Abstract
The purpose of this study was to determine the effects of dietary protein and eating frequency on perceived appetite and satiety during weight loss. A total of 27 overweight/obese men (age 47 ± 3 years; BMI 31.5 ± 0.7 kg/m2) were randomized to groups that consumed an energy-restriction diet (i.e., 750 kcal/day below daily energy need) as either higher protein (HP, 25% of energy as protein, n = 14) or normal protein (NP, 14% of energy as protein, n = 13) for 12 weeks. Beginning on week 7, the participants consumed their respective diets as either 3 eating occasions/day (3-EO; every 5 h) or 6 eating occasions/day (6-EO; every 2 h), in randomized order, for 3 consecutive days. Indexes of appetite and satiety were assessed every waking hour on the third day of each pattern. Daily hunger, desire to eat, and preoccupation with thoughts of food were not different between groups. The HP group experienced greater fullness throughout the day vs. NP (511 ± 56 vs. 243 ± 54 mm · 15 h; P < 0.005). When compared to NP, the HP group experienced lower late-night desire to eat (13 ± 4 vs. 27 ± 4 mm, P < 0.01) and preoccupation with thoughts of food (8 ± 4 vs. 21 ± 4 mm; P < 0.01). Within groups, the 3 vs. 6-EO patterns did not influence daily hunger, fullness, desire to eat, or preoccupation with thoughts of food. The 3-EO pattern led to greater evening and late-night fullness vs. 6-EO but only within the HP group (P < 0.005). Collectively, these data support the consumption of HP intake, but not greater eating frequency, for improved appetite control and satiety in overweight/obese men during energy-restriction-induced weight loss.
INTRODUCTION
Consuming more frequent, higher protein (HP) meals is one of the more commonly practiced dietary strategies among Americans to increase healthy eating and promote weight loss (1,2). Although there is strong support regarding the consumption of increased dietary protein for better appetite control and satiety (3–9), conflicting data exist concerning the effects of eating frequency on these parameters (10–16). Specifically, diets containing HP quantities, ranging from 18 to 35% of daily energy intake, lead to reductions in daily hunger and/or increases in daily fullness compared to those containing lower amounts of dietary protein, ranging from 10 to 15% of daily energy intake (3,8,17–19). With respect to the role of eating frequency on appetite and satiety, several researchers reported reduced hunger and increased fullness with greater eating frequency (11,15), whereas others showed no differences (14,20).
Recently, our research group reported that dietary protein and eating frequency did not affect perceived hunger but altered fullness (21). A HP intake (i.e., 25% of daily energy) led to increased daily fullness, whereas frequent eating (i.e., 6 eating occasions (EO)/day) led to reductions in daily fullness (21). Although these data, obtained using a clinical laboratory-based protocol, support the current literature with respect to the satiating properties of increased protein intake, they refute the concept that greater eating frequency leads to comparable effects. The purpose of this study was to determine the independent and combined effects of increased dietary protein and greater eating frequency on perceived appetite and satiety during weight loss in overweight/obese men, with measurements made while participants maintained their usual, freeliving activities and environment.
METHODS AND PROCEDURES
Participants
This study was part of a 12-week longitudinal protocol designed to examine the effects of normal protein (NP) vs. HP energy-restriction diets on body weight, body composition, and appetite in overweight/obese men. Potential participants for the longitudinal study were recruited through newspaper advertisements. Eligibility was based on the following criteria: (i) men (ii) age ≥21 years; (iii) BMI 25.0–34.9 kg/ m2; (iii) percent body fat >25%; (iv) not dieting; no weight loss/gain (≥4.5 kg) within the past 6 months; (v) nonsmoking; (vi) nondiabetic; (vii) clinically normal blood profiles; and (viii) consistent activity and dietary patterns over the past 3 months (as indicated from the dietary and physical activity questionnaire documenting whether the participant had been following a special diet (e.g., weight loss, high-protein, vegetarian, diabetic, low-fat, or lactose-free) and/or whether the participant’s work, exercise, and/or physical activity had dramatically changed over the past 3 months).
