Abstract
Adams-Oliver syndrome (AOS) is a rare developmental disorder characterized by the presence of aplasia cutis congenita (ACC) of the scalp vertex and terminal limb-reduction defects. Cardiovascular anomalies are also frequently observed. Mutations in five genes have been identified as a cause for AOS prior to this report. Mutations in EOGT and DOCK6 cause autosomal-recessive AOS, whereas mutations in ARHGAP31, RBPJ, and NOTCH1 lead to autosomal-dominant AOS. Because RBPJ, NOTCH1, and EOGT are involved in NOTCH signaling, we hypothesized that mutations in other genes involved in this pathway might also be implicated in AOS pathogenesis. Using a candidate-gene-based approach, we prioritized DLL4, a critical NOTCH ligand, due to its essential role in vascular development in the context of cardiovascular features in AOS-affected individuals. Targeted resequencing of the DLL4 gene with a custom enrichment panel in 89 independent families resulted in the identification of seven mutations. A defect in DLL4 was also detected in two families via whole-exome or genome sequencing. In total, nine heterozygous mutations in DLL4 were identified, including two nonsense and seven missense variants, the latter encompassing four mutations that replace or create cysteine residues, which are most likely critical for maintaining structural integrity of the protein. Affected individuals with DLL4 mutations present with variable clinical expression with no emerging genotype-phenotype correlations. Our findings demonstrate that DLL4 mutations are an additional cause of autosomal-dominant AOS or isolated ACC and provide further evidence for a key role of NOTCH signaling in the etiology of this disorder.
Main Text
Adams-Oliver syndrome (AOS [MIM: 100300]) is a rare developmental disorder with an estimated incidence of 1 in 225,000 live births.1 It is typically characterized by the presence of both aplasia cutis congenita (ACC) of the scalp vertex and terminal limb defects, such as brachydactyly, oligodactyly, syndactyly, hypoplastic nails, or transverse amputations.2 In addition, vascular and cardiac anomalies, comprising pulmonary hypertension, ventricular septum defects, tetralogy of Fallot, and anomalies of the great arteries and their valves are frequently observed.2 In the past few years, mutations in five genes have been described as a cause of AOS. Mutations in EOGT (MIM: 615297) and DOCK6 (MIM: 614219) cause the autosomal-recessive form of AOS, whereas mutations in ARHGAP31 (MIM: 100300), RBPJ (MIM: 614814), and NOTCH1 (MIM: 616028) lead to the autosomal-dominant form of AOS.3–8NOTCH1, EOGT, and RBPJ are all members of the NOTCH signaling pathway. This pathway is conserved throughout metazoan species and is involved in many different tissue-specific cellular processes, including cell-fate determination and neural and hematopoietic stem cell differentiation.9,10 The NOTCH receptors and ligands are composed of several domains, including epidermal growth factor (EGF)-like domains. EOGT is an EGF domain-specific O-linked N-acetylglucosamine (GlcNAc) transferase known to glycosylate NOTCH1 and is therefore highly likely to influence NOTCH signaling through post-translational modification of the extracellular EGF-like repeats.4,10,11 RBPJ is a sequence-specific transcription factor that regulates the transcriptional activity of downstream target genes by binding to the intracellular domain of NOTCH, leading to the recruitment of additional proteins.12 In addition to causing AOS, mutations in NOTCH1 are also known to cause bicuspid aortic valve and thoracic aortic aneurysm.13 NOTCH signaling is activated by two families of ligands, namely Jagged (JAG1 or JAG2) and Delta (DLL1, DLL3, or DLL4). Of these, DLL4 has an essential role in vascular development and angiogenesis, which places it as a prime candidate for AOS, due to the presence of cardiovascular features in AOS-affected individuals.14,15
The study was approved by the appropriate institutional review board, and appropriate informed consents were obtained from human subjects. We performed targeted resequencing of DLL4 on 89 individuals by using HaloPlex Targeted Enrichment (Agilent Technologies) followed by sequencing on MiSeq (Illumina) with 150 bp paired-end reads. An in-house-developed Galaxy-based pipeline was used to process the raw data.16 Variant calling was performed with the Genome Analysis Toolkit Unified Genotyper,17 and variants were annotated and filtered with the in-house-developed database VariantDB.18 We selected unique variants or variants present in the ESP6500 and 1000 Genomes databases with a MAF<1%, prioritizing nonsense, nonsynonymous, splice-site, and indel variants in both a dominant and a recessive model.
