Abstract
Background. In September 2012, the Centers for Disease Control and Prevention (CDC), U.S. Food and Drug Administration (FDA), and state and local partners investigated an outbreak of Salmonella enterica serovar Bredeney linked to peanut butter (PB).
Methods. A case was defined as infection with the outbreak strain of Salmonella Bredeney between June 1, 2012 and October 31, 2012. Food exposure questionnaires were analyzed by the CDC to determine the food vehicle. The FDA reviewed production information from Retail Chain A's sole supplier of PB, Company A. The PB samples collected from case-patients and Company A were tested for Salmonella.
Results. Forty-two case-patients from 20 states were identified. Of 33 case-patients from whom food exposure information was obtained, 25 (76%) shopped at Retail Chain A and 25 (100%) purchased Company A PB. Three state health departments isolated the outbreak strain from opened jars of PB collected from case-patients. The FDA investigators identified multiple deficiencies in current Good Manufacturing Practices (cGMPs) in Company A's manufacturing facility and determined that internal controls were insufficient to prevent shipment of contaminated product. The FDA isolated the outbreak strain of Salmonella Bredeney from implicated product collected at the firm and the environment of the firm's food production facility.
Conclusions. Timely laboratory, investigational, and epidemiologic data led to the voluntary recall of PB by Company A. The FDA suspended Company A's food facility registration, prohibiting the firm from introducing food into interstate commerce. This outbreak underscores the need for effective preventive controls, including robust internal environmental monitoring programs, appropriate action in response to contamination findings, and an improved understanding of food safety at the managerial and corporate levels.
Keywords: food safety, outbreak, peanut butter, Salmonella
Salmonella is one of the leading bacterial causes of foodborne outbreaks in the United States [1]. Historically, a limited number of Salmonella outbreaks have been linked to consumption of peanut butter (PB). The first documented international outbreak of salmonellosis associated with the consumption of PB was reported in 1996 and involved Salmonella enterica serovar Mbandaka in Australia [2, 3]. In the United States, the 2 most noted peanut product and PB-associated outbreaks are the Salmonella Tennessee and Typhimurium outbreaks in 2006 and 2008, respectively [4, 5]. Each outbreak resulted in hundreds of illnesses over many months with high rates of morbidity and significant mortality. Combined, they led to 1429 illnesses, 259 hospitalizations, and 9 deaths. Both outbreaks were characterized by the challenges posed by ingredient-driven outbreaks, because products comprising the same contaminated ingredient may be involved, each with distinct distribution channels and market share.
Salmonella Bredeney is considered an uncommon serotype in the United States, ranking as the 54th most common Salmonella serotype uploaded to PulseNet USA in September 2012. Internationally, 2 outbreaks of Salmonella Bredeney have been reported in the last 10 years: one associated with chicken and the other with meat products in Australia and Northern Ireland, respectively [6]. In the United States, only 1 outbreak of Salmonella Bredeney infections was identified previously, a restaurant-associated outbreak in 1999; however, no vehicle was identified. In this study, we report the latest PB-associated outbreak in the United States, caused by PB contaminated with Salmonella Bredeney [7]. Preliminary findings from this investigation have been previously reported [8].
Multistate bacterial foodborne outbreaks in the United States are usually identified through PulseNet, a national molecular subtyping network for foodborne illness, coordinated by the Centers for Disease Control and Prevention (CDC). In September 2012, the CDC, U.S. Food and Drug Administration's (FDA) Coordinated Outbreak Response and Evaluation Network (CORE), and State and Local Health Departments investigated an outbreak of human illnesses caused by Salmonella Bredeney. Ultimately, 42 persons from 20 states were reported to be infected with the outbreak strain; 61% of case-patients were children under the age of 10 years. The CDC and affected states collected exposure information from case-patients and identified PB purchased from Retail Chain A as the suspect vehicle. Based on the timely epidemiologic findings and rapid analysis, FDA and regulatory state partners gathered additional investigational and laboratory information to confirm the source of the contaminated PB and ultimately implemented control measures to remove the product from the market and stop the outbreak.
