Abstract
The amygdala has long been associated with emotion and motivation, playing an essential part in processing both fearful and rewarding environmental stimuli. How can a single structure be crucial for such different functions? With recent technological advances that allow for causal investigations of specific neural circuit elements, we can now begin to map the complex anatomical connections of the amygdala onto behavioural function. Understanding how the amygdala contributes to a wide array of behaviours requires the study of distinct amygdala circuits.
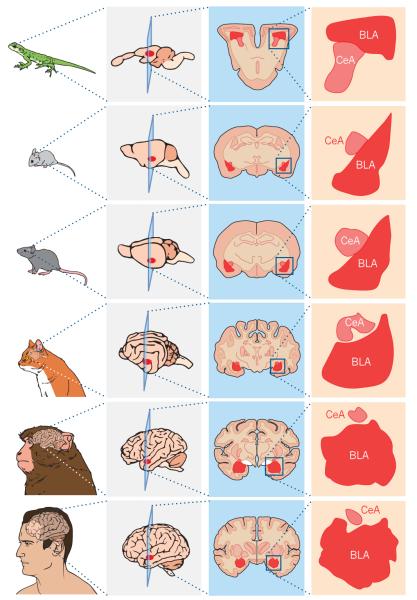
Although humans possess a number of cognitive abilities that differentiate us from other animals, we share emotional behaviours — defined as behavioural responses to emotionally significant stimuli such as food or threats — with other vertebrates. The amygdala is a brain region that is important for emotional processing, the circuitry and function of which has been well-conserved across evolution (Fig. 1), although species differences do exist1. Even non-mammalian species such as reptiles, birds and fish have an amygdala-like brain region with similar circuits and functions to the amygdala in mammals2–5.
Figure 1. Evolution of the amygdala across species.
Primary amygdalar nuclei and basic circuit connections and function are conserved across species. An enlarged image of the basolateral complex of the amygdala (BLA) and central nucleus of the amygdala (CeA) or analogues are shown next to a coronal section from the brains of a lizard, mouse, rat, cat, monkey and human.
Conservation of amygdala circuitry allows findings from one species to inform our appreciation of amygdala functioning in others. Understanding the intricacies of amygdala circuitry is of tremendous importance given that the amygdala is implicated in a wide range of disease states, including addiction, autism and anxiety disorders. Regarding forward translation, features identified using new approaches for neural circuit dissection in rodents have the potential to be directly relevant to humans in well-conserved structures such as the amygdala. For reverse translation, observed correlations between amygdala function and human behaviour can then be brought back to animal models to facilitate the elucidation of underlying mechanisms using systematic, iterative experimentation. The field is ripe for this type of translation, as reflected by the growing emphasis on amygdala research across species (Fig. 2). The collective body of work supports a view of the amygdala as a composite of parallel circuits that affect multiple aspects of emotional behaviour. This Review focuses on recent advances that have been enabled by technologies primarily used in rodents. Readers are directed to recent reviews for in-depth information on the amygdala and fear6, reward7–9, learning-related plasticity10,11, and anatomical or physiological characteristics6,12.
Figure 2. Number of studies on the amygdala.
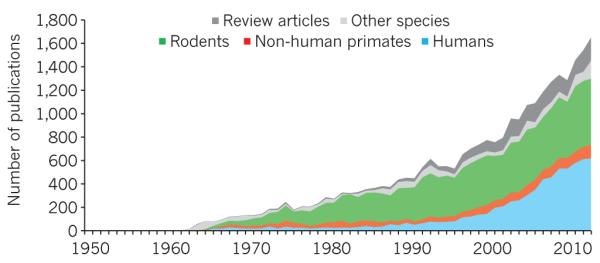
Publications on the amygdala indexed on PubMed between 1950 and 2013 demonstrate the growing interest in amygdala research.
First clues to the role of the amygdala in behaviour
Lesion studies in non-human primates presented the earliest clues that the amygdala was important for emotional reactions to stimuli. In 1888, Brown and Schäfer performed a bilateral ablation of the temporal lobe of a rhesus monkey (Macaca mulatta)13, reporting that: “A remarkable change is … manifested in the disposition of the Monkey … He gives evidence of hearing, seeing, and of the possession of his senses generally, but it is clear that he no longer clearly understands the meaning of the sounds, sights, and other impressions that reach him.” In addition, Brown and Schäfer, and later Klüver and Bucy14, reported reduced aggression, fear and defensive behaviours. Although these lesion effects were intermingled with features that we now attribute to other brain regions, these were the first reports describing the role of a brain region that is important for connecting stimuli with their emotional meaning. In 1956, Weiskrantz15 lesioned the amygdala, demonstrating an impairment in acquiring behavioural responses to shock-predictive cues, concluding that: “the effect of amygdalectomy … is to make it difficult for reinforcing stimuli, whether positive or negative, to become established or to be recognized as such.”
Following these early studies, amygdala lesions in both rodents16,17 and humans18,19 revealed a strong conservation of function across species, most notably an impairment in the recognition of fearful stimuli, and in a type of emotional learning called fear conditioning. Fear is typically studied in the laboratory using a form of associative learning known as Pavlovian conditioning, in which an initially neutral conditioned stimulus (CS) is paired with an aversive unconditioned stimulus (US), such as a footshock, leading the experimental subject to display behavioural signs of fear. This simple behavioural procedure provides a window into basic Pavlovian learning mechanisms that act to enhance survival, and is intimately tied to amygdala function.
What is the amygdala?
Although modest in size, the amygdala is comprised of multiple interconnected nuclei nestled deep in the temporal lobe (Fig. 1). Here we focus on the basolateral complex of the amygdala (BLA; made up of the lateral (LA), basal (BA) and basomedial (BM) cell groups) and the central nucleus of the amygdala (CeA; made up of a lateral (CeL) subdivision and a medial (CeM) subdivision). The BLA consists of glutamatergic principal neurons and inhibitory interneurons. CeA neurons are primarily GABAergic, with the CeL projecting to the CeM. An interconnected sheath of GABAergic neurons, termed the intercalated cells, is also found interposed between the BLA and CeA, providing an important source of inhibition12,20.
A highly simplified view of information flow through the amygdala is as follows. The amygdala receives information about the external environment from the sensory thalamus and sensory cortices, which project strongly to the LA. The LA projects within the BLA to the BA and BM, as well as to the neighbouring CeA. The BLA is reciprocally connected with cortical regions, especially the midline and orbital prefrontal cortices (PFCs), and the hippocampus (HPC), as well as sensory association areas1; in primates, these reciprocal connections extend to primary sensory areas21. Hence, the BLA transmits information widely throughout cortical regions, but its neuronal processing is greatly affected by excitatory projections from these regions. The relative enlargement of the BLA compared with the CeA between rodents and primates might result from the substantial increase in the size of cortical regions that communicate with the BLA in primates22. Predominantly unidirectional outputs of the BLA include the striatum, especially the nucleus accumbens (NAc), and the bed nucleus of the stria terminalis (BNST) and the CeA. In turn, the striatum, BNST and CeA have been considered to mediate the translation of BLA signals to behavioural output. Of note, there are exceptions to this serial model of information flow; the BA and CeA also receive sensory inputs, and the CeA contributes to some behavioural processes independently from the BLA23,24.
