kane K., Dahal K. P., Badawi M. A., Houde M., Hüner N. P., Sarhan F. (2013). Long-term growth under elevated CO2 suppresses biotic stress genes in non-acclimated, but not cold-acclimated winter wheat. Plant Cell Physiol. 54 1751–1768. 10.1093/pcp/pct116 [DOI] [PubMed] [Google Scholar]
- Kebeish R., Niessen M., Thiruveedhi K., Bari R., Hirsch H. J., Rosenkranz R., et al. (2007). Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat. Biotechnol. 25 593–599. 10.1038/nbt1299 [DOI] [PubMed] [Google Scholar]
- Kirschbaum M. U. (2011). Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiol. 155 117–124. 10.1104/pp.110.166819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiber J., Dorn S., Najar-Rodriguez A. J. (2013). Acclimation to elevated CO2 increases constitutive glucosinolate levels of Brassica plants and affects the performance of specialized herbivores from contrasting feeding guilds. J. Chem. Ecol. 39 653–665. 10.1007/s10886-013-0282-3 [DOI] [PubMed] [Google Scholar]
- Kontunen-Soppela S., Parviainen J., Ruhanen H., Brosché M., Keinänen M., Thakur R. C. (2010). Gene expression responses of paper birch (Betula papyrifera) to elevated CO2 and O3 during leaf maturation and senescence. Environ. Poll. 158 959–968. 10.1016/j.envpol.2009.10.008 [DOI] [PubMed] [Google Scholar]
- Kozaki A., Takeba G. (1996). Photorespiration protects C3 plants from photooxidation. Nature 384 557–560. 10.1038/384557a0 [DOI] [Google Scholar]
- Kumari S., Agrawal M., Tiwari S. (2013). Impact of elevated CO2 and elevated O3 on Beta vulgaris L.: pigments, metabolites, antioxidants, growth and yield. Environ. Poll. 174 279–288. 10.1016/j.envpol.2012.11.021 [DOI] [PubMed] [Google Scholar]
- Lavania D., Dhingra A., Siddiqui M. H., Al-Whaibi M. H., Grover A. (2015). Current status of the production of high temperature tolerant transgenic crops for cultivation in warmer climates. Plant Physiol. Biochem. 86 100–108. 10.1016/j.plaphy.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Lavola A., Julkunen-Tiitto R. (1994). The effect of elevated carbon dioxide and fertilization on primary and secondary metabolites in birch, Betula pendula (Roth). Oecologia 99 315–321. 10.1007/BF00627744 [DOI] [PubMed] [Google Scholar]
- Lavola A., Nybakken L., Rousi M., Pusenius J., Petrelius M., Kellomaki S., et al. (2013). Combination treatment of elevated UVB radiation, CO2 and temperature has little effect on silver birch (Betula pendula) growth and phytochemistry. Physiol. Plant. 149 499–514. 10.1111/ppl.12051 [DOI] [PubMed] [Google Scholar]
- Leakey A. D. B., Ainsworth E. A., Bernacchi C. J., Rogers A., Long S. P., Ort D. R. (2009a). Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J. Exp. Bot. 60 2859–2876. 10.1093/jxb/erp096 [DOI] [PubMed] [Google Scholar]
- Leakey A. D. B., Xu F., Gillespie K. M., McGrath J. M., Ainsworth E. A., Ort D. R. (2009b). Genomic basis for stimulated respiration by plants growing under elevated carbon dioxide. Proc. Natl. Acad. Sci. U.S.A. 106 3597–3602. 10.1073/pnas.0810955106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey A. D. B., Uribelarrea M., Ainsworth E. A., Naidu S. L., Rogers A., Ort D. R., et al. (2006). Photosynthesis, productivity and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 140 779–790. 10.1104/pp.105.073957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. D., Wang X. Z., Griffin K. L., Tissue D. T. (2002). Effects of age and ontogeny on photosynthetic responses of a determinate annual plant to elevated CO2 concentration. Plant Cell Environ. 25 359–368. 10.1046/j.0016-8025.2001.00815.x [DOI] [Google Scholar]
- Li P., Sioson A., Mane S. P., Ulanov A., Grothaus G., Heath L. S., et al. (2006). Response diversity of Arabidopsis thaliana ecotypes in elevated [CO2] in the field. Plant Mol. Biol. 62 593–609. 10.1007/s11103-006-9041-y [DOI] [PubMed] [Google Scholar]
