Abstract
Upregulation of regenerating gene 4 (Reg4) is observed in many human gastrointestinal malignancies including colorectal cancer (CRC). We previously reported a Reg4-mediated induction of epidermal growth factor receptor-Akt-AP1 signaling regulating CRC cell apoptosis. However, the role of Reg4 in the regulation of CRC cell division is poorly understood. This study tests the hypothesis that Reg4 induces Akt-GSK3β-β-Catenin-TCF-4 signaling to regulate CRC cell division. In vitro models of human CRC were used to determine the role of Reg4 in regulation of CRC cell division. Cell cycle studies demonstrated that Reg4 treatment significantly decreased CRC cell number in G1 phase and increased in G2 phase. Subsequently Reg4 significantly increased the mitotic index of CRC cells. As assessed by real-time RT-PCR and Western blot analyses, Reg4 significantly increased the expression of cell cycle regulatory genes Cyclin D1 and D3, and associated Cyclin-dependent kinases (CDK4 and CDK6). Reg4-mediated increase in these genes involved a pathway that included an induced Akt activity by increasing phosphorylation of Thr308 and Ser473, a reduced glycogen synthase kinase 3β(GSK-3β) activity by increasing phosphorylation of Ser9, an induced nuclear translocation of β-Catenin by decreasing phosphorylation of Ser33/37/Thr41, and an increased TCF-4 transcriptional activity. Furthermore, antagonism of Reg4-signaling using Reg4-specific mAbs (2H6 and 3E5) and Akt inhibitor significantly decreased, whereas agonism using GSK-3β antagonist (SB216763) significantly increased mitotic index and proliferation of CRC cells. These results identify Reg4 as a key regulator of the CRC cell division and proliferation, hence a potential target of human CRC treatment.
Keywords: gastrointestinal, cyclin, cyclin-dependent kinase, mitotic index, proliferation
INTRODUCTION
Colorectal cancer (CRC) is a commonly diagnosed cancer in both men and women, and is one of the leading causes of cancer-related deaths in the western world [1]. In spite of substantial progress in treatment over the last decade, many new cases of CRC and a large number of CRC-related deaths occur each year [2]. If diagnosed and treated early it is potentially curable. However, novel CRC markers for early diagnosis and targets for successful treatments are still lacking. A better understanding of gene regulation and signal transduction pathways involved in colorectal carcinogenesis may be useful to identify new therapeutic targets for improved treatment outcomes in human CRC.
Regenerating (Reg) gene family belonging to calcium-dependent lectin (C-type lectin) superfamily is involved in differentiation and proliferation of the hepatic, pancreatic, gastric, and intestinal cells. Identified members of the Reg multigene family in human include Reg1α, Reg1β, Reg3α, Reg3β and regenerating gene 4 (Reg4) [3–6]. Increasing evidence suggest that Reg genes are important regulators of GI carcinogenesis. Among these human Reg genes, the upregulation of Reg4 expression was frequently observed in many GI malignancies including gastric [7], pancreatic [8] and CRC [9,10]. In addition, we also reported an induction of Reg4 expression in human CRC samples in comparison to adjacent normal mucosa [11]. An increase in Reg4 expression was also observed in human colon cancer cell lines selected for in vitro resistance to the cancer chemotherapeutic agent 5-FU [10]. Furthermore, higher levels of Reg4 expression and Reg4-mediated genes were found to be associated with an increased resistance to apoptotic death of human CRC cells [11]. These studies suggested an association of Reg4 with poor patient outcomes in human CRC.
Defective Wnt signaling has been associated with human CRC by regulating expression of genes involved in cell division cycle [12,13]. Activation of the Wnt signaling pathway leads to aberrant accumulation of β-Catenin in the nucleus and increased T cell factor (TCF)/LEF transcriptional activities. Without Wnt stimulation, β-Catenin is constantly degraded by the proteasome [14,15]. This degradation strictly depends upon β-Catenin phosphorylation, which occurs in a multiprotein complex composed of tumor suppressor protein adenomatous polyposis coli (APC), Axin, and glycogen synthase kinase 3β (GSK-3β) [14,16,17]. We previously reported an increase in Reg4 expression following second spontaneous mutation in APC gene of APCmin/+ mice, which then developed multiple polyps in the intestine [11]. However, an association between Reg4 and Wnt/APC/β-Catenin signaling has not been established yet. Present study identifies Reg4 as a potent regulator of mitotic division of human CRC cells and its association with Reg4-mediated increase in Akt-GSK-3β-β-Catenin-TCF-4 signaling. Reg4-mediated increase in previously reported Akt activity [18] led to an increased phosphorylation of Ser9 associated with an inactive form of GSK-3β, and an increased nuclear translocation of β-Catenin by decreasing its phosphorylation at Ser33/37/Thr41. Furthermore, Reg4-mediated increase in nuclear β-Catenin induced TCF-4 transcriptional activities to increase expression of cell cycle regulatory genes Cyclin D1 and D3 and associated Cyclin-dependent kinases (CDK4 and CDK6). Along with our previous findings of Reg4-mediated increases in anti-apoptotic genes Bcl-2, Bcl-xL, and Survivin [11,19,20], results of the present study identify Reg4 as an important regulator of CRC growth and a potential target for adjunctive treatments of human CRC.
MATERIALS AND METHODS
Cell Lines and Culture
The human colon adenocarcinoma cell lines HCT116, SW480 and HT29 (American Type Culture Collection, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium (DMEM; Cambrex, Walkersville, MD) containing 10% heat inactivated fetal bovine serum (FBS; HyClone, Logan, UT). Human fetal kidney cell line, 293 (American Type Culture Collection) was cultured in a DMEM adjusted to 2 mM L-glutamine, 1.5 g/L sodium biocarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate and 10% FBS.
Cell Cycle Study
Fluorometric assays were performed to determine the cell population in different phases of cell cycle. The method involved dissolving the cell membrane lipids with the detergent Igepal CA-630, eliminating the cell skeleton and nuclear protein with trypsin, digesting the RNA with RNase and staining the DNA of isolated nuclei with propium iodide (PI). The fluorescence signal from the PI bound to the cell was proportional to its DNA content, and was used to determine the cell population in G1, S and G2/M phases of cell cycle.
Mitotic Assay
The phosphorylation of histone H3 is a useful marker for mitosis [21]. Using a phospho histone H3 (Ser28) monoclonal antibody (mAb), a colorimetric mitotic assay was performed to determine the number of cells undergoing mitosis (Active Motif, Carlsbad, CA). The mitotic index representing the proportion of cells undergoing mitosis within a specified cell population was determined by estimating OD at 450 nm of color developed by cells positive for phospho-histone H3.
