Abstract
Despite advances in surgical technique and clinical care, lung transplantation still remains a short-term solution for the treatment of end-stage lung disease. To date, there has been limited experience in experimental lung transplantation using nonhuman primate models. Therefore, we have endeavored to develop a long-term, nonhuman primate model of orthotopic lung transplantation for the ultimate purpose of designing protocols to induce tolerance of lung grafts. Here, we report our initial results in developing this model and our observation that the nonhuman primate lung is particularly prone to rejection. This propensity toward rejection may be a consequence of 1) upregulated nonspecific inflammation, and 2) a larger number of pre-existing alloreactive memory T cells, leading to augmented deleterious immune responses. Our data show that triple-drug immunosuppression mimicking clinical practice is not sufficient to prevent acute rejection in nonhuman primate lung transplantation. The addition of horse-derived anti-thymocyte globulin and a monoclonal antibody to the IL-6 receptor allowed six out of six lung recipients to be free of rejection for over 120 days.
Introduction
Advances in immunosuppressive management, antibiotic therapy, and surgical technique have made lung transplantation a viable therapeutic option for many patients with end-stage lung diseases such as emphysema, cystic fibrosis, pulmonary fibrosis, and pulmonary hypertension. Although one-year survival rates for lung transplant recipients now exceed 90%, the long-term success of lung transplantation has been limited by chronic rejection, infection, posttransplant malignancy, and drug toxicity, which affect all solid organ recipients to varying degrees (1). It is unlikely that further incremental advances in conventional immunosuppression will significantly impact long-term graft survival. It is therefore important to look toward new paradigms in the management of the transplant patient.
To this end, we have directed our efforts toward creating a high-fidelity, large-animal model of lung transplantation using nonhuman primates (NHPs). Common experience in the laboratory has shown that techniques which are successful in murine species often do not translate to models higher on the phylogenetic tree. Likewise, strategies beneficial to one organ may yield results which are quite dissimilar in another organ. Thus, we feel that clinical progress in the mitigation of rejection and/or the induction of transplant tolerance must be investigated in models that mimic the clinical situation as closely as possible.
In this manuscript, we describe our initial efforts to develop a long-term NHP model of orthotopic lung transplantation that will be useful as a platform for further experimentation in tolerance induction.
Materials and Methods
Animals
Cynomolgus monkeys (Macaca fascicularis) that weighed 4 to 7 kg were used (Charles River Primates, Wilmington, MA). Recipient and donor pairs were selected for compatible ABO blood types and fully mismatched cynomolgus leukocyte (CyLA)-MHC antigens. Genomic DNA was prepared from peripheral blood mononuclear cells and splenocytes. Panels of seventeen microsatellite loci spanning ~5Mb of the MHC region were amplified from the genomic DNA with fluorescent-labeled PCR primers and fragment size analysis was determined. The microsatellite haplotypes for each animal were converted to predicted MHC genotypes based on previous cloning and sequencing work with cynomolgus monkeys. All surgical procedures and postoperative care of animals were performed in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Lung allotransplantation technique
Under general endotracheal anesthesia (isoflurane), the recipient’s chest was entered through a left thoracotomy, and the hilar structures of the native lung were isolated. After heparinization (300 U/kg IV), the native lung was removed. The donor lung was approached through a median sternotomy. After heparinization (300 U/kg) and cannulation of the main pulmonary artery, the heart–lung block was topically cooled with iced-saline and flushed in situ with ~250–500 mL cold Perfadex® solution (XVIVO Perfusion; Englewood, CO), venting through the left atrial appendage. Immediately after harvesting the heart-lung block, the left lung was isolated and surgically prepared for transplantation. The bronchial anastomosis was performed using interrupted 5–0 polyglactin sutures. The left atrial cuff anastomosis was performed with running 6–0 polypropylene sutures. The pulmonary artery anastomosis was performed with running 6–0 or 7–0 polypropylene sutures depending on vessel size. After ventilation and reperfusion of the transplanted lung, the chest was closed over a thoracostomy tube, which was removed once the animal was breathing spontaneously under anesthesia. Recipients and donors received a single-dose of cefazolin (500mg IV) prior to incision, and ketorolac (3mg qd IM), as an anti-platelet agent for the first five postoperative days. Postoperative discomfort was managed with buprenorphine (0.01 mg/kg IM/IV q10–14 h) on a scheduled basis for the first three postoperative days and PRN thereafter.
