Abstract
A Ni-catalyzed method for the coupling of perfluorobenzoates with aryl halides and pseudohalides is described. Aryl iodides, bromides, chlorides, triflates, and tosylates participate in these transformations to afford the products in good yields. Penta-, tetra-, and trifluorinated biaryl compounds are obtained using these newly developed Ni-catalyzed decarboxylative cross-coupling reactions.
Decarboxylative cross-couplings are an attractive strategy for the construction of biaryl bonds because they constitute a greener and more economical alternative to traditional cross-coupling reactions.1 These transformations entail the coupling of aromatic carboxylic acids with aryl halides (or pseudohalides) or organometallic reagents. The carboxylic acids employed in these reactions are bench stable and compatible with multistep reaction sequences in either native or latent form (e.g., from esters, amides, etc.). Furthermore, structurally diverse carboxylic acids are often inexpensive and are prevalent in abundant biomass feedstocks. Additionally, the use of carboxylic acids obviates the formation of stoichiometric organometallic byproducts that are formed in Suzuki–Miyaura, Stille, Kumada, and Negishi reactions.1,2
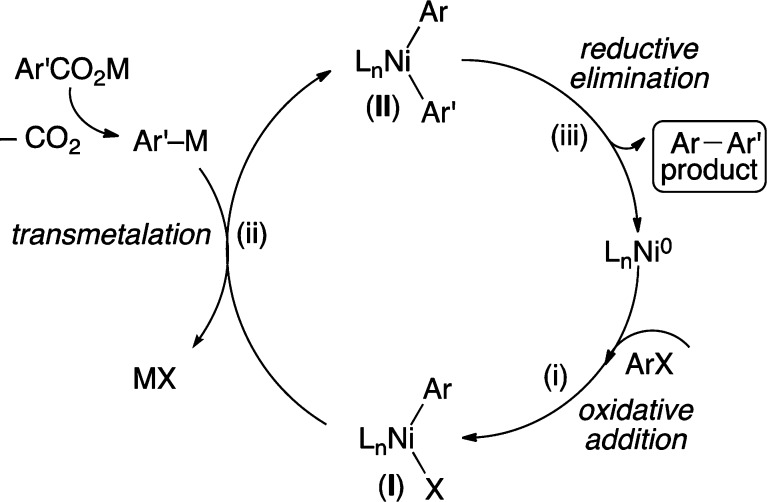
The majority of known methods for the decarboxylative biaryl synthesis utilize precious metal Pd catalysts.1,3,4 In recent years, a growing impetus for environmental and economic sustainability has sparked a desire for the replacement of noble metals such as Pd with their earth-abundant and less expensive counterparts in catalytic transformations.5 To this end, a few examples of Cu-catalyzed decarboxylative biaryl bond formations have surfaced in the literature.6,7 In contrast, examples of similar Ni-catalyzed reactions remain sparse.8,9 To the best of our knowledge, no examples of biaryl synthesis via cross-coupling of benzoic acid derivatives with aryl halides (or pseudohalides) using Ni catalysis have been disclosed. Importantly, a broader range of aromatic electrophiles are known to participate in cross-couplings using Ni catalysis (e.g., sulfonates, carboxylates, carbamates, and carbonates) instead of using Pd (sulfonates) or Cu (halides) catalysis.1,2,5,6,10 As such, the successful implementation of broad and generally applicable Ni-catalyzed decarboxylative reactions will significantly broaden the scope of electrophiles for decarboxylative couplings. A plausible mechanism for the proposed Ni-catalyzed decarboxylative coupling is depicted in Scheme 1. It involves (i) oxidative addition of Ni0 into Ar–X, (ii) transmetalation of Ar′–[M] generated via decarboxylation onto the aryl Ni intermediate (I), and (iii) reductive elimination to release the product and regenerate the Ni0 catalyst.1,11
Scheme 1. Plausible Mechanism for Ni-Catalyzed Decarboxylative Cross-Coupling.
