Abstract
The BN analogue of ortho-benzyne, 1,2-azaborine, is generated by flash vacuum pyroylsis, trapped under cryogenic conditions, and studied by direct spectroscopic techniques. The parent BN-aryne spontaneously binds N2 and CO2, demonstrating its highly reactive nature. The interaction with N2 is photochemically reversible. The CO2 adduct of 1,2-azaborine is a cyclic lactam that undergoes photocleavage thus resulting in overall CO2 splitting.
The chemistry of arynes, aromatic compounds that lack two hydrogen atoms, can be traced back to 1902.[1] Since then, significant progress has turned ortho-benzynes, benzene derivatives that feature a highly strained triple bond, into an invaluable reactive intermediate in all branches of organic synthesis.[2] Formal substitution of the triply bonded carbon atoms of ortho-benzyne by an isoelectronic boron-nitrogen (BN) unit results in unknown 1,2-azaborine (1).[3] The similarity of carbon and its BN derivatives is well documented in chemistry, including the graphite/hexagonal boron nitride, and benzene/1,2-dihydro-1,2-azaborine pairs, including their Dewar forms.[4] However, evidence for the existence of BN-arynes is scarce and indirect as it comes from a self-trapping experiment.[5] Here we show by direct spectroscopic techniques that 1,2-azaborine can exist as reactive intermediate. We found that 1 can be generated by flash vacuum pyrolysis (FVP) from precursor 2 by thermal elimination of tert-butyldimethylsilylchloride and isolated in cryogenic matrices. 1,2-Azaborine spontaneously binds dinitrogen N2 (adduct 3) in a photochemically reversible transformation. The reaction of 1,2-azaborine with carbon dioxide (CO2) followed by irradiation results in CO2 splitting via a cyclic carbamate (4) intermediate. In contrast, the cyclic CO2 adduct of the ortho-benzyne undergoes photodecarboxylation.[6] Our results show that the two heteroelements boron and nitrogen increase and modulate the reactivity compared to the all-carbon analogue. Our research introduces a novel class of reactive intermediates to chemistry. It may prove useful in materials and medicinal chemistry where the boron-nitrogen isoelectronic substitution is sought in order to modify the properties of electronic materials[7] or biologically active compounds.[8]
The thermally induced elimination of trialkylsilyl chlorides from suitable precursors has proven to be a reliable method for the synthesis of iminoboranes.[9] Likewise, FVP of the N-tert-butyldimethylsilyl compound 2[10] (Scheme 1) at oven temperatures of 800–850 °C and isolation of pyrolysis products in a large excess of argon at 6 K yields the corresponding silyl chloride as demonstrated by comparison with the infrared spectrum of an authentic sample. Besides the silyl chloride and the unconverted precursor, at least two sets of additional signals are observed (Supporting Information Figure S1). One set of signals decreases in intensity if increasingly larger amounts of N2 are included into the argon matrix gas, and at the same time a set of new signals appears. The most prominent signal among them is a band at 2266 cm−1. This band is reminiscent of the dinitrogen stretching vibration ν(NN) observed previously by Maier et al. for the borabenzene-dinitrogen adduct. When employing 15N2 the isotopic shift is 75 cm−1, in good agreement with Maier et al.[11] (73.3 cm−1) for borabenzene-dinitrogen and with computations at the CCSD(T)/TZ2P level of theory. The remaining signals of 3 have at most slight isotopic shifts, in agreement with theoretical calculations (Supporting Information Table S1). The computations support assignment to adduct 3 with end-on coordinated dinitrogen (Scheme 1).
Scheme 1.
Generation and trapping of 1,2-azaborine (1) with N2 and CO2 by flash vacuum pyrolysis (FVP) of 2 at 850 °C.
Adduct 3 was found to be photolabile: irradiation with the output of a high pressure mercury lamp (λ > 395 nm) results in disappearance of the set of signals assigned to 3 and the concomitant growth of a set of signals that is assigned to parent 1,2-azaborine 1 (Figure 1). This is the same set of signals that can be observed after thermolysis of 2 in the absence of dinitrogen.
Figure 1.
