Abstract
Background
Lamina propria Th2 cytokines, IL-4 and IL-13, stimulate goblet cell (GC) proliferation and mucin2 (MUC2) production, which protects the intestinal mucosa. Elemental enteral nutrition (EEN) reduces tissue IL-4 and impairs barrier function. Since proanthocyanidins (PAC) stimulate oral mucin levels, we hypothesized that adding PAC to EEN would maintain Th2 cytokines – without stimulating Th1 cytokines - and preserve luminal MUC2 vs. EEN alone.
Materials and Methods
70 mice were randomized to 5 diet groups (14/group): Standard Chow, intragastric EEN, EEN+lowPAC (8 mg), EEN+midPAC (50 mg), or EEN+highPAC (100 mg PAC/kg body weight) for 5 days, starting 2 days after gastric cannulation. Ileal tissue was analyzed for histomorphology and the cytokines IL-4, IL-13, IL-1β, IL-6, TNF-α by ELISA. MUC2 was measured in intestinal washes by western blot.
Results
EEN lowered IL-13 (p<0.05) compared to Standard Chow, while IL-4 did not reach significance (p<0.07). However, EEN+lowPAC and EEN+midPAC increased IL-13 (p<0.05), while EEN+highPAC increased both IL-4 and IL-13 (p<0.05), compared with EEN alone. All EEN diets reduced (P<0.05) crypt depth compared to the Standard Chow group. Compared with Standard Chow, GC numbers and luminal MUC2 were reduced with EEN (p<0.05). These effects were attenuated (p<0.05) with EEN+midPAC and EEN+highPAC. No changes were observed in tissue Th1 cytokines IL-1β, IL-6, and TNF-α.
Conclusions
Adding PACs to EEN reverses the impaired intestinal barrier resulting from EEN by improving the gut mucous layer morphology and function through increased size and number of GC as well as increased levels of MUC2 and ileal IL-4 and IL-13.
Keywords: enteral nutrition, proanthocyanidins, goblet cells, mucin, cytokines
INTRODUCTION
Elemental enteral nutrition (EEN) is a therapeutic option for inflammatory bowel disorders such as Crohns disease 1. Unfortunately, EEN induces well-defined dysfunction of the mucosal immune system, specifically within the gut-associated lymphoid tissue (GALT), and suppresses mucosal barrier function when compared to normal nutrition 2–5. The integrity of the mucosal barrier is critical for maintaining the physical and chemical barrier against food and environmental antigens, including microbes 6. The mucosal barrier is partly dependent upon the physical and compositional characteristics of the mucous layer 7. Dietary compounds that affect this layer may have implications in health through modulation of the intestinal barrier 8.
PAC are a class of polyphenolic compounds widely distributed in plant-derived foods and beverages 9–11 that are associated with the prevention of chronic diseases in epidemiological studies 12–14. However, PAC are minimally absorbed due to non-hydrolyzable bonds between monomeric subunits and a propensity to bind proteins through hydrogen bonding 15. PAC complex salivary glycoproteins, a process that causes astringency in the oral cavity when many fruits and beverages are ingested 16. Complexation induces salivary excretion, hypertrophy of the parotid gland, and a shift in salivary composition to proline-rich glycoproteins in rodents 16, 17. Because of poor absorption, greater than 95% of PAC remain in the intestinal lumen during transit 18, 19 suggesting beneficial dietary effects of PACs may occur through interactions at the mucosal surface of the gastrointestinal tract 8, for example, by influencing secretion of mucins, a class of glycoproteins, in the small intestine 14, 20.
Mucins are secreted by goblet cells (GC) and play a critical role in maintaining mucosal integrity 7. GC, specialized intestinal epithelial cells, migrate up the villi after differentiating from crypt stem cells, turning over with the epithelial layer every 3–5 days. Mucin2 (MUC2) is the most abundant mucin secreted by intestinal GC. The importance of MUC2 is underscored in MUC2−/− mice, in which the deficiency leads to the development of lethal colitis 21. MUC2 secretion is induced by cholinergic stimulation 22, while its production is regulated by the T-helper 2 (Th-2) cytokines IL-4 and IL-13, derived from lamina propria or intraepithelial lymphocytes 23–25.
In this study, we hypothesized that the addition of physiologically relevant doses 26, 27 of cranberry PAC (8–100 mg Gaelic Acid Equivalents (GAE) / kg body weight) to EEN would attenuate the negative effects of EEN on intestinal barrier function as determined by changes in the Th-2 cytokines IL-4 and IL-13, GC number and size, and luminal MUC2. Additionally, we examined potential changes in pro-inflammatory Th1 cytokines (IL-1β, IL-6, and TNF-α) 28, 29 and histomorphometric parameters (e.g., villi length and crypt depth) 30.
MATERIALS AND METHODS
PAC Preparation and Characterization
The methodology for PAC preparation and characterization was previously published 31. Briefly, Non-depectinized cranberry presscake was ground with liquid nitrogen and extracted with 70% acetone (Fisher Scientific, Fair Lawn, NJ). Samples were sonicated and centrifuged at for 10 minutes. The extraction was repeated twice. Acetone was removed by evaporation and the aqueous suspension was solubilized in ethanol (Decon Labs Inc., King of Prussia, PA), followed by centrifugation to eliminate ethanol insoluble material. Cranberry presscake crude extract was loaded on a Sephadex LH-20™ (GE Healthcare, Uppsala, Sweden) column and PAC were isolated by sequential elution with ethanol, ethanol/methanol (1:1) and 80% acetone. Acetone in the last fraction that contained PAC was removed by evaporation under vacuum and re-solubilized in methanol (Fisher Scientific, Fair Lawn, NJ). The total phenolic content of the PAC fraction was determined by the modified Folin-Ciocalteu method and reported as gallic acid equivalents (GAE).