MATERIALS AND METHODS
Case Finding
An outbreak case was defined as infection with Salmonella enterica serovar Bredeney reported to PulseNet with pulsed-field gel electrophoresis (PFGE) XbaI pattern JBXX01.0013, in a person residing in the United States, and with illness onset ranging from June 1, 2012 to September 30, 2012.
Food Exposure Assessment
Using a standardized survey, state public health officials interviewed and collected exposure histories from 33 case-patients. The standardized survey instrument collects food consumption, animal contact, and travel data on over 300 possible exposures during the 7 days before a person's illness onset. Case-patient exposures were analyzed and compared with food consumption data obtained from the Foodborne Diseases Active Surveillance Network (FoodNet) population survey, a population-based survey of respondents in 10 FoodNet sites, collected from May 2006 through April 2007 [9]. Subsequent interviews provided more detailed product information, which, in turn, was used to help identify the manufacturer of the implicated vehicle.
Environmental Investigation
The FDA investigated the firm's peanut roasting and PB manufacturing facilities that produced the implicated PB. The FDA investigators reviewed: quality control and production records; maintenance and installation of equipment records; cleaning and sanitizing procedures; procedures related to receiving of raw materials; product inventory and distribution procedures; and internal testing procedures and results. In addition, FDA investigators inspected firm facilities and collected environmental swabs, raw ingredient samples, and finished peanut and PB products for microbiological analysis.
Microbiological Investigation
Samples from case-patients were cultured for Salmonella, serotyped, and subtyped by PFGE at state public health laboratories using standard methods [10]. Open containers of the implicated PB collected from patients in September 2012 were cultured for bacteria at state public health laboratories. Environmental, peanut, and PB samples collected during the FDA's investigation of the manufacturing facility were cultured for bacteria at FDA laboratories; serotyping and PFGE were performed on food and environmental isolates that yielded Salmonella. Isolates collected by the firm that had already been tested by a third-party laboratory were also obtained and analyzed by PFGE and serotyping by FDA.
RESULTS
Case Finding and Descriptive Epidemiology
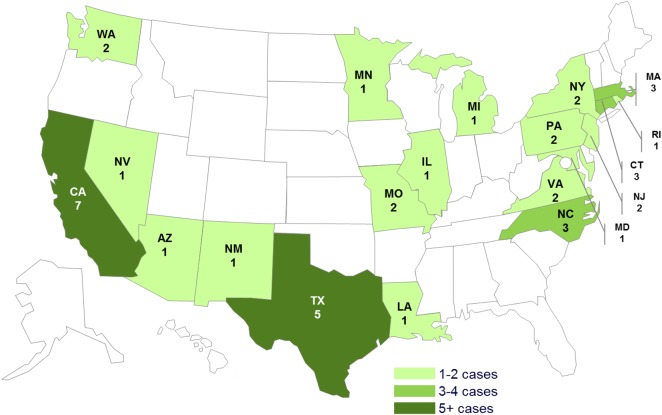
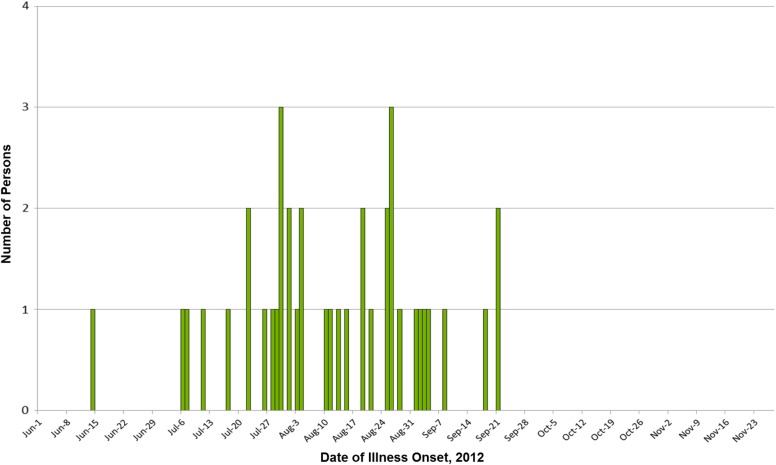
A total of 42 confirmed case-patients were identified in 20 states (Figure 1). Patients had a median age of 7 years (range, <1 year to 79 years); 17 of 41 (41%) were female. Among 39 persons for whom information was available, illness onset dates ranged from June 14, 2012 to September 21, 2012 (Figure 1). Among 36 ill persons for whom information was available, 10 (28%) were hospitalized and no deaths were reported (Figure 2).
