Abstract
Aim:
Aquaporins (AQPs) are the water-channels that play important roles in brain water homeostasis and in cerebral edema induced by brain injury. In this study we investigated the relationship between AQPs and a neuroprotective agent curcumin that was effective in the treatment of brain edema in mice with intracerebral hemorrhage (ICH).
Methods:
ICH was induced in mice by autologous blood infusion. The mice immediately received curcumin (75, 150, 300 mg/kg, ip). The Rotarod test scores, brain water content and brain expression of AQPs were measured post ICH. Cultured primary mouse astrocytes were used for in vitro experiments. The expression of AQP1, AQP4 and AQP9 and NF-κB p65 were detected using Western blotting or immunochemistry staining.
Results:
Curcumin administration dose-dependently reduced the cerebral edema at d 3 post ICH, and significantly attenuated the neurological deficits at d 5 post ICH. Furthermore, curcumin dose-dependently decreased the gene and protein expression of AQP4 and AQP9, but not AQP1 post ICH. Treatment of the cultured astrocytes with Fe2+ (10–100 μmol/L) dose-dependently increased the expression and nuclear translocation of NF-κB p65 and the expression of AQP4 and AQP9, which were partly blocked by co-treatment with curcumin (20 μmol/L) or the NF-κB inhibitor PDTC (10 μmol/L).
Conclusion:
Curcumin effectively attenuates brain edema in mice with ICH through inhibition of the NF-κB pathway and subsequently the expression of AQP4 and AQP9. Curcumin may serve as a potential therapeutic agent for ICH.
Keywords: curcumin, intracerebral hemorrhage, brain edema, NF-κB p65, AQP4, AQP9, astrocytes, Fe2+, PDTC
Introduction
Intracerebral hemorrhage (ICH) causes approximately 10%–20% of all strokes worldwide1 and is associated with a high rate of fatality and morbidity2,3. Spontaneous ICH accounts for 8%–20% of all strokes in high-income countries and has an annual incidence of 24–48 per 100 0004. Less than 50% of patients with ICH survive more than 1 year3. In addition to hemorrhagic volume, the development of brain edema is a major complication associated with ICH. Increasing intracranial pressure (ICP) reduces cerebral blood flow and cerebral perfusion pressure and contributes to a poor prognosis5,6,7. Current therapies for ICH, including medicinal and neurosurgical treatment, do not adequately control brain edema. There is therefore a need for new medicinal therapeutics and methods to be developed to limit post-ICH edema.
Curcumin, or turmeric, which is derived from the root of the plant Curcuma longa Linn, has been used as a treatment for inflammatory conditions in Ayurvedic medicine for centuries8. It has been reported that curcumin attenuates neurobiological deficits in animal models of different neurological disorders, including Parkinson's disease9, brain trauma10, ischemic stroke11, and subarachnoid hemorrhage (SAH)12. The protective and therapeutic effects of curcumin are associated with its anti-inflammatory, anti-oxidative, and anti-apoptotic properties13. Previous studies have demonstrated that curcumin attenuates brain edema and improves neurological outcomes in ICH mice14,15. However, the neuroprotective mechanisms of curcumin in ICH remain poorly understood.
Aquaporins (AQPs), a family of water-channel proteins, play an important role in water movement and homeostasis16. AQP1, AQP4, and AQP9 have been extensively studied in the rodent brain, and AQP4 is the most well-studied water channel17. AQP1 is primarily detected in epithelial cells of the choroid plexus and has been shown to play an important role in cerebrospinal fluid formation and brain water homeostasis18,19. AQP4 is the most abundant water channel in the nervous system and is mainly located on astrocytic endfeet at contacts with cerebral blood vessels. AQP9 is not only a water channel; it also facilitates the transfer of several solutes, including glycerol, urea, and monocarboxylate, suggesting that it plays an additional role in energy metabolism17. Many studies have shown that AQP1, AQP4 and AQP9 are associated with cerebral edema induced by several types of brain injury, including ICH, subarachnoid hemorrhage, ischemic stroke and brain trauma10,20,21,22,23,24,25,26. However, the relationship between curcumin and AQPs has not been studied in ICH. We therefore asked whether curcumin attenuates brain edema by down-regulating AQPs after ICH.
Materials and methods
ICH mouse model
All animal experimental procedures were approved by the ethical committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. C57BL/6 male mice weighing 25–30 g were randomly divided into sham (n=27), ICH (n=27), and curcumin-treated group (n=51), and maintained in a constant environment (temperature: 22±1 °C; humidity: 75%–80%) with a 12 h/12 h light/dark cycle. ICH was induced by autologous blood infusion, as has been described previously27. Mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Mice were then placed in a stereotactic frame (RWD Life Science Co, Shenzhen, China). A 1-mm burr hole was drilled 2.3 mm lateral to the midline and 0.2 mm anterior to bregma. A volume of 30 μL of autologous blood was collected from a tail cut by Hamilton syringe. A needle was then inserted 3.5 mm into the right striatum through the burr hole. A volume of 5 μL of blood was injected into the target point at a rate of 2 μL/min with a microinfusion pump (WPI, Sarasota, FL). The injection was then paused for 7 min before the remaining portion was delivered at the same rate. When the injection was completed, the needle was left in place for 10 min to prevent possible backflow. After withdrawal of the needle, the skin was sutured. Body temperature was maintained at 37 °C throughout all of these procedures.
Drugs and chemicals
Curcumin (Purity≥98%; LKT Laboratories Inc, St Paul, MN, USA) was dissolved in DMSO and injected intraperitoneally at a dose of 75, 150, or 300 mg/kg immediately after ICH was induced. Vehicle mice received intraperitoneal injections of the same volumes of DMSO. For cultured astrocyte experiments, curcumin was dissolved in DMSO and diluted with culture medium to 20 μmol/L.
Ferrous chloride (FeCl2) (Sinopharm Chemical Reagent Co, Ltd, China) was directly diluted with culture medium to the indicated concentration. Pyrrolidine dithiocarbamate (PDTC), a NF-κB inhibitor, was purchased from Beyotime (China) and used at a concentration of 10 μmol/L. All other chemicals used in this study were analytical grade.
Measurement of brain water content
Mice were sacrificed under deep pentobarbital anesthesia on day 3 (d 3) after ICH. Brain tissue was immediately weighed (wet weight) and dehydrated at 100 °C for 24 h. The samples were then reweighed to obtain a dry weight. Tissue water content was calculated with the following formula: [(wet weight-dry weight)/wet weight]×100.
Behavioral testing
The Rotarod test was used to evaluate motor coordination. Before induction of ICH, mice were placed on a rotating horizontal rod and trained first at a slow speed. After the mice were habituated, the speed was accelerated to 40 rounds/min. All mice were trained twice per day. After 3 d of training, animals that could stay on the rod for 5 min were selected for the surgical procedure. The remaining mice were not included in the study. After ICH, selected mice were tested 3 times per day on d 1, d 2, d 3, d 5, d 7, and d 14, and the average latency to falling was documented.