An aliquot of the cranberry presscake PAC fraction was diluted tenfold and a sample was injected onto a Waters Spherisorb® 10 μm ODS2 RP-18 column. The solvents for elution were trifluoroacetic acid/water (0.1%) and methanol. The HPLC system consisted of a Waters automated gradient controller, two Waters 501 HPLC pumps, and a Rheodyne 7125 manual injector. The elution was monitored by a Waters 996 diode array detector using Waters Millennium software for collecting and analyzing three-dimensional chromatograms.
An aliquot of the cranberry presscake PAC fraction was mixed with 2,5-dihydroxybenzoic acid (Aldrich, Milwaukee, WI) and the mixture was applied onto a MALDI-TOF MS stainless steel target and dried at room temperature. Mass spectra were collected on a Bruker Reflex II MALDI-TOF-MS (Billerica, MA) equipped with delayed extraction and a N2 laser (337 nm) in order to characterize the range in degree of polymerization (DP) and nature of interflavan bonds in the cranberry PAC. All preparations were analyzed in the positive ion linear and reflectron mode to detect [M+Na]+ and [M+K]+ molecular ions. MALDI-TOF MS is ideally suited for characterizing PAC because, unlike electrospray ionization in which multiple charge molecular ions create very complex spectral peaks that are often difficult to interpret, this mass spectral technique produces only a singly charged molecular ion for each parent molecule 11.
Animals
All animal experiment protocols were approved by Animal Care and Use Committee of the University of Wisconsin-Madison and the Middleton Veterans Administration Hospital, Madison. Male Institute of Cancer Research (ICR) outbred mice were purchased through Harlan (Indianapolis, IN) and housed in an American Association for Accreditation of Laboratory Animal Care-accredited conventional facility on the V.A Williamson Hospital Campus. The mice were acclimatized for one week in a temperature and humidity controlled environment with a 12h/12h light/dark cycle. The mice were housed 5 per micro isolater-top cages and fed ad libitum Chow (Rodent Diet 5001, LabDiet, PMI Nutrition International, St. Louis, MO) and water for 1 week prior to initiation of study protocol. A description and detailed chemical composition of Rodent Diet 5001 is available at http://labdiet.com/pdf/5001.pdf. Once entering study protocol, the mice were housed individually in metal wire-bottomed cages to prevent coprophagia and ingestion of bedding.
Experimental design
Seventy male ICR mice (6 to 8 wk old) were randomized by weight (n = 14 / diet group) to receive Standard Chow, intragastric EEN or intragastric EEN+PAC [8 mg (EEN+lowPAC), 50 mg (EEN+midPAC) or 100 mg (EEN+highPAC) GAE of PAC/kg body weight]. Animals were anesthetized with intraperitoneal administration of ketamine (100 mg/kg) and acepromazine (10 mg/kg) and gastrostomy was performed. Catheters were tunneled subcutaneously from the gastrostomy site, over the back, finally exiting mid-tail. The mice were partially restrained by the tail for the remainder of the study to protect the catheter during infusions. This partial restraint technique does not induce significant stress in the mice 32. The catheterized mice were connected to infusion pumps and allowed to recover for 48 h while receiving 4 mL/d of saline (0.9%) via the catheter. The mice also received ad libitum Chow (Rodent Diet 5001, LabDiet) and water.
Following the recovery period, animals received their assigned dietary treatments. The Standard Chow fed mice were given ad libitum chow diet and water, and continued to receive 0.9% saline at 4 mL/d via the intragastric catheter. EEN and EEN+PAC fed mice received solution at 4 mL/d (day 1), 7 mL/d (day 2) and 10 mL/d (days 3–5) as well as ad libitum water throughout the study. The EEN solution includes 6.0% amino acids, 35.6% dextrose, electrolytes, and multivitamins, with a non-protein calorie to nitrogen ratio of 126.1 (527.0 kJ/g nitrogen). This value meets the calculated nutrient requirements of mice weighing 30 to 35 g 33.
After 5 d of feeding (7 days post-catheterization), mice were weighed, anesthetized as before, and exsanguinated via left axillary artery transection. The small intestine from each mouse was removed and the lumen rinsed with 20 mL HBSS (Bio Whittaker, Walkersville, MD). The luminal rinse was centrifuged at 2,000 x g for 10 min, and supernatant was aliquoted and frozen at −80°C for MUC2 analysis. Ileal tissue samples were obtained from a 3 cm segment of ileum that excluded Peyer’s patches. Samples for cytokine determination were flash-frozen in liquid N2 with 1% protease inhibitor cocktail (p8340, Sigma-Aldrich, St. Louis, MO) and stored at −80°C until subsequent analysis, while samples for GC analysis were fixed in 4% paraformaldehyde overnight, transferred to 70% ethanol, and stored at 4°C until subsequent histology.
Analysis of ileal cytokines
The flash-frozen small intestine segment from each animal was homogenized in RIPA lysis buffer (Upstate, Lake Placid, NY) containing 1% protease inhibitor cocktail (Sigma-Aldrich). The homogenate was kept on ice for 30 min prior to centrifugation at 16,000 x g for 10 min at 4°C. The supernatant was then stored at −20°C until analysis. Prior to storage, the protein concentration of the supernatant was determined by the Bradford method using BSA as a standard.
Concentrations of IL-4, IL-13, IL-1β, IL-6, and TNF-α were determined in the supernatant using solid phase sandwich ELISA kits (BD Biosciences, San Diego, CA), according to manufacturer’s instructions and identical to our previous work 5, 34. The absorbance at 450 nm was determined using a Vmax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA). The respective cytokine concentrations in the samples were determined by using a 4-parameter logistic fit standard curve (SOFTmax PRO software; Molecular Devices; Sunnyvale, CA) and normalized to total tissue protein content.
