Abstract
Background
Systemic delivery of pharmacologic agents has led to many significant advances in the treatment of neurologic and psychiatric conditions. However, this approach has several limitations, including difficulty penetrating the blood-brain barrier and enzymatic degradation prior to reaching its intended target. Here, we describe the testing of a system allowing intraparenchymal (IPa) infusion of therapeutic agents directly to the appropriate anatomical targets, in a swine model.
New Method
Five male pigs underwent 3.0 T magnetic resonance (MR) guided placement of an IPa catheter into the dorso-medial putamen, using a combined system of the Leksell Stereotactic Arc, a Mayo-developed MRI-compatible pig head frame, and a custom-designed Fred Haer Company (FHC) delivery system.
Results
Our results show hemi-lateral coverage of the pig putamen is achievable from a single infusion point and that the volume of the bolus detected in each animal is uniform (1544±420mm3).
Comparison with Existing Method
The IPa infusion system is designed to isolate the intracranial catheter from bodily-induced forces while delivering drugs and molecules into the brain tissue by convection-enhanced delivery, with minimal-to-no catheter track backflow.
Conclusion
This study presents an innovative IPa drug delivery system, which includes a sophisticated catheter and implantable pump designed to deliver drugs and various molecules in a precise and controlled manner with limited backflow. It also demonstrates the efficacy of the delivery system, which has the potential to radically impact the treatment of a wide range of neurologic conditions. Lastly, the swine model used here has certain advantages for translation into clinical applications.
Keywords: Chronic Drug Delivery System, Intraparenchymal Catheter, Blood Brain Barrier, Central Nervous System, Swine Model
1. INTRODUCTION
Despite significant investment and advances in brain research, neurological and psychiatric disorders remain major causes of disability and are responsible for more hospitalizations and prolonged care than almost all other diseases combined.(Misra et al., 2003) The number of adults affected by devastating central nervous system (CNS) diseases, such as brain tumors, HIV encephalopathy, epilepsy, cerebrovascular disease and neurodegenerative disorders, such as Parkinson’s Disease (PD) exceeds the numbers affected by systemic cancer or heart disease.(Misra et al., 2003)
The principal obstacle in delivering drugs to appropriate brain targets is the biological blood-brain barrier, which drugs must cross in order to be effective against neuronal disease (Begley, 1996; Chen and Liu, 2012). Thus, the limited efficiency and clinical effectiveness of many therapeutics rests not on poor potency, but rather on limited means of drug delivery.(Neuwelt et al., 2008) In addition, precise targeting of drugs may help minimize toxicity and improve treatment efficacy. For example, use of modified adeno-associated viruses (AAV) to introduce glutamic acid decarboxylase (GAD) directly into the subthalamic nucleus (STN) in patients with advanced PD has been demonstrated as a safe and effective treatment.
Delivery limitations and difficulty in sustaining effective drug concentration levels at the target of interest (Buchwald and Bodor, 2001; Habgood et al., 2000; Siegal and Zylber-Katz, 2002; Thorne and Frey, 2001; Witt et al., 2001) interfere in the therapeutic benefit of many systemic pharmaceutical approaches to neurologic disease. A large body of research has focused on developing a method to enhance drug delivery, but the need remains.
Here, we report testing a system designed to provide delivery of drugs in an aqueous solution directly into the brain parenchyma through a chronically implanted intraparenchymal (IPa) catheter. We used a chronic swine model and contrast-enhanced imaging to study catheter patency, accuracy of catheter placement, and coverage of anatomical structures of interest.
2. MATERIALS AND METHODS
2.1 Animals
Five domestic male pigs (30+/−5kg) were used for the study. They were housed individually in a controlled environment with humidity 45%, temperature 70° F and had daily feeding and ad libitum access to water. The study was performed following the National Institutes of Health Guidelines for animal Research (Guide for the Care and Use of Laboratory Animals) and supported and approved by the Mayo Clinic Institutional Animal Care and Use Committee (Protocol A18712).