A total of 58 men began the longitudinal study. In total, 29 men were randomly assigned to each group; 13 men (22%) dropped out during intervention weeks 1–6 due to time constraints and noncompliance. Thus, 45 men were available to begin the eating frequency substudy, and 27 completed all eating frequency procedures. Of the 18 men who did not complete the substudy procedures, 4 dropped out due to time constraints, whereas the remaining 14 participants were noncompliant to the specific eating frequency substudy requirements (see Study Compliance). Data from these participants were excluded from all analyses. When comparing the subject characteristics of the completers vs. noncompleters (i.e., dropouts and excluded), no differences were observed. Thus, the subject characteristics of only those who completed all study procedures are shown in Table 1. No differences in these measurements were found between the protein groups.
Table 1.
subject characteristics of the 27 participants who completed all substudy procedures
| Subject characteristicsa | NP (n = 14) | HP (n = 13) |
|---|---|---|
| Baseline (preintervention) | ||
| Age (years) | 43 ± 4 | 52 ± 4 |
| Height (cm) | 177 ± 2 | 182 ± 2 |
| Weight (kg) | 99.2 ± 3.9 | 105.2 ± 3.8 |
| BMI (kg/m2) | 31.4 ± 1.0 | 31.7 ± 1.0 |
| Start of eating frequency substudy | ||
| Weight (kg) | 93.2 ± 3.9 | 99.0 ± 3.4 |
| Difference from baseline (kg) | −6.0 ± 0.5 | −6.2 ± 0.6 |
| BMI (kg/m2) | 29.5 ± 0.9 | 29.8 ± 0.9 |
| Difference from baseline (kg/m2) | −1.9 ± 0.2 | −1.9 ± 0.2 |
Data expressed as mean ± s.e.m.
HP, higher protein; NP, normal protein.
No differences were observed between NP vs. HP, P < 0.05.
Participants signed an informed consent form approved by the Purdue University biomedical institutional review board and received monetary compensation for completing all study procedures.
Dietary interventions
Longitudinal study
The participants were prescribed a diet that was 750 kcal/day less than their daily energy need estimated as resting energy expenditure × 1.5 activity factor using the Harris Benedict equation for men. This resulted in a daily consumption of 2,290 ± 68 kcal/ day (22). The participants were randomly assigned to the NP diet containing 14% protein (~0.8 g protein/kg/day), 60% carbohydrate, and 26% fat or the HP diet containing 25% protein (~1.4 g protein/kg/day), 49% carbohydrate, and 26% fat (Table 2).
Table 2.
Dietary characteristics of the NP vs. HP diets
| Subject characteristicsa | NP (n = 14) | HP (n = 13) |
|---|---|---|
| Prescribed diet | ||
| Energy content (kcal/day) | 2,236 ± 99 | 2,348 ± 96 |
| Protein (g/day) | 81 ± 3 | 152 ± 6c |
| Carbohydrate (g/day) | 351 ± 17 | 299 ± 14c |
| Fat (g/day) | 64 ± 3 | 66 ± 3 |
| Actual diet (during eating frequency) | ||
| Energy content (kcal/day) | 2,139 ± 109 | 2,350 ± 113 |
| (kcal/meal of the 3-EO) | 713 ± 36b | 783 ± 38b |
| (kcal/meal of the 6-EO) | 356 ± 18 | 392 ± 19 |
| Protein (g/day) | 79 ± 4 | 152 ± 5c |
| (g/meal of the 3-EO) | 26 ± 1b | 51 ± 2b,c |
| (g/meal of the 6-EO) | 13 ± 1 | 25 ± 1c |
| Carbohydrate (g/day) | 334 ± 18 | 299 ± 18c |
| (g/meal of the 3-EO) | 111 ± 6b | 100 ± 6b,c |
| (g/meal of the 6-EO) | 56 ± 3 | 50 ± 3c |
| Fat (g/day) | 62 ± 3 | 66 ± 3 |
| (g/meal of the 3-EO) | 21 ± 1b | 22 ± 1b |
| (g/meal of the 6-EO) | 10 ± 1 | 11 ± 1 |
NP, normal protein; HP, higher protein.
Data expressed as mean ± s.e.m.
Within group, 3 vs. 6-EO, P < 0.05.