- Li X. M., Zhang L. H., Ma L. J., Li Y. Y. (2011). Elevated carbon dioxide and/or ozone concentrations induce hormonal changes in Pinus tabulaeformis. J. Chem. Ecol. 37 779–784. 10.1007/s10886-011-9975-7 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H. K. (1999). The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50 47–65. 10.1146/annurev.arplant.50.1.47 [DOI] [PubMed] [Google Scholar]
- Lindroth R. L., Roth S., Nordheim E. V. (2001). Genotypic variation in response of quaking aspen (Populus tremuloides) to atmospheric CO2 enrichment. Oecologia 126 371–379. 10.1007/s004420000521 [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang J., He C., Duan A. (2014). Genes responsive to elevated CO2 concentrations in triploid white poplar and integrated gene network analysis. PLoS ONE 9:e98300 10.1371/journal.pone.0098300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell D. B., Hammer G. L., McLean G., Messina C., Roberts M. J., Schlenker W. (2013). The critical role of extreme heat for maize production in the United States. Nat. Clim. Change 3 497–501. 10.1038/nclimate1832 [DOI] [Google Scholar]
- Lobell D. B., Schlenker W., Costa-Roberts J. (2011). Climate trends and global crop production since 1980. Science 333 616–620. 10.1126/science.1204531 [DOI] [PubMed] [Google Scholar]
- Long S. P. (1991). Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant Cell Environ. 14 729–739. 10.1111/j.1365-3040.1991.tb01439.x [DOI] [Google Scholar]
- Long S. P., Ainsworth E. A., Rogers A., Ort D. R. (2004). Rising atmospheric carbon dioxide: plants FACE the future. Annu. Rev. Plant Biol. 55 591–628. 10.1146/annurev.arplant.55.031903.141610 [DOI] [PubMed] [Google Scholar]
- Long S. P., Ort D. R. (2010). More than taking the heat: crops and global change. Curr. Opin. Plant Biol. 13 241–248. 10.1016/j.pbi.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Long S. P., Zhu X.-G., Naidu S., Ort D. R. (2006). Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 29 315–330. 10.1111/j.1365-3040.2005.01493.x [DOI] [PubMed] [Google Scholar]
- Loreto F., Centritto M., Barta C., Calfapietra C., Fares S., Monson R. K. (2007). The relationship between isoprene emission rate and dark respiration rate in white poplar (Populus alba L.) leaves. Plant Cell Environ. 30 662–669. 10.1111/j.1365-3040.2007.01648.x [DOI] [PubMed] [Google Scholar]
- Loreto F., Sharkey T. D. (1993). On the relationship between isoprene emission and photosynthetic metabolites under different environmental conditions. Planta 189 420–424. 10.1007/BF00194440 [DOI] [PubMed] [Google Scholar]
- Luo Y., Su B., Currie W. S., Dukes J. S., Finzi A., Hartwig U.et al. (2004). Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54 731–739. 10.1641/0006-3568(2004)054 [DOI] [Google Scholar]
- Markelz R. C., Lai L. X., Vosseler L. N., Leakey A. D. (2014). Transcriptional reprogramming and stimulation of leaf respiration by elevated CO2 concentration is diminished, but not eliminated, under limiting nitrogen supply. Plant Cell Environ. 37 886–898. 10.1111/pce.12205 [DOI] [PubMed] [Google Scholar]
- Markelz R. C., Strellner R. S., Leakey A. D. (2011). Impairment of C4 photosynthesis by drought is exacerbated by limiting nitrogen and ameliorated by elevated [CO2] in maize. J. Exp. Bot. 62 3235–3246. 10.1093/jxb/err056 [DOI] [PubMed] [Google Scholar]
- Matros A., Amme S., Kettig B., Buck-Sorlin G. H., Sonnewald U., Mock H. P. (2006). Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increased resistance against infection with potato virus Y. Plant Cell Environ. 29 126–137. 10.1111/j.1365-3040.2005.01406.x [DOI] [PubMed] [Google Scholar]
- Melillo J. M., McGuire A. D., Kicklighter D. W., Moore B., III, Vorosmarty J., Schloss A. L. (1993). Global climate change and terrestrial net primary production. Nature 363 234–240. 10.1038/363234a0 [DOI] [Google Scholar]
