Abstract
The link of chromatin remodeling to both neurodevelopment and cancer has recently been highlighted by the identification of mutations affecting BAF chromatin-remodeling components, such as ARID1B, in individuals with intellectual disability and cancer. However, the underlying molecular mechanism(s) remains unknown. Here, we show that ARID1B is a repressor of Wnt/β-catenin signaling. Through whole-transcriptome analysis, we find that in individuals with intellectual disability and ARID1B loss-of-function mutations, Wnt/β-catenin target genes are upregulated. Using cellular models of low and high Wnt/β-catenin activity, we demonstrate that knockdown of ARID1B activates Wnt/β-catenin target genes and Wnt/β-catenin-dependent transcriptional reporters in a β-catenin-dependent manner. Reciprocally, forced expression of ARID1B inhibits Wnt/β-catenin signaling downstream of the β-catenin destruction complex. Both endogenous and exogenous ARID1B associate with β-catenin and repress Wnt/β-catenin-mediated transcription through the BAF core subunit BRG1. Accordingly, mutations in ARID1B leading to partial or complete deletion of its BRG1-binding domain, as is often observed in intellectual disability and cancers, compromise association with β-catenin, and the resultant ARID1B mutant proteins fail to suppress Wnt/β-catenin signaling. Finally, knockdown of ARID1B in mouse neuroblastoma cells leads to neurite outgrowth through β-catenin. The data suggest that aberrations in chromatin-remodeling factors, such as ARID1B, might contribute to neurodevelopmental abnormalities and cancer through deregulation of developmental and oncogenic pathways, such as the Wnt/β-catenin signaling pathway.
Introduction
The human BRG1-associated factors (BAF) chromatin-remodeling complex (also known as SWI/SNF-A complex) repositions and alters the structure of nucleosomes, facilitating the activation or repression of gene transcription.1 ARID1, also known as BAF250 and hOsa, is the largest subunit of the BAF complex and has two isoforms: ARID1A and ARID1B.2,3ARID1B is expressed in the human brain and in mammalian embryonic stem cells, and ARID1B-associated BAF complexes are important in the early stages of murine brain development and essential for the pluripotency of mouse embryonic stem cells.4–6 Recent studies have reported overlapping mutational spectrums in BAF subunits, including loss-of-function mutations in ARID1B (MIM: 614556), in both neurodevelopmental disorders, such as Coffin-Siris syndrome (MIM: 135900), and non-syndromic intellectual disability (ID [MIM: 614562]), as well as in numerous cancer entities.7–15 However, the number of studies addressing the molecular mechanisms by which ARID1B functions and how its loss-of-function contributes to neurodevelopmental disorders and cancer is limited. The role of aberrant Wnt/β-catenin signaling in cancer, particularly colorectal cancer, is well established, and the pathway has been implicated in neuronal development. Increased dosage as well as knockout of β-catenin in the brain leads to CNS defects.16–19 β-catenin is phosphorylated in the “β-catenin destruction complex,” a multi-protein complex composed of the adenomatous polyposis coli (APC) gene product, Axin1 and Axin2, and glycogen synthase kinase GSK3β, and earmarked for proteosomal degradation. In the presence of Wnt ligands, this destruction complex is inactivated and β-catenin is stabilized and subsequently translocates to the nucleus to activate transcription of Wnt target genes.20 Wnt target genes control a variety of cellular processes, including proliferation, differentiation, and pluripotency.21
A connection between the BAF chromatin-remodeling complex and Wnt/β-catenin signaling was originally identified by Baker et al., showing that BRG1 interacts with β-catenin to promote target-gene activation.22 These findings have been supported by subsequent research showing that BRG1 is required for Wnt/β-catenin-mediated transcriptional activation of target genes and that loss of BRG1 attenuates aberrant Wnt signaling and prevents Wnt-dependent tumorigenesis in the murine small intestine.23,24 However, a contrasting view has emerged from a recent report suggesting that SNF5, another subunit of the BAF complex and a tumor suppressor, represses Wnt/β-catenin-mediated gene expression and that loss of SNF5 leads to activation of Wnt target genes.25 Interestingly, frequent inactivating mutations in ARID1B were also reported in colorectal cancers driven by aberrant Wnt/β-catenin signaling, and ARID1B-mutated medulloblastomas show activation of the Wnt/β-catenin pathway.7,26 By using individuals with ID who are harboring ARID1B loss-of-function mutations as a departure point and subsequently moving to cellular models to study in detail ARID1B's function, we find that ARID1B represses Wnt/β-catenin signaling in the nucleus at the level of β-catenin through a BRG1-dependent mechanism. Importantly, ARID1B loss-of-function mutants fail to repress Wnt/β-catenin-mediated transcription and, similarly to knockdown of ARID1B, lead to upregulation of target genes of the pathway.
Materials and Methods
Subjects
The six individuals with ID were described in detail in the previous study9 (see Figure S1.) The current study was approved by the institutional ethical review board of the medical faculty of Erlangen-Nürnberg University, and informed consent was obtained from the participants or their legal guardians.
RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR
Total RNA was extracted from untransformed blood lymphocytes from six ID-affected individuals and twelve control individuals with the PAXgene Blood System (Becton Dickinson). The RNA extraction from U2OS, 293T, and HCT116 cells was performed with an RNeasy Mini Kit and a Qiashredder Kit (QIAGEN). cDNA was prepared with a Superscript II Reverse Transcriptase Kit (Invitrogen), according to the manufacturer’s instructions. Expression levels of genes were analyzed by qRT-PCR with predesigned Taqman gene-expression assays (MYC: Hs00153408_m1, LEF1: Hs01547250_m1, TCF7: Hs00175273_m1, ARID1B: Hs00368175_m1, and ARID1A: Hs00195664_m1; Life Technologies) on an ABI 7900HT instrument (Life Technologies). For normalization of results to the mean, the following endogenous controls were used: β-actin (huACTB), β-2 microglobulin (huB2M), acidic ribosomal protein (huPO), and transcription-factor IID (huTBP) (Applied Biosystems). Expression in the six ID-affected individuals was normalized relative to the twelve control individuals, whereas expression in ARID1B siRNA-treated U20S, 293T, and HCT116 cells was normalized to control siRNA-treated cells. Reactions were performed in four replicates. mRNA levels were calculated with the ΔΔCt method. The p values for control and ID-affected individuals and cell lines were calculated with Mann-Whitney and unpaired Student’s t tests, respectively.
RNA-Seq and Bioinformatic Analysis
RNA were amplified with the Ovation RNA-Seq System V2 (Nugen), according to the manufacturer’s instructions. cDNA was purified with the MinElute Reaction Cleanup Kit (QIAGEN), fragmented by ultrasonic shearing with the Covaris S2 System (Covaris), and was subsequently used for automated library construction on a Beckman Coulter SRIworks platform (Beckman Coulter). Sequencing was carried out on a SOLID platform (Life Technologies). The LifeScope analysis suite produced both absolute read counts and reads per kilobase per million reads (RPKM) for 24,364 genes for each individual. Primary component analysis of the RPKM values was performed with Cluster 3.0.27 All subsequent statistical analyses were also performed in R version 2.14.2. Pathway analysis for 452 differentially expressed genes with an expression change of >2-fold in either direction and a p value <0.01 was performed with Ingenuity Systems IPA Software.
Cell Culture, Transfections, and siRNA Sequences
HEK293T cells, human osteosarcoma U2OS cells, and colorectal cancer cell lines HCT116, DLD1, SW480, and CaCo-2 were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin at 37°C in 10% CO2. For the lymphoblastoid cell lines used in Figure 2G, lymphocytes were transformed and immortalized with Epstein-Barr virus and cultured in Roswell Park Memorial Institute 1640 L-glutamin medium with 20% FCS (Biochrom) and 100mg/ml cyclosporin. Previously published siRNAs against GFP, APC, AXIN1, BRG1 (Santa Cruz), and β-catenin, as well as siRNAs targeting human ARID1B 5′-CUCUCUGGUUGCAUCUGUC-3′ (ARID1B) and 5′-GCCGAAUUACAAACGCCAUAU-3′ (ARID1B2), as well as siRNAs targeting ARID1A 5′-CUCAUUGGUUUCACAAGUC-3′ (Thermo Scientific), were transfected with oligofectamin (Invitrogen).28–31 Plasmids were transfected with polyethylenimine. Plasmids and siRNAs were co-transfected via Lipofectamin (Invitrogen).
