Abstract
β-1,2-mannosylation of Candida albicans glycoconjugates has been investigated through the identification of enzymes involved in the addition of β-1,2-oligomannosides (β-Mans) to phosphopeptidomannan and phospholipomannan. β-1,2-oligomannosides are supposed to have virulence properties that they confer to these glycoconjugates. In a previous study, we showed that cell wall mannoproteins (CWMPs) harbor β-Mans in their O-mannosides; therefore, we analyzed their biosynthesis and impact on virulence. In this study, we demonstrate that O-mannans are heterogeneous and that α-mannosylated O-mannosides, which are biosynthesized by Mnt1 and Mnt2 α-1,2-mannosyltransferases, can be modified with β-Mans but only at the nonreducing end of α-1,2-mannotriose. β-1,2-mannosylation of this O-mannotriose depends on growth conditions, and it involves 2 β-1,2-mannosyltransferases, Bmt1 and Bmt3. These Bmts are essential for β-1,2-mannosylation of CWMPs and expression of β-Mans on germ tubes. A bmt1Δ mutant and a mutant expressing no β-Mans unexpectedly disseminated more in BALB/c mice, whereas they had neither attenuated nor enhanced virulence in C57BL/6 mice. In galectin (Gal)3 knockout mice, the reference strain was more virulent than in C57BL/6 mice, suggesting that the β-Mans innate receptor Gal3 is involved in C. albicans fitness during infection.
Keywords: β-1,2-oligomannosides; Candida albicans; fungal virulence; mannosyltransferase; O-mannosylation
Candida albicans is a successful pathogen in immunosuppressed patients, leading to frequent nosocomial infections with high mortality rate [1]. The fungal cell wall and its components are the natural and dynamic interface with the host, forming a moving target in terms of host recognition mechanisms [2]. Cell wall glycans are immunologically active components, which are present either as polysaccharides or glycoconjugates. Amongst its glycan diversity, C. albicans expresses β-1,2-oligomannosides (β-Mans), which are unusual as only evidenced in few nonmammalian eukaryotes and prokaryotes [3]. β-Mans are present in the N-glycan of the cell wall outer layer phosphopeptidomannan (PPM) [4]. Within this molecule, they are linked to a phosphomannose and at the nonreducing end of α-1,2-oligomannosides (Figure 1A). β-Mans are also associated to a cell wall glycolipid named phospholipomannan (PLM) (Figure 1B) [5]. β-Mans biosynthesis involves β-1,2-mannosyltransferases (Bmts), which have distinct substrate specificities and catalyze specific steps of β-1,2-mannosylation [3].
Figure 1.
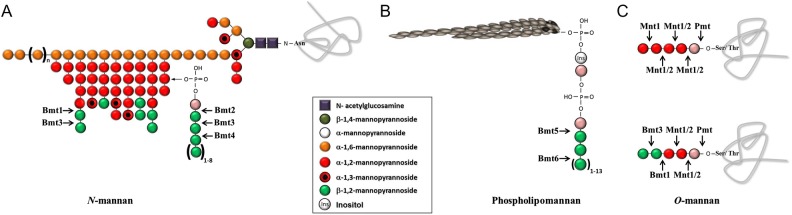
Schematic representation of β-1,2-mannosyltransferase (Bmt)1–6 activities on Candida albicans cell-wall glycoconjugates. (A) β-1,2-oligomannosides (β-Mans), expressed at the nonreducing end of α-1,2-Mans from N-mannan, are biosynthesized by Bmt1 and Bmt3. The Bmt2, Bmt3, and Bmt4 sequentially add β-mannose on phosphomannose linked to α-1,2-Mans of N-mannan. (B) Phospholipomannan β-1,2-mannosylation involves Bmt5 and Bmt6, which transfer β-mannose on the inositol phosphate added to mannose-inositol-phosphoceramide. (C) This illustrates the detailed structure and biosynthetic pathway of the 2 O-mannopentamers: the mannopentaose previously described (top panel) [14, 24] and the mannopentaose partly biosynthesized by Bmt1 and Bmt3 as deduced from this study (bottom panel). The arrows indicate where the enzymes act and which β-mannose is added.
β-Mans are involved in the interplay between C. albicans and its host, for example, mediating adhesion of C. albicans to macrophages and epithelial cells [6, 7], inducing cytokine production [8], and generating protective antibodies against vaginal and disseminated candidiasis [9, 10]. However, very little is known about their relative and respective functions inside the wall. Biological activity of β-Mans depends on their carrier molecule. PPM and PLM phosphomannosides both display β-Mans, but they have distinct immune-modulatory properties [11–13].
In a previous study, we revealed the presence of β-Mans epitopes on cell wall mannoproteins (CWMPs) O-mannan. O-mannosylation is an essential glycosylation process for protein modification, but it is also important for C. albicans virulence [14]. Therefore, we determined which Bmts are responsible for addition of β-Mans on O-mannosides and generated the appropriate mutant to analyze (1) the contribution of N- and O-mannans to the surface expression of β-Mans and (2) the impact of these β-Mans on Candida virulence. We additionally investigated the global role of β-Mans on Candida virulence by generating a mutant expressing no β-Mans.
METHODS
Fungal Strains and Growth Conditions
All strains used are listed in Table 1. Yeast cells were grown in YPD broth (1% yeast extract, 2% bactopeptone, 2% dextrose [Difco]) and grown at 28°C or 37°C. Hyphae were obtained after inoculation of RPMI 1640 medium (Invitrogen) with yeast cells and incubation for 3 hours at 37°C. For animal experiments, cells were grown in YPD at 28°C for 16 hours.
Table 1.
