Abstract
Objective
Chronic subdural hematoma has an increasing incidence and results in high morbidity and mortality. We review here the ten-year experience of a single institution and the literature regarding the treatment and major associations of chronic subdural hematoma (cSDH).
Methods
We retrospectively reviewed all cSDHs surgically treated from 2000 to 2010 at our institution to evaluate duration from admission to treatment, type of treatment, length of stay in critical care, length of stay in the hospital and recurrence. The literature was reviewed with regards to incidence, associations and treatment of cSDH.
Results
From 2000–2008, 44 patients were treated with burr holes. From 2008 to 2010, 29 patients were treated with twist drill evacuation (SEPS). 4 patients from each group were readmitted for reoperation (9% vs. 14%; p=.53). The average time to intervention for SEPS (11.2±15.3 hrs) was faster than for burr holes (40.3±69.1 hrs) (p=.02). The total hospital LOS was shorter for SEPS (9.3±6.8 days) versus burr holes (13.4±10.2 days) (p=.04); both were significantly longer than for a brain tumor patient undergoing craniotomy (7.0±0.5 days, n=94, P<.01).
Conclusion
Despite decreasing lengths of stay over time as treatment for cSDH evolved from burr holes to SEPS, the length of stay for a cSDH is still greater than that of a patient undergoing craniotomy for brain tumor. We noted 11% recurrence in our series of patients, which included individuals who recurred as late as 3 years after initial diagnosis.
Keywords: Burr Hole, Cerebral Atrophy, Cost, Incidence, SEPS, Subdural Hematoma
Introduction
Incidence and Impact
Current trends in an aging population predict that chronic subdural hematoma (cSDH) will surpass primary brain tumors (up to 14 per 100,000/year)1, and metastases (approximately 28 per 100,000/year)2 to become the most common cranial surgical condition once approximately 20 to 25% of the population is greater than 65 years old3. In the United States, this is projected to occur by the year 20304.
The incidence of cSDH has steadily risen in global populations since 1967. It was 1.7 per 100,000 in Helsinki, Finland from 1967 to 19735 and 2.0 per 100,000 in Sweden in 19696. It was 13.1 per 100,000 in Japan from 1986 to 19883 and increased to 20.6 cSDHs per 100,000 by 20057. Among people over 80 years of age, who comprise one-third of the total afflicted3, 5, 6, 8, the incidence is 127.1 per 100,0007. An annual incidence of 20 cases per 100,000 would suggest that ~60,000 Americans will become afflicted with cSDH each year.
Chronic subdural hematoma is neither a trivial nor benign disease. It has a high recurrence rate ranging from 5 to 14%3, 5, 6, 8. Contrary to common belief, it also has a poor prognosis. Patients treated for cSDH are at risk for intracerebral hemorrhage, seizures, and exacerbation of comorbidities associated with the interruption of anticoagulant therapy. Up to 20% of patients have poor neurologic outcomes resulting in significant disability8–11. Perioperative mortality for cSDH ranges from 1.2 to 11%. One-year mortality among elderly patients treated with a drainage intervention is 32% 12. The outcome for cSDH is even worse with conversion to acute SDH6, 8, 11, 13. A recent study observed the mean survival of 209 post-cSDH patients to be 4.4 years, which is significantly shorter (hazard ratio of 1.94, p<0.0002) than the mean of 6.0 years survival computed from actuarial life-tables12. Another retrospective study of 301 patients admitted with cSDH found the mortality risk to be as much as 17 times greater than the general population in the 55–74 age range14. The costs to society for cSDH include not only care costs, but also decreased quality of life for the afflicted patients and their caregivers.
Risk Factors
Unlike acute subdural hematoma, which is nearly always preceded by compelling trauma, 29 to 38% of patients treated surgically for cSDH have no memorable precipitating trauma15, 16. The vast majority have sufficiently mild trauma that there is no loss of consciousness17, suggesting that afflicted patients may have an intrinsic susceptibility.
Several potential predisposing factors for cSDH have been identified. In a Swedish study from 1969 to 1993, alcoholics comprised 14.7% of all patients with cSDH6. Among cSDH patients in the same study, 18% were anticoagulated, and an additional 17% consumed aspirin. Among 1000 surgically treated cSDH patients in Spain, 13% abused alcohol, and 12% were anticoagulated16. Anticoagulation increases the risk of all intracranial hemorrhage by 7 to 10 fold (odds ratio 1.64), with 30% of hemorrhages occurring in the subdural space18.
Cerebral atrophy enables minor stress or trauma (such as a fall rather than a motor vehicle accident) to provoke separation of the dura-arachnoid interface, as is also seen with subdural hygroma19. Cat and dog models suggest that once the dura and arachnoid separate, fibrin, from either serum or exudates, can induce proliferation of granulation tissue on the inner dural surface20. Wilfred Trotter originally hypothesized in 1914 that this proliferation of dural border cells results in production of a neomembrane, and subsequent growth of new vessels directly within the subdural space21. Subsequent studies show that chronic SDH can result from bleeding from these vessels by repeated microhaemorrhage from the neomembrane22.
It has also been hypothesized that atrophy leads to tearing of bridging veins between the rigidly fixed dura and mobile arachnoid layer. Microscopy of post-mortem material demonstrates that the subdural portion of the bridging veins has thinner vessel walls with less collagen, resulting in greater fragility than the subarachnoid portion 23.
The incidence of non-traumatic intracranial hemorrhage resulting from virtually every etiology except for arteriovenous malformations increases with age24. The aged brain is thought to be more susceptible to intracerebral hemorrhage because of the increased incidence of hypertension, arteriosclerosis and angiopathy25. However, many patients with intracranial hemorrhage, and particularly subdural hemorrhage, do not have these findings25.
Previously, we used a volumetric image analysis algorithm to determine the association between atrophy and subsequent cSDH26. Volumetric analysis was performed on CT scans acquired a mean of 209 days prior to cSDH diagnosis in 19 patients. Cerebral atrophy present on these scans was then compared to 76 age matched control patients randomly selected from cSDH-free subjects. There was a higher degree of atrophy in cSDH patients (N = 19, 14.3% ± 5.4%) than in age-matched control patients (N = 76, 11.9% ± 5.5%; p = 0.044). Logistical regression demonstrated that atrophy was found to be a significant predictor of cSDH at all ages (O.R. = 1.11, 95% C.I. = [1.01 1.23], p = 0.05). For younger subjects ≤ 65 years of age (N = 50), atrophy was an even stronger predictor of cSDH (O.R. = 1.17, 95% C.I. = [1.02 1.34], p = 0.026)26.