Analysis of luminal MUC2
Our method of MUC2 analysis was similar to previous work 35–38. Proteins in the intestinal wash fluid (4μL) from each animal were separated by 10% agarose gel by electrophoresis at 150V for 80 min at room temperature. The resolved proteins were transferred to polyvinylidene fluoride membrane using tris-glycine buffer containing 20% methanol at 80V for 60 min at 4°C. The membrane was blocked with 5% nonfat dry milk prepared in Tris buffered saline containing Tween (0.05%) for 1 h at room temperature with constant agitation. Then, the membrane was incubated with mouse anti-human MUC2 (ab-11197, Abcam Inc, Cambridge, MA) primary antibody (diluted 1:2500) overnight at 4°C with constant agitation. The membrane was washed and incubated with stabilized goat anti-mouse IgG-HRP conjugate (sc-2005, Santa Cruz Biotechnology, CA) secondary antibody (diluted 1:20,000) for 1 h at room temperature with constant agitation. After washing, the membrane was incubated with HRP substrate (Super Signal West Femto substrate; Pierce, Rockford, IL) for 5 min and the protein of interest (MUC2) was detected using photographic film. The relative intensities of both the monomeric and dimeric forms of MUC2 were determined together for each sample using NIH ImageJ software (version 1.43, http://rsbweb.nih.gov/ij/); internal controls were used to normalize the densitometry across multiple films.
Histomorphometric analysis
The fixed ileal tissue sections were processed (Tissue-Tek V.I.P, Sakura Finetek, Torrance, CA), and embedded in paraffin. The embedded tissue was cut (5 μm thick), deparaffinized, rehydrated through graded ethanol washes (100% ethanol x 2, 95% ethanol x 2, 70% ethanol x 1, 2 min each) and placed into distilled H20. Samples were stained with periodic acid-schiff (PAS) and counterstained with hematoxylin. GC number was determined by determining the average number of GC present in 15 individual villi per animal. GC size (μm2) was obtained by imaging tissue sections and analyzing individual GC area with NIH ImageJ software (version 1.43, http://rsbweb.nih.gov/ij/). Villi length and crypt depth measurements were determined in 15 villi and crypts. The histomorphometric measurements were performed by two independent, blinded researchers.
Statistical analysis
A fixed effects ANOVA model was fit for each measured parameter using the PROC MIXED function of the statistical software (SAS Software (Version 8), SAS Institute Inc, Cary, NC) to test for significant effects of diet. The correlations between observations between diet groups were modeled using a diagonal covariance structure. For each measured parameter, the model was fit using the untransformed data, and the residuals were evaluated to ensure that standard ANOVA assumptions of constant variance and normality were reasonably met. Transformations of the data were performed if required to improve adherence to these assumptions. Type III tests were then performed to evaluate the significance of the effects of interest for each measured parameter, and least-square means were calculated for the diet groups. Primary effects of interest were differences between the: (1) Standard Chow and EEN groups, (2) EEN and EEN+PAC groups (at each dose), and (3) Standard Chow and EEN+PAC groups (at each dose). The Standard Chow group was included in analysis as a positive control as done in all of our previous work. The data are reported as least-square mean ± standard error of mean (SEM). Statistical significance was accepted at p < 0.05.
RESULTS
PAC characterization by HPLC and MALDI-TOF MS
The cranberry presscake PAC eluted as two unresolved peaks that had absorbance at 280 nm and minor absorbance at 520 nm due to the presence of covalently linked anthocyanin-proanthocyanidin pigments. No peaks were observed with an absorbance max typical of the other classes of cranberry polyphenolic compounds (anthocyanins, hydroxycinnamic acids, and flavonols). The poorly resolved chromatogram at 280 nm is due to structural heterogeneity of cranberry presscake PAC 11.
Reflectron mode MALDI-TOF MS showed masses that correspond to PAC with at least 1A-type interflavan bond in trimers to undecamers. MALDI-TOF MS linear mode spectra had m/z peaks that correspond to cranberry presscake PAC with a range of 3 to 23 degrees of polymerization. The spectra also contained m/z peaks that correspond to covalently linked anthocyanin-proanthocyanidin molecules, ranging from monomers to heptamers (data not shown).
Body Weight Changes
Pre-experiment body weights did not significantly differ between treatment groups. Post-experiment body weights were significantly (p < 0.05) lower in all EEN fed groups compared with standard Chow [Table 2]. The decrease in body weight observed in EEN groups is partly due to absence of bowel fecal content, which we have measured previously at 1–1.5 grams. Post-experiment body weight between EEN fed groups did not differ.
Table 2.
Effects of Feeding Chow, EEN, EEN+lowPAC, EEN+midPAC, and EEN+highPAC Diets on Intestinal Tissue Cytokines, IL-4, IL-13, IL-1β, IL-6, and TNF-α1.
| Chow | EEN | EEN + lowPAC | EEN + midPAC | EEN + highPAC | |
|---|---|---|---|---|---|
| IL-4 (pg/mg Protein) | 6.01 ± 0.56 | 4.48 ± 0.52 | 4.98 ± 0.52 | 5.81 ± 0.52 | 6.99 0.52 ‡ |
| IL-13(pg/mg Protein) | 11.37 ± 1.63 | 7.54 ± 1.42 * | 10.94 ± 1.42 ‡ | 11.83 ± 1.42 ‡ | 13.94 ± 1.79 ‡ |
| IL-6 (pg/mg Protein) | 7.55 ± 0.78 N/S | 6.94 ± 0.75 | 7.19 ± 0.75 | 7.40 ± 0.75 | 7.04 ± 0.73 |
| TNF-α (pg/mg Protein) | 14.74 ± 2.67 N/S | 18.29 ± 2.38 | 17.63 ± 2.46 | 17.69 ± 2.38 | 12.83 ± 2.38 |
| IL-1β (pg/mg Protein) | 167.7 ± 18.06 N/S | 153.7 ± 16.8 | 129.3 ± 18.8 | 166.8 ± 16.8 | 114.7 ± 16.8 |
Values are mean ± SEM, n = 6–14.
denotes P < 0.05 vs Chow.
denotes P < 0.05 vs. EEN.
N/S, non-significant effect across groups.
EEN Elemental Enteral Diet.; EEN+lowPAC, EEN with 8 mg/kg BW PAC; EEN+midPAC, EEN with 50 mg/kg BW PAC; EEN+highPAC, EEN with 100 mg/kg BW.