Candida albicans Strains Used in This Study
| Strain | Parental Strain | Genotype | Reference |
|---|---|---|---|
| SC5314 | Wild type | [50] | |
| BWP17 | RM1000 | arg4::hisG/arg4::hisG;his1::hisG/his1::hisG;ura3Δ::limm434/ura3Δ::limm434, | [51] |
| BWP17 + CIp10 | BWP17 | BWP17 ::RPS10/rps1Δ::CIp10 | This study |
| bmt1Δ + CIp10 | BWP17 | bmt1Δ::ARG4/bmt1Δ::HIS1, RPS10/rps1Δ::CIp10 | [21] |
| bmt1Δ + CIp10-BMT1 | bmt1Δ | As bmt1Δ but RPS10/rps1Δ::CIp10-BMT1 | [21] |
| bmt2Δ + CIp10 | BWP17 | bmt2Δ::ARG4/bmt2Δ::HIS1, RPS10/rps1Δ::CIp10 | [21] |
| bmt3Δ + CIp10 | BWP17 | bmt3Δ::ARG4/bmt3Δ::HIS1, RPS10/rps1Δ::CIp10 | [21] |
| bmt3Δ + CIp10-BMT3 | Bmt3Δ | As bmt3Δ but RPS10/rps1Δ::CIp10-BMT3 | [21] |
| bmt4Δ + CIp10 | BWP17 | bmt4Δ::ARG4/bmt4Δ::HIS1, RPS10/rps1Δ::CIp10 | [22] |
| bmt5Δ + CIp10 | BWP17 | bmt5Δ::ARG4/bmt5Δ::HIS1, RPS10/rps1Δ::CIp10 | [22] |
| bmt6Δ + CIp10 | BWP17 | bmt6Δ::ARG4/bmt6Δ::HIS1, RPS10/rps1Δ::CIp10 | [21] |
| bmt7Δ + CIp10 | BWP17 | bmt7Δ::ARG4/bmt7Δ::HIS1, RPS10/rps1Δ::CIp10 | [21] |
| bmt8Δ + CIp10 | BWP17 | bmt8Δ::ARG4/bmt8Δ::HIS1, RPS10/rps1Δ::CIp10 | [21] |
| bmt9Δ + CIp10 | BWP17 | bmt9Δ::ARG4/bmt9Δ::HIS1, RPS10/rps1Δ::CIp10 | [21] |
| CAI-4 | CAF2-1 | ura3Δ::λimm434/ura3::λimm434 | [52] |
| CAI-4 + CIp10 | CAI-4 | RPS10::rps10CIp10-URA3 | This study |
| bmt1Δ | CAI-4 | As CAI-4 but bmt1Δ::dpl200/bmt1Δ::dpl200 | This study |
| bmt1Δ + CIp10 | bmt1Δ | As bmt1Δ but bmt1Δ::dpl200/bmt1Δ::dpl200, RPS10/rps1Δ::CIp10 | This study |
| bmt2Δ | CAI-4 | As CAI-4 but bmt2Δ::dpl200/bmt2Δ::dpl200 | This study |
| bmt2Δ + CIp10 | bmt2Δ | As bmt2Δ but bmt2Δ::dpl200/bmt2Δ::dpl200, RPS10/rps1Δ::CIp10 | This study |
| bmt2 Δ/bmt5Δ | bmt2Δ | As CAI-4 but bmt2Δ::dpl200/bmt2Δ::dpl200,bmt5Δ::dpl200/bmt5Δ::dpl200 | This study |
| bmt1Δ/bmt2 Δ/bmt5Δ + CIp10 | bmt2 Δ/bmt5Δ | As CAI-4 but bmt1Δ::dpl200/bmt1Δ::dpl200,bmt2Δ::dpl200/bmt2Δ::dpl200, bmt5Δ::dpl200/bmt5Δ::dpl200, RPS10/rps1Δ::CIp10 | This study |
| bmt1Δ + CIp10-BMT1 | bmt1Δ | As bmt1Δ but RPS10/rps1Δ::CIp10-BMT1 | This study |
| NGY112 | NGY111 | As CAI-4 but mnt1-mnt2Δ::hisG/mnt1-mnt2Δ::hisG | [14] |
| mnt1/mnt2Δ + CIp10 | NGY112 | As NGY112 but RPS10/rps10Δ::CIp10 | [14] |
| CAI-4 + CIp10-6xHIS-HWP1 | CAI-4 | As CAI-4 but HWP1/hwp1Δ::CIp10-6xHIS-HWP1 | This study |
| bmt1Δ + CIp10-6xHIS-HWP1 | bmt1Δ | As bmt1Δ but HWP1/hwp1Δ::CIp10-6xHIS-HWP1 | This study |
| bmt2Δ + CIp10-6xHIS-HWP1 | bmt2Δ | As bmt2Δ but HWP1/hwp1Δ::CIp10-6xHIS-HWP1 | This study |
| mnt1Δ/mnt2Δ + CIp10-6xHIS-HWP1 | NGY112 | As NGY112 but HWP1/hwp1Δ::CIp10-6xHIS-HWP1 | This study |
Lectin and Monoclonal Antibody
Biotinylated concanavalin A ([ConA] Sigma-Aldrich) detects terminal α-d-mannosyl residues. Monoclonal antibodies (mAbs) 5B2 (rat-mouse immunoglobulin [Ig] M) is specific for β-Mans with a mannobiose as minimal epitope [15]. The mAb 16B1 is a mouse IgG specific for hyphal wall protein 1 (Hwp1) [16].
Whole-Cell Protein Extraction and Western Blotting
Total extracts were obtained and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as previously described [16, 17]. Membranes were probed with mAb 5B2 and then an alkaline phosphatase-conjugated anti-rat IgM diluted both 1:2000. Enzyme activity was detected with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate substrate (Promega). For lectin staining, membranes were incubated with biotinylated ConA and then horseradish peroxidase-labeled streptavidin (AbCam) diluted both 1:1000. Peroxidase activity was detected with diamidobenzidine (Sigma-Aldrich).
Whole-Cell Hydrolysis and Fluorophore-Assisted-Carbohydrate-Electrophoresis Analysis
Cells were boiled for 1 hour in 10 mM HCl. After cooling and neutralization, the supernatants containing phosphomannosides were harvested by centrifugation. Remaining cells were washed and then incubated for 16 hours at room temperature in 100 mM NaOH. After neutralization, O-mannosides were harvested by centrifugation. Both supernatants were filtered through a 0.22 µm polyvinylidene fluoride filter (Millipore).
Hydrolysates were tagged with 0.15 M 8-amino-naphthalene-1,3,6-trisulfonate ([ANTS] Sigma-Aldrich) as previously described [18]. Electrophoresis of ANTS-labeled oligomannosides was performed on 25%–30% acrylamide gels. Purified oligomannosides from PPM were tagged with ANTS and used as carbohydrate standards. Gels were acquired with the Gel Doc 2000 apparatus (Bio-Rad).
Generation of Candida albicans Cells Expressing 6xHis Hwp1
Primers HWP1-1/HWP1-2 (Table 2) were designed to amplify the promoter and the HWP1 open reading frame (orf19.1321; http://www.candidagenome.org/) missing its last 75 nucleotides. The amplified fragment was cloned in pYES2.1-TOPO (Invitrogen) to fuse the gene to DNA sequence coding for 6xHis. A fragment of pYES2.1-TOPO-6xHIS-HWP1 was amplified using the primers HWP1-1/V5-His (Table 2) and cloned in pCR2.1-TOPO. The sequence coding for 6xHis-Hwp1 was released after digestion with NotI and SacI, gel purified, and ligated into dephosphorylated CIp10. CIp10-6xHIS-HWP1 was linearized with NheI and integrated into HWP1 promoter of Candida cells. Correct integration of the plasmid was confirmed by Southern blot analysis with probes obtained with primers probeFwd/probeRev (Table 2).
Table 2.
