Abstract
LPL is the rate-limiting enzyme for uptake of TG-derived FFA in peripheral tissues, and the enzyme is expressed in the brain and CNS. We previously created a mouse which lacks neuronal LPL. This animal becomes obese on a standard chow, and we observed reduced lipid uptake in the hypothalamus at 3 months preceding obesity. In our present study, we replicated the animal phenotype in an immortalized mouse hypothalamic cell line (N41) to examine how LPL affects expression of AgRP as well as entry and storage of lipids into neurons. We show that LPL is able to modulate levels of the orexigenic peptide AgRP. LPL also exerts effects on lipid uptake into culture neurons, and that uptake of neutral lipid can be enhanced even by mutant LPL lacking catalytic activity. N41 cells also accumulate neutral lipid in droplets, and this is at least in part regulated by LPL. These data in addition to those published in mice with neuron-specific deletion of LPL suggest that neuronal LPL is an important regulator of lipid homeostasis in neurons and that alterations in LPL levels may have important effects on systemic metabolism and neuronal lipid biology.
Keywords: Lipoprotein lipase, AgRP, lipid droplets, apolipoproteins, triglycerides
Introduction
Lipoprotein lipase (LPL) catalyzes the release of free fatty acids (FFA) from triglyceride (TG)-rich lipoproteins and is the rate-limiting enzyme for delivery of lipids in peripheral tissues such as skeletal muscle, heart, and adipose tissue [1, 2]. Normally found anchored to the GPIHBP1 on the luminal vascular endothelium, LPL is expressed in peripheral tissues that store neutral lipids or utilize FFA for energy. LPL is also found in both the central and peripheral nervous systems, although its exact role in these systems is still being elucidated [3-5]. The role of LPL in the brain has become increasingly relevant in recent years as links to regulation of energy homeostasis, Alzheimer's disease, and cognitive development have been identified [6-8]. Additionally, lipids are vital to the developing brain and are important substrates for membrane remodeling [9, 10]. Whether LPL is directly involved in providing these lipids remains to be seen.
In a previous study we generated a mouse with neuron-specific LPL deletion (NEXLPL-/-) to examine LPL's role in neuronal regulation of metabolism and energy homeostasis [6]. This mouse develops obesity by 6 months on a standard chow diet, with AgRP orexigenic peptide levels increased >3-fold at 3 months before obesity onset. Preceding obesity, we also observed reduced uptake of lipoprotein-derived FFA and reduced levels of n-3 long chain polyunsaturated fatty acids (LC-PUFA) in the hypothalamus. We speculate that deletion of neuronal LPL disrupts a major energy homeostasis signaling pathway, with increased AgRP levels underlying the obesity development.
In the current study, we used an immortalized mouse hypothalamic cell line (N41) to probe how LPL regulates neuronal AgRP, lipid uptake, and accumulation. N41 cells represent a clonal population of mouse hypothalamic neurons. These immortalized clonal populations have been published extensively due to their specific expression of relevant markers [11-15]. These commercially-available cells were chosen for our work due to extensive knowledge of the markers that are expressed in this cell line. Importantly for this work N41 cells express AgRP and LPL. We herein show that LPL is directly involved in neutral lipid transport into cultured hypothalamic neurons and that N41 cells store neutral lipid in droplet-like structures.
Materials and Methods DNA constructs
Human LPL cDNA [16] was subcloned into a murine stem-cell retrovirus (MSCV 2.2) vector (EcoRI/XhoI) directly preceding IRES-GFP. The mouse LPL cDNA construct was synthesized by GeneWiz (San Diego, CA) and subcloned into the MSCV 2.2 vector. Catalytically-inactive mLPL cDNA (Gly188Glu) was obtained from Keyclone Technologies (Cincinnati, OH). C-terminal 6xHistidine-tagged wild-type and mutant mLPL were created by PCR mutagenesis in pUC57 plasmid and subcloned into MSCV2.2. Retroviral packaging was done with the pCL-Eco packaging plasmid and pseudotyped with VSV-G. LPL and control shRNA constructs for stable knockdown of LPL were obtained from the University of Colorado Functional Genomics Core.
Cell Culture
mHypoE41 (N41) immortalized mouse hypothalamic neurons were purchased from CELLutions Biosystems (Winnipeg, MB). N41 cells were grown in DMEM containing 1000 mg/L glucose and 10% FBS at 37°C in the presence of 5% CO2. For serum-starving, cells were switched to DMEM containing no FBS for 12-16 hours. The same lot of FBS (#A83DOOE) from Gemini Biotech was used for all experiments (lot# A83D). All viral packaging cells were grown in 4500 mg/L glucose supplemented with 10% FBS in a 5% CO2 environment.