Cell Proliferation Assay
CRC cell proliferation was determined by two assays: (a) MTT assay, and (b) 5-ethynyl-2′-deoxyuridine (EdU) flow cytometry assay. The tetrazolium salt MTT [(3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide)] is reduced in metabolically active cells. By using MTT assay kit (ATCC, Manassas, VA), a colorimetric reduction in tetrazolium salts was used to represent the change in number of proliferation cells. In a different assay set, the rate of CRC cell proliferation was determined using click-iT EdU flow cytometry assay kit (Invitrogen/Molecular Probes, Eugene, OR). EdU, a nucleoside analog to thymidine is incorporated into DNA during active DNA synthesis. EdU in newly synthesized DNA was detected using Alexa Fluor 488 dye. Using BD Accuri C6 flow cytometer, the percentage of Alexa Flour-positive cells in S-phase cell population was analyzed by BD Accuri Cflow sampler (BD Biosciences, San Jose, CA).
Stable Transfection of 293 Cells With Reg4 DNA Sequences
Total RNA isolated from a human CRC sample was reverse transcribed using random hexanucleotide primers (Invitrogen, Carlsbad, CA). Complementary DNA was then used for polymerase chain reaction (PCR) to amplify a DNA sequence encoding Reg4 mature peptide. Specific primers (Forward: 5′-GCGGATCCATGGATAT-CATCATGAGACCC-3′; Reverse: 5′-GCGGATCCCCACCATGGGATATCATCATGAGA-3′) and Taq DNA polymerase (Invitrogen) were used for PCR reaction. The verified PCR product was cloned in the pcDNA 3.1(+) vector, and then used for stable transfection of 293 human fetal kidney cells using FuGENE 6 transfection reagent (Roche, India-polish, IN) and Geneticin (Gibco, Grand Island, NY) in selection media. Human fetal kidney cells 293 were chosen for stable transfection, because non-transfected cells exhibited undetectable level of Reg4 expression.
Western Blot Analysis
Equal amount of cell lysate from different treatment groups was subjected to SDS–PAGE electrophoresis and transferred to PVDF membrane using iBlot gel transfer stacks (Invitrogen). Membranes were incubated with specific primary antibodies including aReg4 4261 [18], TCF-4 (Upstate, Temecula, CA), β-Catenin, Cyclin D1, Cyclin D3, Cyclin E1, CDK2, CDK4, CDK6, GSK-3β (all from Cell Signaling Technology, Danvers, MA), β-Actin, HSP-27 (Santa Cruz Biotech, Santa Cruz, CA) and protein expression was analyzed by detecting specific bands using enhanced chemiluminescence system (GE Healthcare, Buckinghmashire, UK). Following densitometric scanning, net intensity of individual protein bands was normalized either to corresponding housekeeping protein (β-Actin or HSP-27) or total protein bands (for phosphorylated proteins), and values were shown below each band.
Luciferase Reporter Assay
HCT116, SW480, HT29, and 293 cells were transfected with plasmids containing wild type TCF binding sites (TOPflash) and mutated copy of TCF binding sites (FOPflash; Upstate). These plasmids encode the firefly luciferase gene under the control of Thymidine kinase (TK) minimal promoter. Relative luciferase activities in lysates were determined using the Dual-Lucifease Reporter Assay System (Promega Corporation, Madison, WI). The relative luciferase activities in different colon adenocarcinoma cells were estimated as the TOP/FOP ratio.
Real-Time RT-PCR
Total RNA isolated from HCT116, SW480, and HT29 cells using Trizol was reverse transcribed using random hexanucleotide primers (Invitrogen). cDNAs were then used for real-time RT-PCR using Jumpstart Taq DNA polymerase (Sigma, St. Louis, MO) and SYBR Green nucleic acid stain (Molecular Probes). Crossing threshold values for individual genes were normalized to β2-microglobulin. Changes in mRNA expression were expressed as fold change relative to control. Primers used in this study include: β-Actin: 5′-ATCATTGCTCCTGAGCG-3′ and 5′-GCTGATCCACATCTGGAA-3′, Cyclin D1: 5′-TGTTCGTGGCCTCTAAGATG-AA-3′ and 5′-TCGGTGTAGATGCACAGCTTCT-3′, Cyclin D3: 5′-CCGACAGGCCTTGGTCAA-3′ and 5′-GGCAAAGGTATAATCTGTAGCACAGA-3′, Cyclin E1: 5′-AAAGAAG-ATGATGACC-GGGTTTAC-3′ and 5′-GAGCCT-CTGGATGGTGC-AAT-3′.
Statistical Analysis
All values were expressed as the mean ± SEM. Data were analyzed using a two-tailed t test. A P value of <0.05 was considered to indicate statistical significance.
RESULTS
Reg4 Accelerates G1/S-G2/M Phase Transition and Increases Mitotic Index of Human CRC Cells
Cell cycle machinery controls cell proliferation, and cancer is a disease of inappropriate cell proliferation. We previously reported an association of Reg4 expression with increased proliferation of human CRC cells [20]. In this study, we further determined the role of Reg4 in cell cycle regulation. Following an addition of rhR4 (recombinant human Reg4 protein; 200 nM; 24 h) to HCT116 cells, we performed propidium iodide-based flow cytometric analyses to estimate the number of cells in G1, S, and G2/M phases of cell cycle. Reg4 treatments accelerated the cell transition from G1/S to G2/M phase as indicated by a decreased cell number in G1 phase and an increased cell number in G2/M phase (Figure 1: upper and lower left panels). In addition, to determine the number of cells undergoing mitotic division, we performed colorimetric mitotic assays and observed that Reg4 treatments led to a significant increase in mitotic cell population as indicated by an increased mitotic index. These results suggest that Reg4 accelerates transition of CRC cells from G1/S to G2/M phase of cell cycle and induces their division.
Figure 1.