Candida albicans Primers Used in This Study
| Primers | Sequences |
|---|---|
| BMT1 knockout Fwd | CTAAAAAAGGTAAACGAACAATTATATTCCCAAATAATTTCAATCATGTTCATGATCATAAAGGTTCTTATATGATGAAAGTTTTCCCAGTCACGACGTT |
| BMT1 knockout Rev | TCTTTTTCAATTGTCCAAGATGAAATACCATTGGGGATAATTAAATTATAATGTTCACATAAACCTTTATCTAAATACCATGTGGAATTGTGAGCGGATA |
| BMT2 knockout Fwd | CCTTCAGCCCTGTTTGGAAAAGTGTACAAAATAGGCACAAAGTTAAACTTTACACTACTTGCCCTTTGCTTACTTTTGGCATTTTCCCAGTCACGACGTT |
| BMT2 knockout Rev | TAAACCCATAATCATTCAATTCTAATAATTTCAGGTTTGGATCATCAAATAGTATTGACTTTAATAATCCTTTGATATGATGTGGAATTGTGAGCGGATA |
| BMT5 knockout Fwd | GCAGTACCGATTTGCCCCAAAGTCAATATTCACATTTGTGTTTCTATGTTTTGTTGCAATAGTTGTCATAATATCCACATCTTTTCCCAGTCACGACGTT |
| BMT5 knockout Rev | CTGTTTGTTTGCAATAAATATTCAGATAATATCGACTTTAGTATTCCCTTCATGTGTATAATATCAACTGTAGAATCAGATGTGGAATTGTGAGCGGATA |
| probe BMT1 Fwd | ATACAATCATTTAGTCATCAA |
| probe BMT1 Rev | ATACTGGGATAGGGGCGATT |
| probe BMT2 Fwd | GAGAAATGTGGCTGTGGTGA |
| probe BMT2 Rev | TGTTTTTCGGGACCGTATGT |
| probe BMT5 Fwd | GACTCGCCGTTATTGGACAT |
| probe BMT5 Rev | ATTGGCACACCAAAATCCAT |
| probe URA3 Fwd | GCCTCACCAGTAGCACAACGATTA |
| probe URA3 Rev | GCATTCCAACCAGCATCTCTATACC |
| probe HWP1 Fwd | CAACTCCAGCTACTACTCCA |
| probe HWP1 Rev | GTCATTTCAGGAGCAGGAGC |
| probe RPS1 Fwd | ATGGCTGTCGGTAAAAACAAG |
| probe RPS1 Rev | AAAGCCAATAATGAACCCAAG |
| probe ACT1 Fwd | ACCGAAGCTCCAATGAATCCA |
| probe ACT1 Rev | GGATGGACCAGATTCGTCGTA |
| HWP1-1 | CTCGAGTGTACGTAGCTTCTATAG |
| HWP1-2 | TTCAAATGTAGAAATAGGAGC |
| V5-HIS | CTCGAGTGCAGGGCCGCAGCTTGC |
Abbreviations: Fwd, forward; Rev, reverse.
Production and Purification of Secreted 6xHis Hwp1
Cells were grown for 3 hours at 37°C in hyphae-inducing medium, M199 (Invitrogen). Supernatants were cooled and filtered through GF-F membranes (Millipore). 6xHis-Hwp1 was purified using His-GraviTrap column (GE Healthcare Life Sciences) following the manufacturer's information. After dialysis using PD-10 Desalting Columns (GE Healthcare Life Sciences), 6xHis-Hwp1 was analyzed by Western blotting.
Enzymatic Treatments
For α-mannosidase treatment, glycans were solubilized in citrate buffer 50 mM, pH 4.6, and incubated for 8 hours at room temperature with 0.7 units of α-mannosidase (Sigma-Aldrich) from Canavalia ensiformis (jack bean), specific for α-1,2, α-1,3, and α-1,6 terminal mannose residues.
For PNGase F treatment, proteins samples were suspended in sodium phosphate 0.5 M, pH7.5 and incubated with peptide-N-glycosidase F following the manufacturer's recommendation (Sigma-Aldrich).
Indirect Immunofluorescence Assays
Hyphae were fixed with formalin on reaction wells of microscope slides (Thermo Scientific). Wells were blocked with phosphate-buffered saline containing 5% bovine serum albumin and incubated for 1 hour at 37°C with mAb 5B2 or biotinylated ConA diluted 1:500. Wells were washed and incubated with the corresponding RPE-conjugated anti-Ig or fluorescein isothiocyanate-conjugated streptavidin diluted 1:100, respectively.
Slides were examined under a Leica fluorescence microscope. Images were captured and analyzed with a Leica DC camera and its software.
Gene Disruption
All mutants were generated in the CAI-4 background using the mini ura-blaster method as previously described [19] using primers BMTknockoutFwd/BMTknockoutRev (Table 2). Selection URA3 marker was recycled with 5-fluoroorotic acid. Disruption of both alleles for each gene was checked by Southern blot with probes obtained with primers probeFwd/probeRev (Table 2). A BMT1-reconstituted strain was constructed by cloning the BMT1 region (−652 to +870) into CIp10 [20] and transforming Ura–bmt1Δ strain with the resulting linearized CIp10-BMT1. Linearized CIp10 was transformed into CAI-4 and the different mutants. Single integration of these plasmids at the RPS1 locus in the same allele was confirmed by Southern blot analysis with probes obtained with primers probeFwd/probeRev (Table 2).
Animal Experiments
All experiments, approved by the national ethics committee (Reference no. 00374.03), were conducted following the French Guide for the Care and Use of Laboratory Animals and the Guidelines of the European Union.
For survival assays, cells were suspended in sterile physiological saline. For each C. albicans strain, 5 female BALB/c mice (8–10 weeks old) were given intravenous injections of 2.105 colony-forming units (cfu) into the lateral tail vein. Mice were monitored daily and humanely killed when they showed signs of distress.
For determination of organ fungal burdens, cells were suspended in sterile physiological saline. For each C. albicans strain, 5 female BALB/c mice (8–10 weeks old) or 4 C57BL/6 or gal3−/− female mice (8–10 weeks old) were given intraperitoneal injections of 5.107 cfu. Three days after injection, mice were sacrificed and organs (spleen, kidneys and liver) were removed aseptically, weighed, homogenized, and suspended in 5 mL sterile water. Homogenates were plated on Sabouraud chloramphenicol agar and incubated for 24 hours at 37°C. Colony-forming units were counted and reported to the organ's weight.
The results shown are from 3 independent experiments. Survival data were analyzed by the Kaplan-Meier survival analysis. Fungal burdens were analyzed by the Kruskal-Wallis test followed by post hoc testing using the unpaired Mann–Whitney U test. P values <.05 were considered significant.
RESULTS
β-1,2-mannosyltransferases 1 and 3 Are Involved in O-Mannosides β-1,2-Mannosylation
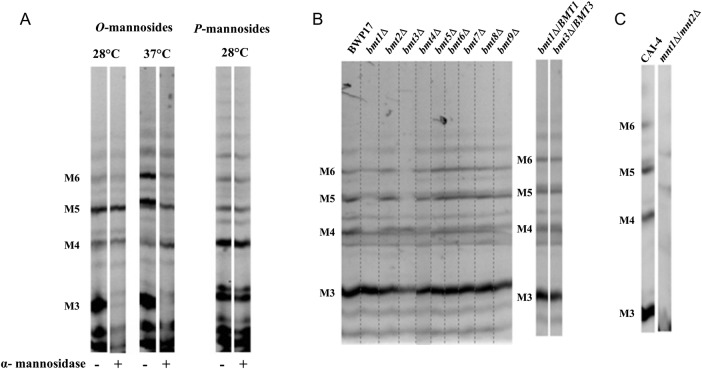
O-mannosides released from whole yeast cells were analyzed by fluorophore-assisted-carbohydrate-electrophoresis (FACE), which separates oligosaccharides according to their degree of polymerization, to the type of saccharide and to their glycosidic linkage [18]. O-mannoside residues depended upon growth temperature (Figure 2A). The O-mannopentaose migrated as a doublet; the lower band was the main mannopentaose recovered at 28°C. This residue and the mannotetraose were the only O-mannosides resistant to α-mannosidase digestion, suggesting that they may contain terminal β-Mans. To confirm the presence of β-Mans in C. albicans O-mannan and to characterize their biosynthesis, we applied the strategy used previously to identify the 6 Bmts involved in the β-1,2-mannosylation of PPM and PLM [21, 22] (Figure 1A and B). O-mannosides from mutants lacking one of the 9 Bmts and their reference strain, grown at 28°C, were analyzed by FACE. All bmtsΔ mutants have similar O-mannosides than their reference strain, except bmt1Δ and bmt3Δ mutants (Figure 2B). These mutants display truncated O-mannosides with an accumulation of a mannotriose and a mannotetraose, respectively. Both mutants were lacking the lower O-mannopentaose band, whereas only the bmt1Δ mutant did not express the O-mannotetraose. Normal O-mannosides were recovered when wild-type copies of BMT1 and BMT3 were reintroduced into the corresponding deletion strains (Figure 2B). The Bmt1 and Bmt3 certainly added the first and the second β-mannose onto the α-linked O-mannotriose, respectively. These 2 Bmts used a substrate biosynthesized by a protein mannosyltransferase isoform [23] and by α1,2-mannosyltransferases (Mnt) 1 and/or 2 [14, 24] and formed a heteropolymer (Figure 1C). As expected, FACE analysis of O-mannosides from the double mnt1Δ/mnt2Δ mutant revealed that, unlike the reference strain, they contained neither a mannotetraose nor a mannopentaose (Figure 2C). Additional bands detected in mnt1Δ/mnt2Δ mutant were certainly nonmannosyl products as previously reported [14]. Reactivity to mAb 5B2 was reduced on CWMPs of mnt1Δ/mnt2Δ mutant compared with the control strain, whereas α-Mans levels were comparable between the 2 strains (Figure 3A).