Striking the optimal balance between the relative rates of decarboxylation and steps (i) and (ii) of the catalytic cycle is critical to minimize the undesired homocoupling of aryl–metal complexes I or of Ar′M (generated from Ar′CO2M). A well-established challenge for decarboxylative cross-coupling reactions is the high temperature required for the extrusion of CO2, which can lead to unwanted side reactions.1 As such, perfluorobenzoates were chosen for the initial studies because these substrates are known to extrude CO2 at moderate temperatures.12 Herein, we describe a method for the Ni-catalyzed intermolecular coupling of benzoates with aryl halides, triflates, and tosylates. Notably, the fluorinated biaryl products of these newly developed transformations find important applications in pharmaceutical and material sciences.13
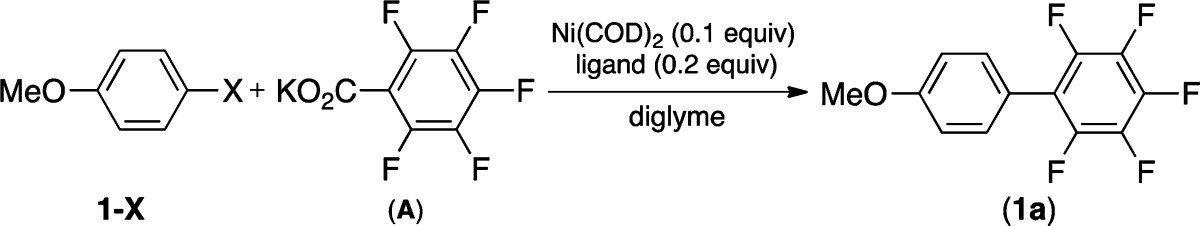
Our studies commenced with the investigation of reaction parameters for the cross-coupling of 4-iodoanisole 1-I with pentafluorophenyl potassium carboxylate (A). The use of Ni(COD)2 was explored in the presence of phosphine ligands used previously in analogous Pd-catalyzed transformations.12 As shown in Table 1, PtBu3 is more effective than PCy3 for these reactions (entries 1 and 2). Interestingly, 1a is obtained in comparable yields in the absence of any ligands (entry 3). In contrast, the use of a ligand is essential to obtain synthetically useful yields of 1a from the reaction of aryl bromides and chlorides. Among the ligands screened, CM-Phos (2-[2-(dicyclohexylphosphino)phenyl]-1-methyl-1H-indole) and DPPF (diphenylphosphinoferrocene) afford the highest yields of the desired products from the reaction of 1-Br and 1-Cl, respectively (entries 9 and 14). Importantly, no product is obtained with any of the halides (1-I, 1-Br, or 1-Cl) in the absence of Ni(COD)2 (entries 4, 10, and 15).14
Table 1. Optimization of Decarboxylative Cross-Coupling.
| entry | X | ligand | temp (°C) | yield of 1aa,b (%) |
|---|---|---|---|---|
| 1 | I | PCy3HBF4 | 140 | 58 |
| 2 | I | PtBu3HBF4 | 140 | 79 |
| 3 | I | none | 140 | 72 |
| 4c | I | PtBu3HBF4 | 140 | 0 |
| 5 | Br | none | 140 | 6 |
| 6 | Br | PCy3HBF4 | 140 | 32 |
| 7 | Br | PtBu3HBF4 | 140 | 13 |
| 8 | Br | CM-Phos | 140 | 55 |
| 9 | Br | CM-Phos | 160 | 62 |
| 10c | Br | CM-Phos | 160 | 0 |
| 11 | Cl | CM-Phos | 140 | 31 |
| 12 | Cl | PtBu3HBF4 | 140 | 0 |
| 13 | Cl | PCy3HBF4 | 140 | <2 |
| 14 | Cl | DPPF | 140 | 51 |
| 15c | Cl | DPPF | 140 | 0 |
General conditions: Ni(COD)2 (0.1 equiv), ligand (0.2 equiv), diglyme, 20 h.
Calibrated GC yields against hexadecane as the internal standard.
General conditions but with no Ni(COD)2.
