Abstract
Aim:
Omi is an ATP-independent serine protease that is necessary for neuronal function and survival. The aim of this study was to investigate the role of protease Omi in regulating differentiation of mouse neuroblastoma cells and to identify the substrate of Omi involved in this process.
Methods:
Mouse neuroblastoma N2a cells and Omi protease-deficient mnd2 mice were used in this study. To modulate Omi and E2F1 expression, N2a cells were transfected with expression plasmids, shRNA plasmids or siRNA. Protein levels were detected using immunoblot assays. The interaction between Omi and E2F1 was studied using immunoprecipitation, GST pulldown and in vitro cleavage assays. N2a cells were treated with 20 μmol/L retinoic acid (RA) and 1% fetal bovine serum to induce neurite outgrowth, which was measured using Image J software.
Results:
E2F1 was significantly increased in Omi knockdown cells and in brain lysates of mnd2 mice, and was decreased in cells overexpressing wild-type Omi, but not inactive Omi S276C. In brain lysates of mnd2 mice, endogenous E2F1 was co-immunoprecipitated with endogenous Omi. In vitro cleavage assay demonstrated that Omi directly cleaved E2F1. Treatment of N2a cells with RA induced marked differentiation and neurite outgrowth accompanied by significantly increased Omi and decreased E2F1 levels, which were suppressed by pretreatment with the specific Omi inhibitor UCF-101. Knockdown of Omi in N2a cells suppressed RA-induced neurite outgrowth, which was partially restored by knockdown of E2F1.
Conclusion:
Protease Omi facilitates neurite outgrowth by cleaving the transcription factor E2F1 in differentiated neuroblastoma cells; E2F1 is a substrate of Omi.
Keywords: Omi, E2F1, neuronal differentiation, N2a cells, mnd2 mice, retinoic acid, UCF-101
Introduction
The ATP-independent serine protease HtrA2 (also known as Omi) is a member of the high-temperature requirement factor A (HtrA) family that was originally identified as a mammalian homolog of the Escherichia coli heat-shock-induced serine protease HtrA/DegP and DegS1. Under normal conditions, Omi is mostly localized in the mitochondria, while some can also be found in the nucleus2,3. Previous studies have concentrated on the pro-apoptotic function of Omi in non-neuronal somatic cells. With apoptotic stimuli, Omi is released from mitochondria into the cytosol, mediating cell death by caspase-dependent or -independent apoptosis4,5,6,7. Besides apoptotic functions, Omi seems to play a physiological role in maintaining cellular homeostasis and promoting neuronal cell survival. Omi-transgenic mice showed normal development without any symptoms of apoptotic cell death8. Moreover, both Omi-knockout mice and mnd2 (motor neuron degeneration 2) mice, which harbor a protease-deficient Omi S276C mutant, exhibited an early onset neurodegeneration and motor abnormalities similar to Parkinson's disease (PD)9,10. Missense mutations that lead to a loss of Omi protease activity have been associated with PD11,12. These studies suggest that intact Omi protease activity is necessary for neuronal function and survival. Recently, increased expression of Omi has been reported during neurogenesis and neuronal development13. However, the mechanism by which Omi functions in neuronal development is completely unknown.
The E2F family of transcription factors comprises eight structurally related E2Fs (E2F1–8) that form as heterodimers with members of the DP family (DP-1 and DP-2). E2F1 forms complexes with DP-1, controlling various genes implicated in G1/S transition that contain the sequence 5′ TTTSSCGC 3′ (S=C or G) in the regulatory regions14,15. The activity of E2F is mediated, in large part, by a direct interaction with members of the pRb family (pRb, p107, p130)16,17. In quiescent cells, non-phosphorylated pRb can bind to E2F, resulting in repressed transcription and enforcing a cell-cycle block; upon entering the cell cycle, pRb is sequentially phosphorylated by cyclin D/cdk4 complexes followed by cyclin E/cdk2 complexes activation, which leads to the dissociation and activation of E2F18,19. E2F1 functions in both proliferation and apoptosis20,21,22,23,24; however, increasing attention has been focused on the function of E2F1 in cell differentiation. The expression of E2F1 is irreversibly downregulated during the differentiation of C2C12 myocytes25. Additionally, the association of GSK3β with E2F1 facilitates nerve growth factor-induced PC12 cell differentiation, and overexpression of E2F1 inhibits neurite outgrowth26.
In this study, we identified that E2F1 is a substrate of Omi and that Omi plays a vital role in promoting neurite outgrowth by cleaving E2F1.
Materials and methods
Animals
mnd2 mice (HtrA2S276C mutant mice, C57BL/6J) and wild-type (WT) mice were obtained from Jackson Laboratory (JAX, Bar Harbor, ME, USA). The protocol to identify the genotypes of the offspring mice was provided by JAX. The mice were bred in a specific pathogen-free environment, and adequate measures were taken to minimize pain and discomfort in compliance with national regulations. All animal experiments were approved by the Animal Welfare Advisory Committee of Soochow University.
Cell culture
HEK293, H1299, and mouse neuroblastoma (N2a) cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (FBS; Gibco) and 100 μg/mL penicillin and 100 μg/mL streptomycin. All cells were cultured at the same passage number for experimental consistency.
RNA interference
The cells were transfected with oligonucleotides mixed with RNAiMax (Invitrogen, La Jolla, CA, USA), according to the manufacturer's instructions. The oligonucleotides targeting Omi sequences (si-Omi) and the negative control (NC) siRNA have been described previously27. The oligonucleotides to target sequences of E2F1 were purchased from Ribobio (Guangzhou, China) and their sequences were as follows: si-E2F1 sense 5′-GGAUCUGGAGACUGACCAU-3′, antisense 5′-AUGGUCAGUCUCCAGAUCC-3′.