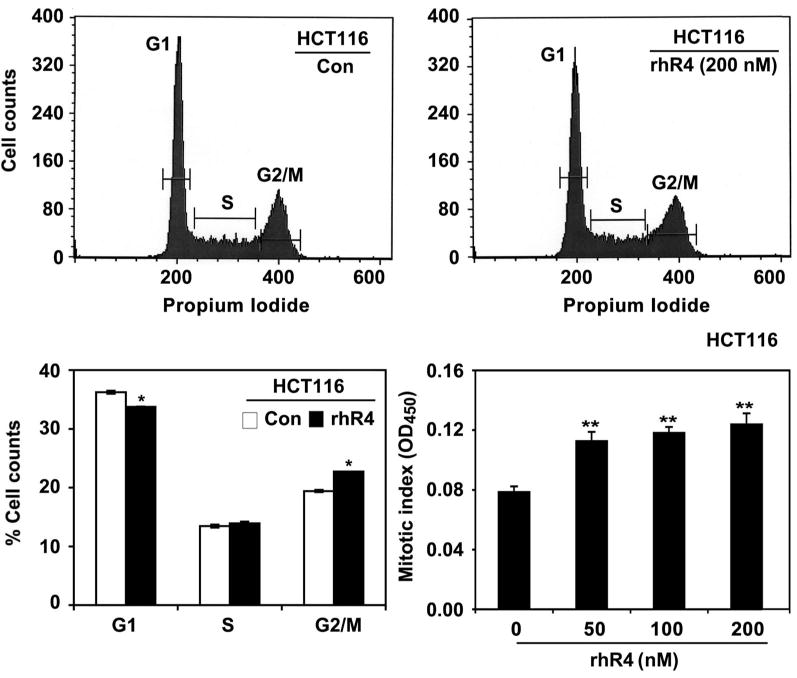
Reg4 accelerates G1/S-G2/M phase transition and increases mitotic index of human CRC cells. Propidium iodide-based flow cytometric analyses were performed to determine the effect of Reg4 on CRC cell population in different phases of cell cycle. Addition of rhR4 (200 nM; 24 h) to HCT116 cells’ media significantly decreased cell number in G1 phase and increased in G2/M phase (upper panels). Lower left panel shows the quantitative analysis of the above-mentioned study. Following colorimetric mitotic assays, rhR4 (200 nM; 24 h) treatment significantly increased the number of HCT116 cells undergoing mitosis as shown by an increased mitotic index (lower right panel); *P < 0.05, **P < 0.01.
Reg4 Increases Expression of β-Catenin and TCF-4 Transcription Factors in Human CRC Cells
Mutations of APC and β-Catenin have previously been implicated in an induced colorectal tumorigenesis [12,13,22]. We previously reported an increase in Reg4 expression following second spontaneous mutation in APC gene of APCmin/+ mice, which then developed multiple polyps in the intestine [11]. However, an association between Reg4 and Wnt/APC/β-Catenin signaling has not been established yet. In the present study, we observed a parallel expression of β-Catenin and Reg4 in different regions of the murine GI tract. Comparatively higher levels of Reg4 expression were observed in the cecum and colon, which corresponded to higher expression of β-Catenin in similar regions, whereas the lower level of Reg4 expression in duodenum paralleled with lower level of β-Catenin expression (Figure 2A). The similarity between Reg4 and β-Catenin expression was also observed in other segments of GI tract (data not shown). This potential relationship between Reg4 and β-Catenin expression was also examined in different human CRC cells. Comparatively higher levels of Reg4 expression in SW480 and HT29 cells were associated with increased protein levels of β-Catenin (total) compared to lower levels of the same proteins in HCT116 cells (Figure 2B). The increased expression of Reg4 in these cell lines was also correlated with the increased expression of TCF-4 transcription factor (Figure 2B). To verify these associations, we stably transfected a human embryonic kidney cell line 293 (that shows a non-detectable level of Reg4 expression) with clones of Reg4 sequence (293-R4). In comparison to 293 cells, 293-R4 cells showed increased protein levels of β-Catenin (total) and TCF-4 transcription factor (Figure 2C). In addition, we transfected HCT116, SW480, HT29 and 293 cells with plasmids containing wild type TCF binding sites (TOPflash) and mutated copy of TCF binding sites (FOPflash), and relative luciferase activity was estimated as the TOP/FOP ratio. The comparative levels of Reg4 expression in these cell lines were correlated with the TOP/FOP ratio suggesting a direct association between Reg4 and TCF-4 transcriptional activities (Figure 2D). These results suggest that Reg4 is an important regulator of β-Catenin and TCF-4 transcriptional activity in human CRC cells.
Figure 2.
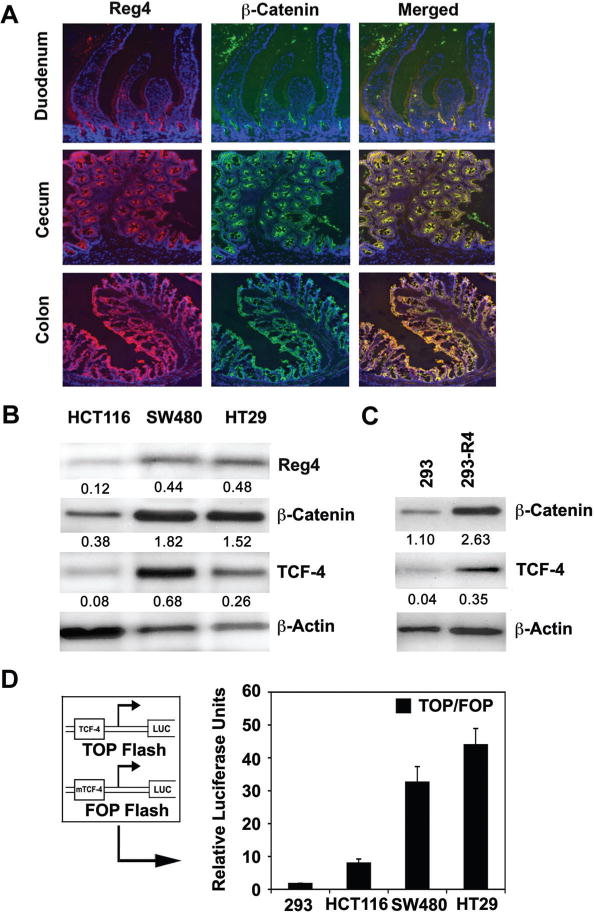
Reg4 expression in murine GI tract and various CRC cell lines parallels with that of β-Catenin and TCF-4 transcription factor. (A) Immunohistochemical staining of tissues from different regions of murine (BALB/c) GI tract exhibited a parallel between Reg4 and β-Catenin expression. Comparatively higher levels of Reg4 in cecum and colon corresponded to higher levels of β-Catenin in similar regions, whereas the lower level of Reg4 in duodenum paralleled with lower β-Catenin expression. (B) Following Western blot analyses similar parallels between Reg4 and β-Catenin expression were also observed in different human CRC cells. Compared to HCT116 cells, SW480 and HT29 cells showed higher levels of Reg4 and β-Catenin protein and were associated with increased protein expression of TCF-4 transcription factor. (C) Compared to non-transfected 293 cells, 293-R4 cells (293 cells stably transfected with Reg4 clones) showed increased protein levels of β-Catenin and TCF-4 transcription factor. (D) Compared to HCT116 cells, higher levels of Reg4 in SW480 and HT29 cells were also associated with increased TCF-4 activity assessed by TOP/FOP ratio using luciferase reporter assay system. HCT116 cells showing comparatively lower expression of Reg4 exhibited lower TOP/FOP ratio. TOP/FOP ratio in 293 human embryonic kidney cells with non-detectable level of Reg4 protein served as negative control.