Astroglial cell culture
Primary cultures of astrocytes were prepared from d 1–3 newborn C57BL/6 mouse brains. After careful removal of the meninges under a dissecting microscope, cerebral tissues were homogenized mechanically and digested in 0.25% trypsin for 10 min. Isolated cells were suspended in Dulbecco's modified Eagle's medium (DMEM) in 10% (v/v) fetal bovine serum (FBS) and plated onto 10-cm dishes at a density of 10×106 cells. Cells were cultured at 37 °C in an incubator with a 5% CO2 air-humidified atmosphere. The culture medium was renewed after 24 h, and then every 2 d thereafter.
Relative quantitative real-time polymerase chain reaction (PCR) analysis
Total RNA was isolated 24 h after ICH from brain blocks, including hematoma and perihematomal tissues, using Trizol reagent (Invitrogen, USA). Real-time PCR was performed using a One Step SYBR® PrimeScript™ PLUS RT-PCR Kit (Takara Bio Inc, China). The primers used to amplify the target genes were as follows: AQP1: 5′-TGCGTTCTGGCCACCACTGAC-3′ (forward), 5′-GATGTCGTCAGCATCCAGGTC-3′ (reverse); AQP4: 5′-CTGGAGCCAGCATGAATCCAG-3′ (forward), 5′-TTCTTCTCTTCTCCACGGTCA-3′ (reverse); AQP9: 5′-CCTTCTGAGAAGGACCGAGCC-3′ (forward), 5′-CTTGAACCACTCCATCCTTCC-3′ (reverse); GADPH: 5′-AGGTCGGTGTGAACGGATTTG-3′ (forward), 5′-TGTAGACCATGTAGTTGAGGTCA-3′ (reverse). All primers were constructed by Invitrogen Corp (Shanghai). Relative levels of mRNA expression were calculated using SDS software (Applied Biosystems, HT7900, USA).
Immunohistological staining
After being deeply anesthetized, mice were perfused with saline followed by fixation with 4% paraformaldehyde (PFA) in 0.1 mol/L phosphate buffer (PBS). Brains were dehydrated in 20%–30% sucrose after an overnight post-fixation in 4% PFA. Brain sections (20 μm) were washed, blocked in 10% bovine serum albumin (BSA) for 30 min, and incubated overnight at 4 °C with primary antibodies: rabbit anti-AQP4 (1:200, sc-20812, Santa Cruz Biotechnology, USA), rabbit anti-AQP9 (1:100, sc-28623, Santa Cruz Biotechnology, USA), and mouse anti-glial fibrillary acidic protein (GFAP) (1:200, MAB360, Millipore, USA). Sections were then washed and incubated with secondary antibodies: Alexa Fluor 488 donkey anti-rabbit IgG (1:500, A21206, Invitrogen, USA) and Alexa Fluor 594 donkey anti-mouse IgG (1:500, A21203, Invitrogen, USA) for 1 h. Microvessels were detected by Texas Red-labeled Lycopersicon Esculentum Lectin (1:200, TL-1176, Vector Laboratories, USA) incubated with primary antibody. A Leica confocal laser-scanning microscope (Leica, Wetzlar, Hesse, Germany) was used to acquire confocal microscopic images.
Immunocytochemistry
Astrocytes were plated onto glass slides. Following treatment with Fe2+ or curcumin, cells were fixed in 4% PFA for 15 min and washed with 0.1 mol/L PBS. After treatment with 0.3% Triton, cells were blocked in 10% BSA for 30 min, and then incubated with primary antibodies overnight at 4 °C. The concentrations of antibodies and other steps for immunocytochemistry were the same as those used for immunohistological staining.
Western blotting
Astrocytes and brain samples (perihematomal tissues at 72 h) were collected in lysis buffer. A nuclear and cytoplasmic protein extraction kit (P0027, Beyotime, China) was used to separate nuclear and cytoplasmic proteins. Protein concentrations were quantified using a bicinchoninic acid protein assay kit (Pierce Biotechnology, USA). Then, 50 μg of protein was loaded onto a 10% polyacrylamide gel for electrophoresis, and then transferred onto a polyvinylidenedifluoride membrane. Blots were blocked with 5% non-fat milk. After being washed, blots were incubated overnight at 4 °C in primary antibody: AQP4 (1:500, sc-20812, Santa Cruz Biotechnology, USA), AQP9 (1:500, sc-28623, Santa Cruz Biotechnology, USA), NF-κB p65 (1:500, sc-372, Santa Cruz Biotechnology, USA), β-actin (1:1000, 8457, Cell Signaling Technology, USA), β-tubulin (1:1000, 2128, Cell Signaling Technology, USA), or Histone H3 (1:1000, 4499, Cell Signaling Technology, USA), followed by 1-h incubation with secondary antibody: Goat anti-rabbit HRP (1:2000, HA-1001-100, HangZhouHuaAn Biotechnology Co, China). Blots were then reacted with SuperSignal West Pico Substrate (ThermoFisher Scientific, USA). Chemiluminescence was detected using an imaging system and quantified using Quantity One software (Bio-Rad, USA).
Statistical analysis
All results were expressed as the mean±SD and analyzed with GraphPad Prism (version 5.01, GraphPad Software, Inc) using one-way analysis of variance followed by a Student-Newman-Keuls test, or a two-way analysis of variance followed by Bonferroni post hoc test. A P-value of <0.05 was considered statistically significant.
