Abstract
Nitric oxide (NO) is one of the 10 smallest molecules found in nature. It is a simple gaseous free radical whose predominant functions is that of a messenger through cGMP. In mammals, NO is synthesized by the enzyme nitric oxide synthase (NOS) of which there are three isoforms. Neuronal (nNOS, NOS1) and endothelial (eNOS, NOS3) are constitutive calcium-dependent forms of the enzyme that regulate neural and vascular function respectively. The third isoform (iNOS, NOS2), is calcium-independent and is inducible. In many tumors, iNOS expression is high, however, the role of iNOS during tumor development is very complex and quite perplexing, with both promoting and inhibiting actions having been described. This review will aim to summarize the dual actions of iNOS-derived NO showing that the microenvironment of the tumor is a contributing factor to these observations and ultimately to cellular outcomes.
Keywords: Nitric oxide, iNOS, Cancer, Apoptosis, Stroma, Cell situation
Graphical abstract
Highlights
-
•
NO is pro- and anti-tumorigenic. High concentrations of NO maybe anti-tumorigenic.
-
•
iNOS produces high concentrations of NO and relates to tumor growth or its inhibition.
-
•
iNOS is associated with cytotoxicity, apoptosis and bystander anti-tumor effects.
-
•
Tumor- and stromal-iNOS, and the ‘cell situation’ contribute to anti or pro-tumor effects.
-
•
Dual role of iNOS is influenced by the cell situation and is environment dependent.
1. Introduction
Nitric oxide (NO) is a free radical gasotransmitter that regulates various biological functions in the body. After it was identified in the 1980s as a vasoactive small molecule, the cardiovascular activities of NO were more notable mainly related to its vascular relaxation function, and to its anti-thrombotic and anti-inflammatory effects [1], [2]. Besides blood flow regulation, NO involvement is recognized in other physiological functions such as neurotransmission, immune-response facilitation and in antipathogenic response [3], [4], [5].
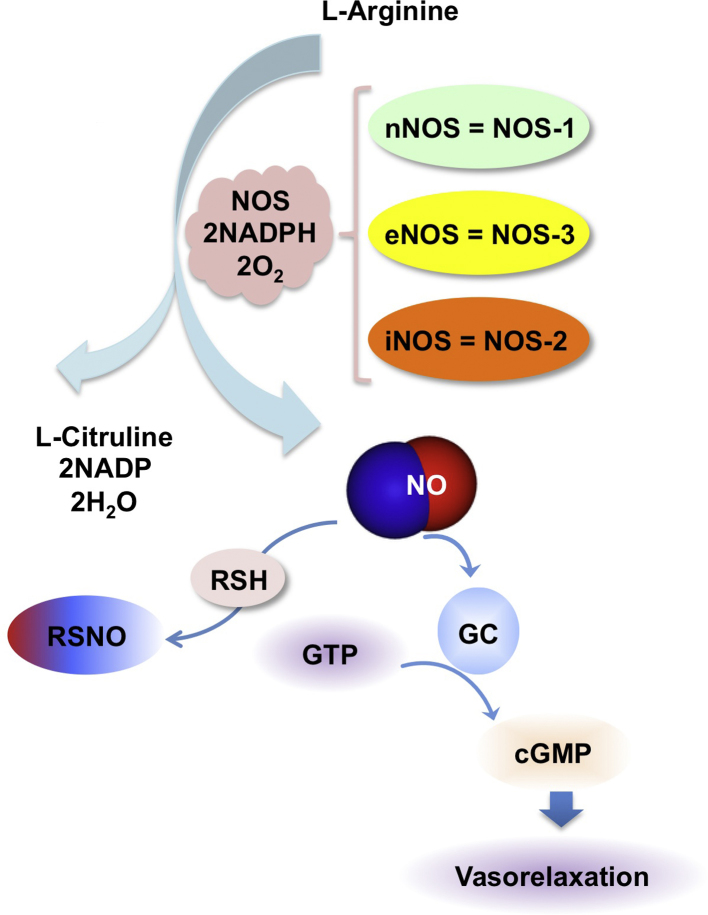
The production of NO in cells under normal physiological conditions occurs by the conversion of l-arginine to l-citrulline by the enzyme nitric oxide synthase (NOS). There are three isoforms of NOS: neuronal NO synthase (nNOS, also known as NOS1), inducible NO synthase (iNOS or NOS2) and endothelial NO synthase (eNOS or NOS3) [3], [6] (Fig. 1). The category called constitutive NOS (cNOS) includes both nNOS and eNOS and when activated only produce nanomolar concentrations of NO for seconds or minutes. However, iNOS the inducible isoform generates higher amounts of NO, in the micromolar range and for longer intervals such as for hours or days [7]. Both cNOS members depend on increases in calcium ion concentrations for activity; hence, produce low amounts of NO for short durations, whereas iNOS is calcium-independent. In general, the expression levels of iNOS in tissues is also a measure of NO generated in that tissue or its surrounding environment [6].
Fig. 1.
Biosynthesis of NO and its mechanism of action. NO is produced by three nitric oxide synthase (NOS) isoforms neuronal, endothelial, and inducible (nNOS, eNOS, and iNOS) that catalyze the oxidation of l-arginine to l-citrulline. NO activates the enzyme sGC to increase cGMP production that has downstream signaling effects. NO also has biological action by modification of protein through S-nitrosylation.
NO was mainly viewed as an oncogenic molecule for several years. However, the biological functions of the NOS enzymes and the activities of NO have been disseminated over the years with a finer eye. This was possible partly due to pharmacological success of NO releasing compounds against heart disease and also to beneficial effects of these compounds when combined with chemotherapy against certain cancers [3], [4]. Among the effects of NO in cancer, it is now evident that NO plays important roles in various stages of carcinogenesis such as DNA damage, oncogene activation, inhibition of DNA repair enzymes and tumor suppressor genes, and the modulation of apoptosis and metastasis [8], [9], [10], [11]. Anti-tumor effects of NO produced by the immune-defense system were demonstrated to function against tumors of different human origins in animal models [12], while implications of pro-tumor effects of NO were made by association with expression of enzymes that produce NO in tumor cells in progressing tumors and metastasized tissue [13]. Over the years a dual role of NO in cancer has been acknowledged [9], [14], [15] and studied with more momentum to dissect the mechanisms leading to these two activities with respect to tumorigenesis [9], [14].
1.1. Basic mechanism of action of NO
In order to appreciate the dual role of NO, its signaling pathway deserves some elaboration. Two major pathways are at play regarding signaling mechansims of NO. One is cGMP-dependent (NO–sGC–cGMP pathway) and the other is cGMP-independent, which is also referred to as the NO oxidative pathway. For the NO–sGC–cGMP signaling pathway in blood vessels, l-arginine is converted by NOS to produce NO which diffuses enzymes to in the lumen and in the walls. In leukocyctes, NO derived from NOS may diffuse across the plasma membrane and cytoplasm. NO reacts with the active site of soluble guanylate cyclase (sGC) and produces cyclic GMP (cGMP). cGMP activates cGMP-dependent Protein Kinase G (PKG), which phosphorylates multiple substrates [16]. The other two major downstream elements that may also be activated are cGMP dependent gated ion channels, and cGMP dependent phosphodiesterase [16]. In platelets, activation of the cGMP dependent kinase phosphorylates a variety of substrates, and is involved in platelet adhesion and aggregation [17]. In general, an increase in cGMP leads to smooth muscle relaxation (Fig. 1), vasorelaxation and decrease of platelet aggregation [17], [18].
The cGMP-independent pathway occurs most commonly through modification of proteins by S-nitrosylation of the cycteine residues [19], [20], [21] (Fig. 1). Such post translational modification also affects transcriptional activity by alteration of DNA binding of the protein, notably the transcription factor NF-κB which loses ability to bind DNA due to such modifications [22], [23]. Hypoxia-inducible factor-1 (HIF-1) estrogen receptor and NF-κB are also redox sensitive transcription factors that are regulated by S-nitrosylation [24]. Signaling events regulated by S-nitrosylation may lead to either progression or inhibition of cancer. S-nitrosylation of NF-κB and matrix metalloproteinase 9 (MMP9) promotes cell death whereas S-nitrosylation of caspase-3, caspase-9, and c-Jun N-terminal kinase prevents activity and inhibits apoptosis [23]. Death receptors were also found to be regulated by for example, nitrosylation of death receptor DR4 and Fas promotes apoptosis via the death-inducing signal complex [25], [26].
NO reacts with oxygen radicals to form peroxynitrite which is a strong oxidative and nitrosative agent, resulting in direct nitrosylation or nitrosation or nitration of signaling proteins. Nitrosative signaling in cancer cells is known to contribute to more proliferation and metastasis, and resistance to therapy and therefore, poor outcome of treatment [27]. On the other hand, apoptotic and necrotic cell death can occur by peroxynitrite via lipid peroxidation, cysteine oxidation, and also by protein nitrosylation [28], [29].
2. More than a tale of two concentrations
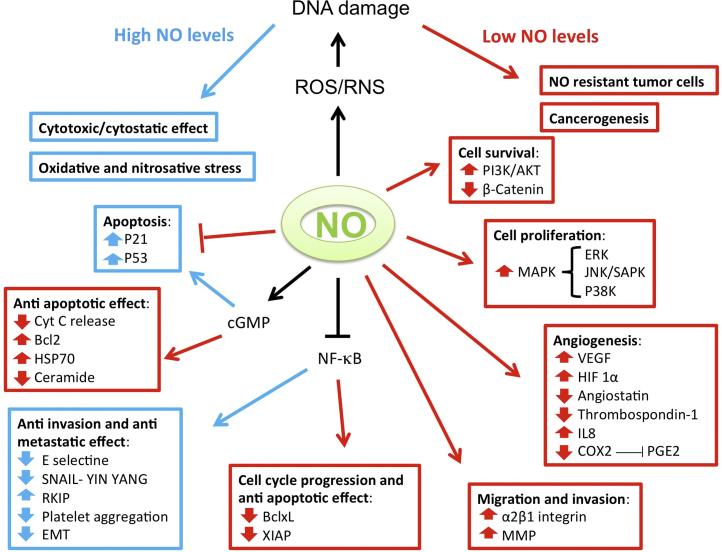
The presence of NOS isoforms and their ability to produce different levels of NO was the basis for the concept that different cellular activities of NO may be concentration dependent [30], [31]. The physiological function of NO is dependent primarily on its concentration. At low concentrations, NO acts as a signaling molecule regulating smooth muscle relaxation and blood flow, neurotransmission, platelet activity, iron homeostasis, cell curvival and proliferation whereas at high concentrations it is believed to modulate immune-mediated anti-tumor activities [32], [33] (Fig. 2). Considering a cancer cell, the cellular outcome is based on additional factors for response such as the cell or tumor type, duration of exposure, NO flux, and immune and vascular cells. Therefore, the update in NO and cancer biology is that the overall effect of NO is an interplay of its activities emanating from its amount from the tumor and from its microenvironment.