Reg4 Inhibits GSK-3β Activity and Induces β-Catenin Nuclear Translocation in Human CRC Cells
β-Catenin is a multifunctional protein that plays an essential role in the transduction of Wnt signals [16,23,24]. In the absence of Wnt signaling, cytosolic β-Catenin is phosphorylated by a protein complex composed of APC, Axin, and GSK-3β that leads to a rapid ubiquitination and proteasomal degradation of β-Catenin [14–16]. However, following induction of Wnt signaling GSK-3β activity is inhibited resulting in reduced phosphorylation of β-Catenin at Ser33/37/Thr41 residues leading to its subsequent release from its destruction protein complex and its further transportation to the nucleus, where it associates with DNA-binding factors to activate target genes involved in the regulation of cell division [25–27]. We previously reported a Reg4-mediated increase in Akt kinase activity in different human CRC cells [20]. In addition, multiple studies have shown Akt-mediated induction of an inactive form of GSK-3β by phosphorylating its Ser9 residues [28]. This constitutes an important event to regulate β-Catenin phosphorylation and its subsequent translocation to the nucleus. Therefore, to establish an association between Reg4-mediated increase in Akt kinase activity and GSK-3β phosphorylation, we added rhR4 (200 nM) to different human CRC cell cultures for 0, 2, 5, 10, 20, 30 and 60 min. We observed an increased phosphorylation of Ser9 of GSK-3β following rhR4 addition in multiple human CRC cell lines including HCT116 (Figure 3A, left panel) and HT29 (Figure 3A, right panel). However, no changes were noted for phosphorylation of Tyr216 of GSK-3β. Since, phosphorylation of Ser9 was associated with an inactive form of GSK-3β, results demonstrated a Reg4-mediated inhibition of GSK-3β activity.
Figure 3.
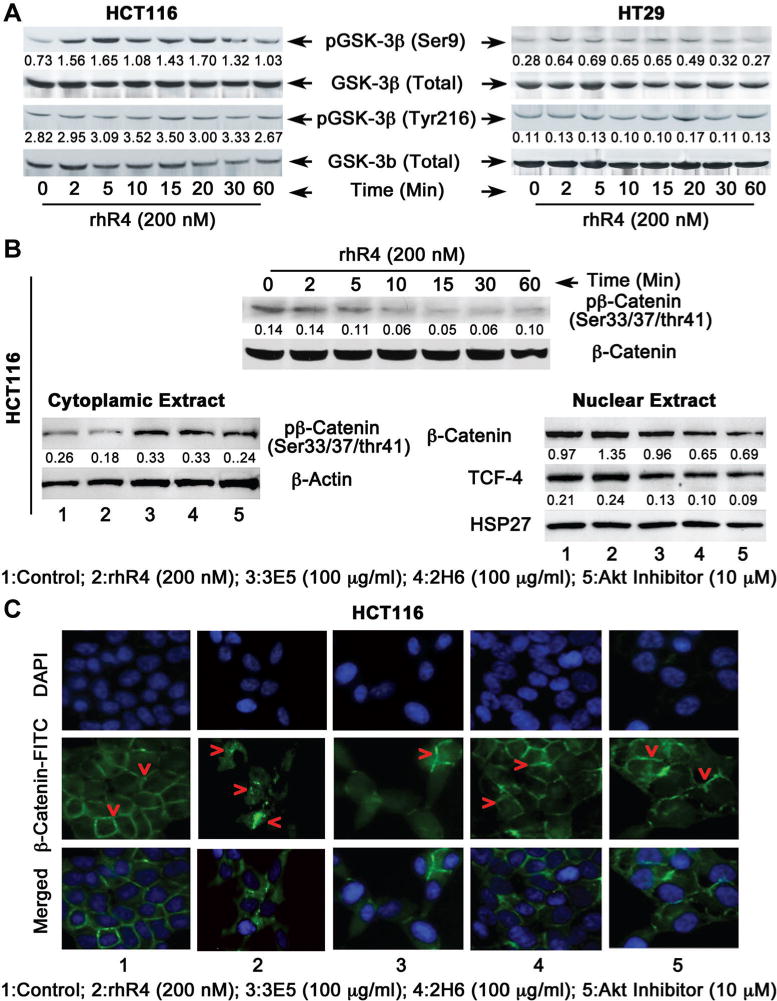
Reg4 inhibits GSK-3β activity and increases β-Catenin nuclear translocation in human CRC cells. (A) Following Western blot analyses addition of rhR4 (200 nM) to HCT116 (left panel) and HT29 (right panel) cells resulted in a time-dependent increase in phosphorylation of Ser9 associated with an inactive form of GSK-3β. However, no changes were observed in phosphorylation of Tyr216 associated with an active form of GSK-3β hence served as a negative control. (B) To determine effects of Reg4 on nuclear translocation of β-catenin, cytoplasmic and nuclear protein fractions were separated from HCT116 cells treated with rhR4 (200 nM), Reg4-specific functional blocking mAbs (3E5 and 2H6: 100 μg/mL) and specific Akt inhibitor (10 μM) for 1 h. Following Western blot analysis (left and right panels), Reg4 treatment led to a decreased expression of phospho-β-catenin in cytoplasmic fraction, however, treatments of 2H6, 3E5, and Akt inhibitor resulted in increased expression of same protein. In nuclear fraction, Reg4 treatments resulted in increased protein levels of β-catenin (total) and TCF-4 transcription factor, however, treatments of 2H6, 3E5 and Akt inhibitor resulted in a decreased levels of these proteins. (C) Following immunocytochemical staining of HCT116 cells, an addition of rhR4 (200 nM; 1 h) led to increased translocation of β-Catenin (shown by red arrows) from peripheral/cytoplasmic region to central nuclear region of the cell. Functional blocking of endogenous Reg4 protein using specific mAbs (2H6 and 3E5) and Akt inhibitor for 1 h resulted in a higher level of β-catenin expression in peripheral/cytoplasmic region of HCT116 cells.