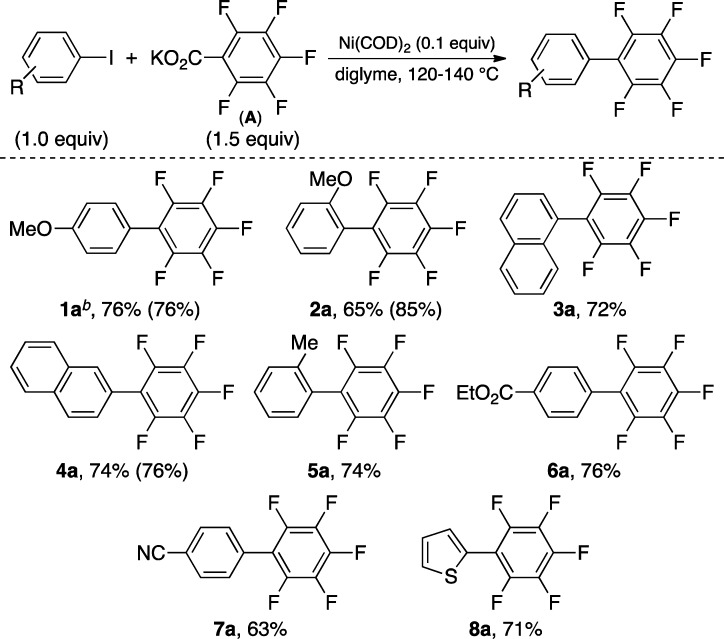
As detailed in Scheme 2, the optimal conditions for the coupling using 1-I (Table 1, entry 3) can be applied toward the use of other aryl iodides. Electron-rich, electron-neutral, and electron-deficient aryl iodides afford the desired products in good yields. ortho-Substituted and heterocyclic iodides also participate effectively in these decarboxylative couplings. The transformation is compatible with a number of synthetically versatile functional groups including ethers, nitriles, and esters.
Scheme 2. Scope of Aryl Iodides.
Conditions: Ni(COD)2 (0.1 equiv), diglyme, 120 °C. Isolated yields are given with GC yields in parentheses (calibrated against hexadecane as the internal standard).
General conditions but at 140 °C.
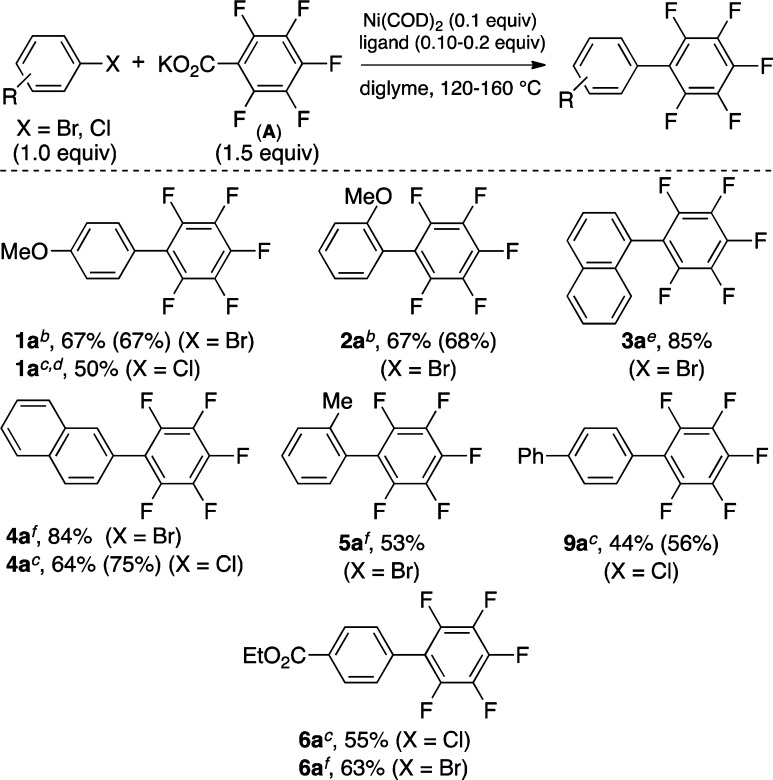
Electronically varied aryl bromides and chlorides also couple with salt A to afford the corresponding biaryl products in modest to good yields (Scheme 3). In general, the efficiency of these reactions is attenuated relative to those with aryl iodides.
Scheme 3. Scope of Aryl Bromides and Chlorides.
Isolated yields are given with GC yields in parentheses (calibrated against hexadecane as the internal standard).
Conditions A: Ni(COD)2 (0.1 equiv), CM-Phos (0.2 equiv), diglyme, 160 °C.
Conditions B: Ni(COD)2 (0.1 equiv), DPPF (0.125 equiv), diglyme, 140 °C.