Fig. 2.
NO modulates various cellular activities by altering multiple pathways. Abbrv: PI3K-AKT: Phosphatidylinositol-3-Kinase and Protein Kinase B; MAPK: Mitogen-activated protein kinase; ERK: Extracellular signal-regulated kinase; JNK: c-Jun N-terminal protein kinase; VEGF: Vascular endothelial growth factor; HIF: Hypoxia inducible factor; COX2: Cytochrome Oxidase subunit 2; IL8: Interleukin 8; PGE2: Prostaglandin E2; Cyt c: Cytochrome c; Bcl-2: B-cell lymphoma 2; HSP70: Heat Shock protein 70; RKIP: Raf kinase inhibitor protein; EMT: Epithelial Mesenchymal transition; Bcl-xl: B-cell lymphoma-extra large XIAP: X-linked inhibitor of apoptosis protein; MMP: Matrixmetalloproteinase; cGMP: Cyclic guanosine monophosphate; NO: nitric oxide.
A low concentration of NO of less than 100 nM prevents certain cell types from apoptosis, and thereby favor tumorigenesis and progression [34], [35]. A sustained low efflux of NO derived from eNOS stimulates the proliferation of the endothelail cells [36]. The low efflux is estimated to be approximately 1–10 nmol/L [31] that lead to such endothelial cell response. Based on several reports of NO measurements in tissues and from exogenous NO donors, the concentrations of NO have been established as low dose (50–100 nM), high dose (400–1000 nM) or intermediate dose (100–400 nM) [37], [38]. High concentrations of NO, more than 500 nM, may be pro-apoptotic, producing cytotoxicity and antitumor activity [33], [39]. High NO levels that produced cytotoxic effects on tumor cells are derived from macrophages, neutrophils, endothelial cells, hepatocytes, cardiac myocytes and chondrocytes [40], [41], [42], [43].
Many exogenous NO donors have been used in obtaining different levels of NO. For example, DETA-NONOate produced high amounts of NO inhibited epithelial-mesenchymal transition (EMT) and invasion of human prostate metastatic cells [44]. Metastatic inhibition was also proposed to occur at high NO levels [45]. Therefore, high levels of NO relating to antitumor and also anti-metastatic properties have raised hopes for therapies.
NO levels and their molecular influences are complicated by threshold amounts. Some exogenous donors have provided accurate measurements of threshold NO exposure and distinct effects on cells. Using spermineNONOate low NO doses of 10–300 nM were obtained in MCF-7 breast cancer cells [46] which induced proliferative events such as ERK phosphorylation and HIF-1α (hypoxia induced factor 1α) accumulation resulting in tumor proliferation and differentiation [46]. Further p53 phosphorylation occurrs at high doses of NO (above 300 nM), generally known to be associated with apoptosis induction. Therefore, in addition to high and low doses, different threshold levels of NO are required for activation or stabilization of key proteins involved in carcinogenesis.
Reactive oxygen species (ROS) also influence threshold NO levels within the tumor microenvironment. ROS may modulate signaling by NO as was observed in both tumor and endothelial cells [39]. Another complication is the development of NO resistant cells through low yet continuous exposure to NO. Such tumor cells show cellular adaptation and enhanced survival by altered threshold levels [47]. NO may also form reactive nitrogen species (RNS) through interaction with the superoxide radical and may cause DNA damage and genotoxicity [48]. In an inflammoroty process, NO and RNS may therefore stimulate carcinogenesis.
Based on in vitro NO release by donors and their in vivo represenation of NO/RNS levels at the sites of tumors, the concentration ranges of NO by NO-donors have also been proposed to be divided into the following two ranges: Moderate concetntration range (100–350 μmol/L) and high concentration range (500–1000 μmol/L) [49]. The normal NO levels are generally below the 50 nmol/L level. The cellular responses of the moderate cconcentration range includes increases in cell proliferation and genomic instability, and decrease in apoptosis and DNA repair [50], [51]. The outcomes for the high concentration range include decreases in cell proliferation and increase in apoptosis, DNA damage and stimulation of DNA-damage signaling pathways leading to ATM/ATR-dependent p53 phosphorylation [52] (Fig. 2).
3. Background iNOS and NO
Since presence of three different NOS enzymes in cells are a reflection of levels of NO produced, reports have demonstrated that the relationship of NO to the dynamics of tumor growth is linked to certain determining features. These are; the expression patterns of NOS isoforms in various types of cells, the amount of NO produced by each isoform in the cell type including the duration, and the chemical interactions of NOS-derived NO in the cell [53]. Among the three NOS enzymes in mammalian systems, iNOS stands apart as it generates more NO than the constitutive members nNOS or eNOS [7], [54], is expressed after cytokine exposure, and more specifically modulates important tumor related processes such as malingnant transformation, angiogenesis, and metastasis [7], [54]. iNOS-derived NO and its tumorigenic or tumoricidal activities are dual roles of considerable interest that are debated in cancer biology [34], [55]. Anti-tumor effects of iNOS have been examined by many researchers despite the volume of tumor promoting reports of NO. The current understanding is that during the phases of tumor growth, the epithelial cells of the growing tumor have tumorigenic properties via iNOS activity while the environmental stroma community, for example, tumor-associated macrophages provide tumoricidal activities also via iNOS, and a competition sets in and dynamics alter with time [56]. When these dynamics are elucidated to a finer detail, therapies may be tailored for the appropriate activity of NO.
3.1. iNOS gene, polymorphisms and basic regulation
In order to understand the role of iNOS in proliferative or anti-tumor kinetics, a brief background of iNOS gene and iNOS regulation is presented first. The human iNOS gene is located on chromosome 17q11.2-q12. There are several sequences with high homology to the iNOS genes in the human genome [57]. Different polymorphisms have been described in the sequence of the human iNOS promoter [58], [59]. Single nucleotide polymorphism of the iNOS gene (NOS2A Ser608Leu) caused a two-fold risk increase for B- and T-cell non-Hodgkin lymphoma and also for the diffuse large B-cell and follicular lymphomas [60], [61]. A penta nucleotide repeat polymorphism -CCTTT (n)- that occurs in the human iNOS promoter at position – 2.5 kb with a median number of 12 CCTTTT-repeats, has been correlated with severity of human diseases including gastric cancer [62], glaucoma [63] and urothelial carcinoma [64], [65].
One advance in the understanding of gene expression regulation involves epigenetics. In particular, DNA methylation at the C-5 positions of cytosine in the CpG dinucleotide occurs as a common epigenetic mechanism leading to gene silencing. In human alveolar macrophages and monocytes CpG motifs near the transcription start site of the iNOS promoter have been shown to be methylated, which means it is an epigenetically silenced iNOS gene [66]. Histone modifications were also found to be low at the iNOS promoter region suggesting low activity of the gene [66].
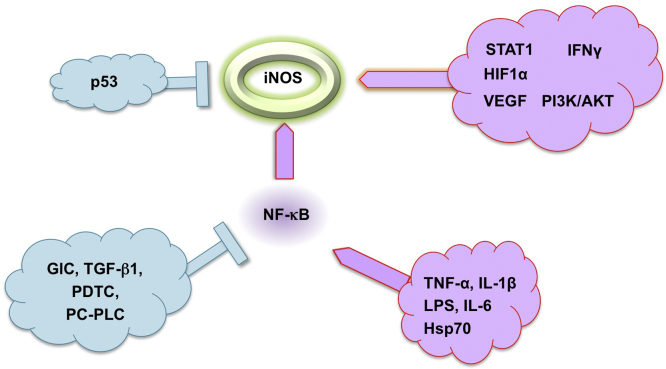
Regarding non-genetic regulatory mechansims, iNOS is primarily regulated at the expressional level by inflammatory cytokines (TNF-α; IL-1β IFN-γ), lipopolysaccharide endotoxin, hypoxia and oxidative stress [67], [68] and more recently, by heat schock protein Hsp70 which may function like a cytokine [69] (Fig. 3). Transcriptional and post-transcriptional mechanisms resulting in the induction of iNOS expression are found to vary in different cell types or species. The most important intracellular signal transduction pathways are the NF-κB and JAK-STAT pathways [70], [71], [72], [73]. Inhibition of iNOS expression by numerous agents, such as glucocorticoids, TGF-β1 and antioxidants have been shown to result from inhibition of NF-κB and STAT-1α activation [74], [75], [76], [77] (Fig. 3). In addition, MAPK pathway contributes to iNOS gene expression involving the activation of transcription factors such as AP1, ATF2, cAMP-responsive elements, NF-κB, and the transcription factors from the Ets family [34], [72], [78]. Post-transcriptional regulation of iNOS gene expression predominantly occurs via mechanisms that influence iNOS mRNA stability [59].
Fig. 3.
The transcription factor NF-κB is central to iNOS regulation. LPS, IL-1β, TNF-α, and IL-6 have been shown to induce iNOS, whereas glucocorticoids (GlC), transforming growth factor-β1 (TGFβ1), antioxidants (e.g. pyrrolidine dithiocarbamate, PDTC) and inhibitors of phosphatidylcholine-specific phospholipase (PC-PLC) have been shown to inhibit iNOS expression by inhibiting NF-κB activation.
4. Tumor iNOS and NO
The roles of NO relating to apoptosis, cell cycle, tumor progression, angiogenesis and metastasis, are currently veiwed at the host end and tumor end since NO was found to be actively associated with tumors as well as the tumor environment, for example the vasculature cells and other stromal cells. In earlier studies in cultured cells high levels of NO that were generated by iNOS transfection in tumor cell lines produced a cytostatic or growth inhibitory effects [79], [80], [81], whereas in animal models, iNOS overexpression produced pro-tumor or anti-tumor effects on tumor growth, depending on the tumor microenvironment and the tumor type [82], [83], [84]. The tumor microenvironment includes stromal cells such as cells of the immune system and vascular tissue, and NO was established to be a component for their activities [84]. Overall, the effect of NO depends on the expression level of iNOS, duration and timing of NO delivery, the microenvironment, the genetic background and the cell type.
4.1. Overexpression of iNOS and anti-tumor activity
Overexpression of iNOS and concomittant changes on cancer cell kinetics were demosntrated to be anti-cancer in potential according to several in vitro and in vivo studies. Prostate cancer cells DU145 and PC3, and colon cancer HT-29 cell produced enhanced amounts of NO upon overexpression of iNOS by gene transfer, which increased these cells' sensitivity to cisplatin-induced cell death [85] or to radiation therapy [86]. In a mouse model of thyroid cancer, iNOS overexpression inhibited tumorigenesis [87]. In animal models of fibrosarcoma, tumor progression slowed down upon expression of iNOS via gene transfer demonstrating potential of tumor growth inhibition by iNOS-derived NO. Introduction of iNOS in conjunction with cytokines IFN-γ in prostate cancer cells also inhibited growth of the cells [88]. iNOS expressing pancreatic cancer cells did not produce tumors or metastases in xenograft mouse models due to NO upregulation and apoptosis [89]. Overexpression by means of other delivery forms of iNOS also produced anti-tumor results. In xenograft models of human colon and ovarian cancers, delivery of iNOS expressing cells by microencapsulation increased apoptosis of xenograft tumors [14]. Retroviral delivery of iNOS in mice produced slowly progressing tumors and fewer lung metastases than control mice transfected with iNOS-negative retrovirus [90]. In contrast, reducing iNOS expression by NOS inhibitors in MDA-MB-231 and T47D estrogen receptor (ER)-negative breast cancer cells led to increase in motility and loss of adhesion implying a role of NO in inhibititng the progression of breast cancers [27].
