Abstract
BACKGROUND
A case of homozygous familial lecithin:cholesterol acyltransferase (LCAT) deficiency with a novel homozygous LCAT missense mutation (replacement of methionine by arginine at position 293 in the amino acid sequence of the LCAT protein) is reported.
METHODS AND RESULTS
The probable diagnosis was suggested by findings of marked high density lipoprotein (HDL) deficiency, corneal opacification, anemia, and renal insufficiency. The diagnosis was confirmed by two dimensional gel electrophoresis of HDL, the measurement of free and esterified cholesterol, and sequencing of the LCAT gene.
CONCLUSIONS
In our view the most important aspects of therapy to prevent the kidney disease that these patients develop is careful control of blood pressure and lifestyle measures to optimize non HDL lipoproteins. In the future replacement therapy by gene transfer or other methods may become available.
Keywords: Apolipoprotein, High density lipoprotein, Lecithin:cholesterol acyltransferase, Corneal opacification, Anemia, Renal disease
A 50-year-old man presented with uncontrolled hypertension, hemolytic anemia, and renal insufficiency with baseline creatinine values around 2.2 mg/dL. He had a long history of proteinuria (3+ for at least 30 years). He was also known to have a low platelet count. Prior ultrasound examinations with Doppler studies of his kidneys showed no evidence of renal artery stenosis, but he did have evidence of multiple renal bilateral cysts, as well as splenomegaly (18 cm in the vertical dimension). His kidney size was normal (13.2 cm on the right and 14.4 cm on the left in the vertical dimension).
The patient had a history of bouts of brief intermittent atrial flutter and atrial fibrillation. At the time of this examination, his electrocardiogram was normal, as was his left ventricular ejection fraction on echocardiogram at 55%– 60%. He had no history of coronary disease or chest pain on exertion. He also had a history of treated Graves’ disease, and his most recent thyroid stimulating hormone level was normal at 1.78 μlU/mL. He had no history of liver disease, and his levels of liver alanine and aspartate transaminases were normal at 18 and 17 U/L, respectively, with a normal alkaline phosphatase of 81 U/L. Other past medical history included meningitis at age 1, a vasectomy in his 30s, and a history of gout. He also had a history of moderate obstructive sleep apnea being treated with continuous positive pressure therapy at night. He had no known allergies.
His parents were first cousins and they had emigrated from Lebanon. His father died at 79 years of age of sudden death (possibly coronary disease), and his mother is alive and well at 80 years of age. An older brother (55 years of age) has a history of arrhythmias, and is known to have a low high density lipoprotein (HDL) cholesterol level of 20 mg/dL. Two sisters (53 and 43 yeas of age) are alive and well. The 53-year-old sister was sampled and had a normal HDL cholesterol of 55 mg/dL, and was not affected. The 43-year-old sister had an HDL cholesterol of 37 mg/dL. Another brother at age 51 has a history of arrhythmias, but an HDL cholesterol of 62 mg/dL. Therefore, the parents were obligate heterozygotes, two siblings (one sister and one brother) are probable heterozygotes, and two siblings are normal. In addition the index case presented here is a homozygote.
The index case was on the following medications at the time of this report (March 2011): extended release diltiazem 90 mg/day, atenolol 25 mg/day, furosemide 40 mg/day, losartan 50 mg/day, terazosin 6 mg/day, allopurinol 200 mg/day for gout, ferrous sulfate 325 mg/day for anemia, and sodium bicarbonate ¼ teaspoon/day.