Viral Packaging and Transduction
To produce retrovirus for stable overexpression of LPL, 6 ug of control or LPL MSCV vector was transfected into Phoenix 293T cells along with 3 ug pCL-Eco and 2 ug VSV-G using 33 uL Lipofectamine 2000 (Life Technologies). Virus was harvested after 48 hours of packaging in a total volume of 6 mL. 1 mL of viral particles was applied to N41 cells overnight. For stable knockdown of LPL, 12 ug of control or LPL shRNA lentiviral vector was transfected into HEK 293FT cells along with 10.8 ug pΔ8.9 and 1.2 ug VSV-G using 45 uL Lipofectamine 2000. Virus was packaged for 48 hours in 6 mL media, and two rounds of transduction were performed. Stably-transduced cells were then selected for 3 days by growth in medium containing 5 ug/mL puromycin.
LPL-targeting siRNA/shRNA constructs
LPL was knocked down in N41 neurons using either siRNA or shRNA targeting. All LPL and control siRNAs were purchased from Ambion (Life Technologies). Control and LPL-targeting siRNA sequences can be found in the supplemental materials section. For siRNA transfection, siRNA was transfected at a concentration of 35 nM with 2 uL/mL RNAiMax Lipofectamine (Life Technologies). Media was changed 16 hours after transfection, and cells were allowed to grow 24 hours before harvest. Sequences for control and LPL-targeting shRNAs can be found in the supplemental materials section.
Measurement of LPL Enzymatic Activity
Heparin-released LPL nzymatic activity was measured using a phospholipid/3H-triolein substrate with human serum as a source of ApoC2 as described previously. [17]
Lipid Uptake Assay
To assess whether N41 cells can take up lipid derived from triglycerides and whether various apolipoproteins have an effect on uptake, an assay was performed by preparing a synthetic phospholipid/TG emulsion containing a 3H triolein tracer. The emulsion was prepared by combining and sonicating the following: 5 mg 1H triolein, 0.25 mg L-phosphatidylcholine, a trace amount of 3H triolein (12.5 uCi total) tracer (Perkin Elmer, NET431001MC), 0.9 mL water, 2 mL 1M tris-HCl (pH 8.0), 800 uL FFA-free BSA (MP Biomedical), and 300 uL KRP. After sonication, the substrate was diluted into DMEM to a final concentration of 85 uM triolein. For cells receiving ApoC2, purified human ApoC2 (MyBioSource) was added to a final concentration of 5 ug/mL. For cells receiving ApoE3 or ApoE4, the recombinant ApoE (MyBioSource) was added to the final diluted substrate at a concentration of 8 ug/mL. Substrate with or without apolipoproteins was applied to serum-starved cells for 2 hours. Cells were washed with 0.1% FFA-free BSA and lysed in 1 mL of RIPA buffer. 800 uL of each lysis fraction was subjected to scintillation counting, while the rest was used for protein normalization.
Analysis of Neutral Lipid Stores in N41 Neurons
For qualitative analysis of lipid droplets in N41 cultures, live cells were stained for 5 minutes with AdipoRed (Lonza) and observed with fluorescent microscopy. For quantitative analysis of neutral lipid content in N41 neurons, coherent anti-stokes Raman spectroscopy (CARS) was employed. The CARS microscope was interfaced with two laser excitation beams. The pump beam was generated from a Ti:sapphire laser (Mira-900, Coherent). The Stokes beam was derived from a Nd:vanadate laser (PicoTrain, HighQ Lasers). The lasers were electronically synchronized at 76 MHz, and the pump and Stokes beams were spatially overlapped on a dichroic mirror (1000dcxr, Chroma) and directed to the laser scanning-microscope. The scanning system included two computer controlled galvanometric mirrors (Fluoview 300, Olympus) and a telescopic lens pair, which projects the excitation beams onto the back aperture of a 60X, 0.75 NA Olympus objective. Signal generated in the sample was detected in the forward direction, captured by a condenser, filtered by a bandpass filter (650 nm, Semrock), and detected by a photomultiplier tube (R3896, Hamamatsu). The images were recorded with FLUOVIEW software (Olympus) and ImageJ was used for data analysis.