Results
Curcumin reduced cerebral edema and attenuated neurological deficits
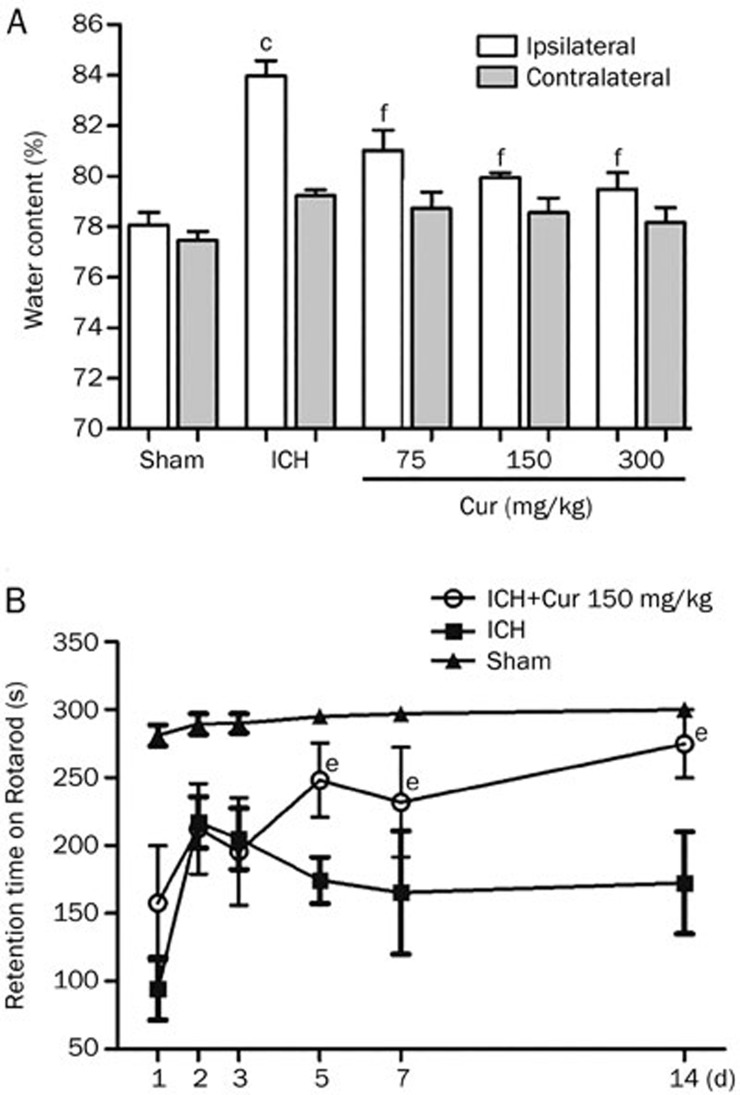
Brain water content was significantly increased on d 3 after ICH in the ipsilateral hemisphere (83.9%±0.6% after ICH vs 78.1%±0.5% in sham, P<0.001). The increase in brain edema following ICH was attenuated in a dose-dependent manner by a single injection of curcumin (75, 150, and 300 mg/kg) administered immediately after ICH induction (81.0%±0.8%, 79.9%±0.2%, 79.5%±0.6%, respectively; P<0.001 vs ICH). Brain water content was not significantly different between any of the treatment groups in the contralateral hemisphere (78.7%±0.6%, 78.5%±0.6%, 78.17%±0.5% vs 79.2%±0.2% in ICH; P>0.05) (Figure 1A).
Figure 1.
Curcumin reduced cerebral edema and attenuated neurological deficits. (A) Curcumin reduced brain edema in a dose-dependent manner at d 3. Curcumin (Cur; 75, 150, or 300 mg/kg) was administered to mice (n=6/group) immediately after ICH. Significant differences were observed between groups in the ipsilateral hemisphere (cP<0.01 vs Sham, fP<0.01 vs ICH), no significant differences were found in the contralateral hemisphere. Values are given as the mean±SD. (B) Neurological deficits were attenuated on the 5th day after ICH, compared to the vehicle-treated ICH group (eP<0.05). On the 14th day, results of Rotarod test were near the level of the sham group. Values are given as the mean±SD from 9 mice/group.
A significant reduction in neurological behavior after ICH was detected in all mice by the Rotarod test (Figure 1B). After treatment with curcumin (150 mg/kg), behavioral outcomes were improved significantly on d 5 (248.2±39.6 s vs174.3±34.2 s, P<0.05), d 7 (231±40.5 s vs 165.4±45.2 s, P<0.05), and d 14 (274.9±35.5 s vs 172.4±26.7 s, P<0.05) after ICH compared to the ICH group.
Curcumin reduced gene expression of AQP4 and AQP9, not AQP1
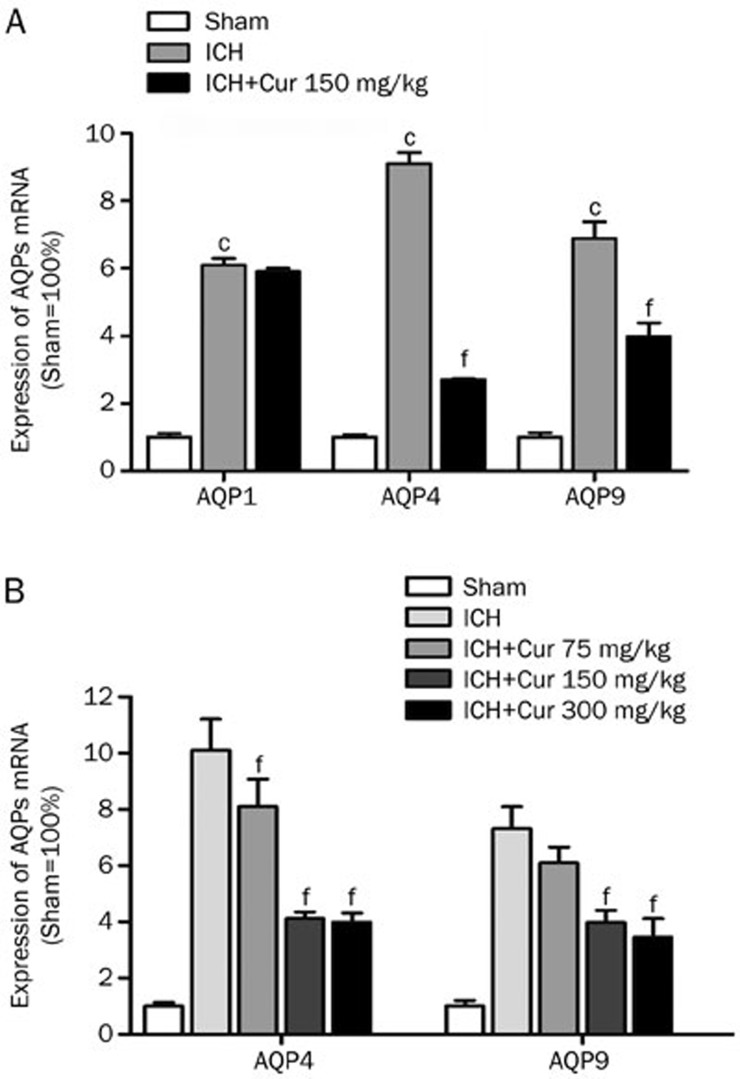
As shown in Figure 2A, AQP1, AQP4, and AQP9 mRNA were increased significantly 24 h post ICH (6.1±0.2, 9.1±0.3, 6.9±0.5-fold increase; P<0.001 vs sham). Curcumin (150 mg/kg) administration reduced gene expression of AQP4 (2.7±0.1 vs 9.1±0.3-fold increase in ICH, P<0.001) and AQP9 (4.0±0.4 vs 6.9±0.5-fold increase in ICH, P<0.001), but not AQP1 (5.9±0.1 vs 6.1±0.2-fold increase in ICH, P>0.05). In contrast, a single injection of curcumin (75, 150, and 300 mg/kg) decreased AQP4 (8.1±1.0, 4.1±0.2, 3.9±0.3 vs 10.1±1.1, P<0.01) and AQP9 (6.1±0.6 vs 7.3±0.8, P>0.05; 4.0±0.4, 3.5±0.7 vs 7.3±0.8, P<0.01) gene expression, in a dose-dependent manner, at 24 h after hemorrhage (Figure 2B).