Lung autotransplantation technique
Under general endotracheal anesthesia (isoflurane), the chest was entered through a left thoracotomy, and the hilar structures of the left lung were exposed sufficiently to allow for vascular clamping. After heparinization (300 U/kg IV), the pulmonary artery and left atrium were clamped, and the lung was removed. The lung was was topically cooled with iced-saline and flushed ex vivo with ~250–500mL cold Perfadex® solution (XVIVO Perfusion; Englewood, Colorado), which drained passively through the atrial cuff. The lung was then re-implanted using interrupted 5–0 polyglactin sutures for the bronchial anastomosis. The left atrial cuff anastomosis was performed with running 6–0 polypropylene sutures. The pulmonary artery anastomosis was performed with running 6–0 or 7–0 polypropylene sutures depending on vessel size. After ventilation and reperfusion of the implanted lung, the chest was closed over a thoracostomy tube, which was removed once the animal was breathing spontaneously under anesthesia. Recipients and donors received a single-dose of cefazolin (500mg IV) prior to incision, and ketorolac (3mg qd IM), as an anti-platelet agent for the first five postoperative days. Postoperative discomfort was managed with buprenorphine (0.01 mg/kg IM/IV q10–14 h) on a scheduled basis for the first three postoperative days and PRN thereafter.
Immunosuppression
Three different immunosuppression regimens were used for the experimental transplants in this study: 1) conventional immunosuppression, 2) conventional immunosuppression with induction therapy, and 3) conventional immunosuppression with induction therapy and anti-IL6R mAb. Conventional immunosuppression consisted of mycophenolate mofitil (200mg PO qD admixed with the monkey’s daily food allotment and provided for consumption ad libitum), tacrolimus (0.1 mg/kg IM bid on post-operative day (POD) 0 and POD 1, then adjusted to achieve target plasma level of 20–30 ng/mL), methylprednisone (40mg IV on POD 0, 40mg IM on POD 1, then tapering over 14 days to 1mg IM qd through the end of the experiment). Tacrolimus levels averaged 23.1 ng/mL among all recipients, and there was no statistically significant difference among the three transplanted groups. Induction therapy consisted of either rabbit anti-thymocyte globulin (rATG) (50 mg/kg IV qd × 3 days) or horse anti-thymocyte globulin (hATG) (50mg/kg IV qd × 3 days) administered on POD -2, POD -1, and POD 0. Anti-IL6R mAb (tocilizumab; Chugai Pharmaceutical Co., Ltd.; Tokyo, Japan) was dosed at 10 mg/kg IV qd on PODs 0, 7, 14, and 21. In addition, 5 monkeys were used in a nontransplant study to assess the effect of hATG and anti-IL6R mAb, alone and in combination, on the level of circulating regulatory T cells. hATG and anti-IL6R mAb were administered in the doses and schedules listed above.
Detection of donor-reactive antibodies
The detection of donor-specific alloantibodies was performed using flow cytofluorometry, as previously described (2). All of our recipients are tested prior to transplantation to ensure that there is no pre-existing alloreactive antibody.
Posttransplant monitoring
All transplanted lung grafts are monitored weekly with chest radiography, beginning with a radiograph immediately after chest closure. Protocol open lung biopsies performed through a limited thoracotomy are scheduled on PODs 14, 28, 50, and 100. Any radiographic signs of graft consolidation are investigated immediately with an additional biopsy.
Histological analyses
Lung biopsies and necropsy specimens were fixed in formalin and stained with hematoxylin and eosin, Masson’s trichrome, and Verhoeff’s stains. Examination of the tissue was performed microscopically by transplant pathologists blinded to the protocol (R.N.S., R.B.C). Acute cellular rejection (ACR) was scored using the grading system promulgated by the International Society of Heart and Lung Transplantation (ISHLT), ranging from 0 (no rejection) to 4 (high-grade rejection) (3).