Induction of iNOS derived NO is believed to occur in photodynamic therapy, which is a recent area for treatment of cutaneous tumors. This procedure induces iNOS as a cytoprotective response. It is envisaged that combination of NO-inhibitors and photodynamic therapy may improve its clinical use [91].
4.2. Bystander anti-tumor effect
Bystander effect referes to a non-targeted response and generally holds signififcance with respect to radiation therapy. However, in gene therapy, it refers to the ability of the transfected cells to transfer death signals to neighboring tumor cells [92]. iNOS gene delivery by designer biomimetic vector and iNOS nanoparticles were found to overexpress iNOS and produce cytotoxicity and cell death in ZR-75-1 breast cancer cells, coupled with killing of bystander cells [93]. Similarly, radiation-induced bystander effects and cell killing are mediated through an iNOS-derived NO [94].
4.3. The mechanisms of anti-tumor effects: cytotoxicity and apoptosis
Typical mechanisms in NO-mediated apoptosis include caspase activation, chromatin condensation, and DNA fragmentation [95], [96]. At the molecular level, high concentrations of NO derived from iNOS in macrophages induce p53 phosphorylation resulting in endotheial cell growth arrest, and higher concentrations and prolonged exposure time induce cell death [37]. Prolonged production of NO has been associated with the release of cytochrome c from the mitochondria, activation of caspase, modulation of anti-apototic Bcl-2 proteins, and increase in p53 expression [97] (Fig. 2). Alternatively, NO may induce the expression of DNA-dependent protein kinase in DNA repair that confers protection against oxidative and nitrosative stress [98]. A recent study has reported that one of the underlying mechanisms by which NO-mediated NF-κB inhibition suppresses tumor cell resistance and metastasis is through inhibition of the downstream targets Snail and the transcription factor Yin Yang 1 (YY1) and induction of Raf-1 kinase inhibitor protein (RKIP) [99] (Fig. 2). Finally, evidence indicating that endothelial-cell derived NO mediates the elimination of disseminated tumor cells is increasing. For example, it has been shown that endothelial cell-produced NO had a cytotoxic effect on disseminating tumor cells [100]. A bystander signaling mechanism which depends on ROS or iNOS derived NO has also been examined to produce cell killing effects [49], however it may depend on the cell situation and environment of the tumor cell.
4.4. iNOS expression and pro-tumor activity
iNOS derived NO have been associated with many tumors and in their progression into metastatsis. iNOS positivity has been reported consistently in human cancers at a variety of sites, including the lung [101] prostate [102], breast [103], bladder [104], oral cavity, esophagus [105] and colon [106]. In each single case, the effects of NO have to be interpreted with regard to the microenvironment of the tumor. Increased iNOS activity has been positively correlated with the degree of malignancy in gynecological tumors [80], gastric cancer, squamous cell carcinoma, hepatocellular carcinoma, melanoma and leukemia [107], while high iNOS expression is associated with favorable prognoses in ovarian [108] and lung cancers [109]. Osteosarcoma of the jaw in patients was associated with over expression of iNOS as deduced by immunohistochemical analysis [110]. Similarly, oral carcinomas and oral hard tissue sarcoma in patients were also associated with iNOS expression. Interesteingly, soft tissue oral sarcomas from this study were negative for iNOS expression [111] and iNOS expression in carcinomas was higher than in sarcomas. In women with estrogen receptor (ER)-negative breast tumors, iNOS expression is an independent marker for prognosis. iNOS positivity related with angiogenesis of tumors, accumulations of p53 mutations and EGFR activation [27]. In other clinical studies of gastric cancer, iNOS correlated with tumor progression and has potential to be of prognostic value [112]. Low levels of expression of iNOS produced tumors in the pancreas and led to liver metastasis and ascites in mice models while higher levels of iNOS expression did not lead to metastasis. When iNOS expression was inhibited, it promoted distant liver metastasis in these mice [113]. Nitric oxide is propososed to remodel the cytoskeleton and lead to metastasis of lung cancer cells with evidence of increased expression of iNOS in these cells.
4.5. Bystander pro-tumor effect and cell situation
NO has context-dependent effects which may be compiled as ‘cell situation’, and when viewed in totality, may affect neoplastic transformation. iNOS derived NO and RNS in cells lead to genomic instability and tumorigenesis in the microenvoronment through a proposed “field effect” where bystander cells are affected. This effect was used to explain BRCA1 downregulation in bystander MCF-10A breast epithelial cells cocultured with iNOS-stimulated macrophages [50]. Further, radiation induced bystander effects include a role of iNOS which appear to be either pro- or anti-tumor depending on the cell situation. Induction of iNOS derived NO is believed to occur in photodynamic therapy, which is a recent area for treatment of cutaneous tumors. This procedure induces iNOS. In animal models of photodynamic therapy, resistance to cell killing has been reported due to increased NO derived from iNOS from breast and prostate tumors [114]. In human prostate cancer cells, there was enhanced proliferation and migration of the photostressed surviving cells, and increased invasion was due to NO-mediated activation of matrix metalloproteinase-9 (MMP-9), an ECM component [114], [115], [116]. Therefore pro-survival influences affecting surviving cells after photo-killing imply that iNOS induction is a cell situation consideration, and may be a secondary response to an altered status.
4.6. Cell situation and iNOS/NO effect
Protective or cytotoxic effects depend on the cells situation. In tumor cell lines with wild type p53, DNA damage by NO and/or modification through nitrosative stresses, as in some transformed cells, induces the accumulation of p53 resulting in apotosis. It was found that p53 binds to the promoter region of the iNOS gene to inhibit protein iNOS expression and further generation of NO [117]. On the other hand, NO also stabilizes p53 leading to apoptosis [118], for example; induction of iNOS expression in tumor cells with wild type p53 resulted in reduced tumor growth [119]. Further, when examined in a p53 negative host, a study showed that iNOS-derived NO suppresses lymphomagenesis by promoting apoptosis and decreasing tumor cell proliferation [120].
Apoptosis induction mediated by NO via iNOS induction also occurs in colorectal [121] and pancreatic cancer cells [122] by IL-2-activated killer lymphocytes upon stimulation with inflammatory cytokines IFN-γ and TNF-α. In general the process was found to require high NO output with a high concentration in the tumor microenvironment.
4.7. iNOS and cell adhesion
NO promotes migration by reduced cell adhesion, and therefore promotes invasion. This occurs through inhibition of integrin expression. In particular NO was found to specifically inhibit α2β1integrin-mediated platelet adhesion to immobilized collagen [123]. Other molecular events leading to induction of iNOS expression and generation of NO include activation of integrin α9β1and src tyrosine kinase activity which enhanced cell migration [124]. In addititon, NO was found to modulate the matrix metalloproteinase-8 (MMP-8) or procollagense expression and its activity, and therefore affected tumor cell invasion (Fig. 2). NO has been implicated in the activation pathways of MMP-1, -2, -3, -8, -9 [125], [126], [127]. NO inhibits the progression of breast cancers since down-regulation of NOS with NOS inhibitors in MDA-MB-231 and T47D cells was found to lead to increase in motility and loss of adhesion [128].
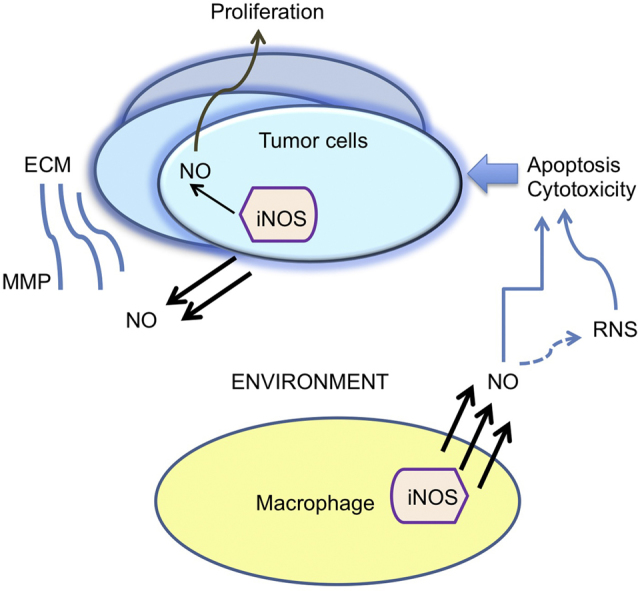
4.8. Pro-tumor effects of stromal iNOS/NO
Besides the tumor cells, the stromal cells of the host produce NO also. These stromal host cells in the tumor micro-environment constitute immune cells such as macrophages, fibroblasts and vascular cells, and include the basement membrane and extracellular matrix as well. There is a constant and dynamic interplay between the tumor cells and the stroma immune cells, which changes according to the change in conditions. In early events of tumorigenesis, macrophages generate high concentrations of NO/RNS to initiate tumor cell apoptosis and destroy emerging transformed cells. When or as the tumor escapes the immune system and grows, macrophages are reprogramed by the tumor microenvironment to support the tumor. Low amounts of NO/RNS are pro-angiogenic and support tumor growth and metastasis by inducing growth factors VEGF and matrix metalloproteinases. Studies in gastric cancer (GC) patients found iNOS expression was associated with distant metastasis, vascular and lymph node development. Approximately 54% of the GC tumors displayed iNOS expression associated with tumor progression and thereby has the potential to indicate poor prognosis in GC patients [129]. In another study, iNOS and COX-2 were induced in gastric cancer cells that were co-cultured with gastric stromal fibroblasts and involved interaction of the IL-6-STAT3 pathway from the fibroblasts to promote tumor growth [130]. p53 status of tumors also plays an important role. Inactivated p53 in fibroblasts may also contribute to iNOS-mediated tumor growth. For example, in a more recent report of co-culture studies with p53 deficient mouse embryonic fibroblasts (MEF) and rectal polyploid carcinoma CMT93 cells, the MEFs produced more iNOS and COX-2 expression, and iNOS-derived NO led to promotion of tumor growth [131]. iNOS may also serve as a biomarker for metastasis of lung cancer. In lung cancer patients with bone metastasis, the circulating metastatic cells were found to have increased iNOS expression contributing to increase in Epithelial Cell Adhesion Molecule (EpCAM) which is associated with metastasis due to reduced cell-cell-adhesion [101].