Gene Expression Analysis
RNA was extracted from N41 cultures using the RNeasy Plus Mini it (Qiagen). For semi-quanitative RT-PCR, the One Step RT-PCR kit (Qiagen) was used in combination with an 18S competimer set (Ambion) for normalization.
Statistics
Variance is presented as SEM. Student t-tests were used to compare differences among groups. P < 0.01 was considered to be statistically-significant.
Results
LPL activity is present in N41 neurons and affects AgRP expression
We first determined whether LPL activity could be detected in cultured N41 neurons. Initial experiments lead to the observation that both heparin-releasable (extracellular) as well as intracellular lipase activity could be measured in our assay (Fig. 1A). To probe whether the activity being detected was directly due to LPL, we conducted lipase activity assays in the absence and presence of purified apoC2—an established activator of LPL activity [18]. Extracellular, heparin-releasable lipase activity was potently-enhanced by the addition of 1 ug/mL and 5 ug/mL apoC2 compared to the control. Activity in the absence of apoC2 was ∼30% of the maximum attainable activity, a result consistent with previous findings that LPL still retains some activity even in the absence of apoC2 with synthetic emulsion substrates [19]. On the other hand, addition of apoC2 up to 5 ug/mL had very little effect on intracellular lipase activity leading us to conclude that the vast majority of intracellular lipase activity was not due to LPL. To further explore the increase in AgRP seen in NEXLPL-/- mice [6], we next examined whether altering gene expression of LPL in cultured N41 neurons could induce changes in AgRP expression. Using several siRNA constructs that target LPL, LPL mRNA was reduced >95% (Fig. 1B and 1C), while heparin-releasable LPL enzymatic activity was reduced to levels below the detection limit of the radiometric assay (Fig. 1D). Concomitantly, AgRP expression was increased 5-fold, mimicking what was seen in vivo (Fig. 1E and 1F).
Figure 1. LPL knockdown increases AgRP expression in N41 neurons.
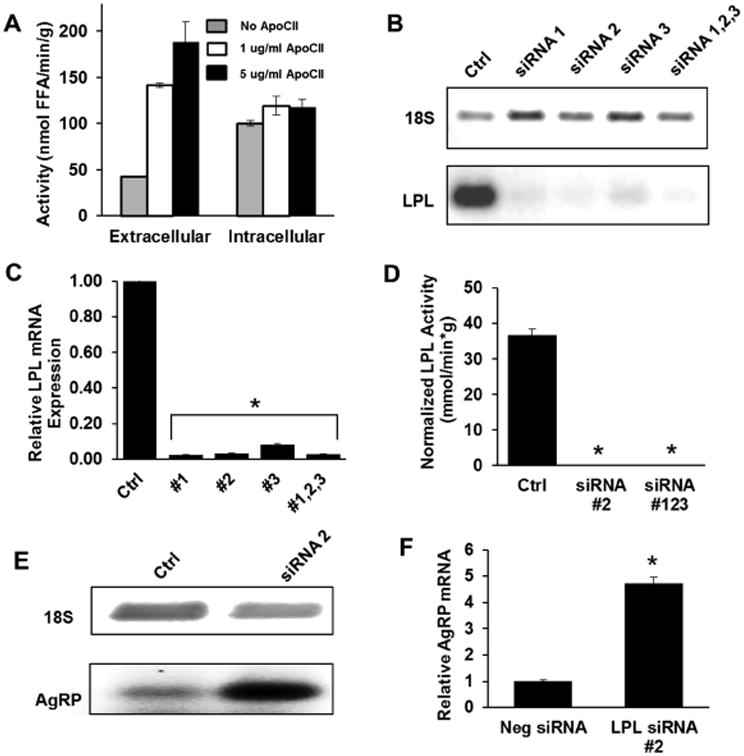
(A) LPL enzymatic activity was assessed from cell-surface heparin-released fractions as well as intracellular lysates with and without purified ApoC2. (B) LPL mRNA expression was assessed using 3 different siRNA constructs. (C) LPL mRNA expression was quantified using ImageJ and normalized to 18S. (D) Cell-surface LPL enzymatic activity was assessed under LPL knockdown. In the siRNA-treated cells, enzymatic activity fell below the detection limit of the assay. (E) RT-PCR gel analysis of AgRP expression in control and LPL siRNA knockdown conditions. (F) Quantified AgRP expression in control and LPL knockdown cells. Results are presented as ±SEM. * indicates p<0.001.