Immunoblot analysis and antibodies
Tissue homogenates and cell extracts were lysed in 1×SDS lysis buffer (25 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 1% NP-40, and 1% sodium deoxycholate) in the presence of a protease inhibitor cocktail (Roche, Basel, Switzerland). Approximately 20 μg of the cell lysates was separated by SDS-PAGE and transferred onto a PVDF membrane (Millipore, Bedford, MA, USA). The following antibodies were used for immunoblot analysis: anti-α-Tubulin (CP06, Calbiochem, San Diego, CA, USA), anti-Omi (AF1458, R&D Systems, Minneapolis, MN, USA), anti-E2F1 (KH95, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-GAPDH (MAB374, Chemicon, Temecula, CA, USA), anti-GFP (sc-9996, Santa Cruz Biotechnology), anti-GST (sc-138, Santa Cruz Biotechnology), anti-HA (sc-7392, Santa Cruz Biotechnology), anti-His (M30111, Abmart, Shanghai, China) and anti-Histone 2B (Q5QNW6, Epitomics, Burlingame, CA, USA). The anti-mouse and anti-rabbit secondary antibodies coupled to horseradish peroxidase were from Amersham Pharmacia Biotech (Peapack, Far Hills, NJ, USA). The proteins were viewed with an ECL detection kit (Amersham Pharmacia Biotech, Arlington Heights, IL, USA).
Plasmids
The Omi-related plasmids have been described previously27. Full-length human E2F1 complementary DNA (cDNA) was amplified using PCR from a human fetal brain cDNA library with the primers 5′-GAGGATCCCCATGGCCTTGGCCGGGGCC-3′ and 5′-GAGAATCCCGAAATCCAGGGGGTGA-3′. The PCR product was inserted in-frame into the pGEX-5x-1 vector at the BamHI/EcoRI sites. The plasmids encoding sh-Omi or the negative control vector (LV3-NC), which contain the green fluorescence protein (GFP) sequence, were purchased from GenePharma (Shanghai, China). The sequences targeting Omi or NC in the plasmid constructs were as follows: LV3-NC 5′-TTCTCCGAACGTGTCACGTTTC-3′ and Omi-mus 5′-GGGAGTTTGTTGTTGCCATGG-3′.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA from mouse brains and N2a cells was isolated using TRIzol reagent (Invitrogen), and the extracted total RNA was reverse-transcribed into cDNA for PCR assays with a TransScript First-Strand cDNA Synthesis Kit (Takara, Otsu, Shiga, Japan). The primer pairs used for qRT-PCR were as follows: mouse β-actin sense 5′-TGTCCACCTTCCAGCAGATGT-3′, antisense 5′-AGCTCAGTAACAGTCCGCCTAGA-3′ mouse E2F1 sense 5′-CTCGACTCCTCGCAGATCG-3′, antisense 5′-GATCCAGCCTCCGTTTCACC-3′ mouse cyclin E1 sense 5′-GTGGCTCCGACCTTTCAG-3′, antisense 5′-CACAGTCTTGTCAATCTTGGCA-3′.
Immunoprecipitation assay
For immunoprecipitation, mnd2 mouse brain hemispheres were lysed in TSPI buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L sodium chloride, 1 mmol/L EDTA, 1% NP-40 and a complete protease inhibitor cocktail). To eliminate cell debris, the lysates were centrifuged at 12 000×g for 30 min at 4 °C. The supernatants were incubated with anti-Omi antibody or normal rabbit immunoglobulin G overnight at 4 °C. After incubation, protein G Sepharose (Roche) was used for precipitation. The beads were washed with TSPI buffer five times, and the binding proteins were eluted with SDS sample buffer for immunoblot analysis.
In vitro proteolytic cleavage assay
GST, GST-E2F1, or β-casein (Sigma, Saint Louis, MO, USA) proteins were incubated in the presence of 1–3 μg of 6×His-tagged Omi in protease cleavage buffer (50 mmol/L Tris-HCl, pH 7.6, 1 mmol/L dithiothreitol) for 1 h at 37 °C. The reactions were stopped with SDS sample buffer, and the samples were boiled for 10 min. The samples were subjected to immunoblot analysis with anti-GST antibody or visualized by Coomassie brilliant blue staining.
GST pulldown assay
An aliquot containing 20 μg of GST or GST-E2F1 that was expressed in E coli strain JM109 was incubated with 30 μg of Glutathione Sepharose 4B (Amersham Biosciences, Piscataway, NJ, USA) for 30 min at 4 °C. Sepharose bound with GST or GST-E2F1 was incubated with 50 μg of His-Omi proteins from the supernatants of the E coli crude extracts in 0.25 mL of HNTG buffer (20 mmol/L HEPES-KOH, pH 7.5, 100 mmol/L NaCl, 0.1% Triton X-100, and 10% glycerol) for 1 h at 4 °C. After incubation, the beads were washed six times with 1 mL of HNTG buffer to remove the non-binding proteins. The bound proteins were eluted using SDS sample buffer and then boiled for 10 min at 95 °C. The samples were then subjected to immunoblot analysis.
Neurite outgrowth assay
N2a cells were plated onto 12-well plates (6×105 cells per well) and cultured overnight in DMEM containing 10% fetal bovine serum; the next day, the medium was replaced by DMEM with 1% fetal bovine serum and supplemented with retinoic acid (RA) at a final concentration of 20 μmol/L for another 48 h to induce neurite outgrowth. The formation of neurites was observed using an inverted IX71 microscope system (Olympus, Tokyo, Japan). The neurite length of each cell was measured by Image J software as previously described28. Each well was evaluated using four different fields under a microscope. The average neurite length of nearly 200 cells is presented as the mean±SEM.