Analysis of ileal cytokines
IL-4 level in the ileal tissue of the EEN group was lower than in the Standard Chow group, almost reaching statistical significance (P = 0.051) [Table 3]. IL-4 levels in the EEN+highPAC group was significantly higher than in the EEN group (P < 0.005), while levels in EEN+lowPAC nor EEN+midPAC groups significantly differed from the EEN group. Additionally, Tissue IL-4 was significantly greater in EEN+highPAC than EEN+lowPAC (P < 0.005).
Table 3.
Effects of Feeding Chow, EEN, EEN+lowPAC, EEN+midPAC, and EEN+highPAC on Intestinal Histomorphometry1.
| Chow | EEN | EEN + lowPAC | EEN + midPAC | EEN + highPAC | |
|---|---|---|---|---|---|
| Villi Length, μm | 171.5 ± 5.49 N/S | 155.5 ± 5.14 | 152.5 ± 5.50 | 157.0 ± 5.50 | 155.4 ± 5.50 |
| Crypt Depth, μm | 79.99 ± 2.73 | 64.83 ± 2.55 * | 66.14 ± 2.73 * | 64.17 ± 2.73 * | 66.17 ± 2.73 * |
| GC/Villi, N | 9.72 ± 0.44 | 7.98 ± 0.36 | 9.15 ± 0.44 ‡ | 9.66 ± 0.48 ‡ | 10.37 ± 0.39 ‡,† |
| GC/Villi Length, N/μm | 0.0598 ± 0.0036 | 0.0522 ± 0.0033 | 0.0598 ± 0.0036 | 0.0619 ± 0.0036 ‡ | 0.0657 ± 0.0036 ‡ |
| GC area, μm2 | 54.49 | 48.56 | 62.57 ‡ | 64.50 ‡ | 61.50 ‡ |
Values are mean ± SEM, n = 8–14.
denotes P < 0.05 vs Chow.
denotes P < 0.05 vs. EEN.
denotes P < 0.05 vs EEN+lowPAC.
N/S, non-significant effect across groups.
EEN Elemental Enteral Diet.; EEN+lowPAC, EEN with 8 mg/kg BW PAC; EEN+midPAC, EEN with 50 mg/kg BW PAC; EEN+highPAC, EEN with 100 mg/kg BW.
EEN significantly reduced IL-13 in the ileal tissue compared to Standard Chow (P < 0.05). IL-13 levels in the EEN+lowPAC (P< 0.05), EEN+midPAC (P < 0.05), and EEN+highPAC (P < 0.005) were significantly higher than in the EEN group alone.
Compared with Standard Chow, EEN did not significantly affect the Th1 cytokines, IL-1β, IL-6, or TNF-α; the addition of PACs at any dose had no effect on these cytokines.
Analysis of GC density and size
While the length of villi were decreased in all EEN fed groups compared with Standard Chow, these changes were not significant. However, there was a significant reduction in crypt depth with all EEN diets (P< 0.05) compared with Standard Chow. The addition of PAC to EEN had no significant effect upon villi length or crypt depth compared with the EEN alone [Table 4].
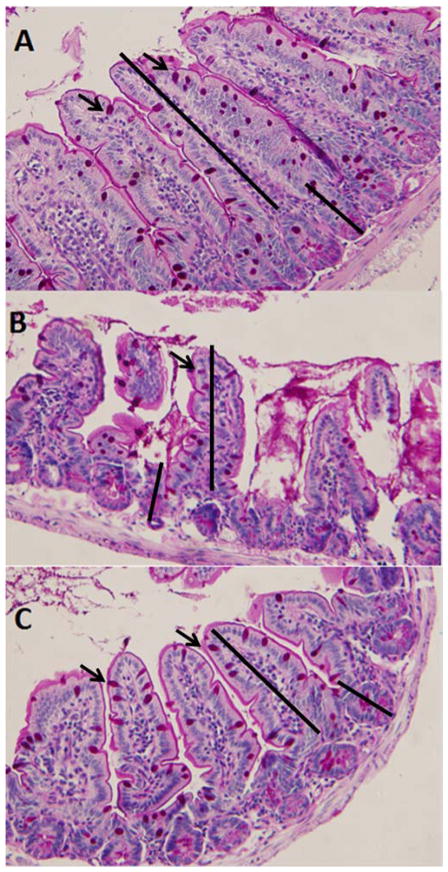
EEN significantly reduced the number of GCs per villi compared with Standard Chow (P < 0.005). EEN+lowPAC (P < 0.05), EEN+midPAC (P < 0.01), and EEN+highPAC (P < 0.0001) significantly increased the number of GCs per villi compared with EEN alone. The number of GCs per villi in the EEN+highPAC was significantly greater than the EEN+lowPAC group (P < 0.05). When adjusted for villi length (GCs/μL villi length) in EEN, there were no significant differences between EEN and Standard Chow in the number of GCs (P = 0.12). However, there were more GCs/villi length in the EEN+midPAC (P = 0.05) and EEN+highPAC (P<0.01) compared with EEN alone. A representative histomorphometric image is shown for Standard Chow, EEN, and EEN+highPAC [Figure 1].
Figure 1. Representative Image of Periodic Acid Shiff-Base (PAS) Stained Ileum Tissue from (A) Chow, (B) EEN, and (C) EEN+highPAC.
Goblet cells are stained pink (denoted by arrows). Measurements of villi length and crypt depth were made as indicated. 20x Zoom.
Although the GC size (μm2) in the EEN group was smaller than in the Standard Chow group, this difference was not significant (P = 0.29) [Table 4]. The GC sizes in the EEN+lowPAC (P < 0.05), EEN+midPAC (P < 0.01), and EEN+highPAC (P < 0.05) groups were significantly greater than EEN alone.