LPL is a mediator of lipid uptake in cultured N41 neurons
Because the role of LPL in the periphery is to provide lipids to tissues, we then explored whether N41 neurons could take up lipid and whether LPL plays a direct role in this process. We first developed several new N41 cell lines. In order to examine the effect of having supra-physiological levels of enzyme present, we created an N41 variant (N41-hLPL) that stably overexpresses human LPL via transduction of an overexpression retrovirus. Heparin-releasable LPL activity in the N41-hLPL cell line is ∼35,000-fold higher than the control (Fig. 2A). At the same time, we developed a stable LPL knockdown cell line (N51-553) that contains stably-integrated LPL-targeting shRNA. This provided a stable LPL knockdown cell line that improved consistency across experiments. In N51-553 neurons, cell-surface heparin-releasable LPL activity was reduced close to the detection limit of the enzymatic assay (Fig. 2B). Next, we developed an assay using a synthetic phospholipid-TG emulsion with a 3H-triolein tracer to probe whether LPL mediates uptake of neutral-lipid-derived FFA. When hLPL was overexpressed, there was a 7-fold increase in TG uptake compared to control cells (Fig. 2C). Conversely, there was a 30% reduction in neutral lipid uptake when LPL was knocked down (Fig. 2D). From these experiments, it was concluded that LPL expression has a profound effect on neutral lipid uptake but does not alter inherent FFA uptake mechanisms in N41 neurons.
Figure 2. LPL is a modulator of neutral-lipid intake in N41 neurons.
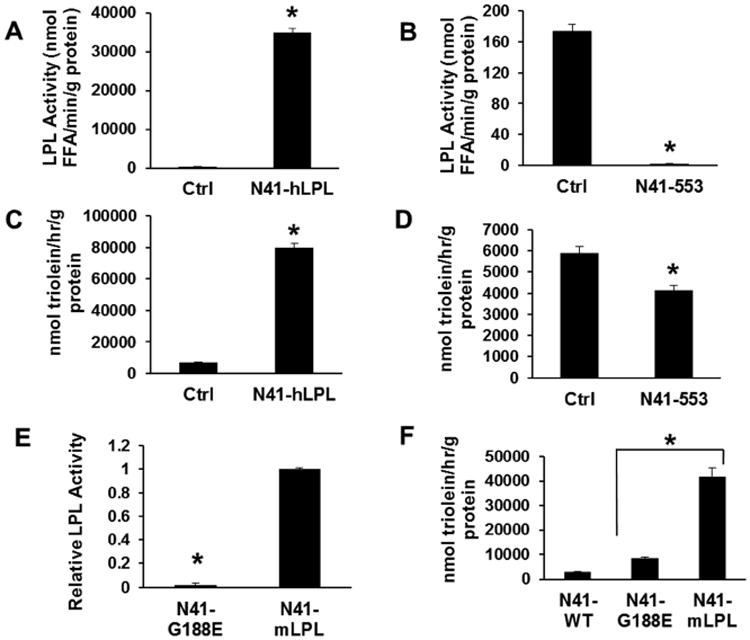
(A) LPL enzymatic activity was assessed in LPL-overexpressing N41 neurons (N41-hLPL). The control cells were transduced with the empty-vector retrovirus. (B) LPL enzymatic activity was assessed in control cells and N41 neurons transduced with LPL-targeting shRNA (N41-553). The control cells were transduced with a scrambled shRNA vector. (C) Lipid uptake was measured in control and N41-hLPL cells using a synthetic phospholipid/trioelin emulsion containing ApoC2. (D) Lipid uptake was assessed in control and N41-553 knockdown cells. (E) The specific activity of catalytically-inactive overexpressed mLPL (N41-G188E) was compared to active overexpressed mLPL (N41-mLPL). (F) Lipid uptake was performed on N41-WT, N41-G188E, and N41-mLPL cells. Overexpression of the inactive mutant enhanced uptake compared to WT cells but did not rescue uptake to the level of active enzyme-overexpressing cells. All error is represented as ±SEM. * indicates p<0.001.
