Abstract
SNPs may restrict cell detoxification activity and be a potential risk factor for cancer chemosensitivity. We evaluated the predictive value of these polymorphisms on the sensitivity of bladder cancer patients to epirubicin and mitomycin chemotherapy instillation as well as their toxicities. SNPs were analyzed by TaqMan genotyping assays in 130 patients treated with epirubicin and 114 patients treated with mitomycin. Recurrence-free survival (RFS) was estimated by the Kaplan-Meier method, and hazard ratios (HRs) and 95% confidence intervals (CIs) of the HRs were derived from multivariate Cox proportional hazard models. GSTP1 rs1695 and GSTO1 rs4925 were also associated with RFS in the epirubicin group. Patients carrying the GSTP1 AG+GG and GSTO1 AC+AA genotypes had an unfavorable RFS. Patients with the GSTP1 AA and GSTO1 CC genotypes had a reduced risk of recurrence after the instillation of epirubicin. In addition, patients with the GSTP1 rs1695 AA genotype had an increased risk of irritative voiding symptoms; while patients with the GSTO1 rs4925 CC genotype had a decreased risk of hematuria. Our results suggest that GSTP1 and GSTO1 polymorphisms are associated with epirubicin treatment outcomes as well as with epirubicin-related toxicity.
Bladder cancer is the most common malignancy of the urinary tract, with 74,690 cases and 15,580 deaths in the United States in 20131. Notwithstanding multidisciplinary advances in its treatment, bladder cancer continues to have an unacceptably high morbidity and mortality2. Approximately 80% of all patients with bladder cancer initially present with superficial tumors, of which 12.5% progress to invasive disease3,4. Symptoms of early bladder cancer that alert patients to seek medical advice include hematuria and urinary frequency. For patients suspected of having bladder cancer, cystoscopy and transurethral resection are used for diagnosis as well as for total endoscopic tumor resection. However, for patients with grade Ta or T1 lesions, tumor recurrence may present a major problem. Twenty percent of patients with low-risk disease and 40% with medium-risk disease will develop tumor recurrence within one year after transurethral resection of the bladder tumor. Nevertheless, patients with high-risk disease will present a greater recurrence rate (90%) at 2 years after transurethral resection5. Intravesical therapy is the most commonly used therapeutic approach for bladder cancer, whereby chemical agents are instilled into the bladder to improve local control and decrease the risk of cancer progression.
Various chemotherapeutics have been administered intravesically to manage superficial bladder cancer. It has been shown that intravesical chemotherapy can effectively reduce disease recurrence within the first 1–5 years after tumor resection6. Chemotherapeutic agents, such as thio-tepa, doxorubicin, epirubicin, mitomycin (MMC), or Bacillus Calmette-Guerin (BCG), often have been utilized as prophylactic treatment to prevent tumor recurrence7,8,9. Although the intravesical instillation of BCG has been shown to be more effective than chemotherapeutic agents for prophylactic treatment7, BCG has the disadvantage of causing various and frequent local or systemic side effects. Besides, BCG is not an easily accessible instillation agent in China. Epirubicin, an anthracycline-containing drug and a stereoisomer of doxorubicin, has been considered the standard treatment for various cancers. Moreover, it has been shown that epirubicin has slightly fewer adverse drug reactions than other agents10. Indeed, Kurth et al.11 have shown a direct relationship between the epirubicin dose and its effect on tumors in situ as well as side effects caused by treatment. Thus, epirubicin is also very frequently used to treat bladder cancer patients due to its few and mild complications. Another standard chemotherapeutic agent, MMC, is the most frequently used chemotherapeutic agent to treat superficial bladder cancer because of its low therapeutic concentration recommended for intravesical infusion8.
The clinical response to chemotherapy or instillation agents is influenced by both genetic and environmental factors. Interindividual differences in pharmacokinetics and pharmacodynamics play a primordial role in the response and toxicity profile of different agents. Furthermore, drug absorption, distribution, metabolism, and excretion are controlled by various genetic factors. Glutathione S-transferases (GSTs) are a class of detoxification enzymes that catalyze the conjugation of potentially damaging chemical mutagens to glutathione and protect against the products of oxidative stress; therefore, they are considered as the most important phase II metabolizing enzymes12. Studies have shown that upregulated GST activity is a hallmark of a malignant bladder cancer phenotype and is involved in the maintenance of the prooxidant–antioxidant balance towards a more reduced state during tumor progression13. GSTP1 has a high level of expression in the bladder and plays a central role in the inactivation of toxic and carcinogenic compounds14. GSTP1 allelic variants may cause an increased susceptibility to oxidative DNA damage and to the accumulation of DNA base adducts15. The tendency of GST polymorphisms to alter carcinogen metabolism is well established, with GSTP1 polymorphisms having been extensively studied in humans. GSTP1 rs1695, which is characterized by an A-to-G transition at nucleotide 313 (codon 105, exon 5), can cause an isoleucine-to-valine change (Ile105Val)16. The GSTP1 rs1695 polymorphism significantly influences the enzymatic activity and is linked to the clinical outcome of patients who receive platinum-based chemotherapy17. The GSTP1 enzyme having Val105 shows a catalytic efficiency for the diol epoxides of polycyclic aromatic hydrocarbons that is seven-fold higher than the isoenzymes having Ile105. Furthermore, the GSTP1 GG genotype was found to be 2–3 times less stable than the AA genotype and was associated with a higher hydrophilic DNA adduct level18.
Both GSTO1 and GSTO2 are composed of 6 exons and are separated by 7.5 kb on chromosome 10q24.3. GSTO2 has 64% aminoacid identity with GSTO119. GSTO1 has glutathione-dependent thiol transfer and dehydroascorbate reductase activities20 and may play a protective role against calcium-induced apoptosis21. GSTO2 also exhibited glutathione-dependent thiol transferase and dehydroascorbate reductase activities. Differently from GSTO1, GSTO2 has a high catalytic activity with 1-chloro-2,4-dinitrobenzene (CDNB) and may play a role in cellular signaling22. GSTO1 rs4925 polymorphism has been found to be associated with age-onset Alzheimer and Parkinson diseases23 and a risk of human cancers, such as hepatocellular carcinoma, cholangiocarcinoma, and breast cancer24. It has recently been suggested that GSTO1 and GSTO2 gene polymorphisms might influence the level of oxidative stress in acute promyelocytic leukemia25. However, GSTO2 rs156697 has been identified as one of the polymorphisms in the GSTO2 gene. The association of GSTO2 polymorphism with human cancers, such as hepatocellular carcinoma, colorectal and ovarian cancer has been demonstrated but it appears not to be significantly associated with cancer risk. Moreover, Djukic et al. have shown that the GSTO1 rs4925 and GSTO2 rs156697 polymorphism is associated with a worse prognosis and a shorter survival in muscle-invasive bladder cancer patients. In the same study, the GSTP1 Ile allele carriers exhibited an increased overall mortality risk12. ATP-binding cassette transporters are transmembrane proteins responsible for most of the drug transport across membranes26. It has been shown that polymorphisms in ATP-binding cassette subfamily B (MDR/TAP), member 1 (ABCB1) may restrict the cell detoxification activity and be a potential risk factor for cancer chemosensitivity27.