As the American population ages, the consumption of aspirin has steadily increased. As of 2005, 43 million American adults, comprising 20% of the people over 18 years old, were taking aspirin on a daily or near-daily basis 27. Among adults over the age of 65 years, 49% were taking aspirin in 2005 compared with only 30% in 1991–428. Among adults over 65 taking aspirin in 2005, 41% had never been told they had any risk factors for cardiovascular disease 27.
While benefits to aspirin are clear in patients with risk factors for, or a history of, vascular thrombotic disease, consumption in asymptomatic adults is still a topic of active investigation29, 30. Two meta-analyses suggest that the risk of hemorrhage is greater than the benefit of thrombotic disease prevention in asymptomatic patients31, 32. Aspirin was found to decrease the risk of particular cancers in one 2011 study33, however a 2012 meta-analysis contradicts this finding32. Meta-analysis by the Antithrombotic Trialists consortium concluded that “in primary prevention without previous disease, aspirin is of uncertain net value, as the reduction in occlusive events needs to be weighed against increase in major bleeds”31.
Based on data from 135,000 patients enrolled in several prospective studies, the risk of intracranial hemorrhage from aspirin use is approximately 0.3 per thousand patients taking aspirin34, with one-third of intracranial hemorrhages being subdural hematomas. Meta-analysis of prospective randomized trials with aspirin versus other agents to prevent secondary vascular events, resulted in finding of a non-significant (possibly due to limited statistical power) trend for increased incidence of hemorrhagic stroke among 17,000 participants31.
More recently, a randomized trial of 28,980 Scots with mild atherosclerosis demonstrated that aspirin use was associated with a trend for increased risk of major hemorrhage (2.0% vs 1.2%, hazard ratio, 1.71, 95% confidence interval, 0.99–2.97). Aspirin profoundly impacts the severity of hemorrhage. Intracranial hemorrhage was fatal in 3 of 4 aspirin patients in the atherosclerosis study35.
Numerous studies provide strong evidence that intracerebral hemorrhage is the most common fatal complication of anticoagulant therapy, with older patients most at risk18, 36–39. The increase in consumption of anticoagulants parallels a corresponding increase in intracranial hemorrhage. Between 1988 and 1999, as the use of warfarin quadrupled, the incidence of anticoagulant-associated intracerebral hemorrhage increased five-fold, to 17% of all intracerebral hemorrhages40. Such hemorrhage has a high mortality, with rates between 46% and 67% reported in three clinical series18, 41, 42. Among patients already unconscious at admission, mortality rates can be as high as 96% at 30 days after the ictus43.
Patients receiving anticoagulants are at increased risk after intracranial hemorrhage and tend to have poorer outcomes than their non-anticoagulated counterparts44. Poorer outcomes may be due to higher rates for delayed enlargement of the hematoma45, recurrent hemorrhage46, 47, or exacerbation of the underlying problem requiring anticoagulation in the first place.
Treatments for cSDH
A craniotomy is the excision of a skull flap over the hematoma to allow surgical drainage or other relief of the collected subdural blood. It is now more commonly used for acute subdural hematomas48, or for chronic hematomas that have coagulated into a solid fibrotic or membranous mass49 and/or calcified50. A 1993 retrospective study of its use for cSDH was performed that found no significant difference in post-operative mortality, neurological outcome as defined by the GCS, or recurrence when comparing craniotomy to burr hole and twist drill craniostomy10. However, a comparison study in 2009 between craniotomies and burr holes found subdural rebleeds to recur more (27.8% to 14.3%) in craniotomy procedures51. Because the majority of the cSDH population is elderly and thus affected by factors including brain atrophy, multiple medications including antiplatelets/anticoagulants, poor cardiovascular health, and increased risk for frequent falls and trauma11, 12, 14, 47, the increased recurrence rate of a surgically invasive procedure may help explain why burr hole and twist drill treatments are more generally popular52, 53.
Burr hole craniostomy is the most broadly popular surgical option for primary chronic subdural hematomas in most reporting countries47, 53–55. 85% of Canadian neurosurgeon respondents to a treatment survey in 2005 preferred single or double burr holes to any other surgical treatment for cSDH55. In 2006, a survey of the UK and Ireland found that 92% of surgeons preferred burr holes53. Burr hole procedures have been found to be competitively efficacious in the elderly, with a recent retrospective 2012 study finding neurological outcome improvement of as much as 83% in the 65–74 age group of the 322 patients by the Rankin Scale47. Most of the current literature regarding burr hole craniostomy is in the refinement of technique. Santarius et al performed a randomized controlled trial comparing draining subdural hematomas relieved by burr hole drilling versus drilling the holes without chronically draining the internal fluid through a catheter. Drainage was theorized to reduce the recurrence of bleeding from a treated hematoma by the removal of fibrinolytic elements in the accumulated fluid22, 56, 57. The presence of a drain was so strongly associated with a reduction in recurrence (9% to 24%) in addition to less stark improvements in long term mortality and post-surgical Rankin scores that the study was stopped and all participants were placed on drainage58. A meta-analysis conducted a year later, however, found no significant difference between postoperative drainage or non-drainage59. A recent 2010 review found literature suggesting the superiority of subperiosteal drainage to direct subdural drainage52. Though the general neurological improvement of subperiosteal drainage patients was generally higher than subdural drainage patients according to a retrospective study in 2012, the former group had triple the recurrence requiring reoperation rate, and no significant difference in outcome could be found between the treatment types60.
Twist drill to drain a cSDH involves inserting a hollow screw in the skull outside the hematoma until it punctures the dura, which will then be drained through the screw by a sterile collecting catheter. It is favored as a new method for treating subdural hematomas because of the reduced need for general anesthesia and the reduced stress of complicated surgery, especially in the elderly61. In 1999, one of the first published studies on its efficacy found it to have a recurrence rate of 26% and an infection rate of 2%62. Recent retrospective studies have demonstrated at least an 80% improvement in neurological status post-treatment using twist drill intervention61, 63. A retrospective study of a modified twist drill technique with post-operative rinsing and closed system drainage used from 2006 to 2010 had a 63.3% rate of resolution with the first operation and an 80.3% rate of significant improvement before additional operations were performed63. Hyperdense hematomas, which are presumably more solid and less liquid than hypodense hemorrhages, were found to require fewer subsequent operations63, though earlier literature theorized that twist drill was more useful for more liquefied hematomas52. Randomized controlled trials have found that the removal of the evacuation catheter after 48 hours is best to prevent complications resulting from sustained bedside drainage64, 65.
Pharmacologic Supplementation to Surgery
In addition to the anesthesia and anticoagulant necessary for general surgery and cSDH craniostomies in particular, there are new and specific drug interventions under review. A 2007–2010 study of 139 patients infused 1 mg/mL tPA into drained burr hole and twist drill patients post-operation as needed if the patient became symptomatic and the drainage was <50mL. This protocol addition significantly reduced the recurrence rate to 0% for both treatments, though the sample sizes were low for the experimental group66.