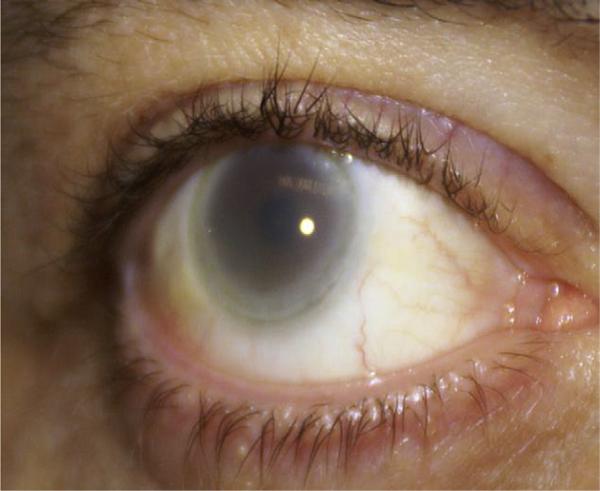
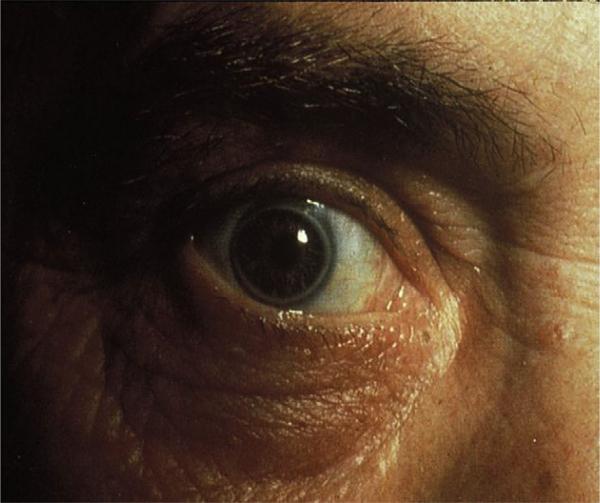
A physical examination showed a height of 75 inches (190 cm) and a weight of 234 pounds (106 kg), body mass index 29.4 kg/m2. His blood pressure was 145/85 mm Hg. He was noted to have striking diffuse marked corneal opacification (Fig. 1), quite different from the arcus senilis observed in a patient with heterozygous familial hypercholesterolemia (Fig. 2). The remainder of his eye examination was normal. He had a low grade 1/VI systolic ejection murmur in the aortic valve area of the heart. His pulses were normal throughout with no bruits. He had no evidence of an enlarged liver, but did have a palpable spleen. He had mild ankle edema.
Figure 1.
Photograph of the marked diffuse corneal opacification in the proband reported here. The opacification is especially marked near the limbal area, forming a circular band resembling arcus senilis.
Figure 2.
Photograph of the arcus senilis in a 50-year-old male patient with heterozygous familial hypercholesterolemia and premature CHD with a documented LDL receptor mutation. Note that despite the arcus senilis, the cornea is clear.
Two years before the current evaluation, he underwent a cardiac stress test with nuclear imaging, which was reported as normal. At the time of this evaluation he underwent a coronary calcium score assessment by computer tomography. This testing showed a calcium score of 90 Agatston units, placing him at the 85th percentile for his age and gender, with 96% of his calcification being in his left anterior descending coronary artery and the remainder in the right coronary artery. He does, therefore, have some evidence of mild coronary artery disease, but is asymptomatic. This degree of coronary calcification has been associated with mild coronary artery plaque burden and moderate coronary heart disease risk.
Laboratory analysis
Laboratory findings from the Joslin Diabetes Center (Boston, MA) showed urinalysis, specific gravity 1.015, 4+ protein, positive for red blood cells, but no white blood cells, pH 5.0; complete blood count: hematocrit 26% (decreased), mean red cell volume 93 (normal), platelet count 79,000/cc (decreased), a normal prothrombin time, normal liver enzymes, and a normal protein electrophoresis. His creatinine was elevated at 2.3 mg/dL (normal, ≤1.4 mg/dL), as was the parathyroid hormone level 115 pg/ml (normal, 10–60 pg/ml). His serum calcium and phosphorus levels were normal at 8.6 mg/dL and 4.2.mg/dL, respectively. His fasting lipid profile was: total cholesterol, 63 mg/dL (very low), triglyceride 73 mg/dL (normal), HDL cholesterol 7 mg/dL (very low), and his calculated low density lipoprotein (LDL) 41 mg/dL. This profile prompted his physicians to send a sample for further analysis to Boston Heart Laboratory, Framingham, MA (www.bostonheartlab.com) and to refer the patient to one of us (E.J.S.).
Results from this laboratory that maintains lipid standardization with the Centers for Disease Control showed: total cholesterol, 71 mg/dL; triglycerides, 139 mg/dL; HDL cholesterol, 7.4 mg/dL; non-HDL, 64 mg/dL; very low density lipoprotein (VLDL) cholesterol, 64 mg/dL; direct LDL and small dense LDL cholesterol, both below detection limits; apolipoprotein (apo), A-I 52 mg/dL (low); apoB, 32 mg/dL (low); and lipoprotein(a), 3 mg/dL (low); using methods described previously.1–4 His calculated VLDL cholesterol was 28 mg/dL and his calculated LDL cholesterol was 36 mg/dL. This discrepancy between calculated values based on the Friedewald formula (VLDL cholesterol = triglyceride/5, and LDL cholesterol = total cholesterol – HDL cholesterol – triglyceride/5) and direct measurements if often observed in patients with abnormal lipoprotein particle composition and unusual lipid disorders.