Separately, whole-genome sequencing (WGS) was performed on family 2, and whole-exome sequencing (WES) was performed on family 6. Genomic DNA was extracted from saliva from the two affected siblings of family 2 and subjected to the Agilent SureSelect Target Enrichment (v.4; 51 Mb) capture process. After library preparation, sequencing was performed on the Illumina HiSeq2000, generating 100 bp paired-end reads. Over 97.5% of target regions were covered by >10×. Reads were mapped against reference genome UCSC hg19 with the Burrows-Wheeler Aligner. Single-nucleotide variants and indels were detected by SAMtools. WES on family 6 was performed as previously described.7 All variants were confirmed by bidirectional Sanger sequencing, and segregation analysis was performed on available family members.
In total, we screened 91 families and identified nine DLL4 (GenBank: NM_019074.3) heterozygous variants (with GRCh37 as a reference build), including nonsense, cysteine-replacing or -creating, and other missense substitutions (Figure 1, Table S1). These results designate mutations in this gene as an additional cause of an autosomal-dominant form of AOS.
Figure 1.
Mutation Analysis
(A) Pedigrees of the families with their respective mutations and nucleotide sequences.
(B) Structure of DLL4 with the structural domains and the identified mutations.
(C) Conservation of specific residues among species and NOTCH ligands.
In two families, we detected nonsense mutations in exon 9 of DLL4, specifically c.1660C>T (p.Gln554∗ [ClinVar: SCV000240088]) in family 1 and c.1672C>T (p.Arg558∗ [ClinVar: SCV000240089]) in family 2. Both stop-gain mutations are predicted to lead to nonsense-mediated decay (NMD) of the mutant mRNA transcript (Figure 1B).19 The severity of the clinical features in the affected family members of both families varied widely and included ACC, syndactyly, and brachydactyly (Figure 2, Table 1). In the proband of family 1 (1-III-3), cardiovascular features were also present, namely tricuspid insufficiency and ventricular septum defect (Table 1). Molecular screening of available family 1 members showed segregation of the mutation in the affected sister (1-III-1) and mother (1-II-2) and absence of the mutation from the unaffected father (1-II-1) and maternal grandmother (1-I-2). Interestingly, the mutation was not detected in the maternal grandfather (1-I-1), who presented with unilateral brachydactyly of the toes (III and IV). We failed to demonstrate the possibility of somatic mosaicism in his blood by a mutation-allele-specific PCR. Nevertheless, somatic mosaicism not affecting the hematopoietic tissue remains a plausible explanation. In family 2, the affected sister (2-II-1) also carried the mutation. Mutation analysis of the parents showed that c.1672C>T was inherited from the father (2-I-1), who did not show obvious signs of AOS. DNA of the paternal grandparents was not available to allow us to investigate whether the mutation in the father occurred de novo.
