Abstract
Radioiodine remnant ablation (RRA) is considered a safe and effective method for eliminating residual thyroid tissue, as well as microscopic disease if at all present in thyroid bed following thyroidectomy. The rationale of RRA is that in the absence of thyroid tissue, serum thyroglobulin (Tg) measurement can be used as an excellent tumor marker. Other considerations are like the presence of significant remnant thyroid tissue makes detection and treatment of nodal or distant metastases difficult. Rarely, microscopic disease in the thyroid bed if not ablated, in the future, could be a source of anaplastic transformation. On the other hand, microscopic tumor emboli in distant sites could be the cause of distant metastasis too. The ablation of remnant tissue would in all probability eliminate these theoretical risks. It may be noted that all these are unproven contentious issues except postablation serum Tg estimation that could be a good tumor marker for detecting early biochemical recurrence in long-term follow-up strategy. Radioactive iodine is administered as a form of “adjuvant therapy” for remnant ablation. There have been several reports with regard to the administered dose for remnant ablation. The first report of a prospective randomized clinical trial was published from India by a prospective randomized study conducted at the All India Institute of Medical Sciences, New Delhi in the year 1996. The study reported that increasing the empirical 131I initial dose to more than 50 mCi results in plateauing of the dose-response curve and thus, conventional high-dose remnant ablation needs critical evaluation. Recently, two important studies were published: One from French group and the other from UK on a similar line. Interestingly, all three studies conducted in three different geographical regions of the world showed exactly similar conclusion. The new era of low-dose remnant ablation has taken a firm scientific footing across the continents.
Keywords: Differentiated thyroid cancer, long-term outcome, low risk, remnant ablation
Special Mention
Dr. Ajit Padhy asked me to write a chapter on “Radioiodine Remnant Ablation: A Critical Review” in January 2013 for Dr. Baum's Therapeutic Nuclear Medicine monogram to be published by Springer. I send him the first draft on March 31, 2013. After 2 weeks when I enquired about the chapter, he told me that after the next issue of WJNM, he shall work on the chapter and make necessary changes and put references in book format. One evening in May 2013, he called me and told that he is impressed with the write up and wants to reproduce the chapter in WJNM, for wider circulation for the benefit of many readers across the world, after taking due permission from the Springer once it is published. The proofreading was done by Dr. Padhy and chapter was ready to be published by July 2013. The most unfortunate thing happened with Dr. Padhy, the Great Soul passed away on 22nd August 2013 at Singapore. He is physically no more among us, but his inspirations and dreams guide a lot of his admirers across the world. To honor his last wish, when I wrote to Dr. John Buscombe, the present Editor-in-Chief of WJNM, he immediately agreed and asked me to get permission from Springer. I am really thankful to Springer to give me permission to reproduce this chapter in WJNM in quickest possible time. This is a small tribute to Dr. Padhy my mentor and guide on fulfilling his last wish to see this article published in WJNM!!.
Introduction
The worldwide incidence of differentiated thyroid cancer (DTC) is increasing many folds except in Sweden where the incidence rates have decreased by about 18% for both males and females.[1] The American Cancer Society's estimates for thyroid cancer in the United States for 2013 are about 60,220 new cases of thyroid cancer (45,310 in women, and 14,910 in men).[2] This was 48,000 in the year 2011.[3] Along with this increasing trend, one can also notice another interesting finding that almost half (48%) of the new cases belong to individuals younger than 45 years of age.[4] Fortunately, the increase in the incidence is limited to early stage papillary thyroid carcinoma (PTC of 2 cm in diameter or smaller).[5] Several factors have been attributed to this phenomenon, namely preclinical detection of microscopic disease in the thyroid gland that may not have any clinical significance due to rampant use of high-resolution ultrasonic probes. Besides, thyroid cytology under ultrasound guidance is now universally available that adds more micropapillary carcinomas; and finally there has been more than three-fold increase in the use of ionizing radiation for medical purposes (radiation is a proven carcinogen as far as thyroid gland is concerned). To add to this debate, the latest publication by Li et al.[6] explored the contribution of enhanced detection to the recent increases in the incidence of thyroid cancer. The authors compared thyroid cancer incidence trends between low- and high-socioeconomic statuses (SES); meaning high SES have more access to medical care than low SES in the United States during the past three decades. The incidence of thyroid cancer of all sizes increased (52% patients had primary tumor 2–4 cm and >4 cm in size) in both SES groups during the period 1980–2010. The incidence increases were similar in metropolitan areas and areas not adjacent to metropolitan areas. They concluded that on the basis of surveillance, epidemiology, and end results data socioeconomic factors and access to medical care do not explain the rising incidence of thyroid cancer. Therefore, it is likely that there is a true increase in thyroid cancer incidence.
The thyroidologists are now chasing smaller and smaller low-risk thyroid malignancies (T1 and T2 tumors) with no prospective randomized trials to guide them, thus creating more chaos than science. Postsurgery radioiodine administration is considered as a form of adjuvant therapy for remnant ablation. In oncology, adjuvant therapy is defined as any form of therapy aimed at reducing the risk of recurrence, thus improving long-term outcome, that is, disease-free and overall survival (OS) by eliminating potential microscopic foci of malignant cells, whose existence and location have not yet been ascertained. It is time to do critical analysis of our current knowledge and plan the future prospects that shall be more evidence based than based on expert opinions.
Definition of Radioiodine Remnant Ablation
Thyroid remnant may be defined as normal thyroid tissue or microscopic disease in the thyroid bed left by the surgeon after total or near-total thyroidectomy. Radioiodine remnant ablation (RRA) is the destruction of this remnant thyroid tissue with the administration of radioactive iodine (RAI). RRA is considered as a safe and effective method for eliminating residual thyroid tissue, as well as microscopic disease if at all present in thyroid bed.[7] Some authors have tried to make a subtle difference between “ormal residual thyroid tissue” from “microscopic disease” present in thyroid bed.[8] They reserve RRA as an adjuvant therapy for later only. It seems separating two entities is more of an academic hair-splitting exercise than any practically relevant issue. We are not talking here the well-defined surgical terms frequently used by Onco-surgeons namely R0 (complete surgical resection), R1 (tumor is saved off, thus microscopic disease left behind), and R2 (gross tumor is left behind) resections. If known disease is left behind following surgery, then further treatment is not technically adjuvant. As, the adjuvant treatment is essentially for a risk, rather than for provable disease. It is accepted that a proportion of patients who received adjuvant therapy will already have been cured by their primary surgery.