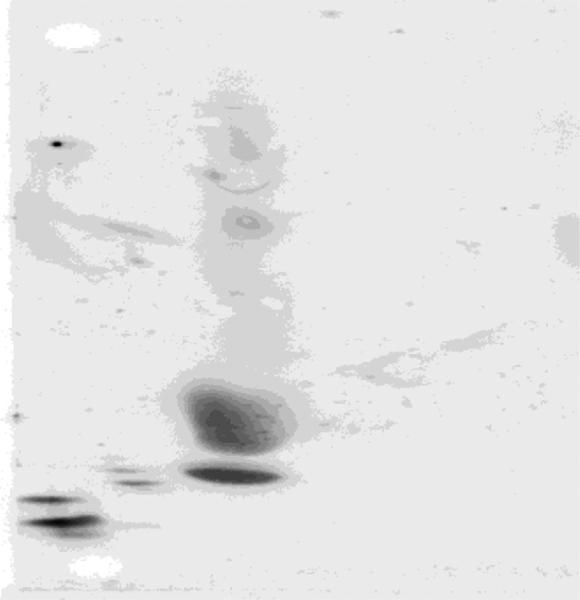
Results of the two-dimensional gel electrophoresis pattern for apoA-I containing lipoproteins (HDL) in plasma in mg/dL showed very small pre-β-1 HDL 11.5 (96% of normal, but with four pre-β bands instead of two, a highly unusual pattern that we have seen previously only in heterozygous and homozygous LCAT deficiency), small α-4 HDL 14 (117% of normal), medium α-3 HDL 18.1 mg/dL (150% of normal), large α-2 HDL 2.1 (5% of normal), and very large α-1 HDL 5.2 (30% of normal) as described previously (Fig. 3).4,5 A normal pattern, a pattern seen in a heart disease patient, and a schematic are shown in Figure 4.4 Sterol analysis carried out as described previously5,6 by gas liquid chromatography showed lathosterol (marker of cholesterol synthesis), and below detection limits, campesterol (marker of cholesterol absorption) 239 umol/mmol of cholesterol (normal), β sitosterol (marker of cholesterol absorption) 211 umol/mmol of cholesterol (normal). The patient was found to have the APOE3/3 (normal) genotype and was –/– for Factor V Leiden.
Figure 3.
The two-dimensional gel electrophoretic pattern of the proband plasma followed by immunoblotting with specific immunopurified apoA-I antibody is shown. We document additional pre-β HDL particles between the predominant pre-β HDL doublet and α-4 HDL. This has been observed in other LCAT-deficient patients.5 The total amount of apoA-I in pre-β 1 HDL particles was 96% of normal. This patient has a very compact dense α-4 HDL band that is 117% of normal in apoA-I concentration, and an increased α-3 HDL band (150% of normal) in terms of its apoA-I concentration. The patient also has a marked decrease in α-2 (5% of normal) and α-1 HDL (30% of normal) in terms of their apoA-I concentrations. The values of the larger particles, although decreased, are somewhat greater than what was observed in other patients with homozygous LCAT deficiency.5
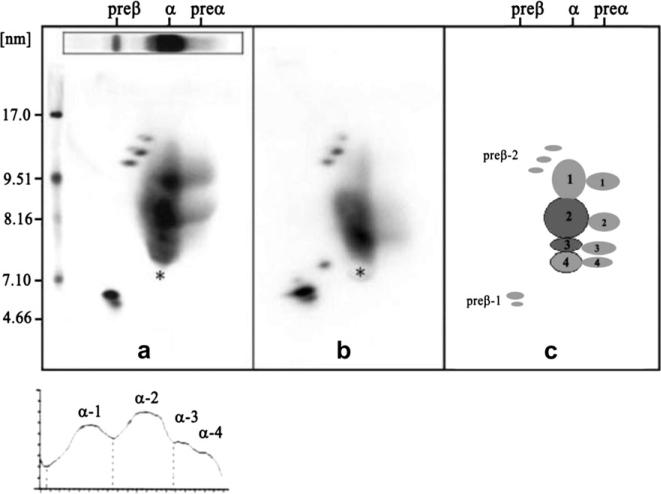
Figure 4.