We further determined the effect of antagonism of Reg4-signaling using Reg4-specific mAbs (3E5 and 2H6) [18] and specific Akt inhibitor (1L6-Hydoxymethyl-chro-inositol-2®-methyl-3-O-octadecylsn-glycerocarbonate, IC50 = 5.0 μM; EMD/Calbiochem, Rockland, MA) on nuclear translocation of β-Catenin. Since phosphorylation of β-Catenin at Ser33/37/Thr41 residues is an important event to determine the fate of β-Catenin in destruction protein complex, we separated cytoplasmic and nuclear protein fractions of HCT116 cells treated with rhR4 (200 nM), 3E5 (100 μg/mL), 2H6 (100 μg/mL) and Akt Inhibitor (10 μM) for 1 h. Following Western blot analyses and densitometric scanning of corresponding protein bands, we observed a decreased phosphoryation of β-Catenin at Ser33/37/Thr41 residues following rhR4 treatment in cytoplasmic protein fraction of cells (Figure 3B, left panel). Treatments of Reg4-specific mAbs (2H6 and 3E5) and specific Akt inhibitor resulted in reverse effects leading to increased phosphorylation of β-Catenin. In nuclear protein fraction of HCT116 cells, rhR4 treatments resulted in increased expression of β-Catenin (total) and TCF-4 proteins, whereas treatments of 3E5, 2H6, and Akt inhibitor led to decreased expression of β-Catenin and TCF-4 proteins (Figure 3B, right panel). Similar results were also noted in other human CRC cells including SW480 and HT29 (data not shown). In order to verify results, we performed immunohistochemical staining of HCT116 from above-mentioned treatment groups using β-Catenin specific antibody (Figure 3C). In control group, most of the β-Catenin staining was observed in peripheral/cytoplasmic region of cells. However, addition of rhR4 (200 nM; 1 h) increased β-Catenin staining towards the nuclear region of cells (Figure 3C). Functional blocking of Reg4 protein using specific mAbs (3E5 and 2H6: 100 μg/mL; 1 h) reversed the effect and higher amount of β-Catenin was observed in peripheral/cytoplasmic regions of the cell. Reduced nuclear localization of β-Catenin was also noted when cells were treated with specific Akt inhibitor (10 μM) for 1 h. These results identify Reg4 as an important regulator of Akt and GSK-3β activity as well as phosphorylation and nuclear translocation of β-Catenin in colorectal tumorigenesis. Results further suggest that Reg4-Akt-GSK-3β-mediated nuclear translocation of β-Catenin induces TCF-4 transcriptional activities, and establishes a crucial event to regulate the expression of genes associated with human CRC cell division.
Reg4 Induces Expression of Cell Cycle Regulatory Genes Cyclin D1 and D3 and Associated Kinases CDK4 and CDK6
Cyclins and CDK are important component of the cell cycle machinery. Activities of the D-type Cyclins (Cyclin D1 and D3) and associated kinases (CDK4 and CDK6) regulate cell cycle progression in mid-late G1 phase [29]. D-Type Cyclins are positive regulators of cell cycle [30]. D-Type Cyclins play a prominent role in differentiation and proliferation of cells and are highly upregulated in various cancers [31]. Furthermore, Cyclin D1 is a known target of Wnt/β-Catenin signaling [22], GSK-3β [32], and TCF-4/LEF transcriptional activities [22]. Since, we observed a Reg4-mediated inactivation of GSK-3β and an increased nuclear translocation of β-Catenin followed by increased TCF-4/LEF transcriptional activities, we next examined the expression of D-type Cyclins as potential downstream target genes of Reg4-Akt-GSK-3β–β-Catenin-TCF-4 signaling pathway. Following real-time RT-PCR and Western blot analyses, we observed a time-dependent increase in Cyclin D1 and D3 expression following the addition of rhR4 (200 nM) to HCT116 and HT29 human CRC cells (Figure 4A and B). Furthermore, addition of rhR4 to the cell cultures also led to an increased expression of CDK4 and CDK6, the known kinase partners of D-type Cyclins. These results suggest that Reg4 is an important regulator of the D-type Cyclins and their kinase partners hence regulate the mitotic division of human CRC cells.
Figure 4.
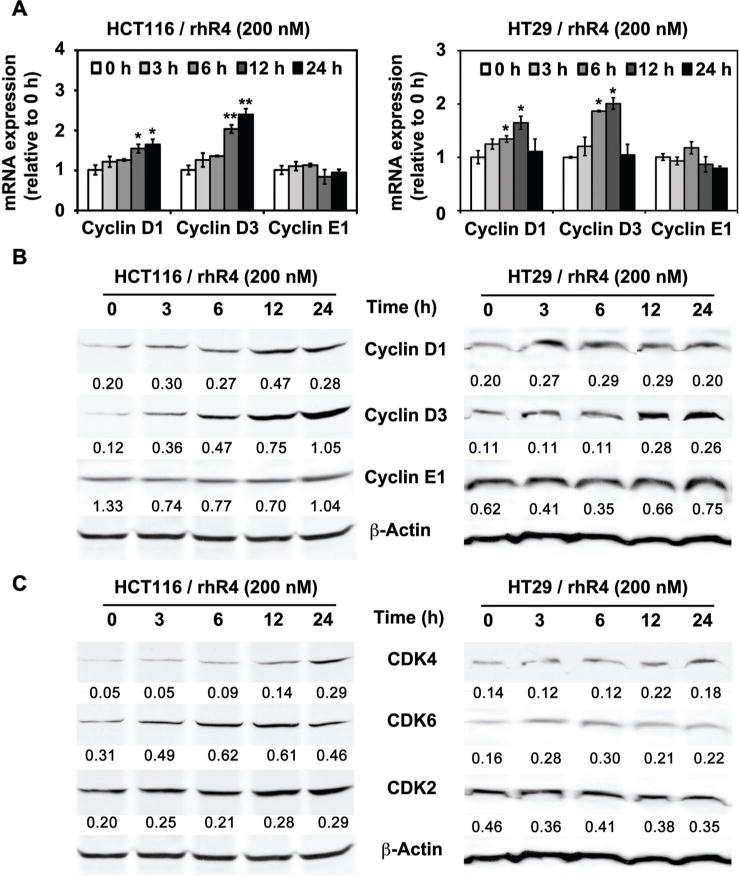
Reg4 induces expression of Cyclin D1, Cyclin D3, CDK4, and CDK6 in human CRC cells. An addition of rhR4 (200 nM) to HCT116 and HT29 cells led to a time-dependent increase in mRNA (panel A) and protein (panel B) expression of Cyclin D1 and Cyclin D3. No change in Cyclin E1 mRNA and protein expression shown in corresponding panels is used as a negative control. In addition, Reg4 treatments led to time-dependent increase in protein expression of CDK4 and 6 (panel C). No change in CDK2 protein expression shown in this panel is used as a negative control; *P < 0.05, **P < 0.01.
Blocking of Reg4-Signaling Reduces the Expression of D-Type Cyclins and the Mitotic Index of Human CRC Cells
Using mice model of CRC xenografts, we previously showed an association of antagonism of Reg4-signaling with a decreased CRC growth, increased animal survival and increased CRC cell susceptibility to IR-induced apoptosis [19]. In this study, we observed an involvement of Akt kinase, GSK-3β, and β-Catenin activities in mediating Reg4-signaling. The Reg4-mediated increase in Akt kinase activity was associated with decreased phosphorylation of GSK-3β resulting in a subsequent increase in nuclear translocation of β-Catenin. Therefore, we next utilized a specific Akt inhibitor as an antagonist and SB216763 [3-(2,4-Dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione, IC50 = 34 nM; Biomol Research Lab, Playmouth Meeting, PA], an inhibitor of GSK-3β as an agonist of Reg4-signaling and examined their effects on expression of D-type Cyclins and mitotic index of human CRC cells. Treatments of specific Akt inhibitor (10 μM; 24 h) to the human CRC cells significantly inhibited the mRNA and protein expression of Cyclin D1 and D3, and subsequently inhibited their mitotic index (Figure 5A–C). Furthermore, an addition of rhR4 (200 nM; 24 h) to human CRC cells pretreated 1 h before with specific Akt inhibitor (10 μM) did not increase the expression of these Cyclins, hence blocked the Reg4-mediated increase in mitotic index of human CRC cells (Figure 5A–C). In addition, the treatments of SB216763 (10 μM; 24 h) positively modulated the Reg4-signaling and led to a significant increase in the expression of D-type Cyclins, hence mitotic index of human CRC cells (Figure 5A–C). These results suggest that Reg4 is a potent regulator of mitotic division of human CRC cells and involves Akt kinase and GSK-3β as important mediators of its signaling.