Analysis of luminal MUC2
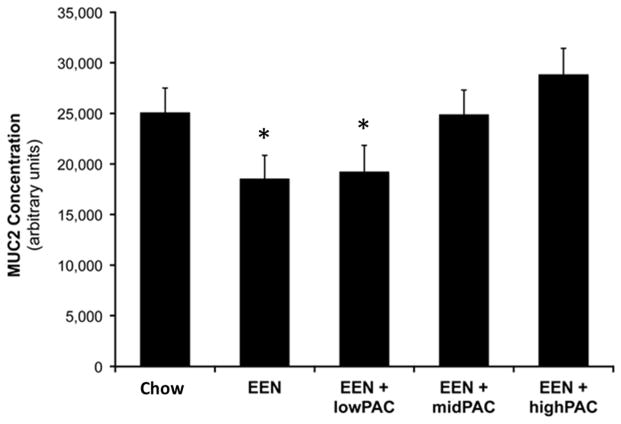
The monomer and dimer observed, at molecular weight markers 250 and 500 kDa respectively, were consistent with other reports of the highly oligomeric structure of intestinal MUC2 35–38. The relative luminal MUC2 [Figure 2] in the EEN and EEN+lowPAC groups was lower than the Standard Chow group, although these differences failed to reach significance (P = 0.057). However, the relative luminal MUC2 in the EEN+highPAC (P <0.005) group was higher than EEN alone, but the EEN+midPAC (P = 0.06) group failed to reach significance. Additionally, the level of MUC2 in the EEN+highPAC was significantly greater than EEN+lowPAC (P < 0.05).
Figure 2.
Effects of Feeding Chow, EEN, EEN+lowPAC, EEN+midPAC, and EEN+highPAC Diets on Intestinal Lumen MUC2 Displayed in Arbitrary Units.
* denotes P < 0.05 vs EEN+highPAC.
DISCUSSION
This study demonstrates that the addition of cranberry PAC to EEN solution improves ileal tissue IL-4 and IL-13 levels, GC number and size, and the secretion of intestinal MUC2, which likely contribute to the impairment of the mucosal barrier integrity previously observed by EEN alone 2, 39. The gastrointestinal mucosa maintains a physical and chemical barrier against 100 trillion resident bacteria as well as food and environmental antigens 6. A number of interrelated factors influence this function, including mucus glycoproteins, antimicrobial molecules, specific and non-specific antibodies, enterocyte tight-junctions, and colonization of a commensal microbiota 40, 41. Dietary intake of the host affects the complex interplay between these factors 42, 43. The route and complexity of nutrition profoundly influences the mucosal immune system, specifically the mucosal associated lymphoid tissue 4, 5, 44. A reduction in dietary intake or complexity, such as those that occur with parenteral nutrition or administration of EEN, decreases the number of lymphocytes in Peyer’s patches and lamina propria, reduces levels of IgA-stimulating Th-2 type cytokines in the gut wall, and reduces levels of intestinal immunoglobulins (primarily IgA) compared to the feeding of a Standard Chow diet or administration of a complex enteral diet containing complex carbohydrates, proteins and fats 4, 34, 44, 45. EEN also increases barrier permeability and significantly suppresses bacterial diversity within the gut 2, 39. While the influence of dietary intake or complexity on mucosal barrier and immunity is appreciated 41, very little is known of the influence of “non-nutritive” dietary compounds such as PAC.
PAC are complex oligomeric polyphenolic compounds widely distributed in fruits, including grapes, cranberries, and apples, and other foods and beverages such as chocolate and wine 9–11. Epidemiological studies suggest PAC may have a wide range of beneficial health effects 12–14. However, PAC are minimally absorbed across the enterocyte layer due to non- hydrolyzable bonds between flavan-3-ol monomeric units and their ability to complex both dietary and endogenous proteins 15. Further, PAC oligomers range in DP from 3 to 30, or more, and therefore have higher molecular weight than other common plant polyphenols. Consequentially, greater than 95% of PAC remain in the intestinal lumen during transit through the gastrointestinal tract 46–48.
Since PAC are poorly absorbed, a number of mechanisms have been investigated to explain their potential beneficial effects. PAC have been shown to exert antioxidant and non-specific antimicrobial functions within the gut 20. Recent animal studies also demonstrate the addition of dietary PAC palliates chemically-induced colitis, although the mechanism of this remains unclear 49–51. Another important effect of PAC is their propensity to complex salivary glycoproteins when ingested, a process that causes the astringency of many fruits and beverages 10. Astringency occurs when PAC crosslink and precipitate salivary glycoproteins and PAC with higher DP have greater effects on crosslinking and precipitation 52. Several biological effects occur in response to astringency including increased salivary excretion, hypertrophy of the parotid gland, and shift in salivary composition to proline rich proteins 16. Within the intestine, in vitro studies demonstrate that intra-epithelial γδ T lymphocytes, in response to PAC, activate and proliferate 53. Interestingly, the level of γδ T cell response also increases with greater DP of PAC. These observations not only suggest that PAC may play an influential role in context of mucosal barrier physiology and immunity, but that DP of PAC may be of importance when investigating their effects. Accordingly, we previously characterized the PAC used in this experiment 31. This analysis allows for the characterization and reliable reproduction of chromatographic fractions for inclusion in experimental treatments.
In this study, we investigated the effects of addition of cranberry PAC to EEN solution on ileal tissue cytokine levels, morphology including GC number and size, and the secretion of the primary glycoprotein MUC2, and explored the effect of physiological doses of PAC on these parameters. We used a chemically defined EEN solution administered via a gastrostomy tube as a model of an elemental enteral diet that we have previously utilized. The EEN administration results in reproducible effects on intestinal (and respiratory) mucosal immunity allowing examination of changes induced with PAC.