- Meng F., Zhang J., Yao F., Hao C. (2014). Interactive effects of elevated CO2 concentration and irrigation on photosynthetic parameters and yield of maize in northeast China. PLoS ONE 9:e98318 10.1371/journal.pone.0098318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A. K., Agrawal S. B. (2014). Cultivar specific response of CO2 fertilization on two tropical mung bean (Vigna radiata L.) cultivars: ROS generation, antioxidant status, physiology, growth, yield and seed quality. J. Agron. Crop Sci. 20 273–289. 10.1111/jac.12057 [DOI] [Google Scholar]
- Mittler R. (2006). Abiotic stress, the field environment and stress combination. Trends Plant Sci. 11 15–19. 10.1016/j.tplants.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Fredricksen M., Hollis K. C., Poroyko V., Shepley D., Galbraith D. W., et al. (2004). Transcript expression profiles of Arabidopsis thaliana grown under controlled conditions and open-air elevated concentrations of CO2 and of O3. Field Crops Res. 90 47–59. 10.1016/j.fcr.2004.07.010 [DOI] [Google Scholar]
- Monteith J. L. (1977). Climate and the efficiency of crop production in Britain. Philos. Trans. R. Soc. B Biol. 281 277–294. 10.1098/rstb.1977.0140 [DOI] [Google Scholar]
- Moore B. D., Cheng S.-H., Sims D., Seemann J. R. (1999). The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ. 22 567–582. 10.1046/j.1365-3040.1999.00432.x [DOI] [Google Scholar]
- Morfopoulos C., Sperlich D., Peñuelas J., Filella I., Llusià J., Medlyn B. E., et al. (2014). A model of plant isoprene emission based on available reducing power captures responses to atmospheric CO2. New Phytol. 203 125–139. 10.1111/nph.12770 [DOI] [PubMed] [Google Scholar]
- Morgan J. A., Lecain D. R., Mosier A. R., Milchunas D. G. (2001). Elevated CO2 enhances water relations and productivity and affects gas exchange in C3 and C4 grasses of the Colorado shortgrass steppe. Global Change Biol. 7 451–466. 10.1046/j.1365-2486.2001.00415.x [DOI] [Google Scholar]
- Morgan J. A., LeCain D. R., Pendall E., Blumenthal D. M., Kimball B. A., Carrillo Y.et al. (2011). C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature 476 202–205. 10.1038/nature10274 [DOI] [PubMed] [Google Scholar]
- Morison J. I. L., Lawlor D. W. (1999). Interactions between increasing CO2 concentration and temperature on plant growth. Plant Cell Environ. 22 659–682. 10.1046/j.1365-3040.1999.00443.x [DOI] [Google Scholar]
- Moroney J. V., Jungnick N., DiMario R. J., Longstreth D. J. (2013). Photorespiration and carbon concentrating mechanisms, two adaptations to high O2, low CO2 conditions. Photosyn. Res. 117 121–131. 10.1007/s11120-013-9865-7 [DOI] [PubMed] [Google Scholar]
- Munne-Bosch S., Queval G., Foyer C. H. (2013). The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol. 161 5–19. 10.1104/pp.112.205690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASA. (2014). Global Climate Change: Vital Signs of the Planet. Available at: http://climate.nasa.gov/400ppmquotes [accessed December 4 2014]. [Google Scholar]
- Naudts K., Van Den Berge J., Farfan E., Rose P., AbdElgawad H., Ceulemans R., et al. (2014). Future climate alleviates stress impact on grassland productivity through altered antioxidant capacity. Environ. Exp. Bot. 99 150–158. 10.1016/j.envexpbot.2013.11.003 [DOI] [Google Scholar]
- Novitskaya L., Trevanion S. J., Driscoll S., Foyer C. H., Noctor G. (2002). How does photorespiration modulate leaf amino acid contents—A dual approach through modelling and metabolite analysis. Plant Cell Environ. 25 821–835. 10.1046/j.1365-3040.2002.00866.x [DOI] [Google Scholar]
- Nowak R. S., Ellsworth D. S., Smith S. D. (2004). Functional responses of plants to elevated atmospheric CO2 - do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol. 162 253–280. 10.1111/j.1469-8137.2004.01033.x [DOI] [Google Scholar]