Figure 2.
Fluorophore-assisted-carbohydrate-electrophoresis (FACE) analysis of β-eliminated O-mannosides of Candida albicans reference strains and mutants. (A) O-mannosides and phosphomannosides (P-mannosides) released by β-elimination and mild acid hydrolysis, respectively, from whole cells of strain SC5314 grown at 28°C and 37°C were analyzed by FACE. Prior to the electrophoresis, α-mannosidase treatment was performed or not to remove terminal α-1,2, α-1,3, and α-1,6 mannosyl residues. (B and C) O-mannosides from mutants lacking 1 of the 9 β-1,2-mannosyltransferases (Bmts), mutants lacking 2 enzymes involved in cell wall O-mannosylation, Mnt1 and Mnt2, and their respective reference strain, BWP17 and CAI-4, respectively, were analyzed by FACE. All of the strains were grown at 28°C before their O-mannosides were released by β-elimination treatment. All the mutants and their reference strains are Ura+ with CIp10 integrated in the same RPS1 allele. Different carbohydrate standards were used to evaluate the monomer number in the oligomannoside chains. Abbreviations: M3, mannotriose; M4, mannotetraose; M5, mannopentaose; M6, mannohexaose; Mnt, α1,2-mannosyltransferase.
Figure 3.
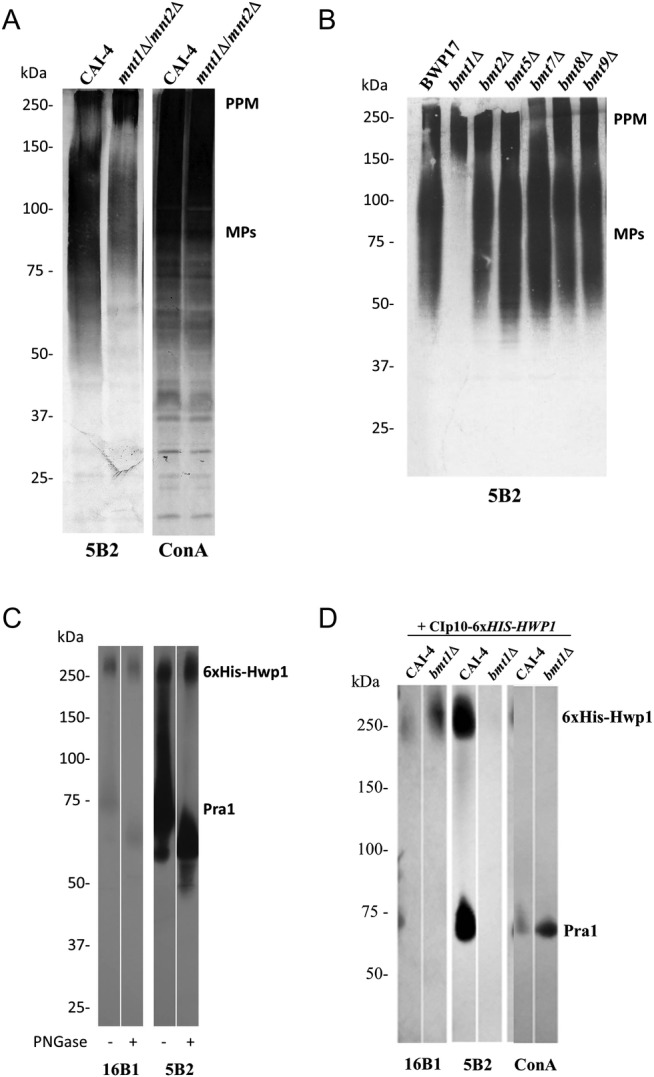
Mapping of β-1,2-oligomannosides epitopes on yeast and hyphal mannoproteins. (A and B) Western blots of whole-cell extracts from different reference strains (CAI-4 and BWP17) and mutants (mnt1Δ/mnt2Δ and bmtsΔ) grown at 28°C were stained with anti-β-1,2-oligomannosides monoclonal antibody (mAb) 5B2 (A and B) and lectin concanavalin A (ConA), specific for terminal α-mannosides (A). Phosphopeptidomannan (PPM) and cell wall mannoproteins (CWMPs) are indicated. (C) Six His-tagged recombinant Hwp1 (6xHis-Hwp1) was produced and secreted in the culture supernatant by CAI-4 strain. Recombinant Hwp1 was purified on nickel column together with a zinc-binding protein, Pra1. The 2 purified hyphal mannoproteins were analyzed by Western blot with mAbs 16B1, specific for Hwp1, and 5B2. Before separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the proteins were treated or not with PNGase F. (D) The 6xHis-Hwp1 and Pra1 were expressed by CAI-4 strain and bmt1Δ mutant, purified on nickel column, and analyzed by Western blot with mAbs 16B1 and 5B2 and lectin ConA. The 2 proteins are indicated. All the mutants and their reference strains are Ura+ with CIp10 integrated in the same RPS1 allele.
BMT1 Deletion Dramatically Affects Mannoproteins β-1,2-Mannosylation
In contrast to PPM, β-Mans epitopes were not detected on CWMPs from the bmt1Δ mutant, whereas CWMPs from the other bmtΔ mutants were β-1,2-mannosylated like the reference strain (Figure 3B). Enhanced staining was obtained for the bmt1Δ mutant with ConA compared with the other strains (data not shown). This was likely to be due to the unmasking of terminal α-mannosyl. These data reveal that CWMPs and PPM are glycosylated in a different manner and that CWMPs β-1,2-mannosylation is only initiated by Bmt1.