Seizures are also a problem in cSDH treatment. Preoperative seizure diagnoses are associated with lower GOS scores post-operation67 and a higher rate of post-operation seizure and death68. Postoperative seizures are also associated with recurrence of the hematoma and the necessity for a repeat operation69. A retrospective 2009 study on the administration of pre-operative anti-epileptic medication did reduce the post-operation seizures but did not significantly affect any measure of post-surgical outcome70.
Corticosteroids are also considered for use to treat cSDH. An observational only literature review of corticosteroid treatment demonstrated recurrence rates of, at most, 26%, and good outcome rates of at least 83%71.
Several studies and meta-analyses have been performed in the recent years comparing the three main treatment modalities, with comparisons between burr hole and twist drill procedures being the majority of recent publications. Several randomized prospective trials have been unable to find a significant difference between burr holes and twist drills in measures of cognitive improvement, mortality, or recurrence/reoperation72–74. A meta-analysis of 830 publications made prior to 2010 defining the utility of each procedure as a function of recurrence, death, and other complications (weighted with death being the worst outcome and no complications being the best) found in favor of burr hole craniostomy by a narrow but significant margin. Of interest in the rough outcome summary is that proportionately, craniotomies resulted in the most deaths, burr holes resulted in the most nonfatal complications, and twist drills had the most recurrence with the least proportion cured59. The increased recurrence of hematomas with twist drills is noticed in several other studies10, 52, 55, 66.
The Subdural Evacuating Port System (SEPS) is a hermetically-sealed closed drainage variant of the twist-drill craniostomy introduced in 200375. It is commonly done under local anesthetic, unlike craniotomies and burr holes, the latter of which can be done under local anesthesia but may be performed under general anesthesia52. Because of the sealed nature of the system, it is frequently done at the bedside without worry of infection75, 76. This is hypothesized to be of great advantage in elderly morbid populations in which cSDH is most prevalent and wreaks multiple sequelae11, 12, 14, 47. In 2010, a case-control study of 129 matched patients with SEPS or burr holes compared radiological changes, recurrence, mortality, seizure, length of stay, and home discharge rates which were all found to have no significant differences77. A retrospective study done afterward comparing successful to failed SEPS treatments in 74 patients had a 74% success rate defined by the need to reoperate (recurrence was 26%). Successful operations were correlated with higher initial rates and total volumes of drainage as well as with hypodense, smaller volume hematomas76, which contrasts with the 2012 Krieg et al finding that twist drills more successfully evacuate hyperdense hematomas on the first operation63. The latest retrospective report of 52 patients found a success by improvement and discharge rate of 73%, with 35% recurrence and 27% recurrence necessitating reoperation. 78% of patients returned to baseline on follow up78. All recent studies have corroborated the theory that SEPS is associated with low comorbidity52, 76–78.
Our research group retrospectively reviewed SEPS (29 patients) and burr holes (44 patients) procedures performed in the Manhattan VA hospital in the New York Harbor Health Care System. Procedures from January 2000 through October 2010 were included. The hospital is not a trauma center, and serves as a neurosurgical referral center for 14 other affiliated hospitals. All patients admitted to the hospital prior to August 2008 underwent burr holes in the operating room and all patients admitted thereafter underwent SEPS (Medtronic) at the bedside in the surgical intensive care unit (SICU) or emergency room. No patient admitted after August of 2008 underwent burr hole drainage of a subdural hematoma.
Patients whose subdural was not first treated by either burr holes or SEPS were excluded. The very first SEPS procedure performed at the institution, which was performed in the operating room with monitored anesthesia care was also excluded. Patients admitted for reasons other than neurological symptoms leading to a diagnosis of chronic subdural hematoma or for treatment of a chronic subdural hematoma were excluded.
A cost analysis was performed based on mean actual cost to the hospital incurred for care rendered in 2010 multiplied by the units of care required for each case. The cost basis for 2010 was used to adjust for inflation from prior years. Costs were $5182 per day in the SICU, $4497 per day on the ward, and $1585 per SEPS kit (materials only). Costs for burr hole procedures in the OR were $2517 for personnel and a total of $838 for disposable equipment, totaling $3355 for each case. All surgeons and anesthesiologists were salaried which resulted in no difference in cost between business day and after hours procedures. Nursing was compensated at a higher rate after hours, which we were unable to account for in our data. When patients were readmitted for recurrent SDH, LOS calculations were performed to reflect the total length of stay, (i.e. a sum of the first and second admissions). All calculable costs of the readmission were taken into account when calculating total costs.
Statistical analysis was performed using standard methodology. The two-sample T-test was used for quantitative analyses including costs and length of stay, and the Fisher exact test was used to compare categorical variables such as recurrence/reoperation. Additionally, a Q-Q plot comparison of SEPS and Burr Hole groups found that the cost data followed a log normal distribution. Therefore, a random sampling method, the Monte Carlo Simulation was used to test the significance of the difference between the two treatment costs by testing if the difference could be replicated if the data was scrambled between the groups.
73 total patients underwent a neurosurgical procedure for evacuation of chronic subdural hematoma during these 10 years of the data inclusion period. 44 patients were treated with burr holes and 29 with SEPS. Patient characteristics were similar between the two groups (Table 1). In the SEPS group, 6 of 29 patients had bilateral SDHs yielding a total of 35 separate SDHs. 23 of these SDHs were treated with a single SEPS placement, 9 were treated with 2 SEPS placements, and 3 were treated with 3 SEPS placements. 25 patients had a single admission and 4 were discharged and readmitted for additional treatment. Three patients underwent craniotomies after SEPS due to insufficient drainage of the hematoma; one craniotomy was performed during the same admission, and 2 were performed on readmission. The other two patients who were readmitted for SDH recurrence underwent repeat SEPS placement which is already accounted for in the above statistics.
Table 1.
Table of patient demographics upon admission to the hospital and discharge after treatment of chronic subdural hematoma
| Characteristics | Burr Holes | SEPS | P |
|---|---|---|---|
| Number of Patients | 44 | 29 | N/A |
| Age ± Standard Deviation | 78.4±8.4 | 76.6±11.03 | .43 |
| Male | 44 (100%) | 44 (100%) | 1.00 |
| Hypertension | 31 (70%) | 23 (79%) | .40 |
| Diabetes | 14 (32%) | 6 (21%) | .30 |
| Anticoagulants | 13 (30%) | 8 (28%) | .86 |
| Intubated for Airway Protection | 3 (7%) | 0 (0%) | .15 |
| Admission Hemiparesis | 26 (59%) | 12 (41%) | .14 |
| Discharge Hemiparesis | 7 (16%) | 3 (10%) | .50 |
| Admission Dysphasia | 13 (30%) | 10 (34%) | .66 |
| Discharge Dysphasia | 6 (14%) | 1 (3%) | .15 |
| Admission Ataxia | 19 (43%) | 12 (41%) | .88 |
| Discharge Ataxia | 11 (25%) | 8 (28%) | .81 |
The Student’s independent two sample t-test was used to calculate the probability value for age, and the χ2 test was used to compare the patient counts for the other variables. There were no significant differences between the treatment groups.