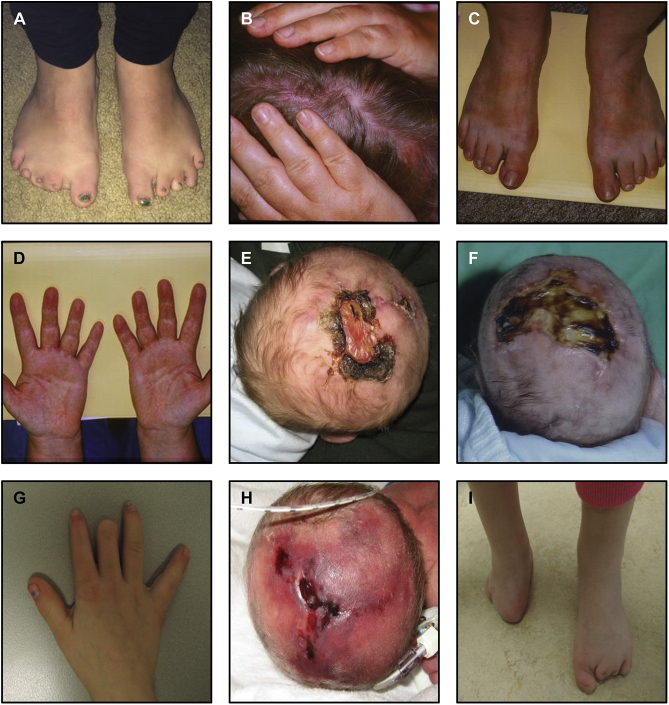
Figure 2.
Clinical Features
(A) Individual 2-II-1 with brachydactyly of the left foot and missing toes on the right foot.
(B) Individual 5-II-17 with a bald area on the scalp.
(C) Individual 5-II-17 with brachydactyly of toes.
(D) Individual 5-II-17 with brachydactyly of fingers.
(E) Individual 5-IV-12 with aplasia cutis congenita.
(F) Individual 6-II-1 with aplasia cutis congenita.
(G) Individual 8-II-1 with short distal phalangus of the middle finger and symphalangism of the index finger on the right hand.
(H) Individual 8-II-1 with aplasia cutis congenita.
(I) Individual 8-II-1 with symbrachydactyly of both feet.
Table 1.
Clinical Features
| Individual | Internal Reference | Mutation Status |
Clinical Features |
|||
|---|---|---|---|---|---|---|
| Scalp Defects | Limb Defects | Cardiovascular Features | Other Remarks | |||
| 1-I-1 | SK039 | − | / | left brachydactyly of 3rd and 4th toes (confirmed by X-ray) |
/ | / |
| 1-II-2 | SK039 | + | / | short distal phalanges (not confirmed by X-ray) | / | / |
| 1-III-1 | SK039 | + | ACC | / | normal echocardiogram | / |
| 1-III-3 | SK039 | + | ACC | brachydactyly, syndactyly of 2nd and 3rd toes on right foot | tricuspid insufficiency, ventricular septum defect | / |
| 2-II-1 | F45 | + | ACC with underlying skull defect | brachydactyly left foot, missing toes on right foot | normal echocardiogram | / |
| 2-II-3 | F45 | + | ACC with underlying skull defect | brachysyndactyly of right foot, severe brachysyndactyly of left foot | normal echocardiogram | small kidneys, mild hypertension |
| 3-I-2 | SK038 | + | ACC | / | / | / |
| 3-II-1 | SK038 | + | ACC | / | / | / |
| 4-II-1 | SK049 | + | ACC | / | / | / |
| 5-II-4 | SK087 | no DNA | ACC | syndactyly of 2nd and 3rd toes, hypoplastic toe nails, brachydactyly of toes | / | / |
| 5-II-14 | SK087 | + | bald area on scalp | / | / | cutis marmorata |
| 5-II-17 | SK087 | + | bald area on scalp | brachydactyly of fingers and toes, syndactyly of 2nd and 3rd toes | / | / |
| 5-III-3 | SK087 | + | bald area on scalp | / | / | / |
| 5-III-21 | SK087 | no DNA | bald area on scalp | / | / | / |
| 5-III-23 | SK087 | no DNA | ACC with underlying skull defect | / | / | / |
| 5-IV-3 | SK087 | + | ACC | / | normal echocardiogram | cutis marmorata, epilepsy, learning difficulties, borderline intellectual function (TIQ 76) at young age, normal IQ at later age, mild periventricular leukomalacia |
| 5-IV-12 | SK087 | no DNA | ACC | / | normal echocardiogram, portal hypertension, esophageal varices | splenomegaly, congenital liver fibrosis |
| 6-I-1 | UK16 | + | ACC | / | no cardiac problems | / |
| 6-II-1 | UK16 | + | ACC | / | normal echocardiogram | / |
| 7-I-2 | SK082 | + | / | / | / | unaffected |
| 7-II-1 | SK082 | + | ACC, delayed ossification | hypoplastic toe nails | no cardiac problems | cutis marmorata, normal chest X-ray at age of 2 months |
| 8-II-1 | SK081 | + | ACC | short distal phalangus of middle finger on the right hand, symphalangism of the index finger, symbrachydactyly of both feet | truncus arteriosus, ventricular septum defect | growth hormone deficiency |
| 9-II-1 | SK013 | + | ACC | / | / | / |
Abbreviations are as follows: +, present; −, absent; ACC, aplasia cutis congenita; /, unknown.