Generation of 1,2-azaborine through irradiation of 3 in Ar/N2 (70:30). (a) IR difference spectrum obtained from the pyrolysis products of 2 frozen at 6 K after two hours of irradiation with visible light (λ > 395 nm, 4 K). The decreasing signals (pointing downwards) belong to dinitrogen adduct 3, increasing signals belong to 1 (blue stars) and 2. (b) Difference spectrum after subsequent annealing for 30 minutes at 12 K (starting from 4 K), showing reversibility of the reaction 3 ⇌ 1 + N2. (c) Computed IR spectrum of 1 at the CCSD(T)/TZ2P level of theory.
After photogenerating 1 from 3, the latter can be regenerated by careful annealing of the matrix from 4 K to 12 K (Figure 1), indicating that the reaction of 1 + N2 is essentially without a barrier. Both 3 and [15N2]-3 behave identically during photolysis and subsequent annealing. As expected, the bands assigned to 1 (Supporting Information Table S2) do not show any shifts in the presence of 15N2.
After the successful generation of 1 and trapping with N2, we used CO2 as another small molecule for studying the reactivity of 1. FVP of 2 and deposition with neat CO2 at 52 K gives new signals in the IR absorption spectrum. The new signals which result from the reaction with CO2 (Fig. 2) can be attributed to the new species 4 by comparison with the computed spectrum (B3LYP/6-311+G**) (Supporting Information Figure S2). Very prominent is the split band at 1874/79 cm−1 that is associated with the carbonyl stretching vibration ν(CO) of the cyclic carbamate. Using isotopically labelled carbon dioxide, C(18O)2 and 13CO2, isotopic shifts (Figure 2) that are in good agreement with calculations (B3LYP/6-311+G**) are observed (Supporting Information Figure S3, Table S3).
Figure 2.
Difference spectra obtained upon irradiation (λ > 305 nm, 4 K) of 4 that was generated by trapping of 1 with neat carbon dioxide at a deposition temperature of 52 K after FVP of 2 at 740 °C. Carbonyl stretching of 4 disappears (bands pointing downwards) upon irradiation with UV light, while new bands for 8 are appearing (bands pointing upwards) for natural (top), C(18O)2 (center), and 13CO2 (bottom) isotopomers.
The carbon analogue of 4 is benzopropiolactone (5) which is known to be in a photochemical equilibrium with the α-oxoketene (6). Prolonged irradiation of 5 leads to ortho-benzyne.[6a, 12] Recently the reversible rearrangement of 6 to the ketene 7 was shown at high temperatures.[13] Irradiation of 4 with UV light (λ > 305 nm) does not result in loss of CO2. Rather, 4 undergoes a ring opening via a retro [2+2] cycloaddition to give 8 that is a carbon analog of 7 (Scheme 1).
All signals of 4 disappear simultaneously while new signals in the range 2305 – 2236 cm−1 of the isocyanate group of 8 and the signals 1965 and 2208 cm−1 of the BO unit[14] appear (Figure 2). The structure of 8 could be confirmed again through use of isotopically labelled carbon dioxide and is in excellent agreement with the corresponding computed isotopic shifts (B3LYP/6-311+G**, Supporting Information Table S4). Further irradiation with λ = 254 nm leads to decomposition of 8 to an unknown product.