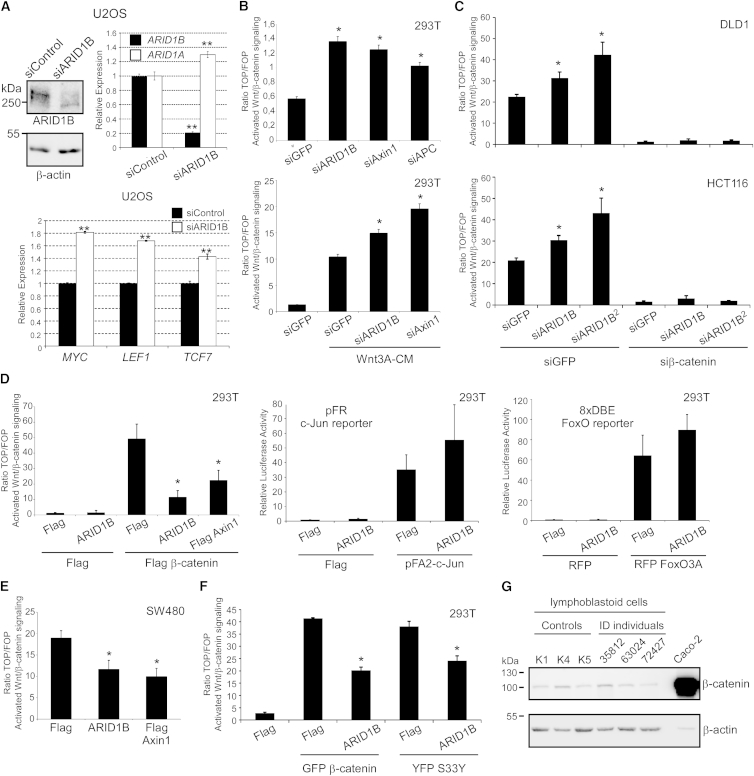
Figure 2.
ARID1B Represses Wnt/β-Catenin Signaling
(A) Western blotting for ARID1B and β-actin (left panels) and qRT-PCR for human ARID1B and ARID1A (right panel) from lysates and with cDNAs, respectively, of U2OS cells transfected with indicated siRNAs. Molecular weights (kDa) are indicated on the left of western-blotting panels. The bottom graph shows qRT-PCR for Wnt/β-catenin target genes MYC, LEF1, and TCF7 with cDNAs from U2OS cells transfected with indicated siRNAs.
(B) Ratio of TOP/FOPFlash luciferase activity measuring β-catenin-dependent transcription in HEK293T cells transfected with indicated siRNAs, in the absence (top panel) or presence (bottom panel) of Wnt3a-conditioned media (Wnt3A-CM) for 7 hr.
(C) Ratio of TOP/FOPFlash in DLD1 (top panel) and HCT116 (bottom panel) cells transfected with indicated combinations of siRNAs.
(D) Luciferase reporter assays in HEK293T cells for Wnt/β-catenin-dependent (TOP/FOPFlash; left panel), c-Jun-dependent (pFR; middle panel), and FoxO-dependent (8xDBE; right panel) reporters after transfection of indicated plasmid combinations. ARID1B refers to T7-tagged ARID1B32 (also see Materials and Methods).
(E) Ratio of TOP/FOPFlash in SW480 cells transfected with indicated plasmids.
(F) Ratio of TOP/FOPFlash in 293T cells co-transfected with GFP β-catenin or a non-degradable β-catenin mutated on serine 33 (YFP S33Y) together with ARID1B or an empty vector (Flag) as controls.
(G) Western blotting for β-catenin and β-actin from lysates of lymphoblastoid cell lines derived from three control indiviudals and three ID-affected individuals as well as from colorectal cancer cells Caco-2. In all panels the cell line used is indicated at the top. Error bars represent SEM from at least three independent experiments, each performed with duplicates. In panel (A), error bars represent SEM from quadruplicates. Single and double asterisks denote a p value of <0.05 and <0.01, respectively, calculated with unpaired Student’s t test, as compared to respective controls indicated in the main text.
Molecular Biology, Plasmids, and Mutagenesis
Standard molecular biology methods were used for cloning N-terminal GFP- and Flag-tagged constructs. Luciferase reporters TOPFlash, FOPFlash, pFR, and 8xDBE, as well as expression plasmids for pFA2-c-Jun, RFP FoxO3A, GFP β-catenin, and Flag Axin, have been described in Dehner et al. and Hadjihannas et al.28,29 T7-tagged ARID1B (T7hOsa2) and Flag-tagged-BRG1 were obtained from Addgene and have been previously described.32 The mutations in ARID1B were generated by mutagenesis PCR with 5′-GCAGAAAGGACTTGATAAGCTTCCTAGAGGATGGGGTCACGATG-3′ (forward) and 5′-CTTATCAAGTCCTTTCTGCACAGCTATGGCCCTTGCTGCTAG-3′ (reverse) primers for ARID1B-2188 and 5′-ACCCAAAGGAGTAGCCCAAGCAAGCCAGTAAGTTCGACAAGCTGCCA-3′ (forward) and 5′-CTTGGGCTACTCCTTTGGGTCTGCAGCGGCGTCCGGGGCAGTCAGAG-3′ (reverse) primers for ARID1B-1777. The sequences of ARID1B plasmids were confirmed by sequencing as described in Hadjihannas et al.33
TOP/FOPFlash Luciferase-Reporter Assays
Cells transfected with TOP/FOPFlash reporters together with plasmids for 24 hr or siRNAs for 48 hr were lysed and luciferase activities were measured on a centro X53 LB 960 microplate Luminometer. For each experiment, TOP/FOPFlash reporters with plasmids and/or siRNAs were transfected in duplets together with an expression plasmid for β-galactosidase (β-gal). After cell lysis, β-gal expression was determined on an ELISA reader and used to normalize luciferase activity for transfection efficiency. Transfection efficiency-normalized luciferase values from TOPFlash and FOPFlash duplets were subsequently averaged and used to calculate the TOP/FOPFlash ratio. TOP/FOPFlash ratios from at least three biological replicates were used to determine SEM and p values, with unpaired Student’s t test, for differences between transfections referred to in the Results.
Immunofluorescence Staining and Microscopy
Cells on coverslips were fixed in 100% methanol at −20°C or 3% paraformaldehyde at room temperature, permeablized with Triton X-100, and blocked in DMEM media containing 10% FCS. Cells were incubated with primary antibodies rabbit polyclonal anti-ARID1B (Millipore), mouse monoclonal anti-ARID1B (Abcam), rabbit polyclonal anti-β-catenin (H102) (Santa Cruz), and rabbit polyclonal PRMT5 (Abcam), followed by washing (1× phosphate-buffered saline) and incubation with appropriate Cy3 conjugated secondary antibodies. Nuclei of stained cells were counterstained with 4,6-diamidino-2phenylindole (DAPI), and coverslips were mounted on glass slides in Mowiol. Coverslips were viewed on a Zeiss Axioplan2 fluorescence microscope, and pictures were acquired with the Metamorph and AxioVision softwares without further enhancement. For proximity ligation assays (PLAs), PLA signals were automatically marked with threshold images by a morphometric analysis module, and the number of signals per cell was calculated by first marking nuclear regions with threshold images of DAPI fluorescence and subsequently determining the number of PLA signals within as well as outside nuclei. For quantification of nuclear puncta size in Figures S4A and S4B, nuclear puncta were automatically marked with threshold images by a morphometric analysis module, and the area in pixels was calculated for every punctum in 20 cells from three independent experiments. Pictures in Figure 4G were processed with the 2D deconvolution module of the Metamorph software. For quantification of Neuro2A differentiation in Figure 5D, the percentage of cells showing neurites with a length double that of the cell body diameter was calculated from images of ten random coverslip fields from three independent experiments.
