Abstract
Different treatment modalities have been proposed in the treatment of early gastric cancer (EGC). Endoscopic resection (ER) is an established treatment that allows curative treatment, in selected cases. In addition, ER allows for an accurate histological staging, which is crucial when deciding on the best treatment option for EGC. Recently, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have become alternatives to surgery in early gastric cancer, mainly in Asian countries. Patients with “standard” criteria can be successfully treated by EMR techniques. Those who meet “expanded” criteria may benefit from treatment by ESD, reducing the need for surgery. Standardized ESD training system is imperative to promulgate effective and safe ESD technique to practices with limited expertise. Although endoscopic resection is an option in patients with EGC, surgical treatment continues to be a widespread therapeutic option worldwide. In this review we tried to point out the treatment modalities for early gastric cancer.
Keywords: Early gastric cancer, Endoscopic submucosal dissection, Endoscopic mucosal resection, Pathological staging, Gastrectomy
Core tip: Gastric cancer is one of the main causes of cancer death. For early gastric cancer (EGC) endoscopic resection is an effective treatment modality for selected cases of EGC. Endoscopic submucosal dissection is designed to provide en bloc R0 resection regardless of size. Gastrectomy is the standard treatment for EGC with suspected lymph node metastases. This review describes the current different treatment modalities for early gastric cancer.
INTRODUCTION
Gastric cancer is one of the leading causes of cancer worldwide, causing high mortality. In Asian countries, the frequency of early gastric cancer (EGC) is far superior to that of Western countries. Currently early gastric cancer (EGC) is defined as one that is limited to the mucosa or submucosa, regardless of the existence of nodal metastases[1]. The incidence of lymph node metastasis in EGC is very low. If the EGC is confined to the mucosa, the incidence is estimated at around 3%. When the EGC reaches the submucosa, rises to nearly 20%[2]. The existence of nodal metastases influence the type of treatment to be used. In these cases, surgical treatment is recommended along with adjuvant therapy. Overall the EGC has a good prognosis, with a 5-year survival rate of over 90%[3]. There are different therapeutic options for the treatment of early gastric cancer. At present, endoscopic resection provides a minimally invasive treatment with a similar efficacy to surgery.
TREATMENT MODALITIES
Therapeutic modalities for EGC range from endoscopic resection to gastrectomy and adjuvant treatments. Therefore, it is essential to perform an adequate staging of cancer as to determine which patients are candidates for either therapy.
ENDOSCOPIC TREATMENT
Endoscopic therapy is a minimally invasive treatment that allows the patient to preserve the entire stomach and maintain a good quality of life. Moreover, the cost is usually less and efficacy comparable to surgery. The degree of difficulty in performing endoscopic resection depends on the location of the lesion in the stomach, being the difficulty higher for resection of lesions localized on the posterior wall and lesser curvature. Depressed type of EGC is the most common. To facilitate the visualization of the lesion mucolytic and defoaming agents are used (e.g., Acetylcysteine and Dimethicone, respectively). Endoscopic therapy is directed to selected patients in whom there is no evidence or risk of lymph node involvement. Endoscopic resection vs ablative technique allows assessing the specimen thus becoming the optimal method of staging for early gastric cancer[4]. Also, endoscopic therapy does not prevent a subsequent surgical therapy if needed. EUS has limited staging accuracy (80%-90%) and therefore would result in unnecessary surgery in up to 10%-20% of patients[5,6]. Endoscopic resection allows the pathologist to assess the depth of invasion, degree of differentiation and lymphatic and vascular involvement, thus allowing for a prediction of the risk of metastases in the lymph nodes. This is crucial for a correct diagnosis and risk stratification of metastasis. The main endoscopic techniques used are endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). According to the histological and morphological findings gastric carcinoma can be divided into differentiated (intestinal) and undifferentiated (diffuse)[7]. The risk for nodal metastasis for differentiated and undifferentiated EGC is around 0.4% and 4%, respectively. Endoscopic resection techniques can be applied, according to “standard” criteria, in patients with lesions resectable en bloc which meet histological criteria (intestinal type adenocarcinoma limited to the mucosa without venous or lymphatic invasion) and morphological criteria (< 20 mm without ulceration; < 10 mm for flat and depressed lesions)[8,9]. When these criteria are met, the risk of lymph node involvement is not more than 1.7%. In addition, “expanded” criteria for endoscopic resection have been defined which include: (1) EGC intestinal type mucosa confined to any size without ulceration; (2) EGC intestinal type confined to the mucosa < 3 cm with ulceration; and (3) EGC intestinal type < 3 cm confined to the upper 0.5 mm from the submucosa (sm1 < 500 μm) without lymphovascular involvement and 4. ECG poorly differentiated, < 2 cm, not ulcerated[10,11]. Expanded criteria for ER reduces the need for gastrectomy in EGC (Table 1). When ER has been performed for poorly differentiated type of EGC results for patients who declined surgical treatment showed: en bloc resection rate 83%, complete resection rate 81%, clinical remission 93%, and recurrence in only 7%[12].