- Ogweno J. O., Song X. S., Shi K., Hu W. H., Mao W. H., Zhou Y. H., et al. (2008). Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J. Plant Growth Regul. 27 49–57. 10.1007/s00344-007-9030-7 [DOI] [Google Scholar]
- Palmroth S., Katul G. G., Maier C. A., Ward E., Manzoni S., Vico G. (2013). On the complementary relationship between marginal nitrogen and water-use efficiencies among Pinus taeda leaves grown under ambient and CO2-enriched environments. Ann. Bot. 111 467–477. 10.1093/aob/mcs268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñuelas J., Sardans J., Estiarte M., Ogaya R., Carnicer J., Coll M., et al. (2013). Evidence of current impact of climate change on life: a walk from genes to the biosphere. Global Change Biol. 19 2303–2338. 10.1111/gcb.12143 [DOI] [PubMed] [Google Scholar]
- Peñuelas J., Sardans J., Rivas-Ubach A., Janssess I. (2012). The human-induced imbalances between C, N and P in Earth’s life system. Global Change Biol. 18 3–6. 10.1111/j.1365-2486.2011.02568.x [DOI] [Google Scholar]
- Pérez-López U., Robredo A., Lacuesta M., Sgherri C., Muñoz-Rueda A., Navari-Izzo F., et al. (2009). The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol. Plant. 135 29–42. 10.1111/j.1399-3054.2008.01174.x [DOI] [PubMed] [Google Scholar]
- Poorter H., Berkel V., Baxter R., den Hertog J., Dijkstra P., Gifford R. M., et al. (1997). The effect of elevated carbon dioxide on the chemical composition and construction costs of leaves of 27 C3 species. Plant Cell Environ. 20 472–482. 10.1046/j.1365-3040.1997.d01-84.x [DOI] [Google Scholar]
- Possell M., Hewitt C. N. (2011). Isoprene emissions from plants are mediated by atmospheric CO2 concentrations. Global Change Biol. 17 1595–1610. 10.1111/j.1365-2486.2010.02306.x [DOI] [Google Scholar]
- Prentice I. C., Farquhar G. D., Fasham M., Goulden M. L., Heimann M., Jaramillo V. J., et al. (2001). “The carbon cycle and atmospheric carbon dioxide,” in Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change, eds Houghton J., Ding Y., Griggs D. (New York, NY: Cambridge University Press; ) 185–237. [Google Scholar]
- Price G. D., Badger M. R., von Caemmerer S. (2011). The prospect of using cyanobacterial bicarbonate transporters to improve leaf photosynthesis in C3 crop plants. Plant Physiol. 155 20–26. 10.1104/pp.110.164681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G., Neukermans J., Vanderauwera S., Van Breusegem F., Noctor G. (2012). Day length is a key regulator of transcriptomic responses to both CO2 and H2O2 in Arabidopsis. Plant Cell Environ. 35 374–387. 10.1111/j.1365-3040.2011.02368.x [DOI] [PubMed] [Google Scholar]
- Ramakrishna A., Ravishankar G. A. (2011). Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 6 1720–1731. 10.4161/psb.6.11.17613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro D. M., Araujo W. L., Fernie A. R., Schippers J. H. M., Mueller-Roeber B. (2012). Action of Gibberellins on growth and metabolism of Arabidopsis plants associated with high concentration of carbon dioxide. Plant Physiol. 160 1781–1794. 10.1104/pp.112.204842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden J. S., Ball M. C. (1996). Growth and photosynthesis of two eucalypt species during high temperature stress under ambient and elevated CO2. Global Change Biol. 2 115–128. 10.1111/j.1365-2486.1996.tb00056.x [DOI] [Google Scholar]
- Rosenstiel T. N., Potosnak M. J., Griffin K. L., Fall R., Monson R. K. (2003). Increased CO2 uncouples growth from isoprene emission in an agroforestry ecosystem. Nature 421 256–259. 10.1038/nature01312 [DOI] [PubMed] [Google Scholar]