Subcellular fractionation assay
N2a cells were cultured in DMEM containing 10% FBS on 12-well plates for 48 h. The collected cells were lysed in fractionation buffer (320 mmol/L sucrose, 3 mmol/L CaCl2, 2 mmol/L MgAc, 0.1 mmol/L EDTA, 1 mmol/L DTT, 0.5 mmol/L phenylmethylsulfonyl fluoride, plus 0.5% NP-40) for 20 min on ice. Then, the samples were spun down at 4 °C for 15 min at 600×g, and the supernatants were collected as the cytoplasmic fractions. The pellets were washed twice with fractionation buffer without NP-40 and were lysed in nuclear lysis buffer (20 mmol/L HEPES, pH 7.9, 25% glycerol, 1.5 mmol/L MgCl2, 280 mmol/L EDTA, 1 mmol/L DTT, 0.5 mmol/L PMSF, and 0.3% NP-40) as the nuclear fractions.
Statistical analysis
Densitometric analysis of immunoblots from three independent experiments was implemented with Adobe Photoshop CS5 (Adobe, San Jose, CA, USA), and the data were analyzed using Origin 6.0 (OriginLab, Northampton, MA, USA). Quantitative data are presented as the mean±SEM. Statistical significance was assessed via one-way ANOVA, and the criterion of significance was set at P<0.05.
Results
Omi represses E2F1 expression
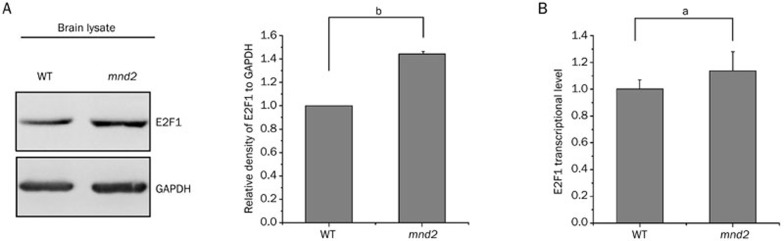
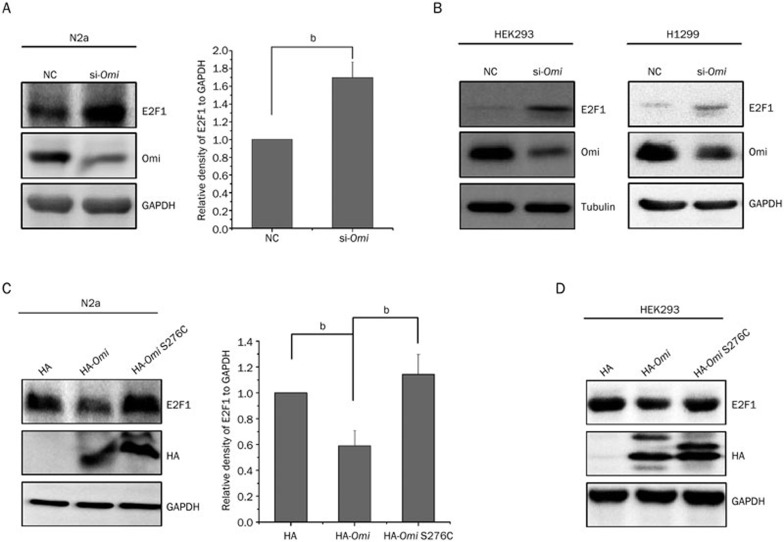
The E2F1-related signaling pathway plays an important role in PD29. To test whether the protein level of E2F1 was altered in mnd2 mice, we performed immunoblot analysis using total brain lysates from 25-d-old mnd2 mice and age-matched WT mice to examine E2F1 levels. As shown in Figure 1A, the E2F1 protein level was significantly increased in mnd2 mouse brains. In addition, we found no alteration in the levels of E2F1 mRNA in the brains of mnd2 mice compared to those in WT mouse brains (Figure 1B), indicating that Omi regulates the expression of E2F1 at the post-transcriptional level. To further confirm the effects of Omi on E2F1, we used small-interfering RNA (siRNA)-mediated silencing to knock Omi down in N2a cells. E2F1 levels were higher in Omi knockdown cells than in the negative control cells (Figure 2A), consistent with the data from in vivo studies (Figure 1A). Similar data were obtained using HEK293 (Figure 2B, left panel) and H1299 cells (Figure 2B, right panel). In N2a cells (Figure 2C) or HEK293 cells (Figure 2D) that were transfected with hemagglutinin (HA)-tagged full-length Omi or the S276C mutant Omi, overexpression of WT Omi resulted in a decreased abundance of E2F1, whereas overexpression of S276C mutant Omi did not (Figure 2C, 2D). These results indicate that Omi represses the protein level of E2F1 dependent on its protease activity.
Figure 1.
Omi represses E2F1 level in vivo. (A) The protein level of E2F1 is increased in mnd2 mouse brain. Brain lysates from 25-d-old mnd2 mice and age-matched WT mice were prepared and immunoblotted with the antibodies indicated. The bar graph on the right shows the band intensities of E2F1 relative to those of GAPDH. Densitometric analyses from three independent experiments were quantified by one-way ANOVA. bP<0.05 vs WT. (B) The mRNA level of E2F1 is not different in mnd2 and control mice brain. Total RNA was extracted from the brains of 25-d-old mnd2 mice and age-matched WT mice. qRT-PCR assays were performed using primers specific for the E2F1 and β-actin genes. n=3–4 per group. aP>0.05; one-way ANOVA.
Figure 2.