Figure 1.
Persons infected with the outbreak strain of Salmonella Bredeney, by state. n = 42 for whom information was reported as of November 28, 2012. Forty-two individuals infected with the outbreak strain of Salmonella Bredeney were reported from 20 states. The number of ill persons identified in each state was as follows: Arizona (1), California (7), Connecticut (3), Illinois (1), Louisiana (1), Massachusetts (3), Maryland (1), Michigan (1), Minnesota (1), Missouri (2), New Jersey (2), New Mexico (1), New York (2), Nevada (1), North Carolina (3), Pennsylvania (2), Rhode Island (1), Texas (5), Virginia (2), Washington (2).
Figure 2.
Persons infected with the outbreak strain of Salmonella Bredeney, by date of illness onset. n = 39 for whom information was reported as of November 28, 2012. Some illness onset dates have been estimated from other reported information.
Outbreak Isolate Characteristics
The outbreak strain had the most common XbaI pattern among all Salmonella Bredeney isolates in PulseNet, with an average of approximately 5 to 8 isolates uploaded per year. Sixty-four percent of all background case-patients were from Texas. There were no historical non-human matches to this pattern specifically, but a variety of meat products, including boneless beef, ground turkey, and chicken breast, yielded Salmonella Bredeney with several different PFGE patterns.
Case Investigations
Interviews of 33 patients using a standard food-consumption survey instrument indicated that consumption of PB (88%) was the only exposure that was reported by a higher proportion of ill persons than would be expected, compared with the FoodNet population survey (58.1%); a binomial probability distribution was used to determine that this difference was statistically significant (p = .00022). Based on these findings, case-patients underwent detailed, directed interviews about the PB eaten during the week before illness onset. Twenty-five (78%) of 33 ill persons interviewed reported shopping at Retail Chain A locations across the United States. Twenty-three (92%) of 25 case patients reported eating Retail Chain A Valencia Creamy PB made with sea salt in the week before becoming ill. Two (8%) of 25 ill persons reported eating Retail Chain A Valencia PB with roasted flaxseed. Retail Chain A PB with sea salt and PB with roasted flaxseed were manufactured by Company A. All 25 (100%) of these case-patients who shopped at Retail Chain A reported eating 1 of 2 store brands of PB manufactured by Company A. Based on the information provided by the CDC, there was substantial epidemiologic evidence to indicate that Retail Chain A Valencia PB was the likely vehicle for this outbreak.
Product Investigation and Further Case Finding
Early epidemiologic data identifying Retail Chain A Valencia PB as the likely source of the outbreak were provided to FDA officials on September 19, 2012. To address the imminent public health risk, FDA, CDC, and the California Department of Public Health (CDPH) worked closely with Retail Chain A's corporate headquarters on September 20, 2012 and September 21, 2012 to share information regarding the investigation and to discuss potential product actions. Late on September 20, 2012, the company voluntarily removed the product from its store shelves and posted a Customer Advisory on its internet page. On September 21, 2012, Retail Chain A confirmed that the sole contract manufacturer of Retail Chain A's Valencia Creamy Salted PB was Company A. Then, on September 22, 2012, after follow-up conversations with the FDA, CDC, and CDPH, Retail Chain A also initiated a voluntary recall of Valencia PB with sea salt. In addition, due to the variety of products manufactured by the firm, the FDA and New Mexico Environment Department (NMED) worked with the producer of the contaminated product, Company A, to determine additional product actions and ensure that any additional potentially harmful product was removed from the marketplace.