Criteria of successful radioiodine remnant ablation
Successful ablation is variably defined by various authors, thus producing highly heterogeneous outcome data (ablation rates) as no standard definition is available. Most groups give primacy to no visible uptake in the thyroid bed in diagnostic scan or, if visible, a percentage uptake <0.1%, 6–8 months after radioiodine therapy, and/or a stimulated serum thyroglobulin (Tg) concentration <1 ng/mL.[9] In our institution (AIIMS), we have strictly defined successful remnant ablation as an absence of visible radioiodine uptake on a diagnostic whole body scan (WBS), with an undetectable or very low detectable (<2 ng/ml) Tg in the absence of antithyroglobulin antibody (ATA), either with thyroid hormone withdrawal (THW) or after recombinant human thyroid-stimulating hormone (rhTSH) stimulation.[10] In addition to the above two parameters, 24 or 48 h RAI uptake of <0.2% is considered as a significant quantitative parameter. When all three criteria are met, long-term chance of recurrence is noted to be significantly low (in approximately 1% of patients only).[11,12]
Justification of Radioiodine Remnant Ablation
The rationale of RRA is that in the absence of thyroid tissue, serum Tg measurement can be used as an excellent tumor marker. However, serum Tg measurement is most sensitive under thyroid-stimulating hormone (TSH) stimulation for the detection of persistent or recurrent disease. Other considerations are like the presence of significant remnant thyroid tissue makes detection and treatment of nodal or distant metastases difficult.[13,14] Rarely, microscopic disease in the thyroid bed if not ablated, in the future, can be a source of anaplastic transformation; microscopic tumor emboli in distant sites could be the cause of distant failure too. Thus, the argument goes, the ablation of remnant tissue, eliminates these theoretical risks. All these are unproven contentious issues except postablation serum Tg estimation that could be a good tumor marker for detecting the early biochemical recurrence in long-term follow-up strategy.
Critical Evidences: Radioiodine Remnant Ablation Reduces Recurrence Rate
One problem with most studies that address the issue of recurrence is the definition of recurrence, which is not clearly stated in the majority of these studies. As technology advances, the definition of recurrence has evolved from symptomatic or palpable disease to radiographic evidence of disease, to identification of small, diseased lymph nodes on sensitive neck ultrasonography or 18 fludeoxyglucose positron emission tomography/computed tomography (CT) imaging, to elevated serum Tg with or without TSH stimulation. The clinical relevance of this detected disease is also hotly debated. Furthermore, the magnitude of recurrence, reported in the literature, is widely varying. One of the major contributors to this confusion is a variable length of follow-up duration-longer the follow-up, higher the chance of reporting a recurrence, than shorter duration of follow-up. Technically speaking, we do not have concrete evidence to say emphatically when to stop the regular follow-up.
Radioactive iodine is administered as a form of “adjuvant therapy” for remnant ablation. RRA is highly controversial, particularly its rampant use in low-risk patients, without any definite proof of efficacy. In oncology, randomized control trials (RCT) generate grade 1 clinical evidences and direct the future therapy. In DTC, there have been no long-term randomized, controlled trials proving the treatment efficacy of adjuvant radioiodine therapy on thyroid cancer-related outcomes. However, the best quality of existing evidence relating to this intervention is observational in nature and subject to methodological limitations. The effect of RRA on long-term outcomes in low-risk PTC could be clarified in a future randomized, controlled trial. In view of low rate of event expected in low-risk papillary thyroid cancer, a primary treatment outcome of thyroid cancer-related mortality is not feasible for use in a randomized, controlled trial. In contrast, the outcome of any thyroid cancer loco-regional recurrence or distant failure may be used as a surrogate measure of success of RRA.
Three major studies from Ohio State University, MD Anderson Cancer Center and Trans-Canadian studies, respectively, have shown RRA use was associated with a significantly decreased risk of locoregional recurrence and distant metastases.[15,16,17] However, these evidences were not reproduced in other publications in low-risk papillary thyroid cancer patients.[18,19,20,21,22,23,24] From the National Thyroid Cancer Treatment Cooperative Study Group (NTCTCSG) data, the only prospective cohort study (of course, nonrandomized), Cooper et al.[25] found that RAI was a significant predictor of disease progression for PTC patients (P = 0.01), after adjusting for disease stage, mean thyrotropin score category, and other variables. However, in subgroup analyses, Cooper et al. found that for low-risk (stage I and II) patients, RAI was not a significant predictor of disease progression, whereas for high-risk (stage III and IV) patients, RAI was a significant predictor of disease progression (P = 0.001), after adjusting for mean thyrotropin score category. Seven retrospective cohort studies of lower-risk patients did not find a statistically significant relationship between RRA and recurrence. A single-institution study by Baudin et al.[22] found that postoperative RRA for lower-risk patients with papillary or follicular microcarcinoma was not associated with decreased recurrence (P value not stated), after adjusting for demographic, tumor, and treatment variables. Palme et al.[21] found that RAI treatment did not significantly predict development of more than 1 recurrence in patients with DTC after adjusting for demographic, disease stage, tumor, treatment, and first recurrence. Pelizzo et al.[19] studied lower risk patients with papillary thyroid microcarcinoma and found that RAI treatment is not associated with a decrease in recurrence (P = NS) after adjusting for demographic, tumor, and treatment variables. In patients with tumors over 5 mm in size, there was no statistically significant difference in recurrence with the combination of near-total thyroidectomy plus RAI as compared with thyroidectomy alone (P = NS). DeGroot et al.[20] also found a nonsignificant association with use of RAI treatment for lower risk patients and decreased recurrence overall or for any of the PTC subgroups analyzed in a multivariable model that adjusted for demographic, disease class, tumor, and treatment variables. Thus, the majority of patients with early stage PTC are unlikely to die from their recurrent disease. It is important to note that in exclusively early stage, papillary thyroid cancer patients treated with bilateral thyroidectomy, the 10-year absolute risk of any recurrence was estimated to be about one in ten; the 10-year risk of locoregional recurrence is 7.3% and that of distant metastatic recurrence 1.3% in this group.[26] Contrast to the earlier seminal publication by Mazzaferri and Jhiang from Ohio State University[15] that reported 20% recurrence in 16-years of median follow-up, a recent study from Italy shows only 0.8% recurrence in low-risk patients, albeit, smaller median follow-up duration of 8-years. Durante et al.[27] conducted a retrospective study of consecutive patients with PTC diagnosed since January 1990 in eight Italian referral centers. Inclusion criteria were negative anti-Tg levels and follow-up for at least 3 years before January 31, 2012. All patients had a total thyroidectomy, and 88% patients received RRA. The study population consisted of 1020 patients, 80% of whom were females. The median tumor size was 15 mm; tumors were multifocal in 36% and bilateral in 25%. Extrathyroidal extension was found in 24% patients, positive lymph nodes in 25%, and distant metastases in 3.2%. The disease recurred in 13 (five were low-risk and eight intermediate-risk patients) of the 948 (1.4%) patients within 8 years, half of them within the first 3 years. This retrospective study provides important data regarding long-term follow-up of patients with PTC; however, there are many serious limitations pointed out such as the study does not provide a definitive rationale for optimal management in terms of whether RRA is required or not (half of their patients had tumors <1.5 cm), completely silent about T4 suppression required or not, and most importantly how long these low-risk patients need regular follow-up is not answered.
The RRA literature for treatment of thyroid cancer has several deficiencies. First, as mentioned above in the absence of RCT, efficacy of RRA in low-risk DTC is fully dependent on retrospective cohort studies.[4] From 1979 till date, there are as many as 16 different staging systems in operation, the latest being TNM system.[28] Third, there is the lack of agreed-upon definitions for “low risk” versus “high risk,” Hence, it is difficult to compare the outcomes for different stages/risks from across studies.[29] Memorial Sloan-Kettering Cancer Centre has introduced third risk group, that is, intermediate risk group too.[30] European Consensus guideline introduced three different terms namely very low-risk, low-risk, and high-risk DTC. Fourth, despite the histological heterogeneity of DTC, some high-risk histological features are well-accepted now;[31] few studies pool histological subtypes in their analyses, whereas others do not, even though the histological subtypes behave differently.[32] Fifth, recurrence and death from thyroid cancer can be seen many years after the initial diagnosis; therefore, outcome data are dependent on the duration of follow-up, which is a definite variable. Finally, as mentioned above, the definition of recurrence is not universal, changes with time and availability of technology for detecting recurrence of disease.[33,34] This makes it difficult to compare study outcomes over a longer period.