More than 90% of patients with a low-grade, noninvasive urothelial tumor (Ta) never progress to having a tumor that invades the basement membrane28. Therefore, it is of greater importance to explore the mechanisms of instillation agent toxicity and complications than the risks of metastasis or death from this tumor. Indeed, previous studies have shown the importance of several polymorphisms within candidate genes that are associated with clinical response and toxicity of instillation agents29.
In order to confirm and further examine the influence of genetic variations on the clinical outcome and toxicity of intravesical instillation agents, the aim of this study was to examine the GSTP1, GSTO1, GSTO2, and ABCB1 polymorphisms in a cohort of Chinese bladder cancer patients treated with epirubicin or MMC.
Methods
In this prospective study, a total of 244 patients diagnosed with nonmuscle invasive bladder cancer were recruited from the First Affiliated Hospital of Nanjing Medical University (Nanjing, China) from May 2007 to October 2012. All patients or patients’ representatives, if direct consent could not be obtained, provided written informed consent to participate in this study, and the study was approved by the Institutional Review Board of Nanjing Medical University (Nanjing, China). All experiments were performed in accordance with relevant guidelines and regulations, and the Institutional Review Board of Nanjing Medical University had approved all experiments. Among them, 130 patients were treated with epirubicin and the other 114 patients were instilled with MMC to prevent cancer recurrence. Prior to recruitment, all subjects were personally interviewed to collect demographic data and clinical characteristics, including age, gender, tobacco use, alcohol use, and self-reported family history of cancer. Patients were excluded from the study if they had a previous history of cancer, had metastasized cancer from other or unknown origins, or were previously subjected to radiotherapy or chemotherapy.
The tumor histological grade was assessed according to the recently published World Health Organization (WHO) consensus30. The pathology slides from radical nephrectomy or core biopsy were independently reviewed by two pathologists and were identified as bladder cancer. According to the histopathological grade (WHO 2004, grading of urothelial papilloma)31, the patients were classified into two subgroups: low risk and high risk. Individuals who smoked daily for more than 1 year were defined as smokers, and the rest were considered as nonsmokers. Individuals who drank alcohol at least three times per week for more than 6 months were defined as drinkers, and the rest were considered as nondrinkers.
Epirubicin or MMC was instilled into the bladder within 24 h after transurethral resection of the bladder tumor. Thereafter, patients were treated with epirubicin (50 mg/week) or mitomycin (30 mg/week) for 8 weeks, and this dosage was maintained for 12 or more months. The survival time was calculated from the date of confirmed diagnosis until the date of the last follow-up or recurrence. The date of recurrence was obtained from inpatient and outpatient records or from the patients’ families via follow-up telephone calls. The patients who did not suffer from recurrence by the last follow-up date were considered as having nonrecurrent disease.
Single nucleotide polymorphism (SNP) selection and genotyping
The SNPs included in this study were selected based on previous studies that demonstrated their association with pharmacosensitivity (GSTP1 rs1695, GSTO1 rs4925, GSTO2 rs156697, ABCB1 rs3747802, and ABCB1 rs3213619). Genomic DNA of each individual was extracted from 150 μL of EDTA-anticoagulated peripheral blood samples by using a DNA extraction kit (Tiangen Biotech, Beijing, China), according to the manufacturer’s instructions. The polymorphisms were genotyped using a TaqMan SNP genotyping assay (Applied Biosystems, Foster City, CA, USA) and a 384-well ABI 7900HT real-time PCR system (Applied Biosystems, Foster City, CA, USA). A 1-μg sample of total DNA was used for genotyping with primers (Invitrogen, Karlsrule, Germany) (shown in the Supplementary data). SDS 2.4 software (Applied Biosystems, Foster City, CA, USA) was used for allelic discrimination. Each sample was run in triplicate. For quality control, four negative controls were included in each plate, and 5% of the samples were randomly selected for repeated genotyping to verify the results; all of the results were 100% consistent. Primers, probes, and reaction conditions for each SNP analysis are available upon request. Amplification was performed under the following conditions: 50 °C for 2 min; 95 °C for 10 min; and 45 cycles of 95 °C for 15 s and 60 °C for 1 min.
Statistical analysis
Recurrence-free survival (RFS) was defined as the time from the first instillation of epirubicin or MMC to the first recurrence of bladder cancer. RFS was estimated by the Kaplan–Meier method, and the log-rank test was used to compare different survival curves. Hazard ratios (HRs) and 95% confidence intervals (CIs) of the HRs were derived from univariate and multivariate Cox proportional hazard models. All analyses were carried out using SPSS 13.0 software (IBM, Armonk, NY, USA). A two-sided P value < 0.05 represented a statistically significant result.
Results
Characteristics of the study population
The frequency distributions of selected characteristics of the cases are shown in Table 1. The median period of instillation treatment was 18 months among the 130 patients who received epirubicin and 17 months among the 114 patients who received MMC regimens. Among all patients, 90 patients had recurrent disease and 5 patients died due to bladder cancer.
Table 1. Patient characteristics.
| Patients (n = 244) | ||
|---|---|---|
| Age (years) | 67 ± 12 | |
| Gender | Male | 189 (77.5%) |
| Female | 55 (22.5%) | |
| Smoking status | Nonsmoker | 190 (77.9%) |
| Smoker | 54 (22.1%) | |
| Drinking status | No Yes | 225 (92.2%) 19 (7.8%) |
| Family history of cancer | No | 239 (98.0%) |
| Yes | 5 (2.0%) | |
| Tumor number | 1 | 171 (70.1%) |
| ≥2 | 73 (29.9%) | |
| Tumor diameter | <3 cm | 217 (88.9%) |
| ≥3 cm | 27 (11.1%) | |
| Tumor grade | Low risk | 143 (58.6%) |
| High risk | 101 (41.4%) |
RFS of patients receiving epirubicin or MMC treatment
The mean survival time (MST) of patients in the epirubicin-treated group was 29.1 months, while the MST in the MMC-treated group was 27.7 months. The association of RFS with different polymorphisms is presented in Table 2. In our study, the two polymorphisms GSTP1 rs1695 and GSTO1 rs4925 were significantly associated with the RFS of patients treated with epirubicin (Table 2). The MST of patients with the GSTP1 AA genotype was 76.7 months, while the MST of patients with the AG or GG genotype was 40.4 and 23.3 months, respectively (the Log-rank P = 0.002) (Fig. 1A). Furthermore, a significant difference in the MST of patients with the AA genotype vs. those with the AG+GG genotype was observed (MST = 40.7 months for the AG+GG genotype, Log-rank P = 0.001) (Fig. 1B). Compared to patients with the AA genotype, patients with the AG+GG genotype exhibited a high risk for bladder cancer recurrence (HR = 3.47, 95% CI = 1.75–6.89). Moreover, patients with the GSTO1 CC genotype were demonstrated to have a longer MST, and this difference was statistically significant (MST = 28.2 months for the AA genotype; 49.0 months for the AC genotype; and 67.4 months for the CC genotype; Log-rank P = 0.019) (Fig. 2A–B). Compared to patients with the AC+AA genotype, patients with the CC genotype had a lower risk for bladder cancer recurrence (HR = 0.51, 95% CI = 0.27–0.95). No significant association was found between the GSTO2 and ABCB1 polymorphisms and the risk of bladder cancer recurrence with intravesical epirubicin chemotherapy (Table 2). In addition, no association of the examined polymorphisms with the RFS of patients treated with MMC was observed (Fig. 3A–B).