Table 1.
Treatments options in a patient with early gastric cancer
| Histology | Mucosal cancer | Submucosal cancer | |||||
| ≤ 10 mm | ≤ 20 mm > 20 mm | ≤ 30 mm > 30 mm | Into the upper third (≤ 30 mm) | Into the middle third (any size) | |||
| (flat/depressed) | (No ulceration) | (Ulceration) | |||||
| Intestinal type | EMR | EMR | ESD | ESD | Surgery | ESD | Surgery |
| Diffuse type | Surgery | Surgery | Surgery | Surgery | Surgery | Surgery | |
| ESD1 | |||||||
Treatment option if the patient decline surgery. EGC: Early gastric cancer; EMR: Endoscopic mucosal resection; ESD: Endoscopic submucosal dissection.
Endoscopic mucosal resection
Currently endoscopic mucosal resection (EMR) is considered as an effective and safe treatment for superficial lesions. Requires specific endoscopic experience and the endoscopist needs to be prepared to try to resolve the possible complications that may arise during the implementation of the technique. Over the last years different EMR techniques have been described[13]: (1) Strip biopsy[14]. This resection technique designed to remove small lesions requires the use of a dual channel endoscope. It simultaneously uses a polypectomy snare and a biopsy forceps to achieve the resection; (2) Endoscopic double snare polypectomy; (3) EMR using a transparent plastic cap, initially developed in 1992 for resection of early oesophageal cancer and later for resection of early gastric cancer[15]; and (4) EMR using a ligation device (Multiband mucosectomy)[16,17] (Figure 1). These last two are the techniques for endoscopic mucosal resection most widely used in the treatment of EGC. However, in lesions greater than 20 mm, recurrence rate may be increased as they might require a piecemeal resection[18]. Therefore, EMR is the procedure of choice in patients with EGC who meet the standard criteria for endoscopic resection. Different studies have shown excellent results using EMR with figures for complete resection and survival at 5 years greater than 85%-90%[19,20]. The risk of local recurrence associated with EMR is variable. If the resection is piecemeal the risk of recurrence rate is set below 35%, being practically nonexistent if en bloc resection was possible[21,22]. In cases of incomplete resection with EMR, gastrectomy might be indicated if the tumor has submucosal or lymphovascular involvement or positive resection margins. However, in cases where the patient is a poor surgical candidate further endoscopic resection could be considered with good results, especially if incomplete resection is due to the presence of positive lateral margins of resection[23,24]. In treatment with EMR a suitable distance of at least 2 mm between the EGC and the edge of the specimen is required to achieve complete resection. Indigo carmine chromoendoscopy is the most useful method to determine the lateral margin of EGC.
Figure 1.
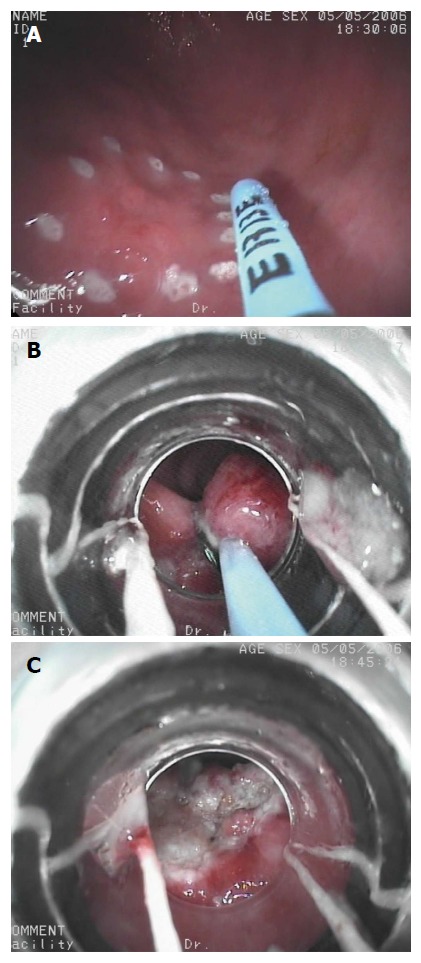
Endoscopic mucosal resection with Multiband Ligator for early gastric cancer. A: Argon plasma coagulation is used for marking early gastric cancer; B: A multiband ligator was used to create a pseudopolyp and it is removed by a minipolypectomy snare using pure coagulating current; C: Residual scar after Multiband Mucosectomy.