Upon carefully examining the best existing long-term observational evidence, we could not confirm a significant, consistent, benefit of RRA in decreasing cause-specific mortality or recurrence in low-risk DTC. However, there is definite evidence of benefit of RRA in high-risk patients, particularly those with T3/T4 tumors; N1 nodal status, gross residual tumor left after surgery (R2 dissection), high-risk histological features, and advanced postsurgical staging of disease on 131I WBS either on diagnostic or posttherapy scan.
Critical evidences: Radioiodine remnant ablation improves disease specific survival or overall survival
American Thyroid Association and European Thyroid Association guidelines cannot recommend for or against RAI ablation after surgery in low-risk DTC patients. Several systematic or meta-analyses were reviewed in the literature to determine whether RAI remnant ablation decreases the risk of thyroid cancer-related death or recurrence in adults who have had grossly complete resection of papillary or follicular thyroid carcinoma.[35,36] In a systematic review, Sacks et al.[37] sought to analyze the evidence for use of RRA to improve survival and to reduce recurrence in patients who are at low risk for recurrence and death from thyroid cancer. Authors performed MEDLINE search for studies published between January 1966 and April 2008 that compared the effectiveness of administering versus not administering RRA for DTC. The majority of studies did not find a statistically significant improvement in mortality or disease-specific survival in those low-risk patients treated with RRA, whereas improved survival was confirmed for high-risk (AJCC stages III and IV) patients. Evidence for RRA decreasing recurrence was mixed with half of the studies showing a significant relationship and half showing no relationship. Author concluded that a majority of very low-risk and low-risk patients, as well as select cases of patients with moderate risk do not demonstrate survival or disease-free survival (DFS) benefit from postoperative RRA, and therefore they recommend against postoperative RRA in these cases.
A recent French study critically evaluated the survival advantages of low-risk DTC patients undergoing RRA. Schvartz et al.[38] identified 1,298 DTC patients who were low-risk and treated between 1975 and 2005. Logistic regressions were used to identify variables associated with RRA and also calculated the propensity score to receive RRA after surgery. They compared OS and DFS according to RRA with the log-rank tests and univariate and multivariate Cox regression models. Analyses stratified on the propensity score were also performed. Median follow-up was 10.3 years. Nine hundred eleven patients received RRA versus 387 patients without RRA after surgery. Using univariate analysis, 10-year OS was found to be 95.8% in patients without RRA versus 94.6% in RRA after surgery (P = 0.006), and 10-year DFS was found to be 93.1% versus 88.7% (P = 0.001). All clinical factors except sex were significantly associated with RRA. Using multivariate Cox analyses, RRA was neither significantly nor independently associated with OS (P = 0.243) and DFS (P = 0.2659). After stratification on propensity score, Cox univariate analyses showed that OS did not differ according to RRA (P = 0.3524), with a hazard ratio for RRA of 0.75 (95% confidence interval 0.40–1.38). Similarly, DFS did not differ (P = 0.48) with a stratified univariate hazard ratio of 1.11 (95% confidence interval 0.73–1.70). They concluded from this sophisticated analyses that there was no proven survival benefit of RRA after surgery in a large cohort of low-risk DTC patients, with a long-term follow-up of 10.3 years.
Another major publication has come from Wurzburg, Germany.[39] The Wurzburg Thyroid Cancer Database was established in 1980. Using this database, the authors analyzed clinical features and survival of 2011 patients with DTC, who had been treated and followed from January 1980 to December 2011. Patients were treated by total thyroidectomy with subsequent 131I ablation, except for 391 who had isolated papillary microcarcinoma and were treated with hemithyroidectomy. The median age at diagnosis was 47.6 years, and the median follow-up was 7.1 years. During the follow-up, 264 patients (13.1%) died. Overall, 14% of the patients had reduced life expectancy. There was no reduction in life expectancy for those younger than age 45.
Role of Low Iodine Diet
Most centers advise a 2-week low iodine diet (LID) prior to the 131I administration.[40,41] The patients are instructed to follow a LID, which basically means avoiding the iodine-rich food and iodine-containing medications.[40] LID is designed to decrease the total body stable iodine concentration prior to radioiodine administration. Urinary iodine excretion is a good marker of the recent dietary iodine intake. According to the World Health Organization's (WHO's) report, the iodine concentrations in the morning urine specimens provide an adequate assessment of the recent dietary iodine intake.[42] WHO recommends the expression of urinary iodine concentration as a simple iodine concentration (μg/L), without the urinary creatinine measurement or 24 h urine collection WHO et al.[42] For the purpose of categorization, urinary iodine concentration <50 μg/L reflects a moderate iodine deficiency, while urinary iodine concentration 50–99 μg/L indicates a mild iodine deficient state and 100–200 μg/L indicates normal iodine status.
Studies demonstrating the efficacy of a LID on postsurgical 131I ablation therapy are contradictory. The proponents of LID argue that iodine deficiency increases the radioiodine uptake in thyroid remnant and also shown longer effective half-life of 131I, both of which theoretically contribute to the increase of the radiation absorbed dose.[5] In fact, using the criteria of no visible uptake in the neck region and negative Tg level, Pluijmen et al.[43] found a significantly higher ablation rate in patients performing a 2-week LID compared to the control group (65% vs. 48%). However, opponents do not agree with these findings. Morris et al.[44] showed no significant difference of ablation rate between 2-week LID patients and those on a regular diet (68.2% vs. 62.0%).
If one critically looks into the country's iodine intake status (iodine deficient, iodine sufficient, or excess iodine intake), one can get fair idea whether stringent LID is required or not. The patients from oriental countries where seafood is a staple diet, or where by law food supplementation with stable iodine is mandatory, definitely need LID. The best way to ensure LID or no LID is by doing spot urinary iodine estimation prior to RRA.
Do We Need Pre-ablation Whole Body Scans?
The utility of radioiodine WBSWBS prior to 131I remnant ablation is controversial. Strong sentiments are shown in its favor as well as against this investigation. Justification for 131I WBS (1) to determine how much residual thyroid tissue has been left after thyroidectomy, (2) to define the presence of functioning metastases, thus accurately staging the disease, (3) to determine whether pre-ablation preparation is adequate for treatment with 131I or not, (4) to determine whether patient is surgically ablated or not, and (5) to ensure the proposed high dose of therapeutic 131I not irradiating a physiological site such as the breasts.
In regard to the arguments against pre-ablation WBS, these can be categorized into six major reasons: (1) Pre-ablation WBS have little to no benefit, (2) there is the potential for stunning, (3) all the information one needs can be obtained by other methods (e.g., large thyroid remnant or gross residual tumor by surgeon's operative note or ultrasonography, lymph node metastases by ultrasound, pulmonary, or mediastinal metastases by chest-X-ray or CT), (4) post-therapy scans are more sensitive and demonstrate all or most of the information that is needed, (5) patient inconvenience and costs are too great, and (6) one shall treat these patients, anyway, regardless of diagnostic WBS and/or uptake values.