Table 2. Association between different polymorphisms and recurrence-free survival of patients.
|
Epirubicin |
MMC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recurrence | All patients | MST * | Log-rank P | Adjusted HR (95% CI) | Recurrence | All patients | MST * | Log-rank P | Adjusted HR (95% CI) | |
| GSTP1 rs1695 | ||||||||||
| AA | 22 | 78 | 76.7 | 0.002 | 1.00 (reference) | 16 | 61 | 64.4 | 0.408 | 1.00 (reference) |
| AG | 26 | 46 | 40.4 | 3.29 (1.63–6.63) | 23 | 51 | 52.6 | 0.63 (0.05–7.46) | ||
| GG | 2 | 6 | 23.3 | 5.18 (1.05–25.62) | 1 | 2 | 62.5 | 1.56 (0.80–3.07) | ||
| AA | 22 | 78 | 76.7 | 0.001 | 1.00 (reference) | 16 | 61 | 64.4 | 0.218 | 1.00 (reference) |
| AG+GG | 28 | 52 | 40.7 | 3.47 (1.75–6.89) | 24 | 53 | 53.9 | 1.54 (0.79–3.01) | ||
| GSTO1 rs4925 | ||||||||||
| CC | 29 | 82 | 67.4 | 0.019 | 1.00 (reference) | 26 | 68 | 55.1 | 0.231 | 1.00 (reference) |
| AC | 15 | 40 | 49.0 | 1.89 (0.94–3.83) | 6 | 30 | 74.5 | 0.34 (0.11–1.09) | ||
| AA | 6 | 8 | 28.2 | 3.23 (1.16-8.94) | 8 | 16 | 45.3 | 1.12 (0.38–3.33) | ||
| CC | 29 | 82 | 67.4 | 0.042 | 1.00 (reference) | 26 | 68 | 55.1 | 0.404 | 1.00 (reference) |
| AC+AA | 21 | 48 | 43.8 | 1.96 (1.05–3.70) | 14 | 46 | 62.8 | 1.20 (0.60–2.44) | ||
| GSTO2 rs156697 | ||||||||||
| CC | 8 | 15 | 35.4 | 0.170 | 1.00 (reference) | 9 | 21 | 51.9 | 0.550 | 1.00 (reference) |
| CT | 16 | 50 | 51.4 | 0.88 (0.31–2.51) | 9 | 38 | 63.2 | 0.75 (0.22–2.62) | ||
| TT | 26 | 65 | 67.7 | 0.51 (0.22–1.22) | 22 | 55 | 55.3 | 1.12 (0.48–2.63) | ||
| CC | 8 | 15 | 35.4 | 0.071 | 1.00 (reference) | 9 | 21 | 51.9 | 0.778 | 1.00 (reference) |
| CT+TT | 42 | 115 | 64.3 | 0.56 (0.25–1.26) | 31 | 93 | 59.9 | 0.94 (0.42–2.14) | ||
| ABCB1 rs3747802 | ||||||||||
| TT | 48 | 126 | 28.1 | 0.580 | 1.00 (reference) | 38 | 111 | 28.0 | 0.105 | 1.00 (reference) |
| TC | 2 | 3 | 71.3 | 0.33 (0.05–1.99) | 2 | 2 | 22.0 | 4.81 (0.99–23.50) | ||
| CC | 0 | 1 | 21.0 | 0.00 | 0 | 1 | 8.0 | 0.00 | ||
| ABCB1 rs3213619 | ||||||||||
| TT | 47 | 114 | 29.1 | 0.335 | 1.00 (reference) | 36 | 103 | 25.8 | 0.323 | 1.00 (reference) |
| TC | 3 | 9 | 32.7 | 0.91 (0.27–3.09) | 3 | 6 | 31.2 | 0.46 (0.06–3.75) | ||
| CC | 0 | 7 | 23.7 | 0.00 | 1 | 5 | 64.0 | 1.17 (0.33–4.17) | ||
*Mean survival time was defined as the mean time before recurrence.
Figure 1.
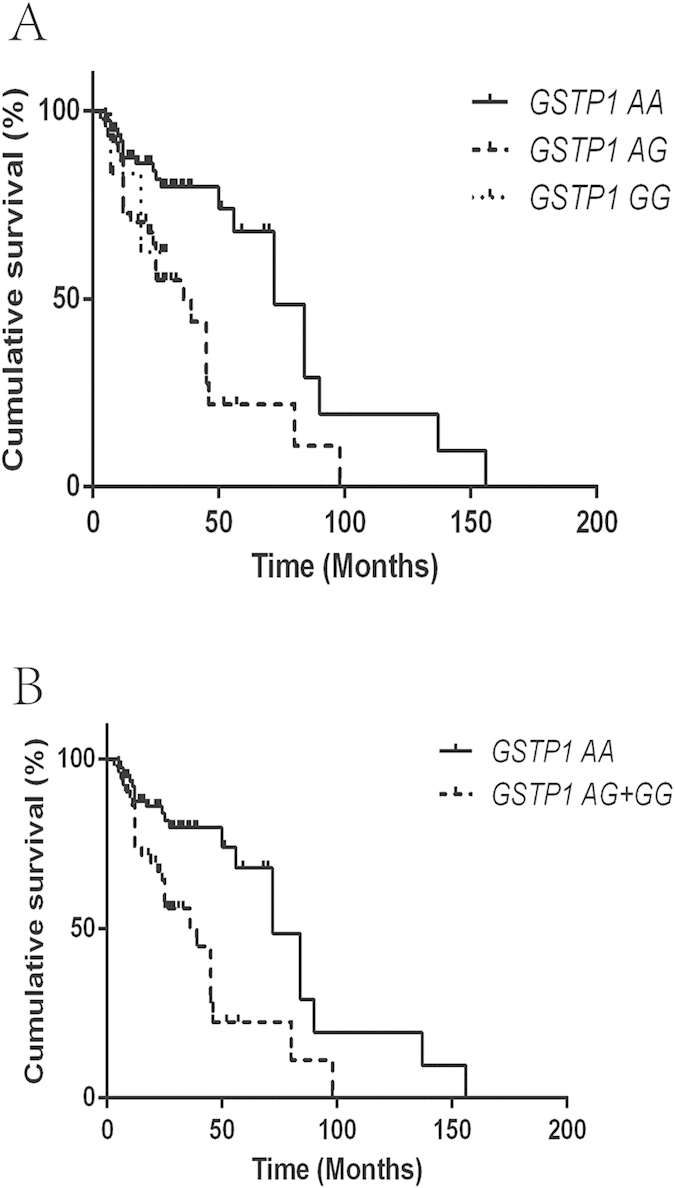
(A) The Kaplan–Meier curve representing the association between the GSTP1 rs1695 genotypes and recurrence-free survival of patients treated with epirubicin. (B) The Kaplan–Meier curve representing the association between the GSTP1 rs1695 AA vs. AG+GG genotype and recurrence-free survival of patients treated with epirubicin.