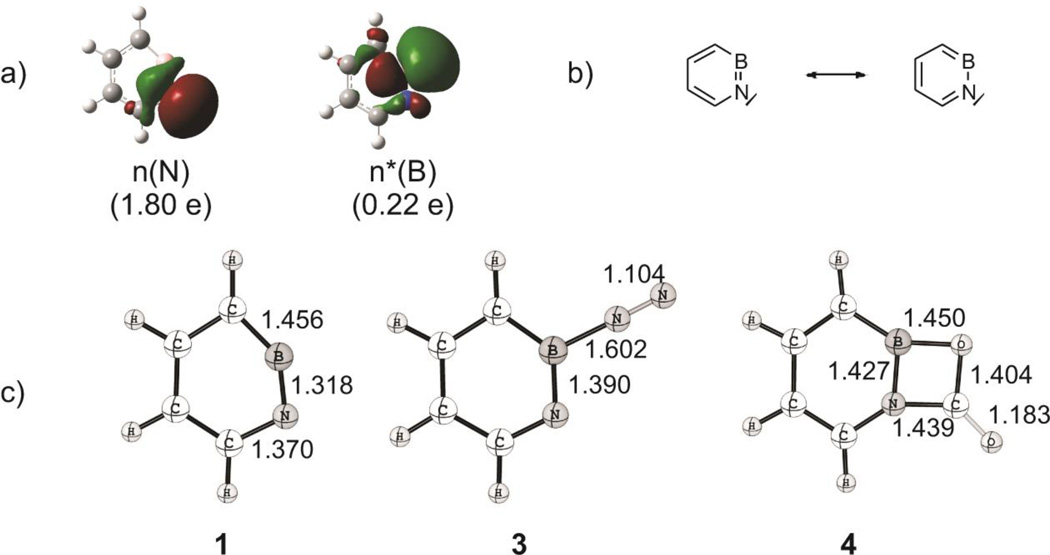
The most remarkable structural feature of azaborine 1 is the distortion of the hexagon with a small angle at N and a wide angle at boron in the singlet ground state, as noted previously.[3, 5] This can be rationalized by the difference in electronegativity: the more electronegative N atom prefers to have a lone pair (HOMO-1) in plane while the more electropositive B atom prefers an empty in-plane orbital (LUMO) that has strong p-character (Figure 3a). The natural bond orbital (NBO) analysis arrives at occupancies of 1.80 e− and 0.22 e− for the lone pair and empty orbitals, respectively, and a pronounced n(N) → n*(B) interaction (E(2) = 27.6 kcal/mol) according to the second-order perturbation estimate of donor-acceptor interaction in the NBO basis. The natural charges obtained from the NBO analysis on N and B are large (−0.79 on N and +0.97 on B) while the Wiberg bond index between B and N is only 1.46. This makes the resonance forms (Figure 3b) a more reasonable description of the electronic structure of 1 than a BN triple bond, despite the isoelectronic relationship with ortho-benzyne. In a sense, the boron and nitrogen centres in 1 may be regarded as an internal frustrated Lewis pair.[15] The empty nB* orbital is responsible for the pronounced Lewis acidity of 1 that allows binding of such a poor Lewis base as N2. According to CCSD(T)/TZ2P+ZPVE computations the binding energy is 6.0 kcal mol–1. The B-N2 distance is 1.602 Å, and the NN distance is hardly elongated (Figure 3c). On the other hand, the endocyclic BN distance is increased from 1.318 Å in 1 to 1.390 Å in 3. Note that the BN distance in tert-butyl(tert-butylimino)borane is 1.258(4) Å and ranges between 1.330–1.340 Å in some of its N-heterocyclic carbene adducts.[9a, 16] With the ambiphilic CO2 molecule both the electron deficient boron and the electron rich nitrogen centers are involved in binding with an overall binding energy of 29 kcal mol–1 at the CCSD(T)/TZ2P + ZPVE(B3LYP/6-311+G**) level of theory.
Figure 3.
a) Natural bond orbitals (NBOs) and their occupation numbers that describe the in-plane part of the BN linkage as computed at the B3LYP/6-311+G** level of theory; b) Resonance forms of 1,2-azaborine; c) Structures and selected bond lengths in Å of molecules 1, 3, and 4 as computed at the CCSD(T)/TZ2P level of theory.
In summary, we have established the first synthesis and characterization of 1,2-azaborine, the BN analogue of ortho-benzyne. 1,2-Azaborine can be generated by flash vacuum thermolysis and can exist under cryogenic matrix isolation conditions as demonstrated by direct spectroscopic techniques. 1,2-Azaborine has a very Lewis acidic boron centre that undergoes reversible binding of dinitrogen molecule and also spontaneously binds CO2. The research described here opens the wide and uncharted field of BN-aryne chemistry and is expected to allow access to novel boron-nitrogen doped molecular architectures of importance in medicinal and materials chemistry.
Supplementary Material
Acknowledgements
The research was supported by the Deutsche Forschungsgemeinschaft and the National Institutes of Health (Grant R01-GM094541). We gratefully thank the bwGRiD project [http://www.bw-grid.de, member of the German D-Grid initiative, funded by Bundesministerium für Bildung und Forschung and Ministerium für Wissenschaft, Forschung und Kunst, Baden-Württemberg)] for the computational resources.