There are a number of flaws in the above-mentioned theories that a diagnostic scan is not necessary. Therefore, no single empiric therapeutic activity, by definition, will fit all. A fairly recent publication from Washington University has demonstrated the value of pre-ablation WBS.[45] These investigators reviewed 355 WBSs and determined whether there were indications that 131I treatment would be modified by knowledge of the pre-treatment findings. Categories in which this would occur included no uptake on the WBS (6%), six or more foci of uptake in the neck (12%), uptake in functioning nodal metastases (14%), functioning distal metastases (4%), activity indicating more than 1 lobe (1.1%), subtotal thyroidectomy meaning thyroid bed uptake 15% in (8%) or uptake in nonthyroidal sites including the breast (14%). Some patients had findings from more than one category.
The American Thyroid Association's[46] guidelines for the management of patients with thyroid nodules and DTC states, “Pre-therapy scans and/or measurement of thyroid bed uptake may be useful when the extent of the thyroid remnant cannot be accurately ascertained from the surgical report or neck ultrasonography, or when the results would alter either the decision to treat or the activity of RAI that is administered. If WBS is performed, pre-therapy scans should utilize low dose 131I (1–3 mCi) or 123I.” Revised ATA guideline recommendation 35: Consider pretherapy diagnostic WBS using rhTSH or THW if expected to change management.[46] Knowledge of the uptake in the remnant and the function of the metastases cannot be known without a preablation WBS. In addition, if stunning is a real phenomenon, would it not be more likely to occur with an insufficient therapeutic activity than a smaller diagnostic dose of 131I is administered? If, diagnostic WBS and radioiodine therapy performed in the same week, stunning is most unlikely phenomena in these patients. In as much as a radiation oncologist properly plans radiotherapy and executes precisely; such precision, in our opinion, is similarly expected from thyroidologists dealing with the largest endocrine malignancy.
Thyroid Hormone Withdrawal or Recombinant Human Thyroid-Stimulating Hormone Stimulation Preparation
Remnant ablation requires TSH stimulation. Thyroid stimulating hormone is secreted from anterior pituitary gland that is tightly controlled by hypothalamus-pituitary-thyroid axis. However, TSH secretion depends on feedback regulation of serum thyroxine level, and to a lesser extent, thyrotropin-releasing hormone status. Withdrawal of thyroid hormone has been the standard procedure over the last few decades to increase serum TSH, thereby enhancing the sensitivity of Tg measurement and increasing radioiodine uptake by tumor tissue.[47] Thyroxine has half-life of 7 days, thus 3–4 weeks' time is necessary to get T4 to a minimum level in the serum. Thyroxin fall is critically dependent on residual thyroid tissue. If significant remnant thyroid tissue is present, it is unlikely that TSH shall rise to a significant level.[48] Second, patients with hypopituitarism may not mount TSH to the desired level. However, there are no controlled studies performed so far to assess adequate levels of endogenous TSH for optimal ablation therapy or follow-up testing. Noncontrolled studies suggest that a TSH of >30 mU/L is associated with increased radioiodine uptake in tumors.[49] Hypothyroidism is well-tolerated by vast majority of younger patients undergoing diagnostic WBS and subsequent RRA; a minority of elderly patients and patients with associated co-morbid conditions do not tolerate hypothyroid features, and needs exogenous TSH stimulation. TSH is a heterodimeric glycoprotein secreted from anterior pituitary gland and consists of two subunits, the alpha and beta subunit. The alpha chain has a 92-amino acid sequence and common to all other glycoprotein hormone namely hCG, FSH, and LH. The beta chain has an 118-amino acid sequence. The beta subunit is unique to TSH and therefore determines its receptor specificity. The alpha subunit is thought to be the effector region responsible for stimulation of adenylate cyclase (involved the generation of cAMP). It was shown that the bioactivity of the new compound was strongly dependent on the degree of sialylation, which is higher than that of normal pituitary TSH. rhTSH (rhTSHa or simply rhTSH) is a synthetic drug, bulk manufactured in Chinese hamster ovary cell line by Genzyme Corporation, Boston, USA modifying alpha subunit and humanizing it, and marketed as “Thyrogen.”
After three successive randomized clinical trials, finally FDA in 1998 approved Thyrogen for stimulated Tg estimation with or without 131I WBS.[50,51,52] Surveillance in detecting occult persistent disease has also been improved using rhTSH. The cut-off value of stimulated Tg is controversial. It is now well-accepted that the stimulated Tg has very high negative predictive value than high positive predictive value. Kloos and Mazzaferri have shown that an rhTSH stimulated Tg <2 ng/mL predicts residual tumor; however, rhTSH-stimulated serum Tg < 0.5 ng/ml have high negative predictive value (about 98%) for a disease-free state.[53]
Next logical step was to use Thyrogen for RRA. Many groups tried to use rhTSH stimulated RRA.[54,55] The preliminary results were very encouraging. The off-level use by international multicenter study finally proved under rhTSH stimulation RRA was as effective as T4 withdrawal method of preparation.[56] Two important findings from this study were the remnant absorbed dose was higher at the same time the whole body dose was significantly lower than conventional method of T4 withdrawal because of renal clearance of radioiodine was intact in rhTSH stimulated RRA. Initially in Europe by EMA and subsequently in USA by FDA approved Thyrogen stimulated RRA. Interestingly, European approval was with the condition, that is, 100 mCi 131I should be used for this purpose, however, FDA did not put any constraint on the amount of 131I required for rhTSH stimulated RRA.
In 2009, Revised ATA recommendation 34 states that remnant ablation can be performed following thyroxine withdrawal or rhTSH stimulation (Recommendation rating: A).
If decided to go ahead with RRA, what should be the administered activity of 131I. It was Professor William Beierwaltes from the University of Michigan, Ann Arbor who vehemently propagated RRA, as he strongly believed in the hypothesis of “adjuvant” effect of radioiodine in DTC.[13] He was probably provoked by a challenging title “Thyroid remnant ablation: Questionable pursuit of an ill-defined goal” published by Snyder et al.[57] Between January 1947 and June 1983, in Beierwaltes’ Center 511 patients were given treatment doses of 131I after surgery for thyroid cancer in the presence of 131I uptake in thyroid remnants. Of 267 patients with radioiodine uptake confined to the thyroid bed, 233 (87%) had ablation from the first dose of 131I ranging from 100 to 200 mCi. In his experience, the higher the percent uptake, the most difficult it was to achieve ablation. In the percentages of successful ablation, there were no significant differences between 131I doses of: 100–149 mCi, 150–174 mCi, 179–199 mCi, and 200 mCi or more. He advocated that 100–149 mCi ablative dose might furnish “adjuvant” therapy for occult metastases. Though he advocated 100–149 mCi for remnant ablation, inadvertently he has also shown that any activity higher than 100 mCi does not improve ablation rate so far the remnant ablation is concerned. Of course, there was no concept of prognostic factor or risk-group analysis in DTC, and then it was just evolving by European Organization for Research on Treatment of Cancer (EORTC). Thyroid Cancer Cooperative Group for the first time presented a prognostic index in 1979 that included all histological subgroups of thyroid carcinomas, and was based on a multivariate analysis of 507 patients’ data with a median follow-up of 40 months.[58] Subsequently, many small cohort studies supported and also opposed 29.9 mCi 131I in RRA; a concept put forth formally by McCowen et al.[59] for logistic reason to avoid hospitalization.