From rodents to humans and back again
The neural circuits underlying Pavlovian fear conditioning have predominantly been explored in rodents. Simple tone–shock pairings produced robust and reproducible changes in amygdala neural responses to the tone25,26, such that neuronal spiking tracks the acquisition and extinction of fear behaviour in response to the tone. Human studies using functional magnetic resonance imaging (fMRI) adapted these simple fear-conditioning tasks and showed that the human amygdala is also activated by fear-conditioned stimuli, and that this activation wanes on extinction27,28. Furthermore, ventromedial prefrontal cortex (vmPFC)–amygdala circuitry was found to mediate fear extinction in both rats29,30 and humans31. By applying tasks designed for rodents to humans, the circuits mediating fear acquisition and extinction were shown to be well conserved.
Not only does fear conditioning activate both the rodent and human amygdala, but these animals also share long-term memory processes for fear memory. When consolidated fear memories of rodents are reactivated during retrieval, they become labile, thereby requiring ‘reconsolidation’32. During this labile state, memories can be modified by interfering with the reconsolidation process using protein-synthesis inhibitors32 or with the non-invasive presentation of non-fearful information in mice, rats and humans33,34.
Reverse translational approaches have become more frequent with the advent of new circuit mapping and manipulation technologies in rodents. One example in which a correlation in humans was taken to causation in rodents comes from fMRI studies in people with generalized anxiety disorder35. The observation of abnormal functional connectivity between the BLA and the CeM in these people inspired the application of optogenetic tools to probe specific projections within mouse amygdala. Optogenetics allows for the rapid and reversible activation or inhibition of neurons by directing light towards neural elements that have been artificially induced to express light-sensitive opsins. In the first demonstration of optogenetic projection-specific manipulation in a freely moving animal, the increase or decrease of transmission between the BLA and the CeA was shown to cause the reduction or augmentation of anxiety-related behaviour36, complementing the fMRI findings.
Any attempt to define the behavioural functions of amygdala neuronal activity is confronted by the dense interconnections among amygdala nuclei and between amygdala nuclei and other brain regions, and by the lack of a predictable distribution of functional cell types. The availability of new neurotechnologies and approaches is overcoming these hurdles and accelerating the mapping of function onto amygdala circuitry, revealing a complex picture of amygdalar control of behaviour. These findings, achieved using optogenetic and pharmacogenetic activation or inhibition, in conjunction with behaviour and electrophysiology, reveal causal relations between amygdala cell types and projections in various behaviours (Fig. 3). As we discuss, the behavioural functions of afferent and efferent projections had not been determined at this level of specificity until the application of these new technologies for circuit manipulation.
Figure 3. Amygdalar circuits that are sufficient to alter behaviour in a diversity of domains.
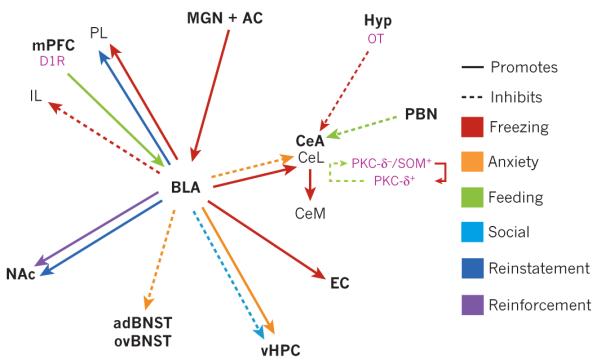
Projection-specific effects as shown by optogenetic or pharmacogenetic manipulation. The solid or dotted lines indicate the promotion or inhibition of certain behaviours. The basolateral complex of the amygdala (BLA) encompasses the lateral and basal nuclei. Specific cell types are shown in pink. For simplicity, projections that are anatomically or electrophysiologically defined but have not been shown to have a causal relationship with behaviour are omitted. This is a selective picture of projections that have been directly manipulated, and is not meant to signify their importance over other anatomical connections. The actual connectivity of the amygdala with other brain regions is considerably more complex. AC, auditory cortex; adBNST, anterodorsal bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; CeL, lateral CeA; CeM, medial CeA; D1R, dopamine 1 receptor; EC, entorhinal cortex; Hyp, hypothalamus; IL, infralimbic; MGN, medial geniculate nucleus; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; OT, oxytocin; ovBNST, oval nucleus of the BNST; PBN, parabrachial nucleus; PKC, protein kinase C; PL, prelimbic; SOM, somatostatin; vHPC, ventral hippocampus.
New insights into circuitry for fear
The investigation of the neural basis of fear learning and expression led to the view that the amygdala is a rapid detector of aversive environmental stimuli and situations, producing affective or behavioural states to allow for adaptive responses to potential threats37,38. Along the way, this line of research uncovered crucial brain mechanisms of associative conditioning, arguably providing us with our best understood neurobehavioural model of learning.
The LA has been the focus of many studies because it has ready access to information about the auditory cue used in conditioning, and lesions of this region block acquisition of conditioned freezing16,17,39. LA neurons develop and maintain excitatory neural responses to the onset of an auditory cue that has been paired with a footshock US25,26,40,41. These responses in vivo probably arise from potentiation of sensory inputs onto LA neurons because CS–US pairings enhance measures of excitatory synaptic plasticity in vivo42 and in acute amygdala slice preparations43–45. In this model, an initially weak afferent carrying sensory information about the CS and a strong afferent carrying US information converge onto individual principal neurons in the LA and, through a Hebbian plasticity mechanism, lead to enhanced strength of the excitatory synapses carrying CS information. This experience-dependent synaptic strengthening allows the presentation of the CS alone to activate LA neurons. The model of CS and US convergence has been explored by studies that took advantage of the temporal specificity of optogenetics to activate neural elements in a time window corresponding to the few seconds of CS or US presentation. The first study expressed the excitatory opsin, channelrhodopsin 2 (ChR2), in LA principal neurons to allow for rapid and reversible activation of these neurons during behaviour. When paired with an auditory CS, this simultaneous photoactivation of LA neurons could be used as a substitute for the footshock US, resulting in conditioned freezing46. The second study showed that brief photoactivation of ChR2-expressing LA axonal terminals from the auditory thalamus (the medial geniculate nucleus, MGN) and the auditory cortex (AC) can substitute for a tone CS when paired with footshock, resulting in conditioned freezing and synaptic potentiation47.
Lesions of the CeA block expression of conditioned fear17,48,49, leading to the hypothesis that projection routes from the LA to the CeA could allow for conditioned freezing behaviour. Notably, the LA does not project directly to the CeM, the proposed output nucleus that projects to non-amygdala regions mediating behavioural and autonomic signs of fear37,50,51. The advent of cell-type- and projection-specific manipulation shed light on information flow from the LA to the CeA, and then within the CeA itself in terms of conditioned fear — information that was unattainable from lesions that remove the entire network. An important step was identifying two subpopulations of neurons within the CeL that have opposing functions and distinct genetic markers. These are neurons in the CeL that are inhibited in response to CS following fear conditioning and express protein kinase C (PKC)δ, termed CeLOFF cells, and neurons that are excited by CS following fear conditioning that are PKCδ−, termed CeLON. Although both subtypes of CeL neurons inhibit one another and both project to the CeM52,53, CeLON neurons respond to a fear CS at a shorter latency than CeLOFF 52, suggesting that conditioned fear responses occur following activation of CeLON neurons that inhibit CeLOFF neurons projecting to CeM output neurons53, thereby promoting freezing through disinhibition52,53.