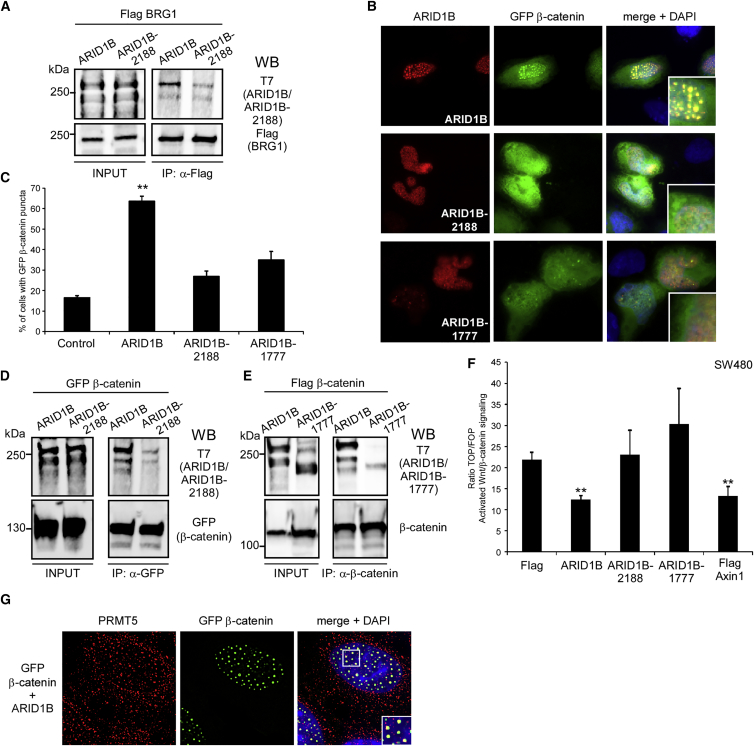
Figure 4.
Mutant ARID1B Proteins Show Compromised Association with BRG1 and β-Catenin and Fail to Repress Wnt/β-Catenin-Dependent Transcription
(A) Western blotting (WB) with indicated antibodies of lysates (INPUT) and Flag immunoprecipitates (IP) from 293T cells co-transfected with Flag BRG1 and T7-tagged ARID1B or ARID1B-2188 mutant, as indicated.
(B) Representative immunofluorescence pictures of U2OS cells co-expressing GFP β-catenin together with ARID1B or mutants ARID1B-2188 and ARID1B-1777 as indicated and stained with an antibody against ARID1B.
(C) Quantification from experiments conducted in (B).
(D) Western blotting (WB) with indicated antibodies of lysates (INPUT) and GFP immunoprecipitates (IP) from 293T cells co-transfected with GFP β-catenin and T7-tagged ARID1B or ARID1B-2188 mutant, as indicated.
(E) Western blotting (WB) with indicated antibodies of lysates (INPUT) and β-catenin immunoprecipitates (IP) from 293T cells co-transfected with Flag β-catenin and T7-tagged ARID1B or ARID1B-1777 mutant, as indicated. Molecular weights (kDa) in panels (A), (D), and (E) are indicated on the left of the panels.
(F) Ratio of TOP/FOPFlash in SW480 cells transfected with indicated plasmids.
(G) Representative immunofluorescence pictures of U2OS cells after 2D image deconvolution co-expressing ARID1B and GFP β-catenin, stained with antibodies against PRMT5. In panels (B) and (G), right columns show merged pictures with DAPI and inlets show magnified nuclear regions. In panel (C), error bars show SEM from 60 nuclei and double asterisks denote p < 0.01 for differences from controls, with unpaired Student’s t test, from two independent experiments. In panel (F), error bars show SEM and (∗) denotes p < 0.05 for differences from control (Flag) from at least three independent experiments.
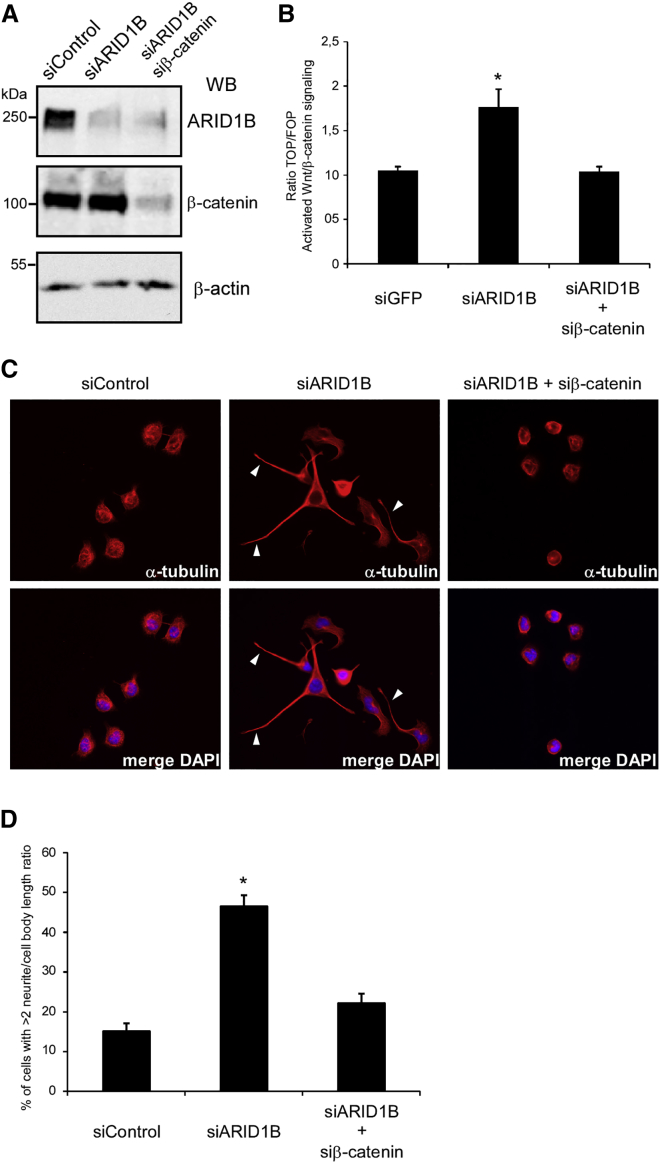
Figure 5.
Knockdown of ARID1B in Neuro2A Cells Leads to Neurite Outgrowth through β-Catenin
(A) Western blotting (WB) for ARID1B, β-catenin and β-actin from lysates of mouse neuroblastoma Neuro2A cells transfected with siRNAs against control, ARID1B or ARID1B, and β-catenin, as indicated. Molecular weights (kDa) are indicated.
(B) Ratio of TOP/FOPFlash luciferase activity measuring β-catenin-dependent transcription in Neuro2A cells transfected with indicated siRNAs.
(C) Representative immunofluorescence pictures of Neuro2A cells transfected with siRNAs against control or ARID1B and β-catenin as indicated, stained for α-tubulin. Arrowheads point to neurites.
(D) Quantification of cells from (C) indicating the percentage of cells exhibiting neurites with twice the length of the cell body. n = number of cells analyzed from three independent experiments. Error bars in (B) and (D) represent SEM from two and three independent experiments, respectively. Asterisks denote statistical significance (p value of <0.05) as compared to respective controls indicated in the main text, calculated with unpaired Student’s t test.
PLA
PLA was carried out with the Duolink In Situ Starter Kit (Sigma) according to the manufacturer’s instructions; mouse monoclonal anti-ARID1B (Abcam) and rabbit polyclonal anti-β-catenin (H102) (Santa Cruz) antibodies were used.
