Abstract
Background and purpose
The current definition of atypical femoral fractures (AFFs) associated with bisphosphonate use includes only de novo fractures. However, in recent years reports of bisphosphonate-associated periprosthetic fractures involving stemmed arthroplasty implants have emerged. In a case series of peri-implant fractures in femurs with plate/screw constructs, we aimed to assess similarities with classical AFFs and how their location may have implications for the pathogenesis and management of AFFs.
Patients and methods
We retrospectively identified 10 patients with 11 peri-implant fractures.
Results
The patients were ambulant women, mean age 80 (70–92) years. Mean duration of bisphosphonate use was 5 (1–10) years. The peri-implant fractures were sustained an average of 4 years (6 months to 9 years) from the time of index surgery. They were all associated with low-energy mechanisms. 8 fractures occurred near the tip of a plate, while 3 traversed the penultimate screwhole of a plate. The peri-implant fractures showed clinical and radiological features of atypicality such as lateral cortical thickening, simple fracture pattern, and lack of comminution. The patients underwent revision surgery, with bone grafting used in all but 1 case. Radiological union was evident after 2–4 months.
Interpretation
Atypical peri-implant fractures of the femur associated with bisphosphonate use may be a new entity. Stress lesions and atypical fractures may tend to develop over stress risers along the operated femur. This finding has implications for the pathogenesis and clinical management of AFFs.
Bisphosphonates form the cornerstone of antiresorptive therapy in the management of post-menopausal osteoporosis. They are used in the treatment of malignant and osteoclast-mediated metabolic bone disease. Their use in patients who have undergone total joint arthroplasty of the lower limb is associated with higher periprosthetic bone mineral density and longer implant survival (Bhandari et al. 2005). Bisphosphonates exert their therapeutic effect by reducing bone turnover and increasing overall mineralization. This translates to increased bone mineral density and bone strength, corresponding clinically to reduced risk of vertebral and non-vertebral fragility fractures (Black et al. 1996).
In recent years, several published reports have described atypical femoral fractures (AFFs) of the proximal femoral diaphysis and subtrochanteric region, in association with bisphosphonate use (Goh et al. 2007, Neviaser et al. 2008, Isaacs et al. 2010). Bisphosphonates are associated with a higher age-adjusted relative risk of AFF in women than in men, which is higher in alendronate users than in risedronate users (Schilcher et al. 2015). Bisphosphonates may cause changes in bone matrix composition and bone mechanical properties, increasing the propensity for accumulation of microdamage. Impaired target remodeling would contribute to the progression of macrocracks. High interfragmentary strain from physiological loads at a thin fracture line may be a mechanical factor in lack of bone healing (Aspenberg et al. 2010).
Periprosthetic/peri-implant fractures are currently excluded from the definition of AFFs. We suggest that peri-implant fractures of the femur with features of atypicality may be linked to bisphosphonate use and that they should be recognized as a clinical entity.
Patients and methods
We present 10 patients from 3 hospitals (Changi General Hospital, Singapore General Hospital, and Tan Tock Seng Hospital, all Republic of Singapore), who presented with 11 atypical peri-implant femoral fractures with a background of bisphosphonate use. These patients were detected when clinical and radiological observations of their femoral fractures revealed similarities with atypical fractures of the native femur. Their case records and radiographs were traced for review.
Results
Our series consisted of 10 patients with 11 peri-implant fractures sustained between 2006 and 2013. The patients were all ambulant Chinese women with a mean age of 80 (70–92) years (Table 1).
Table 1.