Figure 5.
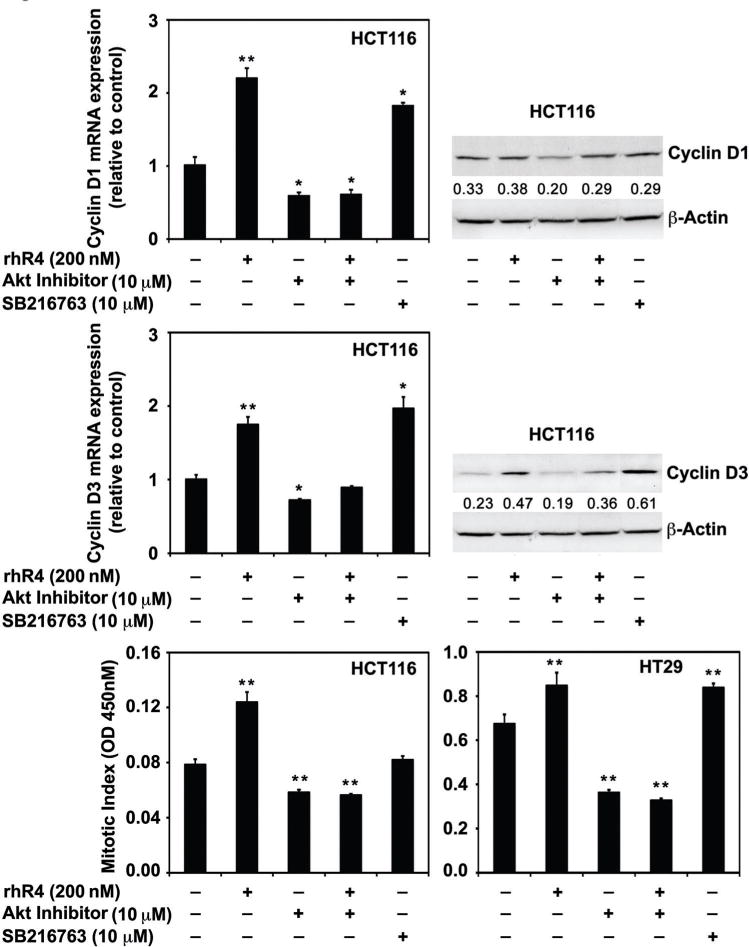
Alteration in Reg4-signaling alters the expression of Cyclin D1 and D3, and the mitotic index of human CRC cells. We used a specific Akt inhibitor as an antagonist and SB216763, an inhibitor of GSK-3β as an agonist of Reg4-signaling. Treatments of specific Akt inhibitor (10 μM; 24 h) to the human CRC cells significantly inhibited the expression of Cyclin D1 and D3, and subsequently inhibited their mitotic index (upper, middle and lower panels). The addition of rhR4 (200 nM; 24 h) to human CRC cells pretreated 1 h before with specific Akt inhibitor (10 μM) did not increase the expression of these Cyclins hence the mitotic index of human CRC cells. In addition, the treatment of SB216763 (10 μM; 24 h) increased the expression of D-type Cyclins, hence mitotic index of human CRC cells; *P < 0.05, **P < 0.01.
Antagonism of Reg4-Signaling Inhibits Human CRC Cell Proliferation
We previously showed that Reg4 induces proliferation of human CRC cells [20]. In the present study, we observed an association of Reg4 with regulation of CRC cell division using a signaling pathway that included Akt kinase and GSK-3β activity. Therefore, we next tested the effects of specific inhibitors of Akt (as an antagonist) and GSK-3β SB216763; as an agonist) on proliferation of human CRC cells using MTT and EdU flow cytometry assays. We also used function blocking mAbs (3E5 and 2H6) specific to Reg4 protein as an inhibitor of Reg4-signaling to determine the role of Reg4 in human CRC cell proliferation. Addition of rhR4 (200 nM; 24 h) to CRC cell cultures induced the rate of cell proliferation (HT29: Figure 6A and B and HCT116: Supplementary Figure 1A and B). Antagonism of Reg4-signaling using Akt inhibitor (10 μM; 24 h) and Reg4-specific mAbs (3E5 and 2H6: 100 μg/mL; 24 h) led to significant decreases in proliferation rate of HT29 (Figure 6A and B) and HCT116 (Supplementary Figure 1A and B) cells. Treatments with SB216763 (10 μM; 24 h) positively increased the CRC cell proliferation rate. However, the addition of rhR4 to the cells pretreated 1 h before with Akt inhibitor (10 μM) could not show the positive effects of Reg4 treatments (HT29: Figure 6A and B and HCT116: Supplementary Figure 1A and B). Taken together, results of this study identify Reg4 as a potent regulator of human CRC cell proliferation via Akt-GSK-3β–β-Catenin-TCF-4 signaling pathway.
Figure 6.