The FDA investigators conducted an extensive investigation of the peanut- and PB-producing facilities of Company A and noted several major current Good Manufacturing Practices (cGMP) deficiencies. A review of the firm's internal microbiological sampling program revealed that the program was insufficient to adequately prevent shipment of contaminated product and that the firm had shipped portions of PB lots that had previously tested positive for multiple Salmonella serotypes. The firm's internal testing program data from May 2012 to September 2012 showed that Salmonella of various serotypes, including Bredeney, had been identified in the manufacturing plant and in the firm's products. Several cGMP deficiencies were observed by investigators that presented many opportunities for cross-contamination of roasted and unroasted nuts from the environment of the plant. These deficiencies included but were not limited to the following: employees handling peanut products were observed wiping gloved hands on street clothes and failed to wash their hands or change gloves; absence of hand-washing sinks in the peanut processing building or packaging areas; birds were observed landing in trailers containing peanuts; and raw, in-shell peanuts were exposed to the elements. In previous inspections between 2007–2012, the FDA noted similar cGMP violations. Company A repeatedly committed to respond to the observations and initiate corrective actions based on the deficiencies noted by the FDA.
Microbiological Investigation
A total of 38 product samples and 481 environmental swabs collected by the inspection team were analyzed by the FDA's Denver District Office laboratory. Salmonella Bredeney with a PFGE pattern indistinguishable from the outbreak strain was isolated from 2 retained lots of PB and from 4 environmental swabs. In addition, multiple Salmonella serotypes including Anatum, Arizonae, Cerro, Kiambu, Mbandaka, Meleagridis, and Montevideo were isolated from 28 environmental swabs, 13 nut butter products, retained product, shelled raw Valencia peanuts, and 1 shelled raw peanut sample at the Company A facility.
Furthermore, 42 of 43 samples from finished nut and nut butter products, which were previously analyzed and found negative for Salmonella by Company A's product sampling program, were collected and analyzed by the FDA and found to be positive for Salmonella Bredeney. Data from the firm's internal testing program indicated that from May to September 2012, multiple Salmonella serotypes were identified in the plant, including Bredeney, Meleagridis, Kiambu, Mbandaka, and Anatum. Finally, the Washington State Department of Agriculture, Virginia, and New Jersey State Departments of Health reported that Salmonella Bredeney with a PFGE pattern indistinguishable from the outbreak strain was isolated from opened jars of PB collected from case-patients' homes.
The FDA investigators determined that, based on Company A facility records, Salmonella serotypes Anatum, Arapahoe, Bredeney, Cerro, Dallgow, Kiambu, Kubacha, Mbandaka, Meleagridis, Newport, and Teddington had been isolated from various nut and nut butter products by Company A's product testing program between June 2009 and August 2012. During this same time period, Company A had at least partially distributed (or cleared for distribution) portions of 11 lots (daily production runs of peanut or almond butter) despite the fact that its own testing program identified Salmonella, including Salmonella Bredeney, in those lots [11].