In addition to the two stop-gain mutations, we identified seven different heterozygous missense mutations: three cysteine-replacing mutations, one cysteine-creating mutation, and three other missense mutations. All identified missense mutations were predicted to be damaging by MutationTaster, PolyPhen-2, and SIFT, and none of the mutations were present in the following public databases: ESP6500, Kaviar,20 1000 Genomes, and ExAC.
In families 3, 4, and 5, cysteine-replacing mutations (c.1365C>G, p.Cys455Trp [ClinVar: SCV000240090]; c.1169G>A, p.Cys390Tyr [ClinVar: SCV000240091]; and c.1168T>C, p.Cys390Arg [ClinVar: SCV000240092], respectively) in exons 8 and 9 of DLL4 were identified. All of these mutations affect critical and highly conserved (up to D. melanogaster) cysteines of the consensus sequence of either the 5th or 7th EGF-like domain (Figure 1C). Similar to the features noted in those affected by the nonsense mutations, the severity of the clinical features of all available affected family members varied greatly, but all showed scalp involvement, including ACC or a bald scalp area (Table 1, Figure 2). The c.1365C>G (p.Cys455Trp) mutation, identified in the proband of family 3 (3-II-1), was confirmed in the affected mother (3-I-2) and in a sister (3-II-2), but no clinical information of the latter was available. We found a mutation replacing the cysteine residue at position 390 but leading to different amino acid substitutions in both family 4 (c.1169G>A, p.Cys390Tyr) and family 5 (c.1168T>C, p.Cys390Arg). The proband of family 4 (4-II-1) showed isolated ACC, and no family members were available for screening. The proband of family 5 (5-IV-3) is part of a large family with multiple affected individuals. The father (5-III-3) and two great aunts (5-II-14 and 5-II-17) carried the c.1168T>C mutation (Figure 1A). They were all only mildly affected, but did have severely affected offspring (Table 1, Figure 2), further illustrating the highly variable clinical expression for DLL4-associated AOS.
In the proband of family 6 (6-II-1), we detected a heterozygous cysteine-creating mutation: c.556C>T (p.Arg186Cys [ClinVar: SCV000240093]). The affected family members both exhibited isolated ACC (Table 1, Figure 2). The arginine at this position is conserved between DLL4 and DLL3 and is conserved across mammalian species (Figure 1C). The affected father (6-I-1) carried the mutation, whereas the unaffected mother (6-I-2) did not.