Figure 2.
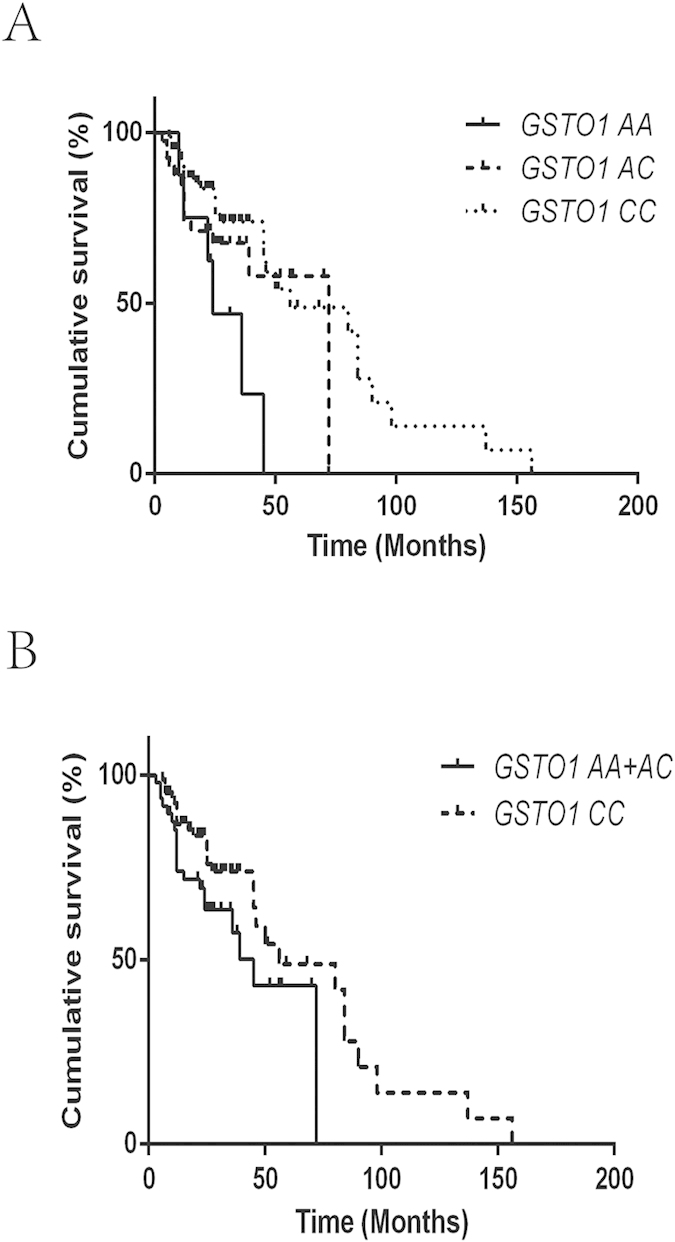
(A) The Kaplan–Meier curve representing the association between the GSTO1 rs4925 genotypes and recurrence-free survival of patients treated with epirubicin. (B) The Kaplan–Meier curve representing the association between the GSTO1 rs4925 AA+AC vs. CC genotype and recurrence-free survival of patients treated with epirubicin.
Figure 3.
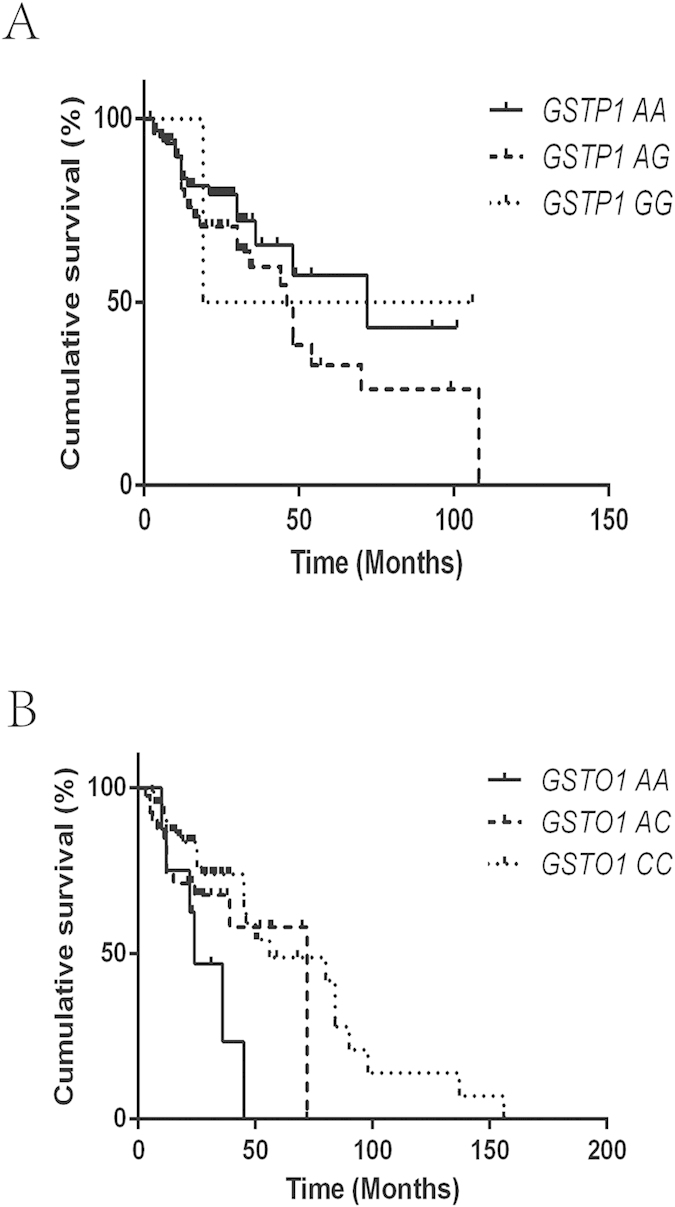
(A) The Kaplan–Meier curve representing the association between the GSTP1 rs1695 genotypes and recurrence-free survival of patients treated with MMC. (B) The Kaplan–Meier curve showing the association between the GSTO1 rs4925 genotypes and recurrence-free survival of patients treated with MMC.