Public Health Response
Product and environmental samples collected by FDA confirmed that both the peanut and PB processing facility were contaminated with Salmonella spp, including the outbreak strain. The FDA, CDC, and NMED presented epidemiological evidence to the firm on Sunday, September 23, 2012 and received notification that Company A would issue a voluntary recall. On September 24, 2012, Company A issued the recall of all nut butter products manufactured on the same process line as the Retail Chain A PB between May 1, 2012 and September 24, 2012 [12]. The recall was expanded to include approximately 100 products (PB and other products, 208 made with nuts and seeds), based on a review of the firm's environmental and finished product testing results indicating multiple positive Salmonella spp findings from May 2012 to September 2012. On October 4, 2012, the recall was expanded again to include all products made in the nut butter production facility between March 1, 2010 and September 24, 2012, adding 139 products to the recall, based on positive laboratory results from 212 samples from Company A's in-house sampling program and positive results from the FDA's finished product testing and environmental samples. This recall expansion did not include anything made in the second plant (the peanut facility) because the FDA had not yet received laboratory results to indicate adulteration in the peanut facility. On October 12, 2012, the recall was expanded a final time to encompass all products made in the firm's nut and nut butter production facilities between March 1, 2010 and September 24, 2012, based on FDA positive testing results matching the outbreak strain and positive testing results from the peanut facility. The October 12, 2012 recall expansion included raw and roasted shelled and in-shell peanuts sold in quantities from 2 ounces to 50 pounds that were within their current shelf life or had no stated expiration date. In total, over 300 products were recalled. On September 27, 2012, the firm reached an agreement with NMED to voluntarily shut down their PB facility until the facility underwent complete cleaning and sanitizing, providing complete food safety and sanitation records, and NMED completing a pre-startup environmental assessment of the firm.
On November 26, 2012, in the interest of public health, the FDA, for the first time, invoked its registration suspension authority granted under the 2009 Food Safety Modernization Act (FSMA). This enables the agency to take action when food manufactured, processed, packed, received, or held by a facility has a reasonable probability of causing serious adverse health consequences or death to humans or animals, and other conditions are met [13]. Specifically, FDA investigators determined that conditions within Company A's facility, including the presence of Salmonella in various locations and current cGMP violations that could lead to cross-contamination between raw materials and finished product, ultimately led to the manufacture and distribution of contaminated product. Furthermore, the firm's history and testing program as well as FDA environmental and product sample testing yielded positive results for several Salmonella serotypes throughout the firm's facilities. The suspension order prohibited the firm from introducing food into interstate or intrastate commerce. As a result, the firm was, in effect, shut down and ceased all activity. On December 21, 2012, the U.S. District Court entered a Consent Decree of Permanent Injunction against Company A. Under the terms of the Decree, Company A was permanently restrained and enjoined from receiving, preparing, processing, packing, holding, or distributing articles of food unless and until the company develops and implemented an FDA-approved sanitation control program, provided FDA the opportunity to inspect the buildings to ensure the company's compliance with the Decree and the Federal Food, Drug, and Cosmetic Act, and received written authorization from FDA to resume its operations. As a result of the Decree and its terms, the Commissioner of the FDA signed an order reinstating the company's food facility registration.
DISCUSSION
In the United States, previous PB outbreaks produced 715 reported case-patients from 48 states in 2006 and 714 reported case-patients from 46 states in 2008 [4, 5, 14]. However, the Salmonella Bredeney PB outbreak of 2012 resulted in far fewer case-patients, 42 from 20 states, most likely because Retail Chain A had, compared with the firms responsible for past outbreaks, a very small market share and diversity of products. The 2008 Salmonella Typhimurium outbreak was instrumental in refocusing national attention on food safety and effective response efforts to foodborne outbreaks [5]. In March 2009, the White House Food Safety Working Group was created to identify actions that could prevent foodborne disease as well as enhance surveillance and regulatory authority [15]. In addition, as a result of the 2006 and 2008 peanut and PB-associated outbreaks, the Grocery Manufacturers Association formed a Salmonella Control Task Force to develop, through a review and synthesis of industry programs and information from literature, a guidance document including 7 elements for the control of Salmonella in the manufacture of low-moisture foods [16]. Two of the 7 control elements in the guidance document, preventing Salmonella spread in the facility and controlling raw materials, were most likely implemented insufficiently in the facility, and this may have led to the outbreak.