Results
Lung autotransplantation
As a necessary control for a new model, one monkey (#M4809) underwent a left lung orthotopic autotransplant without any immunosuppression. Chest radiographs and biopsies on POD 7 and POD 35 showed no evidence of rejection (Figure 1).
Figure 1.
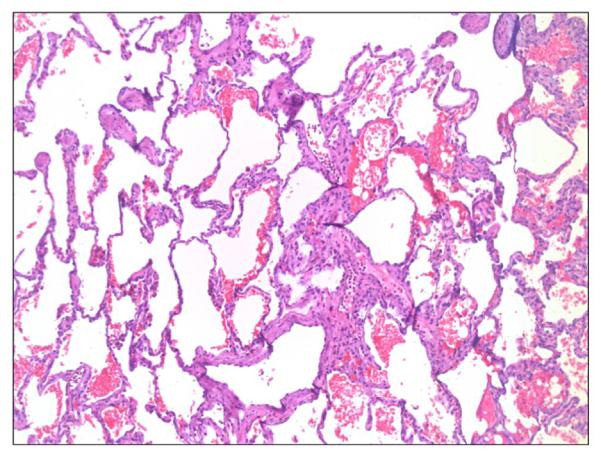
Lung histology showing no evidence for rejection in an autotransplanted lung (#M4809, Day 35, H&E 50X).
Transplantation of an MHC-mismatched lung without immunosuppression
As a necessary control for a new model, one monkey (#M3805) underwent left lung orthotopic transplantation without any immunosuppression. This animal was euthanized on POD 5 with lethargy, cough, and abnormal chest radiograph. As expected, the pathologic examination of this untreated fully MHC-disparate lung at necropsy showed acute rejection (Figure 2). No alloantibody was detected in the serum of this short-lived recipient.
Figure 2.
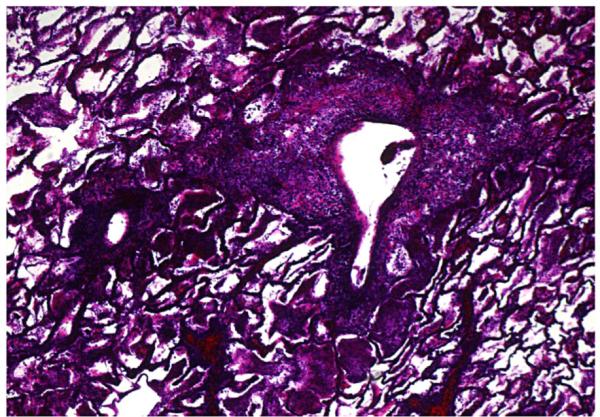
Lung histology showing high-grade rejection of an MHC-mismatched lung in a nonimmunosuppressed recipient (#M3805, Day 5, Verhoeff’s stain 50X).
Transplantation of MHC-mismatched lung allografts with conventional immunosuppression (Group 1)
Five monkeys underwent orthotopic left lung transplants using fully MHC-disparate allografts followed by conventional triple-drug immunosuppression, as described in Methods. One recipient was lost on POD 17 secondary to an anesthetic complication and did not complete the protocol. It has been excluded from analysis. The remaining four monkeys all rejected their lung allografts (while on immunosuppression) on PODs 21, 47, 61, and 97 (Table 1). ISHLT ACR scores at the time of graft loss ranged from 2 to 4. In all of the animals that rejected lung grafts, alloantibody was identified in the serum at the time of rejection (Table 1). Alloantibody was also present in the rejected graft at the time of rejection, but not prior to the development of obvious cellular rejection.
Table 1.