Omi represses E2F1 expression dependent on its protease activity. (A) The protein level of E2F1 is increased by knocking down Omi in N2a cells. N2a cells were transfected with a control siRNA (NC: negtive control) or siRNA that targets Omi (si-Omi) for 72 h. Whole-cell extracts were prepared and immunoblotted with the antibodies indicated. The bar graph on the right shows the band intensities of E2F1 relative to GAPDH. Densitometric analyses from three independent experiments were quantified by one-way ANOVA. bP<0.05 vs NC. (B) The protein level of E2F1 is increased by knocking down Omi in both HEK293 and H1299 cells. (C) The protein level of E2F1 is decreased by transfecting HA-Omi, but not HA alone or HA-Omi S276C in N2a cells. N2a cells were transfected with plasmids encoding HA, HA-Omi, or HA-Omi S276C for 36 h. Whole-cell extracts were prepared and immunoblotted with the antibodies indicated. The bar graph on the right shows the band intensities of E2F1 relative to GAPDH. Densitometric analyses from three independent experiments were quantified by one-way ANOVA. bP<0.05. (D) The protein level of E2F1 is decreased by transfecting HA-Omi, but not HA alone or HA-Omi S276C in HEK293 cells. HEK293 cells were transfected with plasmids encoding HA, HA-Omi, or HA-Omi S276C for 36 h. Whole-cell extracts were prepared and immunoblotted with the antibodies indicated. HA: hemagglutinin.
E2F1 is a substrate of Omi
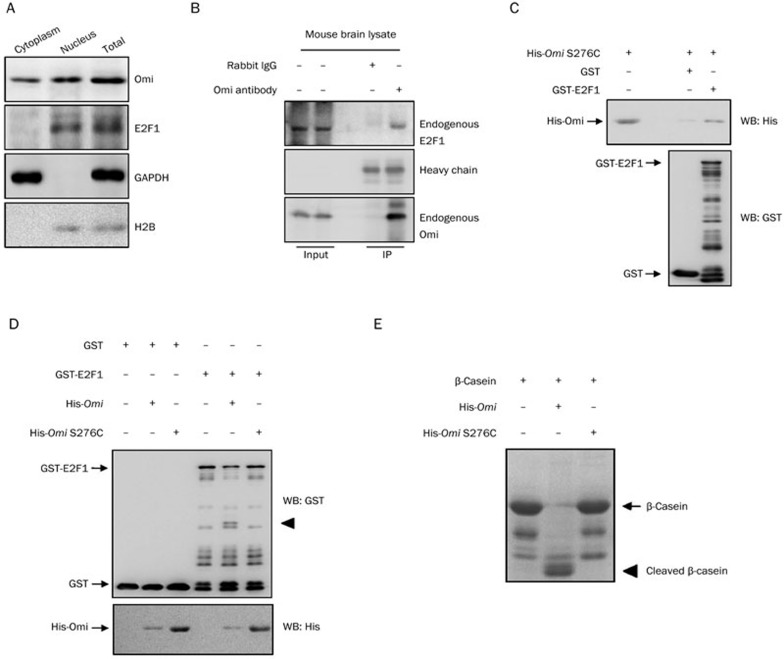
E2F1 is a transcription factor resident in the nucleus, and Omi mainly distributes in the mitochondria and partially in the nucleus1. We therefore performed a subcellular fractionation assay to further identify Omi's subcellular localization, confirming that Omi is both cytoplasmic and nuclear (Figure 3A). These data suggest that there may be a linkage between these two proteins. Because the increase in E2F1 abundance in both Omi-knockdown cells and mnd2 mouse brain is relevant to Omi protease activity and the mRNA level of E2F1 is not changed in mnd2 mice, we wondered whether E2F1 is a substrate of Omi. We therefore examined the interactions between Omi and E2F1. Using an Omi-specific antibody, endogenous E2F1 was co-immunoprecipitated with endogenous Omi from mnd2 mouse brain lysates (Figure 3B). Moreover, GST pulldown assays also showed that GST-E2F1 pulled His-Omi down, whereas GST alone did not (Figure 3C). These data suggest that there is a direct interaction between these two proteins. We next performed in vitro cleavage assays to identify whether Omi could cleave E2F1. In vitro cleavage assays showed that GST-E2F1 was cleaved by WT Omi but not by S276C protease activity-deficient Omi, whereas GST alone was not cleaved by Omi (Figure 3D). In addition, β-casein, a substrate of Omi, was cleaved by WT Omi but not by S276C Omi, demonstrating the protease activity of WT Omi and a loss of protease activity in S276C Omi (Figure 3E). These data indicate that E2F1 is a substrate of Omi.
Figure 3.
E2F1 is a substrate of Omi. (A) Subcellular distribution of Omi and E2F1. A subcellular fractionation assay was performed. The cytoplasmic and nuclear partitions of cell lysates or total cell lysates were separated for immunoblot analysis with the antibodies indicated. (B) Omi interacts with endogenous E2F1 in the brain homogenates of mice. Lysates from mnd2 mouse brain were immunoprecipitated with control IgG or antibodies against Omi. The precipitates were analyzed by immunoblot analysis using the antibodies indicated. (C) In vitro assays were performed to show that GST-E2F1, but not GST alone, interacted with His-Omi. (D) In vitro cleavage assays. In vitro purified GST, GST-E2F1 proteins were incubated with WT or protease-inactive S276C Omi, respectively, for 60 min in protease buffer at 37 °C. The incubated mixtures were subjected to immunoblot analysis with the antibodies indicated. The cleavage product is indicated using an arrow. (E) Omi cleaves the substrate β-casein in vitro. Purified His-Omi or His-Omi S276C was incubated with β-casein for 60 min in protease buffer at 37 °C. After incubation, samples were subjected to SDS-PAGE and visualized using Coomassie bright blue staining. IP: immunoprecipition, WB: Western blot.