Past investigations of Salmonella outbreaks in low-moisture facilities determined the pathogen to be present in plant processing environments where sanitation was substandard [17–19]. An outbreak of Salmonella Agona associated with toasted oat cereal resulted in an investigation of the implicated processing facility and specifically an examination of the potential for cross-contamination and recontamination of processed product. Product contamination was attributed to poor sanitary design and equipment maintenance in processing areas, air-handling systems, ingredient handling and storage, and traffic flow of the plant [17]. The investigators found Salmonella in the plant environment and observed unsanitary conditions, poor employee practices, and other deficiencies in cGMPs, similar to those noted by the FDA investigators during the Salmonella Bredeney outbreak investigation [19]. Furthermore, the entry of Salmonella into a food production plant may lead to the organism becoming established in the plant environment and the subsequent contamination of food [20]. This contamination may persist over many years despite regular cleaning and sanitation procedures.
Investigations of previous PB outbreaks have revealed common antecedents that may have contributed to the contamination and spread of Salmonella within the respective facilities. During the 2006–2007 Salmonella Tennessee outbreak investigation associated with PB, the implicated firm extended its recall to include PB purchased since October 2004 due to the high proportion of case-patients dating back to December 2004, before the initial apparent outbreak period. In addition, the FDA isolated the outbreak strain from environmental samples taken from the firm, and records indicated that Salmonella had been isolated from PB jars in the plant in October 2004 [4]. This evidence suggests that the outbreak could have been related to long-term environmental contamination in the plant.
During the response efforts for the 2008–2009 Salmonella Typhimurium outbreak associated with peanut products manufactured by Company B, FDA investigators inspected the firm's 2 plants in Georgia and found rainwater leakage into storage areas, storage of raw peanuts near roasted peanuts, and possibly inadequate peanut roaster temperatures [5]. These findings suggest that Salmonella may have arrived on raw peanuts or been introduced into the plant and survived processing. Peanuts and peanut products were transferred between the firm's Texas and Georgia facilities, and both had cGMP inadequacies. Most importantly, third-party laboratory testing resulted in positive Salmonella findings on multiple occasions. After the firm retested the product and received negative results, the product was shipped in interstate commerce [21]. Retesting product after an initial positive result is likely to return a false-negative result, and the FDA advises against this practice [11]. The cGMP inadequacies at both plants and a history of Salmonella findings in products suggest that the firm had prolonged exposure to contamination with inadequate corrective actions taken to address the contamination.
Likewise, the 2012 Salmonella Bredeney outbreak investigation at the PB facility revealed multiple cGMP deficiencies and food safety problems. Like the companies implicated in these past 2 Salmonella outbreaks, Company A had similar cGMP deficiencies, including conditions and practices that may have resulted in cross-contamination between raw peanut and processed peanuts. However, the commonality of the likelihood of product contamination from the environment that had been compromised by established Salmonella harborages was likely the most significant contributor to contaminated finished product. In addition, Company A distributed at least a portion of different lots of peanut and almond butter between March 1, 2010 and September 2012, after composite testing of those lots revealed the presence of Salmonella, similar to Company B during the 2008 outbreak. Specifically, when composite testing of a lot was positive for Salmonella, individual containers of product from the positive tested lots were retested, and portions of these lots were distributed based on the retest results. Ultimately, the initial Salmonella-positive composite test results were not properly considered to be evidence of ongoing and pervasive finished product contamination [22]. This pattern of nonaction by the firm in response to positive findings in finished product and from their environmental monitoring program suggests that there is a need for improved understanding of the meaning of microbiological testing results and food safety principles at the managerial level. From a regulatory perspective, there are some processes, such as the Reportable Food Registry, that require industry to report when there is a reasonable probability that an article of food will cause serious adverse health consequences. The more these processes are refined and used, the more they will help ensure an even more prompt and effective regulatory response. These processes are also proving to be instrumental in responding to and in some cases even preventing foodborne outbreaks.