Breast mammography of high density fibroblasts are proposed to produce a stress response and make fibroblasts behave like macrophages based on increased iNOS derived NO production and JNK1 stress kinase signals, leading to an inflammatory process and promoting breast cancer [132]. iNOS expression in tumor parenchyma and stroma in gastric cancer patients showed positive correlation with gastric carcinogenesis, tumor lymphangiogenesis and lymphatic metastases [133]. Treatment with a Toll-like receptor agonist imiquimod in iNOS-knockout mice that were implanted with tumor cells improved tumor suppression compared to control wild-type mice, and led to a better survival rate. Combination of imiquimod and iNOS inhibitor produced similar results [134].
4.9. Anti-tumor effects of stromal iNOS/NO
Anti-tumor activity emanating from stromal iNOS was also demonstrated over the years. An increase in iNOS and iNOS-mediated NO release in stromal macrophages or fibroblasts in the tumor environment was associated with anti-tumor activity in pancreatic cancer [135] and in LPS stimulated macrophages [136]. The interplay and dynamics of tumor-iNOS expression and host-iNOS were also considered, where in wild type and iNOS –/– mice responded differently to iNOS-negative tumor cells; the murine fibrosarcomas in the iNOS –/– mice increased at faster rate than in the wild type host [137]. iNOS were also induced by Lipid A and interleukin 10 which inhibited tumorigenesis in colon and breast cancers in rodent models [138], [139]. Delivery of liposomes containing lipopeptides suppressed murine sarcoma hepatic metastasis by induction of iNOS and NO generation [140]. In human ovarian cancer models, an interesting observation was that only NO-producing macrophages exhibited antitumor activity. IFN-β-transfected tumor cells which were injected into the peritoneal cavity of nude mice produced no tumors compared to control cell injections, and were found to stimulate high NO levels in the murine macrophages [141]. Therefore, host iNOS or stromal iNOS may provide anti-tumor activity.
4.10. Stromal iNOS and cell situation considerations
iNOS from stromal cells may not be indicative of any one effect. A factor that complicates host versus tumor iNOS performance is NO sensitivity of the tumor cells. Different tumor types may have differential NO sensitivity. It was demonstrated that host iNOS deletion increased metastatic tumor growth of M5076 ovarian sarcomas in the lung, whereas in other examples of iNOS–/– mice, a reduced metastasis of B16–BL6 melanomas occurred [142]. A tissue specific sensitivity is hypothesized where in M5076 cells are sensitive to macrophage-induced NO, whereas B16–BL6 cells are insensitive, implying that host iNOS favors tumor progression of B16–BL6 cells. Another contributing factor affecting tumor outcome is tissue type. The pathways regulating iNOS expression are different depending on cell type [143]. In murine breast carcinoma, tumor cell-iNOS decreases the metastatic development whereas stromal cell-iNOS increased metastasis [144]. In contrast, in human cervical intraepithelial neoplasias increased iNOS in the cervical stroma was associated with poor response to IFN-alpha 2B immunotherapy [145]. Platelets are required for the vascular endothelium adhesion to tumor cells. Platelet aggregation prevents tumor cell removal by immunological attack [146]. NO from tumor cells prevent platelet aggregation. The antitumor actions include loss of malignancy due to inhibition of platelet aggregation through a cGMP-dependent mechanism. A metastatic human colorectal carcinoma cell line has lower iNOS activity but higher platelet aggregation compared with a non-metastatic tumor line that is derived from the same patient [147].
Regarding immune suppression activity, NO inhibits the production of IL-12 from macrophages and dendritic cells [148]. It was believed that NO modulates differentiation of T helper cells and therefore controls the T cell response [149], [150], [151] and later it was shown that iNOS from activated T cells selectively modulate T cell differentiation [152].
5. NO molecular pathways and signaling
A collective view of the NO related pathways from the studies reviewed here needs to be highlighted at this point. Increased production of NO may promote tumor progression and metastasis by diect induction of tumor cells proliferation, migration and invasion and indirectly through the expression of angiogenic factors in tumor cells. First, exposure of cells to various oxidants induces MAPK, such as ERK, JNK/SAPK and p38 kinases [153], [154]. A special mention of NFκB is necessary since NF-κB pathway which is a pro-survival mechanism has been found to be a central player and indeed, it is activated in over 50% of all cancers [155], [156]. Promotion of metastasis by NF-κB has been reported through increased epithelial to mesenchymal transition [157], [158]. In breast cancer cells, other pathways are activated, for example phosphatidyinositol 3-kinase (PI3K/Akt), c-Myc and HIF [159], [160]. Others have reported NO activation in β-catenin transcriptional regulation in osteocytes [161] and colorectal cancer cells [162], and in models of colitis [163]. Regarding low level exposure to NO, tumor cells show cellular adaptation and through enhanced survival, particularly by producing IL-10, TGF-β and PGE2 [47].
5.1. Pharmacological inducers of iNOS and cell fate
Statins are cholestererol lowering agents that inhibit HMG-CoA reductase. These agents have been shown to have tumoricidal and apoptotic activity that is mediated by iNOS induction [164]. Epidemiological studies have suggested that statins have anti-cancer activity against many cancers including breast carcinoma [165]. Among the various studies, post-menopausal breast cancer patients using statins had significant benefit when individual classes of statin therapy were used, although not for all classes combined [166]. In MCF-7 breast cancer cells, two representative agents simvastatin and fluvastatin induced iNOS mRNA and protein expression, reduced proliferation and induced apoptosis in these cells. In ER-negative and metastatic triple negative cancer cells, fluvatstatin produced anti-proliferative and anti-invasive effects also by increasing iNOS-derived NO [167]. In contrast pitavastatin reduced iNOS mRNA expression and supprressed intestinal polyp development in APC Min mice models of colon carcinogenesis [168]. Considering the fact that statins are used for cholesterol management for more than two decades, it is important to note that there is no data to suggest that patients with statin regimen are at a greater risk of developing any type of cancer. At the same time, iNOS overexpression or its inhibition are not sole indicators of tumor fate.
Cancer chemopreventive agents that have anti-tumor activity have been examined for iNOS induction and inhibition. Interestingly, in accordance with a dual role of iNOS, compounds that induce iNOS are cancer preventive and compounds that inhibit iNOS are also cancer preventive. Induction of iNOS-derived NO production by fenretinide, a retinamide derivative of vitamin A, is necessary for growth inhibition and promotion of apoptosis of metastatic breast cancer cells. The amount of NO related directly to the instensity of these repsonses. In contrast, reduced iNOS expression by liposomal fenretinide was obtained in animal models of hepatocarcinogenesis [169], [170]. The well known chemotherapeutic agent 5-flurouracil (5-FU) induced iNOS derived NO and induced apoptosis in liver cancer cells. Agents that induced NO formation such as l-arginine improved the sensitivity of the cells to 5-FU [171]. Other pharmacological agents that are NO-donors and are chemopreventive agents, such as the NO-donating Nonsteriodal antiinflammatory drugs (NO-NSAIDs), for example NO-donating aspirin, have demonstrated reduction in cytokine-induced iNOS expression associated with growth inhibition of HT-29 colorectal cancer cells [172], [173]. The same finding was corroborated in azoxymethane-induced colonic tumors in rats where NO donating-aspirin or -ibuprofen inhibited iNOS expressiona and activity, and reduced tumor incidence and multiplicity [174]. It is clear that iNOS and NO affects many types of cells and at various stages. To explain many of these phenomenon from cell and animal models and in human cancers, more studies on the interplay between tumor cells, immune cells, tumor-associated macrophages, endothelial and epithelial cells mediated by iNOS in cancer progression and metastasis are needed.
From a speculative point of view it begins to appear that iNOS is likely an adaptive response to cellular stresses and a mimic of certain cell situations. At the cost of appearing simplistic, it may be envisaged that iNOS has similar role as the heat shock protein Hsp70 which is stress inducible with similar dual roles in cancer, such as promoting tumor cells to survive and regulating antitumor immunity. iNOS may cross talk with Hsp70 [69], and HSP70 and iNOS expressions reflect a collective response affecting cell fate [175]. Perhaps iNOS may be given a similar status as Hsp70.
6. Conclusions and perspectives
During past two decades or so much has been written about the dual nature of NO, which strongly suggests a concentration-dependent relationship between NO expression and biological response. This phenomenon is well accepted in pharmacology. What has been the cornerstone of much debate is the role of iNOS in cancer. Does its expression mean that iNOS-derived NO is carcinogenic or anti-carcinogenic? The current thinking based on observations that iNOS expression is high in a number of tumors and that this correlates with poor survival, has led to the conclusion that induction of this NOS isoform may somehow be related to tumor genesis or that its expression may be used as marker for not so favorable outcomes. However, we believe that such a conclusion is still not warranted as in totality, it appears that the dual role of iNOS is strongly influenced by the cell situation and is environment dependent, with either induction or inhibition of iNOS having anti-cancer potential based on tumor and cell types. We belive that any approaches to iNOS based therapy against cancer may need to be cell situation based and needs a lot more directed studies.
Grant support
This work was supported in part by NIH grant R24 DA018055. The funding agency had no role in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Conflict of interest
The authors have nothing to disclose.
Footnotes
This article belongs to a special issue on Nitric Oxide and Cancer, edited by Jordi Muntané and Benjamin Bonavida.