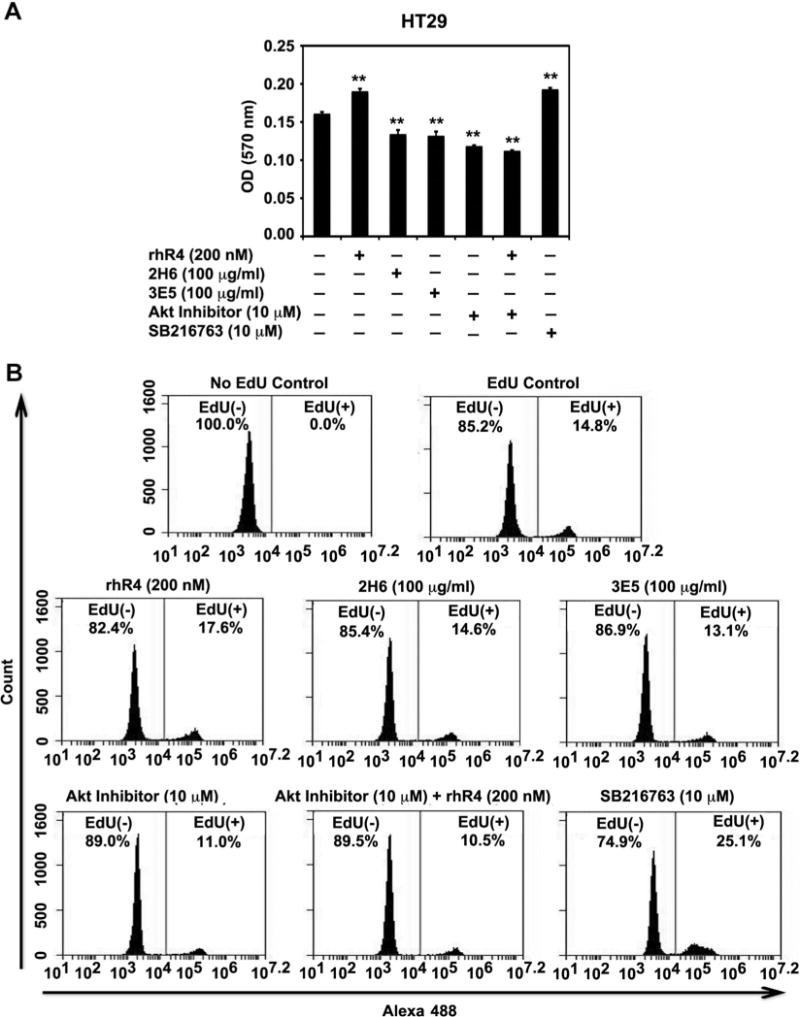
Antagonism of Reg4-signaling inhibits human CRC cell proliferation. Reg4-specific function blocking mAbs (3E5 and 2H6) and Akt inhibitor were used to antagonize Reg4 signaling and tested their effects on human CRC cell proliferation by performing MTT (A) and EdU flow cytometry (B) assays. Treatments of Reg4-specific mAbs (3E5 and 2H6: 100 μg/mL; 24 h) and Akt inhibitor (10 μM; 24 h) led to significant decreases in proliferation rate of HT29 cells. Treatments with rhR4 (200 nM; 24 h) and SB216763 (10 μM; 24 h) positively increased the proliferation rate. However, the addition of rhR4 to HT29 cells pretreated 1 h before with Akt inhibitor (10 μM) did not increase CRC cell proliferation. Results identify Reg4 as a potent regulator of human CRC cell proliferation; *P < 0.05, **P < 0.01.
DISCUSSION
The growth and development of cancer depends on the cancer cells ability to escape the normal controls and check points of cell division cycle. Changes in the components of cell division cycle and apoptotic machineries, or in the signaling pathways that control them allow cancer cells to escape the normal control of cell proliferation and cell death. Understanding and exploiting complexities of cancer cell proliferation and apoptosis holds promise for more effective therapies in the future. Paradoxically, the altered cellular networks of molecular pathways that sustain cancer cell growth and make them resistant to certain therapies may offer new targets for therapy.
Defective Wnt signaling and mutations of APC and β-Catenin have been implicated in human CRC by regulating expression of genes involved in cell division cycle [22]. However, an association of Reg4, a highly expressed gene in human CRC with Wnt/APC/β-Catenin signaling has not been established. This study is first of its kind to show an induced nuclear translocation of β-Catenin in response to a molecule other than Wnt ligands or mutations in APC and β-Catenin. In the present study, we observed that the increased levels of Reg4 in human CRC cells were associated with a decreased phosphorylation of β-Catenin and its subsequent translocation to the nucleus. These Reg4-mediated events were further correlated with increased TCF-4 transcriptional activities including an increased expression of cell cycle regulatory genes Cyclin D1 and D3 and their associated kinases including CDK4 and CDK6.
The division of mammalian cells is regulated at specific points in the cell cycle, particularly at the G1 to S and G2 to M transitions. The proteins encoded by the D-type Cyclins form a complex and functions as a regulatory subunit of CDK4 or CDK6, whose activities are required for cell cycle G1/S transition. Mutations, amplification and overexpression of these genes, which alters cell cycle progression, are observed frequently in a variety of tumors and may contribute to carcinogenesis. Cyclin protein levels oscillate throughout the cell cycle, and their availability is a means of controlling CDK activity and cell proliferation. Cyclin D is degraded through the ubiquitin proteasome pathway in the absence of mitogenic signaling. Ubiquitination of Cyclin D1 is enhanced by phosphorylation at Thr286 by GSK-3β [33]. In this study, we observed Reg4-mediated increases in expression of D-type Cyclins including Cyclin D1 and D3 along with their CDK partners CDK4 and CDK6. Reg4-mediated increase in Cyclin D1 expression indicated a possible involvement of Wnt signaling in Reg4-mediated pathways, however a direct interaction of Reg4 with Wnt ligands or frizzled receptor was not observed. Results suggest that Reg4 activity in human CRC is involved in regulation Cyclin D1 expression, but is independent of a signaling pathway induced by the interaction of Wnt ligands with Frizzled receptor. In addition, Reg4-mediated increase in Cyclin D3 expression raised the possibility of “Notch” signaling involvement in Reg4-mediated pathway, however it needs further exploration to establish an interaction between Reg4 and Notch signaling.
GSK-3β, a key enzyme regulating β-Catenin phosphorylation is a known downstream transducer of the PI3K-Akt signaling pathway [34,35]. GSK-3β is inactivated by phosphorylation in response to PI3K-Akt signaling [36]. We previously reported Reg4-mediated induction of epidermal growth factor receptor (EGFR)-Akt-AP-1 signaling in human CRC cells [20]. In this study, we observed Reg4-mediated regulation of GSK-3β and β-Catenin phosphorylation and TCF4 transcriptional activities. This provided a link between GSK-3β-mediated β-Catenin phosphorylation and Reg4-mediated increase in Akt kinase activity. Results suggested that Reg4-mediated increase in Akt kinase activity might be an important event to inactivate GSK-3β leading to a decreased phosphorylation of β-Catenin and its subsequent release from multiprotein degradation complex. Furthermore, GSK-3β and β-Catenin are involved in regulation of Cyclin D expression and plays vital role in regulation of cell proliferation of many human tumors [22,37]. In the present study, we observed a Reg4-mediated increase in expression of cell cycle regulatory genes Cyclin D1 and D3, and associated kinases in human CRC cells. An addition of specific Akt inhibitor to the cell culture media led to a decreased expression of Cyclin D1 and D3. These results suggested that Reg4-mediated increase in expression of D-type Cyclins might involve Akt-GSK-3β-β-Catenin-TCF4 signaling. In addition, Cyclin D1 promoter also includes binding sequence for AP-1 transcription factor [38], hence Reg4-mediated increase in previously reported AP-1 transcriptional activity might be an important event to regulate Cyclin D1 expression in human CRC cells. These results suggest Reg4 as potent modulators of Akt-GSK-3β-β-Catenin-TCF4 and EGFR-Akt-AP-1 signaling pathways, which often results in altered expression of genes associated with CRC cell proliferation and apoptosis. Therefore, Reg4 and associated signaling pathway(s) could be a potential target for adjunctive treatments of human CRC.