Figure 2.
Curcumin reduced the gene transcription of AQP4 and AQP9, but not AQP1. (A) AQP1, AQP4 and AQP9 mRNA levels were increased significantly at 24 h after ICH (cP<0.01 vs Sham). AQP4 and AQP9 mRNA levels were markedly decreased by curcumin (150 mg/kg) (fP<0.01 vs ICH), but no significant difference was found in AQP1 (P>0.05). Values are given as the mean±SD from 3 mice/group. (B) AQP4 and AQP9 mRNA levels were reduced by curcumin in a dose-dependent manner (fP<0.01 vs ICH). Values are given as the mean±SD from 3 mice/group.
Curcumin inhibited protein expression of AQP4 and AQP9
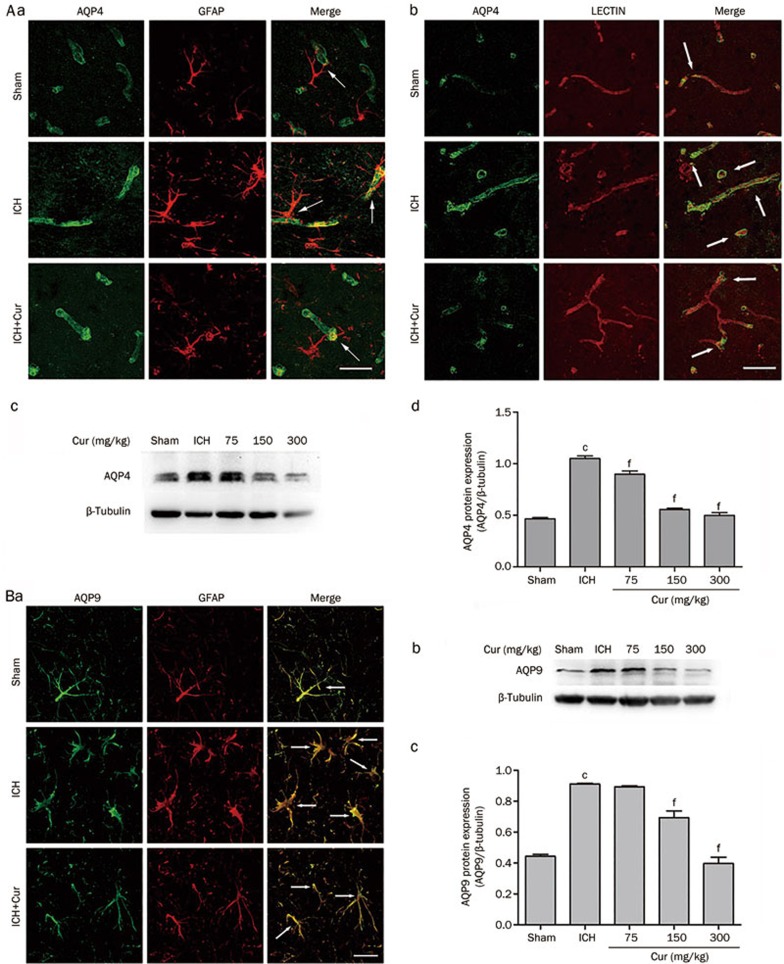
Immunofluorescence staining for AQP4 on d 3 after ICH showed that AQP4 expression was significantly elevated in the perihematomal area and that AQP4 was increased in perivascular astrocyte endfeet (Figure 3Aa and 3Ab). Curcumin (150 mg/kg) decreased AQP4 expression compared to that in the vehicle-treated ICH group. Western blot analysis confirmed that AQP4 protein expression was elevated in the perihematomal area at 72 h (1.05±0.02 vs 0.46±0.01 in sham, P<0.001) following ICH. Curcumin injection (75, 150, and 300 mg/kg) attenuated this post-ICH induction of AQP4 (0.90±0.03, 0.56±0.01, 0.50±0.03 vs 1.05±0.02 in the vehicle-treated ICH group, P<0.001) (Figure 3Ac and 3Ad).
Figure 3.
Curcumin inhibited the protein expression of AQP4 and AQP9 in ICH mice. (A) AQP4 protein expression was increased significantly in the perihematomal area over 3 d after ICH, and especially in perivascular astrocyte endfeet (white arrow). Curcumin treatment (150 mg/kg) decreased AQP4 expression. The results of Western blot analysis showed that AQP4 expression was strongly induced on d 3 after ICH. Curcumin treatment inhibited AQP4 expression in a dose-dependent manner. (B) Immunoreactivity of AQP9 in astrocytes was increased significantly over 3 d after ICH (white arrow). Curcumin treatment (150 mg/kg) reduced AQP9 expression compared to that in the ICH group. AQP9 protein quantitation shown that AQP9 protein expression was markedly increased on d 3 after ICH, and down-regulated by curcumin in a dose-dependent manner. Scale bar=10 μm. Values are mean±SD from 3 mice/group. cP<0.01 vs Sham, fP<0.01 vsICH.
A similar effect was observed in AQP9 expression, in that immunofluorescence showed that AQP9 expression was markedly increased on astrocytes in the perihematomal area and that this effect was inhibited by curcumin administration (150 mg/kg) (Figure 3Ba). Consistent with results obtained by Western blot, the intensity of AQP9 expression was strongly increased after hemorrhage (0.91±0.01 vs 0.44±0.01 in sham, P<0.001). This effect was decreased by curcumin (75, 150, and 300 mg/kg) in a dose-dependent manner (0.89±0.01 vs 0.91±0.01 in ICH group, P>0.05; 0.69±0.04, 0.39±0.04 vs 0.91±0.01 in ICH group, P<0.001) (Figure 3Bb and 3Bc).