References
- 1.Ignarro L.J., Buga G.M., Wood K.S., Byrns R.E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ignarro L.J., Napoli C., Loscalzo J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: an overview. Circ. Res. 2002;90:21–28. doi: 10.1161/hh0102.102330. [DOI] [PubMed] [Google Scholar]
- 3.Geller D.A., Billiar T.R. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev. 1998;17:7–23. doi: 10.1023/a:1005940202801. [DOI] [PubMed] [Google Scholar]
- 4.Quinn A.C., Petros A.J., Vallance P. Nitric oxide: an endogenous gas. Br. J. Anaesth. 1995;74:443–451. doi: 10.1093/bja/74.4.443. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 6.Alderton W.K., Cooper C.E., Knowles R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel T., Feron O. Nitric oxide synthases: which, where, how, and why? J. Clin. Investig. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lala P.K., Orucevic A. Role of nitric oxide in tumor progression: lessons from experimental tumors. Cancer Metastasis Rev. 1998;17:91–106. doi: 10.1023/a:1005960822365. [DOI] [PubMed] [Google Scholar]
- 9.Wink D.A., Ridnour L.A., Hussain S.P., Harris C.C. The reemergence of nitric oxide and cancer. Nitric Oxide: Biol. Chem. 2008;19:65–67. doi: 10.1016/j.niox.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wink D.A., Vodovotz Y., Cook J.A., Krishna M.C., Kim S., Coffin D. The role of nitric oxide chemistry in cancer treatment. Biochemistry. 1998;63:802–809. [PubMed] [Google Scholar]
- 11.Wink D.A., Vodovotz Y., Laval J., Laval F., Dewhirst M.W., Mitchell J.B. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 12.MacMicking J., Xie Q.W., Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 13.Nathan C. Inducible nitric oxide synthase: what difference does it make? J. Clin. Investig. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu W., Liu L.Z., Loizidou M., Ahmed M., Charles I.G. The role of nitric oxide in cancer. Cell Res. 2002;12:311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- 15.Choudhari S.K., Chaudhary M., Bagde S., Gadbail A.R., Joshi V. Nitric oxide and cancer: a review. World J. Surg. Oncol. 2013;11:118. doi: 10.1186/1477-7819-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friebe A., Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ. Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- 17.Dangel O., Mergia E., Karlisch K., Groneberg D., Koesling D., Friebe A. Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. J. Thromb. Haemost.: JTH. 2010;8:1343–1352. doi: 10.1111/j.1538-7836.2010.03806.x. [DOI] [PubMed] [Google Scholar]
- 18.Lincoln T., Cornwell T., Komalavilas P., MacMillan-Crow L.A., Boerth N.J. Academic Press; San Diego: 1996. The Nitric Oxide-Cyclic GMP Signaling System; pp. 257–268. [Google Scholar]
- 19.Lipton S.A., Choi Y.B., Pan Z.H., Lei S.Z., Chen H.S., Sucher N.J. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 20.Stamler J.S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 21.Stamler J.S., Singel D.J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 22.Broillet M.C. S-nitrosylation of proteins. Cell. Mol. Life Sci.: CMLS. 1999;55:1036–1042. doi: 10.1007/s000180050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess D.T., Matsumoto A., Kim S.O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 24.Liu H., Colavitti R., Rovira, Finkel T. Redox-dependent transcriptional regulation. Circ. Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 25.Leon-Bollotte L., Subramaniam S., Cauvard O., Plenchette-Colas S., Paul C., Godard C. S-nitrosylation of the death receptor fas promotes fas ligand-mediated apoptosis in cancer cells. Gastroenterology. 2011;140:2009–2018. doi: 10.1053/j.gastro.2011.02.053. (18 e1-4) [DOI] [PubMed] [Google Scholar]
- 26.Tang Z., Bauer J.A., Morrison B., Lindner D.J. Nitrosylcobalamin promotes cell death via S nitrosylation of Apo2L/TRAIL receptor DR4. Mol. Cell. Biol. 2006;26:5588–5594. doi: 10.1128/MCB.00199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glynn S.A., Boersma B.J., Dorsey T.H., Yi M., Yfantis H.G., Ridnour L.A. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J. Clin. Investig. 2010;120:3843–3854. doi: 10.1172/JCI42059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ducrocq C., Blanchard B., Pignatelli B., Ohshima H. Peroxynitrite: an endogenous oxidizing and nitrating agent. Cell. Mol. Life Sci.: CMLS. 1999;55:1068–1077. doi: 10.1007/s000180050357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabo C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 30.Martinez M.C., Andriantsitohaina R. Reactive nitrogen species: molecular mechanisms and potential significance in health and disease. Antioxid. Redox Signal. 2009;11:669–702. doi: 10.1089/ars.2007.1993. [DOI] [PubMed] [Google Scholar]
- 31.Ridnour L.A., Thomas D.D., Donzelli S., Espey M.G., Roberts D.D., Wink D.A. The biphasic nature of nitric oxide responses in tumor biology. Antioxid. Redox Signal. 2006;8:1329–1337. doi: 10.1089/ars.2006.8.1329. [DOI] [PubMed] [Google Scholar]
- 32.Wink D.A., Mitchell J.B. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 33.Ambs S., Hussain S.P., Harris C.C. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J. 1997;11:443–448. doi: 10.1096/fasebj.11.6.9194524. [DOI] [PubMed] [Google Scholar]
- 34.Lechner M., Lirk P., Rieder J. Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin. Semin. Cancer Biol. 2005;15:277–289. doi: 10.1016/j.semcancer.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Oronsky B., Fanger G.R., Oronsky N., Knox S., Scicinski J. The implications of hyponitroxia in cancer. Transl. Oncol. 2014;7:167–173. doi: 10.1016/j.tranon.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulton D., Gratton J.P., Sessa W.C. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J. Pharmacol. Exp. Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 37.Ridnour L.A., Isenberg J.S., Espey M.G., Thomas D.D., Roberts D.D., Wink D.A. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc. Natl. Acad. Sci. USA. 2005;102:13147–13152. doi: 10.1073/pnas.0502979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isenberg J.S., Ridnour L.A., Perruccio E.M., Espey M.G., Wink D.A., Roberts D.D. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc. Natl. Acad. Sci. USA. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridnour L.A., Thomas D.D., Switzer C., Flores-Santana W., Isenberg J.S., Ambs S. Molecular mechanisms for discrete nitric oxide levels in cancer. Nitric oxide: Biol. Chem. 2008;19:73–76. doi: 10.1016/j.niox.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L.M., Kilbourn R.G., Adams J., Fidler I.J. Role of nitric oxide in lysis of tumor cells by cytokine-activated endothelial cells. Cancer Res. 1991;51:2531–2535. [PubMed] [Google Scholar]
- 41.Jiang H., Stewart C.A., Fast D.J., Leu R.W. Tumor target-derived soluble factor synergizes with IFN-gamma and IL-2 to activate macrophages for tumor necrosis factor and nitric oxide production to mediate cytotoxicity of the same target. J. Immunol. 1992;149:2137–2146. [PubMed] [Google Scholar]
- 42.Xiao L., Eneroth P.H., Qureshi G.A. Nitric oxide synthase pathway may mediate human natural killer cell cytotoxicity. Scand. J. Immunol. 1995;42:505–511. doi: 10.1111/j.1365-3083.1995.tb03687.x. [DOI] [PubMed] [Google Scholar]
- 43.Fukumura D., Yonei Y., Kurose I., Saito H., Ohishi T., Higuchi H. Role in nitric oxide in Kupffer cell-mediated hepatoma cell cytotoxicity in vitro and ex vivo. Hepatology. 1996;24:141–149. doi: 10.1053/jhep.1996.v24.pm0008707254. [DOI] [PubMed] [Google Scholar]
- 44.Baritaki S., Huerta-Yepez S., Sahakyan A., Karagiannides I., Bakirtzi K., Jazirehi A. Mechanisms of nitric oxide-mediated inhibition of EMT in cancer: inhibition of the metastasis-inducer snail and induction of the metastasis-suppressor RKIP. Cell Cycle. 2010;9:4931–4940. doi: 10.4161/cc.9.24.14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aranda E., Lopez-Pedrera C., De La Haba-Rodriguez J.R., Rodriguez-Ariza A. Nitric oxide and cancer: the emerging role of S-nitrosylation. Curr. Mol. Med. 2012;12:50–67. doi: 10.2174/156652412798376099. [DOI] [PubMed] [Google Scholar]
- 46.Thomas D.D., Espey M.G., Ridnour L.A., Hofseth L.J., Mancardi D., Harris C.C. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc. Natl. Acad. Sci. USA. 2004;101:8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alleva D.G., Burger C.J., Elgert K.D. Tumor-induced regulation of suppressor macrophage nitric oxide and TNF-alpha production. Role of tumor-derived IL-10, TGF-beta, and prostaglandin E2. J. Immunol. 1994;153:1674–1686. [PubMed] [Google Scholar]
- 48.Nathan C., Xie Q.W. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 49.Yakovlev V.A. Nitric oxide and genomic stability. In: Bonavida B., editor. Nitric Oxide and Cancer: Pathogenesis and Therapy. Springer International; New York: 2015. pp. 25–38. [Google Scholar]
- 50.Yakovlev V.A. Nitric oxide-dependent downregulation of BRCA1 expression promotes genetic instability. Cancer Res. 2013;73:706–715. doi: 10.1158/0008-5472.CAN-12-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huerta S., Chilka S., Bonavida B. Nitric oxide donors: novel cancer therapeutics (review) Int. J. Oncol. 2008;33:909–927. [PubMed] [Google Scholar]
- 52.Yakovlev V.A., Bayden A.S., Graves P.R., Kellogg G.E., Mikkelsen R.B. Nitration of the tumor suppressor protein p53 at tyrosine 327 promotes p53 oligomerization and activation. Biochemistry. 2010;49:5331–5339. doi: 10.1021/bi100564w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mocellin S., Bronte V., Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med. Res. Rev. 2007;27:317–352. doi: 10.1002/med.20092. [DOI] [PubMed] [Google Scholar]
- 54.Nathan C., Xie Q.W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 55.Singh S., Gupta A.K. Nitric oxide: role in tumour biology and iNOS/NO-based anticancer therapies. Cancer Chemother. Pharmacol. 2011;67:1211–1224. doi: 10.1007/s00280-011-1654-4. [DOI] [PubMed] [Google Scholar]
- 56.Burke A.J., Sullivan F.J., Giles F.J., Glynn S.A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis. 2013;34:503–512. doi: 10.1093/carcin/bgt034. [DOI] [PubMed] [Google Scholar]