Biochemistry
Western blotting and immunoprecipitations were performed as described in Hadjihannas et al.33 The following primary antibodies, rabbit polyclonal anti-ARID1B (Millipore), mouse monoclonal anti-ARID1B (Abcam), rabbit polyclonal anti-ARID1A (Biomol, Hamburg, Germany), mouse monoclonal anti-Flag, mouse monoclonal anti-β-actin (Sigma), rabbit monoclonal Histone H3 (D1H2) (Cell Signaling), mouse monoclonal anti-GFP (Roche), rabbit polyclonal anti-β-catenin (H102) (Santa Cruz), mouse monoclonal anti-T7 (Novagen-Millipore), and mouse monoclonal anti-BRG1 (Santa Cruz), were used according to the manufacturer’s instructions.
Results
Differential Gene Expression between Individuals with ID and Control Groups
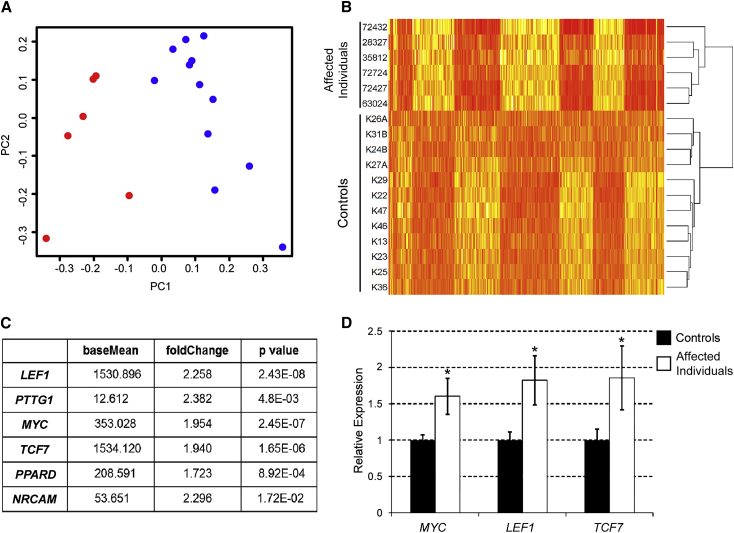
To gain insight into ARID1B’s functions, we set out to explore the effects of ARID1B loss-of-function mutations on gene expression. To this end, we compared the expression profiles of individuals with ID and harboring ARID1B mutations to the expression profiles of control groups by whole-transcriptome analysis (RNA-Seq) of peripheral lymphocytes from fresh blood (Figures S1A and S1B; see Materials and Methods). It has been previously shown that, at the trascriptome level, whole blood shares significant gene-expression-profile similarities with multiple tissues, including the CNS.34–37 Principal component analysis identified distinct expression patterns between individuals with ID and control individuals (Figure 1A). Results were validated by qRT-PCR in a randomly selected subset of ten genes (Figure S1C). Prominent transcriptional changes were observed in 452 genes (Figure 1B). Analysis with Ingenuity Systems’ IPA Software indicated significant changes in a total of 39 biological processes and pathways (Table S1). One of the most significantly altered pathways identified was the Wnt/β-catenin pathway. Although it is likely that some of the other IPA-identified pathways might be involved in the phenotype, we decided to concentrate first on Wnt/β-catenin signaling, given that it is a well-known developmental pathway whose significance in neurogenesis and neuronal and brain development, as well as in CNS defects, is well established. Therefore, we further analyzed changes in Wnt/β-catenin target genes and found significant upregulation of LEF1 (MIM: 153245), PTTG1(MIM: 604147), MYC (MIM: 190080), TCF7 (MIM: 189908), PPARD (MIM: 600409), and NRCAM (MIM: 601581), indicating increased Wnt/β-catenin signaling in individuals with ID (Figure 1C). qRT-PCR for three Wnt/β-catenin target genes, namely MYC, LEF1, and TCF7, confirmed the above results (Figure 1D). These findings indicated that ARID1B represses Wnt/β-catenin-mediated transcription and that loss-of-function mutations in ARID1B lead to derepression ofWnt/β-catenin signaling.
Figure 1.
Differential Gene Expression between ID-Affected Individuals and Control Groups
(A) Principal component analysis of RNA-Seq data showing the first two principal components (PC1 and PC2) of six ID-affected individuals (red) and twelve control individuals (blue) from RPKM values of 24.364 genes.
(B) Heat map showing differential expression patterns between ID-affected and control individuals with 452 genes with a fold change >2 and p value < 0.01 used in pathway analysis. Higher relative expression is indicated by darker hues.
(C) Mean normalized expression values (baseMean), fold change, and negative binomial p value for six Wnt/β-catenin target genes with significant upregulation in ID-affected individuals in comparison to control individuals.
(D) Validation of RNA-Seq data with qRT-PCR in control and ID-affected individuals for three Wnt/β-catenin target genes: MYC, LEF1, and TCF7. Relative expression shows the average values of twelve control individuals (set at a value of 1) and six ID-affected individuals. Error bars represent SEM. Asterisks denote a p value of <0.05.
ARID1B Specifically Represses Wnt/β-Catenin Signaling Downstream of the β-Catenin Destruction Complex
To investigate the role of ARID1B in Wnt/β-catenin signaling, we made use of cell culture models exhibiting low intensity of active Wnt/β-catenin signaling, HEK293T and human osteosarcoma U2OS cells, and human colorectal cancer cells exhibiting intrinsically aberrant Wnt/β-catenin activity due to inactivating mutations in the tumor suppressor APC (MIM: 611731) (SW480, DLD1) or activating mutations in β-catenin (MIM: 116806) itself (HCT116). We measured Wnt/β-catenin-dependent transcription with a well-established luciferase reporter assay (TOP/FOPFlash; see Materials and Methods). We knocked down ARID1B in U2OS cells and confirmed knockdown of ARID1B but not of the related ARID1A (MIM: 603024) (Figure 2A). ARID1B knockdown in these cells led to a significant upregulation of the Wnt/β-catenin target genes MYC, LEF1, and TCF7, in support to the RNA-Seq studies (Figure 2A). Knockdown of ARID1B in HEK293T cells, which, like U2OS cells, exhibit low basal Wnt/β-catenin signaling activity, increased Wnt/β-catenin-dependent transcriptional reporters, similarly to knockdown of the known negative regulators of the pathway Axin1 and APC (Figure 2B and Figure S2A). This increase in Wnt/β-catenin-dependent transcription was also observed in the presence of Wnt3A-conditioned media used to activate the pathway, indicating that ARID1B regulates Wnt/β-catenin signaling even under activating conditions (Figure 2B). Likewise, siRNA-mediated knockdown via two different siRNAs specifically targeting ARID1B but not the related ARID1A further increased Wnt/β-catenin activity in colorectal cancer cell lines DLD1, HCT116, and SW480 (Figure 2C and Figures S2B and S2C). As expected, concurrent knockdown of β-catenin completely abrogated the increase in Wnt/β-catenin activity (Figure 2C).
Reciprocally, overexpression of T7-tagged human ARID1B (hereafter referred to as ARID1B) inhibited Wnt/β-catenin signaling activation by overexpression of Flag β-catenin in both 293T and U2OS cells (Figure 2D and Figure S2D). As expected, overexpression of Axin1 also inhibited Flag β-catenin-induced activity (Figure 2D). However, overexpression of ARID1B could not inhibit transcriptional reporter activities for c-Jun or FoxO, either with or without induction by overexpression of c-Jun or FoxO3A, respectively, indicating that ARID1B specifically represses Wnt/β-catenin-dependent transcription (Figure 2D). In addition, whereas overexpression of ARID1B was also able to inhibit the high Wnt/β-catenin activity in SW480 cells, it did not significantly alter c-Jun and FoxO activities (Figure 2E and Figure S2E).