Fe2+ increased protein expression of AQP4 and AQP9 in astrocytes
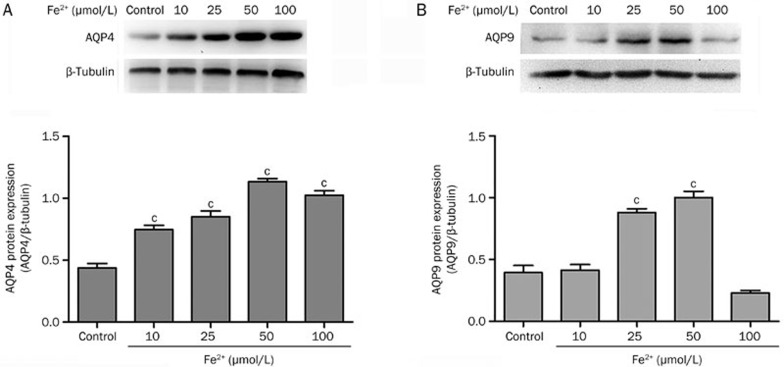
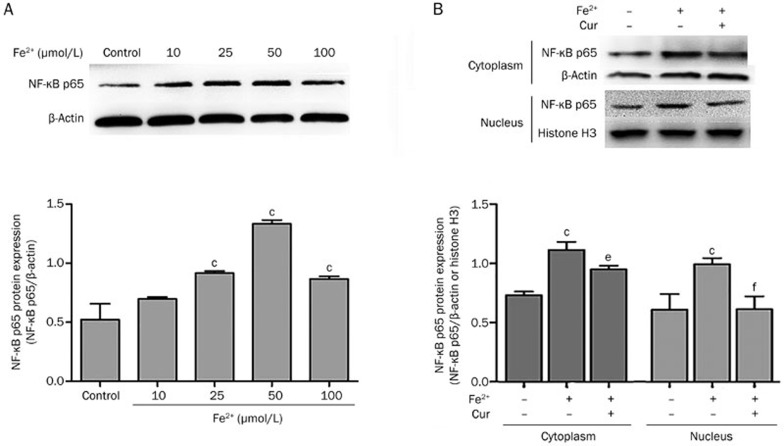
In an in vitro assay, immunofluorescence staining showed that the protein levels of both AQP4 (Figure 6Aa) and AQP9 (Figure 6Ba) were increased in astrocytes at 12 h after treatment with Fe2+(50 μmol/L). Western blot analysis demonstrated that as the concentration of Fe2+(10, 25, 50, and 100 μmol/L) increased, the levels of AQP4 (0.74±0.03, 0.85±0.04, 1.13±0.02, 1.02±0.03 vs 0.44±0.04 in control, P<0.001) and AQP9 (0.41±0.04 vs 0.39±0.06 in control, P>0.05; 0.88±0.03, 1.00±0.05 vs 0.39±0.06 in control, P<0.001) were elevated, reaching a peak at 50 μmol/L (Figure 4).
Figure 4.
Fe2+ increased the protein expression of AQP4 and AQP9 in astrocytes. Representative Western blot at 12 h after treatment with Fe2+ showed that AQP4 (A) and AQP9 (B) protein expression were up-regulated as the concentration of Fe2+ increased, reaching a peak at 50 μmol/L. Values are mean±SD from three independent experiments. cP<0.01 vs control.
Curcumin inhibited the activation of the NF-κB pathway in astrocytes
NF-κB p65 protein expression was increased in astrocytes after treated by Fe2+. As shown in Figure 5A, the level of p65 expression (0.70±0.02 vs0.52±0.13 in control, P>0.05; 0.92±0.02, 1.33±0.03, 0.87±0.02 vs 0.52±0.13 in control, P<0.001) was elevated in a manner following the concentration of Fe2+(10, 25, 50, and 100 μmol/L), reaching a peak at 50 μmol/L. After treatment with 50 μmol/L of Fe2+, expression of p65 was increased in cytoplasm (1.12±0.07 vs 0.73±0.03 in control, P<0.01), and nuclear translocation of p65 was activated (1.00±0.05 vs 0.61±0.13 in control, P<0.01). Co-treatment with curcumin and Fe2+ reduced expression of p65 in cytoplasm (0.95±0.03 vs 1.12±0.07 in Fe2+-only treated group, P<0.05), and nuclear translocation was also inhibited (0.62±0.11 vs 1.00±0.05 in Fe2+-only treatment group, P<0.01) (Figure 5B).
Figure 5.
Curcumin inhibited the activation of the NF-κB pathway in astrocytes. (A) Total p65 protein expression was increased 12 h after treatment with Fe2+, compared to controls, reaching a peak at 50 μmol/L. (B) NF-κB p65 expression in the cytoplasm and nucleus were quantitated, respectively, at 12 h after treatment with Fe2+. After treatment with Fe2+ (50 μmol/L), p65 in the cytoplasm and nucleus were elevated. Following co-treatment with Fe2+ and curcumin (20 μmol/L), p65 expression in the cytoplasm and nucleus was inhibited. Values are mean±SD from three independent experiments. cP<0.01 vs control; eP<0.05, fP<0.01 vs Fe2+.
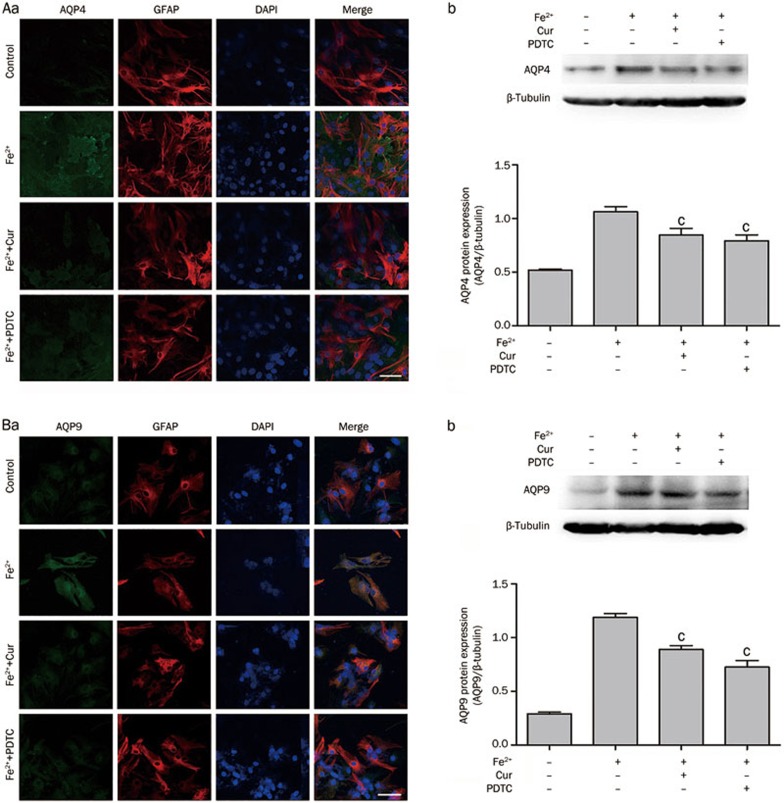
Curcumin down-regulated AQP4 and AQP9 expression via the NF-κB pathway
As mentioned previously, Fe2+ increased AQP4 and AQP9 protein expression in astrocytes. Co-treatment with Fe2+ and curcumin (20 μmol/L) or PDTC (10 μmol/L), a NF-κB inhibitor, reduced Fe2+ induced AQP4 expression, analyzed by immunofluorescence staining. Western blot analysis resulted the same outcomes: Fe2+ and curcumin, 0.84±0.06, vs 1.06±0.05 in the Fe2+-only treatment group, P<0.01; Fe2+ and PDTC, 0.79±0.05, vs1.06±0.05 in the Fe2+-only treatment group, P<0.001 (Figure 6Ab). Similar outcomes were found for AQP9 (Figure 6Bb); AQP9 protein expression was inhibited by curcumin and PDTC: curcumin, 0.89±0.04, vs 1.19±0.04 in the Fe2+-only treatment group, P<0.001; PDTC, 0.73±0.06, vs 1.19±0.04 in the Fe2+-only treatment group, P<0.001.