- 57.Bloch K.D., Wolfram J.R., Brown D.M., Roberts J.D., Jr., Zapol D.G., Lepore J.J. Three members of the nitric oxide synthase II gene family (NOS2A, NOS2B, and NOS2C) colocalize to human chromosome 17. Genomics. 1995;27:526–530. doi: 10.1006/geno.1995.1086. [DOI] [PubMed] [Google Scholar]
- 58.Lu X., Lu P., Xing R.Y., Sun Q.Y., Qiu Z.W., Han L. A short tandem repeat polymorphism in the inducible nitric oxide synthase gene in Chinese population. Yi Chuan Xue Bao: Acta Genet. Sinica. 2002;29:290–293. [PubMed] [Google Scholar]
- 59.Pautz A., Art J., Hahn S., Nowag S., Voss C., Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide: Biol. Chem. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Han X., Zheng T., Lan Q., Zhang Y., Kilfoy B.A., Qin Q. Genetic polymorphisms in nitric oxide synthase genes modify the relationship between vegetable and fruit intake and risk of non-Hodgkin lymphoma. Cancer Epidemiol. Biomarkers Prev. 2009;18:1429–1438. doi: 10.1158/1055-9965.EPI-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fabisiewicz A., Pacholewicz K., Paszkiewicz-Kozik E., Walewski J., Siedlecki J.A. Polymorphisms of DNA repair and oxidative stress genes in B-cell lymphoma patients. Biomed. Rep. 2013;1:151–155. doi: 10.3892/br.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaise M., Miwa J., Suzuki N., Mishiro S., Ohta Y., Yamasaki T. Inducible nitric oxide synthase gene promoter polymorphism is associated with increased gastric mRNA expression of inducible nitric oxide synthase and increased risk of gastric carcinoma. Eur. J. Gastroenterol. Hepatol. 2007;19:139–145. doi: 10.1097/01.meg.0000252637.11291.1d. [DOI] [PubMed] [Google Scholar]
- 63.Motallebipour M., Rada-Iglesias A., Jansson M., Wadelius C. The promoter of inducible nitric oxide synthase implicated in glaucoma based on genetic analysis and nuclear factor binding. Mol. Vis. 2005;11:950–957. [PubMed] [Google Scholar]
- 64.Shen C.H., Wang Y.H., Wang W.C., Jou Y.C., Hsu H.S., Hsieh H.Y. Inducible nitric oxide synthase promoter polymorphism, cigarette smoking, and urothelial carcinoma risk. Urology. 2007;69:1001–1006. doi: 10.1016/j.urology.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 65.Qidwai T., Jamal F. Inducible nitric oxide synthase (iNOS) gene polymorphism and disease prevalence. Scand. J. Immunol. 2010;72:375–387. doi: 10.1111/j.1365-3083.2010.02458.x. [DOI] [PubMed] [Google Scholar]
- 66.Gross T.J., Kremens K., Powers L.S., Brink B., Knutson T., Domann F.E. Epigenetic silencing of the human NOS2 gene: rethinking the role of nitric oxide in human macrophage inflammatory responses. J. Immunol. 2014;192:2326–2338. doi: 10.4049/jimmunol.1301758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 68.Ferreiro C.R., Chagas A.C., Carvalho M.H., Dantas A.P., Jatene M.B., Bento De Souza L.C. Influence of hypoxia on nitric oxide synthase activity and gene expression in children with congenital heart disease: a novel pathophysiological adaptive mechanism. Circulation. 2001;103:2272–2276. doi: 10.1161/01.cir.103.18.2272. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L., Liu Q., Yuan X., Wang T., Luo S., Lei H. Requirement of heat shock protein 70 for inducible nitric oxide synthase induction. Cell. Signal. 2013;25:1310–1317. doi: 10.1016/j.cellsig.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Mankan A.K., Lawless M.W., Gray S.G., Kelleher D., McManus R. NF-kappaB regulation: the nuclear response. J. Cell. Mol. Med. 2009;13:631–643. doi: 10.1111/j.1582-4934.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basak S., Hoffmann A. Crosstalk via the NF-kappaB signaling system. Cytokine Growth Factor Rev. 2008;19:187–197. doi: 10.1016/j.cytogfr.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kleinert H., Wallerath T., Fritz G., Ihrig-Biedert I., Rodriguez-Pascual F., Geller D.A. Cytokine induction of NO synthase II in human DLD-1 cells: roles of the JAK-STAT, AP-1 and NF-kappaB-signaling pathways. Br. J. Pharmacol. 1998;125:193–201. doi: 10.1038/sj.bjp.0702039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ganster R.W., Taylor B.S., Shao L., Geller D.A. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B. Proc. Natl. Acad. Sci. USA. 2001;98:8638–8643. doi: 10.1073/pnas.151239498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kleinert H., Euchenhofer C., Ihrig-Biedert I., Forstermann U. Glucocorticoids inhibit the induction of nitric oxide synthase II by down-regulating cytokine-induced activity of transcription factor nuclear factor-kappa B. Mol. Pharmacol. 1996;49:15–21. [PubMed] [Google Scholar]
- 75.Pascual G., Glass C.K. Nuclear receptors versus inflammation: mechanisms of transrepression. Trends Endocrinol. Metab.: TEM. 2006;17:321–327. doi: 10.1016/j.tem.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Mukaida N., Morita M., Ishikawa Y., Rice N., Okamoto S., Kasahara T. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. J. Biol. Chem. 1994;269:13289–13295. [PubMed] [Google Scholar]
- 77.Tedeschi E., Menegazzi M., Margotto D., Suzuki H., Forstermann U., Kleinert H. Anti-inflammatory actions of St. John's wort: inhibition of human inducible nitric-oxide synthase expression by down-regulating signal transducer and activator of transcription-1alpha (STAT-1alpha) activation. J. Pharmacol. Exp. Ther. 2003;307:254–261. doi: 10.1124/jpet.103.054460. [DOI] [PubMed] [Google Scholar]
- 78.Janssen-Heininger Y.M., Macara I., Mossman B.T. Cooperativity between oxidants and tumor necrosis factor in the activation of nuclear factor (NF)-kappaB: requirement of Ras/mitogen-activated protein kinases in the activation of NF-kappaB by oxidants. Am. J. Respir. Cell Mol. Biol. 1999;20:942–952. doi: 10.1165/ajrcmb.20.5.3452. [DOI] [PubMed] [Google Scholar]
- 79.Lelchuk R., Radomski M.W., Martin J.F., Moncada S. Constitutive and inducible nitric oxide synthases in human megakaryoblastic cells. J. Pharmacol. Exp. Ther. 1992;262:1220–1224. [PubMed] [Google Scholar]
- 80.Thomsen L.L., Lawton F.G., Knowles R.G., Beesley J.E., Riveros-Moreno V., Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994;54:1352–1354. [PubMed] [Google Scholar]
- 81.Thomsen L.L., Miles D.W., Happerfield L., Bobrow L.G., Knowles R.G., Moncada S. Nitric oxide synthase activity in human breast cancer. Br. J. Cancer. 1995;72:41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie K., Huang S., Dong Z., Juang S.H., Gutman M., Xie Q.W. Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. J. Exp. Med. 1995;181:1333–1343. doi: 10.1084/jem.181.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lala P.K., Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 84.Zhang R., Ma A., Urbanski S.J., McCafferty D.M. Induction of inducible nitric oxide synthase: a protective mechanism in colitis-induced adenocarcinoma. Carcinogenesis. 2007;28:1122–1130. doi: 10.1093/carcin/bgl224. [DOI] [PubMed] [Google Scholar]
- 85.Adams C., McCarthy H.O., Coulter J.A., Worthington J., Murphy C., Robson T. Nitric oxide synthase gene therapy enhances the toxicity of cisplatin in cancer cells. J. Gene Med. 2009;11:160–168. doi: 10.1002/jgm.1280. [DOI] [PubMed] [Google Scholar]
- 86.Chung P., Cook T., Liu K., Vodovotz Y., Zamora R., Finkelstein S. Overexpression of the human inducible nitric oxide synthase gene enhances radiation-induced apoptosis in colorectal cancer cells via a caspase-dependent mechanism. Nitric Oxide: Biol. Chem. 2003;8:119–126. doi: 10.1016/s1089-8603(02)00147-7. [DOI] [PubMed] [Google Scholar]
- 87.Soler M.N., Bobe P., Benihoud K., Lemaire G., Roos B.A., Lausson S. Gene therapy of rat medullary thyroid cancer by naked nitric oxide synthase II DNA injection. J. Gene Med. 2000;2:344–352. doi: 10.1002/1521-2254(200009/10)2:5<344::AID-JGM124>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 88.Olson M.V., Lee J., Zhang F., Wang A., Dong Z. Inducible nitric oxide synthase activity is essential for inhibition of prostatic tumor growth by interferon-beta gene therapy. Cancer Gene Ther. 2006;13:676–685. doi: 10.1038/sj.cgt.7700941. [DOI] [PubMed] [Google Scholar]
- 89.Le X., Wei D., Huang S., Lancaster J.R., Jr., Xie K. Nitric oxide synthase II suppresses the growth and metastasis of human cancer regardless of its up-regulation of protumor factors. Proc. Natl. Acad. Sci. USA. 2005;102:8758–8763. doi: 10.1073/pnas.0409581102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Juang S.H., Xie K., Xu L., Wang Y., Yoneda J., Fidler I.J. Use of retroviral vectors encoding murine inducible nitric oxide synthase gene to suppress tumorigenicity and cancer metastasis of murine melanoma. Cancer Biother. Radiopharm. 1997;12:167–175. doi: 10.1089/cbr.1997.12.167. [DOI] [PubMed] [Google Scholar]
- 91.Bhowmick R., Girotti A.W. Cytoprotective signaling associated with nitric oxide upregulation in tumor cells subjected to photodynamic therapy-like oxidative stress. Free Radic. Biol. Med. 2013;57:39–48. doi: 10.1016/j.freeradbiomed.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagasawa H., Little J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 93.McCarthy H.O., Zholobenko A.V., Wang Y., Canine B., Robson T., Hirst D.G. Evaluation of a multi-functional nanocarrier for targeted breast cancer iNOS gene therapy. Int. J. Pharm. 2011;405:196–202. doi: 10.1016/j.ijpharm.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 94.He M., Ye S., Ren R., Dong C., Xie Y., Yuan D. Cytochrome-c mediated a bystander response dependent on inducible nitric oxide synthase in irradiated hepatoma cells. Br. J. Cancer. 2012;106:889–895. doi: 10.1038/bjc.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dimmeler S., Zeiher A.M. Nitric oxide and apoptosis: another paradigm for the double-edged role of nitric oxide. Nitric Oxide: Biol. Chem. 1997;1:275–281. doi: 10.1006/niox.1997.0133. [DOI] [PubMed] [Google Scholar]
- 96.Brune B., von Knethen A., Sandau K.B. Nitric oxide and its role in apoptosis. Eur. J. Pharmacol. 1998;351:261–272. doi: 10.1016/s0014-2999(98)00274-x. [DOI] [PubMed] [Google Scholar]
- 97.Choi B.M., Pae H.O., Jang S.I., Kim Y.M., Chung H.T. Nitric oxide as a pro-apoptotic as well as anti-apoptotic modulator. J. Biochem. Mol. Biol. 2002;35:116–126. doi: 10.5483/bmbrep.2002.35.1.116. [DOI] [PubMed] [Google Scholar]