Between group, NP vs. HP, main effect of diet, P < 0.05.
The participants were provided with menus containing specified quantities of brand-specific food items to purchase and consume. Except for the weeks in which the eating frequency substudy was completed (see below), the menus included a “3-meal + 1-snack” pattern; however, the participants were permitted to follow any eating pattern they preferred as long as all of the prescribed quantities of food were consumed by the end of each day. In addition, the HP group was provided with portioned quantities of cooked pork and egg products comprising 25 and 15% of total protein intake, respectively. The NP group was given portioned quantities of milk and consumed a diet void of striated tissue or eggs. This study incorporated similar dietary procedures and strategies as our previous 12-week intervention study in overweight/obese women (3).
Eating frequency substudy
Beginning at approximately week 7 (range: weeks 4–9) of the intervention, the participants completed the 2-week eating frequency substudy. For the first 3 days (i.e., Monday–Wednesday) of each week, the participants were instructed to consume their respective NP or HP diet as either 3 or 6-EO in randomized order. For the 3-EO pattern, energy intake was equally divided among all 3-EO and consumed every 5 h. During the 6-EO pattern, energy intake was equally divided among all 6-EO and consumed every 2 h. The participants were specifically provided with eating frequency menus containing specific quantities of food to consume at specific times throughout the day depending on which eating pattern they were following that particular week. To document adherence to the diet and eating times, the participants completed daily food and eating logs. Regardless of pattern, days 1 and 2 served as acclimation days, and day 3 was considered the testing day.
Appetite questionnaires
Appetite questionnaires, assessing hunger, fullness, desire to eat, and preoccupation with thoughts of food were loaded onto a handheld personal digital assistant (Palm-Pilot M100; Palm Computing, Sunnyvale, CA). The participants completed the questionnaires every waking hour throughout days 2 and 3 of the 3-EO and 6-EO patterns. A reliable, validated visual analog scale incorporating a 100-mm rating scale with end anchors of “not at all” and “extremely” was used to assess each sensation (23,24).
Study compliance
Study compliance was based on the following criteria collected from day 3 of each eating frequency pattern: (i) at least 15 hourly appetite and satiety questionnaires were completed; (ii) >95% of the food prescribed in each eating occasion was consumed; and (iii) the prescribed food was consumed within 1 h of the prescribed eating time(s). As previously stated, based on this criteria, 14 out of the initial 45 men (who began the eating frequency substudy), did not meet this criteria. Thus, only the data from the 27 participants who met these criteria are reported.
Data and statistical analysis
Perceived hunger, fullness, desire to eat, and preoccupation with thoughts of food responses throughout day 3 of each pattern were each truncated to include a total of 15 time points. Daily, 15-h area under the curve (AUC) was calculated (25). We further divided the 15-h period into three segments: period 1: time 1–6 h; period 2: time 6–11 h; and period 3: 11–15 h and calculated individual AUCs for each outcome with these periods. The trapezoidal rule was utilized for all AUC measurements (22). Morning (i.e., first recorded responses of the day) and late-night (i.e., last recorded response of the day prior to sleep) appetite and satiety responses were also examined. A two-way mixed-factor ANOVA was performed on the appetite and satiety responses to examine between-subject (independent) effects of dietary protein (HP vs. NP) and within-subject (repeated measures) effects of eating frequency (3-EO vs. 6-EO). Due to the differences, albeit non-significant, in participant age and energy content consumed in those following the HP vs. NP diets, we initially included these outcomes as covariates. Neither age nor energy content had a significant impact on any of the appetite and satiety responses; thus, the data are reported without controlling for these variables. Data are expressed as mean ± s.e.m. P < 0.05 is considered statistically significant. Analyses were conducted using the Statistical Package for the Social Sciences (version 16.0; SPSS; Chicago, IL).