In the burr holes group, 6 of 44 patients had bilateral SDHs for a total of 50 SDHs treated. One patient underwent a repeat burr hole procedure during the same hospitalization. 16 of the 44 burr hole subdural evacuations were performed under general anesthesia and the remainder were performed under monitored anesthesia care with local anesthetic. While 6 of 29 patients treated with SEPS and 6 of 44 treated with burr holes had bilateral subdural hematomas, this difference was not significant (p=0.43).
4 patients from the burr hole group and 4 patients from the SEPS group were readmitted for reoperation after initial treatment (9% of patients with burr holes, 14% of SEPS; p=0.53). Details of the readmission cases are described in Table 2. Of the 4 SEPS patients who were readmitted, two were treated with repeat SEPS procedures, and two were treated with craniotomies. Of the 4 burr holes patients who were readmitted, two were treated with SEPS, and one each was treated with craniotomy and repeat burr holes.
Table 2.
Summary of data from patients readmitted for further treatment after SEPS and burr holes (BH)
| Initial | Age | Presenting | Recurrence | Recurrence | Recurrence |
|---|---|---|---|---|---|
| Treatment | (years) | Symptom | Interval | Symptom | Treatment |
| SEPS | 61 | hemiparesis | 31 days | lethargy | SEPS, craniotomy |
| SEPS | 66 | headache | 49 days | slow cognition | craniotomy |
| SEPS | 89 | lethargy | 32 days | headache | SEPS |
| SEPS | 87 | lethargy | 37 days | headache | SEPS |
| BH | 79 | confusion | 46 days | lethargy | burr holes |
| BH | 89 | gait/speech difficulty | 24 days | hemiparesis | craniotomy |
| BH | 85 | hemiparesis | 47 days | dysarthria | SEPS |
| BH | 83 | dysarthria/dysphasia | 841 days | nausea | SEPS |
The average time to intervention for SEPS (11.2±15.9 hrs) was significantly faster than for burr holes (40.3±69.1 hrs) (p=.02) (Figure 1). The total hospital LOS (Figure 2) was significantly shorter for SEPS (9.3±6.8 days) versus burr holes (13.4±10.2 days) (p=.04), although SICU LOS (Figure 3) did not vary significantly (5.7±3.6 days for SEPS vs 4.9±2.1 days for burr holes, p=.30).
Figure 1.
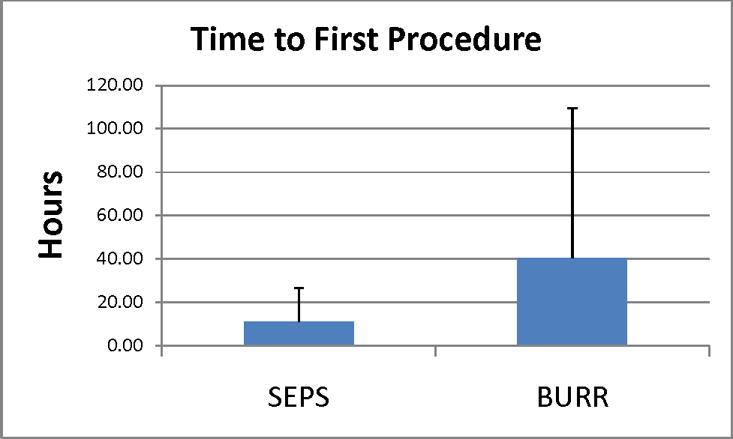
Time to first procedure
Figure 2.
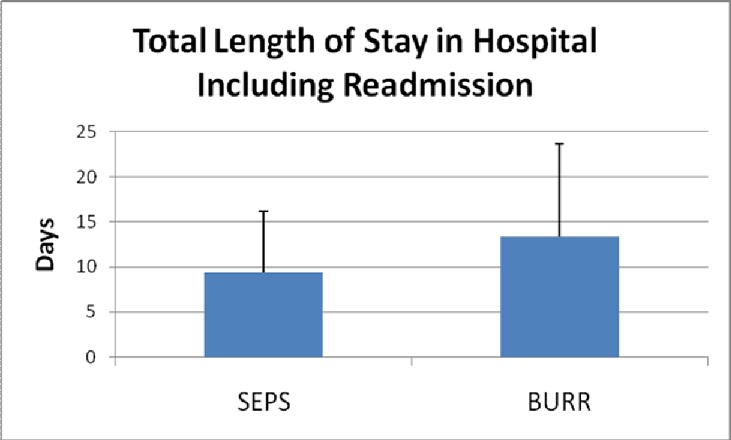
Total length of stay in hospital including readmission
Figure 3.
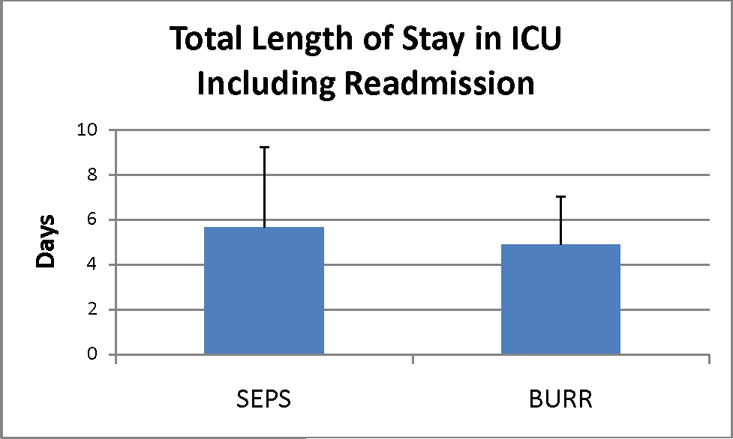
Total length of stay in ICU including readmission
Since the time to intervention was less for SEPS than burr holes, we also calculated the post-procedure length of stay and compared this for the two procedures. The SEPS post-procedure LOS was 8.6±6.6 days, while the burr hole post-procedure LOS was 11.1±9.8 days (T-test heteroscedastic p=0.18).
The length of stay for craniotomy for brain tumor procedures was stable during the years 2000–10, (mean LOS in days by year from 2000 to 2009 were: 6.35, 6.56, 4.38, 5.32, 6.54, 4.42, 4.57, 7.07, 6.67, and 7.33) suggesting that other aspects of patient care not related to the type of surgery did not lead to decreased length of stay. Length of stay was 5.7±1.1 days from 2000–7, and 7.0±0.5 days from 2008–10. The increase in LOS for craniotomy over that time period was not statistically significant either year-over-year or when grouped as before and after 2008.