Figure 6.
Curcumin or PDTC treatment inhibited AQP4 and AQP9 expression induced by Fe2+. (A) Immunofluoresence was performed at 12 h after treatment with Fe2+ (50 μmol/L), and fluorescence intensity of AQP4 was enhanced. After co-treatment with Fe2+ and curcumin (20 μmol/L) or Fe2+ and PDTC (10 μmol/L), AQP4 expression was decreased. The Western blot analysis showed that AQP4 expression was increased at 12 h after treatment with Fe2+ (50 μmol/L) and inhibited by co-treatment with Fe2+ and curcumin (20 μmol/L) or Fe2+ and PDTC (10 μmol/L). (B) The fluorescence intensity of AQP9 was increased at 12 h after treatment with Fe2+ (50 μmol/L) and inhibited by co-treatment with Fe2+ and curcumin (20 μmol/L) or Fe2+ and PDTC (10 μmol/L). AQP9 protein quantitation proved that AQP9 expression was up-regulated at 12 h after treatment with Fe2+ (50 μmol/L) and that this increase was reversed by co-treatment with Fe2+ and curcumin (20 μmol/L) or Fe2+ and PDTC (10 μmol/L). Scale bar=10 μm. Values are mean±SD from three independent experiments. cP<0.01 vs Fe2+.
Discussion
The present study demonstrated that curcumin, a natural polyphenolic compound, attenuated ICH induced cerebral edema and neurological deficits. This effect was associated with reduced activation of the NF-κB pathway, which was activated by Fe2+. It was also associated with the degradation of hemoglobin and decreased expression of AQP4 and AQP9 in astrocytes.
Brain edema and related increases in ICP following ICH contribute to herniation-related deaths and neurological deficits. A higher degree of edema is directly proportional to a poorer prognosis in patients. Perihematomal edema develops immediately after ICH, peaking around the third or fourth day in experimental ICH models, and between d 10–20 in humans28. In our study, d 3 was selected as a time-point for the water content tests. We found that curcumin reduced cerebral edema and improved performance on neurobehavioral tests following ICH. We noted that, in Rotarod tests, curcumin normalized ICH models to almost sham levels over the 2 weeks following ICH. Together with other reports14,15, these results suggest that curcumin attenuates the damage caused by intracerebral hemorrhage by inhibiting matrix metalloproteinase-mediated injury to the blood-brain barrier and cerebral edema. Curcumin therefore has therapeutic potential for treating ICH.
AQP1, AQP4, and AQP9 have been shown to play critical roles in edema formation in different types of brain diseases17. AQP1 expression has been reported to be elevated in human astrocytes after SAH26 and in cortical endothelial cells in rat SAH models29, suggesting an important role in edema formation. Nesic et al reported30 that AQP1 expression was induced in a model of long-term rodent spinal injury, while astrocytic AQP1 expression contributed to the secretion of cerebrospinal fluid during cyst formation and the migration of astrocytes. Neuronal AQP1 expression therefore suggested a role in structural plasticity and neuronal repair. All of these results illustrate the complex pathological roles of AQP1. One study30 also found that hypertonicity affected AQP4, but not AQP1 expression, suggesting that AQP1 and AQP4 expression is regulated by different mechanisms. Several studies have shown that acute brain injuries, including ICH, ischemic stroke, and brain trauma, alter AQP4 and AQP9 expression, and that decreased expression or gene knockout of AQP4 and AQP9 ameliorates brain edema and neurological deficits10,20,21,22,23,24,25,31. The level and duration of AQP4 expression was temporally correlated with the degree of brain edema in a middle cerebral artery (MCA) occlusion model. AQP4 expression has also been observed at 48 h in transient occlusions. In a mild stroke model (30 min of MCA occlusion), up-regulated AQP4 expression was detected for up to 7 d, whereas in a severe stroke model, elevated AQP4 expression persisted for up to 28 d32,33. The pattern of AQP4 expression in cerebrovascular diseases has revealed that this molecule plays a critical role in regulating water movement during brain edema17. AQP9 expression was increased in astrocytes after ischemic injury, but AQP9 up-regulation did not correlate with the degree of brain edema as has been documented in AQP4 studies, revealing that AQP9 does not play a significant role in brain edema after cerebral ischemia. AQP9 does, however, facilitate lactate movement between astrocytes and neurons, suggesting that it plays complex roles in energy metabolism and neurorepair25,32. In the present study, AQP1, AQP4, and AQP9 gene expression were increased after ICH. AQP4 and AQP9, but not AQP1, were decreased after treatment with curcumin. These novel findings suggest that curcumin could be used as an AQP4 and AQP9 inhibitor in the clinical treatment of ICH patients.
Iron, a byproduct of hemoglobin degradation, has been shown to play a major role in ICH-induced brain edema and brain damage34,35,36,37. Iron overload caused by ICH is an important contributor to neurobiological deficits, brain edema, inflammation, neuron apoptosis and brain atrophy. These effects can be reduced with deferoxamine, an iron chelator38,39,40,41,42,43,44,45. However, two studies46,47 have reported that deferoxamine reduces the level of iron, but does not improve outcomes, in a collagenase-induced ICH model. In our experimental design, Fe2+ was added to cultured astrocytes and AQP4 and AQP9 protein expression were strongly induced, suggesting that iron was related to cytotoxic edema. This phenomenon was inhibited by co-treatment of Fe2+with curcumin. These results, in combination with those of Min-chao DAI in 201348, showed that curcumin reduced iron-induced neurotoxicity in primary cortical neurons by attenuating necroptosis and that neuroprotection by curcumin affected not only neurons but also astrocytes.
Previous studies have demonstrated that oxidative stress and inflammation are induced by ICH and the degradation of blood cells, and especially by thrombin and iron28,49. Tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) are increased after ICH or treatments inducing the degradation of blood cells. The transcription factor NF-κB, which regulates IL-1β and TNF-α, is also immediately activated following ICH49. Consistent with these findings, our results show that NF-κB and p65 subunit expression increases as the concentration of Fe2+ increases. In 1995, Singh et al showed that curcumin is a potent inhibitor of NF-κB and that it acts by down-regulating p65 nuclear translocation and AP-1 binding factors50. Recent studies have also shown that curcumin is an NF-κB inhibitor in different diseases, including brain trauma10 and oxygen-glucose deprivation11. As shown in Figure 5, p65 expression in the cytoplasm and nucleus were both down-regulated by curcumin. Co-treatment with Fe2+ and curcumin or PDTC revealed that AQP4 and AQP9 are decreased in co-treated astrocytes compared to those treated with Fe2+ only. We conclude that curcumin reduced AQP4 and AQP9 expression by inhibiting the NF-κB pathway.
