Abstract
Neurological deficits after brain surgery are not uncommon, and correct and prompt differential diagnosis is essential to initiate appropriate treatment. We describe a patient suffering from loss of consciousness due to hyperammonemia, following valproic acid treatment after surgery for an unruptured cerebral aneurysm. A 57-year-old female patient underwent successful aneurysmal neck clipping to correct an unruptured aneurysm. Her postoperative course was good, and she received anti-epileptic therapy (valproic acid) and a soft diet. Within a few days the patient experienced mental deterioration. Her serum valproic acid reached toxic levels (149.40 mg/L), and serum ammonia was fifteen times the upper normal limit (553 mmol/L; normal range, 9-33 mmol/L). After discontinuation of valproic acid and with conservative treatment, the patient recovered without any complications. Valproate-induced hyperammonemic encephalopathy is an unusual but serious neurosurgical complication, and should not be disregarded as a possible cause of neurological deficits after neurovascular surgery. Early diagnosis is crucial, as discontinuation of valproic acid therapy can prevent serious complications, including death.
Keywords: Hyperammonemia, Valproic acid, Encephalopathy
INTRODUCTION
From a neurovascular perspective, the brain is one of the most delicate and complex surgical fields. The postoperative course can vary, and transient or permanent mental deterioration or neurological deficits can occur. These may be due to many factors, such as postoperative vasospasm, delayed hemorrhage, acute infarction, brain swelling, seizure, hypoglycemia, or electrolyte imbalance, and accurate differential diagnosis at this stage is essential.
We present an unusual case of postoperative mental deterioration due to hyperammonemia without signs of hepatic failure. Our case provides evidence that life-threatening hyperammonemic encephalopathy can occur within days of initiation of valproic acid therapy after neurovascular surgery.
CASE REPORT
A 57-year-old female patient was referred to the neurosurgery department by a hospital neurologist, after radiologic imaging for chronic headache revealed a paraclinoid aneurysm. The patient's chronic headache had been managed with conservative treatment (including medication) for several months, and her medical history was otherwise unremarkable.
The patient had no other complaints, her vital signs were stable, and her complete blood count (CBC), routine biochemistry tests, urinalysis, liver function, thyroid function, lipid profile, and creatine kinase levels were normal, as were her erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels. The patient was hepatitis B antibody-positive with a history of hepatitis B vaccination. A neurological examination failed to reveal any deficits; however a 6×5 mm aneurysm was observed on computed tomographic (CT) angiography. There was a postero-medial outpouching at the ophthalmic level with a daughter sac (Fig. 1). Surgical treatment was decided.
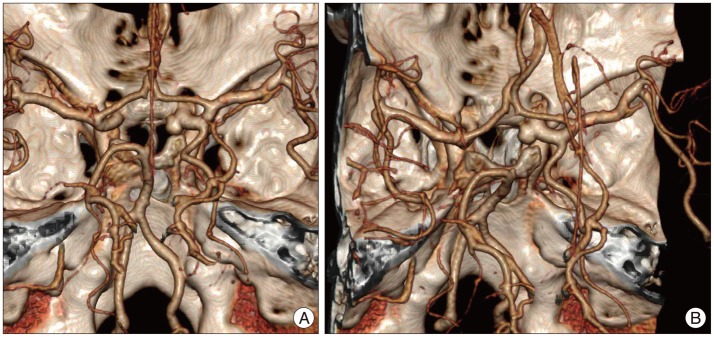
Fig. 1. Preoperative three dimensional computer tomographic angiography (3-D CTA) demonstrating postero-medially directed aneurysm with broad neck and multi-lobulated sac. Aneurysm sac is located in the carotid cave portion of the left proximal internal cerebral artery. A : View from the top. B : View from the right top.
A left-side orbito-zygomatic approach was used to expose the lesion. After careful dissection, the neck of the aneurysm was exposed, and two fenestrated clips were applied. The surgery was completed successfully.
The patient was transferred to a recovery unit, where she recovered fully from anesthesia. She remained alert, and no neurological deficits were observed. Subsequently, she was transferred to a general ward, where she received standard postoperative care. Routine laboratory tests showed that her CBC, serum electrolytes, blood sugar, serum osmolality, liver enzymes, blood urea nitrogen, and creatinine were normal. On postoperative day 1, the patient was administered analgesic and anticonvulsant medication, including 300 mg valproic acid three times a day with a soft diet.
On postoperative day 4, the patient's consciousness suddenly deteriorated. Within hours, her mental state deteriorated to deep confusion. She was transferred to the intensive care unit, where emergency diffusion MRI and CT angiography of the brain were performed. Although the imaging showed evidence of minimal scattered acute infarctions, it failed to identify a definite abnormality that could explain her mental state. A simple chest X-ray and arterial blood gas analysis (ABGA) failed to reveal any respiratory or metabolic problems such as hypoxia, hypercapnia or acidosis. Except for mildly elevated ESR and CRP, the patient's laboratory results contained no abnormal findings. A neurologic examination also failed to identify any abnormalities such as abnormal pupil reflex or hemiparesis, except for the decreased mental status. Moreover, the patient did not perform any seizure-like movements or tremors.