These studies did not, however, directly address the issue of transmission between the LA and the CeA. A recent series of studies capitalized on the advantages of genetic targeting in mice to characterize a neuronal population in the CeL that receives LA input and might be involved in fear conditioning. Using a transgenic mouse line in which Cre recombinase is expressed in somatostatin-positive (SOM+) neurons, the ability of ChR2-expressing LA terminals to activate these cells could be determined. The LA was shown to form functional excitatory synapses on SOM+ and PKCδ− cells in the CeL (CeL:SOM+), and the excitatory strength of these synapses greatly increased as a result of fear conditioning54. This finding is notable because the LA was considered to be the primary site for learning-related plasticity underlying fear conditioning. The presence of experience-dependent potentiation in the CeL indicates that this region is also important for acquisition of the CS–US association, as suggested by earlier studies17,40,52, rather than merely serving as a relay of information from the LA.
The importance of this experience-dependent plasticity onto CeL:SOM+ neurons in fear acquisition was demonstrated using a pharmacogenetic approach to reversibly silence only the CeL:SOM+ neurons during the acquisition phase of fear conditioning. Using a cre-dependent virus to express the inhibitory DREADD receptor hM4Di, an engineered G-protein-coupled receptor activated by its exogenous ligand clozapine N-oxide, the SOM+ cells could be reversibly and selectively inhibited. This inhibition prevented fear acquisition and excitatory synaptic enhancement54. Using the retrograde tracer cholera toxin B injected into SOM+ terminal regions to allow whole cell recording from projection-defined cells, it was revealed that SOM+ cells with learning-induced synaptic enhancement project directly to the periaqueductal grey (PAG) as well as the paraventricular nucleus of the thalamus (PVT)55, bypassing the CeM. Thus, CeL projections to the CeM might not be the only CeL projections that contribute to freezing behaviour after fear conditioning. This evidence revises the view of the CeM as the primary output station of the amygdala and indicates that information at various stages of processing in the CeA is sent to the PAG.
Although pieces of information are continually emerging, these circuit analyses reveal a rich interaction between the LA and CeL — and among neurons within the CeL — in both acquiring and expressing conditioned freezing in response to a fear cue, as well as multiple neural projections from the CeL that may regulate this behavioural response. Fear conditioning to the environment in which footshock is experienced is also mediated by the amygdala. Circuit approaches to contextual fear conditioning are underway; a recent study found that optogenetic inhibition of the BLA terminals in the entorhinal cortex (EC) impairs the acquisition of contextual fear conditioning56, whereas photoexcitation of oxytocin (OT)-releasing fibres from the hypothalamus to the CeL suppresses expression57.
Beyond fear in the amygdala
The robustness of fear conditioning provided a highly replicable behavioural paradigm that allowed researchers to delve deep into the circuit mechanisms underlying this simple behaviour. However, the view that the amygdala is specialized only for fear conditioning is too narrow. Strong evidence supports an integral role for this region in other aversive states, such as anxiety, as well as in reward, providing a challenge for determining how neuronal activity within the amygdala could contribute to each of these distinct processes.
Cell-type- and projection-specific manipulations are providing a way forward. An example comes from the reverse translational study of anxiety described earlier. To probe the role of BLA–CeA projections in anxiety-related behaviour, the excitatory opsin, ChR2, was expressed in BLA neurons. Consistent with previous work, BLA-cell-body activation decreased time spent by mice in the open arms of an elevated plus maze (EPM), indicating an increase in anxiety-like behaviour36. However, on selective excitation of the BLA–CeL pathway an anxiolytic behavioural phenotype of exploration of the EPM open arms was observed. Thus, a glutamatergic BLA projection to the CeA can promote anxiolysis36.
In this study, the identity (that is, ON or OFF cell, PKCδ+ or PKCδ−) of the CeL neurons activated by BLA terminal optical stimulation was not determined. New evidence suggests that these neurons are CeL:PKCδ+; when ChR2 was selectively expressed in CeL:PKCδ+ neurons to allow their direct photoactivation, increased time in the open arms of the EPM was observed58. These data support the notion that a population of BLA neurons preferentially excites CeL:PKCδ+ neurons that lead to reduced anxiety, perhaps through the projection from CeL:PKCδ+ to CeM output neurons. Alternatively, the CeL:PKCδ+ neuronal projection to the BNST, also implicated in anxiety59,60, could mediate anxiolysis. These studies show that the BLA–CeA pathway does not universally promote aversive states. Of note, there are other pathways that originate in the BLA that have since been implicated in bidirectional regulation of anxiety. Optically driving BLA projections to the ventral HPC (vHPC) is anxiogenic61, whereas photostimulation of BLA inputs in the anterodorsal BNST (adBNST) is anxiolytic62. Photostimulation of the BLA–vHPC pathway also decreases social interaction63, an effect perhaps related to the increase in anxiety that is seen following BLA–vHPC activation64.
New findings continue to enlarge our conceptions of CeA circuitry in non-fear behaviour. In a recent study on feeding suppression, genetic targeting of CeL:PKCδ+ neurons was used to selectively express hM4Di to allow for reversible neuronal inhibition. In mice, reducing the activity of CeL:PKCδ+ neurons was found to block decreased feeding stimulated by sickness or unpalatable tastes58. Conversely, optogenetic excitation of CeL:PKCδ+ neurons dramatically decreased feeding in both hungry and sated mice, and this was not a confound of reduced activity or increased anxiety. These results indicate that CeL:PKCδ+ neurons suppress feeding in response to anorexigenic stimuli58.
What inputs might normally serve to drive CeL:PKCδ+ neurons and suppress feeding? Tract tracing methods using cre-dependent retrograde and anterograde viral techniques can reveal the specific inputs and outputs of a given cell type65. Restriction of rabies virus to CeL:PKCδ+ neurons to allow labelling of presynaptic inputs, combined with c-fos immunohistochemistry to measure cellular activation, revealed multiple inputs to CeL:PKCδ+ neurons that are activated by anorexigenic signals, including the BLA, the parabrachial nucleus (PBN) and the insula (IN)58. These findings collectively demonstrate a unique pathway for feeding suppression that relies on CeL circuitry and that was not readily identified using pharmacology or lesion experimental approaches.
The identification of a role for amygdala circuitry in anxiolysis and feeding suppression makes one look at this circuitry differently in comparison to when only considering fear. How could these processes co-exist? Excitatory responses in the BLA to a fear cue activate CeLON:PKCδ− cells, some of which are SOM+, to initiate freezing. By contrast, excitatory responses in the BLA also activate CeLOFF:PKCδ+ cells to promote anxiolysis; to account for these findings, separate functional groups of BLA neurons projecting to CeL are proposed (Fig. 4). One route for the production of freezing and other measures of fear is through inhibition of CeL:PKCδ+ neurons that disinhibit CeM neurons; additional routes through CeL projections to other targets might also mediate conditioned freezing. Thus, the postsynaptic targets that mediate anxyiolysis after excitation of CeL:PKCδ+ neurons may or may not be CeM neurons, and if they are, it is possible they are different CeM neurons to those mediating freezing.