Summary of patient details and mechanism of injury in peri-implant fracture
| Bisphosphonate therapy |
|||||
|---|---|---|---|---|---|
| Case | Age | Comorbidities | type | duration (years) | Mechanism of injury |
| 1 | 88 | Hypertension, diabetes mellitus, colon cancer | Alendronate Risedronate |
4 1 |
Tripped and fell |
| 2 | 77 | Hypertension | Risedronate | 2 | Tripped and fell |
| 3 | 74 | Ischemic heart disease, gout | Risedronate | 4 | Atraumatic right hip pain for several days |
| 4 | 80 | Hypertension, diabetes mellitus, chronic renal impairment | Alendronate | 1 | Heard a crack in the thigh while standing on weighing machine |
| 5 | 74 | Hypertension, gastritis, spinal stenosis | Risedronate | 4 | Heard a crack in the thigh while rising from a chair |
| 6 | 70 | Hypertension, diabetes mellitus, multinodular goiter, rheumatoid arthritis | Alendronate | 10 | Tripped and fell while walking |
| 7 | 90 | Hypertension, dementia | Alendronate | 3 | Both episodes: fell while rising from chair |
| 8 | 74 | Previous cervical cancer | Risedronate | 8 | Used knee to close a drawer |
| 9 | 82 | Hypertension | Risedronate Alendronate |
4 1 |
Persistent left thigh pain after fall; initial radio-graphs normal |
| 10 | 92 | Spinal stenosis st. p. surgery | Alendronate | 10 | Fall from standing height |
Bisphosphonate and steroid use
8 patients were on bisphosphonate therapy at the time of fracture. Patient 2 had last used bisphosphonates 4 years previously, while patient 4 had stopped using bisphosphonate 5 years previously. 4 patients had used only risedronate, while 4 others had only used alendronate. 2 patients had used both alendronate and risedronate at different times. The duration of bisphosphonate use averaged 5 (1–10) years (Table 2).
Table 2.
Bone mineral density (BMD) status of patients
| Case | Year of DEXA scan | Femoral neck T-score | Lumbar spine T-score | Diagnosis |
|---|---|---|---|---|
| 1 | 2006 | −2.6 | −1.6 | Osteoporosis |
| 2 | 2011 | −4 | −2.8 | Osteoporosis |
| 3 | Not available | - | - | - |
| 4 | Not available | - | - | - |
| 5 | 2013 | −2.5 | −2.4 | Osteoporosis |
| 6 | 2011 | −2.2 | −3 | Osteoporosis |
| 7 | 2008 | −1.3 | −1.4 | Osteopenia |
| 8 | Not available | - | - | - |
| 9 | 2013 | −4 | −3.3 | Osteoporosis |
| 10 | Not available | - | - | - |
Patient 6 has a history of rheumatoid arthritis and had been on oral prednisolone for at least 5 years, but she had stopped the year before she sustained her fracture.
Fracture patterns
All 11 fractures were sustained spontaneously or after low-energy trauma such as a fall from standing height. We were unable to obtain a reliable history of prodromal pain due to the retrospective nature of the study. The injuries occurred at a mean of 4 years (6 months to 9 years) after previous surgery to the same femur. At least 4 of the fractures sustained prior to the index surgery (cases 5, 6, 7a, and 7b) showed atypical features such as lateral cortical thickening, simple fracture pattern, and minimal comminution. In cases 6 and 7a, these fractures occurred at the tips of stemmed knee arthroplasty implants (Table 3 and Figure 1).
Table 3.