In families 7, 8, and 9, we found missense variants (c.799C>A, p.Pro267Thr [ClinVar: SCV000240094]; c.361G>C, p.Ala121Pro [ClinVar: SCV000240095]; and c.583T>C, p.Phe195Leu [ClinVar: SCV000240096], respectively) affecting specific residues in functional domains of DLL4; these residues are conserved up to D. melanogaster and in the four most closely related NOTCH-receptor ligands, namely JAG1, JAG2, DLL1, and DLL4 (Figure 1C). Both families 7 and 8 presented with scalp defects, limb defects, and cardiovascular features (Table 1, Figure 2). The parents of proband 7 were reported as unaffected, but the mother (7-I-2), who was a mutation carrier, has a positive family history for cardiac events; this history includes a brother with bicuspid aortic valve with narrowed aorta ascendens and a maternal half-brother who died at birth of an unspecified heart defect. Unfortunately, DNA of the maternal grandparents or other family members was not available. In the proband of family 8 (8-II-1), the c.361G>C (p.Ala121Pro) mutation was shown to be de novo, given that both unaffected parents did not carry the mutation (Figure 1A). The index of family 9 has been described before and shows only ACC.21 In this individual, we identified a heterozygous c.583T>C (p.Phe195Leu) variant. No further information of familial occurrence was available.
The DLL4 variants are distributed across the complete protein, affecting all known structural domains (MNNL [N-terminal domain of NOTCH ligands], DSL [Delta/Serrate/Lag-2], and EGF-like domains), without any obvious mutational hotspots. Recently, the structural basis of the interaction between NOTCH1 and DLL4 has been elaborated.10 The binding of NOTCH1 to DLL4 is coordinated by the interaction between the NOTCH1 EGF-like domain 11 and the DLL4 DSL domain. DLL4-NOTCH1 binding is further modulated by the interaction between NOTCH1 EGF-like domain 12 and the MNNL domain of DLL4. Glycosylation of NOTCH1 is essential and specific for the binding of DLL4.10
Three-dimensional modeling predictions of our DLL4 mutations reveal interesting changes in protein conformation and amino acid functionality (Figure S1). In total, three cysteine-replacing pathogenic variants have been found, two at position 390 (p.Cys390Arg and p.Cys390Tyr), located in the EGF-like domain 5, and one at position 455 (p.Cys455Trp), located in the EGF-like domain 7. In their respective EGF domains, all three cysteines are involved in the formation of a disulfide bond, which will be lost due to the introduction of these pathogenic variants. Furthermore, both arginine and tyrosine residues at position 390 are too bulky to fit in this domain and are therefore likely to disrupt the structure. Hence, structural changes are predicted to occur, which will most likely lead to a loss of function of DLL4.
Both phenylalanine at position 195 and arginine at position 186 are located in the DLL4 DSL domain (Figure 1B), a conserved central domain directly involved in ligand binding to the EGF-like domain 11 of the NOTCH1 receptor.10 It has been shown that the phenylalanine at position 195 is a key interface residue important for binding, and when mutated to an alanine residue, it results in a substantial decrease in NOTCH1 interaction.10 The p.Arg186Cys pathogenic variant is located in the surface loop of the DSL domain. The introduction of a cysteine at this location is not expected to cause steric hindrance; however, the important interaction function of the Arginine186 residue is likely to be lost. In addition, this mutation creates a cysteine, which could dramatically alter the conformation of the protein by forming a novel disulfide bond. Both these DSL domain mutations will likely reduce the binding affinity of DLL4 to NOTCH1.
The Alanine121 residue is located in a beta-strand on the inside of the MNNL domain. Together with the DSL domain, the MNNL domain is involved in the binding of the ligand to EGF-like domain 12 of the NOTCH-receptor and regulates ligand pleiotropy.10 The replacement of Alanine 121 by proline is predicted to disrupt the local structure and function.
Lastly, the Proline267 residue is located in EGF-like domain 2 and makes contact with EGF-like domain 1 in a surface loop. This interaction might be altered by the substitution to threonine.