To explore whether LPL catalytic activity is necessary for lipid uptake in N41 neurons, we overexpressed a inactive LPL (N41-G188E) to see if neutral lipid uptake is enhanced even without enzyme activity present. It has been established that the G188E mutation inactivates the enzyme [20]. The mutant was His-tagged to allow detection by western blot and transduced into N41 cells using a retroviral vector. The specific activity of the mutant was assessed using the enzymatic activity assay and normalized to overexpressed His-tagged wild-type mLPL (N41-mLPL) via Western blot. The G188E mutant was found to be catalytically inactive (Fig. 2F). We then performed the neutral lipid uptake assay with control cells (N41-WT), N41-G188E, and N41-mLPL. There was a 2.8 fold increase in lipid uptake in the catalytically inactive over-expressing cells compared with N41-WT neurons (Fig. 2G). However, lipid uptake was not nearly as high as that observed for active enzyme-overexpressing cells, indicating that a large portion of lipoprotein-dependent uptake depends on the catalytic activity of LPL. Nevertheless, these findings of increased lipid uptake and TG accumulation in hypothalamic cells overexpressing the catalytically inactive mutant provide evidence of some role for LPL in neuronal lipid uptake independent of its catalytic activity.
N41 neurons accumulate neutral lipids in droplet-like structures
Because N41 cells have the ability to take up neutral lipid-derived FFA, we probed whether they might accumulate neutral lipid. Using AdipoRed staining, we observed lipid droplet-like structures in wild-type N41 neurons (Fig. 3A). Additionally, we show that lipid droplet accumulation is remarkably affected by providing cells with oleic acid. Loading N41 cells with FFA lead to a drastic increase in both size and intensity of accumulated lipid droplets (Fig. 3B). To examine whether LPL expression could have a direct effect on the amount of lipid accumulation, we utilized quantitative CARS microscopy to assess neutral lipid content under LPL knockdown and overexpression conditions. This method allows the user to tune the difference frequency between two laser beams into resonance with the vibrational frequency of neutral lipids, enabling the observation of lipid droplets in fixed cells. Under conditions of LPL overexpression (N41-hLPL), neutral lipid accumulation was increased 2-fold compared to the control (Fig. 3C-3E). By contrast, neutral lipid stores were reduced ∼30% under conditions of LPL knockdown (Fig. 3F-3H).
Figure 3. N41 neurons accumulate neutral lipid.
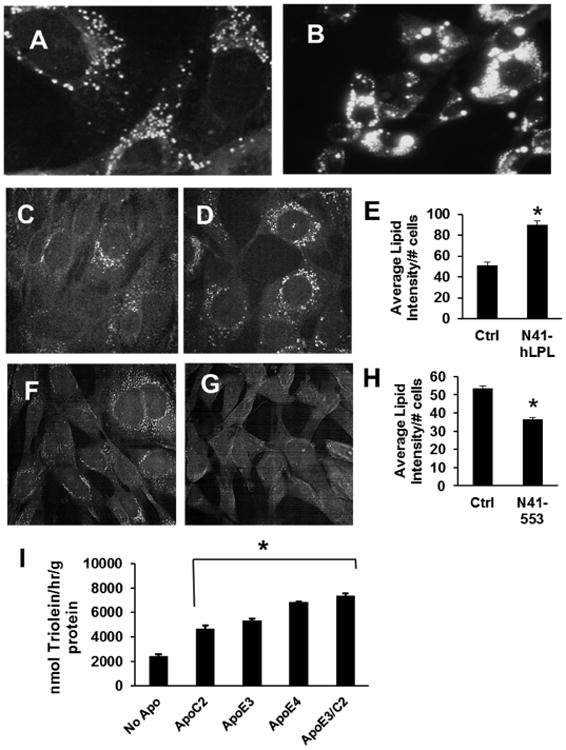
(A) AdipoRed staining of N41-WT cells. (B) N41-WT cells treated with 200 uM oleic acid for 16 hours and stained with AdipoRed. (C) CARS neutral lipid imaging of control cells transduced with empty-vector MSCV retrovirus. (D) CARS neutral lipid imaging of N41-hLPL overexpression cells. (E) Quantification of neutral lipid in control and N41-hLPL neurons. (F) CARS neutral lipid imaging of control cells transduced with non-targeting shRNA. (G) CARS neutral lipid imaging of N41-553 LPL knockdown cells. (H) Quantification of neutral lipid in control and N41-553 neurons. (I) N41-WT cells were subjected to neutral lipid uptake, and purified apolipoproteins included either individually or in combination. Data are presented as ±SEM. * indicates p<0.001.