Fracture details of study population
| Case | Previous surgery, indication | Years previously | Fracture configuration | Treatment |
|---|---|---|---|---|
| 1 | DHS for IT fracture | 9 | Transverse fracture just distal to tip of DHS | DHS removal, IM nailing augmented with bone graft substitute |
| 2 | DHS for IT fracture | 6 | Transverse fracture just distal to tip of DHS | DHS removal, IM nailing augmented with bone graft substitute |
| 3 | Femur plating and bone grafting for femoral shaft fracture | 6 | Transverse fracture through second-most proximal screw hole | Removal of implant, replating, and iliac crest bone grafting |
| 4 | Femur plating for femoral shaft fracture | 6 | Transverse fracture through distal end of plate | Removal of implant, replating, and iliac crest bone grafting |
| 5 | Femur plating for femoral shaft fracture | 0.8 | Transverse fracture through proximal end of plate | Removal of implant, long DHS insertion, and bone grafting with callus material |
| 6 | Femur plating for periprosthetic midshaft fracture | 0.6 | Transverse fracture across most proximal screw hole | Removal of plate and revision plating with long proximal femur locking plate |
| 7a | Femur plating and cerclage wiring for periprosthetic midshaft fracture | 1 | Transverse fracture through second-most proximal screw hole of plate | Removal of plate and revision plating with autologous bone graft from Gerdy’s tubercle |
| 7b | Revision fixation for periprosthetic fracture in 7(a) | 2 | Transverse fracture through second-most proximal screw hole of plate | Removal of implant, open reduction, internal fixation with plate and IM nail and bone graft substitute |
| 8 | Femur plating for femoral shaft fracture | 0.5 | Transverse fracture across distal end of plate | Removal of implant, retrograde femur IM nailing with iliac crest bone grafting |
| 9 | DHS with trochanteric-stabilizing plate for IT fracture | 5 | Transverse fracture just distal to tip of DHS | Removal of implant, long DHS insertion, and iliac crest bone grafting |
| 10 | Distal femur plating for peri-prosthetic supracondylar fracture | 6 | Transverse fracture across most proximal screw hole of plate | Removal of implant and revision plating with bone graft substitute |
DHS: dynamic hip screw; IT fracture: intertrochanteric fracture; IM: intramedullary.
Figure 1.
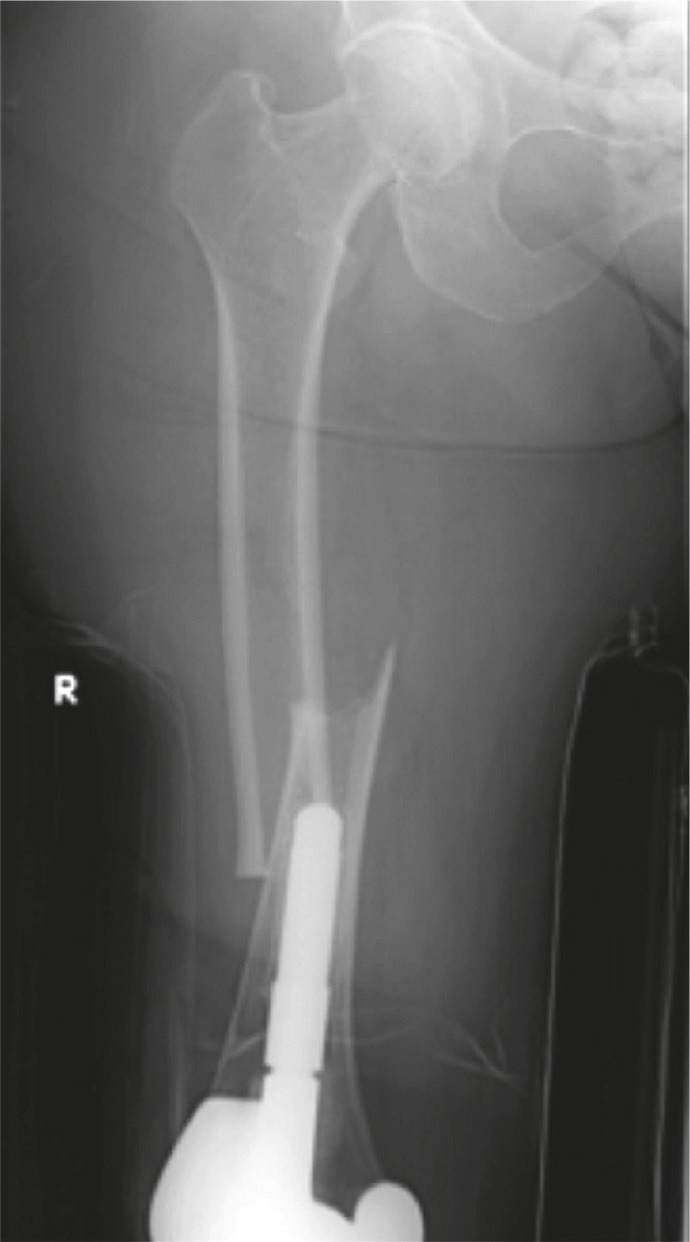
Patient 6 (left panel). Medial spiking and lateral cortical thickening at the transverse fracture site.