Endoscopic submucosal resection
Endoscopic submucosal resection (ESD) is a complex and demanding technique that allows en bloc resection of larger EGC, avoiding piecemeal resection of the EMR and therefore the risk of recurrence[25-27]. Similar to the EMR, its main indication is resection of superficial tumors with no risk of lymph node metastasis. Expanded criteria have been proposed for endoscopic resection with ESD, as with this technique large en bloc resections are possible. ESD for EGC with expanded criteria have long-term survival and outcomes similar to those of patients treated according to the traditional criteria (5-year survival rate 93% and 92%, respectively)[28]. In ESD, the lesion is marked circumferentially, usually by applying soft coagulation current. Then, a solution with saline (0.9% NS), adrenaline and dyes (indigo carmine, methylene blue) is injected into the submucosa allowing distinction between the submucosal and muscular layer. Some authors do not recommend the use of Methylene blue because it is absorbed into the cell nucleus, which results in intense staining that hampers visualization[29]. To avoid the short duration of the lifting effect of submucosal injection, others have suggested the use of substances with a viscosity grade higher than saline (0.9% NS). The use of hyaluronic acid has been proposed but its high price, has conditioned its use[30,31]. Glycerol 10% could be a good and cheap alternative[32]. Finally, the lesion is dissected and removed en bloc using different types of needles, specific for each step of the procedure (Needle Knife, IT Knife, Flex knife, Hook knife, Triangle-tip knife, Dual Knife, Hybrid Knife, Flush knife and others) typically done with coagulating current. Some needles have at the tip an insulating material with a protective function that allows for a safer dissection[33-35] (Figure 2). The main functions that must meet the ESD devices are: marking, injection, precutting, circumferential incision, submucosal disection and hemostasis (Table 2). However the choice of needle depends on the availability, familiarity and personal preference of the endoscopist as there are no studies that demonstrate the superiority of one over the other. Sometimes it can be useful to use a transparent plastic cap on the endoscope tip that allows more control during dissection. Moreover, these devices use cutting currents, coagulation or a mixture of both through electrosurgical generators. CO2 insufflation is recommended because it causes less luminal distension. Furthermore, if there is a perforation the leaking CO2 will rapidly be reabsorbed decreasing the intraperitoneal pressure and then the respiratory compromise[36]. From a technical point of view, ESD is more challenging than EMR and requires more “preparation”. However, the main advantage of the ESD over other techniques is that it allows en bloc resection of larger lesions reducing the rate of local recurrence. As demonstrated in comparative studies between EMR and ESD, with ESD success rates between 95%-98% for in-bloc resection and survival at 5 years of 83%-97%[37,38]. The ESD requires skill and a long learning curve[39-42]. In cases when resection with ESD is incomplete (positive resection margins, invasion of the submucosa or muscularis, lymphovascular invasion or undifferentiated cancer), surgery should be considered (gastrectomy with perigastric nodal resection)[43]. The role of laparoscopic perigastric nodal resection is not clearly established but may be considered as an alternative[44]. ESD can be use in elderly patients as well as in those who require antiplatelet therapy for high risk of thrombosis[2]. Proposed strategy for endoscopic treatment by ESD set 4 levels: capability for EGC detection and knowledge of the indications of ESD, observation of several ESD procedures performed by expert endoscopists, perform dissections in ex-vivo animal models followed by procedures in animal models in vivo and finally performing selected (simple) ESD in humans under expert supervision. Then, continue with training in animal models to acquire more skill. About 20 annual cases of ESD are considered necessary to acquire competence in ESD[2]. In Japan and Korea the incidence of early gastric cancer is significantly higher compared to the West. Therefore in the West, the opportunities to conduct training in gastric EDS are scarce.
Figure 2.
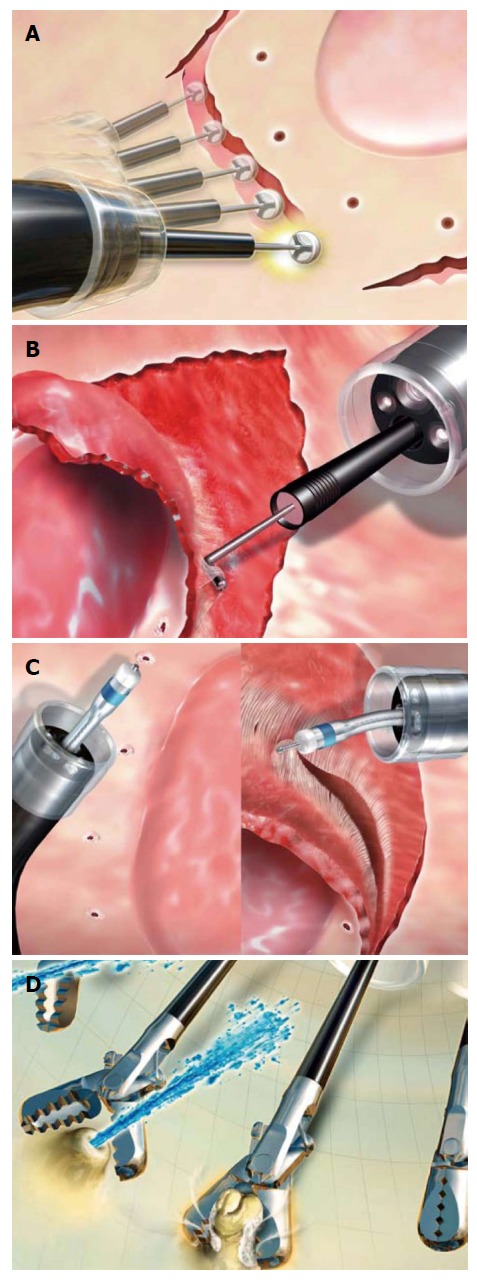
Different types of devices, specific for each step of the Endoscopic submucosal dissection procedure. A: ITknife-2; B: Hook knife; C: Dual knife; D: Grasper for haemostasis. (Courtesy of Olympus Medical Systems,Tokyo, Japan).