2.2 Preoperative Imaging and Targeting
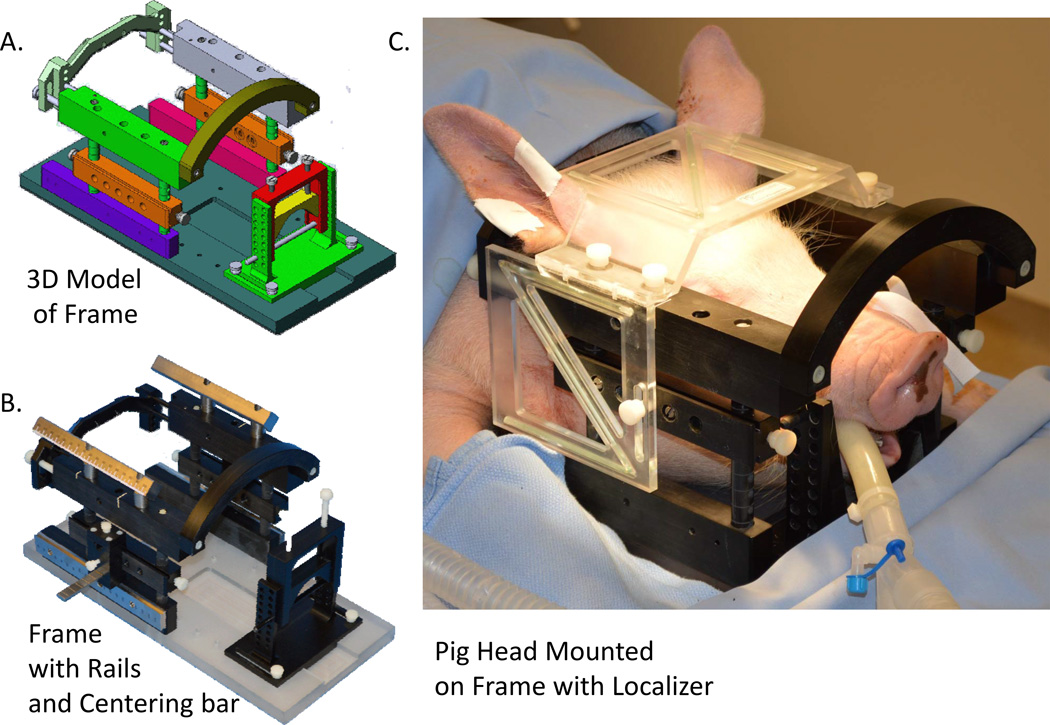
A Mayo Clinic-developed MRI-compatible stereotactic head frame, previously described by our group,(Knight et al., 2013) was used for catheter tip targeting (Figure 1). Preoperative anatomical imaging consisted of a 3D MP-RAGE sequence with 1.5 mm slice thickness, 24 × 24 cm2 field of view and matrix of 512 × 512. Scans were performed on a 3.0T magnetic resonance imaging (MRI) scanner (General Electric Health Care, Signa HDx 16× software with 23mT/m gradient set, Waukasha, WI). A small, custom developed four-channel phased array receive-only coil was used in this experiment. The coil was placed inside the head frame, just above the skull and below the localizer box to provide excellent signal-to-noise ratio (SNR). Stereotactic planning was performed using a custom version of the Compass navigational software (Compass, Rochester MN) that accommodates pig neuroanatomy. Pig-specific MRI data was merged with a pig brain atlas (Felix et al., 1999) using the anterior-posterior commissural line as a reference. Stereotactic coordinates for the catheter tip and implantation trajectory were defined for the left unilateral putamen, centered in the medial-lateral and axial directions, and 2/3 towards the ventral edge in the dorsal-ventral direction.
Figure 1.
A) Three-dimensional illustration of a custom made MRI-compatible stereotactic porcine head frame. Neck bracket provides back support for the head to stabilize the stereotactic position of the head in the frame. Top bars provide a secure placement of the localizer box above the compact, custom-developed four-channel phased array receive-only coil just above the skull. Pin holders allow various adjustments of screw placements into the jawbones to secure the head frame to the swine head with height adjustment capability along the frame posts. Side rails, when assembled with the Leksell stereotactic arc, provide accurate anterior and posterior coordinates for targeting. Snout holder is designed to fix and provide a reliable means for stabilizing the head, allowing versatility in stereotactic adjustments; B) photograph of the stereotactic head frame with additional rails to allow for targeting in the Kopf stereotactic system in addition to the Leksell targeting system; C) stereotactic head frame placed on swine head with localizer box for MRI guided targeting in 3D space.