Conditions B with DPPF (0.2 equiv).
Conditions A but with PtBu3HBF4 as ligand at 120 °C.
Conditions A at 140 °C.
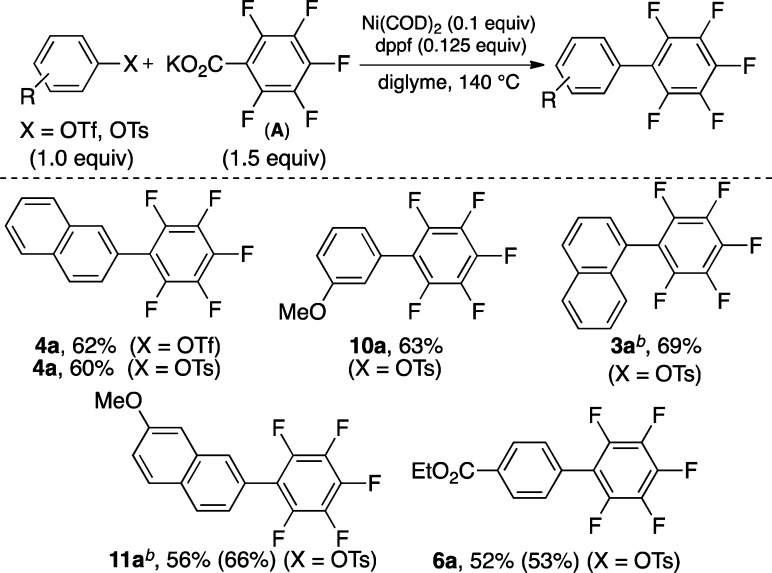
The reaction of pentafluoro salt A with phenolic electrophiles was next examined. These electrophiles avoid the production of undesired halide-containing byproducts and hence are more environmentally desirable.10,15 Scheme 4 illustrates a preliminary scope for the coupling of aryl tosylates with salt A. Electron-neutral, electron-deficient, and moderately electron-rich tosylates couple with A to afford the products in modest to good yields. Additionally, aryl triflates afford the products in yields comparable to those obtained using the corresponding aryl tosylates.
Scheme 4. Scope of Cross-Coupling Using Aryl Triflates and Tosylates.
Conditions: Ni(COD)2 (0.1 equiv), DPPF (0.125 equiv), diglyme, 140 °C. Isolated yields are given with GC yields in parentheses (calibrated against hexadecane as the internal standard).
General conditions but with 2.5 equiv of A.
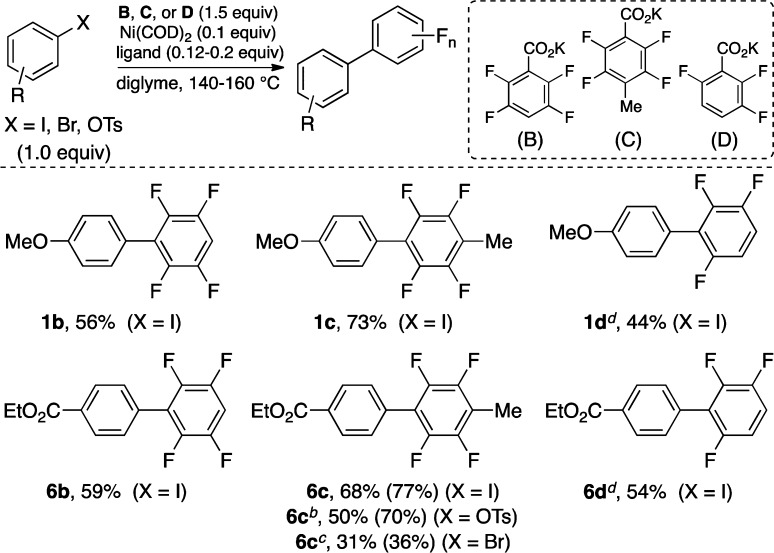
Having explored the preliminary scope for the coupling of aryl halides and tosylates with salt A, we next examined the reaction of aryl electrophiles with other perfluorobenzoates. As shown in Scheme 5, both tetrafluoro- and trifluorophenyl carboxylates couple with electron-rich and electron-deficient iodides to afford the desired products with comparable efficiencies (1b–d and 6b–d). However, the efficiency of the reactions using the trifluorobenzoate salt D is lower than that with tetrafluoro salts B and C. The reactions of the ester-substituted aryl bromides and tosylates with salt C were also explored and compared to the analogous reactions with A. The aryl bromide coupling using the pentafluoro salt A affords product 6a (63%) in higher yield than product 6c (31%) using tetrafluoro salt C (Schemes 3 and 5). However, when the p-tosylethylbenzoate is used, 6c (70%, Scheme 5) is obtained in higher yield than 6a (53%, Scheme 4), suggesting that the leaving group in the electrophile could impact the relative reactivity toward coupling with various carboxylates.1
Scheme 5. Scope of Perfluorobenzoates.