Table 2.
Process steps in endoscopic submucosal dissection treatment of the early gastric cancer
| Process steps | Technique/devices |
| Estimation of lateral extension | Chomoendoscopy (indigo carmine) ± NBI |
| Marking | Mucosal markings are placed 5 mm lateral to the lesion margin |
| Submucosal injection | Injection of saline mixed with diluted epinephrine (1:100000) and indigo carmine into the submucosal layer |
| Mucosal incision (precutting) | A small initial mucosal incision is made to gain access to the submucosal space without to injure the muscularis propria (e.g., by Dual knife) |
| Circumferential incisión | Carried out 5 mm lateral to the mucosal markings (e.g., IT knife) |
| Submucosal dissection | The technique varies among endoscopist |
| Adequate reinjection of fluid into the submucosa | |
| The parallel movement for muscle layer with the IT2 is typically lateral | |
| With the Dual knife forward |
NBI: Narrow band imaging.
ENDOSCOPIC COMPLICATIONS
Various complications after endoscopic treatment for EGC have been described: bleeding, perforation, stenosis, aspiration pneumonia, phlegmonous gastritis, mediastinal emphysema. Of these, the most common is bleeding, the average incidence is set at 9% and usually occur during the process or within 24 h[45]. Depending on the time of onset, bleeding can be classified as: (1) immediate; (2) early (within the procedure); or (3) late (post-procedure). The immediate bleeding is less common in the distal portion of the stomach as the submucosal arteries are of lower caliber[46]. Acute bleeding may obscure the visual field, leading to a higher risk of complications. Therefore, endoscopic hemostasis should be immediately performed. The incidence of delayed bleeding after ESD is below 15%[37] and different factors have been related to its appearance: macroscopic appearance (large size > 40 mm, depressed or flat lesion), location in the middle or upper third, advanced age (> 80 years), limited endoscopic experience, timely procedure or treatment of recurrent lesions[47,48]. Late risk of bleeding after ESD may decrease significantly by prophylactic electrocoagulation of large visible submucosal vessels. This technique is preferable to other types of hemostasis such as clips that can hinder the completion of the procedure[49]. Currently, there is no evidence that the realization of a second-look contributes significantly to reduce the risk of late bleeding following ESD. While it is habitual to advice antisecretory therapy over the following weeks, this practice has not demonstrated benefit in lowering the rate of delayed bleeding[50]. The incidence of perforation ranges from 1%-20% depending on experience[51-53]. The use of dye injection (e.g., Indigo carmine) allows to better identify the muscle layer making ESD a safer technique. Perforation can be diagnosed during or after the procedure (frank perforation or micro-perforation, respectively). However, no evidence of lymph node metastasis and/or peritoneal dissemination caused by gastric perforation has been reported[54]. If a perforation is immediately noticed during the procedure and its size is small, it can be treated endoscopically with clips and broad spectrum antibiotics. In these cases absolute diet is recommended for at least 2 d[55]. Conversely, if the perforation is large, urgent surgery is required. It is possible that CO2 insufflation may reduce the risk of perforation[56]. If free air is found on a plain chest X-ray after the ER (micro-perforation), the management (conservative or surgical) is not conclusively established. The appearance of scar stenosis is uncommon (0.6%-2%) and is associated with extensive resections in the gastric antrum[51]. Local administration triamcinolone can be used as an attempt to prevent this complication[57]. Balloon dilation is the endoscopic treatment most frequently used for this complication, but involves some risk of perforation[58]. Aspiration pneumonia is rare (0.7%-1.5%) and is associated with prolonged procedures.