The discovery of DLL4 mutations in our AOS-affected individuals, in addition to prior identification of mutations in EOGT, RBPJ, and NOTCH1, confirms the key role of the NOTCH signaling pathway in the pathogenesis of AOS. Activation of NOTCH signaling through binding of a NOTCH ligand (JAG1, JAG2, DLL1, DLL3, or DLL4) to one of the NOTCH receptors (NOTCH1, NOTCH2, NOTCH3, or NOTCH4) results in two proteolytic cleavage reactions performed by a member of the ADAM (a disintegrin and metalloproteinase domain-containing protein) family and by the ү-secretase complex. The second cleavage releases the intracellular domain of NOTCH, NICD, which is translocated to the nucleus, where it binds to the coactivator mastermind ligand (MAML) and the DNA binding protein RBPJ to induce transcription of NOTCH target genes.22
DLL4 is a ligand of the NOTCH receptors and is essential for vascular development.12 During embryonic development, protein localization of DLL4 is restricted to the large arteries, whereas in adult stages, the protein is also present in the smaller arteries and microvessels. Haploinsufficiency of Dll4 in mice results in embryonic lethality as a result of profound defects in vascular and arterial development.15 In contrast to what occurs in mice models, human haploinsufficiency of DLL4 does not result in lethality given that we have identified two heterozygous nonsense mutations, which most likely lead to NMD.19 The lethality in mice could be related to the inbred genetic background of the mice23 and potential functional differences in redundancy between humans and mice. The identification of both DLL4 nonsense mutations and several missense mutations in key functional domains of DLL4 that are predicted to disrupt DLL4 integrity suggests that loss of function is the pathogenetic mechanism. Despite DLL4 being described as being essential for vascular development,12 not all individuals with DLL4 mutations presented with cardiovascular features (Table 1), although complete cardiovascular exams have not been performed in all cases.
We did not observe a clear genotype-phenotype correlation between DLL4 mutation and AOS subtype. Furthermore, we observed marked intrafamilial variability in the phenotypic expression. For example, in family 5, the p.Cys390Arg substitution leads to a severe phenotype in the offspring, whereas the parents show isolated ACC. This suggests that other factors are involved in the clinical expression of this disease; these factors could be environmental, but also other genetic and epigenetic influences involved in the NOTCH signaling pathway or other AOS related pathways. In addition to the variable clinical expression, we also observed incomplete penetrance in families 2 and 7, and additional unidentified asymptomatic mutation carriers may exist in other families. Highly variable expressivity and incomplete penetrance, as well as the association with cardiovascular abnormalities, are similar to what has been observed in NOTCH1-related AOS.7,8 In contrast, cerebral and ocular abnormalities, which have been found to be particularly associated with autosomal-recessive DOCK6-related AOS, appear to be uncommon in those autosomal-dominant forms.24
In conclusion, through screening a cohort of 91 families affected with AOS or ACC, we have identified nine heterozygous variants in DLL4, including nonsense, cysteine-replacing or -creating, and other missense mutations, demonstrating that mutations in this gene are an important cause of autosomal-dominant AOS or isolated ACC. Affected individuals show variable clinical expression with no clear genotype-phenotype correlations at present. With the addition of DLL4, four genes involved in NOTCH signaling have now been implicated in AOS, confirming that disruption of this pathway plays a major role in the pathogenesis of AOS.
Acknowledgments
We are grateful to the families who participated in this study. B.L.L. is a senior clinical investigator of the Fund for Scientific Research, Flanders and holds a European Reseach Council starting grant. J.A.N.M. is a predoctoral researcher of the Fund for Scientific Research, Flanders. A.B.S. was supported by the German Academic Exchange Service and the German Research Foundation. This research was supported by funding from the Fund for Scientific Research, Flanders (G.0221.12), the Fondation Leducq, and the European Research Council, a Center for Systems Biology P50 grant (GM076547, NIH), and the Rare Disease Foundation.
Published: August 20, 2015
Footnotes
Supplemental Data include one figure and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2015.07.015.