Summary of results of NHP lung transplantation
| Treatment | Animal Number | Graft Survival (d) | Clinical Outcome | Final Pathology | Alloantibody |
|---|---|---|---|---|---|
| Conventional | M5707 | 21 | Rejection | ACR 4 | + |
| M6207 | 97 | Rejection | ACR 2 | + | |
| M5907 | 61 | Rejection | ACR 2 | + | |
| M7607 | 47 | Rejection | ACR 4 | + | |
| Conventional + ATG | M2908 | 34 | Rejection | ACR 3–4 | + |
| M912 | >120 | Healthy Graft | ACR 0 | − | |
| M4012 | >120 | Healthy Graft | ACR 0 | n/a | |
| Conventional + ATG + anti-IL6R | M4209 | >120 | Healthy Graft | ACR 0–1 | − |
| M5110 | >120 | Healthy Graft | ACR 0 | − | |
| M5410 | >120 | Healthy Graft | ACR 0 | − | |
| M2411 | >120 | Healthy Graft | ACR 0–1 | − | |
| M4711 | >120 | Healthy Graft | ACR 0 | − | |
| M4612 | >120 | Healthy Graft | ACR 0 | n/a |
Transplantation of MHC-mismatched lung allografts with conventional immunosuppression and induction therapy (Group 2)
Four monkeys underwent orthotopic left lung transplants using fully MHC-disparate allografts followed by conventional triple-drug immunosuppression supplemented by induction therapy with either rATG or hATG, as described in Methods. One recipient was lost due to graft infarction on POD 5, and has been excluded from analysis. Of the remaining three monkeys, two recipients receiving hATG maintained healthy grafts with no evidence for rejection (Figure 3) for over 120 days; at which time, they were transferred to other protocols. One of the recipients receiving rATG rejected its graft (while on immunosuppression) on POD34 with moderate to high-grade rejection seen on histology and with alloantibody detected in its serum (Table 1).
Figure 3.
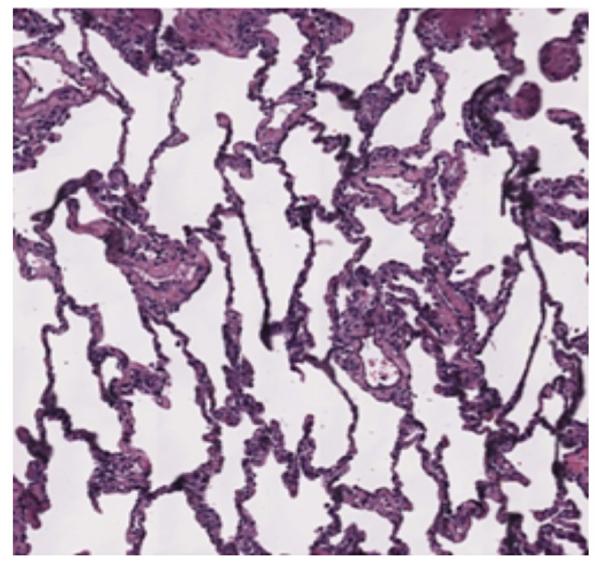
Lung histology showing healthy graft (#M4012 [Group 2], Day 96, H&E 50X).
Transplantation of MHC-mismatched lung allografts with conventional immunosuppression, induction therapy, and anti-IL6R mAb (Group 3)
Seven monkeys underwent orthotopic left lung transplants using fully MHC-disparate allografts followed by conventional triple-drug immunosuppression, supplemented by induction therapy with hATG and anti-IL-6R mAb treatment, as described in Methods. One graft was lost early in the postoperative course due to due infarction, and has been excluded from analysis. All of the remaining six monkeys maintained healthy grafts with no evidence for rejection for over 120 days; at which time, they were transferred to other protocols. No alloantibody was detected in any of these recipients and only two of the grafts showed slight, focal mononuclear cell infiltration (Table 1, Figure 4).
Figure 4.
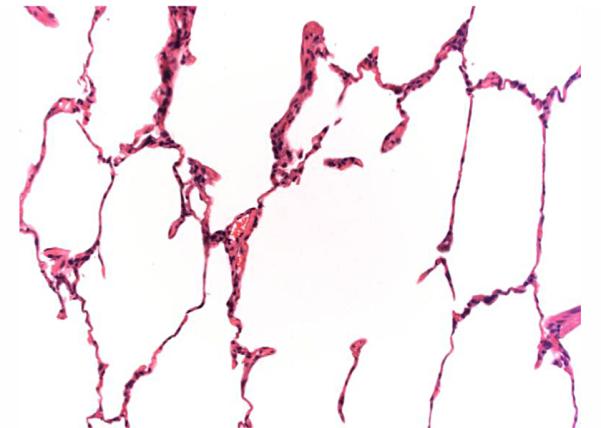
Lung histology showing healthy graft (#M4209 [Group 3], Day 97, H&E 50X).