FOLLOW-UP AFTER ENDOSCOPIC RESECTION
EGC patients treated by endoscopic resection with curative intent, require monitoring to detect local recurrence and metachronous gastric cancer. In patients with EGC who meet “standard” criteria for endoscopic resection, it is advisable to perform an upper gastrointestinal endoscopy yearly. Patients who meet “expanded” criteria, in addition to the annual endoscopy, monitoring can be performed alternating abdominal computed tomography and endoscopic ultrasound every 6 mo for 3 years. The objective of this additional monitoring is to detect lymph node and distant metastases[59].
SURGICAL TREATMENT
Although endoscopic resection is an option in patients with EGC who meet the above criteria, surgical treatment continues to be a widespread therapeutic option worldwide with survival rates at 5 years of 97%[60]. Currently, there are no comparative studies between gastrectomy and endoscopic treatment. However, several results show clinical prognosis to be similar although patients with endoscopic treatment benefit from a shorter hospital stay and lower costs[61,62]. Patients who do not meet the criteria for endoscopic resection have a higher risk of lymph node metastases which forces a gastrectomy with perigastric lymph node excision. Another indication for gastrectomy is the detection during staging of lymph nodes or a high suspicion of their existence. The type of gastrectomy (subtotal gastrectomy or total) is determined by the location of the lesion, reserving the subtotal gastrectomy for EGC located in the lower two thirds of the stomach. Another option is laparoscopic gastrectomy. Laparoscopic gastrectomy was initially reported in Japan in 1994[63]. Open gastrectomy is still performed more frequently in the Western countries than laparoscopic resection even for patients with early stage disease[64]. In Japan, EGC (T1N0 or T2N0) is considered as the only indication for laparoscopic gastrectomy. A recent review that included 22 studies show that laparoscopic gastrectomy vs open gastrectomy offers a similar prognosis with significantly lower postoperative morbidity, lower intraoperative blood use, shorter hospital stay and no increased rates of recurrence. Furthermore conversion rates to open laparoscopic surgery were less than 3%[65].
The surgical outcome of gastric cancer in obese patients is controversial. The number of lymph nodes retrieved is, in these patients, higher[66]. Moreover, obesity is an independent risk factor for developing 30-d postdischarge complications[67].
ADJUVANT THERAPIES
It is known that chronic infection with Helicobacter pylori is a risk factor of developing gastric cancer. Currently, treatment of Helicobacter pylori infection in all patients with EGC is recommended, regardless of the chosen treatment option to reduce the risk of metachronous gastric cancer[68,69]. The need for adjuvant therapy (chemotherapy, radiotherapy) in patients with EGC treated with complete endoscopic resection is debated. Recent guidelines recommend observation, avoiding adjuvant therapy in patients with T1N0 disease without involvement of the resection margins. However, adjuvant treatment is clearly indicated in patients with positive lymph node involvement.
CONCLUSION
Endoscopic resection (EMR/ESD) is a safe and effective staging and therapeutic modality for selected patients with early gastric cancer. Patients with “standard” and “expanded” criteria can be successfully treated by EMR and ESD techniques, respectively. Surgical treatment continues to be a widespread therapeutic option in patients with incomplete endoscopic resection or advanced gastric cancer.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 26, 2015
First decision: June 2, 2015
Article in press: August 28, 2015
P- Reviewer: Murata A, Tarantino G S- Editor: Ma YJ L- Editor: A E- Editor: Jiao XK
References
- 1.Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006;22:561–569. doi: 10.1097/01.mog.0000239873.06243.00. [DOI] [PubMed] [Google Scholar]
- 2.Fernández-Esparrach G, Calderón Á, De-la-Peña J, Díaz-Tasende JB, Esteban JM, Gimeno-García AZ, Herreros-de-Tejada A, Martínez-Ares D, Nicolás-Pérez D, Nogales Ó, et al. Endoscopic submucosal dissection. Sociedad Española de Endoscopia Digestiva (SEED) clinical guideline. Rev Esp Enferm Dig. 2014;106:120–132. doi: 10.4321/s1130-01082014000200007. [DOI] [PubMed] [Google Scholar]