Contributor Information
Bart Loeys, Email: bart.loeys@uantwerpen.be.
Wim Wuyts, Email: wim.wuyts@uantwerpen.be.
Accession Numbers
The ClinVar accession numbers for the variants reported in this paper are SCV000240088, SCV000240089, SCV000240090, SCV000240091, SCV000240092, SCV000240093, SCV000240094, SCV000240095, and SCV00024096.
Web Resources
The URLs for data presented herein are as follows:
1000 Genomes, http://www.1000genomes.org/
Burrows-Wheeler Aligner, http://bio-bwa.sourceforge.net/
ExAC Browser, http://exac.broadinstitute.org/
MutationTaster, http://www.mutationtaster.org/
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
SAMtools, http://samtools.sourceforge.net/
SIFT, http://sift.jcvi.org/
UCSC Genome Browser hg19, http://genome.ucsc.edu/
Supplemental Data
References
- 1.Forrest H., Adams C.P.O. Hereditary deformities in man due to arrested development. J. Hered. 1945;36:3–7. [Google Scholar]
- 2.Snape K.M., Ruddy D., Zenker M., Wuyts W., Whiteford M., Johnson D., Lam W., Trembath R.C. The spectra of clinical phenotypes in aplasia cutis congenita and terminal transverse limb defects. Am. J. Med. Genet. A. 2009;149A:1860–1881. doi: 10.1002/ajmg.a.32708. [DOI] [PubMed] [Google Scholar]
- 3.Hassed S.J., Wiley G.B., Wang S., Lee J.Y., Li S., Xu W., Zhao Z.J., Mulvihill J.J., Robertson J., Warner J., Gaffney P.M. RBPJ mutations identified in two families affected by Adams-Oliver syndrome. Am. J. Hum. Genet. 2012;91:391–395. doi: 10.1016/j.ajhg.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaheen R., Aglan M., Keppler-Noreuil K., Faqeih E., Ansari S., Horton K., Ashour A., Zaki M.S., Al-Zahrani F., Cueto-González A.M. Mutations in EOGT confirm the genetic heterogeneity of autosomal-recessive Adams-Oliver syndrome. Am. J. Hum. Genet. 2013;92:598–604. doi: 10.1016/j.ajhg.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaheen R., Faqeih E., Sunker A., Morsy H., Al-Sheddi T., Shamseldin H.E., Adly N., Hashem M., Alkuraya F.S. Recessive mutations in DOCK6, encoding the guanidine nucleotide exchange factor DOCK6, lead to abnormal actin cytoskeleton organization and Adams-Oliver syndrome. Am. J. Hum. Genet. 2011;89:328–333. doi: 10.1016/j.ajhg.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Southgate L., Machado R.D., Snape K.M., Primeau M., Dafou D., Ruddy D.M., Branney P.A., Fisher M., Lee G.J., Simpson M.A. Gain-of-function mutations of ARHGAP31, a Cdc42/Rac1 GTPase regulator, cause syndromic cutis aplasia and limb anomalies. Am. J. Hum. Genet. 2011;88:574–585. doi: 10.1016/j.ajhg.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Southgate L., Sukalo M., Karountzos A.S., Taylor E.J., Collinson C.S., Ruddy D., Snape K.M., Dallapiccola B., Tolmie J.L., Joss S. Haploinsufficiency of the NOTCH1 Receptor as a Cause of Adams-Oliver Syndrome with Variable Cardiac Anomalies. Circ. Cardiovasc. Genet. 2015 doi: 10.1161/CIRCGENETICS.115.001086. Published online May 11, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stittrich A.B., Lehman A., Bodian D.L., Ashworth J., Zong Z., Li H., Lam P., Khromykh A., Iyer R.K., Vockley J.G. Mutations in NOTCH1 cause Adams-Oliver syndrome. Am. J. Hum. Genet. 2014;95:275–284. doi: 10.1016/j.ajhg.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chillakuri C.R., Sheppard D., Lea S.M., Handford P.A. Notch receptor-ligand binding and activation: insights from molecular studies. Semin. Cell Dev. Biol. 2012;23:421–428. doi: 10.1016/j.semcdb.