- 98.Xu W., Liu L., Smith G.C., Charles l G. Nitric oxide upregulates expression of DNA-PKcs to protect cells from DNA-damaging anti-tumour agents. Nat. Cell Biol. 2000;2:339–345. doi: 10.1038/35014028. [DOI] [PubMed] [Google Scholar]
- 99.Bonavida B., Baritaki S. Dual role of NO donors in the reversal of tumor cell resistance and EMT: downregulation of the NF-kappaB/Snail/YY1/RKIP circuitry. Nitric Oxide: Biol. Chem. 2011;24:1–7. doi: 10.1016/j.niox.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 100.Qiu H., Orr F.W., Jensen D., Wang H.H., McIntosh A.R., Hasinoff B.B. Arrest of B16 melanoma cells in the mouse pulmonary microcirculation induces endothelial nitric oxide synthase-dependent nitric oxide release that is cytotoxic to the tumor cells. Am. J. Pathol. 2003;162:403–412. doi: 10.1016/S0002-9440(10)63835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang L., Liu J., Wang X., Li Z., Zhang X., Cao P. Upregulation of cytoskeleton protein and extracellular matrix protein induced by stromal-derived nitric oxide promotes lung cancer invasion and metastasis. Curr. Mol. Med. 2014;14:762–771. doi: 10.2174/1566524014666140724103147. [DOI] [PubMed] [Google Scholar]
- 102.Klotz T., Bloch W., Volberg C., Engelmann U., Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer. 1998;82:1897–1903. [PubMed] [Google Scholar]
- 103.Vakkala M., Kahlos K., Lakari E., Paakko P., Kinnula V., Soini Y. Inducible nitric oxide synthase expression, apoptosis, and angiogenesis in in situ and invasive breast carcinomas. Clin. Cancer Res. 2000;6:2408–2416. [PubMed] [Google Scholar]
- 104.Swana H.S., Smith S.D., Perrotta P.L., Saito N., Wheeler M.A., Weiss R.M. Inducible nitric oxide synthase with transitional cell carcinoma of the bladder. J. Urol. 1999;161:630–634. [PubMed] [Google Scholar]
- 105.Ohshima H., Tazawa H., Sylla B.S., Sawa T. Prevention of human cancer by modulation of chronic inflammatory processes. Mutat. Res. 2005;591:110–122. doi: 10.1016/j.mrfmmm.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 106.Kojima M., Morisaki T., Tsukahara Y., Uchiyama A., Matsunari Y., Mibu R. Nitric oxide synthase expression and nitric oxide production in human colon carcinoma tissue. J. Surg. Oncol. 1999;70:222–229. doi: 10.1002/(sici)1096-9098(199904)70:4<222::aid-jso5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 107.Pervin S., Singh R., Sen S., Chaudhuri G. Dual role of nitric oxide in cancer biology. In: Bonavida B., editor. Nitric Oxide and Cancer: Prognosis, Prevention and Therapy. Humana Press; New York: 2010. pp. 39–57. [Google Scholar]
- 108.Anttila M.A., Voutilainen K., Merivalo S., Saarikoski S., Kosma V.M. Prognostic significance of iNOS in epithelial ovarian cancer. Gynecol. Oncol. 2007;105:97–103. doi: 10.1016/j.ygyno.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 109.Puhakka A., Kinnula V., Napankangas U., Saily M., Koistinen P., Paakko P. High expression of nitric oxide synthases is a favorable prognostic sign in non-small cell lung carcinoma. APMIS: Acta Pathol. Microbiol. Immunol. Scand. 2003;111:1137–1146. doi: 10.1111/j.1600-0463.2003.apm1111210.x. [DOI] [PubMed] [Google Scholar]
- 110.Chen W.L., Feng H.J., Li J.S., Li H.G. Expression and pathological relevance of inducible nitric oxide synthase in osteosarcoma of the jaws. Int. J. Oral Maxillofac. Surg. 2007;36:541–544. doi: 10.1016/j.ijom.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 111.Augustine D., Sekar B., Murali S., Ramesh M., Madhavan R.N., Patil S.G. Expression of inducible nitric oxide synthase in carcinomas and sarcomas affecting the oral cavity. South Asian J. Cancer. 2015;4:78–82. doi: 10.4103/2278-330X.155686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li L.G., Xu H.M. Inducible nitric oxide synthase, nitrotyrosine and apoptosis in gastric adenocarcinomas and their correlation with a poor survival. World J. Gastroenterol.: WJG. 2005;11:2539–2544. doi: 10.3748/wjg.v11.i17.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang B., Wei D., Crum V.E., Richardson E.L., Xiong H.H., Luo Y. A novel model system for studying the double-edged roles of nitric oxide production in pancreatic cancer growth and metastasis. Oncogene. 2003;22:1771–1782. doi: 10.1038/sj.onc.1206386. [DOI] [PubMed] [Google Scholar]
- 114.Girotti A.W. Tumor-generated nitric oxide as an antagonist of photodynamic therapy. Photochem. Photobiol. Sci. 2015;14:1425–1432. doi: 10.1039/c4pp00470a. [DOI] [PubMed] [Google Scholar]
- 115.Fahey J.M., Girotti A.W. Accelerated migration and invasion of prostate cancer cells after a photodynamic therapy-like challenge: Role of nitric oxide. Nitric Oxide: Biol. Chem. 2015;49:47–55. doi: 10.1016/j.niox.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhowmick R., Girotti A.W. Signaling events in apoptotic photokilling of 5-aminolevulinic acid-treated tumor cells: inhibitory effects of nitric oxide. Free Radic. Biol. Med. 2009;47:731–740. doi: 10.1016/j.freeradbiomed.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Forrester K., Ambs S., Lupold S.E., Kapust R.B., Spillare E.A., Weinberg W.C. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc. Natl. Acad. Sci. USA. 1996;93:2442–2447. doi: 10.1073/pnas.93.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brune B., Schneiderhan N. Nitric oxide evoked p53-accumulation and apoptosis. Toxicol. Lett. 2003;139:119–123. doi: 10.1016/s0378-4274(02)00426-5. [DOI] [PubMed] [Google Scholar]
- 119.Ambs S., Merriam W.G., Ogunfusika M.O., Bennett W.P., Ishibe N., Hussain S.P. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat. Med. 1998;4:1371–1376. doi: 10.1038/3957. [DOI] [PubMed] [Google Scholar]
- 120.Hussain S.P., Trivers G.E., Hofseth L.J., He P., Shaikh I., Mechanic L.E. Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res. 2004;64:6849–6853. doi: 10.1158/0008-5472.CAN-04-2201. [DOI] [PubMed] [Google Scholar]
- 121.Kwak J.Y., Han M.K., Choi K.S., Park I.H., Park S.Y., Sohn M.H. Cytokines secreted by lymphokine-activated killer cells induce endogenous nitric oxide synthesis and apoptosis in DLD-1 colon cancer cells. Cell. Immunol. 2000;203:84–94. doi: 10.1006/cimm.2000.1682. [DOI] [PubMed] [Google Scholar]
- 122.Gansauge S., Nussler A.K., Beger H.G., Gansauge F. Nitric oxide-induced apoptosis in human pancreatic carcinoma cell lines is associated with a G1-arrest and an increase of the cyclin-dependent kinase inhibitor p21WAF1/CIP1. Cell Growth Differ. 1998;9:611–617. [PubMed] [Google Scholar]
- 123.Roberts W., Riba R., Homer-Vanniasinkam S., Farndale R.W., Naseem K.M. Nitric oxide specifically inhibits integrin-mediated platelet adhesion and spreading on collagen. J. Thromb. Haemost.: JTH. 2008;6:2175–2185. doi: 10.1111/j.1538-7836.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- 124.Gupta S.K., Vlahakis N.E. Integrin alpha9beta1 mediates enhanced cell migration through nitric oxide synthase activity regulated by Src tyrosine kinase. J. Cell Sci. 2009;122:2043–2054. doi: 10.1242/jcs.041632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tronc F., Mallat Z., Lehoux S., Wassef M., Esposito B., Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler. Thromb. Vasc. Biol. 2000;20:E120–E126. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- 126.Okamoto T., Akaike T., Nagano T., Miyajima S., Suga M., Ando M. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch. Biochem. Biophys. 1997;342:261–274. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 127.Upchurch G.R., Jr., Ford J.W., Weiss S.J., Knipp B.S., Peterson D.A., Thompson R.W. Nitric oxide inhibition increases matrix metalloproteinase-9 expression by rat aortic smooth muscle cells in vitro. J. Vasc. Surg. 2001;34:76–83. doi: 10.1067/mva.2001.115598. [DOI] [PubMed] [Google Scholar]
- 128.Lahiri M., Martin J.H. Nitric oxide decreases motility and increases adhesion in human breast cancer cells. Oncol. Rep. 2009;21:275–281. [PubMed] [Google Scholar]
- 129.Zhang W., He X.J., Ma Y.Y., Wang H.J., Xia Y.J., Zhao Z.S. Inducible nitric oxide synthase expression correlates with angiogenesis, lymphangiogenesis, and poor prognosis in gastric cancer patients. Hum. Pathol. 2011;42:1275–1282. doi: 10.1016/j.humpath.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 130.Kinoshita H., Hirata Y., Nakagawa H., Sakamoto K., Hayakawa Y., Takahashi R. Interleukin-6 mediates epithelial-stromal interactions and promotes gastric tumorigenesis. PLoS One. 2013;8:e60914. doi: 10.1371/journal.pone.0060914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wada S., Matsushita Y., Tazawa H., Aoi W., Naito Y., Higashi A. Loss of p53 in stromal fibroblasts enhances tumor cell proliferation through nitric-oxide-mediated cyclooxygenase 2 activation. Free Radic. Res. 2015;49:269–278. doi: 10.3109/10715762.2014.997230. [DOI] [PubMed] [Google Scholar]
- 132.Lisanti M.P., Tsirigos A., Pavlides S., Reeves K.J., Peiris-Pages M., Chadwick A.L. JNK1 stress signaling is hyper-activated in high breast density and the tumor stroma: connecting fibrosis, inflammation, and stemness for cancer prevention. Cell Cycle. 2014;13:580–599. doi: 10.4161/cc.27379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Karadayi N., Kandemir N.O., Yavuzer D., Korkmaz T., Gecmen G., Kokturk F. Inducible nitric oxide synthase expression in gastric adenocarcinoma: impact on lymphangiogenesis and lymphatic metastasis. Diagn. Pathol. 2013;8:151. doi: 10.1186/1746-1596-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ito H., Ando T., Ogiso H., Arioka Y., Seishima M. Inhibition of induced nitric oxide synthase enhances the anti-tumor effects on cancer immunotherapy using TLR7 agonist in mice. Cancer Immunol. Immunother.: CII. 2015;64:429–436. doi: 10.1007/s00262-014-1644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Muerkoster S., Wegehenkel K., Arlt A., Witt M., Sipos B., Kruse M.L. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res. 2004;64:1331–1337. doi: 10.1158/0008-5472.can-03-1860. [DOI] [PubMed] [Google Scholar]
- 136.Dong Z., Yang X., Xie K., Juang S.H., Llansa N., Fidler I.J. Activation of inducible nitric oxide synthase gene in murine macrophages requires protein phosphatases 1 and 2A activities. J. Leukoc. Biol. 1995;58:725–732. doi: 10.1002/jlb.58.6.725. [DOI] [PubMed] [Google Scholar]