Conditions: Ni(COD)2 (0.1 equiv), PtBu3HBF4 (0.2 equiv), diglyme, 140 °C. Isolated yields are given with GC yields in parentheses (calibrated against hexadecane as the internal standard).
General conditions but with DPPF as ligand (0.125 equiv).
General conditions but with CM-Phos as ligand at 160 °C.
General conditions but at 160 °C.
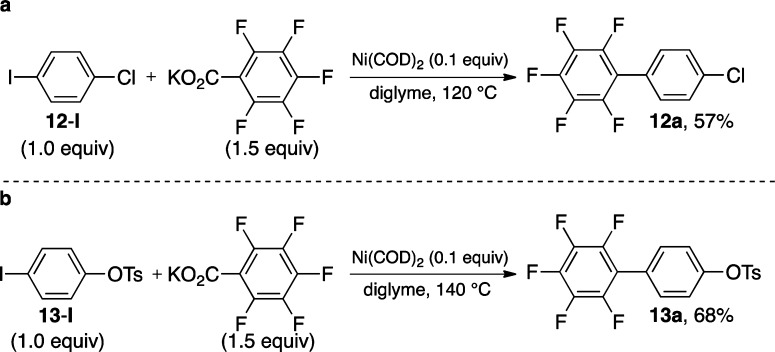
As illustrated in Table 1, aryl iodides participate effectively in these transformations in the absence of a ligand. The superior reactivity of iodides in the absence of ligands can be exploited strategically in sequential cross-couplings using substrates such as 12-I and 13-I (Scheme 6). For example, reaction of 12-I and 13-I with salt A affords products 12a and 13a, respectively, via cross-coupling at the C–I bond. The C–Cl and C–OTs bonds in 12a and 13a can be elaborated using myriad known transformations of aryl halides and tosylates for the synthesis of diverse biaryl motifs.2
Scheme 6. Cross-Couplings with 4-Iodochloride and 4-Iodotosylate.
In summary, the first example of Ni-catalyzed decarboxylative cross-coupling of aryl halides and pseudohalides with aromatic carboxylates is described. These studies set the stage for further explorations toward elucidating the scope of biaryl bond formation using Ni-catalyzed decarboxylative cross-coupling reactions.
Acknowledgments
The authors gratefully acknowledge St. Olaf College, National Institutes of Health (R15 GM107892), and the American Chemical Society Petroleum Research Fund (PRF#52224-UNI3) for funding this research.