Dedicated to Professor Dr. Wolfram Sander on the occasion of his 60th birthday
Footnotes
Supplementary Information for this article is available on the WWW under http://xxx.
References
- 1.Stoermer R, Kahlert B. Ber. Dtsch. Chem. Ges. 1902;35:1633–1640. [Google Scholar]
- 2.a) Tadross PM, Stoltz BM. Chem. Rev. 2012;112:3550–3577. doi: 10.1021/cr200478h. [DOI] [PubMed] [Google Scholar]; b) Gampe CM, Carreira EM. Angew. Chem., Int. Ed. Engl. 2012;51:3766–3778. doi: 10.1002/anie.201107485. [DOI] [PubMed] [Google Scholar]; c) Gampe CM, Carreira EM. Angew. Chem. 2012;124:3829–3842. [Google Scholar]; d) Bhunia A, Yetra SR, Biju AT. Chem. Soc. Rev. 2012;41:3140–3152. doi: 10.1039/c2cs15310f. [DOI] [PubMed] [Google Scholar]; e) Wentrup C. Aust. J. Chem. 2010;63:979–986. [Google Scholar]; f) Kitamura T. Aust. J. Chem. 2010;63:987–1001. [Google Scholar]; g) Winkler M, Sander W. Aust. J. Chem. 2010;63:1013–1047. [Google Scholar]; h) Wenk HH, Winkler M, Sander W. Angew. Chem., Int. Ed. Engl. 2003;42:502–528. doi: 10.1002/anie.200390151. [DOI] [PubMed] [Google Scholar]; i) Pellissier H, Santelli M. Tetrahedron. 2003;59:701–730. [Google Scholar]
- 3.Fazen PJ, Burke LA. Inorg. Chem. 2006;45:2494–2500. doi: 10.1021/ic051649d. [DOI] [PubMed] [Google Scholar]
- 4.a) Bosdet MJD, Piers WE. Can. J. Chem. 2009;87:8–29. [Google Scholar]; b) Campbell PG, Marwitz AJ, Liu SY. Angew. Chem. Int. Ed. Engl. 2012;51:6074–6092. doi: 10.1002/anie.201200063. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Abbey ER, Lamm AN, Baggett AW, Zakharov LN, Liu S-Y. J. Am. Chem. Soc. 2013;135:12908–12913. doi: 10.1021/ja4073436. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Baggett AW, Vasiliu M, Li B, Dixon DA, Liu S-Y. J. Am. Chem. Soc. 2015;137:5536–5541. doi: 10.1021/jacs.5b01916. [DOI] [PubMed] [Google Scholar]; e) Braunschweig H, Hörl C, Mailänder L, Radacki K, Wahler J. Chem. Eur. J. 2014;20:9858–9861. doi: 10.1002/chem.201403101. [DOI] [PubMed] [Google Scholar]; f) Braunschweig H, Geetharani K, Jimenez-Halla JOC, Schaefer M. Angew. Chem., Int. Ed. 2014;53:3500–3504. doi: 10.1002/anie.201309707. [DOI] [PubMed] [Google Scholar]; g) Braunschweig H, Celik MA, Hupp F, Krummenacher I, Mailänder L. Angew. Chem., Int. Ed. Engl. 2015 doi: 10.1002/anie.201500970. n/a-n/a; [DOI] [PubMed] [Google Scholar]; h) Brough SA, Lamm AN, Liu S-Y, Bettinger HF. Angew. Chem., Int. Ed. Engl. 2012;51:10880–10883. doi: 10.1002/anie.201203546. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Bettinger HF, Hauler O. Beilstein J. Org. Chem. 2013;9:761–766. doi: 10.3762/bjoc.9.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller M, Maichle-Mössmer C, Bettinger HF. Angew. Chem., Int. Ed. Engl. 2014;53:9380–9383. doi: 10.1002/anie.201403213. [DOI] [PubMed] [Google Scholar]
- 6.a) Chapman OL, Mattes K, McIntosh CL, Pacansky J, Calder GV, Orr G. J. Am. Chem. Soc. 1973;95:6134–6135. [Google Scholar]; b) Radziszewski JG, Hess BA, Jr, Zahradnik R. J. Am. Chem. Soc. 1992;114:52–57. [Google Scholar]