We were the first group to conduct a prospective randomized clinical trial at All India Institute of Medical Sciences, New Delhi from January 1990 to December 1994 to find out the optimal dose (activity) of 131I for remnant ablation Bal et al.[10] Using a simple randomization technique, 149 patients with remnant thyroid were incorporated into four treatment groups: 30 + 1.5, 50 + 5.4, 89 + 14 and 155 + 29 mCi. Six months to 1 year after treatment, all subjects were reassessed after withdrawing L-thyroxine for 4–6 weeks. A successful ablation was defined as the absence of thyroid bed activity in 5 mCi 131I neck scan at 48 h along with two adjunctive criteria which were the neck uptake of ≤0.2% of the administered activity and the stimulated serum Tg value of <10 ng/mL. Applying the above criteria, we observed complete ablation of thyroid gland remnants 63% in the 30 mCi group, 77.8% in the 50 mCi group, 73.7% in the 90 mCi group, and 76.7% in the 155 mCi group. We had concluded that increasing the empirical 131I initial dose to more than 50 mCi results in plateauing of the dose-response curve and thus, conventional high dose remnant ablation needs critical evaluation. From this study, the upper limit of 131I for RRA was determined, that is, should not be more than 50 mCi. However, the lower limit was not known. Thus, we conducted a second randomized clinical trial to find out the smallest possible effective dose for remnant ablation in cases of differentiated thyroid carcinoma.[60] Between July 1995 and January 2001, 565 patients were randomized into eight groups according to 131I administered activity, starting at 15 mCi and increasing activity in increments of 5 mCi until 50 mCi. In the postrandomization phase, 56 patients were excluded from the study for various reasons, and final analysis was done with 509 patients. The mean age of the patients was 37.5 + 12.7 year with a female to male ratio of 2.6. The surgical procedure was total/near-total thyroidectomy in 72% and subtotal or hemithyroidectomy in the rest. Histology was PTC in 80.6% of patients and follicular thyroid carcinoma in the rest. With one dose of 131I, remnant ablation was achieved in 59.6, 63.6, 81.4, 83.6, 79.4, 78.3, 84.4, and 81.8% of patients in the 15- to 50-mCi groups, respectively (overall ablation rate, 77.6%). The successful ablation rate was statistically different in patients receiving less than 25 mCi of 131I compared with those receiving at least 25 mCi (63 of 102 [61.8%] vs. 332 of 407 [81.6%]; P = 0.006). However, there was no significant intergroup difference in the outcome among patients receiving 25–50 mCi of 131I. Patients receiving at least 25 mCi of 131I had a three times better chance of getting remnant ablation than patients receiving lesser activity of 131I. We concluded that any activity of 131I between 25 and 50 mCi appears to be adequate for remnant ablation.
Subsequently, Hackshaw et al. performed a systematic review comparing the success of remnant ablation after various dose activities of RAI.[61] In pooling data from 16 observational studies (total number of patients 2050), Hackshaw et al. observed that the success rate for ablation using activity of 100 mCi was significantly higher than for 30 mCi, but these findings were not confirmed in a pooled analysis of five randomized, controlled trials. Thus, Hackshaw et al. concluded “from the published data, it is not possible to reliably determine whether ablation success rates using 30 mCi are similar to 100 mCi” and suggested “large randomized trials are needed to resolve the issue and guide clinical practice.”
Soon, we realized that 30 mCi could never give superior remnant ablation rate than 100 mCi of 131I for logical reason, irrespective of many advantages of small dose therapy. The need of the time was to conduct a noninferiority/equivalence trial to prove that whether 30 mCi is equivalent to 100 mCi or not. This time we conducted a third trial; a randomized equivalence/noninferiority trial[62] from January 2001 to December 2006 to determine whether lower administered activities are as effective as 3.7 GBq (100 mCi) of 131I for remnant ablation. The sample size was found to be 450 on the basis of 80% power, alpha of 5% and noninferiority margin of 15% (d = 0.15). We observed that first-dose 131I ablation rates 81.5, 84.9 and 88.5% at 6 months with 25, 50 and 100 mCi (0.93, 1.85, and 3.7 GBq), respectively, of 131I administered activity. The ablation rates were equivalent with the prespecified clinically acceptable noninferiority margin. We conclude that we are probably administering too much 131I for remnant ablation. We were delighted to see two important studies which were published at the time of writing this review: One was by a French group and the other was a HiLo trial from the UK on a similar line −1.1 GBq (30 mCi) versus 3.7 GBq (100 mCi) for remnant ablation in low-risk DTC patients.[63,64] Both the French and the UK trials were conducted from January 2007 to July 2010. Our study was completed by December 2006, and the preliminary data were presented at the 92nd Annual Endocrine Society meeting in San Diego from 19 to 22 June 2010.[65] The major difference was the use of rhTSH in these trials, unlike our study, to prove that rhTSH is equally effective compared with conventional preparation by THW. Surprisingly, all three studies conducted in three different geographical regions of the world showed exactly the same conclusion. The new era of low-dose remnant ablation has taken a firm scientific footing across the continents. The recent meta-analysis based on all RCT on RRA (a class 1 category of evidence) published by Cheng et al. has beautifully summarized [Table 1] the findings and concluded that 30 mCi of 131I is sufficient enough for RRA.[66]
Table 1.
Characteristics of all 10 randomized trials published on RRA and the methods of TSH stimulation (modified from Cheng et al. 2013)[66]
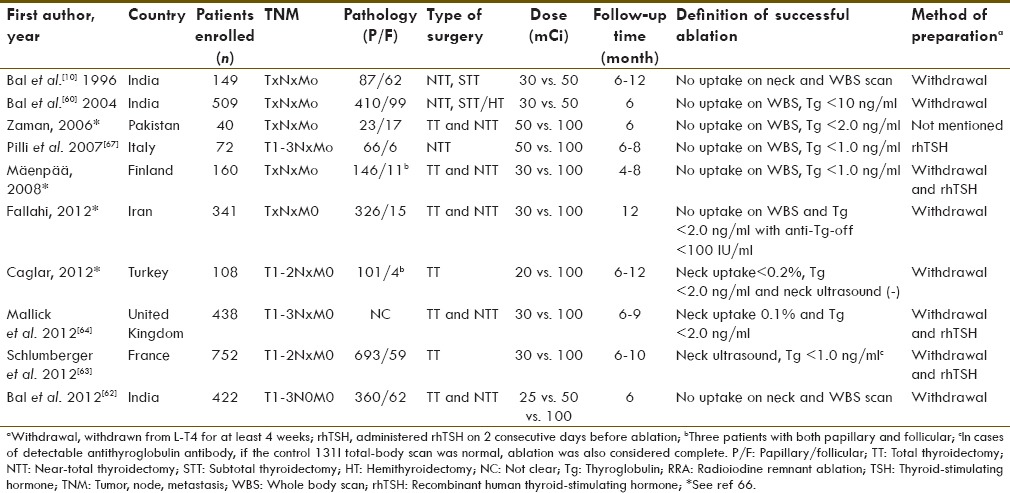
For RRA, rhTSH is Equally Effective Compared with Conventional Preparation by THW. The first single-center randomized controlled trial, including 72 patients with DTC pretreated with recombinant human thyrotropin, Pilli et al. observed that short-term remnant ablation rates were the same (88.9%) after administering 50 mCi, compared with 100 mCi of RAI.[67] Finally, two large high-powered multi-center national randomized trials conducted from France and the UK independently proved noninferiority of Thyrogen stimulated RRA compared to T4 withdrawal RRA with 30 mCi or 100 mCi 131I administration.[63,64]
ATA Guideline versus new evidences for low-dose RRA recommendation 36 states that the minimum activity (30–100 mCi) necessary to achieve successful remnant ablation should be utilized, particularly for low-risk patients (Recommendation rating: B). Now the time has come for all international guideline committees to revise the recommendations for remnant ablation. The new recommendation should advocate 30 mCi of 131I for remnant ablation either under rhTSH stimulation or by THW.