Even accounting for repeat procedures and readmissions, the cost of care was significantly less (p=.05) with SEPS ($48,446±33,226) versus burr holes ($67,227±46,457) (Figure 4) in our single-institution series.
Figure 4.
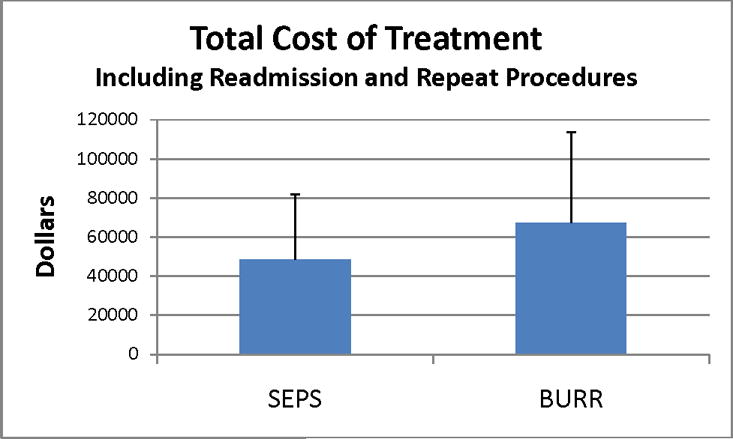
Total cost of treatment including readmission and repeat procedures
Two-thirds of the SDHs in the SEPS group (23 of 35) were successfully treated with a single SEPS placement, while an additional 26% (9 of 35) required 2 SEPS. We generally performed placement of the second SEPS as soon as there was clear evidence that a single SEPS was insufficient. Criteria for placement of a second SEPS included clotting of the drainage tube, cessation of drainage after an initial egress of fluid and a CT scan demonstrating a persistent collection appearing amenable to drainage, along with incomplete resolution of the clinical indications for evacuation.
The craniotomy that was performed on the same admission in a failed SEPS patient, represented a SDH that appeared to be fluid on CT, but was found to have organized clot intraoperatively, and thus in retrospect this patient was a poor candidate for SEPS.
The other two SEPS failures that required readmission and received craniotomies, at 39 and 41 days postoperatively demonstrated multiple loculations that predisposed to recurrence.
Our data suggest that despite a trend toward higher failure rates requiring additional treatment, usage of a bedside technique for evacuation of chronic subdural hemorrhage results in faster treatment, a shorter hospital stay, and less expense to the hospital relative to an equally effective surgical procedure performed in the operating room.
Cost analysis in our study was performed not from perspective, which reflects who is paying, but rather from valuation, which here was determined as actual cost to the hospital. In the Veterans Administration, the majority of patient care costs are directly billed to the United States Government, with a minority of patients having Medicare or private insurances. Our analysis was performed based on actual costs to the hospital for the use of a facility, compensation of personnel, or purchasing of supplies.
In our hospital, the operating room is available on weekends, holidays and evenings through arrangement with the attending surgeon, anesthesia and operating room staff, who are all called in from home. Hence, it is logical that treatment with SEPS at the bedside (mean 11 hours) was considerably faster than mobilizing the team for the operating room (mean 40 hours). We would emphasize however, that similar results would not necessarily be seen in hospitals that are trauma centers, or wherever surgeons, anesthesia and operating room staff are more readily available.
The omission of physician-associated costs from this study was due to the fact that both the neurosurgeons and anesthesiologists involved in these cases were either salaried directly by the VA or through service consulting agreement compensating availability rather than incidence. Since these physicians could be using their time caring for other patients, SEPS represented a considerable savings in “opportunity costs” over burr holes as anesthesiologists were not required at all for the procedure, and surgeons were present for less time since they were not required to wait for anesthesia. One limitation of this study, is that these “opportunity costs” were not directly quantifiable.
Along similar lines, it is conceivable that the cost savings of using SEPS in other institutions where operating room costs are greater than $3354.91 per case would be proportionately greater. We speculate that the mean 4.1 day shorter LOS seen in our study with the SEPS procedure was possibly due to avoidance of general anesthesia. 16 of 44 burr hole drainages in our study were performed under general anesthesia. The mean age of a patient undergoing treatment in this study was 78 years old. The risks of anesthesia are compounded by age, with patients older than 75 years at highest risk for postoperative delirium.79, 80 Interestingly, in the study by Rughani et al, where 7 of 21 SEPS procedures were performed in the operating room, and three of these SEPS were performed under general anesthesia, the difference in LOS between SEPS and burr holes was not significant77.
The first major limitation of this study is its retrospective nature. Our data analysis suggests that the nature of the patients and their disease severity has not changed over time (Table 1). We further validated our data by comparing length of stay of craniotomy for tumor performed by the same teams in the same places over the same time period as subdural drainage. Nonetheless, this is still a retrospective analysis that is not case-controlled, so it is conceivable that an unquantifiable or intangible aspect of patient care has changed over the last ten years and resulted in a decreased LOS for SEPS patients, but not patients undergoing craniotomies for tumor.
The second major limitation of this study is its applicability to other medical centers and patient populations. Our data is drawn from a single hospital’s neurosurgery service, and the population studied is 100% male. The operating room at this facility requires a minimum of two hours notice for procedures on evenings, weekends and holidays. Mean length of stay at this facility may be different than from other medical centers.
Given that length of stay in our facility could be inflated by the time from admission to procedure, we compared post-procedure LOS and found it to be not significantly different between SEPS and burr hole treated patients. While this weakens the generalizability of our study by making it more specific to the VA, and other hospitals that do not have immediate OR access, it emphasizes the point that several factors exist in OR-required procedures, such as time to operation, that increase the cost of the burr hole procedure.
The third limitation of this study is that we are not necessarily comparing absolutely equivalently indicated procedures. There may be risks associated with SEPS that are not seen with burr holes such as increased recurrence77, or conversion to acute hemorrhage via iatrogenic injury.76, 81 Some component of increased risk may be due to case selection. Certainly patients with multiple septations in their chronic subdural or an acute component may not represent ideal candidates for SEPS drainage. While we had four patients that required readmission after SEPS, we factored the cost of that treatment into their care. Our final cost analysis is thus based on the premise that about 10% of patients will fail SEPS and require additional treatment.
The economic ramifications of this study are that it could result in considerable health care cost savings. Given the incidence of chronic subdural hematoma (5/100,000) and the population of the United States (307 million), approximately 15,000 chronic subdurals are treated each year. Assuming that only half of these are candidates for SEPS (no acute hemorrhagic component, relatively few septations), and are treated at hospitals equivalent to ours (non-trauma centers), that is still a savings of more than 150 million dollars per year.