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luca V.C., Jude K.M., Pierce N.W., Nachury M.V., Fischer S., Garcia K.C. Structural biology. Structural basis for Notch1 engagement of Delta-like 4. Science. 2015;347:847–853. doi: 10.1126/science.1261093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogawa M., Sawaguchi S., Kawai T., Nadano D., Matsuda T., Yagi H., Kato K., Furukawa K., Okajima T. Impaired O-linked N-acetylglucosaminylation in the endoplasmic reticulum by mutated epidermal growth factor (EGF) domain-specific O-linked N-acetylglucosamine transferase found in Adams-Oliver syndrome. J. Biol. Chem. 2015;290:2137–2149. doi: 10.1074/jbc.M114.598821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 13.McKellar S.H., Tester D.J., Yagubyan M., Majumdar R., Ackerman M.J., Sundt T.M., 3rd Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2007;134:290–296. doi: 10.1016/j.jtcvs.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 14.Algaze C., Esplin E.D., Lowenthal A., Hudgins L., Tacy T.A., Selamet Tierney E.S. Expanding the phenotype of cardiovascular malformations in Adams-Oliver syndrome. Am. J. Med. Genet. A. 2013;161A:1386–1389. doi: 10.1002/ajmg.a.35864. [DOI] [PubMed] [Google Scholar]
- 15.Gale N.W., Dominguez M.G., Noguera I., Pan L., Hughes V., Valenzuela D.M., Murphy A.J., Adams N.C., Lin H.C., Holash J. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc. Natl. Acad. Sci. USA. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giardine B., Riemer C., Hardison R.C., Burhans R., Elnitski L., Shah P., Zhang Y., Blankenberg D., Albert I., Taylor J. Galaxy: a platform for interactive large-scale genome analysis. Genome Res. 2005;15:1451–1455. doi: 10.1101/gr.4086505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DePristo M.A., Banks E., Poplin R., Garimella K.V., Maguire J.R., Hartl C., Philippakis A.A., del Angel G., Rivas M.A., Hanna M. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandeweyer G., Van Laer L., Loeys B., Van den Bulcke T., Kooy R.F. VariantDB: a flexible annotation and filtering portal for NGS data. Genome Med. 2014;6 doi: 10.1186/s13073-014-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagy E., Maquat L.E. A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem. Sci. 1998;23:198–199. doi: 10.1016/s0968-0004(98)01208-0. [DOI] [PubMed] [Google Scholar]
- 20.Glusman G., Caballero J., Mauldin D.E., Hood L., Roach J.C. Kaviar: an accessible system for testing SNV novelty. Bioinformatics. 2011;27:3216–3217. doi: 10.1093/bioinformatics/btr540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itin P.H., Bargetzi M.C. Aplasia cutis congenita with precancerous transformation - the first case. Why do these scars never develop invasive tumors? Eur. J. Dermatol. 2000;10:181–183. [PubMed] [Google Scholar]
- 22.Bray S.J. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 23.Sigmund C.D. Viewpoint: are studies in genetically altered mice out of control? Arterioscler. Thromb. Vasc. Biol. 2000;20:1425–1429. doi: 10.1161/01.atv.20.6.1425. [DOI] [PubMed] [Google Scholar]
- 24.Sukalo M., Tilsen F., Kayserili H., Müller D., Tüysüz B., Ruddy D.M., Wakeling E., Ørstavik K.H., Snape K.M., Trembath R. DOCK6 mutations are responsible for a distinct autosomal-recessive variant of Adams-Oliver syndrome associated with brain and eye anomalies. Hum. Mutat. 2015;36:593–598. doi: 10.1002/humu.22795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.