In the epirubicin-treated patients, we also found that GSTP1 rs4925 in the G-allele carrier patients was significantly associated with a favorable overall survival (MST = 40.4 months for the AA genotype and MST = 36.7 months for the AG+GG genotype; Log-rank P = 0.018). However, since the number of deaths was so small, we were not able to draw any convincing conclusions (supplementary Table).
Combined effect of selected polymorphisms and the RFS of patients receiving epirubicin treatment
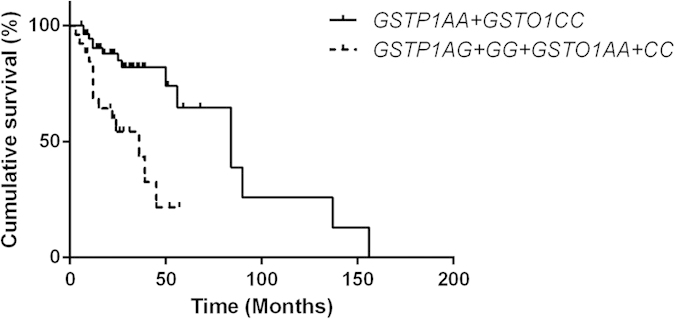
In addition to analyzing the influence of separate SNPs, we also examined the effect of combined protective genotypes (the GSTP1 AA genotype and the GSTO1 CC genotype) on RFS of patients receiving epirubicin treatment (Table 3). A significantly reduced risk of bladder cancer recurrence was found in patients simultaneously carrying the GSTP1 AA and GSTO1 CC genotypes, when compared to patients carrying other combinations of genotypes (Fig. 4) (HR = 0.21, 95% CI = 0.08–0.56).
Table 3. Association between combined GSTP1 and GSTO1 polymorphisms and the recurrence-free survival of patients treated with epirubicin.
|
Epirubicin |
|||
|---|---|---|---|
| MST* | Log-rank P | Adjusted HR (95% CI) | |
| GSTP1 and GSTO1 | |||
| AG+GG and AC+AA | 31.3 | 1.00 (reference) | |
| AG+GG and CC | 43.3 | 0.449 | 0.61 (0.26–1.40) |
| AA and AC+AA | 57.0 | 0.051 | 0.30 (0.09–1.08) |
| AA and CC | 83.4 | 0.001 | 0.21 (0.08–0.56) |
*Mean survival time was defined as the mean time before recurrence.
Figure 4. The Kaplan–Meier curve representing the association between the combined GSTP1 rs1695 and GSTO1 rs4925 genotypes and recurrence-free survival of patients treated with epirubicin.
Polymorphisms and toxicities of epirubicin and MMC
The occurrence of toxic side effects, including hematuria, irritative voiding symptoms, and suprapubic pain, during treatment with instillation agents is presented in Table 4. Interestingly, a significant association was found between the GSTP1 rs1695 and GSTO1 rs4925 genotypes and the prevalence of hematuria and irritative voiding symptoms in the epirubicin-treated group. In our study, the GSTP1 AA genotype was found to cause a five-times higher risk of irritative voiding symptoms than the AG or GG genotype (OR = 0.20, 95% CI = 0.08–0.49; Table 5). Moreover, the GSTO1 CC genotype was found to be associated with a reduced risk of hematuria in patients receiving epirubicin (OR = 0.34, 95% CI = 0.12–0.96; Table 5). No association of combined genotypes with toxic side effects was observed for patients receiving MMC.
Table 4. The frequencies of hematuria, irritative voiding symptoms, and suprapubic pain due to the toxicity of epirubicin or MMC.
|
Epirubicin |
MMC |
|||||
|---|---|---|---|---|---|---|
| Hematuria (%)* | Irritative voiding symptoms (%) | Suprapubic pain (%) | Hematuria (%) | Irritative voiding symptoms (%) | Suprapubic pain (%) | |
| GSTP1 rs1695 | ||||||
| AA | 12 (9.2) | 40 (30.8) | 14 (10.8) | 11 (9.6) | 9 (7.9) | 10 (8.8) |
| AG | 6 (4.6) | 11 (8.5) | 12 (9.2) | 10 (8.8) | 7 (6.1) | 7 (6.1) |
| GG | 1 (0.8) | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| GSTO1 rs4925 | ||||||
| AA | 1 (0.8) | 2 (1.5) | 1 (0.8) | 1 (0.9) | 2 (1.8) | 2 (1.8) |
| AC | 10 (7.7) | 16 (12.3) | 12 (9.2) | 5 (4.4) | 2 (1.8) | 7 (6.1) |
| CC | 8 (6.2) | 34 (26.2) | 13 (10) | 15 (13.2) | 12 (10.5) | 8 (7.0) |
*The number of patients with individual complications versus the number of patients with different instillation agents.
Table 5. Association between the GSTP1 and GSTO1 polymorphisms and the toxicity of epirubicin or MMC.
| Hematuria | Irritative voiding symptoms | Suprapubic pain | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Epirubicin | ||||||
| GSTP1 rs1695 AG+GG/AA | 1.08 (0.38–3.07) | 0.883 | 0.20 (0.08–0.49) | <0.001 | 1.32 (0.52–3.31) | 0.559 |
| GSTO1 rs4925 CC/AA+AC | 0.34 (0.12–0.96) | 0.042 | 1.25 (0.55–2.86) | 0.594 | 0.52 (0.21–1.32) | 0.168 |
| MMC | ||||||
| GSTP1 rs1695 AG+GG/AA | 1.13 (0.42–3.00) | 0.81 | 0.81 (0.27–2.43) | 0.70 | 0.75 (0.26–2.18) | 0.596 |
| GSTO1 rs4925 CC/AA+AC | 2.27 (0.74–6.67) | 0.152 | 2.00 (0.55–7.14) | 0.294 | 0.52 (0.17–1.59) | 0.248 |
Discussion
In the present study, we investigated the possible association between SNPs in the GSTP1, GSTO1, GSTO2, and ABCB1 genes with response to treatment in patients with bladder cancer. To the best of our knowledge, this is the first study to explore the influence of genetic variants on the clinical outcomes and toxic effects in bladder cancer patients treated with epirubicin or MMC. Overall, we found that patients with the GSTP1 rs1695 AA genotype had a lower risk of tumor recurrence when treated with epirubicin, but not with MMC ((AG+GG)/AA: HR = 3.47, 95% CI = 1.75–6.89). In addition, patients carrying two C alleles for GSTO1 rs4925 had a lower risk of bladder cancer recurrence (CC/(AC+AA): HR = 0.51, 95% CI = 0.27–0.95). However, no significant association was found between the GSTO2 and ABCB1 polymorphisms and the risk of bladder cancer recurrence in patients receiving intravesical epirubicin chemotherapy.