Impact of hATG and anti-IL6R mAb, alone and in combination, on circulating regulatory T cells
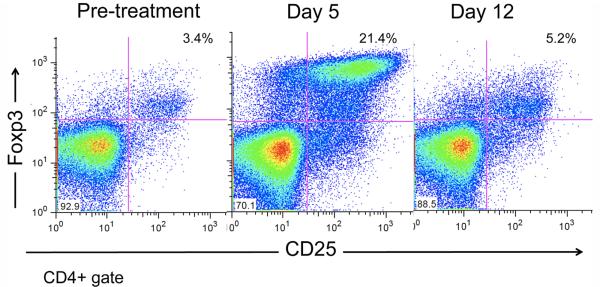
When either hATG (50 mg/kg qd × 3 days) or anti-IL-6RmAb (10 mg/kg q week × 4 week) was administered to a monkey (n=1, each), no significant effect was observed on the numbers of circulating regulatory T cells. However, when both of these reagents were administered concomitantly (n=3), a significant transient increase in circulating T cells was observed (Figures 5 and 6).
Figure 5.
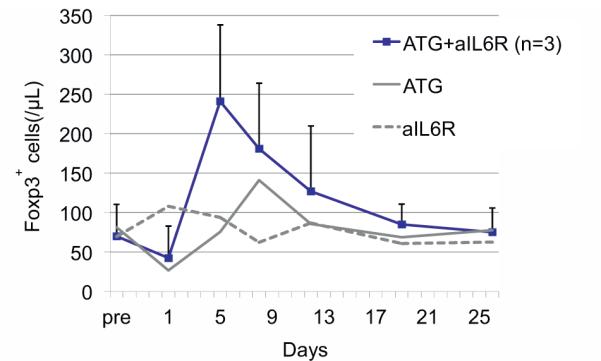
hATG+anti-IL6RmAb (n=3) have asynergistic effect on the expansion of circulating regulatory T cells, compared to either hATG (n=1) or anti-IL6R mAb (n=1) alone.
Figure 6.
Transient increase in CD25+Foxp3+ T lymphocytes after treatment with hATG and anti-ILSR mAb (#M1407).
Discussion
We report here the development of a long-term model of NHP orthotopic transplantation, as a prelude to the development of tolerance induction protocols for clinical lung transplantation. Our initial attempts to transplant lungs using a standard triple-drug regimen (Group I) led to universal graft rejection on immunosuppression. Given that similar immunosuppressive regimens are in successful use in models of kidney and heart transplantation (4,5), we do not believe that lung graft rejection can be attributed to species-specific differences in the immunosuppressive effects of these drugs or drug dosing. However, our data certainly point to an organ-specific difference in the propensity of the NHP lungs to reject, as compared to NHP hearts, NHP kidneys, and human lung allografts. This observation is supported by data from previous short-term studies of NHP lung transplantation, in which rejection also occurred in animals that were immunosuppressed (6,7).
We therefore hypothesize that the nonhuman primate immune system may be more robust than the human counterpart, and that barrier organs in particular may exist in a higher constitutive state of immune activation, making freedom from rejection, and ultimately tolerance, more difficult to achieve. This may be in part due to a relatively high prevalence of alloreactive memory T cells that we have observed in our monkeys, presumably due to past environmental exposures and to the many vaccinations that research NHPs receive (i.e., heterologous immunity) (8,9).
Based on the time course and histology observed on biopsy specimens, graft rejection appears to be driven by an acute cellular immune response, followed by the induction of alloantibody, presumably by a T cell–dependent mechanism. We have not observed grafts in which a cellular immune response has been minimal or absent, which then progress to rejection chronically through an antibody-mediated mechanism, as is common in the clinic.