- 3.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadi A, Draganov P. Endoscopic mucosal resection in the upper gastrointestinal tract. World J Gastroenterol. 2008;14:1984–1989. doi: 10.3748/wjg.14.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanai H, Noguchi T, Mizumachi S, Tokiyama H, Nakamura H, Tada M, Okita K. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut. 1999;44:361–365. doi: 10.1136/gut.44.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hizawa K, Iwai K, Esaki M, Matsumoto T, Suekane H, Iida M. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer? Endoscopy. 2002;34:973–978. doi: 10.1055/s-2002-35851. [DOI] [PubMed] [Google Scholar]
- 7.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt At A Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Min YW, Min BH, Lee JH, Kim JJ. Endoscopic treatment for early gastric cancer. World J Gastroenterol. 2014;20:4566–4573. doi: 10.3748/wjg.v20.i16.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–4498. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 10.Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim do H, Song HJ, Lee GH, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485–493. doi: 10.1016/j.gie.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Kang HJ, Kim DH, Jeon TY, Lee SH, Shin N, Chae SH, Kim GH, Song GA, Kim DH, Srivastava A, et al. Lymph node metastasis from intestinal-type early gastric cancer: experience in a single institution and reassessment of the extended criteria for endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:508–515. doi: 10.1016/j.gie.2010.03.1077. [DOI] [PubMed] [Google Scholar]
- 12.Ryu CB, Kim SG, Jung IS, Kwon KW, Hong SJ, Ko ES, Cho JY, Lee JS, Lee MS, Shim CS, et al. Is It Possible to Perform EMR in Poorly Differentiated Type of Early Gastric Cancer? Gastrointestinal Endosc. 2005;61:AB180. [Google Scholar]
- 13.Marc G, Lopes CV. Endoscopic resection of superficial gastrointestinal tumors. World J Gastroenterol. 2008;14:4600–4606. doi: 10.3748/wjg.14.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tada M, Shimada M, Murakami F, Mizumachi M, Arima K, Yanai H. Development of strip-off biopsy (in Japanese with English abstract) Gastroenterol Endosc. 1984;26:833–839. [Google Scholar]
- 15.Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58–62. doi: 10.1016/s0016-5107(93)70012-7. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y, Hiraishi H, Kanke K, Watanabe H, Ueno N, Ishida M, Masuyama H, Terano A. Treatment of gastric tumors by endoscopic mucosal resection with a ligating device. Gastrointest Endosc. 1999;49:192–199. doi: 10.1016/s0016-5107(99)70485-2. [DOI] [PubMed] [Google Scholar]
- 17.Espinel J, Pinedo E, Rascarachi G. Endoscopic mucosal resection with a multiband ligator for the treatment of Barrett s high-grade dysplasia and early gastric cancer. Rev Esp Enferm Dig. 2009;101:403–407. doi: 10.4321/s1130-01082009000600005. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe S, Koizumi W, Mitomi H, Nakai H, Murakami S, Nagaba S, Kida M, Oida M, Saigenji K. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc. 2002;56:708–713. doi: 10.1067/mge.2002.129085. [DOI] [PubMed] [Google Scholar]
- 19.Takekoshi T, Baba Y, Ota H, Kato Y, Yanagisawa A, Takagi K, Noguchi Y. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352–358. doi: 10.1055/s-2007-1008990. [DOI] [PubMed] [Google Scholar]
- 20.Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, Takeuchi Y, Imanaka K, Yamada T, Yamamoto S, Yamamoto S, et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88–92. doi: 10.1007/s10120-005-0357-0. [DOI] [PubMed] [Google Scholar]
- 21.Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Fukuda K, Fujita Y, Ninomiya K, Tano S, Katurahara M, et al. Risk for local recurrence of early gastric cancer treated with piecemeal endoscopic mucosal resection during a 10-year follow-up period. Surg Endosc. 2012;26:72–78. doi: 10.1007/s00464-011-1830-y. [DOI] [PubMed] [Google Scholar]
- 22.Kojima T, Parra-Blanco A, Takahashi H, Fujita R. Outcome of endoscopic mucosal resection for early gastric cancer: review of the Japanese literature. Gastrointest Endosc. 1998;48:550–554 discussion 554-555. doi: 10.1016/s0016-5107(98)70108-7. [DOI] [PubMed] [Google Scholar]
- 23.Ahn JY, Jung HY, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Choi KD, Song HJ, Lee GH, et al. Natural course of noncurative endoscopic resection of differentiated early gastric cancer. Endoscopy. 2012;44:1114–1120. doi: 10.1055/s-0032-1325676. [DOI] [PubMed] [Google Scholar]
- 24.Nagano H, Ohyama S, Fukunaga T, Seto Y, Fujisaki J, Yamaguchi T, Yamamoto N, Kato Y, Yamaguchi A. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer. 2005;8:149–154. doi: 10.1007/s10120-005-0328-5. [DOI] [PubMed] [Google Scholar]
- 25.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 26.Probst A, Pommer B, Golger D, Anthuber M, Arnholdt H, Messmann H. Endoscopic submucosal dissection in gastric neoplasia - experience from a European center. Endoscopy. 2010;42:1037–1044. doi: 10.1055/s-0030-1255668. [DOI] [PubMed] [Google Scholar]