Next, we determined whether ARID1B represses Wnt/β-catenin-mediated transcription upstream or downstream of the β-catenin destruction complex. For this we activated Wnt/β-catenin signaling in HEK293T cells, which have a functional β-catenin destruction complex, by overexpression of either GFP β-catenin or non-degradable β-catenin harboring a substitution to Tyrosine at Ser33 (YFP S33Y) and assessed the ability of ARID1B to inhibit the resultant Wnt/β-catenin-dependent activation. As shown in Figure 2F, ARID1B was able to inhibit activation of the TOP/FOPFlash reporters in response to both degradable and non-degradable β-catenin to a similar extent. Accordingly, no differences were observed in β-catenin protein amounts between lymphoblastoid cell lines derived from ID-affected individuals with ARID1B mutations and those derived from control subjects (Figure 2G). Taken together, these results show that ARID1B specifically represses Wnt/β-catenin-dependent transcription downstream of the β-catenin destruction complex and that loss of ARID1B leads to upregulation of Wnt target genes in a β-catenin-dependent fashion.
Endogenous and Exogenous ARID1B Associates with β-Catenin
To investigate the mechanism(s) by which ARID1B represses β-catenin-dependent transcription, we employed immunofluorescence microscopy in U2OS cells transiently overexpressing ARID1B and/or GFP β-catenin. When transfected alone, ARID1B localized exclusively in the nucleus of transfected cells and exhibited a granular yet diffuse localization, whereas GFP β-catenin showed a diffuse cellular localization with a preference for the nucleus and occasionally localized in small nuclear puncta (Figure 3A). Strikingly, in a high percentage of cells co-expressing both ARID1B and GFP β-catenin, we observed the formation of well-defined nuclear puncta, which corresponded to a full co-localization, suggesting physical association between the two proteins (Figure 3A; for quantification, also see Figure 4C). Therefore, we investigated the existence of endogenous ARID1B/β-catenin complexes in HCT116 cells, which express relatively high amounts of both proteins. Indeed, immunoprecipitation experiments showed that in nuclear lysates, ARID1B was co-immunoprecipitated with β-catenin but not with control IgG antibodies (Figure 3B). To visualize endogenous ARID1B/β-catenin complexes, we carried out PLAs with antibodies directed against ARID1B and β-catenin.38 In our PLAs, primary ARID1B/β-catenin antibodies raised in different species were used to stain HCT116 cells and were subsequently labeled with appropriate secondary antibodies conjugated with minus and plus oligonucleotides, followed by ligation, amplification, and incorporation of fluorescently labeled oligonucleotides. Resultant fluorescent signals report a close physical proximity between the two primary antibodies and their antigens. We first verified that our antibodies recognized their respective endogenous antigens in HCT116 cells by standard immunofluorescence staining (Figures S3A and S3B). Importantly, PLA for ARID1B/β-catenin showed clear PLA signals in HCT116 cells as assessed by fluorescent microscopy (Figure 3C). Automated signal detection from single-plane pictures showed that, on average, there were around six signals per cell and that over 70% of the signals occurred in the nuclei of HCT116 cells (Figure 3D and Figure S3C). These signals were specific for ARID1B/β-catenin because efficient knockdown of either protein significantly reduced PLA signals per cell (Figures 3C and 3D and Figures S3A and S3B). Taken together, these results clearly demonstrate the existence of endogenous as well as exogenous ARID1B/β-catenin complexes. Moreover, this association seems to take place predominately in the nucleus.
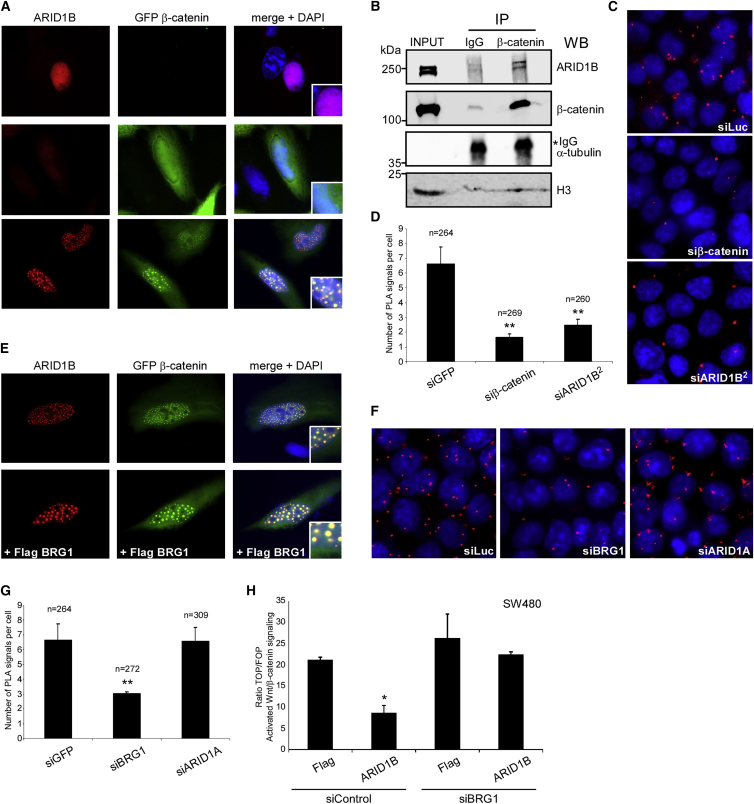
Figure 3.
ARID1B Associates with β-Catenin in Nuclear Puncta and Represses Wnt/β-Catenin-Dependent Transcription through BRG1
(A) Representative immunofluorescence pictures of U2OS cells expressing transfected ARID1B and GFP β-catenin, alone (rows 1 and 2) or together (row3), stained with an antibody against ARID1B. Note that the antibody strongly recognizes overexpressed ARID1B as compared to endogenous ARID1B in these cells.
(B) Western blotting (WB) for indicated endogenous proteins of lysates (INPUT) and IgG (control) or β-catenin immunoprecipitates (IP) from HCT116 nuclei. Enrichment in nuclear proteins was confirmed by the presence of histone H3 (H3) and absence of α-tubulin. Asterisk indicates heavy chains IgG, showing equal loading of IP reactions. Molecular weights (kDa) are indicated on the left of the panels.
(C) Representative immunofluorescence pictures of proximity ligation assays (PLAs) for endogenous ARID1B and β-catenin in HCT116 cells transfected with indicated siRNAs.
(D) Quantification from experiments conducted in (C).
(E) Representative immunofluorescence pictures of U2OS cells co-expressing transfected ARID1B and GFP β-catenin in the absence or presence of co-transfected Flag BRG1 (+ Flag BRG1) stained with an antibody against ARID1B.
(F) Immunofluorescence pictures of PLAs for endogenous ARID1B and β-catenin in HCT116 cells transfected with indicated siRNAs.
(G) Quantification from experiments conducted in (F).
(H) Ratio of TOP/FOPFlash in SW480 cells transfected with indicated plasmids in the presence of co-transfected siRNAs targeting GFP (siControl) or BRG1 (siBRG1). In panels (A) and (E), right columns show merged pictures with DAPI and inlets show magnified nuclear regions. In panels (D) and (G), n shows the number of nuclei analyzed, error bars show SEM, and double asterisks denote p <0.01 for differences from controls (siGFP), with unpaired Student’s t test, from three independent experiments. In panel (H), error bars show SEM and single asterisks denote p< 0.05 for differences from controls (Flag + siControl) from at least three independent experiments.