Omi facilitates neurite outgrowth through E2F1
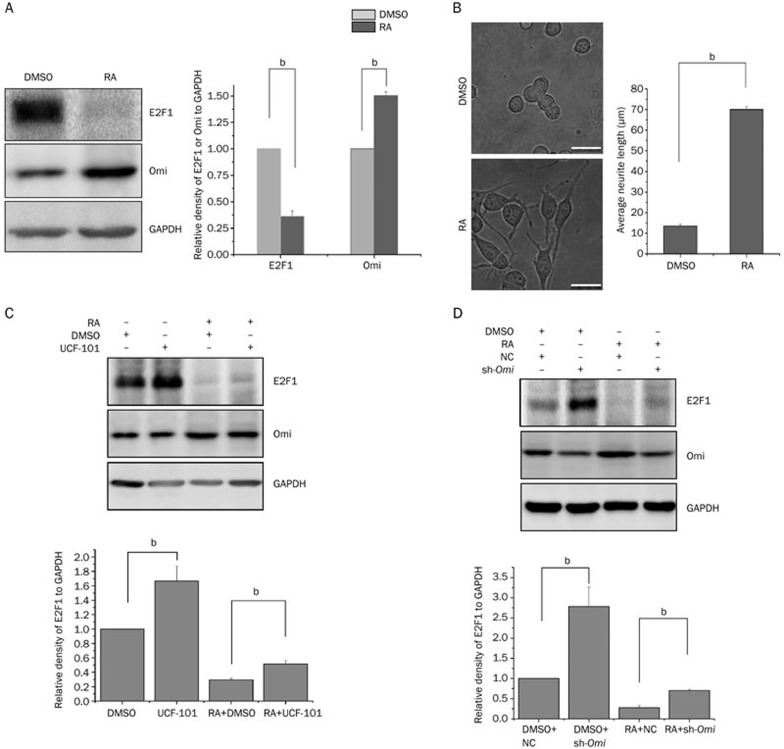
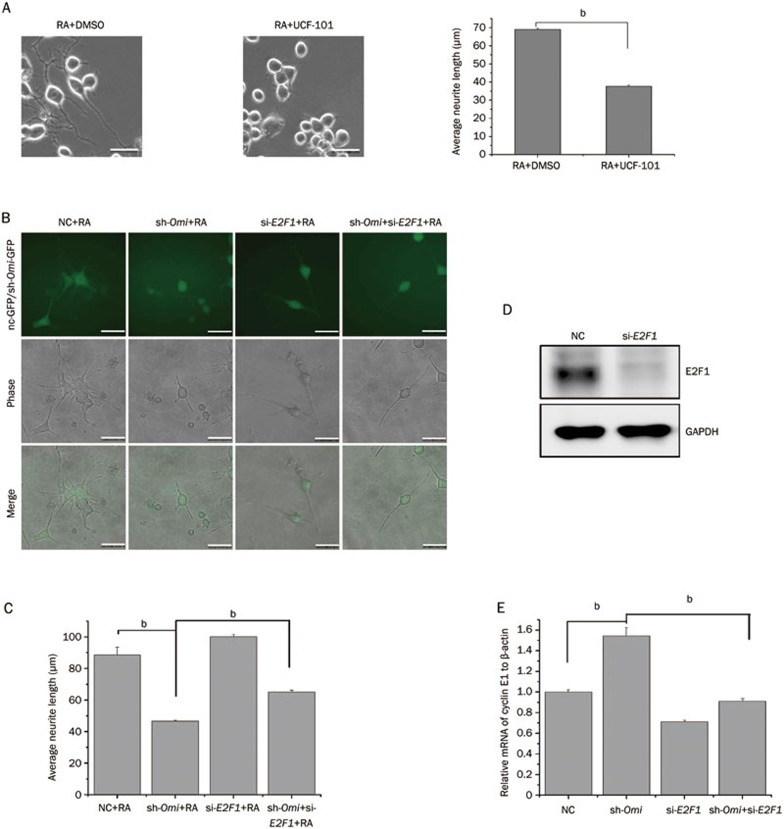
The expression of Omi is increased in NT2 cells after RA treatment and is upregulated in brain tissues with the development of mouse brain, suggesting that Omi may play an important role in neuronal differentiation and development13. We therefore performed a neurite outgrowth assay using N2a cells to address whether Omi regulates neurite outgrowth in association with E2F1 expression. The mouse neuroblastoma cell line (N2a cells) is a commonly used cell line whose differentiation and neurite outgrowth can be induced by RA30,31. In N2a cells, RA treatment in combination with a lower concentration of serum (1%) induced cell differentiation and neurite outgrowth, accompanied by increased Omi and decreased E2F1 levels (Figure 4A). In addition, the average neurite length was significantly increased by RA treatment (Figure 4B). Moreover, in RA-treated N2a cells, inhibition of Omi protease activity with UCF-101 increased E2F1 levels (Figure 4C). Similar results were obtained in cells with Omi knocked down by sh-Omi, demonstrating that the knockdown of Omi increased E2F1 levels (Figure 4D). E2F1 can control cell proliferation and differentiation. Overexpression of E2F1 significantly inhibits neuronal differentiation26. We have found that Omi is increased and E2F1 is decreased in differentiated N2a cells (Figure 4A). We further examined whether Omi activity and E2F1 levels are correlated in differentiated N2a cells to affect neurite outgrowth. Inhibition of Omi protease activity with UCF-101 dramatically decreased the average neurite length (Figure 5A). In addition, knocking down Omi with sh-Omi significantly inhibited neurite outgrowth in N2a cells, and knockdown of E2F1 in Omi knockdown cells partially restored neurite outgrowth (Figure 5B and 5C), suggesting the involvement of E2F1 in the altered neurite outgrowth observed in Omi knockdown cells. The efficiency of si-E2F1 was proved by detecting the protein level of E2F1 in N2a cells (Figure 5D). Finally, to confirm that Omi could affect E2F1 transcriptional activity, we performed qRT-PCR assays to detect the mRNA of E2F1-targeted gene cyclin E1. The mRNA of cyclin E1 was upregulated in sh-Omi transfected N2a cells, and this effect was reversed by knocking down E2F1 in sh-Omi transfected cells (Figure 5E), suggesting that knocking down Omi increases E2F1 activity.