Figure 4. Model of amygdala microcircuits that give rise to behaviour.
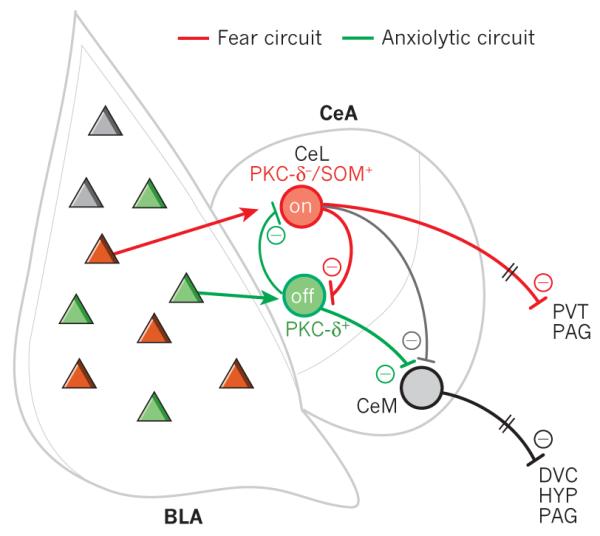
New findings in the amgydala have updated our understanding of these microcircuits. Different populations of basolateral complex of the amygdala (BLA) neurons are proposed to activate distinct populations of lateral central nucleus of the amygdala (CeL) neurons to either promote fear or reduce anxiety. CeM, medial central nucleus of the amygdala; DVC, dorsal vagal complex; PAG, periaqueductal grey; PKC, protein kinase C; PVT, paraventricular nucleus of the thalamus; HYP, hypothalamus; SOM, somatostatin.
In summary, recent studies have demonstrated that the BLA–CeA circuitry is involved in a diverse array of behaviours in addition to those related to fear. The key to understanding the production of these different behaviours is in the functional anatomy.
Adding in reward
The above findings indicate that there must be a diversity of neuronal responses in the BLA, as opposed to only responses to fear cues; in fact, this was well known from in vivo electrophysiological recordings and is necessary to explain the effects of amygdala lesions on other behaviours besides fear conditioning. In parallel to early studies on fear conditioning, amygdala lesions were also found to impair reward-based behaviour66–72. For example, LA lesions prevent amphetamine place preference conditioning70, a procedure in which subjects learn to associate a particular spatial location with the reinforcing effects of the drug, and CeA lesions prevent conditioned orienting responses to reward-predictive cues68. Hence, the same lesions can impair Pavlovian conditioned behaviour in response to cues signalling either a rewarding or an aversive outcome.
Notably, amygdala lesions do not impair all behaviours emitted in response to reward-predictive cues, rather, they impair the ability to respond to cues in the face of changing reward value, leading to the hypothesis that learning mediated by the amygdala is related to the current, relative value of biologically significant outcomes8,73,74. This view is congruent with the broader notion that the amygdala provides information about the ‘state value’ of an organism, defined as the value of the overall situation of an organism at a given moment7.
The BLA and CeA seem to make distinct contributions to the representation of value, as revealed through procedures designed to change the current value of an outcome. BLA lesions impair the ability of value changes in specific reward outcomes to affect behaviour. Thus, the BLA is proposed to represent outcome value along with specific sensory features, allowing for discrimination among multiple outcomes of a similar valence23,72,73. By contrast, the CeA is considered to maintain a more general representation of the motivational significance of an outcome23,73.
Just as BLA neurons develop excitatory responses to a CS paired with aversive outcomes, they show excitatory responses to auditory, visual or olfactory CSs paired with rewarding outcomes, typically sweet liquid or food pellets75–79. As initially proposed for fear conditioning, evidence suggests that reward cue responses develop through long-lasting enhancement of glutamatergic inputs from sensory thalamus onto principal neurons in the LA78. Of note, the acquisition of conditioned responding to both fear and reward cues requires an NMDA-receptor-dependent increase in AMPA-receptor function in LA neurons44,46,78. The end result of this synaptic potentiation is that the cue is able to drive spiking in LA neurons, which in turn activates neuronal populations in downstream regions that contribute to the cue-triggered behaviour. Clearly, learning about environmental stimuli that predict the occurrence of food and other rewards is adaptive, as is learning about potential aversive outcomes. Thus, learning across a valence continuum of positive (rewarding) to negative (punishing or aversive) outcomes engages the amygdala.
Amygdala neurons encode valence
Because both fear and reward cues recruit BLA neurons, these findings raise the question of how processing of fear and reward cues by amygdala networks is organized. For example, would neurons with excitatory responses to fear-predictive cues also show excitatory responses to reward-predictive cues, or is there segregation of positive- and negative-valenced signals? This question was addressed by directly comparing neural responses in the same subjects following training on both appetitive and aversive tasks. These within-subject comparisons in rodents and non-human primates consistently reveal populations of valence-selective neurons79–85 such that some neurons excited by a fear cue do not respond to a reward cue, or show inhibition in the presence of the reward cue, and vice versa. By training subjects on two parallel cue outcome associations, with one cue followed by a rewarding outcome, and the other followed by an aversive outcome, and then reversing the cues assigned to each outcome, it was revealed that a substantial proportion of cue-selective neurons encode the outcome with which it is currently paired, not the sensory features of the cue itself81,82. Outcome-specific neuronal populations are consistent with valence-sensitive neuronal populations that are responsive to one valence, but not another. The finding that different BLA populations respond to a fear cue after learning than after extinction, when that cue signals no footshock86, can also be interpreted as valence encoding. The observance of valence-specific neuronal activity is congruent with the notion that the amygdala is concerned with the relative value of the outcome, the occurrence of which is signalled by the cue.
Notably, valence-encoding is complemented by salience-encoding; some BLA units show excitatory responses to both fear and reward cues80,83,85, and these responses are correlated with measures of autonomic nervous system activation83. The salience of a stimulus is defined as the intensity of a stimulus and is a second dimension along which stimuli are encoded, in line with common models of emotion87. The salience responses may contribute to processes of arousal and attention that enhance processing within the amygdala or in target regions. This may be reflected behaviourally in better performance in real-time and in enhanced learning. A role in signalling the salience of stimuli is in agreement with the suggested contributions of the BLA in attention88,89 and in enhancing memory storage in downstream regions90–92. The amygdala projects to basal forebrain cholinergic systems and midbrain dopaminergic systems, two means by which these effects may be mediated. For example, CeA projections to midbrain dopaminergic regions are required for conditioned orienting93. Furthermore, projections to sensory cortical regions, including primary visual cortex in primates21, may allow amygdala attentional signals to modulate stimulus processing94. In addition, findings suggest that attention modulates amygdala valence signals95.