Even though the radiologic findings, such as mild narrowing of the internal cerebral artery (Fig. 2) and a minimal acute infarct in the cortex and subcortical white matter of the left frontal and temporal lobes, could not conclusively explain the patient's mental deterioration, she was thought to be suffering from transient cerebral ischemia due to postoperative vasospasm. 3-H therapy (hypervolemia, hemodilution, hypertension) was initiated. Oral diet and medications were withdrawn, and an intravenous vasodilator was started with sufficient hydration to maintain a strictly positive balance. The patient began to regain consciousness one day after starting treatment, and after two more days she was almost fully conscious, with only some confusion remaining.
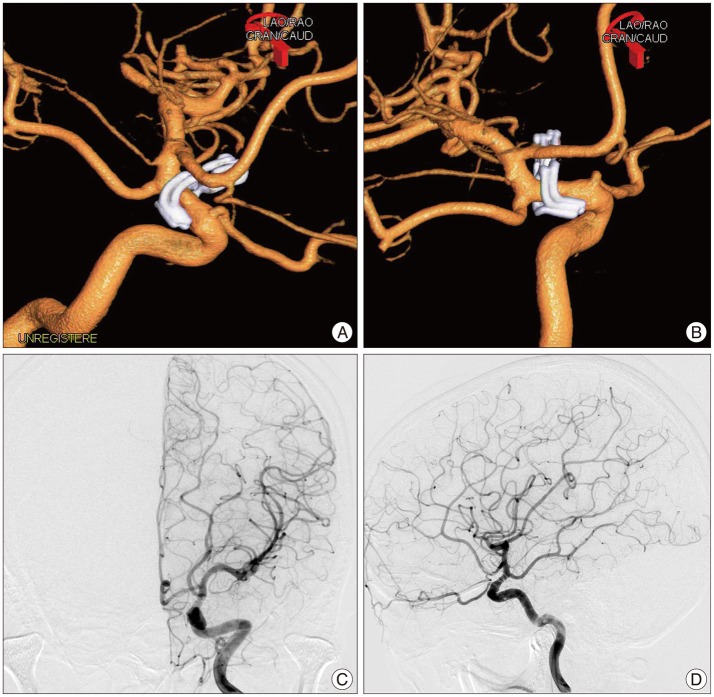
Fig. 2. Postoperative left three dimensional digital subtraction angiography (3-D DSA) (A and B). Demonstrating complete obliteration of carotid cave aneurysm sac with preservation of ophthalmic artery using two fenestrated clips. A : Anterior-oblique view. B : Medial view. Postoperative conventional DSA (C and D) demonstrating mild narrowing of the internal cerebral artery at the clipping portion. However, no definite vasospasm is observed at the whole left cerebral vascular trees. C : Anterior-posterior view. D : Lateral view.
The patient was transferred to a general ward. A soft diet and oral medication were restarted. Two days later, her mental status deteriorated again. Diffusion MRI and CT angiography were repeated but showed no significant abnormal findings. As before, laboratory tests and physical examination failed to reveal any cause of the deterioration. However, on this occasion we measured the blood levels of valproic acid, which turned out to be toxic (149.40 mg/L; therapeutic range, 50-100 mg/L). We then checked blood ammonia level, which were also markedly elevated (553 mmol/L; normal range, 9-33 mmol/L). At the same time, liver enzymes and other markers of hepatic function, such as g-glutamyl transferase, aspartate transaminase, alanine transaminase, and alkaline phosphatase, as well as ABGA, were all within the normal ranges.
Valproic acid was discontinued, and the patient received oxygen, thiamine and a lactulose enema. She regained full consciousness within 24 hours, and the ammonia levels returned to normal within 48 hours. The patient recovered completely without any complications. Our diagnosis was hyperammonemic encephalopathy induced by valproic acid, following neurovascular surgery for an unruptured aneurysm.
DISCUSSION
Valproic acid is a broad-spectrum anti-epileptic drug (AED) with several neurological indications2). In particular, it is used as a first-line drug in brain surgery or brain trauma patients in our department, to prevent postoperative or post-traumatic seizures. Its initial loading dose is 20-30 mg/kg administered at 6-10 mg/kg/min2), followed by a maintenance dose of 900-1200 mg/day. Valproate has a number of recognized side effects, including hepatotoxicity, renal toxicity, thrombocytopenia, blood dyscrasia, coagulopathy and teratogenicity3,5). Therefore, it is important to monitor blood chemistry and the drug's blood concentration.
Hyperammonemia induced by valproic acid has been reported before2,5). Although several explanations have been proposed, the key mechanism seems to be valproate-induced inhibition of hepatic carbamoylphosphate synthetase I, the first enzyme of the urea cycle 7,8). Mildly elevated blood ammonia levels have been observed in 16-52% of patients receiving valproic acid 5,9), with a reported incidence of 20% for asymptomatic and 5% for symptomatic cases5). The experience with our patient shows that even a short treatment (in this case three days) with a standard dose can increase serum valproate to toxic levels.