The β-1,2-mannosylation of a highly O-glycosylated CWMP, the Hwp1 [25], was then studied. After replacement of the glycosylphosphatidylinositol signal sequence with a 6xHis tag, recombinant Hwp1 was purified from culture supernatants of CAI-4 and bmt1Δ strains by affinity chromatography (Figure 3B). CAI-4 6xHis-Hwp1 was assessed by Western blot analysis using mAb 16B1 (Figure 3C). Protein staining showed that 6xHis-Hwp1 copurified with another protein of 75 kDa (data not shown). Mass spectrometry analysis of this protein, which was performed by the SICaPS platform of IMAGIF (www.imagif.cnrs.fr), confirmed that it was not a digestion product of Hwp1 but was the hyphal cell wall protein Pra1 (pH Regulated Antigen 1) [26]. The Pra1 is secreted by hyphal cells [27], and thus it was not surprising to recover it from culture supernatants. Furthermore, Pra1 is a zinc-binding protein [28], with affinity for other divalent cations, which may explain why Pra1 copurified on the nickel column. Western blot analysis with mAb 5B2 showed that similar to Hwp1, Pra1 was β-1,2-mannosylated; however, in contrast to Hwp1 [16], Pra1 had β-Mans epitopes in both O- and N-glycans (Figure 3C). PNGase treatment of Pra1 resulted in a clear molecular weight shift of this protein (Figure 3C) and a reduction in mAb 5B2 specificity.
Deletion of BMT1 deprived 6xHis-Hwp1 and Pra1 of their β-Mans epitopes (Figure 3D). Similar results were obtained with other anti-β-Mans antibodies (data not shown). Staining with mAb 16B1 and ConA confirmed that CAI-4 and bmt1Δ expressed approximately equal amounts of proteins. Taken together, these results suggest that, as for yeast CWMPs, initiation of β-Mans expression on hyphal CWMPs is dependent on Bmt1.
β-1,2-mannosyltransferase 1 Is Responsible for Surface Expression of Hyphal β-1,2-oligomannosides
We showed previously that single BMT deletions have no effect on β-Mans surface expression [21]. Considering that β-Mans biosynthesis is initiated by at least 3 Bmts, it is not surprising that single deletion has no impact on surface expression of β-Mans. Phosphopeptidomannan differs between yeast and hyphal cells [29], with reduced amounts of β-Mans in PPM phosphomannosides of hyphal cells. Therefore, we assessed whether deletion of BMT1 would affect exposure of β-Mans on hyphae. Staining of hyphal cells from selected strains with mAb 5B2 confirmed that, compared with CAI-4, the bmt1Δ mutant expressed β-Mans on the mother cells but not on the lateral walls of germ tubes (Figure 4). Expression of β-Mans in germ tubes was recovered by the reintroduction of BMT1. In contrast, bmt2Δ and bmt5Δ mutants expressed β-Mans at the surface of both germ tubes and mother cells (data not shown). This immunofluorescence confirmed that, in contrast to mother cells, germ tubes do not express β-Mans from N-glycan phosphomannosides and PLM (Figure 1A and B) on their surface. The mnt1Δ/mnt2Δ mutant expressed, even if at a reduced level, β-Mans on germ tubes (Figure 4), certainly at the nonreducing ends of N-glycans (Figure 1A). These data show that the glycosylation motifs on the cell wall surface vary between germ tubes and mother cells. The β-Mans expressed on the germ tube surface were therefore synthesized by Bmt1 and Bmt3 and correspond to β-Mans from O-mannans and N-mannans at the nonreducing end of α-1,2-Mans (Figure 1A and C).
Figure 4.
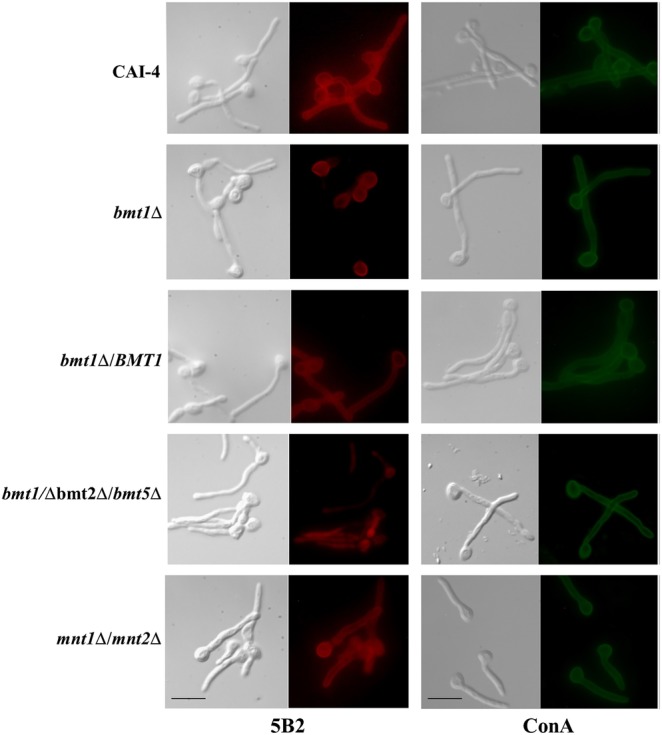
Mapping of β-1,2-oligomannosides epitopes expressed at the surface of hyphae. Indirect immunofluorescence assays were performed on hyphae from bmt1Δ, bmt1Δ/BMT1, bmt1Δ bmt2Δ bmt5Δ, and mnt1Δ mnt2Δ mutants and their reference strain, CAI-4, using anti-β-1,2-oligomannosides monoclonal antibody (mAb) 5B2 and lectin concanavalin A (ConA). Evan's blue was used as counterstain to decrease the nonspecific background fluorescence and to visualize nonstained cells, which display then red fluorescence in their cytoplasm (bmt1Δ/bmt2Δ/bmt5Δ mutant with mAb 5B2). For each strain, a micrograph of phase contrast and a fluorescence micrograph with the mentioned antibody or lectin are shown. Scale bars = 10 µm. All of the mutants and their reference strains are Ura+ with CIp10 integrated in the same RPS1 allele.