What might lend a cell its functional phenotype? No obvious anatomical segregation of neurons that are sensitive to the reward or fear cue has been detected with electrophysiology83,96, demonstrating that neurons encoding two very different signals are intermingled within this structure. But wiring must still be fundamental to cell phenotype. A given BLA principal neuron, for example, is likely to be associated with reward or fear by virtue of its distinct connectivity, including both the pattern of specific inputs to that neuron and its projections to effector regions for expression of the adaptively appropriate behaviour. Amygdala neuronal pairs sensitive to stimuli of the same valence are more likely to show correlated neural activity than neuronal pairs sensitive to opposite valences, supporting the idea of functional networks96.
Membership in a memory network
How do these functional networks for fear, reward or anxiety arise? Molecular genetic studies in mice have investigated the size, stability and required initial conditions for the formation of memory ensembles after fear conditioning. Using expression of the activity-dependent gene Arc to visualize activated LA neurons, the proportion of principal neurons participating in the fear memory trace is estimated to be 15–25%97,98. Although there may be a larger deterministic population, final membership in the trace can be biased by enhancing the activity level of individual LA neurons by multiple methods, including CREB overexpression97 and brief optical activation immediately before tone–footshock pairing, which takes advantage of the temporal precision of optogenetics to prove that activity enhancement need only be present immediately before training98. Selective ablation after training of CREB-overexpressing neurons abolished conditioned freezing, providing strong evidence that these neurons are recruited into the memory trace99. Expressing the excitatory DREADD hM3Dq in a sparse population of LA neurons and activating these receptors during fear conditioning enhanced fear memory and biased the inclusion of these neurons into the fear trace. When the hM3Dq receptors are activated without presentation of the CS, the conditioned freezing response is partially recapitulated98. Importantly, similar mechanisms act for reward learning; the acquisition and maintenance of a cocaine-conditioned place preference depends on the recruitment of a small population of neurons in the LA100. These studies provide evidence that subsets of LA neurons stably participate in fear and reward memory traces, and there is similar evidence for ensembles in the BA101.
Although there do seem to be similarities in the cellular mechanisms that promote inclusion into memory networks, does that mean that any LA neuron could be either a ‘fear’ or ‘reward’ neuron, or are there constraints on ensemble membership? A recent study102 provides evidence that valence-specific ensembles in the BLA (including the LA), once formed, are immutable, which does not directly demonstrate that there are anatomical constraints on initial ensemble formation, but is congruent with that possibility. This study used ‘memory trace tagging’ approaches to determine whether a given neuron is limited to representing one valence or shows equipotentiality. ChR2 expression was limited to BLA or HPC neurons in the dentate gyrus (DG) that expressed c-fos on either fear (footshock) or reward (mate exposure) conditioning, thereby labelling a network of neurons that presumably constitute part of the memory trace. Later, optical activation of these networks supported either approach or avoidance following fear or reward conditioning, respectively, in a real-time place preference (RTPP) task, establishing the ‘valence’ of the labelled network. To examine the reversibility of valence assignment, a network of a given valence was activated during subsequent retraining with outcomes of the opposite valence. Whereas pairing an ensemble of DG neurons activated with one valence with the opposite valence US could produce a ‘switch’ in the valence of the tagged ensemble, this was not true for BLA ensembles102. It seems likely that this ‘fixed valence’ feature derives from the distinct wiring of BLA neurons into positive- and negative-valenced networks, and is in agreement with the observations made within the awake recording studies already discussed. Together, these findings raise the question of what the cellular identity and anatomical connectivity of those positive or negative valence ensembles might be.
Although reward and fear networks in the BLA seem to occupy an overlapping spatial location, a recent study using c-fos activation patterns paints a different picture for the CeA. By taking advantage of the different time courses for stimulation of c-fos mRNA and protein production, the CeA neuronal populations activated by two oppositely valenced stimuli were visualized using fluorescence immunohistochemistry and in situ hybridization within mice. Neurons activated by morphine were located primarily in the CeL, whereas neurons activated by footshock were mainly found within the CeM103. Although this study provides intriguing new information on the activation of CeA populations in a valence-specific manner, earlier studies already reviewed indicate that neurons within both the CeL and CeM contribute to diverse behaviours, so the functional implications of this result remain to be determined.
Valence encoding in BLA inhibitory networks
An important key to understanding neural diversity is determining how individual neurons act within a circuit, and electrophysiology provides one means to query the role of a neuron. However, a drawback of in vivo extracellular recording studies is that the neuronal subtype, such as projection neuron or interneuron, can only be inferred from physiological measures. The ability to target opsin expression in defined neuronal subpopulations, in combination with electrophysiology, is overcoming this issue and has allowed us to gain new understanding of the inhibitory processes in the amygdala.
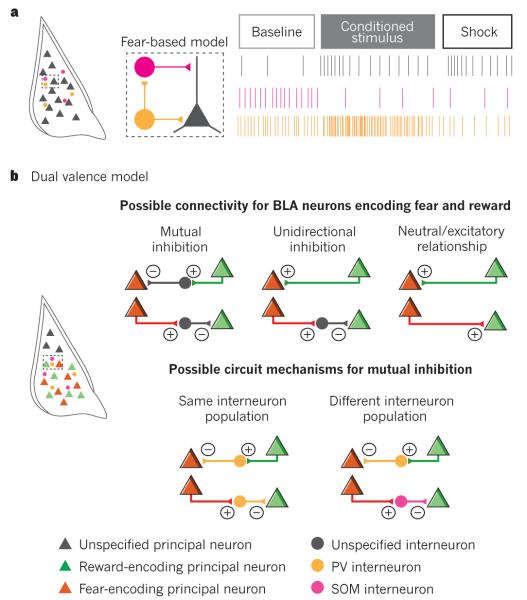
As described above, interactions among CeL inhibitory neurons, including inhibitory neurons that send projections outside the CeL itself, are crucial for the production of behavioural measures of fear. By contrast, in the BLA, inhibition entails suppression of excitatory principal projection neurons by local inhibitory interneurons and intercalated neurons. A recent study harnessed the power of optogenetics to study inhibitory BLA interneurons by temporally restricting excitation or inhibition of parvalbumin (PV) or SOM interneurons to the US footshock or the auditory CS, and observing the effect on fear conditioning104. Intriguing evidence of two different local circuit mechanisms that disinhibit principal neurons was found: during US presentation, PV neurons that provide perisomatic innervation of BLA principal neurons are inhibited by footshock, thereby directly disinhibiting BLA principal neurons; during the CS, PV neurons inhibit SOM interneurons that selectively contact principal neuron dendrites to disinhibit input-driven activity (Fig. 5a). Unit recordings were made during behaviour, and comparisons between these naturally occurring and optically evoked waveforms were used to convincingly ascribe neural correlates of behaviour to spike activity of PV and SOM interneurons. The electrophysiological activity of a subset of these interneurons during the CS and the US was the same as predicted by the model constructed from the optogenetic manipulations, providing support for the proposed local circuit mechanisms104. Thus, biologically significant stimuli and the cues that predict them change the firing of inhibitory interneurons, the activity of which gates the responsiveness of principal neurons.
Figure 5. Interneuron and principal neuron interactions within the basolateral complex of the amygdala (BLA).
a, Model of how interneurons expressing parvalbumin (PV) and somatostatin (SOM) interact with principal neurons to mediate fear conditioning89. b, The heterogeneity in PV interneuron responses is consistent with the diverse functionality of BLA principal neurons and raises the question of how BLA principal neurons may interact locally. Depicted are simplified scenarios for these interactions.