Figure 4.
Omi facilitates neurite outgrowth through E2F1. (A) N2a cells were treated with DMSO or RA for 48 h. Whole-cell extracts were prepared and immunoblotted with the antibodies indicated. (B) A neurite outgrowth assay was performed, showing that RA significantly induced neurite outgrowth in N2a cells. The bar graph shows the average neurite length of each group measured by Image J software (bar, 20 μm; number of cells, approximately 200; bP<0.05, one-way ANOVA). (C) UCF-101 was added to N2a cells at a final concentration of 20 μmol/L for 30 min before DMSO or RA treatment for another 48 h. Whole-cell extracts were prepared and immunoblotted with the antibodies indicated. (D) N2a cells transfected with NC (negtive control) or sh-Omi plasmids were treated with DMSO or RA for 48 h. Whole-cell extracts were prepared and immunoblotted with the antibodies indicated. RA: retinoic acid.
Figure 5.
Omi activity and E2F1 level are correlated in regulation of neurite outgrowth. (A) A neurite outgrowth assay was performed showing that UCF-101, an Omi activity inhibitor, inhibits neurite outgrowth in N2a cells. UCF-101 was added to N2a cells at a final concentration of 20 μmol/L for 30 min before RA treatment for another 48 h. The bar graph shows the average neurite length of each group measured by Image J software (bar, 20 μm; number of cells, approximately 200; bP<0.05, one-way ANOVA). (B) A neurite outgrowth assay was performed, showing that knocking down Omi by sh-Omi containing a GFP sequence inhibited neurite outgrowth in N2a cells and knocking down E2F1 in sh-Omi-transfected cells restored neurite outgrowth. Scale bar, 20 μm; (C) The bar graph shows the average neurite length of each group measured by Image J software (number of GFP-positive cells, approximately 200; bP<0.05, one-way ANOVA). (D) The effect of si-E2F1 was proved by detecting the protein level of E2F1. N2a cells were transfected with a control siRNA (NC) or siRNA that targets E2F1 (si-E2F1) for 72 h. Whole-cell extracts were prepared and immunoblotted with the E2F1 antibody. (E) The mRNA of E2F1-targeted gene cyclin E1 was upregulated in sh-Omi transfected N2a cells, and this effect was reversed by knocking down E2F1. qRT-PCR assays were performed using primers specific for cyclin E1 and β-actin genes. n=3 per group. bP<0.05; one-way ANOVA.
Discussion
Lines of evidence suggest that some neurodegenerative diseases, such as Alzheimer's disease, multiple sclerosis and PD, are related to neural differentiation disorders32,33,34. The characteristics of PD are progressive degeneration of dopaminergic neurons in the substantia nigra of the midbrain and other brain regions, and visible intracellular inclusions, known as Lewy bodies35. A missense mutation, G2019S, of a PD-associated protein, LRRK2, leads to reduced neurite complexity in dopaminergic neurons36. Overexpression of another PD-related protein, α-synuclein, also causes reduced neurite length37,38. Omi is a protein associated with PD11,12. The expression of Omi in the brain increases with mouse age, indicating an association of Omi with neuronal development13. In this study, we demonstrated that Omi regulates neuronal differentiation. Knocking down Omi or inhibiting Omi protease activity by UCF-101 in N2a cells reduces neurite outgrowth. Furthermore, increasing levels of Omi, along with decreasing levels of E2F1 (an important regulator of cell differentiation), were observed in N2a cells after differentiation was induced with RA. Moreover, knocking down Omi or inhibiting its protease activity affects neurite outgrowth. Thus, our findings suggest that Omi regulates E2F1 levels to influence neurite outgrowth.
Previous reports have indicated that Omi does locate in the nuclei1. To date, extensive studies have revealed the mitochondrial and cytoplasmic substrates of Omi and shown the function of Omi in these subcellular sites4,39,40; however, the nuclear substrates and functions of Omi have not been fully explored. Omi regulates transcription by processing the transcription factor, p73, or the transcriptional regulator, Wilm's suppressor protein2,3, to regulate apoptosis. In this study, we identified the transcription factor, E2F1, as a novel substrate of Omi. E2F1 plays a vital role in cell proliferation and differentiation. E2F1 contributes to cell proliferation by facilitating the passage of cells through the G1/S checkpoint41,42. As withdrawing from the cell cycle is an important step before cell differentiation and E2F1 functions in cell proliferation, the regulation of E2F1 protein levels or transcriptional activity are critical for cell differentiation. In the nervous system, E2F1 is involved in neuronal differentiation. E2F1 significantly decreases in differentiated neuronal cells25,43,44. Thus, the factors that affect E2F1 functions are able to influence neuronal differentiation. In mouse neuroblastoma cells, overexpression of necdin, a factor that interacts with E2F1 and represses E2F1 activity, leads to neuronal differentiation45. In vivo mouse models also suggest that E2F1 is critical for central nervous system development46. Those studies suggest an involvement of E2F1 in neuronal differentiation. E2F1 can be degraded through the ubiquitin-proteasome system by its E3 ligase SCFskp2 and cleaved by calpain47,48. In our study, we found that Omi interacts with and cleaves E2F1 to regulate E2F1 levels. After RA induction, an increase of Omi in N2a cells results in a decrease of E2F1, leading to neurite outgrowth in N2a cells.
In summary, our findings reveal the role of Omi in N2a cell differentiation. E2F1 is a novel substrate of Omi that is regulated by Omi in N2a cell differentiation.
Author contribution
Qi MA and Guang-hui WANG designed the experiments; Qi MA performed most of the experiments; Qing-song HU and Ran-jie XU performed parts of the experiments; Qi MA, Xue-chu ZHEN and Guang-hui WANG analyzed the data; Qi MA and Guang-hui WANG wrote the manuscript.