- Ruiz-Vera U. M., Siebers M., Gray S. B., Drag D. W., Rosenthal D. M., Kimball B. A., et al. (2013). Global warming can negate the expected CO2 stimulation in photosynthesis and productivity for soybean grown in the Midwestern United States. Plant Physiol. 162 410–423. 10.1104/pp.112.211938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryle G. J. A., Powell C. E., Tewson V. (1992). Effect of elevated CO2 on the photosynthesis, respiration and growth of perennial ryegrass. J. Exp. Bot. 43 811–818. 10.1093/jxb/43.6.811 [DOI] [Google Scholar]
- Sage R. F. (2004). The evolution of C4photosynthesis. New Phytol. 161 341–370. 10.1111/j.1469-8137.2004.00974.x [DOI] [PubMed] [Google Scholar]
- Sage R. F., Pearcy W. R., Seemann J. R. (1987). The nitrogen use efficiency of C3 and C4 plants. III Leaf nitrogen effects on the activity of carboxylating enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol. 85 355–359. 10.1104/pp.85.2.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Parra C., Aguirreolea J., Sánchez-Díaz M., JoséIrigoyen J., Morales F. (2012). Climate change (elevated CO2, elevated temperature and moderate drought) triggers the antioxidant enzymes response of grapevine cv. Tempranillo, avoiding oxidative damage. Physiol. Plant. 144 99–110. 10.1111/j.1399-3054.2011.01524.x [DOI] [PubMed] [Google Scholar]
- Sanz-Sáez, Á., Erice G., Aranjuelo I., Aroca R., Ruíz-Lozano J. M., Aguirreolea J., et al. (2013). Photosynthetic and molecular markers of CO2-mediated photosynthetic downregulation in nodulated alfalfa. J. Integr. Plant Biol. 55 721–734. 10.1111/jipb.12047 [DOI] [PubMed] [Google Scholar]
- Schwanz P., Polle A. (1998). Antioxidative systems, pigment and protein contents in leaves of adult Mediterranean oak species (Quercus pubescens and Q. ilex) with lifetime exposure to elevated CO2. New Phytol. 140 411–423. 10.1111/j.1469-8137.1998.00290.x [DOI] [PubMed] [Google Scholar]
- Schwanz P., Polle A. (2001). Differential stress responses of antioxidative systems to drought in pendunculate oak (Quercus robur) and maritime pine (Pinus pinaster) grown under high CO2 concentrations. J. Exp. Bot. 52 133–143. 10.1093/jexbot/52.354.133 [DOI] [PubMed] [Google Scholar]
- Sekmen A. H., Ozgur R., Uzilday B., Turkan I. (2014). Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot. 99 141–149. 10.1016/j.envexpbot.2013.11.010 [DOI] [Google Scholar]
- Sharkey T. D. (1988). Estimating the rate of photorespiration in leaves. Physiol. Plant. 73 147–152. 10.1111/j.1399-3054.1988.tb09205.x [DOI] [Google Scholar]
- Sharkey T. D., Loreto F., Delwiche C. F. (1991). High carbon dioxide and sun/shade effects on isoprene emission from oak and aspen tree leaves. Plant Cell Environ. 14 333–338. 10.1111/j.1365-3040.1991.tb01509.x [DOI] [Google Scholar]
- Sharkey T. D., Singsaas E. L. (1995). Why plants emit isoprene. Nature 374 769 10.1038/374769a0 [DOI] [Google Scholar]
- Singh A., Agrawal M. (2015). Effects of ambient and elevated CO2 on growth, chlorophyll fluorescence, photosynthetic pigments, antioxidants, and secondary metabolites of Catharanthus roseus (L.) G Don. grown under three different soil N levels. Environ. Sci. Poll. Res. Int. 22 3936–3946. 10.1007/s11356-014-3661-6 [DOI] [PubMed] [Google Scholar]
- Smirnoff N., Wheeler G. L. (2000). Ascorbic acid in plants: biosynthesis and function. Crit. Rev. Plant Sci. 19 267–290. 10.1016/S0735-2689(00)80005-2 [DOI] [PubMed] [Google Scholar]
- Stitt M., Krapp A. (1999). The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ. 22 583–621. 10.1046/j.1365-3040.1999.00386.x [DOI] [Google Scholar]
- Sun Z., Niinemets Ü., Hüve K., Noe S. M., Rasulov B., Copolovici L., et al. (2012). Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Global Change Biol. 18 3423–3440. 10.1111/j.1365-2486.2012.02789.x [DOI] [Google Scholar]