ARID1B Associates with β-Catenin and Represses Wnt/β-Catenin-Dependent Transcription through BRG1
Both ARID1B and β-catenin bind directly to the ATP-dependent helicase BRG1 (encoded by SMARCA4 [MIM: 603254]), an enzymatic subunit of the BAF complex, albeit to different regions.32,39 We observed that co-expression of Flag BRG1 led to significantly larger exogenous ARID1B/β-catenin nuclear puncta in U2OS cells, suggesting that BRG1 might mediate association between the two proteins (Figure 3E and Figures S4A and S4B). We therefore assessed the effect of BRG1 knockdown on the endogenous ARID1B/β-catenin complexes in HCT116 by PLA. Efficient knockdown of BRG1 significantly reduced ARID1B/β-catenin PLA signals (Figures 3F and 3G and Figure S4C). Knockdown of BRG1 had no effect on the protein amounts of ARID1B or β-catenin, ruling out the possibility that reduced ARID1B/β-catenin association was due to reduced protein amounts (Figure S4C). Transfection of a siRNA specifically targeting ARID1A did not affect ARID1B/β-catenin PLA signals, consistent with the fact that ARID1B and ARID1A are part of mutually exclusive BAF remodeling complexes (Figures 3F and 3G and Figure S4D).3 Importantly, knockdown of BRG1 alleviated the inhibitory effect of ARID1B on Wnt/β-catenin signaling activation in SW480 cells (Figure 3H). These results suggest that BRG1 mediates the association between β-catenin and an ARID1B-based BAF complex to repress β-catenin-mediated transcription.
Mutant ARID1B Proteins Show Compromised Association with BRG1 and β-Catenin and Fail to Repress Wnt/β-Catenin-Dependent Transcription
Mutations of ARID1B occurring in individuals with Coffin-Siris syndrome and non-syndromic ID, as well as in cancers, result in truncated ARID1B proteins.11 In ID, the smallest truncation in ARID1B reported to date occurs in an individual (individual 4 in Figure S1B) carrying a de novo, heterozygous deletion (c.6463_6473del, p.Ser2155Leufs∗33) of ten nucleotides in exon 20 of ARID1B (GenBank: NM_020732.3). This mutation deletes only 30% of the highly conserved amino acid sequence within the C terminus of ARID1B in the EDH2 domain (Eld/Osa homology domain 2), which, intriguingly, has been previously shown to mediate interaction with BRG1 (Figure S1A).32,39 This transcript is not subject to nonsense-mediated decay given that it was readily detectable with RT-PCR from the RNA sample of the affected individual (Figure S5A). We generated an ARID1B expression plasmid harboring this mutation (ARID1B-2188) and found that the resultant protein was only weakly co-immunoprecipitated with Flag BRG1 as compared to wild-type ARID1B (Figure 4A). In addition to the ARID1B-2188 mutant, we generated an ARID1B expression plasmid harboring a mutation (c.5329A>T; p.Lys1777∗) found also in exon 20 in an individual with Coffin-Siris syndrome and which, similarly to the majority of truncating ARID1B mutations found in cancers, deletes the EDH2 domain in its entirety (ARID1B-1777) and assessed their co-localization with GFP β-catenin in U2OS cells (see Figure S1A). Whereas wild-type and mutant ARID1B proteins localized similarly when expressed alone, the mutants exhibited significantly reduced ability to form nuclear GFP β-catenin puncta as compared to wild-type ARID1B (Figures 4B and 4C and Figure S5B). As compared to ARID1B, both ARID1B-2188 and ARID1B-1777 showed reduced co-immunoprecipitation with co-expressed β-catenin in HEK293T cells and could not repress Wnt/β-catenin-dependent transcription in SW480 cells (Figures 4D–4F). These data demonstrate that ARID1B mutations leading to partial or complete deletion of the BRG1-binding domain compromise association with β-catenin and ARID1B-mediated repression of Wnt/β-catenin signaling.
ARID1B/β-Catenin Interaction Sites Are Enriched for the Negative Regulator of Transcription PRMT5
The fact that ARID1B formed nuclear puncta with GFP β-catenin and inhibited the GFP β-catenin-induced activation of reporters in U2OS indicated that this association is repressive in nature (Figure 3A and Figure S2D). We therefore explored the composition of the ARID1B/β-catenin puncta in U2OS cells. Using immunofluorescence staining and 2D deconvolution microscopy, we found that PRMT5 (protein arginine N-methyltransferase 5), which promotes a closed chromatin structure and was previously found among ARID1B-interacting proteins, was highly enriched in ARID1B/β-catenin nuclear puncta (Figure 4G).40,41 However, histone H3 acetylated on lysine 9 (acetylH3K9) or on lysine 27 (acetylH3K27), histone H3 tri-methylated on lysine 4 (H3K4me3), all of which are associated with transcriptionally active chromatin, as well as histone H3 tri-methylated on lysine 9 (H3K9me3), histone H3 symmetrically dimethylated on arginine 8 (H3R8me2s), and histone H4 symmetrically dimethylated on arginine 3 (H4R3me2s), all of which are associated with a repressive chromatin state, were absent from ARID1B/β-catenin nuclear puncta (data not shown). This finding suggests that either another histone modification(s) is responsible for repression or that the chromatin architecture within or surrounding these puncta is altered in an as-of-yet-unknown way, and further studies are required to describe the mechanism(s) involved.
Knockdown of ARID1B Leads to Neurite Outgrowth in a β-Catenin-Dependent Fashion
Substantial evidence from various model systems points to an important role for Wnt signaling in neurite outgrowth and axonal guidance.42 Previous studies have shown that active forms of β-catenin promote neurite outgrowth in human neuroblastoma lines and that β-catenin upregulates the expression of neuronal cell adhesion molecules, NRCAM and L1, that in turn stimulate neurite outgrowth.43–45 Interestingly, NRCAM was one of the genes that we found to be upregulated in individuals with ARID1B-associated ID (Figure 1C). To determine whether regulation of β-catenin-dependent transcription by ARID1B is relevant to the function of Wnt signaling in neurite outgrowth, we performed ARID1B knockdown experiments in mouse neuroblastoma Neuro2A cells. Efficient knockdown of ARID1B in these cells activated β-catenin-dependent transcription (Figures 5A and 5B). After knockdown of ARID1B, approximately 48% of Neuro2A cells, versus 14% of control cells, exhibited pronounced neurite outgrowth with neurites extending to twice the length of the cell body diameter (Figures 5C and 5D). Importantly, concurrent knockdown of β-catenin blocked both the increase of β-catenin-dependent transcription as well as the neurite outgrowth induced by knockdown of ARID1B (Figure 5). These results indicate that loss of ARID1B modulates neurite behavior in a β-catenin-dependent fashion and is supportive of an ARID1B function in brain and neuronal physiology.
Discussion
Collectively, our findings illustrate that, in humans, ARID1B is a repressor of the Wnt/β-catenin pathway and that inactivation of ARID1B leads to elevated Wnt/β-catenin-dependent transcription of target genes. Additionally, regulation of β-catenin-mediated transcription by ARID1B appears to control neurite outgrowth in mouse neuroblastoma cells, underscoring the physiological relevance of the mechanism reported here. We propose that ARID1B associates with β-catenin via BRG1, thereby recruiting negative regulators of transcription, such as PRMT5, to inhibit Wnt/β-catenin-dependent transcription. Although it has been previously shown that BRG1 acts as a co-activator of Wnt/β-catenin signaling, our data support the notion that combinatorial assembly of distinct BAF complexes could determine their functional specificity, resulting in either activation or repression of associated signaling pathways.11,22,46 It has been recently reported that loss of SNF5, a BAF chromatin-remodeling subunit and tumor suppressor, leads to derepression of Wnt target genes by decreasing the ability of TCF4 (encoded by TCF7L2 [MIM: 602228]) to bind and repress target genes.25 Although we do not formally exclude the possibility that the mechanism for derepression of Wnt-target genes after loss of ARID1B might also involve decreased TCF4-associated repressive activity, our results showing association between ARID1B and β-catenin and the effects of ARID1B on TOP/FOPFlash reporters are in favor of a β-catenin-dependent mechanism. Furthermore, although we have shown that knockdown of the related protein ARID1A does not interfere with the association between ARID1B and β-catenin, our preliminary data suggest that ARID1A itself can also associate with GFP β-catenin in nuclear puncta, indicating that ARID1A might operate in a similar fashion as ARID1B in Wnt/β-catenin-dependent transcription (data not shown).