Since the first evidence that GSTs are involved in the response to chemotherapy32, the inconsistent nature of this relationship has been investigated both in vivo and in vitro33,34. GSTs catalyze the first step in the formation of mercapturic acids, originating from the elimination pathways of toxic compounds as well as chemotherapy and instillation agents. A study by Stoehlmacher et al.17 has suggested that GSTP1 may be an important player in the metabolism of platinum drugs in colorectal cancer patients. Furthermore, apoptosis inhibition mediated by the GSTP1–JNK interaction has been found to be the key mechanism in the progression of bladder cancer35. Previous studies have reported an effective role of GSTP1 rs1695 in terms of the clinical outcome of breast cancer patients36 and a significant association between high GSTP1 expression of tumor cells and reduced sensitivity to chemotherapy37,38. The results of our study showed that patients with the GSTP1 AA genotype had a significantly reduced risk of tumor recurrence after receiving epirubicin. Similarly, others have shown that the GSTP1 AA genotype also diminishes the risk of chemoresistance to doxorubicin in osteosarcoma patients39.
Unlike other GSTs, GSTO1 has a unique structure and function. Rather than having a catalytic serine or tyrosine residue in the active site, GSTO1 utilizes a hyperreactive catalytic cysteine nucleophile40. It has been shown that the GSTO1 CC genotype may modify the risk of developing breast cancer, urothelial carcinoma, and other tumors41. In addition, it has been shown that after melanoma cancer cells are treated with a GSTO1 inhibitor, they have a heightened sensitivity to the cytotoxic effects of cisplatin42. GSTO1 rs4925 AC genotype creates a non-conservative amino acid change from hydrophobic to hydrophilic residue, causing the thiol transferase activity of the variant type to reduce to 75% of the wild type, indicating the rs4925 C allele may decrease protection against cellular oxidation stresses caused by instillation agents43. Therefore, it may be inferred that the biological effects of the rs4925 CC genotype can be explained by inefficient dehydroascorbate reductase capacity in response to improved chemotherapy sensitivity, which results in induced apoptosis of bladder tumor cells. Our results showed that patients with the GSTO1 CC genotype had a significantly abridged risk of bladder tumor recurrence (CC/(AC+AA): HR = 0.51, 95% CI = 0.27–0.95) after treatment with epirubicin, which was in agreement with the results of these studies.
In the present study, we also examined the joint effect of multiple genotypes on the risk of bladder cancer recurrence. Based on the genotyping analysis results of selected SNPs in the GSTP1 and GSTO1 genes and their association with the treatment outcome, we conducted a combined polymorphism analysis. The results of this combined analysis demonstrated that patients carrying both the GSTP1 rs1695 AA and GSTO1 rs4925 CC genotypes had a significantly decreased risk of bladder cancer recurrence, when compared with the combined increased risk genotypes (the GSTP1 AG+GG genotype and the GSTO1 AC+AA genotype). This result suggests that crosstalk between these two GST genes might modulate the susceptibility toward bladder cancer recurrence in patients receiving epirubicin. No significant associations of analyzed SNPs were observed in patients treated with MMC. One of the reasons for these findings may be that GSTs and ABCB1 do not participate in the metabolism of MMC. It has been clearly demonstrated that NQO1, but not the genes we mentioned, plays a pivotal role in MMC activation44.
Toxicity is the main dose-limiting factor in cancer therapy. Thus, it is important to assess qualitative and pharmacogenetic associations of the induced side effects when comparing different treatments. Several clinical studies have shown moderate-to-severe acute complications following the instillation of epirubicin or MMC45,46. In this study, we found that patients with the GSTP1 rs1695 AA genotype had a 5-fold higher risk of irritative voiding symptoms than patients with the GSTP1 AG+GG genotype in the epirubicin-treated group. Nevertheless, GSTP1 rs1695 did not influence the outcome of the MMC-treated group. These findings are in agreement with the results reported in breast cancer patients by Zhang et al.29. GSTP1 is associated with the regulation of stress signaling and resistance to apoptosis by mechanisms independent of its catalytic activity; and redox-active GSTP1 components inhibit c-JNK, which triggers the apoptotic cascade in the cell. Namely, it has been clearly shown that GSTP1 is involved in the regulation of the JNK signaling pathway in order to protect bladder epidermal cells from epirubicin peroxidation toxicity47. Assuming that the GSTP1 rs1695 AA genotype leads to decreased gene function, it may mimic the inhibition of GSTP1 and confer susceptibility to irritative voiding symptoms. Besides, we also found that patients carrying the GSTO1 rs4925 AA+AC genotype had an almost 3-fold higher risk of hematuria compared to patients with the CC genotype. Kim et al. have demonstrated that GSTO1 might protect against drug toxicity via the regulation of ATP synthase activity48. GSTO1 would presumably result in deficient dehydroascorbic acid reductase activity and a lower ascorbic acid level in bladder cancer49. As a synergistic nutrient of antineoplastic drugs, ascorbic acid may increase the cytotoxic effects of some anticancer drugs; in particular, anthracyclines are related to free radical formation because of their cytotoxic activities50,51. Consequently, associated with decreased dehydroascorbate reductase activity, the GSTO1 rs4925 wildtype CC genotype would reduce lower amount of ascorbic acid than other rs4925 genotypes, which would decrease more free radical production of epirubicin and thus decease the toxicity of instillation drugs. In this study, we did not observe any association of the examined polymorphisms with the antitoxicity of MMC.
Taken together, our results suggest that the GSTP1 rs1695 and GSTO1 rs4925 polymorphisms are associated with clinical outcomes of epirubicin treatment but not with MMC treatment. Additionally, the combined effects of GSTP1 and GSTO1 polymorphisms showed a significant influence on the RFS in patients receiving epirubicin treatment. Moreover, the GSTP1 and GSTO1 polymorphisms were also associated with epirubicin-related toxicities. If our findings are to be confirmed in a larger cohort of patients, the stratification of patients according to the selected SNP genotypes could possibly provide the basis for their individualized treatment.