By adding ATG induction therapy (Group II), we were able to partially address the issue of rejection, with two out of three grafts remaining healthy for over 120 days. We suspect that the benefit derived from ATG is due to both its general anti–T cell effect, and its ability to deplete or modulate alloreactive memory T cells. ATG has also been shown to facilitate the generation of regulatory T cells (10,11), and this effect is reported to be enhanced by concomitant tacrolimus administration (12). Interestingly, the two healthy grafts were induced with hATG, whereas the rejected graft was induced with rATG. Additional studies would be needed to determine whether there is a true difference between these two ATGs, and whether hATG in combination with triple-drug immunosuppression would be sufficient to prevent rejection reliably.
However, based in part on the in vivo data presented here (Figures 5 and 6) showing that the combined administration of hATG and anti-IL-6R resulted in a measurable increase in peripheral regulatory T cells, we elected to add an anti-IL-6R mAb to the above hATG regimen. This humanized mAb inhibits IL-6 binding to membrane-bound IL-6R (CD126) and to its soluble form (sIL-6R) (13). Its potential beneficial effects on the graft are multiple. First, IL-6 is known to be a potent pro-inflammatory cytokine and general activator of innate immunity (14–19). As such, it is able to reinforce an antigen-specific immune response. Second, blockade of the IL-6 pathway has been reported to inhibit the activation and survival of alloreactive CD4+ memory T cells (20,21). Third, IL-6 blockade has been shown to prevent the differentiation of CD4+ T cells into a TH17 phenotype, while at the same time enhancing the activation/expansion of regulatory T cells (22,23). This is consistent with the data presented here showing that the combination of IL-6 blockade and ATG therapy leads to a transient increase in peripheral regulatory T cells.
The addition of the anti-IL6-R mAb allowed the reliable transplantation of MHC-mismatched lungs, with six out of six transplants surviving with healthy grafts for over 120 days (Group III). Again, our data do not conclude whether the anti-IL-6RmAb is necessary in addition to hATG to allow reliable transplantation. However, if rATG and hATG are considered collectively, the regimen which includes the anti-IL-6R mAb is clearly superior.
In the course of developing this long-term NHP model of orthotopic lung transplantation, we have observed that the cynomolgus monkey lung is particularly prone to rejection, making this model a rigorous platform for pre-clinical investigation. Nonetheless, this NHP lung transplant model has far greater fidelity than any current murine model or heterotopic tissue model, and represents an incremental improvement over our own orthotopic inbred swine model, which differs substantially from human beings, particularly in terms of the ease with which tolerance can be induced. We also have generated limited evidence to show that that triple-drug therapy, along with hATG induction and IL-6R blockade, prevents graft rejection. While not initially foreseen or intended, the stringency of our NHP model will make it likely that any tolerance induction protocol successful in the monkey, will be easily translated into clinical practice.
Acknowledgments
This work was supported by NIH grants P01HL67110, U19AI066705, P01HL018646, the American Society of Transplant Surgeons-Wyeth Collaborative Scientist Award (J.S.A), and a Scholarship to Study Abroad from the Yoshida Scholarship Foundation (A.A.).