Compared to Chow, EEN produces significantly fewer total GCs per villi. However, when normalizing the GC numbers over villi length (GC number/μm), there were no differences between EEN and the Standard Chow diet. There were also no significant differences in GC size and villi length between Standard Chow and EEN, although EEN reduced the average measurement of both parameters. Interestingly, the number of GCs per villi length was significantly preserved in the EEN+midPAC and EEN+highPAC groups compared to EEN alone. GCs normally undergo hypertrophy and hyperplasia in response to IL-4 and IL-13, which act through the IL-4 receptor α and IL-13 receptor α1, respectively 23, 54, 55. Our data shows that EEN lowered ileal IL-4 and IL-13 levels compared with Standard Chow. The addition of PAC to the EEN diet maintained IL-4 and IL-13, but did not significantly affect the cytokines IL-1β, TNF-α, or IL-6 [Table 3]. Since GC differentiate, migrate up the villi, and slough off every 3–5 days, these findings suggests the addition of PAC to the EEN diet alters the rate of cellular differentiation of progenitor crypt stem cells to GC likely via changes in Th-2 type cytokines observed 4. The data also suggest PAC induce the observed effect through Th2 mediated immunity consistent with a previous study showing a similar IL-4 effect in colonic tissue following ingestion of proanthocyanidins 50. Additionally, while the effects on the Th1 cytokine TNF-α were not significant across treatment groups, the trend of reduced tissue TNF-α level with increasing doses of PAC was consistent with previous work 56.
Simultaneously, EEN suppressed the concentration of MUC2 within the lumen, although this change did not reach significance. Functionally MUC2 forms the viscous mucin layer which overlays the intestinal surface, allowing smooth passage of digesta. From an immunological stand point, secreted antimicrobial proteins and peptides from Paneth cells as well as secretory IgA (sIgA) localize and are concentrated in this layer 57. These mucin glycoproteins also provide endogenous flora with a consistent nutrient source. The observed decrease in luminal MUC2 may increase susceptibility to bacterial opportunistic pathogens or intestinal inflammation, since others have shown that MUC2−/− mice are at increased risk for spontaneous colitis 21. The addition of PAC at the EEN+midPAC and EEN+highPAC doses maintained MUC2 to levels observed in the Standard Chow group.
Cranberry PAC administration at physiologic doses 26, 27 counteracts many of the changes associated with EEN administration. One limitation of the current study is that we do not address the source of the Th2 cytokines, although studies investigating changes to tissue lymphocytes and whether a mechanism similar to astringency is responsible for these observations effects are planned. Overall, this study supports the hypothesis that reduced enteral stimulation results in the impairment of mucosal integrity and gut barrier function through the reduction in the mucin component. The current work demonstrates that the administration of EEN produces lower levels of the Th2 stimulating cytokine IL-13, lower GC number and size, and lower luminal MUC2 levels in the ileum. The addition of cranberry PAC to this diet, at physiologic doses, attenuates these changes and likely normalizes mucosal integrity. This suggests that a non-nutritional dietary component such as PAC may influence health without being absorbed from the gastrointestinal tract.
Supplemental Table 1.
Formulation of EEN Solution
| Component | Amount (per 1 L) |
|---|---|
| Dextrose | 356.0 g |
| Amino acids (Clinisol) | 60.0 g |
| Sodium chloride | 32.0 mEq |
| Sodium phosphate | 36 mmol |
| Potassium chloride | 16 mEq |
| Calcium gluconate | 37.5 mEq |
| Potassium acetate | 44.0 mEq |
| Magnesium sulfate | 8.0 mEq |
| Manganese | 0.8 mg |
| Copper | 0.5 μg |
| Zinc | 2.0 mg |
| Vitamin C | 200 mg |
| Vitamin A | 3300 IU |
| Vitamin D3 | 200 IU |
| Thiamine | 6 mg |
| Riboflavan | 3.6 mg |
| Pyridoxine HCl | 6 mg |
| Niacinamide | 40 mg |
| Folic Acid | 600 mcg |
| Biotin | 60 mcg |
| Cyanocobalamin | 5 mcg |
| Vitamin E (dl-α-tocopheryl Acetate) | 10 IU |
| Vitamin K1 | 150 mcg |
| Dexpanthenol | 15 mg |
CLINICAL RELEVANCY.
Multiple components of the intestinal mucosal barrier including secreted mucus and antimicrobial compounds maintain the host-bacterial relationship within the gut lumen. Elemental enteral nutrition adversely affects mucus production and secretion impairing the most basic level of gut immunity – barrier function. The addition of a complex, unabsorbed phytochemical, proanthocyanidins, to elemental nutrition improves this aspect of mucosal defense.
Acknowledgments
The research was supported by National Institute of Health (NIH) Grant (R01 GM53439), and by the Reed Research Group. The project described was supported by Award Number I01BX001672 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development.
Rodrigo P Feliciano is recipient of BD fellowship (SFRH/BD/73067/2010) from Fundação para a Ciência e a Tecnologia, Portugal. We would like to thank Jennifer J. Meudt and Michael P. Shea for their technical assistance, and Dr. Martha Vestling for her assistance with mass spectrometry.
ABBREVIATIONS
- DP
degree of polymerization
- EEN
elemental enteral nutrition
- GAE
gallic acid equivalents
- GC
goblet cells
- GALT
gut-associated lymphoid tissue
- ICR
Institute of Cancer Research
- MALDI-TOF MS
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MUC2
mucin2
- PAC
proanthocyanidin
- PAS
periodic acid-schiff
- sIgA
secretory IgA
- Th-2
T-helper 2 lymphocytes
Footnotes
STATEMENT OF AUTHORS’ CONTRIBUTIONS TO MANUSCRIPT
J.F.P., A.F.H, D.S., J.D.R., K.A.K., and C.G.K. designed research; J.F.P., A.F.H., R.P.F., and D.A.R. conducted research; J.F.P. analyzed data, and performed statistical analysis; J.F.P., D.S., J.D.R., and K.A.K wrote paper. J.F.P. had primary responsibility for final content; All authors have read and approved the final manuscript.
The contents of this article do not represent the views of the Veterans Affairs or the United States Government.