RESULTS
Dietary characteristics during the eating frequency substudy
The dietary characteristics during the eating frequency substudy are reported in Table 2. No differences in 24-h energy intake were observed when comparing the prescribed vs. actual intakes between diet groups. By design, the HP group consumed more protein vs. NP (26 ± 1 vs. 15 ± 1% of energy intake as protein; 1.54 ± 0.03 vs. 0.84 ± 0.03 g/kg body weight/day at the time of testing; P < 0.001). The HP group also consumed less carbohydrate vs. NP (51 ± 1 vs. 62 ± 1 % of total intake as carbohydrate; P < 0.001). No difference in dietary fat consumption was observed between the HP vs. NP groups (both groups: 26 ± 1 % of total intake as fat; nonsignificant (NS); Table 2). Additionally, no differences in total energy intake, protein, carbohydrate, or fat consumption were observed between the 3 vs. 6-EO patterns (Table 2).
Perceived appetite
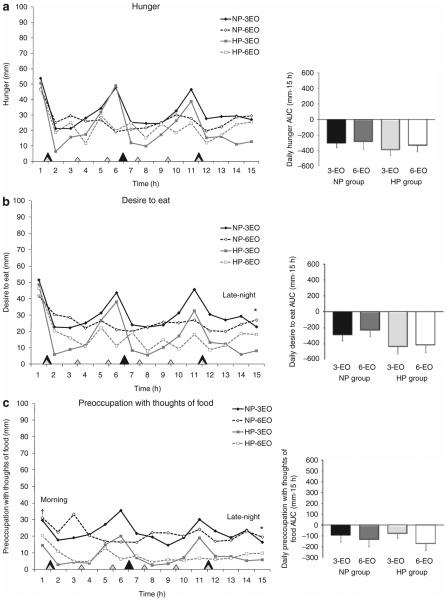
Hourly and AUC appetite responses are shown in Figure 1a–c. No differences in daily hunger (−361 ± 74 vs. −294 ± 71 mm · 15 h; NS), desire to eat (−425 ± 76 vs. −260 ± 74 mm · 15 h; NS), or preoccupation with thoughts of food (−126 ± 46 vs. −114 ± 45 mm · 15 h; NS) were observed between HP vs. NP groups nor were there any differences when examined according to specific time periods (data not shown). Although no differences in morning (i.e., waking) hunger and desire to eat were observed between groups, preoccupation with thoughts of food tended to be lower in the HP (17 ± 5 mm) vs. NP group (30 ± 5 mm; P = 0.067). Late-night desire to eat and preoccupation with thoughts of food were lower in the HP vs. NP groups (desire to eat: 13 ± 4 vs. 27 ± 4 mm, P < 0.01; preoccupation with thoughts of food: 8 ± 4 vs. 21 ± 4 mm; P < 0.01).
Figure 1.
Perceived (a) hunger, (b) desire to eat, and (c) preoccupation with thoughts of food throughout the day following the 3 vs. 6-EO patterns in NP vs. HP diet groups. Closed diamonds, NP group following the 3-EO pattern; open diamonds, NP group following the 6-EO pattern; closed squares, HP group following the 3-EO pattern; and open squares, HP group following the 6-EO pattern. Main effect of group: HP vs. NP at specific time points, *P < 0.01; main effect of group: HP vs. NP at specific time points, †P = 0.067. EO, eating occasions; HP, higher protein; NP, normal protein.
Regardless of groups, no differences were observed between the 3 vs. 6-EO patterns with respect to daily hunger (−346 ± 48 vs. −309 ± 63 mm · 15 h; NS), desire to eat (−363 ± 55 vs. −322 ± 63 mm · 15 h; NS), or preoccupation with thoughts of food (−87 ± 38 vs. −152 ± 44 mm · 15 h; NS). No other differences were observed between eating frequencies.