It has been published that ARID1A- and ARID1B-containing chromatin-remodelling complexes are important for cell cycle progression through regulation of MYC expression.30,47 As seen in our RNA-seq study, MYC, along with other Wnt target genes, was upregulated in ID-affected individuals and in ARID1B knockdown cells, suggesting that ARID1B complexes might indeed impact cell cycle progression. Taken together with previous work showing that Wnt/β-catenin signaling is regulated during the cell cycle, the present study raises the possibility that the connection between ARID1 protein complexes and the cell cycle is driven at least in part through the Wnt/β-catenin pathway.33,48 In light of this possibility and while this paper was under revision, Ruijtenberg and van den Heuvel demonstrated that SWI/SNF remodeling complexes regulate lineage-specific cell cycle arrest during differentiation by activating the expression of negative cell cycle regulators but repressing positive regulators.49 This raises the question as to how this complex is targeted to intended chromatin locations for negative or positive cell cycle regulation. Our finding that ARID1B can associate with, and inhibit, β-catenin nuclear activity might shed light on how chromatin-remodeling complexes are targeted to specific loci.
In the past few years, several studies have reported the presence of apparent loss-of-function mutations in chromatin-remodeling factors in both developmental syndromes associated with ID and in several human cancers.11 This ongoing identification of de novo mutations in ID and somatic mutations in cancer of the very same factors suggests an epigenetic convergence of the two pathologies. Despite this convergence, the two pathologies are phenotypically disparate and current datasets do not indicate that ID-affected individuals are at a greater risk for developing cancer, possibly due to the young age of mutation-carrying individuals. Given that Wnt/β-catenin signaling plays instrumental roles both during development and carcinogenesis, its involvement after ARID1B loss of function described in the present work could help us understand the molecular mechanisms underlying ID and cancer. We speculate that the seemingly different pathological outcomes of ARID1B mutations in individuals with ID versus in those with cancer could either be due to accompanying cooperative mutations in the latter and/or due to tissue-specific deregulation of epigenetically regulated pathways. Finally, our results offer a departure point from which to further investigate the mechanisms by which mutations in ARID1B, as well as in other chromatin-remodeling components, are involved in ID and cancer through derepression of developmental and oncogenic pathways.
Acknowledgments
We thank the affected individuals and their families for their cooperation. We also thank Andreas Winterpacht for reagents and Sabine Endele, Christian Thiel, Katharina Brauburger, Francesca Pasutto, and Kristin Kessler for their useful advice. We are indebted to Martina Brückner, Angelika Diem, Petra Rothe, and Olga Zwenger for excellent technical assistance. This work was supported in part by the German Intellectual Disability Network (MRNET) through a grant from the German Ministry of Education and Research to A.R. (01GS08160) and by the Interdisciplinary Center for Clinical Research Erlangen, project E16.
Published: September 3, 2015
Footnotes
Supplemental Data include five figures and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2015.08.002.
Accession Numbers
The accession number for the RNA-Seq data in this paper is BioProject: PRJNA289690.
Web Resources
The URLs for data presented herein are as follows:
Ensembl Genome Browser, http://www.ensembl.org/index.html
ExAC Browser, http://exac.broadinstitute.org/
NCBI Short Read Archive, http://www.ncbi.nlm.nih.gov/sra
OMIM, http://www.omim.org/
UCSC Genome Browser, http://genome.ucsc.edu/
Supplemental Data
References
- 1.Ho L., Crabtree G.R. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins R.T., Furukawa T., Tanese N., Treisman J.E. Osa associates with the Brahma chromatin remodeling complex and promotes the activation of some target genes. EMBO J. 1999;18:7029–7040. doi: 10.1093/emboj/18.24.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Nagl N.G., Wilsker D., Van Scoy M., Pacchione S., Yaciuk P., Dallas P.B., Moran E. Two related ARID family proteins are alternative subunits of human SWI/SNF complexes. Biochem. J. 2004;383:319–325. doi: 10.1042/BJ20040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer L.A., Lee T.I., Cole M.F., Johnstone S.E., Levine S.S., Zucker J.P., Guenther M.G., Kumar R.M., Murray H.L., Jenner R.G. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores-Alcantar A., Gonzalez-Sandoval A., Escalante-Alcalde D., Lomelí H. Dynamics of expression of ARID1A and ARID1B subunits in mouse embryos and in cells during the cell cycle. Cell Tissue Res. 2011;345:137–148. doi: 10.1007/s00441-011-1182-x. [DOI] [PubMed] [Google Scholar]
- 6.Yan Z., Wang Z., Sharova L., Sharov A.A., Ling C., Piao Y., Aiba K., Matoba R., Wang W., Ko M.S. BAF250B-associated SWI/SNF chromatin-remodeling complex is required to maintain undifferentiated mouse embryonic stem cells. Stem Cells. 2008;26:1155–1165. doi: 10.1634/stemcells.2007-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cajuso T., Hanninen U.A., Kondelin J., Gylfe A.E., Tanskanen T., Katainen R., Pitkanen E., Ristolainen H., Kaasinen E., Taipale M. Exome sequencing reveals frequent inactivating mutations in ARID1A, ARID1B, ARID2 and ARID4A in microsatellite unstable colorectal cancer. Int. J. Cancer. 2014;135:611–623. doi: 10.1002/ijc.28705. [DOI] [PubMed] [Google Scholar]
- 8.Halgren C., Kjaergaard S., Bak M., Hansen C., El-Schich Z., Anderson C.M., Henriksen K.F., Hjalgrim H., Kirchhoff M., Bijlsma E.K. Corpus callosum abnormalities, intellectual disability, speech impairment, and autism in patients with haploinsufficiency of ARID1B. Clin. Genet. 2012;82:248–255. doi: 10.1111/j.1399-0004.2011.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoyer J., Ekici A.B., Endele S., Popp B., Zweier C., Wiesener A., Wohlleber E., Dufke A., Rossier E., Petsch C. Haploinsufficiency of ARID1B, a member of the SWI/SNF-a chromatin-remodeling complex, is a frequent cause of intellectual disability. Am. J. Hum. Genet. 2012;90:565–572. doi: 10.1016/j.ajhg.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadoch C., Hargreaves D.C., Hodges C., Elias L., Ho L., Ranish J., Crabtree G.R. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 2013;45:592–601. doi: 10.1038/ng.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronan J.L., Wu W., Crabtree G.R. From neural development to cognition: unexpected roles for chromatin. Nat. Rev. Genet. 2013;14:347–359. doi: 10.1038/nrg3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santen G.W., Aten E., Sun Y., Almomani R., Gilissen C., Nielsen M., Kant S.G., Snoeck I.N., Peeters E.A., Hilhorst-Hofstee Y. Mutations in SWI/SNF chromatin remodeling complex gene ARID1B cause Coffin-Siris syndrome. Nat. Genet. 2012;44:379–380. doi: 10.1038/ng.2217. [DOI] [PubMed] [Google Scholar]
- 13.Sausen M., Leary R.J., Jones S., Wu J., Reynolds C.P., Liu X., Blackford A., Parmigiani G., Diaz L.A., Jr., Papadopoulos N. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat. Genet. 2013;45:12–17. doi: 10.1038/ng.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens P.J., Tarpey P.S., Davies H., Van Loo P., Greenman C., Wedge D.C., Nik-Zainal S., Martin S., Varela I., Bignell G.R., Oslo Breast Cancer Consortium (OSBREAC) The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsurusaki Y., Okamoto N., Ohashi H., Kosho T., Imai Y., Hibi-Ko Y., Kaname T., Naritomi K., Kawame H., Wakui K. Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat. Genet. 2012;44:376–378. doi: 10.1038/ng.2219. [DOI] [PubMed] [Google Scholar]