BMT1 Deletion Affects Candida albicans Virulence
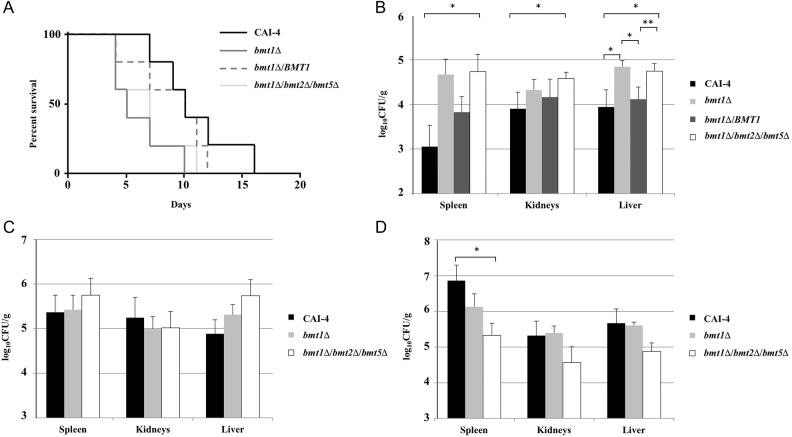
To investigate the possible involvement of β-Mans to O-mannosides virulence [14, 30], we analyzed the survival of the bmt1Δ mutant in a murine model of invasive candidosis [31]. BALB/c mice were chosen because they have been widely used to identify factors that contribute to C. albicans virulence. Mice received a tail vein injection of either bmt1Δ mutant or its reference strain for mortality studies. Deletion of BMT1 reduced the survival of BALB/c mice, suggesting that the bmt1Δ mutant displays hypervirulence (Figure 5A). Similar to β-Mans epitope biosynthesis, wild-type virulence was partially recovered by complementing the mutant with a single copy of BMT1. To analyze the global role of β-Mans on virulence, we generated a strain devoid of β-Mans. We deleted BMT2 and BMT5, coding for Bmts that initiate the synthesis of β-Mans on PPM phosphomannosides and PLM (Figure 1A and B) in the bmt1Δ mutant background. We confirmed by Western blot analysis (data not shown) and immunofluorescence (Figure 4) that the bmt1Δ bmt2Δ bmt5Δ mutant had no β-Man in its cell wall. This mutant was as virulent as the bmt1Δ mutant (Figure 5A). To rule out faster elimination of reference strain by blood cells or serum components, we used a second murine model of invasive candidosis. Intraperitoneal injection of C. albicans has been used in different studies as a systemic infection model to measure tissue dissemination of the yeast [32–34]. Comparable results to the survival assay were obtained when spleen, kidneys, and liver fungal burdens were determined following BALB/c mice intraperitoneal infection (Figure 5B). Organs, particularly spleen and liver, contained more fungal cells after mice challenge with bmt1Δ mutant than reference strain (Figure 5B). All independent experiments gave similar results, and even with the strong heterogeneity of the mutant fungal burdens between mice, the differences between the bmt1Δ mutant and the reference strain were significant in the liver. Restoration of the native BMT1 gene into bmt1Δ mutant attenuated significantly its dissemination. The differences between the bmt1Δ bmt2Δ bmt5Δ mutant and the wild-type strain were significant in the different organs analyzed.
Figure 5.
Virulence assays are shown. (A) Cumulative mortality of mice given injections of 4 × 105 cells from bmt1Δ, bmt1Δ/BMT1, and bmt1Δ bmt2Δ bmt5Δ mutants and their reference strain, CAI-4. Difference between CAI-4 and bmt1Δ or bmt1Δ bmt2Δ bmt5Δ mutants were significant (P < .05). Fungal burdens of different Candida albicans strains in the spleen, kidney, and liver of BALB/c (B), C57BL/6 (C), and Gal3−/− (D) mice, 3 days postintraperitoneal infection. Mice received intraperitoneal injection of 5 × 107 CAI-4, bmt1Δ, and bmt1Δ bmt2Δ bmt5Δ cells. The results shown are from 3 independent experiments. *, P < .05; **, P < .01. All the mutants and their reference strains are Ura+ with CIp10 integrated in the same RPS1 allele.
The lectin, galectin-3 (Gal3), has been described as a β-Mans receptor [35], and it is proposed to induce killing of C. albicans [36]. Spleen, kidneys, and liver fungal burdens in Gal3−/− mice and C57BL/6 control mice were therefore analyzed following intraperitoneal infection with the bmt1Δ and bmt1Δ bmt2Δ bmt5Δ mutants and the control strain. The 2 mutants had the tendency to disseminate less than the wild-type strain in liver of C57BL/6 mice, but the difference was not significant (Figure 5C). However, dissemination in the spleen and the kidneys was similar for the 3 strains. It is interesting to note that spleen from Gal3−/− mice infected with CAI-4 contained significantly higher fungal burdens than those from C57BL/6 mice (P < .05) (Figure 5C and D). In contrast, no significant difference was observed with the 2 bmtsΔ mutants. Gal3−/− mice displayed lower fungal burdens in the spleen when infected with the bmt1Δ/bmt2Δ/bmt5Δ mutant (Figure 5D).
DISCUSSION
Surface mannoproteins have specific functions related to C. albicans virulence including adhesion to host tissues, biofilm formation, and triggering of the immune system [37]. Some of these virulence attributes are conferred through proteins N- and/or O-glycans [14, 38]. Emphasis has then been placed on the role of the cell wall glycans in the interplay between C. albicans and its host. The commensal/pathogen transition is critically dependent on the biological activities of specific oligomannosides, including β-Mans that are recognized by the soluble lectin Gal3 [35]. These epitopes have immunomodulatory properties [8, 11] and seem to be related to C. albicans infections [10, 39]. Structural studies performed by Shibata et al [4] have described β-Mans in PPM N-glycans, whereas Trinel et al [5] characterized β-Mans in the PLM glycolipid, a member of the mannose-inositol-phosphoceramide family. We have later identified the 6 Bmts responsible for their biosynthesis [21, 22].
We have shown that β-Mans epitopes are widely distributed among the different CWMPs [16]. We also demonstrated that, in contrast to PPM, O-mannosylation is part of the C. albicans β-1,2-mannosylation process. We report here the presence of β-Mans and their biosynthetic process in C. albicans O-mannan. Two mutants, bmt1Δ and bmt3Δ, had truncated O-mannosides with an accumulation of O-mannotriose and O-mannotetraose, respectively. Alpha-mannosidase treatment of O-mannosides from wild-type strain digests all mannosides containing less than 4 mannose residues, showing that a heteropolymer rather than a homopolymer made up α-mannosidase-resistant O-mannans. The Bmt1 is known to add β-mannose on α-1,2-Mans in N-mannan, initiating then a heteropolymer composed of α-1,2-Man with terminal β-Mans (Figure 1A) [21]. This Bmt1 substrate would also be available on O-mannosides after Mnt1 and possibly Mnt2 activities (Figure 3D) [14, 24]. The mutant that lacks these 2 enzymes was deprived of β-mannosylated O-mannopentaose and expressed less β-Mans on CWMPs than the reference strain. As for β-Mans at the nonreducing end of PPM α-1,2-Mans [21], Bmt3 adds the second β-mannose.
We highlighted heterogeneity of O-mannosides released from yeast cells, and we demonstrated variability in the β-Mans content determined by growth temperature. The β-Mans expression is complex because yeasts grown at 37°C express less β-Mans on N-mannans than yeasts grown at 28°C [40, 41]. C. albicans cells display 2 O-mannopentaoses (Man5) mainly composed of an α-mannosidase-sensitive Man5 at 37°C and an α-mannosidase-resistant Man5 at 28°C. These data suggest that β-Mans from both N- and O-mannans undergo the same temperature-dependent regulation.
β-1,2-oligomannosides at the nonreducing end of α-1,2-Mans are major CWMPs β-Mans in both yeasts and hyphae. Yeast CWMPs from bmt1Δ mutant did not harbor β-Mans epitopes, and 2 purified hyphal proteins, Hwp1 and Pra1, were no more β-1,2-mannosylated when expressed in this mutant. Lack of Bmt1 activity has been reported to have no impact on yeast surface expression of β-Mans [21], certainly due to expression of alternative sources of β-Mans such as those present on PLM and N-mannans phosphomannosides. We were surprised to find that surface expression of β-Mans on germ tubes was initiated only by Bmt1. β-1,2-mannosylation of Hwp1 and Pra1 was not affected in the bmt2Δ mutant (data not shown), but it was diminished in the mnt1Δ/mnt2Δ mutant, suggesting that hyphae-specific N-mannans contain β-Mans only at the nonreducing end of α-1,2-Mans. Supporting this hint, others have observed presence of non-β-1,2-mannosylated phosphomannose in hyphae [42].