Perceived fullness (satiety)
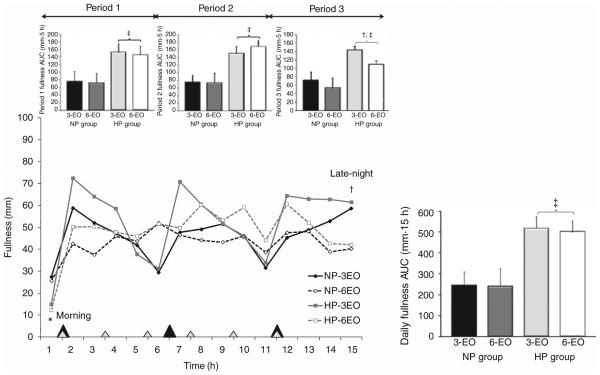
Hourly and AUC fullness responses are shown in Figure 2. The HP group experienced greater daily fullness vs. NP (511 ± 56 vs. 243 ± 54 mm · 15 h; P < 0.005). According to specific time periods, the HP group exhibited greater fullness during periods 1–3 vs. NP (all comparisons; P < 0.005; Figure 2). In contrast, morning fullness was lower in the HP group (14 ± 4 mm) vs. NP (26 ± 4 mm; P < 0.05). No differences in late-night fullness were observed between groups.
Figure 2.
Perceived fullness throughout the day following the 3 vs. 6-EO patterns in normal vs. higher protein diet groups. Closed diamonds, NP group following the 3-EO pattern; open diamonds, NP group following the 6-EO pattern; closed squares, HP group following the 3-EO pattern; and open squares, HP group following the 6-EO pattern. Main effect of group: HP vs. NP at specific time points, *P < 0.05; protein × eating frequency interaction: HP-3EO vs. HP-6EO at specific time points; †P < 0.005; main effect of group: HP vs. NP, ‡P < 0.005. EO, eating occasions; HP, higher protein; NP, normal protein.
Within diet groups, no differences in daily fullness were observed between the 3 vs. 6-EO patterns (Figure 2). However, compared to 3-EO, the 6-EO pattern led to reduced evening fullness (i.e., period 3; Figure 2; P < 0.005) and late-night fullness (3-EO: 57 ± 4 vs. 6-EO: 39 ± 5 mm; P < 0.005) but only within the HP group. No difference in morning fullness was observed between eating frequencies.
DISCUSSION
We examined the impact of consuming frequent, HP meals on perceived appetite and satiety during weight loss in over-weight and obese men. HP intake led to greater fullness throughout the day along with reductions in late-night desire to eat and preoccupation with thoughts of food compared to an NP diet. In contrast, greater eating frequency had little impact on these outcomes unless combined with an HP diet. Taken together, these data support the approach of consuming HP intake, but not eating more frequently, for improved appetite control and satiety in overweight and obese men during weight loss.
The dietary interventions in this study are similar in nature to that of our previous study, comparing protein quantities of 14 vs. 25% of daily energy as protein in 3 vs. 6-EO patterns (21). In our previous study, we found that dietary protein and eating frequency had no effect on daily perceived hunger but significantly altered satiety throughout the day. Although HP intake led to greater daily fullness, eating more frequently led to reductions in daily fullness (21).
This study extends these findings to include (i) a controlled-feeding approach within a free-living environment; (ii) energy restriction; and (iii) longer assessment periods of 15 h. Using this approach, the participants were permitted to maintain their habitual, daily living activity patterns but were required to consume only the portioned quantities of food prescribed and/or provided to them, at specific times. Thus, the participants were unable to respond to any changes in perceived appetite and/or satiety by increasing and/or decreasing energy intake. This design allowed for the specific assessment of whether protein and eating frequency alter perceived appetite and satiety without the potential confounding effects of reduced activity patterns (as observed in controlled-feeding laboratory trials) and changes in ingestive behavior (i.e., compensatory feeding) during weight loss. The findings from our current study are similar to our previous study (21) in that increased dietary protein led to increased fullness throughout the morning, afternoon, and evening hours compared to eating an NP diet, whereas the perceived sensations of appetite during these segments were not altered with dietary protein (21). When comparing these findings to other protein studies, both meal-related and daily fullness are consistently increased with HP meals and/or diets (3–5,7,17,18,26,27). In contrast, although the majority of acute, meal-related studies illustrate reductions in post-meal appetite following higher vs. NP meals (4,5,7,26–28), diet-related, intervention studies find no significant changes in daily appetite (3,17,21). These findings suggest that appetite-related drive to eat is influenced more by physiological, environmental, social, and behavioral factors than by the energy content and macronutrient composition of the diet (29). On the other hand, satiety appears to be driven less by environmental, social, and behavioral factors and more by meal-related factors such as increased dietary protein (5,7).