References
- 1.Isaacs KL, Lewis JD, Sandborn WJ, Sands BE, Targan SR. State of the art: IBD therapy and clinical trials in IBD. Inflamm Bowel Dis. 2005;11(Suppl 1):S3–12. doi: 10.1097/01.mib.0000184852.84558.b2. [DOI] [PubMed] [Google Scholar]
- 2.Mosenthal AC, Xu D, Deitch EA. Elemental and intravenous total parenteral nutrition diet-induced gut barrier failure is intestinal site specific and can be prevented by feeding nonfermentable fiber. Crit Care Med. 2002;30(2):396–402. doi: 10.1097/00003246-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Kudsk K, Gomez F, Kang W, Ueno C. Enteral feeding of a chemically defined diet preserves pulmonary immunity but not intestinal immunity: the role of lymphotoxin beta receptor. JPEN J Parenter Enteral Nutr. 2007;(6):477–481. doi: 10.1177/0148607107031006477. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Kudsk KA, DeWitt RC, Tolley EA, Li J. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999;229(5):662–668. doi: 10.1097/00000658-199905000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermsen J, Gomez F, Sano Y, Kang W, Maeshima Y, Kudsk K. Parenteral feeding depletes pulmonary lymphocyte populations. JPEN J Parenter Enteral Nutr. 2009;33(5):535–540. doi: 10.1177/0148607109332909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guamer F. Role of intestinal flora in health and disease. Nutr Hosp. 2007;22(Suppl 2):14–19. [PubMed] [Google Scholar]
- 7.Johansson ME, Ambort D, Pelaseyed T, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011;68(22):3635–3641. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scalbert A, Déprez S, Mila I, Albrecht AM, Huneau JF, Rabot S. Proanthocyanidins and human health: systemic effects and local effects in the gut. Biofactors. 2000;13(1–4):115–120. doi: 10.1002/biof.5520130119. [DOI] [PubMed] [Google Scholar]
- 9.Hellstrom JK, Torronen AR, Mattila PH. Proanthocyanidins in common food products of plant origin. J Agric Food Chem. 2009;57(17):7899–7906. doi: 10.1021/jf901434d. [DOI] [PubMed] [Google Scholar]
- 10.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 11.Reed JD, Krueger CG, Vestling MM. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry. 2005;66(18):2248–2263. doi: 10.1016/j.phytochem.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81(1 Suppl):317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 13.Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol. 2005;16(1):77–84. doi: 10.1097/00041433-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45(4):287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 15.Hagerman AE, Butler LG. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981;256(9):4494–4497. [PubMed] [Google Scholar]
- 16.Mehansho H, Ann DK, Butler LG, Rogler J, Carlson DM. Induction of proline-rich proteins in hamster salivary glands by isoproterenol treatment and an unusual growth inhibition by tannins. J Biol Chem. 1987;262(25):12344–12350. [PubMed] [Google Scholar]
- 17.Mehansho H, Carlson DM. Induction of protein and glycoprotein synthesis in rat submandibular glands by isoproterenol. J Biol Chem. 1983;258(10):6616–6620. [PubMed] [Google Scholar]
- 18.Gonthier MP, Donovan JL, Texier O, Felgines C, Remesy C, Scalbert A. Metabolism of dietary procyanidins in rats. Free Radic Biol Med. 2003;35(8):837–844. doi: 10.1016/s0891-5849(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 19.Reed JD. Nutritional toxicology of tannins and related polyphenols in forage legumes. J Anim Sci. 1995;73(5):1516–1528. doi: 10.2527/1995.7351516x. [DOI] [PubMed] [Google Scholar]
- 20.Cos P, De Bruyne T, Hermans N, Apers S, Berghe DV, Vlietinck AJ. Proanthocyanidins in health care: current and new trends. Curr Med Chem. 2004;11(10):1345–1359. doi: 10.2174/0929867043365288. [DOI] [PubMed] [Google Scholar]
- 21.Bergstrom KS, Kissoon-Singh V, Gibson DL, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6(5):e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusuf S, Nok AJ, Ameh DA, Adelaiye AB, Balogun EO. Quantitative changes in gastric mucosal glycoproteins: effect of cholinergic agonist and vagal nerve stimulation in the rat. Neurogastroenterol Motil. 2004;16(5):613–619. doi: 10.1111/j.1365-2982.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- 23.Finkelman FD, Shea-Donohue T, Goldhill J, et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 24.Fallon PG, Jolin HE, Smith P, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17(1):7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8(6):339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 26.Knaze V, Zamora-Ros R, Luján-Barroso L, et al. Intake estimation of total and individual flavan-3-ols, proanthocyanidins and theaflavins, their food sources and determinants in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2011:1–14. doi: 10.1017/S0007114511006386. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Chung SJ, Song WO, Chun OK. Estimation of daily proanthocyanidin intake and major food sources in the U.S. diet. J Nutr. 2011;141(3):447–452. doi: 10.3945/jn.110.133900. [DOI] [PubMed] [Google Scholar]
- 28.Kume H, Okazaki K, Yamaji T, Sasaki H. A newly designed enteral formula containing whey peptides and fermented milk product protects mice against concanavalin A-induced hepatitis by suppressing overproduction of inflammatory cytokines. Clin Nutr. 2012;31(2):283–289. doi: 10.1016/j.clnu.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Bastarache JA, Ware LB, Girard TD, Wheeler AP, Rice TW. Markers of Inflammation and Coagulation May Be Modulated by Enteral Feeding Strategy. JPEN J Parenter Enteral Nutr. 2012 Feb 7; doi: 10.1177/0148607111433054. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aydogan A, Kismet K, Kilicoglu B, et al. Effects of various enteral nutrition solutions on bacterial translocation and intestinal morphology during the postoperative period. Adv Ther. 2007;24(1):41–49. doi: 10.1007/BF02849991. [DOI] [PubMed] [Google Scholar]