- Takagi D., Inoue H., Odawara M., Shimakawa G., Miyake C. (2014). The Calvin cycle inevitably produces sugar-derived reactive carbonyl methylglyoxal during photosynthesis: a potential cause of plant diabetes. Plant Cell Physiol. 55 333–340. 10.1093/pcp/pcu007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N., Jin B., Wang Q., Hao H., Ceulemans R., Kuang T., et al. (2009). No detectable maternal effects of elevated CO2 on Arabidopsis thaliana over 15 generations. PLoS ONE 4:e6035 10.1371/journal.pone.0006035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N., Wang J., Chen T., Wu X., Wang Y., Lin J. (2006). Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 172 92–103. 10.1111/j.1469-8137.2006.01818.x [DOI] [PubMed] [Google Scholar]
- Thomas R. B., Reid C. D., Ybema R., Strain B. R. (1993). Growth and maintenance components of leaf respiration of cotton grown in elevated carbon dioxide partial pressure. Plant Cell Environ. 16 539–546. 10.1111/j.1365-3040.1993.tb00901.x [DOI] [Google Scholar]
- Timm S., Bauwe H. (2013). The variety of photorespiratory phenotypes—employing the current status for future research directions on photorespiration. Plant Biol. 15 737–747. 10.1111/j.1438-8677.2012.00691.x [DOI] [PubMed] [Google Scholar]
- Tingey D. T., McKane R. B., Olszyk D. M., Johnson M. G., Rygiewicz P. T., Lee E. H. (2003). Elevated CO2 and temperature alter nitrogen allocation in Douglas-fir. Global Change Biol. 9 1038–1050. 10.1046/j.1365-2486.2003.00646.x [DOI] [Google Scholar]
- Tognetti R., Johnson J. D., Michelozzi M., Raschi A. (1998). Response of foliar metabolism in mature trees of Quercus pubescens and Quercus ilex to long-term elevated CO2. Environ. Exp. Bot. 39 233–245. 10.1016/S0098-8472(98)00013-6 [DOI] [Google Scholar]
- Trowbridge A. M., Asensio D., Eller A. S., Way D. A., Wilkinson M. J., Schnitzler J. P., et al. (2012). Contribution of various carbon sources toward isoprene biosynthesis in poplar leaves mediated by altered atmospheric CO2 concentrations. PLoS ONE 7:e32387 10.1371/journal.pone.0032387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban O., Hrstka M., Zitová M., Holišová P., Šprtová M., Klem K., et al. (2012). Effect of season, needle age and elevated CO2 concentration on photosynthesis and Rubisco acclimation in Picea abies. Plant Physiol. Biochem. 58 135–141. 10.1016/j.plaphy.2012.06.023 [DOI] [PubMed] [Google Scholar]
- Vaughan M. M., Huffaker A., Schmelz E. A., Dafoe N. J., Christensen S., Sims J., et al. (2014). Effects of elevated [CO2] on maize defence against mycotoxigenic Fusarium verticillioides. Plant Cell Environ. 37 2691–2706. 10.1111/pce.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S., Evans J. R. (1991). Determination of the average partial pressure of CO2 in chloroplasts from leaves of several C3 plants. Aust. J. Plant Physiol. 18 287–305. 10.1071/PP9910287 [DOI] [Google Scholar]
- von Caemmerer S., Furbank R. T. (2003). The C4 pathway: an efficient CO2 pump. Photosyn. Res. 77 191–207. 10.1023/A:1025830019591 [DOI] [PubMed] [Google Scholar]
- Wang J., Cheung M., Rasooli L., Amirsadeghi S., Vanlerberghe G. C. (2014). Plant respiration in a high CO2 world: how will alternative oxidase respond to future atmospheric and climatic conditions? Can. J. Plant Sci. 94 1091–1101. 10.4141/cjps2013-176 [DOI] [Google Scholar]
- Wang X., Taub D. R., Jablonski L. M. (2015). Reproductive allocation in plants as affected by elevated carbon dioxide and other environmental changes: a synthesis using meta-analysis and graphical vector analysis. Oecologia 177 1075–1087. 10.1007/s00442-014-3191-4 [DOI] [PubMed] [Google Scholar]
- Ward J. K., Kelly J. K. (2004). Scaling up evolutionary responses to elevated CO2: lessons from Arabidopsis. Ecol. Lett. 7 427–440. 10.1111/j.1461-0248.2004.00589.x [DOI] [Google Scholar]