The FDA investigation of Company A's facility and records showed that they had positive environmental and product test results before the outbreak. Company A also failed to take effective corrective actions in response to these positive results. Although the original source of the Salmonella strain is unknown, it is clear that failure to control Salmonella in the processing environment, failure to take appropriate corrective actions, and lack of institutional food safety knowledge played a major role. This, and previous outbreaks, highlight common antecedents for contamination that led to illness from contaminated PB. This third U.S. PB-associated outbreak that affected product produced as far back as March 2010 underscores the need for effective preventive controls, including robust environmental monitoring programs, appropriate action in response to contamination findings, and an improved understanding of food safety at the managerial and corporate levels.
Outbreak response efforts serve as opportunities to affect institutional change aimed at enhancing food safety. However, they must also provide lessons learned with the intention of improving the timeliness of determining the culprit of any given outbreak and removing it from the market. When comparing the 3 Salmonella outbreaks linked to PB, one thing that stands out is the timeliness of the response and resolution of the Salmonella Bredeney outbreak. The FDA Coordinated Outbreak Response and Evaluation (CORE) Network was designed to effectively and efficiently coordinate the response efforts of federal, state, and local partners by providing a common forum to share and evaluate epidemiologic, laboratory, and investigational findings with the ultimate goal of protecting public health. The FDA continuously evaluates outbreak detection, response, and prevention efforts to fine tune processes and strategies, maximizing both response speed and accuracy. In the aftermath of the Salmonella Bredeney outbreak, the FDA engaged in an educational outreach initiative, targeting private industry partners to improve the understanding of food safety at the managerial and corporate levels and highlight the importance of effective preventive controls, robust environmental monitoring programs, and appropriate response to contamination findings. The FDA continues to pursue inspectional activities for this regulated industry, guided by the lessons learned from past outbreaks to prevent future nut butter outbreaks. Finally, the FDA is engaged in research to obtain additional information on typical levels of the pathogen in PB through dose-response assessments.
CONCLUSION
The FDA, CDC, and state and local health agencies collaborated successfully to remove the contaminated product from the market to protect public health and end the outbreak. Furthermore, the collaborating partners produced evidence that allowed the FDA to suspend Company A's food facility registration. This suspension order prohibited the firm from introducing food into interstate commerce. This is the first use of this new authority under the FSMA. Results from the investigation of the company implicated in this Salmonella Bredeney outbreak echoed findings from investigations of other PB outbreaks in the United States within the past decade. In particular, a common theme observed among all companies responsible for these outbreaks was a need for developing and applying effective preventive controls, including robust environmental monitoring programs, appropriate action in response to positive findings, and an improved understanding of food safety by corporate leadership.
Acknowledgments
The response efforts to the Salmonella Bredeney outbreak included numerous public health officials at local and state health departments and public health laboratories in the United States. The assistance of the California Department of Food and Agriculture and the New Mexico Environment Department are especially appreciated.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Food and Drug Administration.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Scallan E, Hoekstra RM, Angulo FJ et al. Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis 2011; 17:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheil W, Carmeron S, Heaton S et al. Human salmonellosis and peanut butter. Comm Dis Intell 1996; 20:326. [Google Scholar]
- 3.Scheil W, Carmeron S, Dalton C et al. A South Australian Salmonella Mbdanka outbreak investigation using a database to select controls. Aust N Z J Public Health 1998; 22:536–9. [DOI] [PubMed] [Google Scholar]
- 4.Sheth AN, Hoekstra M, Patel N et al. A national outbreak of Salmonella serotype tennessee infections from contaminated peanut butter: a new food vehicle for salmonellosis in the United States. Clin Infect Dis 2011; 53:356–62. [DOI] [PubMed] [Google Scholar]
- 5.Cavallaro E, Date K, Medus C et al. Salmonella typhimurium infections associated with peanut products. N Eng J Med 2011; 365:601–10. [DOI] [PubMed] [Google Scholar]
- 6.Moore JE, Murray L, Fanning S et al. Comparison of phenotypic and genotypic characteristics of Salmonella Bredeney associated with a poultry-related outbreak of gastroenteritidis in Northern Ireland. J Infect 2003; 47:33–9. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). Notes from the field: Salmonella bredeney infections linked to a brand of peanut butter-United States, 2012. MMWR Morb Mortal Wkly Rep 2013; 62:107. [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Multistate outbreak of Salmonella Bredeney infections linked to peanut butter manufactured by Sunland, Inc. (final update), 2012. Available at: http://www.cdc.gov/salmonella/bredeney-09-12/index.html Accessed 19 March 2013.