Supporting Information Available
Experimental details and spectroscopic and analytical data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Gooßen L. J.; Rodríguez N.; Gooßen K. Angew. Chem., Int. Ed. 2008, 47, 3100–3120. [DOI] [PubMed] [Google Scholar]; b Weaver J. D.; Recio A.; Grenning A. J.; Tunge J. A. Chem. Rev. 2011, 111, 1846–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Rodríguez N.; Gooßen L. J. Chem. Soc. Rev. 2011, 40, 5030–5048. [DOI] [PubMed] [Google Scholar]; d Dzik W. I.; Lange P. P.; Gooßen L. J. Chem. Sci. 2012, 3, 2671–2678. [Google Scholar]; e Gooßen L. J.; Gooßen K. Top. Organomet. Chem. 2013, 44, 121–141. [Google Scholar]; f Park K.; Lee S. RSC Adv. 2013, 3, 14165–14182. [Google Scholar]
- a Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; de Meijere A., Diederich F., Eds.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]; b Seechurn C. C. C. J.; Kitching M. O.; Colacot T. J.; Snieckus V. Angew. Chem., Int. Ed. 2012, 51, 5062–5085. [DOI] [PubMed] [Google Scholar]
- For Pd-catalyzed decarboxylative biaryl bond formation, see:; a Hu P.; Zhang M.; Jie X.; Su W. Angew. Chem., Int. Ed. 2012, 51, 227–231. [DOI] [PubMed] [Google Scholar]; b Zhang F.-Z.; Greaney M. F. Angew. Chem., Int. Ed. 2010, 49, 2768–2771. [DOI] [PubMed] [Google Scholar]; c Xie K.; Yang Z.; Zhou X.; Li X.; Wang S.; Tan Z.; An X.; Guo C.-C. Org. Lett. 2010, 12, 1564–1567. [DOI] [PubMed] [Google Scholar]; d Wang C.; Piel I.; Glorius F. J. Am. Chem. Soc. 2009, 131, 4194–4195. [DOI] [PubMed] [Google Scholar]; e Cornella J.; Lu P.; Larrosa I. Org. Lett. 2009, 11, 5506–5509. [DOI] [PubMed] [Google Scholar]; f Voutchkova A.; Coplin A.; Leadbeater N. E.; Crabtree R. H. Chem. Commun. 2008, 6312–6314. [DOI] [PubMed] [Google Scholar]
- For Rh- and Ru-catalyzed reactions, see:; a Neely J. M.; Rovis T. J. Am. Chem. Soc. 2014, 136, 2735–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhang J.; Liu J.-F.; Ugrinov A.; Pillai A. F. X.; Sun Z.-M.; Zhao P. J. Am. Chem. Soc. 2013, 135, 17270–17273. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang J.; Liu B.; Zhao H.; Wang J. Organometallics 2012, 31, 8598–8607. [Google Scholar]; d Sun Z. M.; Zhang J.; Zhao P. Org. Lett. 2010, 12, 992–995. [DOI] [PubMed] [Google Scholar]; e Austeri M.; Linder D.; Lacour J. Adv. Synth. Catal. 2010, 352, 3339–3347. [Google Scholar]; f Austeri M.; Linder D.; Lacour J. Chem.—Eur. J. 2008, 14, 5737–5741. [DOI] [PubMed] [Google Scholar]; g Burger E. C.; Tunge J. A. Org. Lett. 2004, 6, 2603–2605. [DOI] [PubMed] [Google Scholar]
- a Nakamura E.; Hatakeyama T.; Ito S.; Ishizuka K.; Ilies L.; Nakamura M. Org. React. 2013, 83, 1–209. [Google Scholar]; b Yamaguchi J.; Muto K.; Itami K. Eur. J. Org. Chem. 2013, 1, 19–30. [Google Scholar]; c Tekale S. U.; Jadhav V. B.; Pagore V. P.; Kauthale S. S.; Gaikwad D. D.; Pawar R. P. Mini-Rev. Org. Chem. 2013, 10, 281–301. [Google Scholar]; d Cahiez G.; Moyeux A. Chem. Rev. 2010, 110, 1435–1462. [DOI] [PubMed] [Google Scholar]
- For Cu-catalyzed decarboxylative biaryl bond formation, see:; a Ponpandian T.; Muthusubramanian S. Tetrahedron Lett. 2012, 53, 4248–4252. [Google Scholar]; b Shang R.; Fu Y.; Wang Y.; Xu Q.; Yu H.-Z.; Liu L. Angew. Chem., Int. Ed. 2009, 48, 9350–9354. [DOI] [PubMed] [Google Scholar]