Interestingly, the in vivo activity of PV and SOM interneurons is not limited to the role suggested by the above model. Of note, in vivo recordings found that a substantial subset of interneurons responded to the CS and US in an opposite way to that predicted by the model104, yet another example of neuronal response diversity. This is consistent with a view of parallel opposing circuits in the BLA, in which distinct populations of inhibitory neurons may govern the activity of BLA neurons mediating positive and negative valenced associations.
Together, the findings support proposed roles for inhibitory neurons in shaping functional networks in the amygdala6,12. Neuronal networks in the BLA may actively suppress other networks, the output of which would be incompatible with current behavioural requirements by feed forward inhibition onto a principal neuron through an intervening interneuron activated by a neighbouring principal neuron encoding a different valence or behaviour. To flexibly switch between different behavioural states, mutual inhibition among parallel competing networks is an attractive solution, although there may be other means of interaction among competing networks (Fig. 5). It is probable that long-range excitatory synapses, for example from the cortex, onto inhibitory interneurons (or GABAergic intercalated populations29) are crucial for the suppression of opposing networks105 (for simplicity, external afferent influences are not depicted in Fig. 5b). Other means to inhibit opposing networks include changes in synaptic input; extinction, for example, induces heterosynaptic inhibition of thalamic inputs onto LA neurons105. In addition, the effect of interneurons on principal neurons is dynamic. New evidence indicates that inhibitory synapses onto principal cells change with experience; extinction training increases PV+ perisomatic synaptic contacts on principal neurons that are activated during fear learning106, an example of how inhibition can remodel a network as the value of a cue changes.
Valence, amygdala circuits and behaviour
Given its access to primary sensory information, the amygdala seems well suited to rapidly process and transmit information regarding the positive or negative valence of stimuli to bias behaviour in an adaptive manner. The evidence reviewed above suggests that networks within the amygdala are organized into distinct neural circuits for positive- and negative-valenced stimuli. How do these circuits come to affect behaviour?
To answer this question, we must map functionality onto the anatomical connections within the amygdala itself and onto projections from the amygdala. For fear conditioning, functional circuit mapping is relatively well developed, as described above. In the case of reward-related behaviours, locomotion, directed approach and manipulation of objects in the environment in order to obtain or interact with the reward are usually required. These types of coordinated, but flexible action patterns, engage corticostriatal circuitry. The BLA projects robustly to the NAc107, which mediates motivated responding to reward-predictive cues for both natural and drug rewards; hence the BLA–NAc projection could allow information regarding the current value of cues to affect reward-related behaviour, as suggested by earlier findings66,67,108. These ideas can be directly assessed using projection-specific manipulations during behaviour. The first findings along these lines reported that the BLA–NAc projection is in itself sufficient to support positive reinforcement, as demonstrated by intracranial self-stimulation109,110 and RTPP, in which entry into a particular spatial location triggered stimulation of BLA–NAc terminals109. More recently, optical inhibition of the BLA–NAc projection (as well as the BLA–prelimbic (PL) projection) was found to prevent reinstatement of responding in reaction to a cocaine-paired cue in an animal model of relapse, further supporting a role for this projection in cue–reward associations111. These findings demonstrate that some BLA neurons involved in reward project to the NAc. Perhaps a subset of these receive efferents from the PFC; photoactivation of mPFC terminals in the BLA has been reported to increase instrumental responses for food112. Collectively these studies have begun to define amygdala circuits that contribute to reward-related behaviour, although the circuit analysis is not as far along for reward as it is for fear.
As function continues to be mapped onto amygdala circuitry, our understanding of how neural signals in the amygdala affect behaviour will continue to grow. A wide range of behavioural changes has been achieved by optogenetic or pharmacogenetic manipulation of the projections studied so far (Fig. 3). Two observations can be made: first, multiple projections leading from the amgydala affect a single behaviour, and second, behaviours of different or opposite valence are affected by projections between the same two brain regions. Both conditioned freezing and anxiety exemplify the first observation. In the case of anxiety, for example, the finding that three different BLA efferents influence anxiety-like behaviour36,61,62 suggests that even at the level of the comparatively simple system of the amygdala, neural control of a single behaviour is not reduced to one serial pathway but is normally accomplished by multiple circuits, although these circuits potentially interact. The second observation that the same pathway may affect very different behaviours is exemplified by the role of the BLA–PL pathway in both fear and relapse to reward seeking. In each of these behavioural procedures111,113, photoinhibition of the pathway impaired the behavioural effects of the conditioned cue; this pathway may be valence-independent, or may carry mixed fibres. These possibilities can be distinguished using the types of approaches discussed here.
This new functional map supports a view of the amygdala as a composite of parallel circuits that contribute to multiple behavioural states. Although there may be a substantial degree of overlap among the circuit elements, the circuits are differentiated by the specific details of their neural connections, both afferent and efferent, and the patterns of activation of those connections. Hence, to fully understand the relationship between neurons that transmit information about the valence of a stimulus and subsequent behaviour, neural circuit analysis is key. This way of thinking about the amygdala is different from past conceptions of it as a fear hub or as a circuit providing a readout of positive or negative affect in simple terms. Instead, the emphasis is on understanding the behaviourally relevant functions of paths of information flow through these regions, including how diverse, primarily sensory, inputs might interact locally to produce varied downstream functional effects.
Although we make the case that the amygdala contributes to a diverse set of behaviours, as revealed by the fine-grained analysis of circuits, the notion of valence remains a useful heuristic. Sensitivity to the valence of a stimulus, of whether it is good or bad, is crucially important to ensure appropriate behavioural responses that promote approach and the acquisition of food, safety and social partners, and in alternative circumstances, that promote vigilance, avoidance or aggression towards threats. Each of the behaviours affected by amygdala manipulations falls somewhere along the continuum of ‘good’ to ‘bad’. We note that organizing adaptive behaviours only along the single dimension of valence is certainly an oversimplification, but this may capture an essential feature of these diverse behaviours that engages the amygdala.
Looking forward
Recently, we have made great strides in delineating the functional microcircuitry of the amygdala using the new technologies of optogenetics, pharmacogentics and viral-based tract tracing that take advantage of gene-targeting. Crucially, these approaches have been used in partnership with state-of-the-art electrophysiology and careful behavioural analysis. This programme of circuit analysis is equally applicable to the study of other neural systems and is a way forward towards deeper knowledge of the functions that emerge from neural circuits.
As we look to the future of research on amygdala circuits, we consider areas that deserve attention. It is important to define amygdala functional microcircuitry in preclinical models of human behavioural disorders. Animal and human studies implicate the amygdala in anxiety disorders, autism and addiction. In the case of addiction, chronic alcohol use alters neural transmission in the CeA, and these changes have been linked to excessive alcohol use114. Furthermore, alcohol and drug seeking triggered by auditory cues requires the BLA115,116, whereas memory traces related to the smell and taste of alcohol that drive relapse are stored in the CeA117. In the case of autism, understanding the mediation of social interaction by the amygdala is highly relevant64,118, adding momentum to this line of research63.