Surface cell wall molecules are important because they can directly interact with the host. However, β-Mans biosynthesized by Bmt1 apparently have no detrimental impact on the host, because mutants lacking Bmt1 were more or equally virulent than control strains in 2 different genetic background mice, BALB/c and C57/BL6 mice, respectively. Wild-type strain clearly disseminated more in organs of C57BL/6 mice, whereas differences between mutants were lower between the 2 genetic background mice. BALB/c and C57BL/6 mice can produce different immunological responses to C. albicans infections that can impact yeast virulence and dissemination [43, 44]. Likewise, male and female mice might display different immune responses [45]. We then used only female mice in this study. Putative sex-associated differences in the regulation of β-Mans-dependent C. albicans virulence should be analyzed in upcoming studies.
Our data strongly suggest that Bmt1-specific β-Mans are a potential avirulence factor, whose presence attenuates pathogen-mediated damage. As revealed by ConA staining, deletion of Bmt1 unmasks α-1,2-Mans, which are virulence attributes of C. albicans [46]. We ruled out that remaining β-Mans in PPM and PLM phosphomannosides of bmt1Δ mutant have the potential to impact a more virulent phenotype by generating bmt1Δ/bmt2Δ/bmt5Δ mutant. This strain, which contains no β-Mans, had an equivalent virulence to the bmt1Δ mutant. C. albicans yeast cell wall extracts were reported to induce murine vasculitis, which is associated with inflammatory response that is increased in the absence of β-Mans [47]. Decrease surface expression of β-Mans at 37°C can be considered as a mechanism favoring exposure of α-1,2-Mans and then the transition from commensal to pathogen.
Kohatsu et al [35, 36] have shown that Gal3, which is a soluble lectin reported to bind β-Mans, could induce death of C. albicans strains expressing β-Mans of the PPM acid stable and labile fractions. Recent studies have demonstrated that this lectin helps mice to overcome Candida systemic infections [48]. Galectin-3 may be secreted by neutrophils to aid opsonization and phagocytosis of yeast cells [49]. However, incubation of bmt1Δ and bmt1Δ/bmt2Δ/bmt5Δ mutants and their reference strain with human neutrophils did not confirm this hypothesis, and these mutants were found to be more susceptible to these phagocytes (data not shown). Comparing dissemination in C57BL/6 and Gal3−/− mice, we showed that the wild-type strain was more virulent in the knockout mice, whereas the mutants had similar (bmt1Δ mutant) or reduced (bmt1Δ/bmt2Δ/bmt5Δ mutant) virulence. These data indicate that Gal3 potentiates the elimination of C. albicans; however, in Gal3−/− deficient cells, β-Mans expressing strains of C. albicans are more virulent.
CONCLUSIONS
Our data show that β-Mans expression is under the control of regulatory mechanisms that favor exposure of α-1,2-Mans and their associated virulence and facilitate the yeast to counteract Gal3-mediated host defenses. Regulation of Gal3 expression by the host and regulation of β-Mans expression by C. albicans may be crucial events controlling the balance between saprophytic and parasitic status of the yeast.
Acknowledgments
We gratefully acknowledge Professor A. P. Mitchell (Carnegie Mellon University, Pittsburgh, PA), Professor A. J. P. Brown (Department of Molecular and Cell Biology, Aberdeen, UK), and Professor R. Robert (GEIHP, Univesrité d'Angers, France), for providing pDDB57 and CIp10 plasmids and mAb 16B1. We are indebted to Annick Masset for excellent technical assistance. This work has benefited from the facilities and expertise of the SICaPS platform of IMAGIF (Centre de Recherche de Gif-www.imagif.cnrs.fr). We thank the animal facility Département Hospitalo-Universitaire de Recherche Expérimentale from IMPRT-IFR114 for the maintenance of mice.
Financial support. This work was supported by the Agence Nationale de la Recherche (grant ANR-09-MIE-031-01); the European project AllFun from the 7th Framework Programme-Health (grant 260338), and the College Doctoral Lille Nord de France (Ouverture Internationale des Etudes et de la Formation Doctorale en Région Nord-Pas de Calais). N. A. R. G. was also supported by the Wellcome Trust (grants 080088, 086827, 075470, and 099215).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Courjol F, Jouault T, Fradin C. Modulation of the host response to control invasive fungal infections. In: Vandeputte ATCaP, ed. Antifungals: From Genomics to Resistance and the Development of Novel Agents. Poole, UK: Caister Academic Press, 2015: 237–65. [Google Scholar]
- 2.Klis FM, de Groot P, Hellingwerf K. Molecular organization of the cell wall of Candida albicans. Med Mycol 2001; 39(Suppl 1):1–8. [PubMed] [Google Scholar]
- 3.Fabre E, Hurtaux T, Fradin C. Mannosylation of fungal glycoconjugates in the Golgi apparatus. Curr Opin Microbiol 2014; 20:103–10. [DOI] [PubMed] [Google Scholar]
- 4.Shibata N, Kobayashi H, Suzuki S. Immunochemistry of pathogenic yeast, Candida species, focusing on mannan. Proc Jpn Acad Ser B Phys Biol Sci 2012; 88:250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trinel PA, Maes E, Zanetta JP et al. Candida albicans phospholipomannan, a new member of the fungal mannose inositol phosphoceramide family. J Biol Chem 2002; 277:37260–71. [DOI] [PubMed] [Google Scholar]
- 6.Dalle F, Jouault T, Trinel PA et al. Beta-1,2- and alpha-1,2-linked oligomannosides mediate adherence of Candida albicans blastospores to human enterocytes in vitro. Infect Immun 2003; 71:7061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fradin C, Jouault T, Mallet A et al. Beta-1,2-linked oligomannosides inhibit Candida albicans binding to murine macrophage. J Leukoc Biol 1996; 60:81–7. [DOI] [PubMed] [Google Scholar]
- 8.Jouault T, Lepage G, Bernigaud A et al. Beta-1,2-linked oligomannosides from Candida albicans act as signals for tumor necrosis factor alpha production. Infect Immun 1995; 63:2378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han Y, Morrison RP, Cutler JE. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun 1998; 66:5771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin H, Dziadek S, Bundle DR, Cutler JE. Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc Jpn Acad Ser B Phys Biol Sci 2008; 105:13526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devillers A, Courjol F, Fradin C et al. Deficient beta-mannosylation of Candida albicans phospholipomannan affects the proinflammatory response in macrophages. PloS One 2013; 8:e84771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fradin C, Bernardes ES, Jouault T. Candida albicans phospholipomannan: a sweet spot for controlling host response/inflammation. Semin Immunopathol 2015; 37:123–30. [DOI] [PubMed] [Google Scholar]
- 13.Hobson RP, Munro CA, Bates S et al. Loss of cell wall mannosylphosphate in Candida albicans does not influence macrophage recognition. J Biol Chem 2004; 279:39628–35. [DOI] [PubMed] [Google Scholar]