LPL-independent lipid uptake mechanisms exist in N41 cells
Although levels of LPL expression directly affected the amount of neutral lipid that accumulates in N41 neurons, we still observed neutral lipid in N41-553 knockdown cells in the absence of catalytic activity on the surface of these cells. As such, we went further to demonstrate other possible routes of lipid uptake that might be independent of LPL. Using the neutral lipid uptake assay, we saw pronounced increase in lipid uptake in N41-WT cells when several different apolipoproteins were included in the substrate. While inclusion of apoC2 in the substrate enhanced uptake 2-fold compared to substrate lacking apolipoproteins, we were also able to observe apoE-mediated lipid entry into N41 neurons (Fig. 3I). When the substrate included apoE3 only (the most common isoform of this apolipoprotein in human plasma) lipid uptake was enhanced 2-fold, while apoE4 lead to a ∼3-fold increase in lipid uptake. The highest levels of lipid uptake were achieved when cells were given both apoC2 and apoE in the uptake substrate. These findings may partially explain why neutral lipid accumulation is only reduced ∼30% in N41-553 knockdown cells.
Discussion
LPL has been studied in the CNS for several decades; however, its exact role remains elusive. It has been hard to study the enzyme in vivo—especially in humans. N41 immortalized hypothalamic neurons provide a unique opportunity to conduct high-throughput studies to elucidate the molecular/cellular role of LPL in energy homeostasis. Here we show that the in vivo phenotype of neuron-specific LPL knockout mice can be reconstituted in a hypothalamic cell line. As is seen in NEXLPL(-/-) mice, when LPL expression is reduced in these cells, AgRP expression increases. In NEXLPL(-/-) mice, levels of n-3/n-6 long-chain polyunsaturated fatty acids LC-PUFA) are markedly reduced preceding obesity [6]. Because these species are widely used for signaling in the CNS, we speculate that LPL might act as a sensor in AgRP neurons; FFA released from LPL-catalyzed hydrolysis might signal to suppress AgRP expression with the result being suppressed appetite. When this sensing mechanism is absent (i.e. LPL knockdown), AgRP expression remains high and food intake is increased. As such, we plan to test this hypothesis in N41 cells.
Although its primary role in the periphery is to affect lipid uptake in tissues, it remains unknown whether LPL performs a similar role in the CNS—especially in neurons. Previous work showed that mice lacking neuronal LPL exhibit reduced lipid uptake in the hypothalamus when injected with radiolabeled chylomicrons [6]. Here we show that LPL is a mediator of lipid uptake in cultured N41 neurons, and reduced lipid uptake is observed when LPL is decreased. This uptake is enhanced by the presence of apoC2 which indicates a significant role for LPL catalytic activity in the neutral lipid uptake process. Interestingly, we also observe some lipid uptake when catalytically-inactive LPL is overexpressed. It is well-known that LPL can enhance uptake of lipoprotein remnants in the periphery by acting as a bridging ligand with members of the low-density lipoprotein receptor (LDLR) family [21-23]. LPL can act as a molecular bridge between the lipoprotein particle and proteoglycans, thereby anchoring the particle to cell surfaces where it can be rapidly internalized by members of LDLR. This enhancement of lipid uptake by LPL has been shown to be independent of its catalytic activity. Finally, because ATGL is unchanged regardless of LPL expression (data not shown), it is likely that inherent rates of lipolysis of stored neutral lipid are the same between all cell types studied. Thus, the fact that N41-553 cells still contain abundant neutral lipid stores cannot be explained by differences in ATGL expression. Plin2 is up-regulated in N41-553 (data not shown), suggesting that these cells might increase production of neutral lipid storage machinery to compensate for loss of LPL.
We also observed storage of neutral lipids in N41, and the accumulation of neutral lipid in N41 cells is at least partially dependent on LPL. Previous work has shown that neurons can produce and store LDs during autophagy, and there is evidence that certain neurons may store cholesterol, presumably in the form of esterified cholesterol, in droplet-like structures [24-26]. Because droplets are observed even in N41 cells with no detectable cell-surface LPL activity, it is likely that these cells have apoE-mediated mechanisms in place to take up lipid independent of LPL. Indeed, we observed apoE-mediated lipid entry into N41 neurons. At present, it is unknown what role lipid droplets play in hypothalamic neurons. There is evidence that, under conditions of glucose deprivation, neuronal metabolism can be shifted to other substrates including pyruvate, glutamate, lactate, aspartate, ketone bodies, and other fuels [27]. Whether neurons use fatty acids as a fuel source during times of starvation remains to be seen, and we are investigating whether β-oxidation occurs in cultured neurons. It is also possible that neurons use lipid-droplet-derived FFA for signaling or as substrates of phospholipid synthesis during membrane remodeling.