- 137.Wei D., Richardson E.L., Zhu K., Wang L., Le X., He Y. Direct demonstration of negative regulation of tumor growth and metastasis by host-inducible nitric oxide synthase. Cancer Res. 2003;63:3855–3859. [PubMed] [Google Scholar]
- 138.Onier N., Hilpert S., Reveneau S., Arnould L., Saint-Giorgio V., Exbrayat J.M. Expression of inducible nitric oxide synthase in tumors in relation with their regression induced by lipid A in rats. Int. J. Cancer. 1999;81:755–760. doi: 10.1002/(sici)1097-0215(19990531)81:5<755::aid-ijc15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 139.Kundu N., Dorsey R., Jackson M.J., Guiterrez P., Wilson K., Fu S. Interleukin-10 gene transfer inhibits murine mammary tumors and elevates nitric oxide. Int. J. Cancer. 1998;76:713–719. doi: 10.1002/(sici)1097-0215(19980529)76:5<713::aid-ijc17>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 140.Xie K., Fidler I.J. Therapy of cancer metastasis by activation of the inducible nitric oxide synthase. Cancer Metastasis Rev. 1998;17:55–75. doi: 10.1023/a:1005956721457. [DOI] [PubMed] [Google Scholar]
- 141.Xu L., Xie K., Fidler I.J. Therapy of human ovarian cancer by transfection with the murine interferon beta gene: role of macrophage-inducible nitric oxide synthase. Hum. Gene Ther. 1998;9:2699–2708. doi: 10.1089/hum.1998.9.18-2699. [DOI] [PubMed] [Google Scholar]
- 142.Shi Q., Xiong Q., Wang B., Le X., Khan N.A., Xie K. Influence of nitric oxide synthase II gene disruption on tumor growth and metastasis. Cancer Res. 2000;60:2579–2583. [PubMed] [Google Scholar]
- 143.Kleinert H., Pautz A., Linker K., Schwarz P.M. Regulation of the expression of inducible nitric oxide synthase. Eur. J. Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 144.Gauthier N., Lohm S., Touzery C., Chantome A., Perette B., Reveneau S. Tumour-derived and host-derived nitric oxide differentially regulate breast carcinoma metastasis to the lungs. Carcinogenesis. 2004;25:1559–1565. doi: 10.1093/carcin/bgh158. [DOI] [PubMed] [Google Scholar]
- 145.Machado F.A., Abdalla D.R., Montes L., Etchebehere R.M., Michelin M.A., Murta E.F. An evaluation of immune system cell infiltrate in the cervical stroma of patients with grade III cervical intraepithelial neoplasia after treatment with intralesional alpha-2B interferon. Eur. J. Gynaecol. Oncol. 2014;35:20–25. [PubMed] [Google Scholar]
- 146.Mehta P. Potential role of platelets in the pathogenesis of tumor metastasis. Blood. 1984;63:55–63. [PubMed] [Google Scholar]
- 147.Gasic G.J., Gasic T.B., Stewart C.C. Antimetastatic effects associated with platelet reduction. Proc. Natl. Acad. Sci. USA. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xiong H., Zhu C., Li F., Hegazi R., He K., Babyatsky M. Inhibition of interleukin-12 p40 transcription and NF-kappaB activation by nitric oxide in murine macrophages and dendritic cells. J. Biol. Chem. 2004;279:10776–10783. doi: 10.1074/jbc.M313416200. [DOI] [PubMed] [Google Scholar]
- 149.Nath N., Chattopadhyay M., Kodela R., Tian S., Vlismas P., Boring D. Modulation of stress genes expression profile by nitric oxide-releasing aspirin in Jurkat T leukemia cells. Biochem. Pharmacol. 2010;79:1759–1771. doi: 10.1016/j.bcp.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 150.Lee J.J., Chang Y.L., Lai W.L., Ko J.Y., Kuo M.Y., Chiang C.P. Increased prevalence of interleukin-17-producing CD4(+) tumor infiltrating lymphocytes in human oral squamous cell carcinoma. Head Neck. 2011;33:1301–1308. doi: 10.1002/hed.21607. [DOI] [PubMed] [Google Scholar]
- 151.Niedbala W., Alves-Filho J.C., Fukada S.Y., Vieira S.M., Mitani A., Sonego F. Regulation of type 17 helper T-cell function by nitric oxide during inflammation. Proc. Natl. Acad. Sci. USA. 2011;108:9220–9225. doi: 10.1073/pnas.1100667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jianjun Y., Zhang R., Lu G., Shen Y., Peng L., Zhu C. T cell-derived inducible nitric oxide synthase switches off Th17 cell differentiation. J. Exp. Med. 2013;210:1447–1462. doi: 10.1084/jem.20122494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Biswas N., Mahato S.K., Chowdhury A.A., Chaudhuri J., Manna A., Vinayagam J. ICB3E induces iNOS expression by ROS-dependent JNK and ERK activation for apoptosis of leukemic cells. Apoptosis. 2012;17:612–626. doi: 10.1007/s10495-011-0695-9. [DOI] [PubMed] [Google Scholar]
- 154.Siegert A., Rosenberg C., Schmitt W.D., Denkert C., Hauptmann S. Nitric oxide of human colorectal adenocarcinoma cell lines promotes tumour cell invasion. Br. J. Cancer. 2002;86:1310–1315. doi: 10.1038/sj.bjc.6600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grivennikov S.I., Karin M. Inflammation and oncogenesis: a vicious connection. Curr. Opin. Genet. Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gochman E., Mahajna J., Shenzer P., Dahan A., Blatt A., Elyakim R. The expression of iNOS and nitrotyrosine in colitis and colon cancer in humans. Acta Histochem. 2012;114:827–835. doi: 10.1016/j.acthis.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 157.Huber M.A., Azoitei N., Baumann B., Grunert S., Sommer A., Pehamberger H. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Investig. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Min C., Eddy S.F., Sherr D.H., Sonenshein G.E. NF-kappaB and epithelial to mesenchymal transition of cancer. J. Cell. Biochem. 2008;104:733–744. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 159.Prueitt R.L., Boersma B.J., Howe T.M., Goodman J.E., Thomas D.D., Ying L. Inflammation and IGF-I activate the Akt pathway in breast cancer. Int. J. Cancer. 2007;120:796–805. doi: 10.1002/ijc.22336. [DOI] [PubMed] [Google Scholar]
- 160.Lo H.W., Hsu S.C., Ali-Seyed M., Gunduz M., Xia W., Wei Y. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 161.Santos A., Bakker A.D., Zandieh-Doulabi B., de Blieck-Hogervorst J.M., Klein-Nulend J. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem. Biophys. Res. Commun. 2010;391:364–369. doi: 10.1016/j.bbrc.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 162.Liu Y., Borchert G.L., Phang J.M. Polyoma enhancer activator 3, an ets transcription factor, mediates the induction of cyclooxygenase-2 by nitric oxide in colorectal cancer cells. J. Biol. Chem. 2004;279:18694–18700. doi: 10.1074/jbc.M308136200. [DOI] [PubMed] [Google Scholar]
- 163.Wang H., Zhang R., Wen S., McCafferty D.M., Beck P.L., MacNaughton W.K. Nitric oxide increases Wnt-induced secreted protein-1 (WISP-1/CCN4) expression and function in colitis. J. Mol. Med. 2009;87:435–445. doi: 10.1007/s00109-009-0445-4. [DOI] [PubMed] [Google Scholar]
- 164.Kotamraju S., Williams C.L., Kalyanaraman B. Statin-induced breast cancer cell death: role of inducible nitric oxide and arginase-dependent pathways. Cancer Res. 2007;67:7386–7394. doi: 10.1158/0008-5472.CAN-07-0993. [DOI] [PubMed] [Google Scholar]
- 165.Ahern T.P., Lash T.L., Damkier P., Christiansen P.M., Cronin-Fenton D.P. Statins and breast cancer prognosis: evidence and opportunities. Lancet Oncol. 2014;15:e461–e468. doi: 10.1016/S1470-2045(14)70119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cauley J.A., McTiernan A., Rodabough R.J., LaCroix A., Bauer D.C., Margolis K.L. Statin use and breast cancer: prospective results from the Women's Health Initiative. J. Natl. Cancer Inst. 2006;98:700–707. doi: 10.1093/jnci/djj188. [DOI] [PubMed] [Google Scholar]
- 167.Kanugula A.K., Gollavilli P.N., Vasamsetti S.B., Karnewar S., Gopoju R., Ummanni R. Statin-induced inhibition of breast cancer proliferation and invasion involves attenuation of iron transport: intermediacy of nitric oxide and antioxidant defence mechanisms. FEBS J. 2014;281:3719–3738. doi: 10.1111/febs.12893. [DOI] [PubMed] [Google Scholar]
- 168.Teraoka N., Mutoh M., Takasu S., Ueno T., Yamamoto M., Sugimura T. Inhibition of intestinal polyp formation by pitavastatin, a HMG-CoA reductase inhibitor. Cancer Prev. Res. 2011;4:445–453. doi: 10.1158/1940-6207.CAPR-10-0028. [DOI] [PubMed] [Google Scholar]
- 169.Simile M.M., Pagnan G., Pastorino F., Brignole C., De Miglio M.R., Muroni M.R. Chemopreventive N-(4-hydroxyphenyl)retinamide (fenretinide) targets deregulated NF-{kappa}B and Mat1A genes in the early stages of rat liver carcinogenesis. Carcinogenesis. 2005;26:417–427. doi: 10.1093/carcin/bgh315. [DOI] [PubMed] [Google Scholar]
- 170.Simeone A.M., Ekmekcioglu S., Broemeling L.D., Grimm E.A., Tari A.M. A novel mechanism by which N-(4-hydroxyphenyl)retinamide inhibits breast cancer cell growth: the production of nitric oxide. Mol. Cancer Ther. 2002;1:1009–1017. [PubMed] [Google Scholar]
- 171.Jiang J., Liu J., Zhu J., Yang C., Zhang A. Mechanism of apoptotic effects induced by 5-fluorouracil on human liver carcinoma Bel7402 cell line. Chin. Med. J. 2002;115:968–971. [PubMed] [Google Scholar]
- 172.Spiegel A., Hundley T.R., Chen J., Gao J., Ouyang N., Liu X. NO-donating aspirin inhibits both the expression and catalytic activity of inducible nitric oxide synthase in HT-29 human colon cancer cells. Biochem. Pharmacol. 2005;70:993–1000. doi: 10.1016/j.bcp.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 173.Williams J.L., Nath N., Chen J., Hundley T.R., Gao J., Kopelovich L. Growth inhibition of human colon cancer cells by nitric oxide (NO)-donating aspirin is associated with cyclooxygenase-2 induction and beta-catenin/T-cell factor signaling, nuclear factor-kappaB, and NO synthase 2 inhibition: implications for chemoprevention. Cancer Res. 2003;63:7613–7618. [PubMed] [Google Scholar]
- 174.Rao C.V., Reddy B.S., Steele V.E., Wang C.X., Liu X., Ouyang N. Nitric oxide-releasing aspirin and indomethacin are potent inhibitors against colon cancer in azoxymethane-treated rats: effects on molecular targets. Mol. Cancer Ther. 2006;5:1530–1538. doi: 10.1158/1535-7163.MCT-06-0061. [DOI] [PubMed] [Google Scholar]
- 175.Grasso S., Scifo C., Cardile V., Gulino R., Renis M. Adaptive responses to the stress induced by hyperthermia or hydrogen peroxide in human fibroblasts. Exp. Biol. Med. 2003;228:491–498. doi: 10.1177/15353702-0322805-12. [DOI] [PubMed] [Google Scholar]