- 14.Munro CA, Bates S, Buurman ET et al. Mnt1p and Mnt2p of Candida albicans are partially redundant alpha-1,2-mannosyltransferases that participate in O-linked mannosylation and are required for adhesion and virulence. J Biol Chem 2005; 280:1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinel PA, Faille C, Jacquinot PM et al. Mapping of Candida albicans oligomannosidic epitopes by using monoclonal antibodies. Infect Immun 1992; 60:3845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fradin C, Slomianny MC, Mille C et al. Beta-1,2 oligomannose adhesin epitopes are widely distributed over the different families of Candida albicans cell wall mannoproteins and are associated through both N- and O-glycosylation processes. Infect Immun 2008; 76:4509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227:680–5. [DOI] [PubMed] [Google Scholar]
- 18.Goins TL, Cutler JE. Relative abundance of oligosaccharides in Candida species as determined by fluorophore-assisted carbohydrate electrophoresis. J Clin Microbiol 2000; 38:2862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson RB, Davis D, Enloe BM, Mitchell AP. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruptions. Yeast 2000; 16:65–70. [DOI] [PubMed] [Google Scholar]
- 20.Murad AM, Lee PR, Broadbent ID et al. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 2000; 16:325–7. [DOI] [PubMed] [Google Scholar]
- 21.Mille C, Bobrowicz P, Trinel PA et al. Identification of a new family of genes involved in beta-1,2-mannosylation of glycans in Pichia pastoris and Candida albicans. J Biol Chem 2008; 283:9724–36. [DOI] [PubMed] [Google Scholar]
- 22.Mille C, Fradin C, Delplace F et al. Members 5 and 6 of the Candida albicans BMT family encode enzymes acting specifically on β-mannosylation of the phospholipomannan cell-wall glycosphingolipid. Glycobiology 2012; 22:1332–42. [DOI] [PubMed] [Google Scholar]
- 23.Prill SK, Klinkert B, Timpel C et al. PMT family of Candida albicans: five protein mannosyltransferase isoforms affect growth, morphogenesis and antifungal resistance. Mol Microbiol 2005; 55:546–60. [DOI] [PubMed] [Google Scholar]
- 24.Díaz-Jiménez DF, Mora-Montes HM, Hernández-Cervantes A et al. Biochemical characterization of recombinant Candida albicans mannosyltransferases Mnt1, Mnt2 and Mnt5 reveals new functions in O- and N-mannan biosynthesis. Biochem Biophys Res Commun 2012; 419:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staab JF, Bahn YS, Tai CH et al. Expression of transglutaminase substrate activity on Candida albicans germ tubes through a coiled, disulfide-bonded N-terminal domain of Hwp1 requires C-terminal glycosylphosphatidylinositol modification. J Biol Chem 2004; 279:40737–47. [DOI] [PubMed] [Google Scholar]
- 26.Sentandreu M, Elorza MV, Sentandreu R, Fonzi WA. Cloning and characterization of PRA1, a gene encoding a novel pH-regulated antigen of Candida albicans. J Bacteriol 1998; 180:282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo S, Hartmann A, Dahse HM et al. Secreted pH-regulated antigen 1 of Candida albicans blocks activation and conversion of complement C3. J Immunol 2010; 185:2164–73. [DOI] [PubMed] [Google Scholar]
- 28.Citiulo F, Jacobsen ID, Miramón P et al. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog 2012; 8:e1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibata N, Suzuki A, Kobayashi H, Okawa Y. Chemical structure of the cell-wall mannan of Candida albicans serotype A and its difference in yeast and hyphal forms. Biochem J 2007; 404:365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouabhia M, Schaller M, Corbucci C et al. Virulence of the fungal pathogen Candida albicans requires the five isoforms of protein mannosyltransferases. Infect Immun 2005; 73:4571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacCallum DM. Mouse intravenous challenge models and applications. Methods Mol Biol 2012; 845:499–509. [DOI] [PubMed] [Google Scholar]
- 32.Felk A, Kretschmar M, Albrecht A et al. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect Immun 2002; 70:3689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kretschmar M, Bertsch T, Göller M et al. Parameters for determination of Candida albicans virulence in murine peritonitis. Mycoses 1999; 42(Suppl 2):19–24. [PubMed] [Google Scholar]
- 34.Thewes S, Kretschmar M, Park H et al. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol Microbiol 2007; 63:1606–28. [DOI] [PubMed] [Google Scholar]
- 35.Fradin C, Poulain D, Jouault T. Beta-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect Immun 2000; 68:4391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohatsu L, Hsu DK, Jegalian AG et al. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol 2006; 177:4718–26. [DOI] [PubMed] [Google Scholar]
- 37.Gow NA, Hube B. Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol 2012; 15:406–12. [DOI] [PubMed] [Google Scholar]
- 38.Bates S, Hughes HB, Munro CA et al. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J Biol Chem 2006; 281:90–8. [DOI] [PubMed] [Google Scholar]
- 39.Dromer F, Chevalier R, Sendid B et al. Synthetic analogues of beta-1,2 oligomannosides prevent intestinal colonization by the pathogenic yeast Candida albicans. Antimicrob Agents Chemother 2002; 46:3869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinel PA, Jouault T, Cutler JE, Poulain D. Beta-1,2-mannosylation of Candida albicans mannoproteins and glycolipids differs with growth temperature and serotype. Infect Immun 2002; 70:5274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goto K, Okawa Y. Activity and stability of alpha- and beta-mannosyltransferases in Candida albicans cells cultured at high temperature and at low pH. Biol Pharm Bull 2008; 31:1333–6. [DOI] [PubMed] [Google Scholar]
- 42.Shibata N, Fukasawa S, Kobayashi H et al. Structural analysis of phospho-d-mannan-protein complexes isolated from yeast and mold form cells of Candida albicans NIH A-207 serotype A strain. Carbohydr Res 1989; 187:239–53. [DOI] [PubMed] [Google Scholar]
- 43.Schofield DA, Westwater C, Balish E. Divergent chemokine, cytokine and beta-defensin responses to gastric candidiasis in immunocompetent C57BL/6 and BALB/c mice. J Med Microbiol 2005; 54:87–92. [DOI] [PubMed] [Google Scholar]
- 44.Staab JF, Datta K, Rhee P. Niche-specific requirement for hyphal wall protein 1 in virulence of Candida albicans. PLoS One 2013; 8:e80842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstein Y, Ran S, Segal S. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J Immunol 1984; 132:656–61. [PubMed] [Google Scholar]
- 46.Hall RA, Bates S, Lenardon MD et al. The Mnn2 mannosyltransferase family modulates mannoprotein fibril length, immune recognition and virulence of Candida albicans. PLoS Pathog 2013; 9:e1003276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shinohara H, Nagi-Miura N, Ishibashi K et al. Beta-mannosyl linkages negatively regulate anaphylaxis and vasculitis in mice, induced by CAWS, fungal PAMPS composed of mannoprotein-beta-glucan complex secreted by Candida albicans. Biol Pharm Bull 2006; 29:1854–61. [DOI] [PubMed] [Google Scholar]
- 48.Linden JR, De Paepe ME, Laforce-Nesbitt SS, Bliss JM. Galectin-3 plays an important role in protection against disseminated candidiasis. Med Mycol 2013; 51:641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linden JR, Kunkel D, Laforce-Nesbitt SS, Bliss JM. The role of galectin-3 in phagocytosis of Candida albicans and Candida parapsilosis by human neutrophils. Cell Microbiol 2013; 15:1127–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 1984; 198:179–82. [DOI] [PubMed] [Google Scholar]
- 51.Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol 1999; 181:1868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993; 134:717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]