Additional Information
How to cite this article: Deng, X. et al. GSTP1 and GSTO1 single nucleotide polymorphisms and the response of bladder cancer patients to intravesical chemotherapy. Sci. Rep. 5, 14000; doi: 10.1038/srep14000 (2015).
Supplementary Material
Acknowledgments
This work was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Program for Development of Innovative Research Team in the First Affiliated Hospital of Nanjing Medical University, the Provincial Initiative Program for Excellency Disciplines of Jiangsu Province, and the National Natural Science Foundation of China [Grant numbers 81171963 and 81201571].
Footnotes
Author Contributions Conceived and designed the experiments: X.D., Q.L. and X.Y. Performed the experiments: X.L. and X.D. Analyzed the data: X.D., C.Q. and C.Y. Contributed reagents/materials/analysis tools: X.L. and Y.C. Wrote the paper: X.D. and R.Z. All authors reviewed the manuscript.
References
- Siegel R., Ma J., Zou Z. & Jemal A. Cancer statistics, 2014. CA Cancer J Clin 64, 9–29, 10.3322/caac.21208 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang H. H. et al. Recurrence of inflammatory myofibroblastic tumor in bladder secondary to prostate treated with laparoscopic radical cystectomy. Med Sci Monit 18, CS63–66 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum B. L. & Torti F. M. Adjuvant intravesicular pharmacotherapy for superficial bladder cancer. J Natl Cancer Inst 83, 682–694 (1991). [DOI] [PubMed] [Google Scholar]
- Donat S. M. Evaluation and follow-up strategies for superficial bladder cancer. Urol Clin North Am 30, 765–776 (2003). [DOI] [PubMed] [Google Scholar]
- Shelley M. D., Mason M. D. & Kynaston H. Intravesical therapy for superficial bladder cancer: a systematic review of randomised trials and meta-analyses. Cancer Treat Rev 36, 195–205, 10.1016/j.ctrv.2009.12.005 (2010). [DOI] [PubMed] [Google Scholar]
- Saika T. et al. Two instillations of epirubicin as prophylaxis for recurrence after transurethral resection of Ta and T1 transitional cell bladder cancer: a prospective, randomized controlled study. World J Urol 28, 413–418, 10.1007/s00345-009-0502-1 (2010). [DOI] [PubMed] [Google Scholar]
- Lamm D. L. et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guerin for transitional-cell carcinoma of the bladder. N Engl J Med 325, 1205–1209, 10.1056/NEJM199110243251703 (1991). [DOI] [PubMed] [Google Scholar]
- Witjes J. A. & Hendricksen K. Intravesical pharmacotherapy for non-muscle-invasive bladder cancer: a critical analysis of currently available drugs, treatment schedules, and long-term results. Eur Urol 53, 45–52, 10.1016/j.eururo.2007.08.015 (2008). [DOI] [PubMed] [Google Scholar]
- Witjes J. A. Topic issue on new treatments in bladder cancer. World J Urol 27, 285–287, 10.1007/s00345-009-0390-4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali-el-Dein B., Nabeeh A., el-Baz M., Shamaa S. & Ashamallah A. Single-dose versus multiple instillations of epirubicin as prophylaxis for recurrence after transurethral resection of pTa and pT1 transitional-cell bladder tumours: a prospective, randomized controlled study. Br J Urol 79, 731–735 (1997). [DOI] [PubMed] [Google Scholar]
- Kurth K. et al. Phase 1/2 study of intravesical epirubicin in patients with carcinoma in situ of the bladder. J Urol 146, 1508–1512, discussion 1512–1503 (1991). [DOI] [PubMed] [Google Scholar]
- Djukic T. I. et al. Glutathione S-transferase T1, O1 and O2 polymorphisms are associated with survival in muscle invasive bladder cancer patients. PLoS One 8, e74724, 10.1371/journal.pone.0074724 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen C. L. et al. Glutathione S-transferase activity and subunit composition in transitional cell cancer and mucosa of the human bladder. Urology 49, 644–651 (1997). [DOI] [PubMed] [Google Scholar]
- Hengstler J. G., Arand M., Herrero M. E. & Oesch F. Polymorphisms of N-acetyltransferases, glutathione S-transferases, microsomal epoxide hydrolase and sulfotransferases: influence on cancer susceptibility. Recent Results Cancer Res 154, 47–85 (1998). [DOI] [PubMed] [Google Scholar]
- Wu K., Wang X., Xie Z., Liu Z. & Lu Y. Glutathione S-transferase P1 gene polymorphism and bladder cancer susceptibility: an updated analysis. Mol Biol Rep 40, 687–695, 10.1007/s11033-012-2109-7 (2013). [DOI] [PubMed] [Google Scholar]
- Ali-Osman F., Akande O., Antoun G., Mao J. X. & Buolamwini J. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J Biol Chem 272, 10004–10012 (1997). [DOI] [PubMed] [Google Scholar]
- Stoehlmacher J. et al. Association between glutathione S-transferase P1, T1, and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst 94, 936–942 (2002). [DOI] [PubMed] [Google Scholar]
- Ryberg D. et al. Genotypes of glutathione transferase M1 and P1 and their significance for lung DNA adduct levels and cancer risk. Carcinogenesis 18, 1285–1289 (1997). [DOI] [PubMed] [Google Scholar]
- Whitbread A. K., Tetlow N., Eyre H. J., Sutherland G. R. & Board P. G. Characterization of the human Omega class glutathione transferase genes and associated polymorphisms. Pharmacogenetics 13, 131–144, 10.1097/01.fpc.0000054062.98065.6e (2003). [DOI] [PubMed] [Google Scholar]
- Girardini J., Amirante A., Zemzoumi K. & Serra E. Characterization of an omega-class glutathione S-transferase from Schistosoma mansoni with glutaredoxin-like dehydroascorbate reductase and thiol transferase activities. Eur J Biochem 269, 5512–5521 (2002). [DOI] [PubMed] [Google Scholar]
- Dulhunty A., Gage P., Curtis S., Chelvanayagam G. & Board P. The glutathione transferase structural family includes a nuclear chloride channel and a ryanodine receptor calcium release channel modulator. J Biol Chem 276, 3319–3323, 10.1074/jbc.M007874200 (2001). [DOI] [PubMed] [Google Scholar]
- Wang L. et al. Cloning, expression and characterization of human glutathione S-transferase Omega 2. Int J Mol Med 16, 19–27 (2005). [PubMed] [Google Scholar]
- Li Y. J. et al. Glutathione S-transferase omega-1 modifies age-at-onset of Alzheimer disease and Parkinson disease. Hum Mol Genet 12, 3259–3267, 10.1093/hmg/ddg357 (2003). [DOI] [PubMed] [Google Scholar]