- 7.a) Jaska CA, Emslie DJH, Bosdet MJD, Piers WE, Sorensen TS, Parvez M. J. Am. Chem. Soc. 2006;128:10885–10896. doi: 10.1021/ja063519p. [DOI] [PubMed] [Google Scholar]; b) Hatakeyama T, Hashimoto S, Seki S, Nakamura M. J. Am. Chem. Soc. 2011;133:18614–18617. doi: 10.1021/ja208950c. [DOI] [PubMed] [Google Scholar]; c) Hatakeyama T, Hashimoto S, Oba T, Nakamura M. J. Am. Chem. Soc. 2012;134:19600–19603. doi: 10.1021/ja310372f. [DOI] [PubMed] [Google Scholar]
- 8.a) Baldock C, de BGJ, Rafferty JB, Stuitje AR, Rice DW. Biochem. Pharmacol. 1998;55:1541–1549. doi: 10.1016/s0006-2952(97)00684-9. [DOI] [PubMed] [Google Scholar]; b) Baker SJ, Tomsho JW, Benkovic SJ. Chem. Soc. Rev. 2011;40:4279–4285. doi: 10.1039/c0cs00131g. [DOI] [PubMed] [Google Scholar]; c) Baggett AW, Cournia Z, Han MS, Patargias G, Glass AC, Liu S-Y, Nolen BJ. ChemMedChem. 2012;7:1286–1294. doi: 10.1002/cmdc.201200104. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Knack DH, Marshall JL, Harlow GP, Dudzik A, Szaleniec M, Liu S-Y, Heider J. Angew. Chem., Int. Ed. Engl. 2013;52:2599–2601. doi: 10.1002/anie.201208351. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Montalbano F, Cal PMSD, Carvalho MABR, Goncalves LM, Lucas SD, Guedes RC, Veiros LF, Moreira R, Gois PMP. Org. Biomol. Chem. 2013;11:4465–4472. doi: 10.1039/c3ob40614h. [DOI] [PubMed] [Google Scholar]
- 9.a) Paetzold P, Plotho CV, Schmid G, Boese R, Schrader B, Bougeard D, Pfeiffer U, Gleiter R, Schüfer W. Chem. Ber. 1984;117:1089–1102. [Google Scholar]; b) Paetzold P. In: Adv. Inorg. Chem. Emeléus HJ, Sharpe AG, editors. Vol. 31. Academic Press; 1987. pp. 123–170. [Google Scholar]; c) Paetzold P. Phosphorus Sulfur Silicon Relat. Elem. 1994;93:39–50. [Google Scholar]
- 10.Marwitz AJV, Matus MH, Zakharov LN, Dixon DA, Liu S-Y. Angew. Chem., Int. Ed. Engl. 2009;48:973–977. doi: 10.1002/anie.200805554. [DOI] [PubMed] [Google Scholar]
- 11.Maier G, Reisenauer HP, Henkelmann J, Kliche C. Angew. Chem., Int. Ed. Engl. 1988;27:295–296. [Google Scholar]
- 12.Chapman OL, McIntosh CL, Pacansky J, Calder GV, Orr G. J. Am. Chem. Soc. 1973;95:4061–4062. [Google Scholar]
- 13.Koch R, Blanch RJ, Wentrup C. J. Org. Chem. 2014;79:6978–6986. doi: 10.1021/jo5011087. [DOI] [PubMed] [Google Scholar]
- 14.a) Lanzisera DV, Andrews L. J. Phys. Chem. A. 1997;101:1482–1487. [Google Scholar]; b) Bettinger HF. Organometallics. 2007;26:6263–6267. [Google Scholar]
- 15.a) Kehr G, Schwendemann S, Erker G. Top. Curr. Chem. 2013;332:45–84. doi: 10.1007/128_2012_373. [DOI] [PubMed] [Google Scholar]; b) Stephan DW. Acc. Chem. Res. 2015;48:306–316. doi: 10.1021/ar500375j. [DOI] [PubMed] [Google Scholar]
- 16.Braunschweig H, Ewing WC, Geetharani K, Schäfer M. Angew. Chem., Int. Ed. Engl. 2015;54:1662–1665. doi: 10.1002/anie.201409699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.