We also examined several important time points in the daily initiation and/or cessation of eating during weight loss. One of the more unexpected findings was that of the reduced morning (i.e., waking) fullness observed in the higher vs. NP group, which might indicate a habitualization to dietary protein. However, upon closer examination, the reduced fullness response was completely reversed upon eating the first meal (i.e., eating occasion #1; Figure 2). We also found that late-night desire to eat and preoccupation with thoughts of food, two perceptions that are increased when dieting (30), were reduced in the group that consumed the HP vs. NP weight-loss diet. These findings indicate a reduced motivational drive to eat with increased dietary protein (30,31) and suggest that HP intake might lead to reduced late-night snacking and overeating in the evening, which are two leading contributors to poor compliance when following a weight-loss diet (32).
Regarding the impact of eating frequency on appetite control and satiety, our previous study indicated no beneficial effects of eating more frequently throughout the day (21). In fact, frequent eating (i.e., eating every 2 h) led to reduced fullness throughout the day compared to eating every 4 h (21). Although our current study found no differences in overall appetite and satiety when comparing 3 vs. 6-EO, evening and late-night fullness were reduced following the 6-EO pattern; however, this was only observed when consuming an HP diet. Besides our current and past studies, only one other study has examined these effects when providing 3 vs. 6-EO (33). The participants were free-living and consumed diets 700 kcal/day below energy needs over an 8-week period. Energy content and timing of the eating occasions were varied and appetite was only assessed for 6 h during the controlled-feeding, clinical testing trials (33). Regardless of the differences among study designs, no differences in hunger, fullness, desire to eat, or prospective food consumption were observed between eating frequency patterns (33). Taken together, the overall findings support that eating more frequently does not enhance appetite control and satiety in overweight and obese individuals during weight loss.
Several study limitations have been identified. By only examining the impact of eating frequency during 2 weeks of the 12-week intervention, we were unable to assess long-term changes in appetite, body weight, or body composition. This approach was used due to the uncertainty that the participants would be able to adhere to the strict meal times (i.e., specifically with eating every 2 h) in a free-living environment for 12 weeks. This concern was substantiated by the fact that only 60% of the participants who started the eating frequency substudy were able to adhere to the eating guidelines. Of those who were noncompliant, 90% were specifically unable to follow the 6-EO pattern. The lack of compliance might have occurred due to the unfamiliarity with a 6-EO pattern, eating every 2 h. This adds further support that eating more frequently is not the most appropriate or feasible eating strategy.
Utilizing a free-living study design prevented performing blood collections and hormonal analyses necessary to identify proposed mechanisms. According to our previous controlled-feeding, laboratory-based study (21), consuming higher vs. normal dietary protein in 3 vs. 6-EO patterns leads to elevations in the satiety hormone peptide YY. Reductions in the appetite-stimulating hormone ghrelin following HP vs. NP intake have also been reported (4,34,35).
The dietary interventions in our current and previous studies (21,26) incorporate animal-based protein sources from egg and pork in the HP diets but eliminate these sources and all other striated tissue sources in the NP diets. With this design, we are unable to ascertain whether the protein effects on appetite and satiety are due to the increase in dietary protein (i.e., protein quantity) in the form of eggs and pork or whether it is the unique amino acid profile (i.e., protein quality) of these protein sources that contribute to the differences. Thus, more research is needed before generalizing these findings to other protein sources including, but not limited to, milk or soy-based protein sources.
We would like to mention that the differential effects observed following the HP vs. NP diets may be partly attributed to the reduction in carbohydrate consumption (i.e., 60–49% of energy as carbohydrate) prescribed in the HP diet instead of simply the increased quantity of protein consumed (i.e., 14–25% of energy as protein). Many of the studies comparing HP vs. NP diets keep dietary fat constant between diets, in order to control for energy density, and thus reduce carbohydrate content (3–7,9,17,18,26,27). Based on this approach, it has been suggested that the effects of HP diets might actually be due to the reduced carbohydrate content, not increased protein. However, in a recent study, Weigle et al. (8) addressed this issue by examining the effects of increased protein intake while maintaining carbohydrate intake and adjusting fat content accordingly. This study observed similar findings to that of HP/lower carbohydrate diets in that the HP/constant carbohydrate diet led to increases in satiety, reductions in daily energy intake, and greater weight loss compared to the diet containing lower protein but similar carbohydrate content (8).