2.3 Chronic Intraparenchymal Drug Delivery System
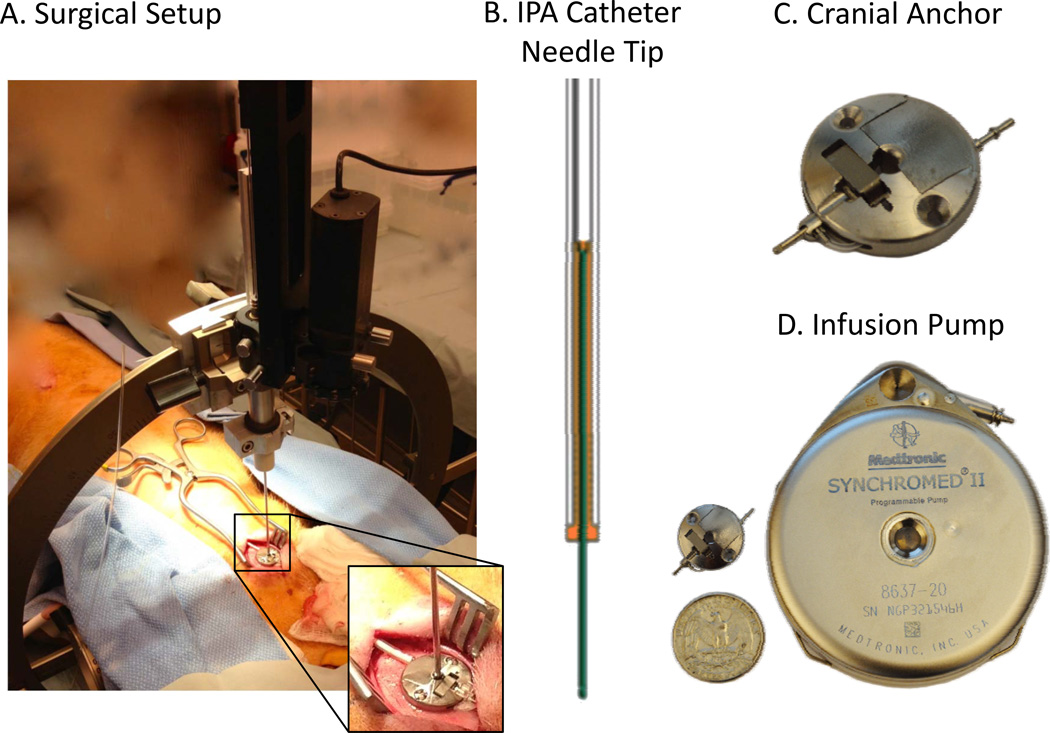
The chronic intraparenchymal drug delivery system, developed by Medtronic Inc, consists of four components: a pump device, a cranial anchor, cranial catheter and an external programmer (Figure 2A–D). The Synchromed® II pump (Figure 2D), provides a drug reservoir while also controlling the amount and rate of drug infusion to the target. The pump is connected to the cranial catheter, forming a fluid pathway to an infusate exit port of the cranial catheter at the target in the brain tissue. The connection is established through a cranial anchor that secures the catheter relative to a cranial burr hole. The cranial anchor (Figure 2D) is designed so that the cranial catheter is isolated from any loads transmitted from the catheter connecting the anchor to the abdominally positioned pump. The use of two isolated catheters prevents bodily-induced forces such as physical movement from interfering with the depth or position of the cranial catheter; such forces could potentially result in catheter retraction, moving the infusion site away from the intended target.
Figure 2.