- 27.Cho KB, Jeon WJ, Kim JJ. Worldwide experiences of endoscopic submucosal dissection: not just Eastern acrobatics. World J Gastroenterol. 2011;17:2611–2617. doi: 10.3748/wjg.v17.i21.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotoda T, Iwasaki M, Kusano C, Seewald S, Oda I. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010;97:868–871. doi: 10.1002/bjs.7033. [DOI] [PubMed] [Google Scholar]
- 29.Chan EP, Kaltenbach T, Rouse RV, Soetikno R. Potential hazards of submucosal injection of methylene blue. Am J Gastroenterol. 2012;107:633–634. doi: 10.1038/ajg.2011.462. [DOI] [PubMed] [Google Scholar]
- 30.Yeh RW, Triadafilopoulos G. Submucosal injection: safety cushion at what cost? Gastrointest Endosc. 2005;62:943–945. doi: 10.1016/j.gie.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto H, Yube T, Isoda N, Sato Y, Sekine Y, Higashizawa T, Ido K, Kimura K, Kanai N. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251–256. doi: 10.1016/s0016-5107(99)70234-8. [DOI] [PubMed] [Google Scholar]
- 32.Uraoka T, Fujii T, Saito Y, Sumiyoshi T, Emura F, Bhandari P, Matsuda T, Fu KI, Saito D. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736–740. doi: 10.1016/s0016-5107(05)00321-4. [DOI] [PubMed] [Google Scholar]
- 33.Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kwon R, Mamula P, Rodriguez S, Shah RJ, Wong Kee Song LM, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11–18. doi: 10.1016/j.gie.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 34.Toyonaga T, Nishino E, Man-I M, East JE, Azuma T. Principles of quality controlled endoscopic submucosal dissection with appropriate dissection level and high quality resected specimen. Clin Endosc. 2012;45:362–374. doi: 10.5946/ce.2012.45.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui N, Akahoshi K, Nakamura K, Ihara E, Kita H. Endoscopic submucosal dissection for removal of superficial gastrointestinal neoplasms: A technical review. World J Gastrointest Endosc. 2012;4:123–136. doi: 10.4253/wjge.v4.i4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nonaka S, Saito Y, Takisawa H, Kim Y, Kikuchi T, Oda I. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc. 2010;24:1638–1645. doi: 10.1007/s00464-009-0824-5. [DOI] [PubMed] [Google Scholar]
- 37.Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 38.Kosaka T, Endo M, Toya Y, Abiko Y, Kudara N, Inomata M, Chiba T, Takikawa Y, Suzuki K, Sugai T. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc. 2014;26:183–191. doi: 10.1111/den.12099. [DOI] [PubMed] [Google Scholar]
- 39.Oda I, Odagaki T, Suzuki H, Nonaka S, Yoshinaga S. Learning curve for endoscopic submucosal dissection of early gastric cancer based on trainee experience. Dig Endosc. 2012;24 Suppl 1:129–132. doi: 10.1111/j.1443-1661.2012.01265.x. [DOI] [PubMed] [Google Scholar]
- 40.Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa N, Lopes C, Moreira-Dias L. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69:350–355. doi: 10.1016/j.gie.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 41.Deprez PH, Bergman JJ, Meisner S, Ponchon T, Repici A, Dinis-Ribeiro M, Haringsma J. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy. 2010;42:853–858. doi: 10.1055/s-0030-1255563. [DOI] [PubMed] [Google Scholar]
- 42.Vázquez-Sequeiros E, de Miquel DB, Olcina JR, Martín JA, García M, Lucas DJ, Garrido E, González C, Blanco AP, Arnau MR, et al. Training model for teaching endoscopic submucosal dissection of gastric tumors. Rev Esp Enferm Dig. 2009;101:546–552. doi: 10.4321/s1130-01082009000800005. [DOI] [PubMed] [Google Scholar]
- 43.Jung H, Bae JM, Choi MG, Noh JH, Sohn TS, Kim S. Surgical outcome after incomplete endoscopic submucosal dissection of gastric cancer. Br J Surg. 2011;98:73–78. doi: 10.1002/bjs.7274. [DOI] [PubMed] [Google Scholar]
- 44.Abe N, Takeuchi H, Ohki A, Yanagida O, Masaki T, Mori T, Sugiyama M. Long-term outcomes of combination of endoscopic submucosal dissection and laparoscopic lymph node dissection without gastrectomy for early gastric cancer patients who have a potential risk of lymph node metastasis. Gastrointest Endosc. 2011;74:792–797. doi: 10.1016/j.gie.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390–396. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]
- 46.Toyonaga T, Nishino E, Hirooka T, Ueda C, Noda K. Intraoperative bleeding in endoscopic submucosal dissection in the stomach and strategy for prevention and treatment. Dig Endosc. 2006;18:S123–S127. [Google Scholar]
- 47.Okada K, Yamamoto Y, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Chino A, Tsuchida T, Fujisaki J, et al. Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc. 2011;25:98–107. doi: 10.1007/s00464-010-1137-4. [DOI] [PubMed] [Google Scholar]