In 8 of the injuries, the fracture line was at or just beyond the tip of the plate (Figure 2). In 3 of the injuries, the fracture was sustained through the second-most proximal screw hole of a plate. In 2 of these cases, the penultimate screw hole was the last functioning screw hole at the end of the plate (Figure 3). The fractures showed some radiographic features of atypicality such as (1) thickening of the lateral femoral cortex, (2) simple transverse or short oblique fracture pattern, and (3) absence of comminution.
Figure 2.
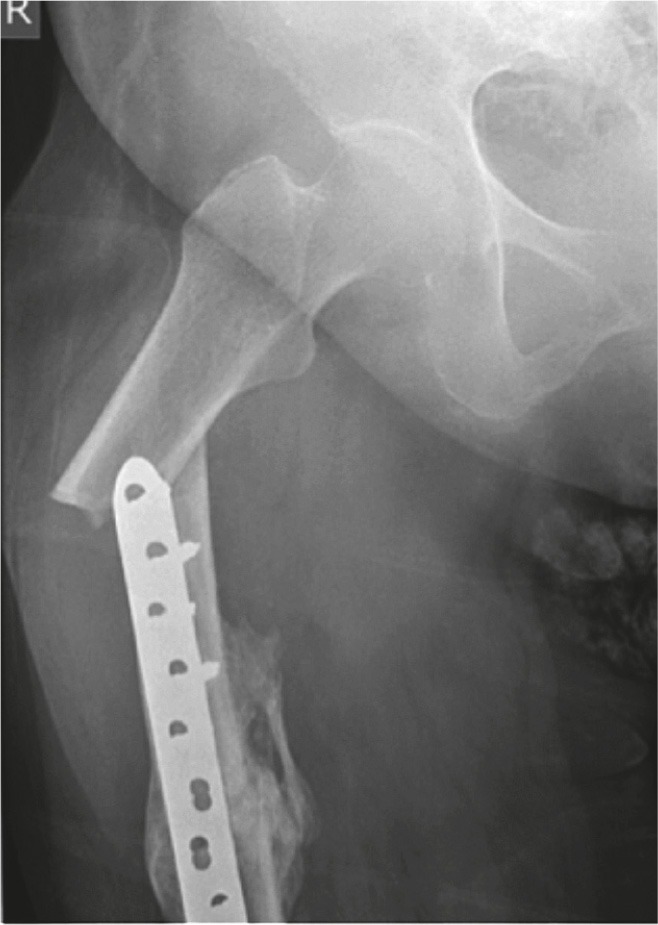
Patient 5. Atypical peri-implant fracture at the proximal end of a plate used to fix a midshaft fracture of the femur.
Figure 3.
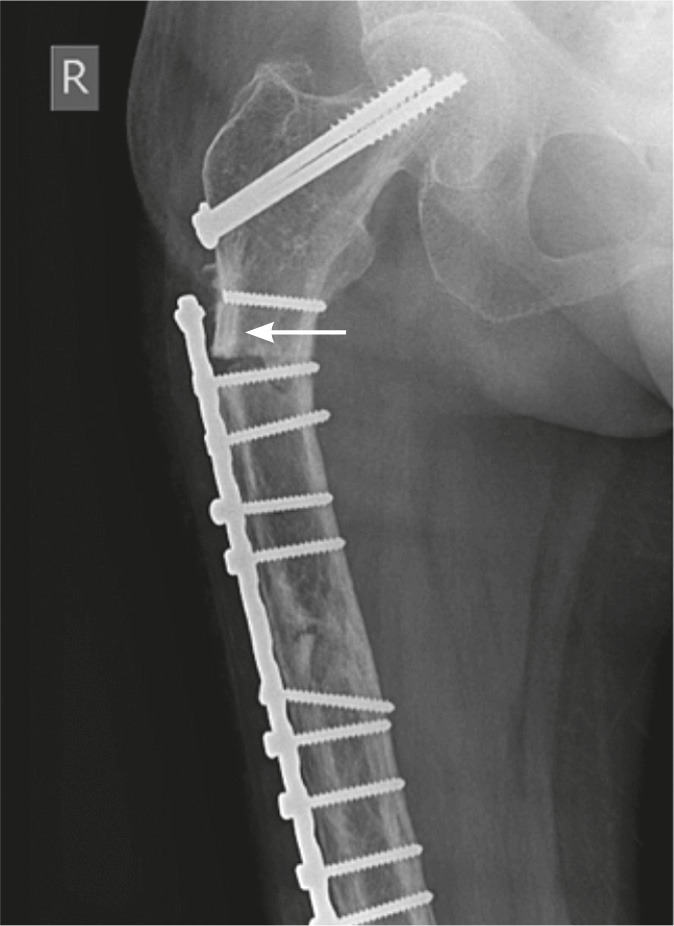
Patient 3. Fracture through the penultimate screw hole. The proximal screw had fractured earlier, leaving it uninvolved in the tension-band construct. The patient also had previous cancellous screw insertion. Lateral cortical thickening is evident (arrow).
Incidentally, we detected lateral cortical thickening in the opposite native femur of patient 10 during radiological investigation (Figure 4). She was asymptomatic and was advised not to bear weight on that limb.
Figure 4.

Patient 10. A. A transverse fracture through the top-most screw hole of the right femur plate shows minimal comminution. The native left femur shows lateral cortical thickening (box). B. The “dreaded black line” can be seen in the area of cortical thickening in this magnified image of the left femur shaft.
Laboratory and histological results
Biochemical tests for malignancy and metabolic disease were negative for all patients except patient 8. Initial investigations during her admission had shown normocalcemia, but she was found to have hypercalcemia with possible hyperparathyroidism 1 year after fixation of her peri-implant fracture.
Histology samples from the fracture site were obtained from patients 1, 6, 7, and 9. These showed reactive inflammatory tissue and were not malignant. Intraoperative tissue cultures from the fracture sites obtained from patients 1, 9, and 10 were negative for bacterial growth.
Treatment and response
We performed intramedullary nailing with bone grafting for 3 of the fractures. We did revision plating in 7 of the cases, with concurrent bone grafting in 6 instances. Patient 7 underwent both intramedullary nailing and plating with bone graft the second time she sustained a peri-implant fracture. At outpatient follow-up, the fractures showed radiological evidence of union 2–4 months postoperatively.
All patients stopped bisphosphonate therapy. We started patients 3, 4, and 9 on strontium postoperatively. We gave additional pharmacological therapy to some patients after consultation with an endocrinologist. Patients 6, 7, 8, and 9 had vitamin D supplementation, with patients 7 and 9 also receiving teriparatide.
Discussion
In 2010, a task force of the American Society for Bone and Mineral Research outlined the major and minor criteria for atypical femoral fractures (Shane et al. 2010). The case definition was subsequently revised to highlight the lateral cortical reaction and the lateral origin of a fracture of simple pattern (Shane et al. 2014). Both case definitions excluded peri-prosthetic fractures (amongst other exceptions).
After having found case reports of bisphosphonate-associated periprosthetic fractures (see below), we suggest that atypical fractures are not confined to native femurs but can also occur in operated femurs with arthroplasty implants and plate-screw constructs. Here we discuss the possible implications of our findings for the pathogenesis and clinical management of atypical fractures.
We have identified 5 papers featuring 7 cases of bisphosphonate-associated periprosthetic fractures (Sayed-Noor and Sjödén 2009, Curtin and Fehring 2011, Cross et al. 2012, Chen and Bhattacharyya 2012, Schaeffer et al. 2012). 5 cases involved cemented total hip arthroplasty (THA) implants while the remaining 2 involved uncemented THA implants. All the cases featured unicortical breaks of the lateral cortex around the distal tip of the femoral stem. The literature illustrates how an atypical periprosthetic fracture can present as a simple fracture line that runs across the end of an intramedullary prosthesis. Similar examples involving stemmed total knee arthroplasty implants can be seen in our patients’ index fractures, for cases 6 and 7a.
Among our patients, we noticed a different pattern of fracture, which occurs just distal to the tip of a plate or through the screw hole at the ends of a plate. We use the term “peri-implant fracture” instead of periprosthetic fracture to differentiate our findings from the existing case reports. Both patterns of implant/prosthesis-linked fracture share clinical and radiographic similarities with atypical fractures of the native femur. The fractures were sustained after negligible trauma, and showed lateral cortical thickening and a simple fracture pattern with minimal comminution.
AFFs have been described as a variant of stress or insufficiency fracture, “stress fracture(s) evolving under systemic influence” (Koh et al. 2011). They are like insufficiency fractures, as they occur after relatively low impact. The bilateral tendency and delayed progress in healing suggest intrinsic deficiencies of the bone tissue beyond local stress factors. AFFs also share features with stress fractures, such as prodromal pain before outright fracture, and localized cortical proliferation.
In contrast to the medial-sided compression failure seen in typical subtrochanteric stress fractures, atypical fractures in both operated and native femurs can be attributed to tensile failure (Isaacs et al. 2010). Lesions are localized to the lateral femoral cortex, with none occurring in the area of compression. Atypical lesions tend to cluster over the lateral subtrochanteric region, where tensile forces are highest along the femoral shaft (Koh et al. 2011). Another subgroup can be found distributed more distally along the femoral shaft. The pathophysiology of subtrochanteric AFFs may be more closely related to mechanical stress than more distal AFFs (Koeppen et al. 2013). Lower limb geometry has been shown to affect the location of AFFs (Saita et al. 2014). Mechanical factors can thus be seen to play a determinant role in the development of AFFs.
Implants concentrate stress at the plate-bone junctions due to differential stiffness. Short-stemmed prostheses and laterally positioned plates shift the fulcrum of the bending forces acting on the tension side of the femoral cortex to the edges of the construct. Dynamic strains under tension may be accentuated, contributing to local microdamage that overwhelms the impaired healing capacity of the antiresorptive-treated bone. These areas may then become more susceptible to chronic stress reactions and subsequent atypical fractures.
Fixing of a femoral shaft fracture with a lateral plate would concentrate stress at the ends of the plate, which are then possibly at risk of fracture. It may therefore be desirable to ensure that the plate does not end in an area of high tensile stress, such as the subtrochanteric region or the point of maximal bowing of a femoral shaft, where the inherent bony resistance may be weaker. According to Koch’s model, the forces along the lateral aspect of the femur taper distally from the highly tensile subtrochanteric region, becoming compressive along the distal lateral metaphysis. Ending a plate in the lower metaphyseal region may thus reduce the risk of sustaining implant-related atypical fractures. Alternatively, applying an intramedullary nail that spans the length of the bone may avoid the problem of stress concentration at the ends of a plate construct.
Atypical fractures of the native femur are more likely to occur after 2 or more years of bisphosphonate use and within 1 year of the last prescription (Schilcher et al. 2011). While patients 2 and 4 did not match this profile of bisphosphonate use, this does not negate the findings of atypicality on radiographs of their peri-implant fractures. Classical AFFs can occur without an established association with bisphosphonate use, and we acknowledge that this may also be the case for some atypical peri-implant fractures.
As no previous association between bisphosphonates and atypical peri-implant fractures has been made, our study was retrospective and not systematic or systemic. We acknowledge that there may have been an element of observational bias, and these fractures are likely to be rare. However, we believe that recognition of this clinical entity would be a first step in further identification—and that it could improve our understanding of bone metabolism and bisphosphonate therapy.
Acknowledgments
JYYL and TS: data collection and preparation of manuscript. TSH, JSBK, EBKK, and DTCC: data collection, editing of manuscript, and supervision.
No competing interests declared.
References
- Aspenberg P, Schilcher J, Fahlgren A. Histology of an undisplaced femoral fatigue fracture in association with bisphosphonate treatment . Acta Orthop. 2010;81(5):460–462. doi: 10.3109/17453674.2010.492766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari M, Bajammal S, Guyatt GH, et al. Effect of bisphosphonates on periprosthetic bone mineral density after total joint arthroplasty. A meta-analysis . J Bone Joint Surg Am. 2005;87(2):293–301. doi: 10.2106/JBJS.D.01772. [DOI] [PubMed] [Google Scholar]
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group . Lancet. 1996;348(9041):1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- Chen F, Bhattacharyya T. Periprosthetic fracture of the femur after long-term bisphosphonate use. JBJS Case Connect. 2012;2(e21) doi: 10.2106/JBJS.CC.K.00085. [DOI] [PubMed] [Google Scholar]
- Cross MB, Nam D, van der Meulen MC, Bostrom M PG. A rare case of a bisphosphonate-induced peri-prosthetic femoral fracture . J Bone Joint Surg Br. 2012;94(7):994–7. doi: 10.1302/0301-620X.94B7.28778. [DOI] [PubMed] [Google Scholar]
- Curtin BM, Fehring TK. Bisphosphonate fractures as a cause of painful total hip arthroplasty . Orthopedics. 2011;34(12):939–44. doi: 10.3928/01477447-20111021-36. [DOI] [PubMed] [Google Scholar]
- Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution . J Bone Joint Surg Br. 2007;89(3):349–53. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- Isaacs JD, Shidiak L, Harris IA, Szomor ZL. Femoral insufficiency fractures associated with prolonged bisphosphonate therapy . Clin Orthop Relat Res. 2010;468(12):3384–92. doi: 10.1007/s11999-010-1535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical fractures . Acta Orthop. 2013;84(6):561–4. doi: 10.3109/17453674.2013.866193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J SB, Goh SK, Png MA, Ng A CM, Howe TS. Distribution of atypical fractures and cortical stress lesions in the femur: implications on pathophysiology . Singapore Med J. 2011;52(2):77–80. [PubMed] [Google Scholar]
- Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use . J Orthop Trauma. 2008;22(5):346–50. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- Saita Y, Ishijima M, Mogami A, et al. The fracture sites of atypical femoral fractures are associated with the weight-bearing lower limb alignment . Bone. 2014;66:105–10. doi: 10.1016/j.bone.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Sayed-Noor AS, Sjödén GO. Case reports: two femoral insufficiency fractures after long-term alendronate therapy . Clin Orthop Relat Res. 2009;467(7):1921–6. doi: 10.1007/s11999-009-0725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer JF, Attarian DE, Wellman SS. Periprosthetic femoral insufficiency fracture in a patient on long-term bisphosphonate therapy. The Duke Orthop J. 2012;2(1):66–9. [Google Scholar]
- Schilcher J, Michaëlsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Eng J Med. 2011;364:1728–37. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- Schilcher J, Koeppen V, Aspenberg P, Michaëlsson K. Risk of atypical femoral fracture during and after bisphosphonate use: Full report of a nationwide study. Acta Othop. 2015;86(1):100–107. doi: 10.3109/17453674.2015.1004149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane E, Burr D, Ebeling PR, et al. American Society for Bone and Mineral Research. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research . J Bone Miner Res. 2010;25(11):2267–94. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research . J Bone Miner Res. 2014;29(1):1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]