In conclusion, alterations of LPL expression directly affect AgRP expression levels in N41 hypothalamic neurons thereby mimicking what is seen in vivo in NEXLPL(-/-) mice. Additionally, LPL is a major modulator of neutral lipid-derived FFA entry into these cultured cells, and lipid taken up by N41 cells can be stored as neutral lipid. Finally, LPL is at least, in part, responsible for the amount of stored lipid, although LPL-independent lipid entry mechanisms do exist.
Supplementary Material
Table S1: Primer sequences used for one-step RT-PCR and qPCR:
Table S2: Control and LPL-targeting siRNA sequences:
Table S3: Control and LPL-targeting shRNA sequences:
Highlights.
LPL levels modulate AgRP expression in cultured mouse hypothalamic cells.
LPL mediates neutral lipid uptake in cultured hypothalamic cells.
LPL facilitates entry of lipid into neurons independent of catalytic activity.
Cultured hypothalamic neurons store neutral lipid in droplet-like structures.
Acknowledgments
Grant Support:The authors would like to thank Ganna Bilousova for providing the overexpression viral constructs and packaging cells. This work was supported by National Institutes of Health Grant R01-NIDDK089309 to RHE as well as an ADA Basic Science Research Award to RHE. EOP acknowledges support from National Institutes of Health grant P41-RR01192 (Laser Microbeam and Medical Program, LAMMP)
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. American journal of physiology Endocrinology and metabolism. 2009;297:E271–288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. The New England journal of medicine. 1989;320:1060–1068. doi: 10.1056/NEJM198904203201607. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg IJ, Soprano DR, Wyatt ML, Vanni TM, Kirchgessner TG, Schotz MC. Localization of lipoprotein lipase mRNA in selected rat tissues. Journal of lipid research. 1989;30:1569–1577. [PubMed] [Google Scholar]
- 4.Wang H, Eckel RH. Lipoprotein lipase in the brain and nervous system. Annual review of nutrition. 2012;32:147–160. doi: 10.1146/annurev-nutr-071811-150703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huey PU, Marcell T, Owens GC, Etienne J, Eckel RH. Lipoprotein lipase is expressed in cultured Schwann cells and functions in lipid synthesis and utilization. Journal of lipid research. 1998;39:2135–2142. [PubMed] [Google Scholar]
- 6.Wang H, Astarita G, Taussig MD, Bharadwaj KG, DiPatrizio NV, Nave KA, Piomelli D, Goldberg IJ, Eckel RH. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metab. 2011;13:105–113. doi: 10.1016/j.cmet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xian X, Liu T, Yu J, Wang Y, Miao Y, Zhang J, Yu Y, Ross C, Karasinska JM, Hayden MR, Liu G, Chui D. Presynaptic defects underlying impaired learning and memory function in lipoprotein lipase-deficient mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4681–4685. doi: 10.1523/JNEUROSCI.0297-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong HL, Dong WJ, Rostad SW, Marcovina SM, Albers JJ, Brunzell JD, Vuletic S. Lipoprotein Lipase (LPL) is Associated with Neurite Pathology and Its Levels Are Markedly Reduced in the Dentate Gyrus of Alzheimer's Disease Brains. J Histochem Cytochem. 2013;61:857–868. doi: 10.1369/0022155413505601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain research. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 10.Rapoport SI. In vivo fatty acid incorporation into brain phosholipids in relation to plasma availability, signal transduction and membrane remodeling. Journal of molecular neuroscience : MN. 2001;16:243–261. doi: 10.1385/JMN:16:2-3:243. discussion 279-284. [DOI] [PubMed] [Google Scholar]
- 11.Mayer CM, Belsham DD. Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of resistance and apoptosis through adenosine 5′ monophosphate-activated protein kinase activation. Endocrinology. 2010;151:576–585. doi: 10.1210/en.2009-1122. [DOI] [PubMed] [Google Scholar]
- 12.Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AM, Shkreta L. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology. 2004;145:393–400. doi: 10.1210/en.2003-0946. [DOI] [PubMed] [Google Scholar]