Of great interest is determining how information from the amygdala affects downstream cortical circuits. Much research indicates that neural connectivity with the orbital prefrontal cortex is important for updating cue values after changes in their associated outcome8,119,120. In addition to the orbital prefrontal cortex, BLA neurons project strongly to the medial PFC. The function of BLA signals in these cortical regions is less likely to be closely tied to discrete behavioural output. Instead, these BLA–cortical projections are proposed to mediate the impact of Pavlovian associations on decision making7,121. In addition, electrophysiological recordings have uncovered dynamic experience-dependent interactions between the amygdala, PFC and HPC in the entrainment of oscillations of different frequencies122,123, but their causal role in behaviour is not clear. Selective projection manipulations may reveal the circuit and behaviour impacts of these long-range interactions. New studies manipulating specific amygdala projections to the cortex are beginning to address these issues111,113.
Increasingly, the model systems available for circuit analysis will include primates as genetically accessible non-human primate models are developed and viral-mediated tools for neural manipulation are optimized, allowing for further investigation of species similarities and differences in amygdala function. Moving forward, it is crucial to complement causal manipulations with measurements of neural activity during a wide variety of behaviours across multiple species.
Although we champion the ability to manipulate circuit components that can be isolated with genetic or anatomical features, the existing tools still have limitations that prevent a comprehensive understanding of these circuits. Genetically encodable tools for neural manipulation allow far greater targeting specificity than before, but it is unlikely that all of the neurons that project from one region to another, or that share a genetic marker, have identical functions. Thus, we may still be observing a ‘majority vote’ for a given behavioural readout when manipulating any circuit component, and only more specific targeting strategies will reveal the functional minority populations. Along these lines, the synchrony and timing of most photostimulation experiments are not physiological and could disturb important rhythmic interactions across distal networks in ways we do not understand. To solve these issues, we need a greater library of molecular markers and tools to target combinatorial expression patterns, and we need the means to induce more naturalistic activity patterns in neurons. Basic science insights into molecules, synapses, cells and circuits will need to be synthesized to achieve this level of understanding.
Acknowledgements
P.H.J. acknowledges funding from US National Institutes of Health grants DA015096, AA014925, AA17072. K.M.T. is a New York Stem Cell Foundation-Robertson Investigator and acknowledges funding from the JPB Foundation, PIIF, PNDRF, NARSAD Young Investigator Award, Whitehead Career Development Chair, and NIH grant MH102441. We thank K. Vitale for input regarding interneurons and network selection, G. Calhoon and P. Namburi for input on Fig. 5, R. Keiflin for assistance with Fig. 3, B. Saunders for comments on our text, J. Gabrieli for input on human amygdala research, I. Choi for assistance illustrating Fig. 1 and all the members of our laboratories for valuable discussion.
Footnotes
The authors declare no competing financial interests.
References
- 1.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 2.Jarvis ED, et al. Avian brains and a new understanding of vertebrate brain evolution. Nature Rev. Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston JB. Further contributions to the study of the evolution of the forebrain. J. Comp. Neurol. 1923;35:337–481. [Google Scholar]
- 4.Kappers CUA, Huber GC, Crosby EC. Including Man. Macmillan; 1936. The Comparative Anatomy of the Nervous System of Vertebrates. [Google Scholar]
- 5.Lanuza E, Belekhova M, Martínez-Marcos A, Font C, Martínez-García F. Identification of the reptilian basolateral amygdala: an anatomical investigation of the afferents to the posterior dorsal ventricular ridge of the lizard Podarcis hispanica. Eur. J. Neurosci. 1998;10:3517–3534. doi: 10.1046/j.1460-9568.1998.00363.x. [DOI] [PubMed] [Google Scholar]
- 6.Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison SE, Salzman CD. Re-valuing the amygdala. Curr. Opin. Neurobiol. 2010;20:221–230. doi: 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray EA. The amygdala, reward and emotion. Trends Cogn. Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Stamatakis AM, et al. Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology. 2014;76:320–328. doi: 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pape H-C, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrlich I, et al. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Brown S, Schäfer E. An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Phil. Trans. R. Soc. B. 1888;179:303–327. [Google Scholar]
- 14.Klüver H, Bucy P. ‘Psychic blindness’ and other symptoms following bilateral temporal lobectomy in Rhesus monkeys. Am. J. Physiol. 1937;119:352–353. [Google Scholar]
- 15.Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J. Comp. Physiol. Psychol. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- 16.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J. Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J. Comp. Physiol. Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 18.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 19.Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 20.Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Freese JL, Amaral DG. The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. J. Comp. Neurol. 2005;486:295–317. doi: 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- 22.Chareyron LJ, Banta Lavenex P, Amaral DG, Lavenex P. Stereological analysis of the rat and monkey amygdala. J. Comp. Neurol. 2011;519:3218–3239. doi: 10.1002/cne.22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J. Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur. J. Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- 25.Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 26.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. This is a seminal study showing the increased responding of LA neurons to a CS after fear conditioning.
- 27.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 28.Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- 29.Amano T, Unal CT, Paré D. Synaptic correlates of fear extinction in the amygdala. Nature Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 31.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 32.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 33.Monfils M-H, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch. Gen. Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tye KM, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. This was the first study to use optogenetic projection-specific manipulations; it showed that activation or inhibition of BLA projections to the CeL nucleus could cause anxiolytic or anxiogenic effects on behaviour, respectively.
- 37.Davis M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 38.LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 39.Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn. Mem. 2001;8:156–163. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins DR, Paré D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS+ and CS. Learn. Mem. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maren S. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. Eur. J. Neurosci. 2000;12:4047–4054. doi: 10.1046/j.1460-9568.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- 42.Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 43.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. Along with ref. 42, this was the first evidence to show synaptic enhancement onto LA neurons after fear conditioning.
- 44.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 46.Johansen JP, et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc. Natl Acad. Sci. USA. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nabavi S, et al. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol. Behav. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- 49.Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav. Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- 50.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viviani D, et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 52.Ciocchi S, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 53.Haubensak W, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. Together with ref. 52 this study identified functionally and genetically distinct populations of neurons in the CeL in the expression of conditioned fear.
- 54.Li H, et al. Experience-dependent modification of a central amygdala fear circuit. Nature Neurosci. 2013;16:332–339. doi: 10.1038/nn.3322. This article reports that experience-dependent plasticity occurs at LA–CeL:SOM+ synapses, demonstrating that amygdala plasticity occurs in more than just the LA.
- 55.Penzo MA, Robert V, Li B. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J. Neurosci. 2014;34:2432–2437. doi: 10.1523/JNEUROSCI.4166-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sparta DR, et al. Inhibition of projections from the basolateral amygdala to the entorhinal cortex disrupts the acquisition of contextual fear. Front. Behav. Neurosci. 2014;8:129. doi: 10.3389/fnbeh.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knobloch HS, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 58.Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nature Neurosci. 2014;17:1240–1248. doi: 10.1038/nn.3767. This study showed that PKCδ+ neurons suppress feeding and are anxiolytic, and using a ‘cre-out’ strategy demonstrated opposing functions for PKCδ+ and PKCδ− neurons.