In 2001, Cheng et al51 reported that oral administration of up to 8 g/d of curcumin resulted in safe bioactivity; serum concentration peaked at 1–2 h and gradually declined over 12 h. Following on these results, which support the use of curcumin as a strong anti-inflammation and anti-oxidative stress agent, curcumin was tested in several clinical trials over a decade. Positive effects were observed in patients with pre-malignant lesions51, colorectal cancer52, Alzheimer's disease53, colorectal neoplasia54, chronic periodontitis55, radiation dermatitis56, mastitis57, aphthous stomatitis58 and osteoarthritis59, but not in patients with psoriasis vulgaris60. Unfortunately, at the present time, there are no reports of clinical trials aimed at studying the effect of curcumin on ICH. Well-designed clinical trials are needed to study and verify the neuroprotective effect of curcumin in ICH patients.
Conclusions
The results of this study suggest that curcumin attenuates ICH-induced brain edema by inhibiting AQP4 and AQP9, but not AQP1, and that it acts through the NF-κB signaling pathway. Curcumin is an effective anti-inflammatory agent and may therefore have therapeutic potential for the treatment of ICH.
Authors contribution
Liu-guan BIAN, Bao-feng WANG, and Zhen-wen CUI participated in the study design and manuscript preparation; Bao-feng WANG, Zhen-wen CUI, Zhi-hong ZHONG, and Yu-hao SUN performed the experiments; Bao-feng WANG, Zhen-wen CUI, Qing-fang SUN, and Guo-yuan YANG analyzed data; Liu-guan BIAN was responsible for supervision and funding. All authors read and approved the final version of the manuscript.
Acknowledgments
This research was supported by SJTU Medicine-Engineering Research Fund (Grant No YG2012MS06; YG2014QN17), National Natural Science Foundation of China (Grant No 81471176) and Young Scientists Fund of the National Natural Science Foundation of China (Grant No 81301045)
References
- van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–81. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:660–7. doi: 10.1136/jnnp-2013-306476. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–69. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- Arima H, Wang JG, Huang Y, Heeley E, Skulina C, Parsons MW, et al. Significance of perihematomal edema in acute intracerebral hemorrhage: the INTERACT trial. Neurology. 2009;73:1963–8. doi: 10.1212/WNL.0b013e3181c55ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiex R, Tsirka SE. Brain edema after intracerebral hemorrhage: mechanisms, treatment options, management strategies, and operative indications. Neurosurg Focus. 2007;22:E6. doi: 10.3171/foc.2007.22.5.7. [DOI] [PubMed] [Google Scholar]
- Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86:272–8. doi: 10.3171/jns.1997.86.2.0272. [DOI] [PubMed] [Google Scholar]
- Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin — from molecule to biological function. Angew Chem Int Ed Engl. 2012;51:5308–32. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- Jiang TF, Zhang YJ, Zhou HY, Wang HM, Tian LP, Liu J, et al. Curcumin ameliorates the neurodegenerative pathology in A53T alpha-synuclein cell model of Parkinson's disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J Neuroimmune Pharmacol. 2013;8:356–69. doi: 10.1007/s11481-012-9431-7. [DOI] [PubMed] [Google Scholar]
- Laird MD, Sukumari-Ramesh S, Swift AE, Meiler SE, Vender JR, Dhandapani KM. Curcumin attenuates cerebral edema following traumatic brain injury in mice: a possible role for aquaporin-4. J Neurochem. 2010;113:637–48. doi: 10.1111/j.1471-4159.2010.06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HJ, Shang CZ, Peng DW, Xu J, Xu PX, Zhan L, et al. Curcumin attenuates ischemia-like injury induced IL-1beta elevation in brain microvascular endothelial cells via inhibiting MAPK pathways and nuclear factor-kappaB activation. Neurol Sci. 2014;35:1387–92. doi: 10.1007/s10072-014-1718-4. [DOI] [PubMed] [Google Scholar]
- Kuo CP, Lu CH, Wen LL, Cherng CH, Wong CS, Borel CO, et al. Neuroprotective effect of curcumin in an experimental rat model of subarachnoid hemorrhage. Anesthesiology. 2011;115:122–38. doi: 10.1097/ALN.0b013e31823306f0. [DOI] [PubMed] [Google Scholar]
- Shishodia S. Molecular mechanisms of curcumin action: gene expression. Biofactors. 2013;39:37–55. doi: 10.1002/biof.1041. [DOI] [PubMed] [Google Scholar]
- King MD, McCracken DJ, Wade FM, Meiler SE, Alleyne CH, Jr., Dhandapani KM. Attenuation of hematoma size and neurological injury with curcumin following intracerebral hemorrhage in mice. J Neurosurg. 2011;115:116–23. doi: 10.3171/2011.2.JNS10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Dai M, Wang Y, Wang W, Sun Q, Yang GY, et al. Neuroprotection and sensorimotor functional improvement by curcumin after intracerebral hemorrhage in mice. J Neurotrauma. 2011;28:2513–21. doi: 10.1089/neu.2011.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22:367–78. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Badaut J, Ashwal S, Obenaus A. Aquaporins in cerebrovascular disease: a target for treatment of brain edema. Cerebrovasc Dis. 2011;31:521–31. doi: 10.1159/000324328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–70. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcienega II, Brunet JF, Bloch J, Badaut J. Cell locations for AQP1, AQP4 and 9 in the non-human primate brain. Neuroscience. 2010;167:1103–14. doi: 10.1016/j.neuroscience.2010.02.059. [DOI] [PubMed] [Google Scholar]
- Xu M, Su W, Xu QP. Aquaporin-4 and traumatic brain edema. Chin J Traumatol. 2010;13:103–10. [PubMed] [Google Scholar]
- Zhong Z, Wang B, Dai M, Sun Y, Sun Q, Yang G, et al. Carvacrol alleviates cerebral edema by modulating AQP4 expression after intracerebral hemorrhage in mice. Neurosci Lett. 2013;555:24–9. doi: 10.1016/j.neulet.2013.09.023. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wu P, Su J, Xiang J, Cai D, Dong Q. Effects of Aquaporin-4 on edema formation following intracerebral hemorrhage. Exp Neurol. 2010;223:485–95. doi: 10.1016/j.expneurol.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang M, Qiu GP, Zhuo F, Yu WH, Sun SQ, et al. Aquaporin 9 in rat brain after severe traumatic brain injury. Arq Neuropsiquiatr. 2012;70:214–20. doi: 10.1590/s0004-282x2012000300012. [DOI] [PubMed] [Google Scholar]