- 13.Cai F, Gyulkhandanyan AV, Wheeler MB, Belsham DD. Glucose regulates AMP-activated protein kinase activity and gene expression in clonal, hypothalamic neurons expressing proopiomelanocortin: additive effects of leptin or insulin. The Journal of endocrinology. 2007;192:605–614. doi: 10.1677/JOE-06-0080. [DOI] [PubMed] [Google Scholar]
- 14.Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) alpha to ERbeta in clonal hypothalamic neurons. Molecular endocrinology. 2006;20:2080–2092. doi: 10.1210/me.2006-0027. [DOI] [PubMed] [Google Scholar]
- 15.Cheng H, Isoda F, Belsham DD, Mobbs CV. Inhibition of agouti-related peptide expression by glucose in a clonal hypothalamic neuronal cell line is mediated by glycolysis, not oxidative phosphorylation. Endocrinology. 2008;149:703–710. doi: 10.1210/en.2007-0772. [DOI] [PubMed] [Google Scholar]
- 16.Schlaepfer IR, Eckel RH. Plasma triglyceride reduction in mice after direct injections of muscle-specific lipoprotein lipase DNA. Diabetes. 1999;48:223–227. doi: 10.2337/diabetes.48.1.223. [DOI] [PubMed] [Google Scholar]
- 17.Jensen DR, Knaub LA, Konhilas JP, Leinwand LA, MacLean PS, Eckel RH. Increased thermoregulation in cold-exposed transgenic mice overexpressing lipoprotein lipase in skeletal muscle: an avian phenotype? Journal of lipid research. 2008;49:870–879. doi: 10.1194/jlr.M700519-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinomiya M, Mclean LR, Jackson RL. Chain-Length Dependence of Phosphatidylcholine Hydrolysis Catalyzed by Lipoprotein-Lipase - Effect of Apolipoprotein-C-Ii. Journal of Biological Chemistry. 1983;258:4178–4180. [PubMed] [Google Scholar]
- 19.Bengtsson G, Olivecrona T. Lipoprotein-Lipase - Some Effects of Activator Proteins. Eur J Biochem. 1980;106:549–555. doi: 10.1111/j.1432-1033.1980.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 20.Shibasaki M, Bujo H, Takahashi K, Murakami K, Unoki H, Saito Y. Catalytically inactive lipoprotein lipase overexpression increases insulin sensitivity in mice. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2006;38:491–496. doi: 10.1055/s-2006-949530. [DOI] [PubMed] [Google Scholar]
- 21.Mulder M, Lombardi P, Jansen H, van Berkel TJ, Frants RR, Havekes LM. Heparan sulphate proteoglycans are involved in the lipoprotein lipase-mediated enhancement of the cellular binding of very low density and low density lipoproteins. Biochem Biophys Res Commun. 1992;185:582–587. doi: 10.1016/0006-291x(92)91664-c. [DOI] [PubMed] [Google Scholar]
- 22.Mulder M, Lombardi P, Jansen H, van Berkel TJ, Frants RR, Havekes LM. Low density lipoprotein receptor internalizes low density and very low density lipoproteins that are bound to heparin sulfate proteoglycans via lipoprotein lipase. The Journal of biological chemistry. 1993;268:9369–9375. [PubMed] [Google Scholar]
- 23.Eisenberg S, Sehayek E, Olivecrona T, Vlodavsky I. Lipoprotein-Lipase Enhances Binding of Lipoproteins to Heparan-Sulfate on Cell-Surfaces and Extracellular-Matrix. Journal of Clinical Investigation. 1992;90:2013–2021. doi: 10.1172/JCI116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh R. Hypothalamic lipophagy and energetic balance. Aging. 2011;3:934–942. doi: 10.18632/aging.100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, Cuervo AM, Singh R. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. 2011;14:173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenger C, Pincon A, Hanse M, Royer L, Comte A, Koziel V, Olivier JL, Pillot T, Yen FT. Brain region-specific immunolocalization of the lipolysis-stimulated lipoprotein receptor (LSR) and altered cholesterol distribution in aged LSR+/- mice, Journal of neurochemistry. 2012;123:467–476. doi: 10.1111/j.1471-4159.2012.07922.x. [DOI] [PubMed] [Google Scholar]
- 27.Amaral AI. Effects of hypoglycaemia on neuronal metabolism in the adult brain: role of alternative substrates to glucose. Journal of inherited metabolic disease. 2013;36:621–634. doi: 10.1007/s10545-012-9553-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Primer sequences used for one-step RT-PCR and qPCR:
Table S2: Control and LPL-targeting siRNA sequences:
Table S3: Control and LPL-targeting shRNA sequences:


