APSM was found to be feasible and effective in optimizing participation in desired daily life activities in women with CFS.
MeSH TERMS: activities of daily living; fatigue syndrome, chronic; outcome assessment (health care); personal satisfaction; relaxation; self care
Abstract
OBJECTIVE. The objective of this study was to evaluate the effectiveness of an activity pacing self-management (APSM) intervention in improving performance of daily life activities in women with chronic fatigue syndrome (CFS).
METHOD. A total of 33 women with CFS (age 41.1 ± 11.2 yr) were randomly allocated to APSM (experimental group; n = 16) or relaxation (control group; n = 17). Main outcome measures included the Canadian Occupational Performance Measure (COPM; primary) and Checklist Individual Strength (CIS).
RESULTS. COPM scores changed significantly over time in both groups (p = .03). The change in Satisfaction scores showed a significant difference in favor only of APSM (effect size = 0.74 [0.11, 1.4]). CIS scores decreased significantly in the experimental group only (p < .01).
CONCLUSION. APSM was found to be feasible and effective in optimizing participation in desired daily life activities in women with CFS. Replication in a larger sample with long-term follow-up is required.
Chronic fatigue syndrome (CFS) is a disabling illness affecting children, young people, and adults, with an estimated prevalence in adults in primary care of up to 6.4% (Fukuda et al., 1994; Johnston, Brenu, Staines, & Marshall-Gradisnik, 2013; Maquet, Demoulin, & Crielaard, 2006). The most commonly experienced symptoms are fatigue, pain, and cognitive problems (Anderson, Jason, Hlavaty, Porter, & Cudia, 2012; Goudsmit, Nijs, Jason, & Wallman, 2012). People with CFS may experience restricted performance in a variety of life domains, including employment, education, personal care, home care, leisure, and social relationships, although their symptoms are not always visible to others in their environment (Anderson et al., 2012; Taylor et al., 2010).
Possible nonpharmacological strategies to treat CFS include activity pacing or modification, graded exercise therapy (GET), and cognitive–behavioral therapy (CBT; Afari & Buchwald, 2003; Maquet et al., 2006; Pemberton & Cox, 2014). Activity pacing, a strategy to encourage people with CFS to be active within their physical and mental limits, is related to the energy envelope theory in that effort is made to maintain expended energy within the “envelope” of perceived available energy levels by moderating activity and conserving energy (Goudsmit & Howes, 2008; Goudsmit et al., 2012; Jason, Brown, & Brown, 2013). The rationale for activity pacing can be found in several strategies observed in people with CFS: reduced activity levels resulting from and in anticipation of fatigue (Nijs et al., 2011; Vercoulen et al., 1997), lowered activity peaks followed by very long rest periods (van der Werf, Prins, Vercoulen, van der Meer, & Bleijenberg, 2000), and ability to perform short periods of light to moderate activity without exacerbating symptoms (Clapp et al., 1999; Cook et al., 2005). Several randomized controlled trials (RCTs) have shown positive effects of treatment programs based on activity pacing for people with CFS (Cox, 2002; Crawley et al., 2013; Goudsmit et al., 2012; Jason et al., 2013; Pemberton & Cox, 2014; Taylor, 2004). The findings of these studies are in line with those of a pilot study in which several of the current authors participated (Nijs et al., 2009).
However, the available evidence regarding activity pacing self-management (APSM) as a sole treatment strategy in people with CFS is too limited to support its use (Goudsmit et al., 2012). The PACE trial (White et al., 2011) showed that an adapted pacing intervention, compared with CBT or GET, did not improve fatigue and physical functioning in people with CFS. However, rather than APSM, the PACE trial used an adapted pacing intervention that restricted people to live within their limits to clearly delineate pacing from CBT and GET in the trial setting. APSM, in contrast, encourages both reintroduction of and increases in activity. Studies of diverse methodological quality have found decreased fatigue levels and somatic symptoms and increased self-efficacy and quality of life after activity pacing as part of a multicomponent program for CFS (Cox, 2002; Crawley et al., 2013; Goudsmit, Ho-Yen, & Dancey, 2009; Taylor, 2004).
Thus, the findings regarding the effects of APSM are confusing and require further study. In addition, no studies have yet reported the effect of an activity pacing program focusing on client-centered outcome measures assessing desired and important daily life activities. This shortcoming is important because clients’ personal needs and desires are coming to the fore, and individually tailored interventions are becoming the standard.
In this study, we examined whether the performance of and satisfaction with desired and important daily life activities of people with CFS would improve in response to an APSM program compared with relaxation therapy (RT). We formed three hypotheses: (1) Activity performance and satisfaction would improve in both intervention groups, (2) increases in performance and satisfaction would be greater for the APSM group than the RT group, and (3) subjective fatigue would improve in both groups.
Method
Sample
All participants were diagnosed by an experienced internist (Greta Moorkens) according to the Centers for Disease Control and Prevention criteria for chronic fatigue syndrome (Fukuda et al., 1994) using serial physical examination and laboratory measurements. Clients diagnosed with CFS were placed on a waiting list for multidisciplinary rehabilitation. Native Dutch-speaking women with CFS between 18 and 65 yr old were eligible for study participation. This study focused on women only because it was part of a larger study that included physiological measures for which pooling of gender data is not feasible. Potential participants were excluded if they had been treated with activity pacing or CBT before or had already entered the multidisciplinary rehabilitation program for CFS at their local hospital.
The study was approved by the ethics committee of the University Hospital of Antwerp, and all participants signed an informed consent. The study protocol was registered at ClinicalTrials.gov (NCT01512342).
Required sample size was calculated with G*Power Version 3.1.7 (Faul, Erdfelder, Lang, & Buchner, 2007) using the pilot sample’s change scores on the Canadian Occupational Performance Measure (COPM; Law et al., 2005; Nijs et al., 2009). With a significance level of .05 and a power of .80, the total sample size needed to be at least 17 participants.
Procedure
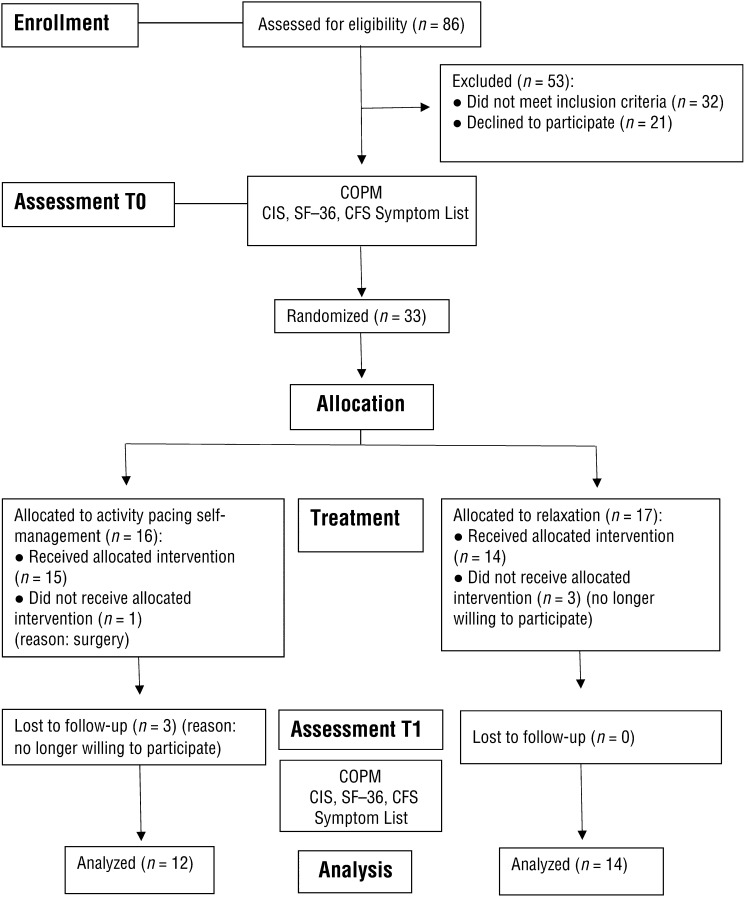
The study had a single-blind RCT design with baseline and posttreatment assessment (Figure 1). Potential participants were extracted from the waiting list for multidisciplinary rehabilitation and informed about the study through an information leaflet sent by mail. The researchers later contacted potential participants by telephone to confirm inclusion and exclusion criteria and verified their willingness to participate.
Figure 1.
Flowchart of the study (based on Turner et al., 2012).
Note. CFS = chronic fatigue syndrome; CIS = Checklist Individual Strength; COPM = Canadian Occupational Performance Measure.
Pre- and postintervention assessment was performed by a blinded researcher (Daphne Kos or Jo Nijs) who was not involved in the treatment programs. To prevent test order and administration bias, participants completed the questionnaires in random order and alone without input from the researcher.
For randomization, participants were asked to blindly pick a folded paper marked with “self-management” or “relaxation” out of a box. An independent researcher (not involved in data collection or analyses) notified participants about the type of intervention they would receive and informed all participants that the intervention could help decrease their feelings of fatigue; participants were therefore unaware of their group allocation.
Three individual therapy sessions of 60–90 min/wk for 3 consecutive wk were provided by an occupational therapist (Inge Van Eupen, activity pacing) or a physical therapist (Deborah Van Cauwenbergh, relaxation) in the center. Participants were asked not to change or initiate any pharmaceutical intervention during the study period. We did not record the types of medication used but did monitor changes in medication use in the study period.
Intervention
Activity Pacing Self-Management.
The APSM program consists of a stabilization phase and a grading phase (Nijs, Paul, & Wallman, 2008; Nijs et al., 2009). The stabilization phase focuses on coaching clients in how to perform daily life activities within the limits of their actual capacity. Daily life activities were defined as all responsibilities and desired activities in the areas of personal and child care, domestic care, productivity, and leisure. To appropriately pace activities, participants were instructed to estimate their current physical and mental capabilities (in terms of activity duration) before commencing an activity, keeping in mind the fluctuating nature of their symptoms.
The activity duration advised within the program was 25%–50% lower than the capacity participants reported to account for any overestimations. Each activity block was interspersed with breaks, with the length of each break equal to the duration of the activity. Breaks were defined as relative periods of rest, with the participant just relaxing or performing a different type of light activity. The emphasis on breaks is based on the observation that recovery from physical exertion is prolonged in people with CFS (Ickmans, Meeus, De Kooning, Lambrecht, & Nijs, 2014; Ickmans, Meeus, De Kooning, Lambrecht, Pattyn, & Nijs, 2014; Paul, Wood, Behan, & Maclaren, 1999). The process of restructuring activity patterns involves significant behavioral change for people with CFS, and facilitation of this process can be beneficial (Abraham & Michie, 2008). Participants received education in the form of a booklet with evidence-based information on factors influencing fatigue and strategies to cope with fatigue and pace activities. Participants were asked to keep a diary recording the type of activity and time spent on all activities throughout the day during 7 days to increase their awareness and guide implementation of coping strategies.
Once clients are able to control their daily life activities without excessive feelings of fatigue, the grading phase can start. In this phase, activity and exercise level are increased gradually. Participants conferred with the therapist to set relevant and achievable personal activity and exercise goals, based on the prioritized activities reported in the COPM and diary results (e.g., “During two of my lunch breaks next week, I will walk for 400 m” or “For 2 days next week, I will prepare a warm meal in two time slots [preparation in the morning, finalization in the late afternoon]”). Strategies to reach these goals were discussed and performed in real life between two sessions. During the next session, participants reflected on activity performance, facilitators, and barriers and adapted their strategies and goals accordingly, whenever relevant and appropriate.
Relaxation Therapy.
Stress is an important factor in the persistence of fatigue; therefore, relaxation may be a crucial component in the treatment of CFS (Bassi, Amital, Amital, Doria, & Shoenfeld, 2008). The relaxation program for this study comprised education about the role of stress in CFS biology and the opportunities that stress management provides. This information was also provided in the form of an evidence-based information booklet. Participants were instructed how to apply stress management techniques, including Jacobson relaxation skills, Schultz relaxation skills, visualization, and other techniques, depending on their individual preferences for one or more of the techniques (Lehrer, 1996). Participants also completed a stress reaction diary to record activities or events that evoked stress. The therapist and participant discussed this diary during the session, and the therapist provided the participant with activities to improve coping in similar future stress events. The mode, duration, and frequency of the relaxation therapy sessions were identical to those in the APSM program.
Outcome Measures
The primary outcome measure was the COPM. Other measures were the Checklist Individual Strength (CIS), the SF–36 (Ware, Snow, Kosinski, & Gandek, 1993), and the CFS Symptom List.
The COPM is a standardized outcome measure frequently used by occupational therapists to assess clients’ perceived performance of and satisfaction with relevant daily life activities (Law et al., 2005). Using a semistructured interview, the therapist explores problems in performing daily activities in the domains of self-care, productivity, and leisure. The mean score for all activities in the Performance and Satisfaction subscales is calculated. At follow-up, clients rescore the same activities, blinded to earlier scores. The COPM is a reliable, valid, and responsive instrument, and change scores of 1.4 for Performance and 1.9 for Satisfaction are considered clinically important (Carswell et al., 2004; Dedding, Cardol, Eyssen, Dekker, & Beelen, 2004; Eyssen, Beelen, Dedding, Cardol, & Dekker, 2005; Eyssen et al., 2011).
The CIS is a self-report instrument that measures diverse aspects of fatigue (Vercoulen, Alberts, & Bleijenberg, 1999). The questionnaire lists 20 statements, and clients score the extent to which each statement is appropriate for his or her situation during the past 2 weeks on a 7-point Likert scale. A total score is calculated (20–140), as well as four subscale scores: Subjective Experience of Fatigue (8–56), Reduction in Concentration (5–35), Reduction in Motivation (4–28), and Reduction in Activity (3–21). The CIS has acceptable reliability and validity (Vercoulen et al., 1997). A minimal detectable change score of 18% was found in a sample of people with multiple sclerosis (Rietberg, Van Wegen, & Kwakkel, 2010).
The SF–36 assesses functional status and quality of life. The assessment consists of 36 questions or statements in eight subscales: (1) General Health, (2) Physical Functioning, (3) Role–Physical, (4) Bodily Pain, (5) Vitality, (6) Social Functioning, (7) Role–Emotional, and (8) Mental Health. Health change reflects the change in health status compared with 1 yr previously. For comparison, a transformed score is calculated so that the scores of all subscales range from 0 to 100. The SF–36 has shown adequate reliability and validity in a wide variety of populations (McHorney, Ware, Lu, & Sherbourne, 1994; McHorney, Ware, & Raczek, 1993).
The CFS Symptom List is a self-report instrument to assess symptom severity in CFS. The severity of 19 frequently reported symptoms, such as pain, fatigue, attention disorders, and muscle weakness, are scored on a visual analog scale (0–100 mm). The total score is the mean of all 19 severity scores. The CFS Symptom List has adequate psychometrics (Nijs & Thielemans, 2008).
Statistical Analyses
Data were analyzed with IBM SPSS Statistics Version 20.0 (IBM Corporation, Armonk, NY). Shapiro–Wilk normality testing showed a normal distribution for the COPM and SF–36 but not for the CIS (total score and subscales) and CFS Symptom List. Therefore, we used parametric statistics for the COPM and SF–36 and nonparametric analyses for the CIS and CFS Symptom List.
Parametric analyses included 2 (Condition: Experimental, Control) × 2 (Time: Preintervention, Postintervention) mixed-model analysis of variance based on the univariate F statistic. Condition is a between-subjects factor, and Time is a within-subjects factor. Effect sizes of significant between-subjects effects are expressed as Cohen’s d (Cohen, 1988) with a 95% confidence interval.
Nonparametric analyses were used to interpret differences within groups using Wilcoxon signed rank tests and between groups with Mann–Whitney U tests. Effect sizes of significant between-subjects effects are calculated as z score divided by the square root of n (Corder & Foreman, 2009). Effect sizes of 0.20 are considered small, 0.50 moderate, and 0.80 large (Cohen, 1988).
Instead of performing corrections for comparisons of multiple outcome measures, all actual p values are reported, as recommended (Feise, 2002; Rothman, 1990). Statistical significance was set at α = .05 and β = .20. Clinical relevance was set at a minimum difference score of 2 points for COPM Performance and Satisfaction (Eyssen et al., 2011); the proportion of participants with a change score in COPM Performance and Satisfaction of 2 or more points was calculated to evaluate clinical relevance.
Results
The sample consisted of 33 women with CFS (n = 16 in the experimental group, n = 17 in the control group; Figure 1). No significant differences were found between groups for any variable (Table 1). Dropouts (25% in the experimental group, 18% in the control group) did not significantly differ from participants who completed the study on these baseline variables, and consequently no intention-to-treat analysis was performed. None of the participants reported initiating or altering other treatments during the study period, except for one dropout who had to undergo surgery.
Table 1.
Baseline Participant Characteristics
| Variable | Activity Pacing Self-Management Group | Relaxation Therapy Group | p |
|---|---|---|---|
| n | 16 | 17 | |
| Age, yr (M ± SD) | 39.3 ± 11.4 | 40.8 ± 11.1 | .983 |
| COPM Performance (M ± SD) | 4.1 ± 1.5 | 4.8 ± 1.4 | .656 |
| COPM Satisfaction (M ±SD) | 3.9 ± 2.0 | 4.3 ± 1.8 | .663 |
| CIS Subjective Fatigue (median, IQR) | 51 (6.5) | 54 (10) | .231 |
| SF–36 Vitality subscale (M ± SD) | 32.5 ± 14.4 | 27.7 ± 12.5 | .311 |
| CFS Symptom List total score (median, IQR) | 54.5 (27.1) | 63.7 (27.1) | .345 |
Note. CFS = chronic fatigue syndrome; CIS = Checklist Individual Strength; COPM = Canadian Occupational Performance Measure; IQR = interquartile range; M = mean; SD = standard deviation.
COPM Performance and Satisfaction scores changed significantly over time in both groups; the APSM and RT groups differed only on the change in Satisfaction scores, with a moderate to high effect size (0.74 [0.11, 1.40]) in favor of the APSM group (Table 2). Some SF–36 subscales showed significant Time effects, but these effects did not differ between groups, with one exception: A significant Time × Group effect was found for Role–Emotional, with increased scores in the experimental group; participants in the control group scored worse (effect size = 1.21).
Table 2.
Baseline and Postintervention Assessment of Participants Who Completed the Study, Including Time Effects, Time × Group Effects, and Effect Sizes for Postintervention Differences Between Groups (N = 26)
| Scale | Group | T0 Score | T1 Score | Time Effect | Time × Group Effect | Effect Size Between Groups (Cohen’s d [CI]) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | F | p | F | p | |||
| COPM Performance | exp | 4.1 | 1.5 | 5.6 | 1.4 | 5.4 | .03* | 2.1 | .16 | 0.34 [−0.2, 0.9] |
| cont | 4.8 | 1.4 | 5.1 | 1.5 | ||||||
| COPM Satisfaction | exp | 3.9 | 2.1 | 5.7 | 1.9 | 5.1 | .03* | 6.8 | .02* | 0.74 [0.1, 1.4] |
| cont | 4.3 | 1.8 | 4.5 | 1.5 | ||||||
| SF–36 Physical Functioning | exp | 46.3 | 21.9 | 53.2 | 20.9 | 0.1 | .78 | 0.1 | .78 | NS |
| cont | 41.2 | 19.0 | 45.0 | 12.7 | ||||||
| SF–36 Role–Physical | exp | 12.5 | 27.4 | 36.4 | 39.3 | 5.7 | .03* | 2.6 | .12 | NS |
| cont | 4.4 | 9.8 | 11.5 | 28.2 | ||||||
| SF–36 Bodily Pain | exp | 45.1 | 21.1 | 48.0 | 24.8 | 0.1 | .76 | 1.2 | .28 | NS |
| cont | 40.3 | 17.5 | 40.4 | 15.5 | ||||||
| SF–36 General Health | exp | 35.2 | 19.4 | 42.5 | 19.0 | 5.6 | .03* | 0.6 | .44 | NS |
| cont | 35.4 | 23.2 | 39.0 | 20.1 | ||||||
| SF–36 Vitality | exp | 29.1 | 11.4 | 38.6 | 14.0 | 10.6 | .00* | 1.0 | .32 | NS |
| cont | 30.0 | 12.2 | 35.0 | 15.3 | ||||||
| SF–36 Social Functioning | exp | 43.2 | 18.0 | 53.4 | 19.4 | 4.6 | .04* | 0.4 | .54 | NS |
| cont | 37.5 | 21.7 | 43.1 | 21.7 | ||||||
| SF–36 Role–Emotional | exp | 72.7 | 32.8 | 93.9 | 20.1 | 0.1 | .71 | 5.7 | .03* | 1.21 [0.3, 1.9] |
| cont | 66.7 | 38.5 | 51.3 | 46.4 | ||||||
| SF–36 Mental Health | exp | 63.3 | 11.1 | 69.5 | 10.6 | 2.5 | .13 | 2.1 | .17 | NS |
| cont | 57.8 | 23.4 | 58.2 | 21.9 | ||||||
| SF–36 Health Change | exp | 56.8 | 27.6 | 47.7 | 34.4 | 1.5 | .24 | 0.6 | .44 | NS |
| cont | 63.5 | 28.2 | 61.5 | 21.9 | ||||||
Note. CI = 95% confidence interval; cont = control group (relaxation therapy); COPM = Canadian Occupational Performance Measure; exp = experimental group (activity pacing self-management); M = mean; NS = not significant; SD = standard deviation; T0 = baseline assessment; T1 = postintervention assessment.
p < .05.
CIS total and subscale scores decreased significantly in the experimental group but not in the control group (Table 3). All subscale scores show a trend toward statistically significant differences between groups, with effect sizes ranging from 0.36 to 0.41, except for Experience of Subjective Fatigue, which decreased similarly in both groups (p = .32). On the CFS Symptom List, one symptom—mood swings—showed a significant decrease in the experimental group compared with the control group at postintervention assessment (p = .03, effect size = 0.45).
Table 3.
Nonparametric Analyses of Variables at Baseline and Postintervention Assessment, Differences Within Groups and Between Groups, and Effect Sizes
| Scale | Group | T0 | T1 | Change Within Group (p)a | Change Between Groups (p)b | Effect Size Between Groups at T1c | ||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | |||||
| Checklist Individual Strength | ||||||||
| Total | exp | 112 | 15.5 | 91 | 18.0 | .00* | .05 | 0.41 |
| cont | 120 | 10.5 | 107 | 26.5 | .59 | |||
| Reduction in Concentration | exp | 27 | 6.8 | 23 | 5.0 | .01* | .06 | 0.38 |
| cont | 31 | 6.5 | 29 | 7.0 | .05 | |||
| Reduction in Activity | exp | 16.5 | 5.0 | 12 | 6.0 | .00* | .07 | 0.37 |
| cont | 16 | 4.0 | 14 | 4.0 | .10 | |||
| Reduction in Motivation | exp | 17.5 | 7.8 | 14 | 8.0 | .01* | .08 | 0.36 |
| cont | 18 | 7.0 | 18 | 7.5 | .72 | |||
| Experience of Subjective Fatigue | exp | 51 | 6.5 | 43 | 11.0 | .00* | .32 | NS |
| cont | 54 | 10.0 | 48 | 12.5 | .11 | |||
| Chronic Fatigue Syndrome Symptom List | ||||||||
| Total score | exp | 53.9 | 26.7 | 55.1 | 28.9 | .21 | .82 | NS |
| cont | 58.8 | 28.7 | 44 | 43.2 | .32 | |||
| Mood swings | exp | 46.5 | 45.8 | 10 | 15.5 | .02* | .03 | 0.45 |
| cont | 63 | 50.0 | 57 | 59.0 | .97 | |||
| Recurrent flulike symptoms | exp | 48 | 59.0 | 26 | 62.0 | .02* | .17 | NS |
| cont | 74 | 54.0 | 60 | 49.0 | .27 | |||
| Attention deficits | exp | 68 | 31.5 | 40 | 51.0 | .04* | .26 | NS |
| cont | 79 | 31.5 | 67.5 | 55.0 | .07 | |||
| Personality changes | exp | 54.5 | 54.8 | 11 | 17.0 | .04* | .21 | NS |
| cont | 55 | 42.5 | 28 | 63.0 | .35 | |||
| Sleep disturbances | exp | 65 | 61.8 | 47 | 60.0 | .07 | .97 | NS |
| cont | 55 | 57.0 | 34 | 60.0 | .45 | |||
| Muscle weakness | exp | 54.5 | 36 | 52 | 63.5 | .07 | .88 | NS |
| cont | 67 | 26.5 | 59 | 19 | .13 | |||
| Intolerance to bright light | exp | 46 | 66 | 46 | 72 | .09 | .65 | NS |
| cont | 35 | 71.5 | 42 | 73 | .48 | |||
| Memory disturbances | exp | 65 | 26.3 | 39 | 53 | .09 | .19 | NS |
| cont | 79 | 25.5 | 74 | 64 | .07 | |||
| Calculation difficulties | exp | 50 | 50.5 | 39 | 58.5 | .34 | .17 | NS |
| cont | 72 | 27 | 66.5 | 54.8 | .07 | |||
| Muscle pain | exp | 62 | 38.3 | 62 | 54.5 | .55 | .20 | NS |
| cont | 70 | 26 | 74 | 16 | .88 | |||
| Headaches | exp | 21.5 | 76.5 | 31 | 53 | .48 | .78 | NS |
| cont | 25.5 | 54.8 | 31.5 | 63 | .66 | |||
| Sore throat | exp | 22.5 | 50.8 | 4 | 41 | .24 | .24 | NS |
| cont | 37 | 64.3 | 42 | 62.8 | .95 | |||
| Fatigue | exp | 72 | 13 | 71 | 55.5 | .64 | .94 | NS |
| cont | 77 | 20.5 | 63 | 23.8 | .12 | |||
| Fatigue after physical activity | exp | 75 | 33.3 | 70 | 25 | .89 | .21 | NS |
| cont | 81 | 24 | 84.5 | 20 | .75 | |||
| Frequent use of the wrong word | exp | 68.5 | 21.3 | 53 | 62 | .50 | .57 | NS |
| cont | 55 | 39 | 54 | 54 | .33 | |||
| Unrefreshing sleep | exp | 73 | 43 | 52 | 63.5 | .34 | .27 | NS |
| cont | 74 | 32 | 71 | 23 | .93 | |||
| Cold hands and feet | exp | 56 | 38.8 | 66 | 32.5 | .64 | .88 | NS |
| cont | 80 | 23.5 | 74 | 73 | .28 | |||
| Abdominal pain | exp | 57.5 | 81.5 | 31 | 82 | .41 | .59 | NS |
| cont | 52 | 66.5 | 59 | 65 | .42 | |||
| Dyspnea on exertion | exp | 72 | 55.8 | 53 | 73 | .72 | .29 | 0.06 |
| cont | 76 | 34.5 | 77 | 53 | .48 | |||
Note. cont = control group (relaxation therapy); exp = experimental group (activity pacing self-management); IQR = interquartile range; M = mean; NS = not significant; SD = standard deviation; T0 = baseline assessment; T1 = postintervention assessment.
Based on Wilcoxon signed rank test. bBased on Mann–Whitney U test. cEffect size = z score divided by the square root of n.
p < .05.
On the COPM, 33% of the APSM group showed a clinically relevant change in Performance, and 42% showed a clinically relevant change in Satisfaction. Of the RT group, only 14% showed a clinically relevant change in Performance, and none showed a clinically relevant change in Satisfaction.
Discussion
This study is the first to show that APSM as a sole treatment, compared with relaxation therapy, was effective in improving the perceived performance of and satisfaction with desired daily life activities in a sample of women with CFS. In addition, the study results suggest that APSM was more effective than RT in improving fatigue.
Although performance changed significantly over time in both groups (confirming Hypothesis 1), performance did not differ between groups at reassessment (inconsistent with Hypothesis 2). Clinically relevant changes in performance occurred in both groups, although to a higher extent in the experimental group (33% vs. 14% of participants with COPM Performance change scores of 2 points or more). The relaxation and stress management techniques offered to control participants may have improved performance of daily life activities as well; such effects have not been studied in relation to relaxation as a sole treatment of CFS.
It was to be expected that performance of daily life activities would not have reached the most optimal levels immediately after the intervention. More time than the intervention period of 3 wk used in this study may have been necessary for participants to make the practical arrangements required to maximize performance (e.g., acquisition of needed equipment, alterations in work schedules, installation of home adaptations). With a longer treatment program or a longer follow-up period, we might have observed the evolution of Performance scores. However, participants in this study entered a multidisciplinary rehabilitation program after the reassessment, and therefore follow-up scores would have been contaminated by the additional intervention.
Experimental group participants did experience increased satisfaction with their occupational performance compared with the control group, consistent with Hypothesis 2. Participants who were trained in activity pacing self-management seemed to have learned superior coping strategies for dealing with limitations in daily life activities or their illness in general compared with control participants, findings that are consistent with those of a similar study (Cox, 2002). Experimental group participants may have reappraised their daily life activities, altering the value they placed on those activities, in turn resulting in improved satisfaction with performance of desired activities (Persson, Andersson, & Eklund, 2011). The increased Role–Emotional scores on the SF–36 and decreased levels of mood swings on the CFS Symptom List in the APSM group compared with the RT group may be related to this observation. However, the current study design does not allow us to draw conclusions about the causality of these relationships.
The decrease in fatigue scores, confirming Hypothesis 3, is in accordance with findings from other studies of behavioral treatments for CFS, including fatigue amelioration in response to cognitive–behavioral therapy (Prins et al., 2001; White et al., 2011; Wiborg, Knoop, Prins, & Bleijenberg, 2011), graded exercise therapy (Wallman, Morton, Goodman, Grove, & Guilfoyle, 2004; White et al., 2011), and activity pacing (Goudsmit et al., 2009; Nijs et al., 2009). In addition, the finding that APSM was effective for participants with CFS adds to the findings from the PACE trial (White et al., 2011) showing that adaptive pacing alone was not effective in treating CFS. The adaptive pacing intervention studied in the PACE trial differs conceptually from the activity pacing intervention in the current study (Goudsmit et al., 2012). Activity pacing self-management uses the principle of activity pacing but incorporates a grading phase to gradually increase activity and exercise levels. Still, more studies comparing the effects of activity pacing with established CFS treatments like GET and CBT are warranted. However, we advocate APSM not as a stand-alone treatment for CFS, but rather as part of a multidisciplinary rehabilitation program that also includes comprehensive client education, counseling, exercise therapy, and stress management and sleep management interventions (Goudsmit et al., 2012; Nijs, Mannerkorpi, Descheemaeker, & Van Houdenhove, 2010).
The activity pacing self-management program was well tolerated in our sample; no adverse effects were reported, and the dropout rate was limited. This finding is in line with the earlier reports of Shepherd (2001) based on a large-scale survey.
Study Limitations
This study has several limitations that limit the generalizability of its results. Participants may have been unusually motivated to participate in an intervention program; they were on a waiting list for multidisciplinary rehabilitation and consequently had already made initial steps toward behavior change (Prochaska & Velicer, 1997). Although both groups were drawn from the waiting list and this client profile is usually the target group for these kinds of interventions in clinical practice, conclusions may not be extended to the entire CFS population. People recently diagnosed with CFS may benefit to a lesser degree from this program if they are in a mourning process or are unable to perform any activity until first undergoing a restoration phase. Acceptance of the condition has been shown to be important for well-being (Van Damme, Crombez, Van Houdenhove, Mariman, & Michielsen, 2006) and response to CBT (Brooks, Rimes, & Chalder, 2011; Poppe, Petrovic, Vogelaers, & Crombez, 2013) in people with CFS. None of the participants in our study had been diagnosed with CFS within the previous 3 months. We did not assess exact duration of symptoms; still, the evidence supporting the impact of symptom duration on conservative treatment outcomes in CFS is limited (Crawley et al., 2013).
Furthermore, to be able to participate in the intervention, participants had to attend the clinic for both assessment and treatment sessions, a total of five visits. We acknowledge that this approach may have excluded people who were severely affected by CFS (i.e., mostly bedridden) from participating, hence favoring those with less severe symptoms (Carruthers et al., 2011). A home-delivered program would overcome this travel barrier and would have the additional advantage of allowing performance of, training in, and advice regarding activities to take place in the authentic situation. The feasibility, effectiveness, and economic analysis of home-delivered APSM should be part of future research.
The study had a small sample, although it met the a priori required sample size. To allow firm conclusions, the study should be replicated with a larger sample.
Implications for Occupational Therapy Practice
The results of this study have the following implications for occupational therapy practice:
A short APSM program may be feasible and effective for women with chronic fatigue syndrome when combined with a multidisciplinary rehabilitation program.
This study showed the intervention’s effectiveness in the short term; long-term follow-up studies are needed.
The personalized and graded approach in the APSM may be important components to support clients with CFS in their coping process and can be applied in other rehabilitation domains.
The COPM and activity diaries are useful tools in detecting and prioritizing desired daily life activity goals in CFS.
It is to be determined whether a combination of APSM and relaxation techniques would lead to increased effectiveness in activity performance and satisfaction.
Conclusion
The APSM program shows evidence of being a feasible and effective intervention to optimize performance of and satisfaction with desired daily life activities and to decrease fatigue in women with CFS. Although the findings are promising for a condensed intervention of three treatment sessions, the findings require replication in a larger sample, and the long-term effects of the intervention need to be studied in future research.
Acknowledgments
The authors thank all participants for their contribution to this study. Staff of the University Hospital of Antwerp and students are acknowledged for facilitating and providing support in data collection. This work was supported by the research council of Artesis Plantijn University College, Antwerp, Belgium (Grant PWO G848).
Contributor Information
Daphne Kos, Daphne Kos, PhD, OT, is Assistant Professor, Department of Rehabilitation Sciences, Neuromotor Research Group, KU Leuven–University of Leuven, Belgium; Lecturer, Division of Occupational Therapy, Department of Health and Social Care, Artesis Plantijn University College, Antwerp, Belgium; and Member, Pain in Motion Research Group, Brussels, Belgium; daphne.kos@faber.kuleuven.be.
Inge van Eupen, Inge van Eupen, OT, is Lecturer, Division of Occupational Therapy, Department of Health and Social Care, Artesis Plantijn University College, Antwerp, Belgium.
Jill Meirte, Jill Meirte, PT, is PhD Researcher, Faculty of Medicine and Health Sciences, Department of Rehabilitation Sciences and Physiotherapy, University of Antwerp, Antwerp, Belgium. At the time of the study, she was Lecturer, Artesis Plantijn University College, Antwerp, Belgium.
Deborah Van Cauwenbergh, Deborah Van Cauwenbergh, PT, is PhD Researcher, Faculty of Medicine and Health Sciences, Department of Rehabilitation Sciences and Physiotherapy, University of Antwerp, Antwerp, Belgium. At the time of the study, she was Lecturer, Artesis Plantijn University College, Antwerp, Belgium.
Greta Moorkens, Greta Moorkens, PhD, MD, is Associate Professor, Department of General Internal Medicine of University of Antwerp, Belgium; and Warrant-Manager, University Hospital, Antwerp, Belgium.
Mira Meeus, Mira Meeus, PhD, PT, is Associate Professor, Faculty of Medicine and Health Sciences, Department of Rehabilitation Sciences and Physiotherapy, University of Antwerp, Belgium; Associate Professor, Faculty of Medicine and Health Sciences, Department of Rehabilitation Sciences and Physiotherapy, Ghent University, Ghent, Belgium; and Member, Pain in Motion Research Group, Brussels, Belgium. At the time of the study, she was Lecturer, Artesis Plantijn University College, Antwerp, Belgium.
Jo Nijs, Jo Nijs, PhD, PT, is Associate Professor, Departments of Human Physiology and Physiotherapy, Faculty of Physical Education and Physiotherapy, Vrije Universiteit, Brussels, Belgium; Physiotherapist, Department of Physical Medicine and Physiotherapy, University Hospital, Brussels, Belgium; and Member, Pain in Motion Research Group, Brussels, Belgium. At the time of the study, he was Lecturer, Artesis Plantijn University College, Antwerp, Belgium.
References
- Abraham C., & Michie S. (2008). A taxonomy of behavior change techniques used in interventions. Health Psychology, 27, 379–387. http://dx.doi.org/10.1037/0278-6133.27.3.379 [DOI] [PubMed] [Google Scholar]
- Afari N., & Buchwald D. (2003). Chronic fatigue syndrome: A review. American Journal of Psychiatry, 160, 221–236. http://dx.doi.org/10.1176/appi.ajp.160.2.221 [DOI] [PubMed] [Google Scholar]
- Anderson V. R., Jason L. A., Hlavaty L. E., Porter N., & Cudia J. (2012). A review and meta-synthesis of qualitative studies on myalgic encephalomyelitis/chronic fatigue syndrome. Patient Education and Counseling, 86, 147–155. http://dx.doi.org/10.1016/j.pec.2011.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi N., Amital D., Amital H., Doria A., & Shoenfeld Y. (2008). Chronic fatigue syndrome: Characteristics and possible causes for its pathogenesis. Israel Medical Association Journal, 10, 79–82. [PubMed] [Google Scholar]
- Brooks S. K., Rimes K. A., & Chalder T. (2011). The role of acceptance in chronic fatigue syndrome. Journal of Psychosomatic Research, 71, 411–415. http://dx.doi.org/10.1016/j.jpsychores.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Carruthers B. M., van de Sande M. I., De Meirleir K. L., Klimas N. G., Broderick G., Mitchell T., . . . Stevens S. (2011). Myalgic encephalomyelitis: International consensus criteria. Journal of Internal Medicine, 270, 327–338. http://dx.doi.org/10.1111/j.1365-2796.2011.02428.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell A., McColl M. A., Baptiste S., Law M., Polatajko H., & Pollock N. (2004). The Canadian Occupational Performance Measure: A research and clinical literature review. Revue Canadienne d’Ergothérapie, 71, 210–222. http://dx.doi.org/10.1177/000841740407100406 [DOI] [PubMed] [Google Scholar]
- Clapp L. L., Richardson M. T., Smith J. F., Wang M., Clapp A. J., & Pieroni R. E. (1999). Acute effects of thirty minutes of light-intensity, intermittent exercise on patients with chronic fatigue syndrome. Physical Therapy, 79, 749–756. [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Cook D. B., Nagelkirk P. R., Peckerman A., Poluri A., Mores J., & Natelson B. H. (2005). Exercise and cognitive performance in chronic fatigue syndrome. Medicine and Science in Sports and Exercise, 37, 1460–1467. http://dx.doi.org/10.1249/01.mss.0000179921.48404.ef [DOI] [PubMed] [Google Scholar]
- Corder G. W., & Foreman D. I. (2009). Nonparametric statistics for non-statisticians: A step-by-step approach. Hoboken, NJ: Wiley; http://dx.doi.org/10.1002/9781118165881.ch5 [Google Scholar]
- Cox D. (2002). Chronic fatigue syndrome: An evaluation of an occupational therapy inpatient intervention. British Journal of Occupational Therapy, 65, 461–468. http://dx.doi.org/10.1177/030802260206501005 [Google Scholar]
- Crawley E., Collin S. M., White P. D., Rimes K., Sterne J. A., & May M. T.; CFS/ME National Outcomes Database. (2013). Treatment outcome in adults with chronic fatigue syndrome: A prospective study in England based on the CFS/ME National Outcomes Database. QJM: Monthly Journal of the Association of Physicians, 106, 555–565. http://dx.doi.org/10.1093/qjmed/hct061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedding C., Cardol M., Eyssen I. C. J. M., Dekker J., & Beelen A. (2004). Validity of the Canadian Occupational Performance Measure: A client-centred outcome measurement. Clinical Rehabilitation, 18, 660–667. http://dx.doi.org/10.1191/0269215504cr746oa [DOI] [PubMed] [Google Scholar]
- Eyssen I. C., Beelen A., Dedding C., Cardol M., & Dekker J. (2005). The reproducibility of the Canadian Occupational Performance Measure. Clinical Rehabilitation, 19, 888–894. http://dx.doi.org/10.1191/0269215505cr883oa [DOI] [PubMed] [Google Scholar]
- Eyssen I. C., Steultjens M. P., Oud T. A., Bolt E. M., Maasdam A., & Dekker J. (2011). Responsiveness of the Canadian Occupational Performance Measure. Journal of Rehabilitation Research and Development, 48, 517–528. http://dx.doi.org/10.1682/JRRD.2010.06.0110 [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., & Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. http://dx.doi.org/10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Feise R. J. (2002). Do multiple outcome measures require p-value adjustment? BMC Medical Research Methodology, 2, 8 http://dx.doi.org/10.1186/1471-2288-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Straus S. E., Hickie I., Sharpe M. C., Dobbins J. G., & Komaroff A.; International Chronic Fatigue Syndrome Study Group. (1994). The chronic fatigue syndrome: A comprehensive approach to its definition and study. Annals of Internal Medicine, 121, 953–959. http://dx.doi.org/10.7326/0003-4819-121-12-199412150-00009 [DOI] [PubMed] [Google Scholar]
- Goudsmit E. M., & Howes S. (2008). Pacing: A strategy to improve energy management in chronic fatigue syndrome. Health Psychology Update, 17, 46–52. [Google Scholar]
- Goudsmit E. M., Ho-Yen D. O., & Dancey C. P. (2009). Learning to cope with chronic illness: Efficacy of a multi-component treatment for people with chronic fatigue syndrome. Patient Education and Counseling, 77, 231–236. http://dx.doi.org/10.1016/j.pec.2009.05.015 [DOI] [PubMed] [Google Scholar]
- Goudsmit E. M., Nijs J., Jason L. A., & Wallman K. E. (2012). Pacing as a strategy to improve energy management in myalgic encephalomyelitis/chronic fatigue syndrome: A consensus document. Disability and Rehabilitation, 34, 1140–1147. http://dx.doi.org/10.3109/09638288.2011.635746 [DOI] [PubMed] [Google Scholar]
- Ickmans K., Meeus M., De Kooning M., Lambrecht L., & Nijs J. (2014). Recovery of upper limb muscle function in chronic fatigue syndrome with and without fibromyalgia. European Journal of Clinical Investigation, 44, 153–159. http://dx.doi.org/10.1111/eci.12201 [DOI] [PubMed] [Google Scholar]
- Ickmans K., Meeus M., De Kooning M., Lambrecht L., Pattyn N., & Nijs J. (2014). Can recovery of peripheral muscle function predict cognitive task performance in chronic fatigue syndrome with and without fibromyalgia? Physical Therapy, 94, 511–522. http://dx.doi.org/10.2522/ptj.20130367 [DOI] [PubMed] [Google Scholar]
- Jason L. A., Brown M., & Brown A. (2013). Energy conservation/envelope theory interventions to help patients with myalgic encephalomyelitis/chronic fatigue syndrome. Fatigue: Biomedicine, Health and Behavior, 1, 27–42. http://dx.doi.org/10.1080/21641846.2012.733602 [Google Scholar]
- Johnston S., Brenu E. W., Staines D. R., & Marshall-Gradisnik S. (2013). The adoption of chronic fatigue syndrome/myalgic encephalomyelitis case definitions to assess prevalence: A systematic review. Annals of Epidemiology, 23, 371–376. http://dx.doi.org/10.1016/j.annepidem.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Law M., Baptiste S., Carswell A., McColl M. A., Polatajko H. J., & Pollock N. (2005). Canadian Occupational Performance Measure manual (4th ed.). Toronto, Ontario, Canada: Canadian Occupational Therapy Association. [Google Scholar]
- Lehrer P. (1996). Varieties of relaxation methods and their unique effects. International Journal of Stress Management, 3, 1–15. http://dx.doi.org/10.1007/BF01857884 [Google Scholar]
- Maquet D., Demoulin C., & Crielaard J. M. (2006). Chronic fatigue syndrome: A systematic review. Annales de Réadaptation et de Médecine Physique, 49, 337–347, 418–427. http://dx.doi.org/10.1016/j.annrmp.2006.03.011 [DOI] [PubMed] [Google Scholar]
- McHorney C. A., Ware J. E. Jr., Lu J. F., & Sherbourne C. D. (1994). The MOS 36-Item Short-Form Health Survey (SF–36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical Care, 32, 40–66. http://dx.doi.org/10.1097/00005650-199401000-00004 [DOI] [PubMed] [Google Scholar]
- McHorney C. A., Ware J. E. Jr., & Raczek A. E. (1993). The MOS 36-Item Short-Form Health Survey (SF–36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care, 31, 247–263. http://dx.doi.org/10.1097/00005650-199303000-00006 [DOI] [PubMed] [Google Scholar]
- Nijs J., Aelbrecht S., Meeus M., Van Oosterwijck J., Zinzen E., & Clarys P. (2011). Tired of being inactive: A systematic literature review of physical activity, physiological exercise capacity and muscle strength in patients with chronic fatigue syndrome. Disability and Rehabilitation, 33, 1493–1500. http://dx.doi.org/10.3109/09638288.2010.541543 [DOI] [PubMed] [Google Scholar]
- Nijs J., Mannerkorpi K., Descheemaeker F., & Van Houdenhove B. (2010). Primary care physical therapy in people with fibromyalgia: Opportunities and boundaries within a monodisciplinary setting. Physical Therapy, 90, 1815–1822. http://dx.doi.org/10.2522/ptj.20100046 [DOI] [PubMed] [Google Scholar]
- Nijs J., Paul L., & Wallman K. (2008). Chronic fatigue syndrome: An approach combining self-management with graded exercise to avoid exacerbations. Journal of Rehabilitation Medicine, 40, 241–247. http://dx.doi.org/10.2340/16501977-0185 [DOI] [PubMed] [Google Scholar]
- Nijs J., & Thielemans A. (2008). Kinesiophobia and symptomatology in chronic fatigue syndrome: A psychometric study of two questionnaires. Psychology and Psychotherapy: Theory, Research and Practice, 81, 273–283. http://dx.doi.org/10.1348/147608308X306888 [DOI] [PubMed] [Google Scholar]
- Nijs J., van Eupen I., Vandecauter J., Augustinus E., Bleyen G., Moorkens G., & Meeus M. (2009). Can pacing self-management alter physical behavior and symptom severity in chronic fatigue syndrome? A case series. Journal of Rehabilitation Research and Development, 46, 985–996. http://dx.doi.org/10.1682/JRRD.2009.01.0007 [DOI] [PubMed] [Google Scholar]
- Paul L., Wood L., Behan W. M., & Maclaren W. M. (1999). Demonstration of delayed recovery from fatiguing exercise in chronic fatigue syndrome. European Journal of Neurology, 6, 63–69. http://dx.doi.org/10.1046/j.1468-1331.1999.610063.x [DOI] [PubMed] [Google Scholar]
- Pemberton S., & Cox D. L. (2014). Experiences of daily activity in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) and their implications for rehabilitation programmes. Disability and Rehabilitation, 36, 1790–1797. http://dx.doi.org/10.3109/09638288.2013.874503 [DOI] [PubMed] [Google Scholar]
- Persson D., Andersson I., & Eklund M. (2011). Defying aches and revaluating daily doing: Occupational perspectives on adjusting to chronic pain. Scandinavian Journal of Occupational Therapy, 18, 188–197. http://dx.doi.org/10.3109/11038128.2010.509810 [DOI] [PubMed] [Google Scholar]
- Poppe C., Petrovic M., Vogelaers D., & Crombez G. (2013). Cognitive behavior therapy in patients with chronic fatigue syndrome: The role of illness acceptance and neuroticism. Journal of Psychosomatic Research, 74, 367–372. http://dx.doi.org/10.1016/j.jpsychores.2013.02.011 [DOI] [PubMed] [Google Scholar]
- Prins J. B., Bleijenberg G., Bazelmans E., Elving L. D., de Boo T. M., Severens J. L., . . . van der Meer J. W. M. (2001). Cognitive behaviour therapy for chronic fatigue syndrome: A multicentre randomised controlled trial. Lancet, 357, 841–847. http://dx.doi.org/10.1016/S0140-6736(00)04198-2 [DOI] [PubMed] [Google Scholar]
- Prochaska J. O., & Velicer W. F. (1997). The transtheoretical model of health behavior change. American Journal of Health Promotion, 12, 38–48. http://dx.doi.org/10.4278/0890-1171-12.1.38 [DOI] [PubMed] [Google Scholar]
- Rietberg M. B., Van Wegen E. E. H., & Kwakkel G. (2010). Measuring fatigue in patients with multiple sclerosis: Reproducibility, responsiveness and concurrent validity of three Dutch self-report questionnaires. Disability and Rehabilitation, 32, 1870–1876. http://dx.doi.org/10.3109/09638281003734458 [DOI] [PubMed] [Google Scholar]
- Rothman K. J. (1990). No adjustments are needed for multiple comparisons. Epidemiology (Cambridge, Mass.), 1, 43–46. http://dx.doi.org/10.1097/00001648-199001000-00010 [PubMed] [Google Scholar]
- Shepherd C. (2001). Pacing and exercise in chronic fatigue syndrome. Physiotherapy, 87, 395–396. http://dx.doi.org/10.1016/S0031-9406(05)65457-0 [Google Scholar]
- Taylor R. R. (2004). Quality of life and symptom severity for individuals with chronic fatigue syndrome: Findings from a randomized clinical trial. American Journal of Occupational Therapy, 58, 35–43. http://dx.doi.org/10.5014/ajot.58.1.35 [DOI] [PubMed] [Google Scholar]
- Taylor R. R., O’Brien J., Kielhofner G., Lee S. W., Katz B., & Mears C. (2010). The occupational and quality of life consequences of chronic fatigue syndrome/myalgic encephalomyelitis in young people. British Journal of Occupational Therapy, 73, 524–530. http://dx.doi.org/10.4276/030802210X12892992239233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L., Shamseer L., Altman D. G., Weeks L., Peters J., Kober T., . . . Moher D. (2012). Consolidated Standards of Reporting Trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database of Systematic Reviews (Online), 11, MR000030 http://dx.doi.org/10.1002/14651858.MR000030.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme S., Crombez G., Van Houdenhove B., Mariman A., & Michielsen W. (2006). Well-being in patients with chronic fatigue syndrome: The role of acceptance. Journal of Psychosomatic Research, 61, 595–599. http://dx.doi.org/10.1016/j.jpsychores.2006.04.015 [DOI] [PubMed] [Google Scholar]
- van der Werf S. P., Prins J. B., Vercoulen J. H., van der Meer J. W., & Bleijenberg G. (2000). Identifying physical activity patterns in chronic fatigue syndrome using actigraphic assessment. Journal of Psychosomatic Research, 49, 373–379. http://dx.doi.org/10.1016/S0022-3999(00)00197-5 [DOI] [PubMed] [Google Scholar]
- Vercoulen J., Alberts M., & Bleijenberg G. (1999). The Checklist Individual Strength (CIS). Gedragstherapie, 32, 131–136. [Google Scholar]
- Vercoulen J. H., Bazelmans E., Swanink C. M., Fennis J. F., Galama J. M., Jongen P. J., . . . Bleijenberg G. (1997). Physical activity in chronic fatigue syndrome: Assessment and its role in fatigue. Journal of Psychiatric Research, 31, 661–673. http://dx.doi.org/10.1016/S0022-3956(97)00039-3 [DOI] [PubMed] [Google Scholar]
- Wallman K. E., Morton A. R., Goodman C., Grove R., & Guilfoyle A. M. (2004). Randomised controlled trial of graded exercise in chronic fatigue syndrome. Medical Journal of Australia, 180, 444–448. [DOI] [PubMed] [Google Scholar]
- Ware J., Snow K., Kosinski M., & Gandek B. (1993). SF–36 Health Survey: Manual and interpretation guide. Boston: Health Institute, New England Medical Center. [Google Scholar]
- White P. D., Goldsmith K. A., Johnson A. L., Potts L., Walwyn R., DeCesare J. C., . . . Sharpe M.; PACE Trial Management Group. (2011). Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomised trial. Lancet, 377, 823–836. http://dx.doi.org/10.1016/S0140-6736(11)60096-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg J. F., Knoop H., Prins J. B., & Bleijenberg G. (2011). Does a decrease in avoidance behavior and focusing on fatigue mediate the effect of cognitive behavior therapy for chronic fatigue syndrome? Journal of Psychosomatic Research, 70, 306–310. http://dx.doi.org/10.1016/j.jpsychores.2010.12.011 [DOI] [PubMed] [Google Scholar]