A) Surgical setup consisting of a custom-designed Fred Hare Company (FHC) drive and Leksell Stereotactic Arc. The inset shows a cranial anchor secured on the swine skull; B) schematic drawing of IPA catheter needle tip with unique structural configurations to limit backflow along the catheter; C) picture of the cranial anchor shown in (A). This anchor is designed to connect the cranial catheter implanted in the brain to the catheter connected to the Synchromed® II pump while limiting changes in depth or position due to bodily-induced physical forces; D) Medtronic Synchromed® II pump.
The cranial catheter (Figure 2B) used in the drug delivery system has unique structural configurations that minimize backflow and promote spherical drug distribution. The primary catheter is assembled with a flexible tubular body that holds a rigid needle. The needle consists of a nonporous outer surface with a sealed distal tip. As the catheter is lowered into the brain tissue, only the narrower needle diameter penetrates the tissue nearest the target, thus creating a good seal between the small outer diameter of needle and the surrounding tissue. The step formed between the larger catheter body and smaller diameter of the needle tip helps create a barrier to backflow. Additionally, the IPa catheter can be trimmed, which allows manipulation of catheter length, and potential translation to a variety of pump implantation sites and targets of interest.
2.4 Surgical Procedure
Anesthesia was induced with Telazol (5 mg/kg i.m.) and Xylazine (2 mg/kg i.m.), followed by intubation and isoflurane (1.5–3%) maintenance throughout the remainder of the procedure. Vital signs (heart rate and temperature) were monitored continuously, and respiration was maintained at 12 breaths per minute throughout the procedure. Following the preoperative imaging, a Leksell stereotactic arc was mounted onto the custom stereotactic head frame.
A 15cm incision was made in the (ipsilateral) lower quadrant abdominal wall to make a pocket for the pump implant. The pump catheter was tunneled in the subcutaneous space from the head incision to the flank incision and connected to the cranial anchor. Prior to implantation, the SynchroMed® II pump was primed with sterile Phosphate-buffered saline (PBS) and programmed with a delivery rate of 0.1µl/min.
A Burr hole, 4–6 mm diameter, was drilled in line with the stereotactic coordinates and the dura punctured to expose the surface of the brain. The catheter delivery system consisted of a custom-designed FHC drive and Leksell adaptor, mounted on the Leksell stereotactic arc (Figure 2A).
Using a hollow stylet, the cranial catheter was introduced to the target at a rate of 1mm per minute. The stylet was retracted and the cranial catheter was secured to the skull using the cranial anchor (Figure 2C). PBS was infused into the catheter and hollow-stylet using a table-side Medfusion 3500 syringe pump at a rate of 0.3ml/hr during introduction and retraction. This fluid flow prevents any fluid (blood) entering the catheter during placement, which could potentially clot and block the needle tip.
A 100µl bolus was delivered from the pump (10µl/min over 10 minutes) to infuse a contrast agent through the catheter into the brain and to flush any possible blood from the tip. A postoperative 3D MP-RAGE T1-weighted anatomical MRI was obtained to confirm the location of the catheter tip and continuity of the connections, as visualized by the Magnevist® bolus (Figure 3B). This ensured that any potential issue with contrast infusion between postoperative days 7 and 10 was not present in the immediate postoperative period.
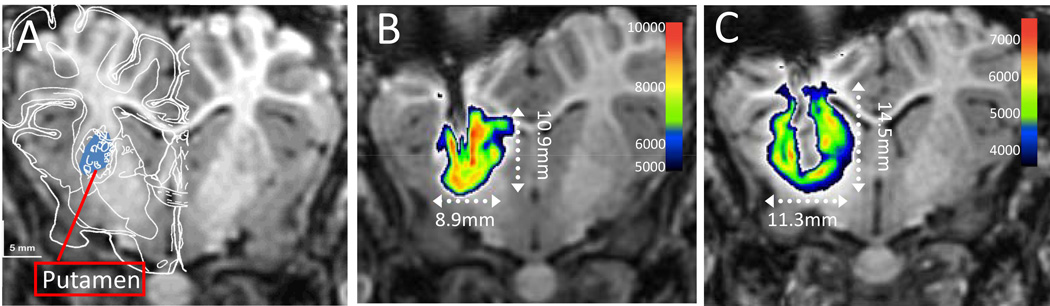
Figure 3.