Another important aspect of eating frequency not included in this study pertains to the examination of infrequent eating (i.e., <3-EO) and/or skipping meals, which is suggested to negatively influence appetite control (10,16). Future studies including this end of the eating frequency spectrum would provide comprehensive evidence concerning the impact of eating frequency on the regulation of appetite and food intake.
In summary, during weight loss, HP intake improved daily satiety and evening appetite control, whereas greater eating frequency had relatively no impact on these outcomes. These data suggest that an energy-restriction diet containing a moderate increase in dietary protein consumed in 3-EO leads to better appetite control and satiety in overweight and obese men.
ACKNOWLEDGMENTS
This study was funded by the National Pork Board and the American Egg Board—Egg Nutrition Center. Additional support was provided by the Purdue University Ingestive Behavior Research Center (postdoctoral fellowship (H.J.L.); and, the Indiana Clinical and Translational Sciences Institute, funded in part by grant # RR 02576 from the National Institutes of Health, National Center for Research Resources.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Group NPDN . NPD Finds Fewer Americans Dieting But More Eating “Better for You” Foods: Healthier Eating Trend Contributes to Obesity Leveling Off. NPD; Rosemont: 2008. [Google Scholar]
- 2.Popkin BM, Duffey KJ. Does hunger and satiety drive eating anymore? Increasing eating occasions and decreasing time between eating occasions in the United States. Am J Clin Nutr. 2010;91:1342–1347. doi: 10.3945/ajcn.2009.28962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 4.Leidy HJ, Mattes RD, Campbell WW. Effects of acute and chronic protein intake on metabolism, appetite, and ghrelin during weight loss. Obesity (Silver Spring) 2007;15:1215–1225. doi: 10.1038/oby.2007.143. [DOI] [PubMed] [Google Scholar]
- 5.Westerterp-Plantenga MS, Nieuwenhuizen A, Tomé D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 6.Smeets AJ, Soenen S, Luscombe-Marsh ND, Ueland Ø , Westerterp-Plantenga MS. Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1, and peptide tyrosine-tyrosine concentrations following a single high-protein lunch. J Nutr. 2008;138:698–702. doi: 10.1093/jn/138.4.698. [DOI] [PubMed] [Google Scholar]
- 7.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373–385. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- 8.Weigle DS, Breen PA, Matthys CC, et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41–48. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- 9.Veldhorst M, Smeets A, Soenen S, et al. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav. 2008;94:300–307. doi: 10.1016/j.physbeh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Stote KS, Baer DJ, Spears K, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85:981–988. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speechly DP, Rogers GG, Buffenstein R. Acute appetite reduction associated with an increased frequency of eating in obese males. Int J Obes Relat Metab Disord. 1999;23:1151–1159. doi: 10.1038/sj.ijo.0801046. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins DJ, Wolever TM, Vuksan V, et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med. 1989;321:929–934. doi: 10.1056/NEJM198910053211403. [DOI] [PubMed] [Google Scholar]
- 13.Solomon TP, Chambers ES, Jeukendrup AE, Toogood AA, Blannin AK. The effect of feeding frequency on insulin and ghrelin responses in human subjects. Br J Nutr. 2008;100:810–819. doi: 10.1017/S000711450896757X. [DOI] [PubMed] [Google Scholar]
- 14.Taylor MA, Garrow JS. Compared with nibbling, neither gorging nor a morning fast affect short-term energy balance in obese patients in a chamber calorimeter. Int J Obes Relat Metab Disord. 2001;25:519–528. doi: 10.1038/sj.ijo.0801572. [DOI] [PubMed] [Google Scholar]