- Badaut J. Aquaglyceroporin 9 in brain pathologies. Neuroscience. 2010;168:1047–57. doi: 10.1016/j.neuroscience.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Badaut J, Hirt L, Granziera C, Bogousslavsky J, Magistretti PJ, Regli L. Astrocyte-specific expression of aquaporin-9 in mouse brain is increased after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:477–82. doi: 10.1097/00004647-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Badaut J, Brunet JF, Grollimund L, Hamou MF, Magistretti PJ, Villemure JG, et al. Aquaporin 1 and aquaporin 4 expression in human brain after subarachnoid hemorrhage and in peritumoral tissue. Acta Neurochir Suppl. 2003;86:495–8. doi: 10.1007/978-3-7091-0651-8_101. [DOI] [PubMed] [Google Scholar]
- Rynkowski MA, Kim GH, Komotar RJ, Otten ML, Ducruet AF, Zacharia BE, et al. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat Protoc. 2008;3:122–8. doi: 10.1038/nprot.2007.513. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Yatsushige H, Ostrowski RP, Tsubokawa T, Colohan A, Zhang JH. Role of c-Jun N-terminal kinase in early brain injury after subarachnoid hemorrhage. J Neurosci Res. 2007;85:1436–48. doi: 10.1002/jnr.21281. [DOI] [PubMed] [Google Scholar]
- Nesic O, Lee J, Unabia GC, Johnson K, Ye Z, Vergara L, et al. Aquaporin 1 — a novel player in spinal cord injury. J Neurochem. 2008;105:628–40. doi: 10.1111/j.1471-4159.2007.05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Xiang J, Wu P, Su J, Ding H, Tang Y, et al. The role of aquaporin 4 in apoptosis after intracerebral hemorrhage. J Neuroinflammation. 2014;11:184. doi: 10.1186/s12974-014-0184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Mde C, Hirt L, Bogousslavsky J, Regli L, Badaut J. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J Neurosci Res. 2006;83:1231–40. doi: 10.1002/jnr.20819. [DOI] [PubMed] [Google Scholar]
- Badaut J, Ashwal S, Tone B, Regli L, Tian HR, Obenaus A. Temporal and regional evolution of aquaporin-4 expression and magnetic resonance imaging in a rat pup model of neonatal stroke. Pediatr Res. 2007;62:248–54. doi: 10.1203/PDR.0b013e3180db291b. [DOI] [PubMed] [Google Scholar]
- Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38:759–62. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, et al. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104:305–12. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–93. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- Qing WG, Dong YQ, Ping TQ, Lai LG, Fang LD, Min HW, et al. Brain edema after intracerebral hemorrhage in rats: the role of iron overload and aquaporin 4. J Neurosurg. 2009;110:462–8. doi: 10.3171/2008.4.JNS17512. [DOI] [PubMed] [Google Scholar]
- Xie Q, Gu Y, Hua Y, Liu W, Keep RF, Xi G. Deferoxamine attenuates white matter injury in a piglet intracerebral hemorrhage model. Stroke. 2014;45:290–2. doi: 10.1161/STROKEAHA.113.003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama T, Okauchi M, Hua Y, Keep RF, Xi G. Deferoxamine reduces neuronal death and hematoma lysis after intracerebral hemorrhage in aged rats. Transl Stroke Res. 2013;4:546–53. doi: 10.1007/s12975-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun HJ, Kim DW, Yi HJ, Kim YS, Kim EH, Hwang SJ, et al. Effects of statin and deferoxamine administration on neurological outcomes in a rat model of intracerebral hemorrhage. Neurol Sci. 2012;33:289–96. doi: 10.1007/s10072-011-0733-y. [DOI] [PubMed] [Google Scholar]
- Hatakeyama T, Okauchi M, Hua Y, Keep RF, Xi G. Deferoxamine reduces cavity size in the brain after intracerebral hemorrhage in aged rats. Acta Neurochir Suppl. 2011;111:185–90. doi: 10.1007/978-3-7091-0693-8_31. [DOI] [PubMed] [Google Scholar]
- Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke. 2010;41:375–82. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okauchi M, Hua Y, Keep RF, Morgenstern LB, Xi G. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke. 2009;40:1858–63. doi: 10.1161/STROKEAHA.108.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Hua Y, Keep RF, He Y, Wang J, Wu J, et al. Deferoxamine reduces brain swelling in a rat model of hippocampal intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:13–8. doi: 10.1007/978-3-211-09469-3_3. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–8. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- Warkentin LM, Auriat AM, Wowk S, Colbourne F. Failure of deferoxamine, an iron chelator, to improve outcome after collagenase-induced intracerebral hemorrhage in rats. Brain Res. 2010;1309:95–103. doi: 10.1016/j.brainres.2009.10.058. [DOI] [PubMed] [Google Scholar]
- Auriat AM, Silasi G, Wei Z, Paquette R, Paterson P, Nichol H, et al. Ferric iron chelation lowers brain iron levels after intracerebral hemorrhage in rats but does not improve outcome. Exp Neurol. 2012;234:136–43. doi: 10.1016/j.expneurol.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai MC, Zhong ZH, Sun YH, Sun QF, Wang YT, Yang GY, et al. Curcumin protects against iron induced neurotoxicity in primary cortical neurons by attenuating necroptosis. Neurosci Lett. 2013;536:41–6. doi: 10.1016/j.neulet.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629–52. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–3. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4:354–64. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottumukkala SN, Koneru S, Mannem S, Mandalapu N. Effectiveness of sub gingival irrigation of an indigenous 1% curcumin solution on clinical and microbiological parameters in chronic periodontitis patients: a pilot randomized clinical trial. Contemp Clin Dent. 2013;4:186–91. doi: 10.4103/0976-237X.114874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JL, Heckler CE, Ling M, Katz A, Williams JP, Pentland AP, et al. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res. 2013;180:34–43. doi: 10.1667/RR3255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshariani R, Farhadi P, Ghaffarpasand F, Roozbeh J. Effectiveness of topical curcumin for treatment of mastitis in breastfeeding women: a randomized, double-blind, placebo-controlled clinical trial. Oman Med J. 2014;29:330–4. doi: 10.5001/omj.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh RA, Bagewadi AS. Comparison of effectiveness of curcumin with triamcinolone acetonide in the gel form in treatment of minor recurrent aphthous stomatitis: A randomized clinical trial. Int J Pharm Investig. 2014;4:138–41. doi: 10.4103/2230-973X.138346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrotin Y, Gharbi M, Dierckxsens Y, Priem F, Marty M, Seidel L, et al. Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complement Altern Med. 2014;14:159. doi: 10.1186/1472-6882-14-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurd SK, Smith N, VanVoorhees A, Troxel AB, Badmaev V, Seykora JT, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: A prospective clinical trial. J Am Acad Dermatol. 2008;58:625–31. doi: 10.1016/j.jaad.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]