- 59.Jennings JH, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim S-Y, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Felix-Ortiz AC, et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim S-Y, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 2014;34:586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allsop SA, Vander Weele CM, Wichmann R, Tye KM. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front. Behav. Neurosci. 2014;8:241. doi: 10.3389/fnbeh.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl Acad. Sci. USA. 2010;107:21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- 67.Everitt BJ, Cador M, Robbins TW. Interactions between the amygdala and ventral striatum in stimulus-reward associations: studies using a second-order schedule of sexual reinforcement. Neuroscience. 1989;30:63–75. doi: 10.1016/0306-4522(89)90353-9. This study, along with ref. 66, provided early evidence that amygdala projections to the NAc mediate the effects of Pavlovian stimuli predictive of reward on behaviour.
- 68.Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J. Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J. Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hiroi N, White NM. The lateral nucleus of the amygdala mediates expression of the amphetamine-produced conditioned place preference. J. Neurosci. 1991;11:2107–2116. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- 72.Málková L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J. Neurosci. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Baxter MG, Murray EA. The amygdala and reward. Nature Rev. Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 75.Sanghera MK, Rolls ET, Roper-Hall A. Visual responses of neurons in the dorsolateral amygdala of the alert monkey. Exp. Neurol. 1979;63:610–626. doi: 10.1016/0014-4886(79)90175-4. [DOI] [PubMed] [Google Scholar]
- 76.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 77.Tye KM, Janak PH. Amygdala neurons differentially encode motivation and reinforcement. J. Neurosci. 2007;27:3937–3945. doi: 10.1523/JNEUROSCI.5281-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. This study demonstrated a causal relationship between synaptic potentiation in the amygdala and cue–reward learning, and showed amygdala neurons increase responses in vivo with cue–reward learning.
- 79.Uwano T, Nishijo H, Ono T, Tamura R. Neuronal responsiveness to various sensory stimuli, and associative learning in the rat amygdala. Neuroscience. 1995;68:339–361. doi: 10.1016/0306-4522(95)00125-3. [DOI] [PubMed] [Google Scholar]
- 80.Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. In this study, electrophysiological recordings showed that different populations of primate amygdala neurons encoded visual stimuli that predicted positive or negative outcomes.
- 82.Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J. Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. This was the first electrophysiological recording study demonstrating the ability of amygdala neurons to track changing outcomes across a reversal task.
- 83.Shabel SJ, Janak PH. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc. Natl Acad. Sci. USA. 2009;106:15031–15036. doi: 10.1073/pnas.0905580106. This study suggested that populations of amygdala neurons that encoded positive and negative outcomes were only partially non-overlapping; the overlapping population may encode salience.
- 84.Shabel SJ, Schairer W, Donahue RJ, Powell V, Janak PH. Similar neural activity during fear and disgust in the rat basolateral amygdala. PLoS ONE. 2011;6:e27797. doi: 10.1371/journal.pone.0027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sangha S, Chadick JZ, Janak PH. Safety encoding in the basal amygdala. J. Neurosci. 2013;33:3744–3751. doi: 10.1523/JNEUROSCI.3302-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herry C, et al. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 87.Russell JA. A circumplex model of affect. J. Personal. Soc. Psychol. 1980;39:1161–1178. [Google Scholar]
- 88.Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn. Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 89.Roesch MR, Esber GR, Li J, Daw ND, Schoenbaum G. Surprise! Neural correlates of Pearce-Hall and Rescorla-Wagner coexist within the brain. Eur. J. Neurosci. 2012;35:1190–1200. doi: 10.1111/j.1460-9568.2011.07986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 91.Huff ML, Miller RL, Deisseroth K, Moorman DE, LaLumiere RT. Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc. Natl Acad. Sci. USA. 2013;110:3597–3602. doi: 10.1073/pnas.1219593110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Popescu AT, Saghyan AA, Paré D. NMDA-dependent facilitation of corticostriatal plasticity by the amygdala. Proc. Natl Acad. Sci. USA. 2007;104:341–346. doi: 10.1073/pnas.0609831104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han JS, McMahan RW, Holland P, Gallagher M. The role of an amygdalo-nigrostriatal pathway in associative learning. J. Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 95.Peck CJ, Salzman CD. Amygdala neural activity reflects spatial attention towards stimuli promising reward or threatening punishment. eLife. 2014;3:e04478. doi: 10.7554/eLife.04478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang W, et al. Functional circuits and anatomical distribution of response properties in the primate amygdala. J. Neurosci. 2013;33:722–733. doi: 10.1523/JNEUROSCI.2970-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han J-H, et al. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 98.Yiu AP, et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 99.Han J-H, et al. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. This study provided causal evidence for a stable fear memory engram in the LA by ablating a small proportion of LA neurons overexpressing CREB.
- 100.Hsiang H-LL, et al. Manipulating a ‘cocaine engram’ in mice. J. Neurosci. 2014;34:14115–14127. doi: 10.1523/JNEUROSCI.3327-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 102.Redondo RL, et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 2014;513:426–430. doi: 10.1038/nature13725. This study used neuronal tagging to express ChR2 in valence-specific networks, demonstrating that positive and negative valenced networks in the BLA cannot be reversed to the opposite valence by retraining.
- 103.Xiu J, et al. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nature Neurosci. 2014;17:1552–1559. doi: 10.1038/nn.3813. [DOI] [PubMed] [Google Scholar]
- 104.Wolff SBE, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. Demonstration of unique roles for PV+ and SOM+ interneurons in combination with in vivo electrophysiology in behaving mice to provide new evidence for inhibitory networks contributing to fear conditioning.
- 105.Cho J-H, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron. 2013;80:1491–1507. doi: 10.1016/j.neuron.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trouche S, Sasaki JM, Tu T, Reijmers LG. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron. 2013;80:1054–1065. doi: 10.1016/j.neuron.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat–an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- 108.Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Britt JP, et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stuber GD, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front. Behav. Neurosci. 2013;7:213. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Land BB, et al. Medial prefrontal D1 dopamine neurons control food intake. Nature Neurosci. 2014;17:248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Senn V, et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 114.Roberto M, Gilpin NW, Siggins GR. The central amygdala and alcohol: role of γ-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb. Perspect. Med. 2012;2:a012195. doi: 10.1101/cshperspect.a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buffalari DM, See RE. Amygdala mechanisms of Pavlovian psychostimulant conditioning and relapse. Curr. Top. Behav. Neurosci. 2010;3:73–99. doi: 10.1007/7854_2009_18. [DOI] [PubMed] [Google Scholar]
- 116.Chaudhri N, Woods CA, Sahuque LL, Gill TM, Janak PH. Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur. J. Neurosci. 2013;38:2751–2761. doi: 10.1111/ejn.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barak S, et al. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nature Neurosci. 2013;16:1111–1117. doi: 10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baron-Cohen S, et al. The amygdala theory of autism. Neurosci. Biobehav. Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 119.Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 120.Morrison SE, Saez A, Lau B, Salzman CD. Different time courses for learning-related changes in amygdala and orbitofrontal cortex. Neuron. 2011;71:1127–1140. doi: 10.1016/j.neuron.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 122.Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nature Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seidenbecher T, Laxmi TR, Stork O, Pape H-C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]