A) Pre-operative anatomical MRI with pig brain atlas overlay.(Felix et al., 1999) The right putamen is shown in blue; B) post-operative anatomical MRI. The contrast bolus is shown in pseudocolor. C) Post-operative anatomical MRI at postoperative day 10 after 3 days of Magnevist® infusion. The contrast infusion is shown in pseudocolor.
2.5 Catheter Patency Imaging
Seven days after the surgery, the PBS was transcutaneously removed from the pump reservoir and replaced with a 5 mM solution of gadolinium-based MRI contrast agent in PBS (Magnevist®쏺, Bayer Healthcare). The pump was re-programmed to deliver the gadolinium agent to the brain at a rate of 0.3 ul/min. A final 3D MP-RAGE MRI of the animal’s brain was taken after 3 days of continuous delivery of the Magnevist®.
2.6 Bolus Analysis
In order to analyze the shape and volume of the Magnevist® bolus, the area of high contrast within the MRI was segmented by voxel threshholding at an intensity corresponding to the bolus while excluding the surrounding white matter. The region of interest was 3-D rendered and the volume was measured. All analyses were performed using Amira software (Visualization Sciences Group, Burlington, MA).
3. RESULTS
3.1 Animal model
Five male pigs underwent 3.0 T magnetic resonance (MR) guided placement of an IPa catheter into the dorso-medial putamen, using the Leksell stereotactic arc, a Mayo-developed MRI-compatible pig head frame, and a custom-designed FHC, Inc. delivery system. Simultaneously, the Synchromed® II pump was embedded into the ipsilateral lower quadrant abdominal wall and connected to the intracranial catheter by the cranial anchor through the subcutaneous space from the head incision to the flank incision. No MRI indication of hemorrhage or edema was observed at the infusion site in any of the subjects. No fever or other adverse events occurred during the 10-day protocol. A small infection was observed in the first pig, near the suture site on the lower abdominal wall above the implanted Synchromed® II pump after post-operative day 10. The infection was determined to have occurred due to failed sterile procedure during surgery, or invasion of infectious bacteria from the natural housing environment. The infection was minimal and treated successfully with antibiotics with no subsequent adverse events. No abnormal behavior changes were observed after the surgery or the Magnevist® infusion. Overall, the execution of the implant resulted in a low rate of complications. There were no deaths and no neurological deficits, confirming the safety of the surgical procedure.
3.2 Imaging Confirmation of Catheter Positioning
The target selected for catheter implantation was within the putamen, based on preoperative imaging centered in the medial-lateral and axial directions and 2/3 of the way toward the ventral edge in the dorsal-ventral direction. The target region is demonstrated by atlas overlay(Felix et al., 1999) and highlighted in blue in Figure 3A. Postoperative MR imaging (Figure 3B) demonstrated accurate placement of the catheter tip into the putamen area and coverage of the putaminal region by the contrast bolus (pseudocolored).