The relationship between valproate-induced hyperammonemia and hyperammonemia-induced encephalopathy is not fully understood, and there have been conflicting reports2,5,8). DeWolfe et al.2) found that the hyperammonemia was transient and not associated with changes in consciousness, while others have suggested that hyperammonemia can cause encephalopathy1,2). Elevated serum ammonia can inhibit glutamate uptake by astrocytes and exacerbate extracellular glutamate accumulation, leading to excitotoxic neuronal injury1,6). Another hypothesis suggests that elevated serum ammonia is conjugated in the brain with alpha-ketoglutarate to produce glutamate. This could lead to alpha-ketoglutarate depletion and a subsequent block in the Krebs cycle, which could precipitate cell damage and neuronal death1,4). Elevated serum ammonia has also been reported to increase the risk of seizure due to excessive activation of N-methyl-D-aspartic acid-type glutamate receptors8).
Known risk factors for VHE include congenital enzymatic defects of the urea cycle, underlying liver disease, long-term valproic acid treatment, concomitant anti-epileptic therapy, and strict vegetarianism1,5). To these, Tsia and Chen8) added three major risk factors : initiation of valproate treatment, high initial dose, and catabolic state.
At therapeutic concentrations, 90-95% of serum valproic acid is bound to serum protein. Consequently, a decrease in protein binding capacity caused by conditions such as metabolic acidosis, low albumin level, and older age could lead to unusually high serum valproic acid concentrations8). Postoperatively, patients may present with metabolic acidosis and low albumin levels, which, following Tsia and Chen8), which may have exposed our patient to two risk factors at the same time : initiation of treatment and catabolic state. Because there is a high probability that AED therapy with valproic acid will be initiated in most neurosurgical and brain trauma patients, we may suppose that such patients may have a higher risk of valproate-induced hyperammonemic encephalopathy (VHE).
Primary treatment for VHE is immediate discontinuation of valproic acid. Hydration, sodium phenylacetate, lactulose, and L-carnitine may also be of benefit8). When ammonia levels are in excess of 680 µg/dL (400 µmol/L) hemodialysis could be an additional therapeutic option1,8). In the case of our patient, the hemodilutional effect of the 3-H therapy and discontinuation of oral valproic acid after the first episode of mental deterioration were sufficient to allow her to regain full consciousness.
CONCLUSION
The occurrence of neurological deficits following brain surgery should prompt the physician to make an accurate differential diagnosis. Serum ammonia levels should be part of the basic work-up in all patients presenting with mental deterioration after brain surgery or trauma, even when their liver function is normal and valproic acid has been administered at normal doses and for a short period of time. Early diagnosis of VHE and prompt treatment can prevent potentially life-threatening complications.
References
- 1.Chou HF, Yang RC, Chen CY, Jong YJ. Valproate-induced hyperammonemic encephalopathy. Pediatr Neonatol. 2008;49:201–204. doi: 10.1016/S1875-9572(09)60010-3. [DOI] [PubMed] [Google Scholar]
- 2.DeWolfe JL, Knowlton RC, Beasley MT, Cofield S, Faught E, Limdi NA. Hyperammonemia following intravenous valproate loading. Epilepsy Res. 2009;85:65–71. doi: 10.1016/j.eplepsyres.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Eubanks AL, Aguirre B, Bourgeois JA. Severe acute hyperammonemia after brief exposure to valproate. Psychosomatics. 2008;49:82–83. doi: 10.1176/appi.psy.49.1.82. [DOI] [PubMed] [Google Scholar]
- 4.Felipo V, Butterworth RF. Neurobiology of ammonia. Prog Neurobiol. 2002;67:259–279. doi: 10.1016/s0301-0082(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 5.Garg R, Sunder RA. Valproate-induced hyperammonemia and seizures : perioperative concerns. Paediatr Anaesth. 2011;21:1084–1085. doi: 10.1111/j.1460-9592.2011.03633.x. [DOI] [PubMed] [Google Scholar]
- 6.Hamer HM, Knake S, Schomburg U, Rosenow F. Valproate-induced hyperammonemic encephalopathy in the presence of topiramate. Neurology. 2000;54:230–232. doi: 10.1212/wnl.54.1.230. [DOI] [PubMed] [Google Scholar]
- 7.Segura-Bruna N, Rodriguez-Campello A, Puente V, Roquer J. Valproate-induced hyperammonemic encephalopathy. Acta Neurol Scand. 2006;114:1–7. doi: 10.1111/j.1600-0404.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsai MF, Chen CY. Valproate-induced hyperammonemic encephalopathy treated by hemodialysis. Ren Fail. 2008;30:822–824. doi: 10.1080/08860220802272613. [DOI] [PubMed] [Google Scholar]
- 9.Verrotti A, Trotta D, Morgese G, Chiarelli F. Valproate-induced hyperammonemic encephalopathy. Metab Brain Dis. 2002;17:367–373. doi: 10.1023/a:1021918104127. [DOI] [PubMed] [Google Scholar]