Acknowledgments
This work was supported, in part, by the National Natural Sciences Foundation of China (No 31330030 and 81371393), the National High-Tech Research and Development Program of China 973-projects (2011CB504102), the Natural Science Foundation of Jiangsu Province (BK2012181) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Gray CW, Ward RV, Karran E, Turconi S, Rowles A, Viglienghi D, et al. Characterization of human HtrA2, a novel serine protease involved in the mammalian cellular stress response. Eur J Biochem. 2000;267:5699–710. doi: 10.1046/j.1432-1327.2000.01589.x. [DOI] [PubMed] [Google Scholar]
- Hartkamp J, Carpenter B, Roberts SG. The Wilms' tumor suppressor protein WT1 is processed by the serine protease HtrA2/Omi. Mol Cell. 2010;37:159–71. doi: 10.1016/j.molcel.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabese M, Mazzoletti M, Vikhanskaya F, Broggini M. HtrA2 enhances the apoptotic functions of p73 on bax. Cell Death Differ. 2008;15:849–58. doi: 10.1038/cdd.2008.7. [DOI] [PubMed] [Google Scholar]
- Yang QH, Church-Hajduk R, Ren J, Newton ML, Du C. Omi/HtrA2 catalytic cleavage of inhibitor of apoptosis (IAP) irreversibly inactivates IAPs and facilitates caspase activity in apoptosis. Genes Dev. 2003;17:1487–96. doi: 10.1101/gad.1097903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blink E, Maianski NA, Alnemri ES, Zervos AS, Roos D, Kuijpers TW. Intramitochondrial serine protease activity of Omi/HtrA2 is required for caspase-independent cell death of human neutrophils. Cell Death Differ. 2004;11:937–9. doi: 10.1038/sj.cdd.4401409. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Takahashi-Niki K, Akagi T, Hashikawa T, Takahashi R. Mitochondrial protease Omi/HtrA2 enhances caspase activation through multiple pathways. Cell Death Differ. 2004;11:208–16. doi: 10.1038/sj.cdd.4401343. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell. 2001;8:613–21. doi: 10.1016/s1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- Liu MJ, Liu ML, Shen YF, Kim JM, Lee BH, Lee YS, et al. Transgenic mice with neuron-specific overexpression of HtrA2/Omi suggest a neuroprotective role for HtrA2/Omi. Biochem Biophys Res Commun. 2007;362:295–300. doi: 10.1016/j.bbrc.2007.07.118. [DOI] [PubMed] [Google Scholar]
- Jones JM, Datta P, Srinivasula SM, Ji W, Gupta S, Zhang Z, et al. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature. 2003;425:721–7. doi: 10.1038/nature02052. [DOI] [PubMed] [Google Scholar]
- Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, et al. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol. 2004;24:9848–62. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, et al. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson's disease. Hum Mol Genet. 2005;14:2099–111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- Bogaerts V, Nuytemans K, Reumers J, Pals P, Engelborghs S, Pickut B, et al. Genetic variability in the mitochondrial serine protease HTRA2 contributes to risk for Parkinson disease. Hum Mutat. 2008;29:832–40. doi: 10.1002/humu.20713. [DOI] [PubMed] [Google Scholar]
- Liu ML, Liu MJ, Shen YF, Ryu H, Kim HJ, Klupsch K, et al. Omi is a mammalian heat-shock protein that selectively binds and detoxifies oligomeric amyloid-beta. J Cell Sci. 2009;122:1917–26. doi: 10.1242/jcs.042226. [DOI] [PubMed] [Google Scholar]
- Martin K, Trouche D, Hagemeier C, Kouzarides T. Regulation of transcription by E2F1/DP1. J Cell Sci Suppl. 1995;19:91–4. doi: 10.1242/jcs.1995.supplement_19.13. [DOI] [PubMed] [Google Scholar]
- Farnham PJ, Slansky JE, Kollmar R. The role of E2F in the mammalian cell cycle. Biochim Biophys Acta. 1993;1155:125–31. doi: 10.1016/0304-419x(93)90001-s. [DOI] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–62. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 2000;14:2393–409. doi: 10.1101/gad.813200. [DOI] [PubMed] [Google Scholar]
- Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2:910–7. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- Field SJ, Tsai FY, Kuo F, Zubiaga AM, Kaelin WG, Livingston DM, et al. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–61. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- Kowalik TF, DeGregori J, Schwarz JK, Nevins JR. E2F1 overexpression in quiescent fibroblasts leads to induction of cellular DNA synthesis and apoptosis. J Virol. 1995;69:2491–500. doi: 10.1128/jvi.69.4.2491-2500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Matsumura I, Ezoe S, Satoh Y, Sakamaki T, Albanese C, et al. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Mol Cell. 2002;9:1017–29. doi: 10.1016/s1097-2765(02)00522-1. [DOI] [PubMed] [Google Scholar]
- Stender JD, Frasor J, Komm B, Chang KC, Kraus WL, Katzenellenbogen BS. Estrogen-regulated gene networks in human breast cancer cells: involvement of E2F1 in the regulation of cell proliferation. Mol Endocrinol. 2007;21:2112–23. doi: 10.1210/me.2006-0474. [DOI] [PubMed] [Google Scholar]
- Zheng C, Ren Z, Wang H, Zhang W, Kalvakolanu DV, Tian Z, et al. E2F1 Induces tumor cell survival via nuclear factor-kappaB-dependent induction of EGR1 transcription in prostate cancer cells. Cancer Res. 2009;69:2324–31. doi: 10.1158/0008-5472.CAN-08-4113. [DOI] [PubMed] [Google Scholar]
- Wang J, Helin K, Jin P, Nadal-Ginard B. Inhibition of in vitro myogenic differentiation by cellular transcription factor E2F1. Cell Growth Differ. 1995;6:1299–306. [PubMed] [Google Scholar]