- 16.Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D.H., McMahon A.P., Sommer L., Boussadia O., Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 17.Chenn A., Walsh C.A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 18.Lie D.C., Colamarino S.A., Song H.J., Désiré L., Mira H., Consiglio A., Lein E.S., Jessberger S., Lansford H., Dearie A.R., Gage F.H. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 19.Wisniewska M.B. Physiological role of β-catenin/TCF signaling in neurons of the adult brain. Neurochem. Res. 2013;38:1144–1155. doi: 10.1007/s11064-013-0980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polakis P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Barker N., Hurlstone A., Musisi H., Miles A., Bienz M., Clevers H. The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. EMBO J. 2001;20:4935–4943. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin C.T., Curtis C.D., Davis R.B., Muthukumar V., Magnuson T. The chromatin-remodeling enzyme BRG1 modulates vascular Wnt signaling at two levels. Proc. Natl. Acad. Sci. USA. 2011;108:2282–2287. doi: 10.1073/pnas.1013751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holik A.Z., Young M., Krzystyniak J., Williams G.T., Metzger D., Shorning B.Y., Clarke A.R. Brg1 loss attenuates aberrant wnt-signalling and prevents wnt-dependent tumourigenesis in the murine small intestine. PLoS Genet. 2014;10:e1004453. doi: 10.1371/journal.pgen.1004453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora-Blanco E.L., Mishina Y., Tillman E.J., Cho Y.J., Thom C.S., Pomeroy S.L., Shao W., Roberts C.W. Activation of β-catenin/TCF targets following loss of the tumor suppressor SNF5. Oncogene. 2014;33:933–938. doi: 10.1038/onc.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones D.T., Jäger N., Kool M., Zichner T., Hutter B., Sultan M., Cho Y.J., Pugh T.J., Hovestadt V., Stütz A.M. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488:100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Hoon M.J., Imoto S., Nolan J., Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 28.Dehner M., Hadjihannas M., Weiske J., Huber O., Behrens J. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J. Biol. Chem. 2008;283:19201–19210. doi: 10.1074/jbc.M710366200. [DOI] [PubMed] [Google Scholar]
- 29.Hadjihannas M.V., Brückner M., Behrens J. Conductin/axin2 and Wnt signalling regulates centrosome cohesion. EMBO Rep. 2010;11:317–324. doi: 10.1038/embor.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagl N.G., Jr., Patsialou A., Haines D.S., Dallas P.B., Beck G.R., Jr., Moran E. The p270 (ARID1A/SMARCF1) subunit of mammalian SWI/SNF-related complexes is essential for normal cell cycle arrest. Cancer Res. 2005;65:9236–9244. doi: 10.1158/0008-5472.CAN-05-1225. [DOI] [PubMed] [Google Scholar]
- 31.Helming K.C., Wang X., Wilson B.G., Vazquez F., Haswell J.R., Manchester H.E., Kim Y., Kryukov G.V., Ghandi M., Aguirre A.J. ARID1B is a specific vulnerability in ARID1A-mutant cancers. Nat. Med. 2014;20:251–254. doi: 10.1038/nm.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue H., Furukawa T., Giannakopoulos S., Zhou S., King D.S., Tanese N. Largest subunits of the human SWI/SNF chromatin-remodeling complex promote transcriptional activation by steroid hormone receptors. J. Biol. Chem. 2002;277:41674–41685. doi: 10.1074/jbc.M205961200. [DOI] [PubMed] [Google Scholar]
- 33.Hadjihannas M.V., Bernkopf D.B., Brückner M., Behrens J. Cell cycle control of Wnt/β-catenin signalling by conductin/axin2 through CDC20. EMBO Rep. 2012;13:347–354. doi: 10.1038/embor.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Codina-Solà M., Rodríguez-Santiago B., Homs A., Santoyo J., Rigau M., Aznar-Laín G., Del Campo M., Gener B., Gabau E., Botella M.P. Integrated analysis of whole-exome sequencing and transcriptome profiling in males with autism spectrum disorders. Mol. Autism. 2015;6:21. doi: 10.1186/s13229-015-0017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregor A., Oti M., Kouwenhoven E.N., Hoyer J., Sticht H., Ekici A.B., Kjaergaard S., Rauch A., Stunnenberg H.G., Uebe S. De novo mutations in the genome organizer CTCF cause intellectual disability. Am. J. Hum. Genet. 2013;93:124–131. doi: 10.1016/j.ajhg.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan P.F., Fan C., Perou C.M. Evaluating the comparability of gene expression in blood and brain. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2006;141B:261–268. doi: 10.1002/ajmg.b.30272. [DOI] [PubMed] [Google Scholar]
- 37.Tylee D.S., Kawaguchi D.M., Glatt S.J. On the outside, looking in: a review and evaluation of the comparability of blood and brain “-omes”. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2013;162B:595–603. doi: 10.1002/ajmg.b.32150. [DOI] [PubMed] [Google Scholar]
- 38.Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K.J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L.G., Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 39.Hurlstone A.F., Olave I.A., Barker N., van Noort M., Clevers H. Cloning and characterization of hELD/OSA1, a novel BRG1 interacting protein. Biochem. J. 2002;364:255–264. doi: 10.1042/bj3640255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue H., Giannakopoulos S., Parkhurst C.N., Matsumura T., Kono E.A., Furukawa T., Tanese N. Target genes of the largest human SWI/SNF complex subunit control cell growth. Biochem. J. 2011;434:83–92. doi: 10.1042/BJ20101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L., Pal S., Sif S. Protein arginine methyltransferase 5 suppresses the transcription of the RB family of tumor suppressors in leukemia and lymphoma cells. Mol. Cell. Biol. 2008;28:6262–6277. doi: 10.1128/MCB.00923-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endo Y., Rubin J.S. Wnt signaling and neurite outgrowth: insights and questions. Cancer Sci. 2007;98:1311–1317. doi: 10.1111/j.1349-7006.2007.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conacci-Sorrell M.E., Ben-Yedidia T., Shtutman M., Feinstein E., Einat P., Ben-Ze’ev A. Nr-CAM is a target gene of the beta-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Dev. 2002;16:2058–2072. doi: 10.1101/gad.227502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gavert N., Conacci-Sorrell M., Gast D., Schneider A., Altevogt P., Brabletz T., Ben-Ze’ev A. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J. Cell Biol. 2005;168:633–642. doi: 10.1083/jcb.200408051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sangkhathat S., Nara K., Kusafuka T., Yoneda A., Fukuzawa M. Artificially accumulated beta-catenin inhibits proliferation and induces neurite extension of neuroblastoma cell line NB-1 via up-regulation of trkA. Oncol. Rep. 2006;16:1197–1203. [PubMed] [Google Scholar]
- 46.Son E.Y., Crabtree G.R. The role of BAF (mSWI/SNF) complexes in mammalian neural development. Am. J. Med. Genet. C. Semin. Med. Genet. 2014;166C:333–349. doi: 10.1002/ajmg.c.31416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagl N.G., Jr., Zweitzig D.R., Thimmapaya B., Beck G.R., Jr., Moran E. The c-myc gene is a direct target of mammalian SWI/SNF-related complexes during differentiation-associated cell cycle arrest. Cancer Res. 2006;66:1289–1293. doi: 10.1158/0008-5472.CAN-05-3427. [DOI] [PubMed] [Google Scholar]
- 48.Davidson G., Shen J., Huang Y.L., Su Y., Karaulanov E., Bartscherer K., Hassler C., Stannek P., Boutros M., Niehrs C. Cell cycle control of wnt receptor activation. Dev. Cell. 2009;17:788–799. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Ruijtenberg S., van den Heuvel S. G1/S Inhibitors and the SWI/SNF Complex Control Cell-Cycle Exit during Muscle Differentiation. Cell. 2015;162:300–313. doi: 10.1016/j.cell.2015.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.