- 15.Speechly DP, Buffenstein R. Greater appetite control associated with an increased frequency of eating in lean males. Appetite. 1999;33:285–297. doi: 10.1006/appe.1999.0265. [DOI] [PubMed] [Google Scholar]
- 16.Smeets AJ, Westerterp-Plantenga MS. Acute effects on metabolism and appetite profile of one meal difference in the lower range of meal frequency. Br J Nutr. 2008;99:1316–1321. doi: 10.1017/S0007114507877646. [DOI] [PubMed] [Google Scholar]
- 17.Lejeune MP, Kovacs EM, Westerterp-Plantenga MS. Additional protein intake limits weight regain after weight loss in humans. Br J Nutr. 2005;93:281–289. doi: 10.1079/bjn20041305. [DOI] [PubMed] [Google Scholar]
- 18.Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord. 2004;28:57–64. doi: 10.1038/sj.ijo.0802461. [DOI] [PubMed] [Google Scholar]
- 19.Moran LJ, Luscombe-Marsh ND, Noakes M, et al. The satiating effect of dietary protein is unrelated to postprandial ghrelin secretion. J Clin Endocrinol Metab. 2005;90:5205–5211. doi: 10.1210/jc.2005-0701. [DOI] [PubMed] [Google Scholar]
- 20.Jackson SJ, Leahy FE, Jebb SA, et al. Frequent feeding delays the gastric emptying of a subsequent meal. Appetite. 2007;48:199–205. doi: 10.1016/j.appet.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Leidy HJ, Armstrong CL, Tang M, Mattes RD, Campbell WW. The influence of higher protein intake and greater eating frequency on appetite control in overweight and obese men. Obesity (Silver Spring) 2010;18:1725–1732. doi: 10.1038/oby.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris J, Benedict F. A Biometric Study of Basal Metabolism in Man. Carnegie Institute of Washington; Washington, D.C.: 1919. [Google Scholar]
- 23.Stubbs RJ, Hughes DA, Johnstone AM, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84:405–415. doi: 10.1017/s0007114500001719. [DOI] [PubMed] [Google Scholar]
- 24.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 25.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr. 1986;43:167–172. doi: 10.1093/ajcn/43.1.167. [DOI] [PubMed] [Google Scholar]
- 26.Leidy HJ, Bossingham MJ, Mattes RD, Campbell WW. Increased dietary protein consumed at breakfast leads to an initial and sustained feeling of fullness during energy restriction compared to other meal times. Br J Nutr. 2009;101:798–803. doi: 10.1017/s0007114508051532. [DOI] [PubMed] [Google Scholar]
- 27.Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr. 2006;83:89–94. doi: 10.1093/ajcn/83.1.89. [DOI] [PubMed] [Google Scholar]
- 28.Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Wansink B. Mindless Eating—Why We Eat More Than We Think. Bantam-Dell; New York, NY: 2006. [Google Scholar]
- 30.Jones N, Rogers PJ. Preoccupation, food, and failure: an investigation of cognitive performance deficits in dieters. Int J Eat Disord. 2003;33:185–192. doi: 10.1002/eat.10124. [DOI] [PubMed] [Google Scholar]
- 31.Timmerman GM, Gregg EK. Dieting, perceived deprivation, and preoccupation with food. West J Nurs Res. 2003;25:405–418. doi: 10.1177/0193945903025004006. [DOI] [PubMed] [Google Scholar]
- 32.Waller SM, Vander Wal JS, Klurfeld DM, et al. Evening ready-to-eat cereal consumption contributes to weight management. J Am Coll Nutr. 2004;23:316–321. doi: 10.1080/07315724.2004.10719374. [DOI] [PubMed] [Google Scholar]
- 33.Cameron JD, Cyr MJ, Doucet E. Increased meal frequency does not promote greater weight loss in subjects who were prescribed an 8-week equi-energetic energy-restricted diet. Br J Nutr. 2009:1–4. doi: 10.1017/S0007114509992984. [DOI] [PubMed] [Google Scholar]
- 34.Foster-Schubert KE, Overduin J, Prudom CE, et al. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 2008;93:1971–1979. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowen J, Noakes M, Clifton PM. Appetite hormones and energy intake in obese men after consumption of fructose, glucose and whey protein beverages. Int J Obes (Lond) 2007;31:1696–1703. doi: 10.1038/sj.ijo.0803665. [DOI] [PubMed] [Google Scholar]