3.3 Characterization of the anatomical extent of gadolinium delivery to the putamen under chronic, steady state delivery conditions
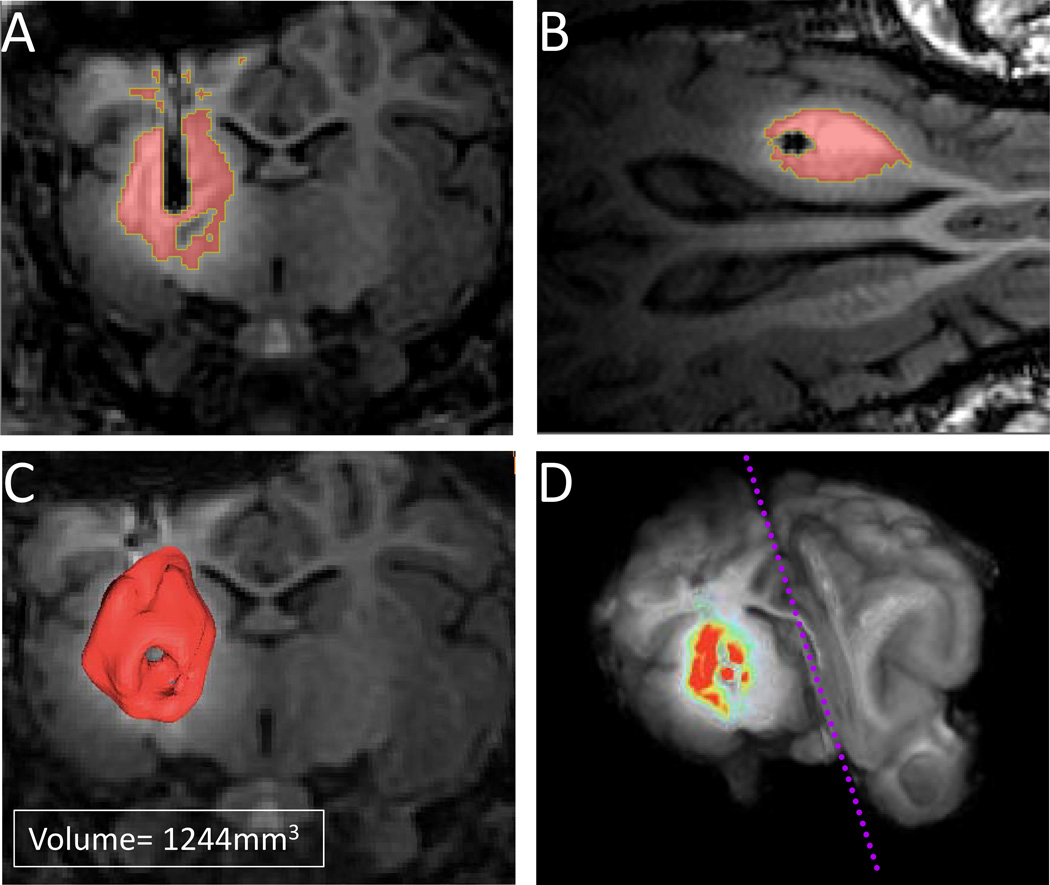
Next, we analyzed the size and distribution of the 3 day Magnevist® infusion using MR imaging. Infusion areas were defined by thresholding regions of high contrast within the MRI (Figure 3C). Figure 4 shows the three-dimensional renderings of the area of gadolinium contrast agent delivery in one representative pig. All targets were localized within the putamen, ensuring coverage of the entire putamen region in all five pigs. However, variability in distribution of the contrast bolus was observed between pigs (Figure 4A–D). On average, the shape of the bolus was rounded with the majority of the contrast surrounding the target. Additionally, the volume of the bolus was consistent (1544±420mm3).
Figure 4.
A) Coronal view of the Magnevist® infusion in a subject at postoperative day 10 after 3 days of Magnevist® infusion. The volumes demonstrate the pattern of drug spread from the injection area. The entire putamen region was covered in all subjects with an average volume of average volume (1544±420mm3); B) axial view of the Magnevist® infusion at postoperative day 10 after 3 days of Magnevist® infusion; C) 3D-rendered contrast infusion in a representative subject; D) pseudocolored contrast infusion superimposed on a 3D pig brain.
4. DISCUSSION
4.1 Intraparenchymal drug administration
Intraparenchymal drug administration has several advantages over vascular drug delivery. IPa bypasses the difficulties of permeating the blood-brain barrier, resulting in direct delivery to the target and minimal loss of drug concentration. Because the drug is localized within the brain parenchyma and cerebrospinal fluid (CSF), smaller doses may be sufficient for clinical treatment, potentially attenuating systemic toxicity. In addition, drugs introduced into CSF have a longer half-life than do drugs introduced into plasma, due to limited protein binding and reduced enzymatic degradation.
4.2 Advantages of the IPa infusion system
The Medtronic Synchromed® II implantable pump allows targeted, sustained and controlled intracranial drug delivery. This is of critical importance for many chemotherapeutic and other agents with narrow therapeutic ranges, whereby the effective drug concentration is very close to the concentration at which dose-limiting toxicity occurs. A reservoir in the implantable infusion system can be refilled by sub-cutaneous injection and is capable delivering drugs at different infusion rates over extended periods of time. Furthermore, the drug delivery rate can be controlled via external, handheld computers.