- 9.Centers for Disease Control and Prevention (CDC). Foodborne Active Surveillance Network (FoodNet) Population Survey Atlas of Exposures. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2006–2007; Available at: http://www.cdc.gov/foodnet/surveys/FoodNetExposureAtlas0607_508.pdf. [Google Scholar]
- 10.Nataro IP, Bopp CA, Fields PI et al. eds. Manual of Clinical Microbiology. 9th ed. Washington, DC: ASM Press, 2007. [Google Scholar]
- 11.US Food and Drug Administration. Establishment Inspection Report: 17 September–16 October 2012. Available at: http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofGlobalRegulatoryOperationsandPolicy/ORA/ORAElectronicReadingRoom/UCM324793.pdf Accessed 16 January 2014.
- 12.US Food and Drug Administration b. Sunland Nut and Seed Product Recalls. Available at: http://www.fda.gov/Safety/Recalls/MajorProductRecalls/SunlandNutSeedProductRecalls/default.htm Accessed 19 March 2013.
- 13.US Food and Drug Administration. Letter to Sunland Inc. Concerning Suspension of Food Facility Registration; Notice of Opportunity for Hearing. 26 November 2012c. Available at: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectronicReadingRoom/UCM329370.htm Accessed 28 February 2013.
- 14.Centers for Disease Control and Prevention (CDC). Multistate outbreak of Salmonella infections associated with peanut butter and peanut butter-containing products--United States, 2008–2009. MMWR Morb Mortal Wkly Rep 2009; 58:85–90. [PubMed] [Google Scholar]
- 15.President's Food Safety Working Group. Available at: http://www.foodsafetyworkinggroup.gov/Home.htm. Accessed 28 February 2013.
- 16.Scott V, Chen Y, Freier T et al. Control of Salmonella in low-moisture foods I: minimizing entry of Salmonella into a processing facility. Food Prot Trend 2009; 29:342–53. [Google Scholar]
- 17.Breuer T. CDC investigations: the May 1998 outbreak of Salmonella Agona linked to cereal. Cereal Foods World 1999; 44:185–6. [Google Scholar]
- 18.Ehret E. Salmonella in peanut products: understanding the risk and controlling the process. Overview of a 2007 voluntary recall. In: GMA Rapid Response Symposium, Arlington, VA; 2009. [Google Scholar]
- 19.Podolak R, Enache E, Stone W et al. Sources and risk factors for contamination, survival, persistence, and heat resistance of Salmonella in low-moisture foods. J Food Prot 2010; 73:1919–36. [DOI] [PubMed] [Google Scholar]
- 20.Hilbert F, Smulders FJ, Chopra-Dewasthaly R et al. Salmonella in the wildlife-human interface. Food Res Int 2012; 45:603–8. [Google Scholar]
- 21.US Food and Drug Administration. Report of a Department of Health and Human Services/Food and Drug Administration plant inspection to the Peanut Corporation of America, 9 January–5 February 2009. Available at: http://www.fda.gov/downloads/AboutFDA/CentersOffices/ORA/ORAElectronicReadingRoom/UCM109834.pdf Accessed 28 February 2013.
- 22.US Food and Drug Administration. Guidance for Industry: Testing for Salmonella Species in Human Foods and Direct-Human-Contact Animal Foods. March 2012a. Available at: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm295271.htm Accessed 19 February 2015.