Supplementary Material
Acknowledgments
We thank Dr. Kathleen Sheehan, director of the Hybridoma Center at Washington University School of Medicine, St. Louis for technical help with hybridoma maintenance and antibody purification. This study was supported by NIH grants DK060106, AI069390, and DK052574 to B.K.D.
Grant sponsor: NIH; Grant numbers: DK060106; AI069390; DK052574
Abbreviations
- CRC
colorectal cancer
- Reg
regenerating
- Reg4
regenerating gene 4
- TCF
T cell factor
- GSK-3β
glycogen synthase kinase 3β
- APC
adenomatous polyposis coli
- CDK
cyclin-dependent kinase
- PI
propium Iodide
- mAb
monoclonal antibody
- MTT
[(3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide)]
- EdU
5-ethynyl-2′-deoxyuridine
- PCR
polymerase chain reaction
Footnotes
Conflicts of Interest: The authors have no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Van Cutsem E, Dicato M, Wils J, et al. Adjuvant treatment of colorectal cancer (current expert opinion derived from the Third International Conference: Perspectives in Colorectal Cancer, Dublin, 2001) Eur J Cancer. 2002;38:1429–1436. doi: 10.1016/s0959-8049(02)00122-3. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Lasserre C, Simon MT, Ishikawa H, et al. Structural organization and chromosomal localization of a human gene (HIP/PAP) encoding a C-type lectin overexpressed in primary liver cancer. Eur J Biochem. 1994;224:29–38. doi: 10.1111/j.1432-1033.1994.tb19991.x. [DOI] [PubMed] [Google Scholar]
- 4.Laurine E, Manival X, Montgelard C, et al. PAP IB, a new member of the Reg gene family: Cloning, expression, structural properties, and evolution by gene duplication. Biochim Biophys Acta. 2005;1727:177–187. doi: 10.1016/j.bbaexp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Hartupee JC, Zhang H, Bonaldo MF, Soares MB, Dieckgraefe BK. Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: Reg IV. Biochim Biophys Acta. 2001;1518:287–293. doi: 10.1016/s0167-4781(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 6.Dieckgraefe BK, Crimmins DL, Landt V, et al. Expression of the regenerating gene family in inflammatory bowel disease mucosa: Reg Ialpha upregulation, processing, and antiapoptotic activity. J Investig Med. 2002;50:421–434. doi: 10.1136/jim-50-06-02. [DOI] [PubMed] [Google Scholar]
- 7.Oue N, Mitani Y, Aung PP, et al. Expression and localization of Reg IV in human neoplastic and non-neoplastic tissues: Reg IV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. J Pathol. 2005;207:185–198. doi: 10.1002/path.1827. [DOI] [PubMed] [Google Scholar]
- 8.Takehara A, Eguchi H, Ohigashi H, et al. Novel tumor marker REG4 detected in serum of patients with resectable pancreatic cancer and feasibility for antibody therapy targeting REG4. Cancer Sci. 2006;97:1191–1197. doi: 10.1111/j.1349-7006.2006.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Lai M, Lv B, et al. Overexpression of Reg IV in colorectal adenoma. Cancer Lett. 2003;200:69–76. doi: 10.1016/s0304-3835(03)00460-9. [DOI] [PubMed] [Google Scholar]
- 10.Violette S, Festor E, Pandrea-Vasile I, et al. Reg IV, a new member of the regenerating gene family, is overexpressed in colorectal carcinomas. Int J Cancer. 2003;103:185–193. doi: 10.1002/ijc.10788. [DOI] [PubMed] [Google Scholar]
- 11.Bishnupuri KS, Luo Q, Korzenik JR, et al. Dysregulation of Reg gene expression occurs early in gastrointestinal tumorigenesis and regulates anti-apoptotic genes. Cancer Biol Ther. 2006;5:1714–1720. doi: 10.4161/cbt.5.12.3469. [DOI] [PubMed] [Google Scholar]
- 12.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 13.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 14.Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- 15.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis–a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 17.Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 18.Li A, Crimmins DL, Luo Q, et al. Expression of a novel regenerating gene product, Reg IV, by high density fermentation in Pichia pastoris: Production, purification, and characterization. Protein Expr Purif. 2003;31:197–206. doi: 10.1016/s1046-5928(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 19.Bishnupuri KS, Luo Q, Sainathan SK, et al. Reg IV regulates normal intestinal and colorectal cancer cell susceptibility to radiation-induced apoptosis. Gastroenterology. 138:616–626. 626 e611–626 e612. doi: 10.1053/j.gastro.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bishnupuri KS, Luo Q, Murmu N, Houchen CW, Anant S, Dieckgraefe BK. Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology. 2006;130:137–149. doi: 10.1053/j.gastro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Goto H, Tomono Y, Ajiro K, et al. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem. 1999;274:25543–25549. doi: 10.1074/jbc.274.36.25543. [DOI] [PubMed] [Google Scholar]
- 22.Tetsu O, McCormick F. Beta-catenin regulates expression of Cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 23.Morin PJ. beta-Catenin signaling and cancer. Bioessays. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.Barth AI, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: Interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- 25.Molenaar M, van de Wetering M, Oosterwegel M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 26.Behrens J, von Kries JP, Kuhl M, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 27.Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 28.Sharma M, Chuang WW, Sun Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J Biol Chem. 2002;277:30935–30941. doi: 10.1074/jbc.M201919200. [DOI] [PubMed] [Google Scholar]
- 29.Hirai H, Roussel MF, Kato JY, Ashmun RA, Sherr CJ. Novel INK4 proteins, p19 and p18, are specific inhibitors of the Cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 31.Bartkova J, Lukas J, Strauss M, Bartek J. Cyclin D3: Requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene. 1998;17:1027–1037. doi: 10.1038/sj.onc.1202016. [DOI] [PubMed] [Google Scholar]
- 32.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates Cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diehl JA, Zindy F, Sherr CJ. Inhibition of Cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 34.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 36.Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: Properties, functions, and regulation. Chem Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi-Yanaga F, Sasaguri T. GSK-3beta regulates Cyclin D1 expression: A new target for chemotherapy. Cell Signal. 2008;20:581–589. doi: 10.1016/j.cellsig.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Shiozawa T, Miyamoto T, Kashima H, Nakayama K, Nikaido T, Konishi I. Estrogen-induced proliferation of normal endometrial glandular cells is initiated by transcriptional activation of Cyclin D1 via binding of c-Jun to an AP-1 sequence. Oncogene. 2004;23:8603–8610. doi: 10.1038/sj.onc.1207849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