- 31.Feliciano RP, Shea MP, Shanmuganayagam D, Krueger CG, Howell AB, Reed JD. Comparison of Isolated Cranberry (Vaccinium macrocarpon Ait.) Proanthocyanidins to Catechin and Procyanidins A2 and B2 for Use as Standards in the 4-(Dimethylamino)cinnamaldehyde Assay. J Agric Food Chem. 2012;60(18):4578–4585. doi: 10.1021/jf3007213. [DOI] [PubMed] [Google Scholar]
- 32.Sitren HS, Heller PA, Bailey LB, Cerda JJ. Total parenteral nutrition in the mouse: development of a technique. JPEN J Parenter Enteral Nutr. 1983;7(6):582–586. doi: 10.1177/0148607183007006582. [DOI] [PubMed] [Google Scholar]
- 33.Nutrition SoLA, Nutrition CoA, Agriculture Bo, Council NR. Nutrient Requirements of Laboratory Animals. 4. The National Academies Press; 1995. Revised Edition, 1995. [PubMed] [Google Scholar]
- 34.Sano Y, Gomez F, Hermsen J, et al. Parenteral nutrition induces organ specific alterations in polymeric immunoglobulin receptor levels. J Surg Res. 2008;149(2):236–242. doi: 10.1016/j.jss.2007.12.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aksoy N, Thornton DJ, Corfield A, Paraskeva C, Sheehan JK. A study of the intracellular and secreted forms of the MUC2 mucin from the PC/AA intestinal cell line. Glycobiology. 1999;9(7):739–746. doi: 10.1093/glycob/9.7.739. [DOI] [PubMed] [Google Scholar]
- 36.Asker N, Axelsson MA, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998;273(30):18857–18863. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- 37.Axelsson MA, Asker N, Hansson GC. O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J Biol Chem. 1998;273(30):18864–18870. doi: 10.1074/jbc.273.30.18864. [DOI] [PubMed] [Google Scholar]
- 38.Larsson JM, Karlsson H, Crespo JG, et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm Bowel Dis. 2011;17(11):2299–2307. doi: 10.1002/ibd.21625. [DOI] [PubMed] [Google Scholar]
- 39.Kajiura T, Takeda T, Sakata S, et al. Change of intestinal microbiota with elemental diet and its impact on therapeutic effects in a murine model of chronic colitis. Dig Dis Sci. 2009;54(9):1892–1900. doi: 10.1007/s10620-008-0574-6. [DOI] [PubMed] [Google Scholar]
- 40.Ouellette AJ, Selsted ME. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10(11):1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 41.Fukatsu K, Kudsk KA. Nutrition and gut immunity. Surg Clin North Am. 2011;91(4):755–770. vii. doi: 10.1016/j.suc.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hermsen J, Sano Y, Kudsk K. Food fight! Parenteral nutrition, enteral stimulation and gut-derived mucosal immunity. Langenbecks Arch Surg. 2009;394(1):17–30. doi: 10.1007/s00423-008-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierre JF, Heneghan AF, Tsao FH, et al. Route and type of nutrition and surgical stress influence secretory phospholipase A2 secretion of the murine small intestine. JPEN J Parenter Enteral Nutr. 2011;35(6):748–756. doi: 10.1177/0148607111414025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39(1):44–51. doi: 10.1097/00005373-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 45.King B, Li J, Kudsk K. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132(12):1303–1309. doi: 10.1001/archsurg.1997.01430360049009. [DOI] [PubMed] [Google Scholar]
- 46.Deprez S, Mila I, Huneau JF, Tome D, Scalbert A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid Redox Signal. 2001;3(6):957–967. doi: 10.1089/152308601317203503. [DOI] [PubMed] [Google Scholar]
- 47.Baba S, Osakabe N, Natsume M, Terao J. Absorption and urinary excretion of procyanidin B2 [epicatechin-(4beta-8)-epicatechin] in rats. Free Radic Biol Med. 2002;33(1):142–148. doi: 10.1016/s0891-5849(02)00871-7. [DOI] [PubMed] [Google Scholar]
- 48.Donovan JL, Manach C, Rios L, Morand C, Scalbert A, Remesy C. Procyanidins are not bioavailable in rats fed a single meal containing a grapeseed extract or the procyanidin dimer B3. Br J Nutr. 2002;87(4):299–306. doi: 10.1079/bjnbjn2001517. [DOI] [PubMed] [Google Scholar]
- 49.Wang YH, Yang XL, Wang L, et al. Effects of proanthocyanidins from grape seed on treatment of recurrent ulcerative colitis in rats. Can J Physiol Pharmacol. 2010;88(9):888–898. doi: 10.1139/y10-071. [DOI] [PubMed] [Google Scholar]
- 50.Li XL, Cai YQ, Qin H, Wu YJ. Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS-induced ulcerative colitis. Can J Physiol Pharmacol. 2008;86(12):841–849. doi: 10.1139/Y08-089. [DOI] [PubMed] [Google Scholar]
- 51.Yoshioka Y, Akiyama H, Nakano M, et al. Orally administered apple procyanidins protect against experimental inflammatory bowel disease in mice. Int Immunopharmacol. 2008;8(13–14):1802–1807. doi: 10.1016/j.intimp.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Vidal S, Francis L, Guyot S, et al. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. Journal of the Science of Food and Agriculture. 2003;83(6):564–573. [Google Scholar]
- 53.Holderness J, Hedges JF, Daughenbaugh K, et al. Response of gammadelta T Cells to plant-derived tannins. Crit Rev Immunol. 2008;28(5):377–402. doi: 10.1615/critrevimmunol.v28.i5.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steenwinckel V, Louahed J, Lemaire MM, et al. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J Immunol. 2009;182(8):4737–4743. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 55.Iwashita J, Sato Y, Sugaya H, Takahashi N, Sasaki H, Abe T. mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-alpha through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol Cell Biol. 2003;81(4):275–282. doi: 10.1046/j.1440-1711.2003.t01-1-01163.x. [DOI] [PubMed] [Google Scholar]
- 56.Wang YH, Ge B, Yang XL, et al. Proanthocyanidins from grape seeds modulates the nuclear factor-kappa B signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. Int Immunopharmacol. 2011;11(10):1620–1627. doi: 10.1016/j.intimp.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 57.Meyer-Hoffert U, Hornef MW, Henriques-Normark B, et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57(6):764–771. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]