- Zhou F, Zhang L, Wang A, Song B, Gong K, Zhang L, et al. The association of GSK3 beta with E2F1 facilitates nerve growth factor-induced neural cell differentiation. J Biol Chem. 2008;283:14506–15. doi: 10.1074/jbc.M706136200. [DOI] [PubMed] [Google Scholar]
- Li B, Hu Q, Wang H, Man N, Ren H, Wen L, et al. Omi/HtrA2 is a positive regulator of autophagy that facilitates the degradation of mutant proteins involved in neurodegenerative diseases. Cell Death Differ. 2010;17:1773–84. doi: 10.1038/cdd.2010.55. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Tomoda T, Chang J, Takaki M, Zhan C, Morita M, et al. DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum Mol Genet. 2006;15:3313–23. doi: 10.1093/hmg/ddl407. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Breunig JJ, Depboylu C, Rouaux C, Michel PP, Alvarez-Fischer D, et al. The pRb/E2F cell-cycle pathway mediates cell death in Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104:3585–90. doi: 10.1073/pnas.0611671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PY, Lin YC, Chang CL, Lu HT, Chin CH, Hsu TT, et al. Functional decreases in P2X7 receptors are associated with retinoic acid-induced neuronal differentiation of Neuro-2a neuroblastoma cells. Cell Signal. 2009;21:881–91. doi: 10.1016/j.cellsig.2009.01.036. [DOI] [PubMed] [Google Scholar]
- Ma X, Fei E, Fu C, Ren H, Wang G. Dysbindin-1, a schizophrenia-related protein, facilitates neurite outgrowth by promoting the transcriptional activity of p53. Mol Psychiatry. 2011;16:1105–16. doi: 10.1038/mp.2011.43. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206:165–71. doi: 10.1016/s0022-510x(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Gai WP, Blessing WW, Blumbergs PC. Ubiquitin-positive degenerating neurites in the brainstem in Parkinson's disease. Brain. 1995;118 (Pt 6):1447–59. doi: 10.1093/brain/118.6.1447. [DOI] [PubMed] [Google Scholar]
- Li B, Yamamori H, Tatebayashi Y, Shafit-Zagardo B, Tanimukai H, Chen S, et al. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol. 2008;67:78–84. doi: 10.1097/nen.0b013e318160c5db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–66. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Ramonet D, Daher JP, Lin BM, Stafa K, Kim J, Banerjee R, et al. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One. 2011;6:e18568. doi: 10.1371/journal.pone.0018568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Hashimoto M, Hsu LJ, Mackowski B, Rockenstein E, Mallory M, et al. Reduced neuritic outgrowth and cell adhesion in neuronal cells transfected with human alpha-synuclein. Mol Cell Neurosci. 2001;17:141–50. doi: 10.1006/mcne.2000.0923. [DOI] [PubMed] [Google Scholar]
- Winner B, Regensburger M, Schreglmann S, Boyer L, Prots I, Rockenstein E, et al. Role of alpha-synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J Neurosci. 2012;32:16906–16. doi: 10.1523/JNEUROSCI.2723-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DW, Nam MK, Rhim H. The serine protease HtrA2 cleaves UCH-L1 and inhibits its hydrolase activity: implication in the UCH-L1-mediated cell death. Biochem Biophys Res Commun. 2011;415:24–9. doi: 10.1016/j.bbrc.2011.09.148. [DOI] [PubMed] [Google Scholar]
- Kieper N, Holmstrom KM, Ciceri D, Fiesel FC, Wolburg H, Ziviani E, et al. Modulation of mitochondrial function and morphology by interaction of Omi/HtrA2 with the mitochondrial fusion factor OPA1. Exp Cell Res. 2010;316:1213–24. doi: 10.1016/j.yexcr.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshita S, Terada Y, Nakashima O, Kuwahara M, Sasaki S, Marumo F. Regulation of the G1/S transition phase in mesangial cells by E2F1. Kidney Int. 1999;56:1238–41. doi: 10.1046/j.1523-1755.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- DeGregori J, Kowalik T, Nevins JR. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–24. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma-Hara M, Taniura H, Uetsuki T, Niinobe M, Yoshikawa K. Regulation and deregulation of E2F1 in postmitotic neurons differentiated from embryonal carcinoma P19 cells. Exp Cell Res. 1999;251:442–51. doi: 10.1006/excr.1999.4593. [DOI] [PubMed] [Google Scholar]
- Fajas L, Landsberg RL, Huss-Garcia Y, Sardet C, Lees JA, Auwerx J. E2Fs regulate adipocyte differentiation. Dev Cell. 2002;3:39–49. doi: 10.1016/s1534-5807(02)00190-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Taniura H, Yoshikawa K. Ectopic expression of necdin induces differentiation of mouse neuroblastoma cells. J Biol Chem. 2002;277:42128–35. doi: 10.1074/jbc.M205024200. [DOI] [PubMed] [Google Scholar]
- Tsume M, Kimura-Yoshida C, Mochida K, Shibukawa Y, Amazaki S, Wada Y, et al. Brd2 is required for cell cycle exit and neuronal differentiation through the E2F1 pathway in mouse neuroepithelial cells. Biochem Biophys Res Commun. 2012;425:762–8. doi: 10.1016/j.bbrc.2012.07.149. [DOI] [PubMed] [Google Scholar]
- Harper JW, Elledge SJ. Skipping into the E2F1-destruction pathway. Nat Cell Biol. 1999;1:E5–7. doi: 10.1038/8952. [DOI] [PubMed] [Google Scholar]
- Zyskind JW, Wang Y, Cho G, Ting JH, Kolson DL, Lynch DR, et al. E2F1 in neurons is cleaved by calpain in an NMDA receptor-dependent manner in a model of HIV-induced neurotoxicity. J Neurochem. 2015;132:742–55. doi: 10.1111/jnc.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]