An important characteristic of this system is its unique catheter tip, which was designed to prevent backflow. Backflow can spread therapeutic agents into unwanted areas along the catheter length, which is especially undesirable when the agent is limited or expensive. In addition, within most drug delivery systems, the infusion pressure may drop to the level of CSF pressure, which compromises convention-enhanced drug delivery and can result in limited drug distribution into the target tissue. In contrast, the system described here did not demonstrate any significant backflow.
4.3 Limitations of the system
There are several potential complications associated with IPa drug delivery that warrant thorough examination. These include increased extracellular fluid in brain tissue with the potential for build-up and some displacement of tissue over time. The chronic effects of limited tissue displacement are currently unknown. Additionally, the effectiveness of drug diffusion through the brain parenchyma can vary with size of the molecules that make up the infused pharmaceutical agent.(Buchwald and Bodor, 2001) Increasing the concentration of the infused agent can enhance molecular distribution by increasing the diffusion gradient.(Kroll et al., 1996) However, significant increases in the distribution of drugs within the brain requires delivery by sustaining a pressure gradient to create bulk fluid movement in the brain space(Bobo et al., 1994) as is the intent of the infusion system demonstrated here.
4.4 Potential usage and contribution of the IPa infusion system for future studies
The IPa infusion system can be used to deliver different classes of molecules to treat a wide range of neurologic and psychiatric conditions. For example, the treatment of PD with oral levodopa has many limitations, including difficulty in monitoring effective dosage level and adverse effects. Direct delivery to the brain has the potential to circumvent these complications.
As previously noted, the IPa infusion system allows delivery of substances that would otherwise fail to cross the blood-brain barrier. For example, glial cell line-derived neurotrophic factor (GDNF) has proven to be effective in improving motor functions of patients with PD, but it cannot be administered orally. In contrast, a study of unilateral intraputaminal GDNF infusion in 10 patients with PD for six months, showed significant improvement in bilateral motor functions and quality of life.(Slevin et al., 2005)
Another area of particular promise is the delivery of small interfering RNA (siRNA) directly into the brain for the treatment of conditions involving the accumulation of abnormal proteins.(Chen and Zhaori, 2011) This approach has potential therapeutic implications for neurodegenerative conditions ranging from Huntington’s disease to Alzheimer’s disease.(Davidson and Paulson, 2004; Howard et al., 1989; Imarisio et al., 2008; Singer et al., 2005; Wang et al., 2005)
Finally, the study of the IPa drug delivery system in a large animal model has implications not only for basic science but also for translational research. For example, a critical component of neuroscience research is confirmation of molecular mechanisms via knock down or overexpression of proteins in target-specific tissue. This step can establish whether a specific molecule is necessary and/or sufficient to enact a particular neural process within specific brain regions, a question that is important in the identification of disease mechanisms and new drug targets. Currently available techniques lack the target specificity to definitively determine these mechanisms. Lastly, there is value in testing the long-term safety and clinical efficacy of new drugs in a large animal model prior to human clinical application. With its large gyrencephalic brain, the swine model used here represents a valuable tool for pre-clinical infusion device research and studying the long-term effects of new agents.
5. CONCLUSION
The results of this study demonstrate the capabilities of a long-term intraparenchymal drug delivery system with the potential to revolutionize treatment of a wide variety of neurologic and psychiatric conditions. The large animal model represents a translational research advance for testing the potential applications of this new system. This opens the door for the development of new pharmacological therapies previously considered untenable due to imprecise control over their distribution and their limited ability to permeate the blood-brain barrier.
Contributor Information
Inyong Kim, Email: kim.inyong@mayo.edu.
Seungleal Paek, Email: paek.seungleal@mayo.edu.
Brian D. Nelson, Email: brian.d.nelson@medtronic.com.
Emily J. Knight, Email: knight.emily@mayo.edu.
Michael P. Marsh, Email: marsh.michael@mayo.edu.
Allan J. Bieber, Email: bieber.allan@mayo.edu.
Kevin E. Bennet, Email: kbennet@mayo.edu.
Kendall H. Lee, Email: lee.kendall@mayo.edu.