Reduced hospital time of 4.1 days per patient treated with SEPS versus burr holes, again assuming that half of the 15,000 patients per year in the United States would benefit from SEPS, results in 30,750 saved hospital days.
Further validation of our data with a prospective randomized trial would establish whether SEPS is superior to burr holes in the aspects we have evaluated.
Summary.
As the American population ages and cerebral atrophy becomes increasingly common, the incidence of chronic subdural hematoma will continue to rise. Treatment strategies for cSDH include craniotomy, burr-hole craniostomy, and twist drill procedures. The optimal management of these patients remains controversial.
Acknowledgments
DB was supported by an American Heart Association Medical Student Research Fellowship. CS was supported by the AOA Carolyn L. Kuckein Medical Student Summer Research Fellowship.
References
- 1.Filippini G. Epidemiology of primary central nervous system tumors. Handb Clin Neurol. 2012;104:3–22. doi: 10.1016/B978-0-444-52138-5.00001-3. [DOI] [PubMed] [Google Scholar]
- 2.Gavrilovic IT, Posner JB. Brain metastases: Epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 3.Kudo H, Kuwamura K, Izawa I, Sawa H, Tamaki N. Chronic subdural hematoma in elderly people: Present status on awaji island and epidemiological prospect. Neurol Med Chir (Tokyo) 1992;32:207–9. doi: 10.2176/nmc.32.207. [DOI] [PubMed] [Google Scholar]
- 4.Center PR. Aging of america. 2011 http://www.prcdc.org/300million/The_Aging_of_America/.
- 5.Foelholm R, Waltimo O. Epidemiology of chronic subdural haematoma. Acta Neurochir (Wien) 1975;32:247–50. doi: 10.1007/BF01405457. [DOI] [PubMed] [Google Scholar]
- 6.Mellergard P, Wisten O. Operations and re-operations for chronic subdural haematomas during a 25-year period in a well defined population. Acta Neurochir (Wien) 1996;138:708–13. doi: 10.1007/BF01411476. [DOI] [PubMed] [Google Scholar]
- 7.Karibe H, Kameyama M, Kawase M, Hirano T, Kawaguchi T, Tominaga T. epidemiology of chronic subdural hematomas. No Shinkei Geka. 2011;39:1149–53. [PubMed] [Google Scholar]
- 8.Frontera JA, de los Reyes K, Gordon E, Gowda A, Grilo C, Egorova N, et al. Trend in outcome and financial impact of subdural hemorrhage. Neurocrit Care. 2011;14:260–6. doi: 10.1007/s12028-010-9418-2. [DOI] [PubMed] [Google Scholar]
- 9.De Jesus O, Pacheco H, Negron B. Chronic and subacute subdural hematoma in the adult population. The puerto rico experience. P R Health Sci J. 1998;17:227–33. [PubMed] [Google Scholar]
- 10.Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma: The role for craniotomy reevaluated. Neurosurgery. 1993;33:67–72. doi: 10.1227/00006123-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Iantosca MR, Simon RH. Chronic subdural hematoma in adult and elderly patients. Neurosurg Clin N Am. 2000;11:447–54. [PubMed] [Google Scholar]
- 12.Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: Not a benign disease. J Neurosurg. 2011;114:72–6. doi: 10.3171/2010.8.JNS10298. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran R, Hegde T. Chronic subdural hematomas–causes of morbidity and mortality. Surg Neurol. 2007;67:367–72. doi: 10.1016/j.surneu.2006.07.022. discussion 72–3. [DOI] [PubMed] [Google Scholar]
- 14.Dumont TM, Rughani AI, Goeckes T, Tranmer BI. Chronic subdural hematoma: A sentinel health event. World Neurosurg. 2012 doi: 10.1016/j.wneu.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: Clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo) 2001;41:371–81. doi: 10.2176/nmc.41.371. [DOI] [PubMed] [Google Scholar]
- 16.Gelabert-Gonzalez M, Iglesias-Pais M, Garcia-Allut A, Martinez-Rumbo R. Chronic subdural haematoma: Surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107:223–9. doi: 10.1016/j.clineuro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Deci DM. Chronic subdural hematoma presenting as headache and cognitive impairment after minor head trauma. W V Med J. 2004;100:106–7. [PubMed] [Google Scholar]
- 18.Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke. 1995;26:1471–7. doi: 10.1161/01.str.26.8.1471. [DOI] [PubMed] [Google Scholar]
- 19.Lee KS. The pathogenesis and clinical significance of traumatic subdural hygroma. Brain Inj. 1998;12:595–603. doi: 10.1080/026990598122359. [DOI] [PubMed] [Google Scholar]
- 20.Apfelbaum RI, Guthkelch AN, Shulman K. Experimental production of subdural hematomas. J Neurosurg. 1974;40:336–46. doi: 10.3171/jns.1974.40.3.0336. [DOI] [PubMed] [Google Scholar]
- 21.Trotter Chronic subdural haemorrhage of traumatic origin and its relationship to pachymeningitis haemorrhagica interna. Br J Surg. 1914;2:271–91. [Google Scholar]
- 22.Fujisawa H, Ito H, Saito K, Ikeda K, Nitta H, Yamashita J. Immunohistochemical localization of tissue-type plasminogen activator in the lining wall of chronic subdural hematoma. Surg Neurol. 1991;35:441–5. doi: 10.1016/0090-3019(91)90177-b. [DOI] [PubMed] [Google Scholar]
- 23.Yamashima T, Friede RL. Why do bridging veins rupture into the virtual subdural space? J Neurol Neurosurg Psychiatry. 1984;47:121–7. doi: 10.1136/jnnp.47.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang MC, Chang Y, Hylek EM, Rosand J, Greenberg SM, Go AS, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141:745–52. doi: 10.7326/0003-4819-141-10-200411160-00005. [DOI] [PubMed] [Google Scholar]
- 25.Vasilevko V, Passos GF, Quiring D, Head E, Kim RC, Fisher M, et al. Aging and cerebrovascular dysfunction: Contribution of hypertension, cerebral amyloid angiopathy, and immunotherapy. Ann N Y Acad Sci. 2010;1207:58–70. doi: 10.1111/j.1749-6632.2010.05786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang AI, Balser DS, Mikheev A, Offen S, Huang JH, Babb J, et al. Cerebral atrophy is associated with development of chronic subdural haematoma. Brain Inj. 2012 doi: 10.3109/02699052.2012.698364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soni A. Aspirin use among the adult u.S. Noninstitutionalized population, with and without indicators of heart disease. 2005;2007 [Google Scholar]