Rationale of low-dose radioiodine remnant ablation
There are several advantages to both the patient and healthcare provider for using a lower activity of radioiodine, including less time in isolation, a shorter hospital stay (when local or national regulations deem this necessary), reduced exposure of radioiodine to the environment, and lower financial cost. Furthermore, radioiodine ablation is associated with an increased risk of second primary malignancies; the lower the activity administered, the lower the risk. The risks of radioiodine therapy must be considered. A large multicenter cohort study analyzed the risk of secondary malignancies in 6,841 patients with DTC (62% were treated with radioiodine).[68] The investigators observed a significant 30% increased risk of second primary malignancies in patients treated with radioiodine, and there appeared to be a linear relationship between the cumulative dose and solid tumors (4% increased risk/GBq 131I). An estimated 53 solid tumors are expected among 10,000 patients after 10 years if they were treated with 100 mCi compared with only 16 if they had been treated with 30 mCi. Although these data are estimates from an extrapolation of higher cumulative doses of radioiodine, and not actual data from patients receiving 100 mCi 131I, they should provoke thyroidologists to consider that there may be risks - however small it may be associated with administration of radioiodine for remnant ablation.[69,70]
Thyroid-Stimulating Hormone Suppression Issues in Low-Risk Differentiated Thyroid Cancer Patients
A prospective cohort study[71] of 2,936 patients found that OS improved significantly when the TSH was suppressed to undetectable levels in patients with NTCTCSG stage III or IV disease and suppressed to the subnormal to undetectable range in patients with NTCTCSG stage II disease; however, in the latter group, there was no incremental benefit from suppressing TSH to undetectable levels. Suppression of TSH was not beneficial in patients with stage I disease. In another study, there was a positive association between serum TSH levels and the risk for recurrent disease and cancer-related mortality.[72,73]
Antithyroglobulin antibody recommendation 40 states that initial TSH suppression to below 0.1 mU/L is recommended for high-risk and intermediate-risk thyroid cancer patients, while maintenance of the TSH at or slightly below the lower limit of normal (0.1–0.5 mU/L) are appropriate for low-risk patients. Similar recommendations apply to low-risk patients who have not undergone remnant ablation, that is, serum TSH 0.1–0.5 mU/L (Recommendation rating: B)
Conclusions
The benefit of radioiodine in younger patients with smaller tumors (B4 cm) is less clear, and potential risks of therapy need to be thoroughly considered. We must individualize radioiodine therapy-(a) use 30 mCi 131I for RRA either with rhTSH stimulation or THW, the lowest effective dose of radioiodine, in low-risk patients; (b) for older patients with larger tumors (>4 cm), and patients with lymph node involvement, may significantly benefit from RRA, probably with higher dose of radioiodine (>50 mCi). The current reality is that in the absence of randomized clinical trial to prove or disprove the beneficial long-term effect of RRA, the decision-making about RRA in low-risk thyroid carcinoma is difficult and complex. Thus, till definite evidence is generated from high quality future study, one must limit the known harmful effect of radiation, and the good clinical practice must follow the basic principle of primum non nocere (Latin phrase that means “first, do no harm”).
Acknowledgment
We are grateful to Springer for giving permission to reproduce this article in World Journal of Nuclear Medicine. The original publication was a book chapter in “Therapeutic Nuclear Medicine” edited by Prof. Richard P. Baum.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–31. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society: Cancer Facts and Figures (2013) [Last accessed on 2013 Mar 31]. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/docume.nts/document/acspc-036845.pdf .
- 3.American Cancer Society: Cancer Facts and Figures (2011) [Last accessed on 2013 Mar 31]. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf .
- 4.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–7. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 5.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 6.Li N, Du XL, Reitzel LR, Xu L, Sturgis EM. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: Review of incidence trends by socioeconomic status within the surveillance, epidemiology, and end results registry, 1980–2008. Thyroid. 2013;23:103–10. doi: 10.1089/thy.2012.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzaferri E. A randomized trial of remnant ablation - In search of an impossible dream? J Clin Endocrinol Metab. 2004;89:3662–4. doi: 10.1210/jc.2004-0739. [DOI] [PubMed] [Google Scholar]
- 8.Filetti S, Tuttle RM, Sherman SI. Medical management of differentiated epithelial cell thyroid cancer. In: Braverman LE, Cooper DS, editors. Werner and Ingebar's Thyroid: A Fundamental and Clinical Text. 10th ed. Philadelphia: Lippincort William and Wilkins; 2013. pp. 725–43. [Google Scholar]
- 9.Duntas LH, Cooper DS. Review on the occasion of a decade of recombinant human TSH: Prospects and novel uses. Thyroid. 2008;18:509–16. doi: 10.1089/thy.2007.0331. [DOI] [PubMed] [Google Scholar]
- 10.Bal C, Padhy AK, Jana S, Pant GS, Basu AK. Prospective randomized clinical trial to evaluate the optimal dose of 131 I for remnant ablation in patients with differentiated thyroid carcinoma. Cancer. 1996;77:2574–80. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2574::AID-CNCR22>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 11.Schlumberger M, Berg G, Cohen O, Duntas L, Jamar F, Jarzab B, et al. Follow-up of low-risk patients with differentiated thyroid carcinoma: A European perspective. Eur J Endocrinol. 2004;150:105–12. doi: 10.1530/eje.0.1500105. [DOI] [PubMed] [Google Scholar]
- 12.Han JM, Kim WB, Yim JH, Kim WG, Kim TY, Ryu JS, et al. Long-term clinical outcome of differentiated thyroid cancer patients with undetectable stimulated thyroglobulin level one year after initial treatment. Thyroid. 2012;22:784–90. doi: 10.1089/thy.2011.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beierwaltes WH, Rabbani R, Dmuchowski C, Lloyd RV, Eyre P, Mallette S. An analysis of “ablation of thyroid remnants” with I-131 in 511 patients from 1947–1984: Experience at University of Michigan. J Nucl Med. 1984;25:1287–93. [PubMed] [Google Scholar]
- 14.Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–63. doi: 10.1210/jcem.86.4.7407. [DOI] [PubMed] [Google Scholar]
- 15.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 16.Samaan NA, Maheshwari YK, Nader S, Hill CS, Jr, Schultz PN, Haynie TP, et al. Impact of therapy for differentiated carcinoma of the thyroid: An analysis of 706 cases. J Clin Endocrinol Metab. 1983;56:1131–8. doi: 10.1210/jcem-56-6-1131. [DOI] [PubMed] [Google Scholar]
- 17.Simpson WJ, Panzarella T, Carruthers JS, Gospodarowicz MK, Sutcliffe SB. Papillary and follicular thyroid cancer: Impact of treatment in 1578 patients. Int J Radiat Oncol Biol Phys. 1988;14:1063–75. doi: 10.1016/0360-3016(88)90381-1. [DOI] [PubMed] [Google Scholar]
- 18.Podnos YD, Smith DD, Wagman LD, Ellenhorn JD. Survival in patients with papillary thyroid cancer is not affected by the use of radioactive isotope. J Surg Oncol. 2007;96:3–7. doi: 10.1002/jso.20656. [DOI] [PubMed] [Google Scholar]
- 19.Pelizzo MR, Boschin IM, Toniato A, Piotto A, Bernante P, Pagetta C, et al. Papillary thyroid microcarcinoma (PTMC): Prognostic factors, management and outcome in 403 patients. Eur J Surg Oncol. 2006;32:1144–8. doi: 10.1016/j.ejso.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71:414–24. doi: 10.1210/jcem-71-2-414. [DOI] [PubMed] [Google Scholar]
- 21.Palme CE, Waseem Z, Raza SN, Eski S, Walfish P, Freeman JL. Management and outcome of recurrent well-differentiated thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:819–24. doi: 10.1001/archotol.130.7.819. [DOI] [PubMed] [Google Scholar]
- 22.Baudin E, Travagli JP, Ropers J, Mancusi F, Bruno-Bossio G, Caillou B, et al. Microcarcinoma of the thyroid gland: The Gustave-Roussy Institute experience. Cancer. 1998;83:553–9. doi: 10.1002/(sici)1097-0142(19980801)83:3<553::aid-cncr25>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 23.Handkiewicz-Junak D, Wloch J, Roskosz J, Krajewska J, Kropinska A, Pomorski L, et al. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med. 2007;48:879–88. doi: 10.2967/jnumed.106.035535. [DOI] [PubMed] [Google Scholar]
- 24.Chow SM, Yau S, Kwan CK, Poon PC, Law SC. Local and regional control in patients with papillary thyroid carcinoma: Specific indications of external radiotherapy and radioactive iodine according to T and N categories in AJCC 6 th edition. Endocr Relat Cancer. 2006;13:1159–72. doi: 10.1677/erc.1.01320. [DOI] [PubMed] [Google Scholar]
- 25.Cooper DS, Specker B, Ho M, Sperling M, Ladenson PW, Ross DS, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737–44. doi: 10.1089/thy.1998.8.737. [DOI] [PubMed] [Google Scholar]
- 26.Hay ID. Management of patients with low-risk papillary thyroid carcinoma. Endocr Pract. 2007;13:521–33. doi: 10.4158/EP.13.5.521. [DOI] [PubMed] [Google Scholar]
- 27.Durante C, Montesano T, Torlontano M, Attard M, Monzani F, Tumino S, et al. Papillary thyroid cancer: Time course of recurrences during postsurgery surveillance. J Clin Endocrinol Metab. 2013;98:636–42. doi: 10.1210/jc.2012-3401. [DOI] [PubMed] [Google Scholar]
- 28.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: A review and comparison. Ann Surg. 2007;245:366–78. doi: 10.1097/01.sla.0000250445.92336.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow SM, Law SC, Mendenhall WM, Au SK, Chan PT, Leung TW, et al. Papillary thyroid carcinoma: Prognostic factors and the role of radioiodine and external radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52:784–95. doi: 10.1016/s0360-3016(01)02686-4. [DOI] [PubMed] [Google Scholar]
- 30.Shaha AR. Implications of prognostic factors and risk groups in the management of differentiated thyroid cancer. Laryngoscope. 2004;114:393–402. doi: 10.1097/00005537-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Podnos YD, Smith D, Wagman LD, Ellenhorn JD. Radioactive iodine offers survival improvement in patients with follicular carcinoma of the thyroid. Surgery. 2005;138:1072–6. doi: 10.1016/j.surg.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Lal G, O’Dorisio T, McDougall R, Weigel RJ. Cancer of the head and neck. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, McKenna WG, editors. Abeloff's Clinical Oncology. Philadelphia: Churchill Livingstone Elsevier; 2008. p. 148. [Google Scholar]
- 33.Pacini F, Molinaro E, Castagna MG, Agate L, Elisei R, Ceccarelli C, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–73. doi: 10.1210/jc.2002-021925. [DOI] [PubMed] [Google Scholar]
- 34.Torlontano M, Attard M, Crocetti U, Tumino S, Bruno R, Costante G, et al. Follow-up of low risk patients with papillary thyroid cancer: Role of neck ultrasonography in detecting lymph node metastases. J Clin Endocrinol Metab. 2004;89:3402–7. doi: 10.1210/jc.2003-031521. [DOI] [PubMed] [Google Scholar]
- 35.Sawka AM, Thephamongkhol K, Brouwers M, Thabane L, Browman G, Gerstein HC. Clinical review 170: A systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004;89:3668–76. doi: 10.1210/jc.2003-031167. [DOI] [PubMed] [Google Scholar]
- 36.Sawka AM, Brierley JD, Tsang RW, Thabane L, Rotstein L, Gafni A, et al. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol Metab Clin North Am. 2008;37:457–80, x. doi: 10.1016/j.ecl.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Sacks W, Fung CH, Chang JT, Waxman A, Braunstein GD. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: A systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid. 2010;20:1235–45. doi: 10.1089/thy.2009.0455. [DOI] [PubMed] [Google Scholar]
- 38.Schvartz C, Bonnetain F, Dabakuyo S, Gauthier M, Cueff A, Fieffé S, et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J Clin Endocrinol Metab. 2012;97:1526–35. doi: 10.1210/jc.2011-2512. [DOI] [PubMed] [Google Scholar]
- 39.Verburg FA, Mäder U, Tanase K, Thies ED, Diessl S, Buck AK, et al. Life expectancy is reduced in differentiated thyroid cancer patients ≥45 years old with extensive local tumor invasion, lateral lymph node, or distant metastases at diagnosis and normal in all other DTC patients. J Clin Endocrinol Metab. 2013;98:172–80. doi: 10.1210/jc.2012-2458. [DOI] [PubMed] [Google Scholar]
- 40.Park JT, 2nd, Hennessey JV. Two-week low iodine diet is necessary for adequate outpatient preparation for radioiodine rhTSH scanning in patients taking levothyroxine. Thyroid. 2004;14:57–63. doi: 10.1089/105072504322783858. [DOI] [PubMed] [Google Scholar]
- 41.Tomoda C, Uruno T, Takamura Y, Ito Y, Miya A, Kobayashi K, et al. Reevaluation of stringent low iodine diet in outpatient preparation for radioiodine examination and therapy. Endocr J. 2005;52:237–40. doi: 10.1507/endocrj.52.237. [DOI] [PubMed] [Google Scholar]
- 42.WHO; UNICEF; ICCIDD. WHO Document WHO/NHD/01.1. Geneva: World Health Organisation; 2001. Assessment of the iodine deficiency disorders and monitoring their elimination. [Google Scholar]
- 43.Pluijmen MJ, Eustatia-Rutten C, Goslings BM, Stokkel MP, Arias AM, Diamant M, et al. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2003;58:428–35. doi: 10.1046/j.1365-2265.2003.01735.x. [DOI] [PubMed] [Google Scholar]
- 44.Morris LF, Wilder MS, Waxman AD, Braunstein GD. Reevaluation of the impact of a stringent low-iodine diet on ablation rates in radioiodine treatment of thyroid carcinoma. Thyroid. 2001;11:749–55. doi: 10.1089/10507250152484583. [DOI] [PubMed] [Google Scholar]
- 45.Van Nostrand D, Aiken M, Atkins F, Moreau S, Garcia C, Acio E, et al. The utility of radioiodine scans prior to iodine 131 ablation in patients with well-differentiated thyroid cancer. Thyroid. 2009;19:849–55. doi: 10.1089/thy.2008.0419. [DOI] [PubMed] [Google Scholar]
- 46.American Thyroid Association (ATA); Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 47.Goldman JM, Line BR, Aamodt RL, Robbins J. Influence of triiodothyronine withdrawal time on 131 I uptake postthyroidectomy for thyroid cancer. J Clin Endocrinol Metab. 1980;50:734–9. doi: 10.1210/jcem-50-4-734. [DOI] [PubMed] [Google Scholar]
- 48.Bal CS, Kumar A, Chandra P, Dwivedi SN, Pant GS. A prospective clinical trial to assess the efficacy of radioiodine ablation as an alternative to completion thyroidectomy in patients with differentiated thyroid cancer undergoing sub-total thyroidectomy. Acta Oncol. 2006;45:1067–72. doi: 10.1080/02841860500418377. [DOI] [PubMed] [Google Scholar]
- 49.Edmonds CJ, Hayes S, Kermode JC, Thompson BD. Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodine. Br J Radiol. 1977;50:799–807. doi: 10.1259/0007-1285-50-599-799. [DOI] [PubMed] [Google Scholar]
- 50.Meier CA, Braverman LE, Ebner SA, Veronikis I, Daniels GH, Ross DS, et al. Diagnostic use of recombinant human thyrotropin in patients with thyroid carcinoma (phase I/II study) J Clin Endocrinol Metab. 1994;78:188–96. doi: 10.1210/jcem.78.1.8288703. [DOI] [PubMed] [Google Scholar]
- 51.Ladenson PW, Braverman LE, Mazzaferri EL, Brucker-Davis F, Cooper DS, Garber JR, et al. Comparison of administration of recombinant human thyrotropin with withdrawal of thyroid hormone for radioactive iodine scanning in patients with thyroid carcinoma. N Engl J Med. 1997;337:888–96. doi: 10.1056/NEJM199709253371304. [DOI] [PubMed] [Google Scholar]
- 52.Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999;84:3877–85. doi: 10.1210/jcem.84.11.6094. [DOI] [PubMed] [Google Scholar]
- 53.Kloos RT, Mazzaferri EL. A single recombinant human thyrotropin-stimulated serum thyroglobulin measurement predicts differentiated thyroid carcinoma metastases three to five years later. J Clin Endocrinol Metab. 2005;90:5047–57. doi: 10.1210/jc.2005-0492. [DOI] [PubMed] [Google Scholar]
- 54.Robbins RJ, Tuttle RM, Sonenberg M, Shaha A, Sharaf R, Robbins H, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin. Thyroid. 2001;11:865–9. doi: 10.1089/105072501316973127. [DOI] [PubMed] [Google Scholar]
- 55.Berg G, Lindstedt G, Suurküla M, Jansson S. Radioiodine ablation and therapy in differentiated thyroid cancer under stimulation with recombinant human thyroid-stimulating hormone. J Endocrinol Invest. 2002;25:44–52. doi: 10.1007/BF03343960. [DOI] [PubMed] [Google Scholar]
- 56.Hänscheid H, Lassmann M, Luster M, Thomas SR, Pacini F, Ceccarelli C, et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: Procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med. 2006;47:648–54. [PubMed] [Google Scholar]
- 57.Snyder J, Gorman C, Scanlon P. Thyroid remnant ablation: Questionable pursuit of an ill-defined goal. J Nucl Med. 1983;24:659–65. [PubMed] [Google Scholar]
- 58.Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, et al. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15:1033–41. doi: 10.1016/0014-2964(79)90291-3. [DOI] [PubMed] [Google Scholar]
- 59.McCowen KD, Adler RA, Ghaed N, Verdon T, Hofeldt FD. Low dose radioiodide thyroid ablation in postsurgical patients with thyroid cancer. Am J Med. 1976;61:52–8. doi: 10.1016/0002-9343(76)90030-9. [DOI] [PubMed] [Google Scholar]
- 60.Bal C, Chandra P, Kumar A, Dwivedi S. A randomized equivalence trial to determine the optimum dose of iodine-131 for remnant ablation in differentiated thyroid cancer. Nucl Med Commun. 2012;33:1039–47. doi: 10.1097/MNM.0b013e32835674af. [DOI] [PubMed] [Google Scholar]
- 61.Hackshaw A, Harmer C, Mallick U, Haq M, Franklyn JA. 131 I activity for remnant ablation in patients with differentiated thyroid cancer: A systematic review. J Clin Endocrinol Metab. 2007;92:28–38. doi: 10.1210/jc.2006-1345. [DOI] [PubMed] [Google Scholar]
- 62.Bal C, Chandra P, Kumar A, Dwivedi S. A randomized equivalence trial to determine the optimum dose of iodine-131 for remnant ablation in differentiated thyroid cancer. Nucl Med Commun. 2012;33:1039–47. doi: 10.1097/MNM.0b013e32835674af. [DOI] [PubMed] [Google Scholar]
- 63.Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–73. doi: 10.1056/NEJMoa1108586. [DOI] [PubMed] [Google Scholar]
- 64.Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366:1674–85. doi: 10.1056/NEJMoa1109589. [DOI] [PubMed] [Google Scholar]
- 65.Bal CS, Chandra P, Kumar A, Dwivedi SN. A randomized non-inferiority trial to determine the optimum dose of radioiodine for remnant ablation in differentiated thyroid cancer. Endocr Rev. 2010;31(Suppl 1):S1674. abstract. [Google Scholar]
- 66.Cheng W, Ma C, Fu H, Li J, Chen S, Wu S, et al. Low- or high-dose radioiodine remnant ablation for differentiated thyroid carcinoma: A meta-analysis. J Clin Endocrinol Metab. 2013;98:1353–60. doi: 10.1210/jc.2012-3682. [DOI] [PubMed] [Google Scholar]
- 67.Pilli T, Brianzoni E, Capoccetti F, Castagna MG, Fattori S, Poggiu A, et al. A comparison of 1850 (50 mCi) and 3700 MBq (100 mCi) 131-iodine administered doses for recombinant thyrotropin-stimulated postoperative thyroid remnant ablation in differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:3542–6. doi: 10.1210/jc.2007-0225. [DOI] [PubMed] [Google Scholar]
- 68.Rubino C, de Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89:1638–44. doi: 10.1038/sj.bjc.6601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–46. doi: 10.1002/cncr.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sawka AM, Thabane L, Parlea L, Ibrahim-Zada I, Tsang RW, Brierley JD, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: A systematic review and meta-analysis. Thyroid. 2009;19:451–7. doi: 10.1089/thy.2008.0392. [DOI] [PubMed] [Google Scholar]
- 71.Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229–42. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 72.Hovens GC, Stokkel MP, Kievit J, Corssmit EP, Pereira AM, Romijn JA, et al. Associations of serum thyrotropin concentrations with recurrence and death in differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:2610–5. doi: 10.1210/jc.2006-2566. [DOI] [PubMed] [Google Scholar]
- 73.Brabant G. Thyrotropin suppressive therapy in thyroid carcinoma: What are the targets? J Clin Endocrinol Metab. 2008;93:1167–9. doi: 10.1210/jc.2007-2228. [DOI] [PubMed] [Google Scholar]