Abbreviations
- ACR
acute cellular rejection
- hATG
horse-derived anti-thymocyte globulin
- ISHLT
International Society for Heart and Lung Transplantation
- NHP
nonhuman primate
- POD
postoperative day
- rATG
rabbit-derived anti-thymocyte globulin
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth adult lung and heart-lung transplant report–2013; focus theme: Age. J Heart Lung Transplant. 2013;32:965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Boskovic S, Kawai T, Smith RN, et al. Monitoring antidonor alloantibodies as a predictive assay for renal allograft tolerance/long-term observations in nonhuman primates. Transplantation. 2006;82:819–825. doi: 10.1097/01.tp.0000234786.26511.a4. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Yamada Y, Boskovic S, Aoyama A, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012;12:330–340. doi: 10.1111/j.1600-6143.2011.03795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Yamada Y, Boskovic S, Atif M, Tonsho M, Nadazdin O, et al. Renal allograft tolerance can be achieved in non-human primates via delayed mixed-hematopoietic chimerism and alefacept treatment. Abstract , American Transplant Congress, Seattle, 2013, published in Am J Transplant. 2013;13:149. [Google Scholar]
- 6.Hausen B, Gummert J, Berry GJ, et al. Prevention of acute allograft rejection in nonhuman primate lung transplant recipients: Induction with chimeric anti-interleukin-2 receptor monoclonal antibody improves the tolerability and potentiates the immunosuppressive activity of a regimen using low doses of both microemulsion cyclosporine and 40-O-(2-hydroxyethyl)-rapamycin. Transplantation. 2000;69:488–496. doi: 10.1097/00007890-200002270-00005. [DOI] [PubMed] [Google Scholar]
- 7.Hausen B, Ikonen T, Briffa N, et al. Combined immunosuppression with cyclosporine (neoral) and SDZ RAD in non-human primate lung transplantation: Systematic pharmacokinetic-based trials to improve efficacy and tolerability. Transplantation. 2000;69:76–86. doi: 10.1097/00007890-200001150-00015. [DOI] [PubMed] [Google Scholar]
- 8.Nadazdin O, Boskovic S, Murakami T, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3:86ra51. doi: 10.1126/scitranslmed.3002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadazdin O, Boskovic S, Murakami T, et al. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am J Transplant. 2010;10:1375–1384. doi: 10.1111/j.1600-6143.2010.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4 + CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111:3675–3683. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: Induction of CD4 + CD25 + Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17:2844–2853. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 12.Sewgobind VD, van der Laan LJ, Kho MM, et al. The calcineurin inhibitor tacrolimus allows the induction of functional CD4CD25 regulatory T cells by rabbit anti-thymocyte globulins. Clin Exp Immunol. 2010;161:364–377. doi: 10.1111/j.1365-2249.2010.04183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 14.de Hooge AS, van de Loo FA, Koenders MI, et al. Local activation of STAT-1 and STAT-3 in the inflamed synovium during zymosan-induced arthritis: exacerbation of joint inflammation in STAT-1 gene-knockout mice. Arthritis Rheum. 2004;50:2014–2023. doi: 10.1002/art.20302. [DOI] [PubMed] [Google Scholar]
- 15.Lissilaa R, Buatois V, Magistrelli G, et al. Although IL-6 trans-signaling is sufficient to drive local immune responses, classical IL-6 signaling is obligate for the induction of T cell-mediated autoimmunity. J Immunol. 2010;185:5512–5521. doi: 10.4049/jimmunol.1002015. [DOI] [PubMed] [Google Scholar]
- 16.Nowell MA, Richards PJ, Fielding CA, et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: Implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006;54:2084–2095. doi: 10.1002/art.21942. [DOI] [PubMed] [Google Scholar]
- 17.Nowell MA, Richards PJ, Horiuchi S, et al. Soluble IL-6 receptor governs IL-6 activity in experimental arthritis: Blockade of arthritis severity by soluble glycoprotein 130. J Immunol. 2003;171:3202–3209. doi: 10.4049/jimmunol.171.6.3202. [DOI] [PubMed] [Google Scholar]
- 18.Nowell MA, Williams AS, Carty SA, et al. Therapeutic targeting of IL-6 trans signaling counteracts STAT3 control of experimental inflammatory arthritis. J Immunol. 2009;182:613–622. doi: 10.4049/jimmunol.182.1.613. [DOI] [PubMed] [Google Scholar]
- 19.Wong PK, Quinn JM, Sims NA, van Nieuwenhuijze A, Campbell IK, Wicks IP. Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum. 2006;54:158–168. doi: 10.1002/art.21537. [DOI] [PubMed] [Google Scholar]
- 20.Jones GW, McLoughlin RM, Hammond VJ, et al. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J Immunol. 2010;184:2130–2139. doi: 10.4049/jimmunol.0901528. [DOI] [PubMed] [Google Scholar]
- 21.Curnow SJ, Scheel-Toellner D, Jenkinson W, et al. Inhibition of T cell apoptosis in the aqueous humor of patients with uveitis by IL-6/soluble IL-6 receptor trans-signaling. J Immunol. 2004;173:5290–5297. doi: 10.4049/jimmunol.173.8.5290. [DOI] [PubMed] [Google Scholar]
- 22.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 23.Kimura A, Kishimoto T. IL-6: Regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]