- 28.Control CfD. Prevalence of aspirin use to prevent heart disease – wisconsin, 1991, and michigan, 1994. 1997;46:498–502. [PubMed] [Google Scholar]
- 29.Mukherjee D. Review: Aspirin does not reduce cv events but may reduce non-fatal stroke in peripheral artery disease. Evid Based Med. 2009;14:172–3. doi: 10.1136/ebm.14.6.173. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee D. Review: Aspirin reduces vascular events but increases bleeding in primary and secondary prevention. Evid Based Med. 2009;14:172–3. doi: 10.1136/ebm.14.6.172. [DOI] [PubMed] [Google Scholar]
- 31.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Erqou S, Sattar N, et al. Effect of aspirin on vascular and nonvascular outcomes: Meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:209–16. doi: 10.1001/archinternmed.2011.628. [DOI] [PubMed] [Google Scholar]
- 33.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 34.Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: A randomized controlled trial. JAMA. 2010;303:841–8. doi: 10.1001/jama.2010.221. [DOI] [PubMed] [Google Scholar]
- 36.Duff IF, Shull WH. Fatal hemorrhage in dicumarol poisoning; with report of necropsy. J Am Med Assoc. 1949;139:762–6. doi: 10.1001/jama.1949.02900290008003. [DOI] [PubMed] [Google Scholar]
- 37.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–4. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 38.Silverstein A. Neurological complications of anticoagulation therapy: A neurologist’s review. Arch Intern Med. 1979;139:217–20. [PubMed] [Google Scholar]
- 39.Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120:700–5. doi: 10.1016/j.amjmed.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flaherty ML, Kissela B, Woo D, Kleindorfer D, Alwell K, Sekar P, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68:116–21. doi: 10.1212/01.wnl.0000250340.05202.8b. [DOI] [PubMed] [Google Scholar]
- 41.Franke CL, de Jonge J, van Swieten JC, Op de Coul AA, van Gijn J. Intracerebral hematomas during anticoagulant treatment. Stroke. 1990;21:726–30. doi: 10.1161/01.str.21.5.726. [DOI] [PubMed] [Google Scholar]
- 42.Radberg JA, Olsson JE, Radberg CT. Prognostic parameters in spontaneous intracerebral hematomas with special reference to anticoagulant treatment. Stroke. 1991;22:571–6. doi: 10.1161/01.str.22.5.571. [DOI] [PubMed] [Google Scholar]
- 43.Sjoblom L, Hardemark HG, Lindgren A, Norrving B, Fahlen M, Samuelsson M, et al. Management and prognostic features of intracerebral hemorrhage during anticoagulant therapy: A swedish multicenter study. Stroke. 2001;32:2567–74. doi: 10.1161/hs1101.098523. [DOI] [PubMed] [Google Scholar]
- 44.Flaherty ML. Anticoagulant-associated intracerebral hemorrhage. Semin Neurol. 2010;30:565–72. doi: 10.1055/s-0030-1268866. [DOI] [PubMed] [Google Scholar]
- 45.Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–64. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- 46.Majeed A, Kim YK, Roberts RS, Holmstrom M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke. 2010;41:2860–6. doi: 10.1161/STROKEAHA.110.593087. [DOI] [PubMed] [Google Scholar]
- 47.Borger V, Vatter H, Oszvald A, Marquardt G, Seifert V, Guresir E. Chronic subdural haematoma in elderly patients: A retrospective analysis of 322 patients between the ages of 65–94 years. Acta Neurochir (Wien) 2012 doi: 10.1007/s00701-012-1434-x. [DOI] [PubMed] [Google Scholar]
- 48.Li LM, Kolias AG, Guilfoyle MR, Timofeev I, Corteen EA, Pickard JD, et al. Outcome following evacuation of acute subdural haematomas: A comparison of craniotomy with decompressive craniectomy. Acta Neurochir (Wien) 2012 doi: 10.1007/s00701-012-1428-8. [DOI] [PubMed] [Google Scholar]
- 49.Kim JH, Kang DS, Kong MH, Song KY. Chronic subdural hematoma treated by small or large craniotomy with membranectomy as the initial treatment. J Korean Neurosurg Soc. 2011;50:103–8. doi: 10.3340/jkns.2011.50.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imaizumi S, Onuma T, Kameyama M, Naganuma H. Organized chronic subdural hematoma requiring craniotomy—five case reports&mdashdash. Neurol Med Chir (Tokyo) 2001;41:19–24. doi: 10.2176/nmc.41.19. [DOI] [PubMed] [Google Scholar]
- 51.Mondorf Y, Abu-Owaimer M, Gaab MR, Oertel JM. Chronic subdural hematoma–craniotomy versus burr hole trepanation. Br J Neurosurg. 2009;23:612–6. doi: 10.3109/02688690903370297. [DOI] [PubMed] [Google Scholar]
- 52.Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Anderson K, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev. 2012;35:155–69. doi: 10.1007/s10143-011-0349-y. discussion 69. [DOI] [PubMed] [Google Scholar]
- 53.Santarius T, Lawton R, Kirkpatrick PJ, Hutchinson PJ. The management of primary chronic subdural haematoma: A questionnaire survey of practice in the united kingdom and the republic of ireland. Br J Neurosurg. 2008;22:529–34. doi: 10.1080/02688690802195381. [DOI] [PubMed] [Google Scholar]
- 54.Nayil K, Ramzan A, Sajad A, Zahoor S, Wani A, Nizami F, et al. Subdural hematomas: An analysis of 1181 kashmiri patients. World Neurosurg. 2012;77:103–10. doi: 10.1016/j.wneu.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Cenic A, Bhandari M, Reddy K. Management of chronic subdural hematoma: A national survey and literature review. Can J Neurol Sci. 2005;32:501–6. doi: 10.1017/s0317167100004510. [DOI] [PubMed] [Google Scholar]
- 56.Santarius T, Hutchinson PJ. Chronic subdural haematoma: Time to rationalize treatment? Br J Neurosurg. 2004;18:328–32. doi: 10.1080/02688690400004845. [DOI] [PubMed] [Google Scholar]
- 57.Tahsim-Oglou Y, Beseoglu K, Hanggi D, Stummer W, Steiger HJ. Factors predicting recurrence of chronic subdural haematoma: The influence of intraoperative irrigation and low-molecular-weight heparin thromboprophylaxis. Acta Neurochir (Wien) 2012;154:1063–7. doi: 10.1007/s00701-012-1334-0. discussion 8. [DOI] [PubMed] [Google Scholar]
- 58.Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: A randomised controlled trial. Lancet. 2009;374:1067–73. doi: 10.1016/S0140-6736(09)61115-6. [DOI] [PubMed] [Google Scholar]