- Marahatta S. B. et al. Polymorphism of glutathione S-transferase omega gene and risk of cancer. Cancer Lett 236, 276–281, 10.1016/j.canlet.2005.05.020 (2006). [DOI] [PubMed] [Google Scholar]
- Pongstaporn W., Pakakasama S., Sanguansin S., Hongeng S. & Petmitr S. Polymorphism of glutathione S-transferase Omega gene: association with risk of childhood acute lymphoblastic leukemia. J Cancer Res Clin Oncol 135, 673–678, 10.1007/s00432-008-0501-4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. F. et al. Clinical pharmacogenetics and potential application in personalized medicine. Curr Drug Metab 9, 738–784 (2008). [DOI] [PubMed] [Google Scholar]
- Zubor P., Lasabova Z., Hatok J., Stanclova A. & Danko J. A polymorphism C3435T of the MDR-1 gene associated with smoking or high body mass index increases the risk of sporadic breast cancer in women. Oncol Rep 18, 211–217 (2007). [PubMed] [Google Scholar]
- Koya M. P., Simon M. A. & Soloway M. S. Complications of intravesical therapy for urothelial cancer of the bladder. J Urol 175, 2004–2010, 10.1016/S0022-5347(06)00264-3 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang B. L. et al. Polymorphisms of GSTP1 is associated with differences of chemotherapy response and toxicity in breast cancer. Chin Med J (Engl) 124, 199–204 (2011). [PubMed] [Google Scholar]
- Oosterhuis J. W. et al. Histological grading of papillary urothelial carcinoma of the bladder: prognostic value of the 1998 WHO/ISUP classification system and comparison with conventional grading systems. J Clin Pathol 55, 900–905 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. & Leong A. S. Histologic grading of noninvasive papillary urothelial tumors: validation of the 1998 WHO/ISUP system by immunophenotyping and follow-up. Am J Clin Pathol 121, 679–687, 10.1309/0KAT-YHQB-JD5X-HQ8J (2004). [DOI] [PubMed] [Google Scholar]
- Schisselbauer J. C. et al. Characterization of glutathione S-transferase expression in lymphocytes from chronic lymphocytic leukemia patients. Cancer Res 50, 3562–3568 (1990). [PubMed] [Google Scholar]
- Mavis C. K., Morey Kinney S. R., Foster B. A. & Karpf A. R. Expression level and DNA methylation status of glutathione-S-transferase genes in normal murine prostate and TRAMP tumors. Prostate 69, 1312–1324, 10.1002/pros.20976 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick D. S. et al. Cancer chemotherapy and drug metabolism. Drug Metab Dispos 33, 1083–1096, 10.1124/dmd.105.004374 (2005). [DOI] [PubMed] [Google Scholar]
- Pljesa-Ercegovac M. et al. Enhanced GSTP1 expression in transitional cell carcinoma of urinary bladder is associated with altered apoptotic pathways. Urol Oncol 29, 70–77, 10.1016/j.urolonc.2008.10.019 (2011). [DOI] [PubMed] [Google Scholar]
- Romero A. et al. Glutathione S-transferase P1 c.313A > G polymorphism could be useful in the prediction of doxorubicin response in breast cancer patients. Ann Oncol 23, 1750–1756, 10.1093/annonc/mdr483 (2012). [DOI] [PubMed] [Google Scholar]
- Sun N. et al. MRP2 and GSTP1 polymorphisms and chemotherapy response in advanced non-small cell lung cancer. Cancer Chemother Pharmacol 65, 437–446, 10.1007/s00280-009-1046-1 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goekkurt E. et al. Polymorphisms of glutathione S-transferases (GST) and thymidylate synthase (TS)–novel predictors for response and survival in gastric cancer patients. Br J Cancer 94, 281–286, 10.1038/sj.bjc.6602891 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. et al. Predictive potential of ABCB1, ABCC3, and GSTP1 gene polymorphisms on osteosarcoma survival after chemotherapy. Tumour Biol 35, 9897–9904, 10.1007/s13277-014-1917-x (2014). [DOI] [PubMed] [Google Scholar]
- Board P. G. et al. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J Biol Chem 275, 24798–24806, 10.1074/jbc.M001706200 (2000). [DOI] [PubMed] [Google Scholar]
- Xu Y. T. et al. Genetic polymorphisms in Glutathione S-transferase Omega (GSTO) and cancer risk: a meta-analysis of 20 studies. Sci Rep 4, 6578, 10.1038/srep06578 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi K. et al. Potent and selective inhibitors of glutathione S-transferase omega 1 that impair cancer drug resistance. J Am Chem Soc 133, 16605–16616, 10.1021/ja2066972 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Kagawa T. et al. Functional characterization of two variant human GSTO 1-1s (Ala140Asp and Thr217Asn). Biochem Biophys Res Commun 301, 516–520 (2003). [DOI] [PubMed] [Google Scholar]
- Phillips R. M., Burger A. M., Fiebig H. H. & Double J. A. Genotyping of NAD(P)H:quinone oxidoreductase (NQO1) in a panel of human tumor xenografts: relationship between genotype status, NQO1 activity and the response of xenografts to Mitomycin C chemotherapy in vivo(1). Biochem Pharmacol 62, 1371–1377 (2001). [DOI] [PubMed] [Google Scholar]
- Shelley M. D. et al. Intravesical bacillus Calmette-Guerin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Rev, CD003231, 10.1002/14651858.CD003231 (2003). [DOI] [PubMed] [Google Scholar]
- Onrust S. V., Wiseman L. R. & Goa K. L. Epirubicin: a review of its intravesical use in superficial bladder cancer. Drugs Aging 15, 307–333 (1999). [DOI] [PubMed] [Google Scholar]
- Adler V. et al. Regulation of JNK signaling by GSTp. EMBO J 18, 1321–1334, 10.1093/emboj/18.5.1321 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Kim S. H., Kim J., Kim H. & Yim J. Glutathione s-transferase omega 1 activity is sufficient to suppress neurodegeneration in a Drosophila model of Parkinson disease. J Biol Chem 287, 6628–6641, 10.1074/jbc.M111.291179 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. et al. Glutathione S-transferase omega genes in Alzheimer and Parkinson disease risk, age-at-diagnosis and brain gene expression: an association study with mechanistic implications. Mol Neurodegener 7, 13, 10.1186/1750-1326-7-13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromberg A. et al. Ascorbate exerts anti-proliferative effects through cell cycle inhibition and sensitizes tumor cells towards cytostatic drugs. Cancer Chemother Pharmacol 67, 1157–1166, 10.1007/s00280-010-1418-6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka D. et al. Vitamin C-driven epirubicin loading into liposomes. Int J Nanomedicine 8, 3573–3585, 10.2147/IJN.S47745 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