- Watanabe C. K., Sato S., Yanagisawa S., Uesono Y., Terashima I., Noguchi K. (2014). Effects of elevated CO2 on levels of primary metabolites and transcripts of genes encoding respiratory enzymes and their diurnal patterns in Arabidopsis thaliana: possible relationships with respiratory rates. Plant Cell Physiol. 55 341–357. 10.1093/pcp/pct185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way D. A., Oren R., Kroner Y. (2015). The space-time continuum: the effects of elevated CO2 and temperature on trees and the importance of scaling. Plant Cell Environ. 38 991–1007. 10.1111/pce.12527 [DOI] [PubMed] [Google Scholar]
- Wilkinson M. J., Monson R. K., Trahan N., Lee S., Brown E., Jackson R. B, et al. (2009). Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Global Change Biol. 15 1189–1200. 10.1111/j.1365-2486.2008.01803.x [DOI] [Google Scholar]
- Wingler A., Quick W. P., Bungard R. A., Bailey K. J., Lea P. J., Leegood R. C. (1999). The role of photorespiration during drought stress: an analysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant Cell Environ. 22 361–373. 10.1046/j.1365-3040.1999.00410.x [DOI] [Google Scholar]
- Xu Z. Z., Shimizu H., Ito S., Yagasaki Y., Zou C. J., Zhou G. S., et al. (2014). Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North China grassland. Planta 239 421–435. 10.1007/s00425-013-1987-9 [DOI] [PubMed] [Google Scholar]
- Xu Z. Z., Shimizu H., Yagasaki Y., Ito S., Zheng Y. R., Zhou G. S. (2013a). Interactive effects of elevated CO2, drought, and warming on plants. J. Plant Growth Regul. 32 692–707. 10.1007/s00344-013-9337-5 [DOI] [Google Scholar]
- Xu Z. Z., Yu Z. W., Zhao J. Y. (2013b). Theory and application for the promotion of wheat production in china: past, present and future. J. Sci. Food Agrc. 93 2339–2350. 10.1002/jsfa.6098 [DOI] [PubMed] [Google Scholar]
- Xu Z. Z., Zhou G. S. (2006). Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 224 1080–1090. 10.1007/s00425-006-0281-5 [DOI] [PubMed] [Google Scholar]
- Xu Z. Z., Zhou G. S. (2011). Responses of photosynthetic capacity to soil moisture gradient in perennial rhizome grass and perennial bunchgrass. BMC Plant Biol. 11:21 10.1186/1471-2229-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. Z., Zhou G. S., Shimizu H. (2009a). Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J. Exp. Bot. 60 3737–3749. 10.1093/jxb/erp216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. Z., Zhou G. S., Shimizu H. (2009b). Effects of soil drought with nocturnal warming on leaf stomatal traits and mesophyll cell ultrastructure of a perennial grass. Crop Sci. 49 1843–1851. 10.2135/cropsci2008.12.0725 [DOI] [Google Scholar]
- Xu Z. Z., Zhou G. S., Shimizu H. (2010). Plant responses to drought and rewatering. Plant Signal. Behav. 5 649–654. 10.4161/psb.5.6.11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. Z., Zhou G. S., Wang Y. H. (2007). Combined effects of elevated CO2 and soil drought on carbon and nitrogen allocation of the desert shrub Caragana intermedia. Plant Soil 301 87–97. 10.1007/s11104-007-9424-0 [DOI] [Google Scholar]
- Yan K., Chen W., Zhang G., Xu S., Liu Z., He X., et al. (2010). Elevated CO2 ameliorated oxidative stress induced by elevated O3 in Quercus mongolica. Acta Physiol. Plant. 32 375–385. 10.1007/s11738-009-0415-z [DOI] [Google Scholar]
- Yin X. W. (2002). Responses of leaf nitrogen concentration and specific leaf area to atmospheric CO2 enrichment: a retrospective synthesis across 62 species. Global Change Biol. 8 631–642. 10.1046/j.1365-2486.2002.00497.x [DOI] [Google Scholar]
- Zavala J. A., Nabity P. D., Delucia E. H. (2013). An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu. Rev. Entomol. 58 79–97. 10.1146/annurev-ento-120811-153544 [DOI] [PubMed] [Google Scholar]
- Zinta G., AbdElgawad H., Domagalska M. A., Vergauwen L., Knapen D., Nijs I., et al. (2014). Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Global Change Biol. 20 3670–3685. 10.1111/gcb.12626 [DOI] [PubMed] [Google Scholar]