- 59.Lega BC, Danish SF, Malhotra NR, Sonnad SS, Stein SC. Choosing the best operation for chronic subdural hematoma: A decision analysis. J Neurosurg. 2010;113:615–21. doi: 10.3171/2009.9.JNS08825. [DOI] [PubMed] [Google Scholar]
- 60.Bellut D, Woernle CM, Burkhardt JK, Kockro RA, Bertalanffy H, Krayenbuhl N. Subdural drainage versus subperiosteal drainage in burr-hole trepanation for symptomatic chronic subdural hematomas. World Neurosurg. 2012;77:111–8. doi: 10.1016/j.wneu.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 61.Ramnarayan R, Arulmurugan B, Wilson PM, Nayar R. Twist drill craniostomy with closed drainage for chronic subdural haematoma in the elderly: An effective method. Clin Neurol Neurosurg. 2008;110:774–8. doi: 10.1016/j.clineuro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 62.Emonds N, Hassler WE. New device to treat chronic subdural hematoma–hollow screw. Neurol Res. 1999;21:77–8. doi: 10.1080/01616412.1999.11740897. [DOI] [PubMed] [Google Scholar]
- 63.Krieg SM, Aldinger F, Stoffel M, Meyer B, Kreutzer J. Minimally invasive decompression of chronic subdural haematomas using hollow screws: Efficacy and safety in a consecutive series of 320 cases. Acta Neurochir (Wien) 2012;154:699–705. doi: 10.1007/s00701-012-1294-4. discussion. [DOI] [PubMed] [Google Scholar]
- 64.Ibrahim I, Maarrawi J, Jouanneau E, Guenot M, Mertens P, Sindou M. evacuation of chronic subdural hematomas with the twist-drill technique: Results of a randomized prospective study comparing 48-h and 96-h drainage duration. Neurochirurgie. 2010;56:23–7. doi: 10.1016/j.neuchi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Sindou M, Ibrahim I, Maarrawi J. Chronic sub-dural hematomas: Twist drill craniostomy with a closed system of drainage, for 48 hours only, is a valuable surgical treatment. Acta Neurochir (Wien) 2010;152:545–6. doi: 10.1007/s00701-009-0489-9. [DOI] [PubMed] [Google Scholar]
- 66.Neils DM, Singanallur PS, Wang H, Tracy P, Klopfenstein J, Dinh D, et al. Recurrence-free chronic subdural hematomas: A retrospective analysis of the instillation of tissue plasminogen activator in addition to twist drill or burr hole drainage in the treatment of chronic subdural hematomas. World Neurosurg. 2011 doi: 10.1016/j.wneu.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 67.Rabinstein AA, Chung SY, Rudzinski LA, Lanzino G. Seizures after evacuation of subdural hematomas: Incidence, risk factors, and functional impact. J Neurosurg. 2010;112:455–60. doi: 10.3171/2009.7.JNS09392. [DOI] [PubMed] [Google Scholar]
- 68.Battaglia F, Lubrano V, Ribeiro-Filho T, Pradel V, Roche PH. incidence and clinical impact of seizures after surgery for chronic subdural haematoma. Neurochirurgie. 2012 doi: 10.1016/j.neuchi.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012 doi: 10.1007/s00701-012-1399-9. [DOI] [PubMed] [Google Scholar]
- 70.Grobelny BT, Ducruet AF, Zacharia BE, Hickman ZL, Andersen KN, Sussman E, et al. Preoperative antiepileptic drug administration and the incidence of postoperative seizures following bur hole-treated chronic subdural hematoma. J Neurosurg. 2009;111:1257–62. doi: 10.3171/2009.6.JNS0928. [DOI] [PubMed] [Google Scholar]
- 71.Berghauser Pont LM, Dirven CM, Dippel DW, Verweij BH, Dammers R. The role of corticosteroids in the management of chronic subdural hematoma: A systematic review. Eur J Neurol. 2012 doi: 10.1111/j.1468-1331.2012.03768.x. [DOI] [PubMed] [Google Scholar]
- 72.Horn EM, Feiz-Erfan I, Bristol RE, Spetzler RF, Harrington TR. Bedside twist drill craniostomy for chronic subdural hematoma: A comparative study. Surg Neurol. 2006;65:150–3. doi: 10.1016/j.surneu.2005.05.030. discussion 3–4. [DOI] [PubMed] [Google Scholar]
- 73.Gokmen M, Sucu HK, Ergin A, Gokmen A, Bezircio Lu H. Randomized comparative study of burr-hole craniostomy versus twist drill craniostomy; surgical management of unilateral hemispheric chronic subdural hematomas. Zentralbl Neurochir. 2008;69:129–33. doi: 10.1055/s-2007-1004587. [DOI] [PubMed] [Google Scholar]
- 74.Muzii VF, Bistazzoni S, Zalaffi A, Carangelo B, Mariottini A, Palma L. Chronic subdural hematoma: Comparison of two surgical techniques. Preliminary results of a prospective randomized study. J Neurosurg Sci. 2005;49:41–6. discussion 6–7. [PubMed] [Google Scholar]
- 75.Asfora WT, Schwebach L. A modified technique to treat chronic and subacute subdural hematoma: Technical note. Surg Neurol. 2003;59:329–32. doi: 10.1016/s0090-3019(03)00039-9. discussion 32. [DOI] [PubMed] [Google Scholar]
- 76.Kenning TJ, Dalfino JC, German JW, Drazin D, Adamo MA. Analysis of the subdural evacuating port system for the treatment of subacute and chronic subdural hematomas. J Neurosurg. 2010;113:1004–10. doi: 10.3171/2010.5.JNS1083. [DOI] [PubMed] [Google Scholar]
- 77.Rughani AI, Lin C, Dumont TM, Penar PL, Horgan MA, Tranmer BI. A case-comparison study of the subdural evacuating port system in treating chronic subdural hematomas. J Neurosurg. 2010;113:609–14. doi: 10.3171/2009.11.JNS091244. [DOI] [PubMed] [Google Scholar]
- 78.Singla A, Jacobsen WP, Yusupov IR, Carter DA. Subdural evacuating port system (seps)-minimally invasive approach to the management of chronic/subacute subdural hematomas. Clin Neurol Neurosurg. 2012 doi: 10.1016/j.clineuro.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 79.Brown NA, Zenilman ME. The impact of frailty in the elderly on the outcome of surgery in the aged. Adv Surg. 2010;44:229–49. doi: 10.1016/j.yasu.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 80.Ansaloni L, Catena F, Chattat R, Fortuna D, Franceschi C, Mascitti P, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg. 2010;97:273–80. doi: 10.1002/bjs.6843. [DOI] [PubMed] [Google Scholar]
- 81.Reinges MH, Hasselberg I, Rohde V, Kuker W, Gilsbach JM. Prospective analysis of bedside percutaneous subdural tapping for the treatment of chronic subdural haematoma in adults. J Neurol Neurosurg Psychiatry. 2000;69:40–7. doi: 10.1136/jnnp.69.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]