- For Fe-catalyzed decarboxylative cross-couplings, see:; a Rong G.; Liu D.; Lu L.; Yan H.; Zheng Y.; Chen J.; Mao J. Tetrahedron 2014, 70, 5033–5037. [Google Scholar]; b Zhao J.; Zhou W.; Han J.; Li G.; Pan Y. Tetrahedron Lett. 2013, 54, 6507–6510. [Google Scholar]; c Zhao J.; Fang H.; Han J.; Pan Y. Beilstein J. Org. Chem. 2013, 9, 1718–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Yang H.; Yan H.; Sun P.; Zhu Y.; Lu L.; Liu D.; Rong G.; Mao J. Green Chem. 2013, 15, 976–981. [Google Scholar]; e Bi H.-P.; Chen W.-W.; Liang Y.-M.; Li C.-J. Org. Lett. 2009, 11, 3246–3249. [DOI] [PubMed] [Google Scholar]
- For Ni-catalyzed decarboxylative cross-coupling for construction of bonds other than the biaryl bond, see:; a Yang K.; Zhang C.; Wang P.; Zhang Y.; Ge H. Chem.—Eur. J. 2014, 20, 7241–7244. [DOI] [PubMed] [Google Scholar]; b Wu Y.; Liu L.; Yan K.; Xu P.; Gao Y.; Zhao Y. J. Org. Chem. 2014, 79, 8118–8127. [DOI] [PubMed] [Google Scholar]; c Zuo Z.; Ahneman D. T.; Chu L.; Terrett J. A.; Doyle A. G.; MacMillan D. W. C. Science 2014, 345, 437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Choe J.; Yang J.; Park K.; Palani T.; Lee S. Tetrahedron Lett. 2012, 53, 6908–6912. [Google Scholar]
- For Ni-catalyzed decarbonylative and decarboxylative C–H arylation, see:; a Yang K.; Wang P.; Zhang C.; Kadi A. A.; Fun H. K.; Zhang Y.; Lu H. Eur. J. Org. Chem. 2014, 34, 7586–7589. [Google Scholar]; b Amaike K.; Muto K.; Yamaguchi J.; Itami K.; Zhang C.; Wang P.; Zhang Y.; Ge H. J. Am. Chem. Soc. 2012, 134, 13573–13576. [DOI] [PubMed] [Google Scholar]
- a Mesganaw T.; Garg N. K. Org. Process Res. Dev. 2013, 17, 29–39. [Google Scholar]; b So C. M.; Kwong F. Y. Chem. Soc. Rev. 2011, 40, 4963–4972. [DOI] [PubMed] [Google Scholar]; c Rosen B. M.; Quasdorf K. W.; Wilson D. A.; Zhang N.; Resmerita A.-M.; Garg N. K.; Percec V. Chem. Rev. 2011, 111, 1346–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Li B.-J.; Yu D.-G.; Sun C.-L.; Shi Z.-J. Chem.—Eur. J. 2011, 17, 1728–1759. [DOI] [PubMed] [Google Scholar]; e Kozhushkov S. I.; Potukuchi H. K.; Ackermann L. Catal. Sci. Technol. 2013, 3, 562–571. [Google Scholar]; f Yu D.-G.; Li B.-J.; Shi Z.-J. Acc. Chem. Res. 2010, 43, 1486–1495. [DOI] [PubMed] [Google Scholar]; g Antoft-Finch A.; Blackburn T.; Snieckus V. J. Am. Chem. Soc. 2009, 131, 17750–17752. [DOI] [PubMed] [Google Scholar]
- Fromm A.; van Wüllen C.; Hackenberger D.; Gooßen L. J. J. Am. Chem. Soc. 2014, 136, 10007–10023. [DOI] [PubMed] [Google Scholar]
- Shang R.; Xu Q.; Jiang Y.-Y.; Wang Y.; Liu L. Org. Lett. 2010, 12, 1000–1003. [DOI] [PubMed] [Google Scholar]
- a Okamoto T.; Nakahara K.; Saeki A.; Seki S.; Oh J. H.; Akkerman H. B.; Bao Z.; Matsuo Y. Chem. Mater. 2011, 23, 1646–1649. [Google Scholar]; b Amii H.; Uneyama K. Chem. Rev. 2009, 109, 2119–2183. [DOI] [PubMed] [Google Scholar]; c Babudri F.; Farinola G. M.; Naso F.; Ragni R. Chem. Commun. 2007, 1003. [DOI] [PubMed] [Google Scholar]
- NiBr2(DME) and/or NiCl2(PCy3)2 can be used instead of Ni(COD)2 for iodides, bromides, and tosylates. See Table 1 in Supporting Information for details.
- a Gooßen L. J.; Rodríguez N.; Linder C. J. Am. Chem. Soc. 2008, 130, 15248. [DOI] [PubMed] [Google Scholar]; b Gooßen L. J.; Rodríguez N.; Lange P. P. Angew. Chem., Int. Ed. 2010, 49, 1111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.