References
- Begley DJ. The blood-brain barrier: principles for targeting peptides and drugs to the central nervous system. The Journal of pharmacy and pharmacology. 1996;48:136–146. doi: 10.1111/j.2042-7158.1996.tb07112.x. [DOI] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald P, Bodor N. A simple, predictive, structure-based skin permeability model. J Pharm Pharmacol. 2001;53:1087–1098. doi: 10.1211/0022357011776478. [DOI] [PubMed] [Google Scholar]
- Chen SH, Zhaori G. Potential clinical applications of siRNA technique: benefits and limitations. Eur J Clin Invest. 2011;41:221–232. doi: 10.1111/j.1365-2362.2010.02400.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Advanced drug delivery reviews. 2012;64:640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Paulson HL. Molecular medicine for the brain: silencing of disease genes with RNA interference. Lancet Neurol. 2004;3:145–149. doi: 10.1016/S1474-4422(04)00678-7. [DOI] [PubMed] [Google Scholar]
- Felix B, Leger ME, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP. Stereotaxic atlas of the pig brain. Brain Res Bull. 1999;49:1–137. doi: 10.1016/s0361-9230(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Habgood MD, Begley DJ, Abbott NJ. Determinants of passive drug entry into the central nervous system. Cellular and molecular neurobiology. 2000;20:231–253. doi: 10.1023/a:1007001923498. [DOI] [PubMed] [Google Scholar]
- Howard MA, 3rd, Gross A, Grady MS, Langer RS, Mathiowitz E, Winn HR, Mayberg MR. Intracerebral drug delivery in rats with lesion-induced memory deficits. J Neurosurg. 1989;71:105–112. doi: 10.3171/jns.1989.71.1.0105. [DOI] [PubMed] [Google Scholar]
- Imarisio S, Carmichael J, Korolchuk V, Chen CW, Saiki S, Rose C, Krishna G, Davies JE, Ttofi E, Underwood BR, Rubinsztein DC. Huntington's disease: from pathology and genetics to potential therapies. Biochem J. 2008;412:191–209. doi: 10.1042/BJ20071619. [DOI] [PubMed] [Google Scholar]
- Knight EJ, Min HK, Hwang SC, Marsh MP, Paek S, Kim I, Felmlee JP, Abulseoud OA, Bennet KE, Frye MA, Lee KH. Nucleus accumbens deep brain stimulation results in insula and prefrontal activation: a large animal FMRI study. PLoS One. 2013;8:e56640. doi: 10.1371/journal.pone.0056640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll RA, Pagel MA, Muldoon LL, Roman-Goldstein S, Neuwelt EA. Increasing volume of distribution to the brain with interstitial infusion: dose, rather than convection, might be the most important factor. Neurosurgery. 1996;38:746–752. discussion 52–4. [PubMed] [Google Scholar]
- Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2003;6:252–273. [PubMed] [Google Scholar]
- Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, Pardridge W, Rosenberg GA, Smith Q, Drewes LR. Strategies to advance translational research into brain barriers. Lancet neurology. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- Siegal T, Zylber-Katz E. Strategies for increasing drug delivery to the brain: focus on brain lymphoma. Clinical pharmacokinetics. 2002;41:171–186. doi: 10.2165/00003088-200241030-00002. [DOI] [PubMed] [Google Scholar]
- Singer O, Marr RA, Rockenstein E, Crews L, Coufal NG, Gage FH, Verma IM, Masliah E. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–1349. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Frey WH., 2nd Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clinical pharmacokinetics. 2001;40:907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- Wang YL, Liu W, Wada E, Murata M, Wada K, Kanazawa I. Clinico-pathological rescue of a model mouse of Huntington's disease by siRNA. Neurosci Res. 2005;53:241–249. doi: 10.1016/j.neures.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Witt KA, Gillespie TJ, Huber JD, Egleton RD, Davis TP. Peptide drug modifications to enhance bioavailability and blood-brain barrier permeability. Peptides. 2001;22:2329–2343. doi: 10.1016/s0196-9781(01)00537-x. [DOI] [PubMed] [Google Scholar]