- 48.Shiba M, Higuchi K, Kadouchi K, Montani A, Yamamori K, Okazaki H, Taguchi M, Wada T, Itani A, Watanabe T, et al. Risk factors for bleeding after endoscopic mucosal resection. World J Gastroenterol. 2005;11:7335–7339. doi: 10.3748/wjg.v11.i46.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179–183. doi: 10.1055/s-2007-995530. [DOI] [PubMed] [Google Scholar]
- 50.Ono S, Kato M, Ono Y, Nakagawa M, Nakagawa S, Shimizu Y, Asaka M. Effects of preoperative administration of omeprazole on bleeding after endoscopic submucosal dissection: a prospective randomized controlled trial. Endoscopy. 2009;41:299–303. doi: 10.1055/s-0029-1214530. [DOI] [PubMed] [Google Scholar]
- 51.Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc. 2013;25 Suppl 1:71–78. doi: 10.1111/j.1443-1661.2012.01376.x. [DOI] [PubMed] [Google Scholar]
- 52.Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763–770. doi: 10.1016/j.gie.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907–912. doi: 10.1111/j.1440-1746.2011.07039.x. [DOI] [PubMed] [Google Scholar]
- 54.Ikehara H, Gotoda T, Ono H, Oda I, Saito D. Gastric perforation during endoscopic resection for gastric carcinoma and the risk of peritoneal dissemination. Br J Surg. 2007;94:992–995. doi: 10.1002/bjs.5636. [DOI] [PubMed] [Google Scholar]
- 55.Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video) Gastrointest Endosc. 2006;63:596–601. doi: 10.1016/j.gie.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 56.Dellon ES, Hawk JS, Grimm IS, Shaheen NJ. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc. 2009;69:843–849. doi: 10.1016/j.gie.2008.05.067. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto S, Kobayashi M, Takeuchi M, Sato Y, Narisawa R, Aoyagi Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1389–1393. doi: 10.1016/j.gie.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 58.Tsunada S, Ogata S, Mannen K, Arima S, Sakata Y, Shiraishi R, Shimoda R, Ootani H, Yamaguchi K, Fujise T, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc. 2008;67:979–983. doi: 10.1016/j.gie.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 59.Gotoda T, Jung HY. Endoscopic resection (endoscopic mucosal resection/ endoscopic submucosal dissection) for early gastric cancer. Dig Endosc. 2013;25 Suppl 1:55–63. doi: 10.1111/den.12003. [DOI] [PubMed] [Google Scholar]
- 60.Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc. 2009;23:1759–1763. doi: 10.1007/s00464-008-0198-0. [DOI] [PubMed] [Google Scholar]
- 61.Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukuda K, Saito H, Tatebe S. Evaluation of a pylorus-preserving gastrectomy for patients preoperatively diagnosed with early gastric cancer located in the middle third of the stomach. Surg Today. 2010;40:228–233. doi: 10.1007/s00595-009-4043-4. [DOI] [PubMed] [Google Scholar]
- 62.Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, Kim do H, Lee JH, Kim MY, Kim BS, Oh ST, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942–948. doi: 10.1016/j.gie.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 63.Kitano S, Tomikawa M, Iso Y, Hashizume M, Moriyama M, Sugimachi K. Laparoscopy-assisted devascularization of the lower esophagus and upper stomach in the management of gastric varices. Endoscopy. 1994;26:470–473. doi: 10.1055/s-2007-1009006. [DOI] [PubMed] [Google Scholar]
- 64.Bamboat ZM, Strong VE. Minimally invasive surgery for gastric cancer. J Surg Oncol. 2013;107:271–276. doi: 10.1002/jso.23237. [DOI] [PubMed] [Google Scholar]
- 65.Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg. 2012;256:39–52. doi: 10.1097/SLA.0b013e3182583e2e. [DOI] [PubMed] [Google Scholar]
- 66.Attaallah W, Uprak K, Javadov M, Yegen C. Impact of body mass index on number of lymph nodes retrieved in gastric cancer patients. Hepatogastroenterology. 2014;61:2425–2427. [PubMed] [Google Scholar]
- 67.Jeong O, Kyu Park Y, Ran Jung M, Yeop Ryu S. Analysis of 30-day postdischarge morbidity and readmission after radical gastrectomy for gastric carcinoma: a single-center study of 2107 patients with prospective data. Medicine (Baltimore) 2015;94:e259. doi: 10.1097/MD.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwon YH, Heo J, Lee HS, Cho CM, Jeon SW. Failure of Helicobacter pylori eradication and age are independent risk factors for recurrent neoplasia after endoscopic resection of early gastric cancer in 283 patients. Aliment Pharmacol Ther. 2014;39:609–618. doi: 10.1111/apt.12633. [DOI] [PubMed] [Google Scholar]
- 69.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M; Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]