Two-dimensional gel electrophoresis patterns followed by immunoblotting with monospecific apoA-I antibody of a normal subject (left) and of a patient with premature heart disease (center) in the center, and with a schematic of the individual HDL particles (right). The electrophoresis is carried out in the horizontal dimension to separate pre-β, α, and pre-α particles, and in the vertical dimension using a 4%–30% gradient gel (particles of 5–12 nm in diameter, followed by immunoblotting with specific apoA-I antibody). The pattern from the patient with premature coronary heart disease in the center has decreased large HDL. All of the particles shown contain apoA-I, but only the α-3 and α-2 HDL particles contain appreciable amounts of apoA-II.
Other parameters include C reactive protein 1.5 mg/L (normal), lipoprotein associated phospholipase A2 126 ng/ml (normal), NT-pro brain natriuretic peptide 245 pg/dL (slightly increased), glycosylated hemoglobin 4.7% (normal), insulin 19.9 μIU/mL (moderately increased), liver alanine and aspartate transaminases 18 and 17 U/L, respectively (normal), alkaline phosphatase 81 U/L (normal), blood urea nitrogen 67.2 mg/dL (elevated), creatinine 2.9 mg/dL (elevated), uric acid 6 mg/dL (normal), and thyroid stimulating hormone 1.78 מIU/mL (normal). All of these latter assays were carried out on an automated COBAS analyzer using reagents obtained from Roche Diagnostics (Indianapolis, IN). Based on the finding of normal liver enzymes and triglyceride levels, and the presence of apoA-I in plasma at a concentration approximately 50% of normal, with all HDL particles being present, although with a marked decrease in the large α 1 and α 2 HDL particles, the tentative diagnosis of LCAT deficiency was made (Fig. 3).
The above results prompted a further referral of a sample to the Lipid Metabolism Laboratory at Tufts University. Results from this laboratory indicated that lipoprotein X was present in plasma, and only 19% of plasma cholesterol was found to be esterified (normal, 70%). On ultracentrifugation of plasma at its own density of 1.006 g/ml, the cholesterol concentration in the supernatant fraction was 41 mg/dL, with 30 mg/dL in the infranatant fraction, for a true LDL cholesterol value of 23 mg/dL (very low). Plasma cholesterol esterification rate in μg/ml/hr of cholesteryl ester was 8% of normal (mean of three determinations), measured as previously described, consistent with the diagnosis of LCAT deficiency.7,8
Sequencing of the LCAT gene from isolated DNA by our contract DNA sequencing facility in this case showed a homozygous missense mutation with a T-to-G change in exon 6 of the LCAT gene resulting in the replacement of a methionine with an arginine at position 293 of the amino acid sequence of the LCAT protein, resulting in significant loss of function. To our knowledge, this mutation has not been reported previously.9,10
Discussion
Severe HDL deficiency can result in markedly different phenotypes depending on the underlying etiology. End stage liver disease with cirrhosis can also cause severe HDL deficiency, and this is also due to LCAT deficiency secondary to hepatocellular failure.11 In the setting of severe hypertriglyceridemia secondary to lipoprotein lipase deficiency, there is lack of lipolysis, and very lipid poor HDL that is hypercatabolized. These patients are at increased risk of pancreatitis. However, none of these conditions were present in the index case.
The most severe form of HDL deficiency is familial apoA-I deficiency, first described by Schaefer et al in 1982.12 These patients have undetectable plasma apoA-I levels in the homozygous state, HDL cholesterol levels less than 5 mg/dL, and normal triglyceride and LDL cholesterol levels. They may have tubo-eruptive xanthomas, and usually develop premature coronary artery disease by age 40 years.1,2,12 These patients have various mutations resulting in lack of apoA-I production (deletions, rearrangements, and premature stop codons). However, this diagnosis is ruled in the index because his plasma apoA-I concentration was 52 mg/dL (~50% of normal).
Another form of severe HDL deficiency is homozygous Tangier disease, first described by Fredrickson1,2 in 1961, due to defects in ATP binding cassette transfer protein A1 (ABCA1), responsible for cellular cholesterol efflux. These patients have only pre-β-1 HDL in their plasma, usually have both HDL cholesterol and apoA-I levels of less than 5 mg/dL, moderate hypertriglyceridemia, and LDL cholesterol levels that are approximately 50% of normal. These patients have hepato-splenomegaly, enlarged orange tonsils, and cholesteryl ester and β carotene deposition in macrophages throughout the body. They usually develop premature CHD before 60 years of age.1,2 The index case reported here had an HDL pattern by two-dimensional gel electrophoresis that was not compatible with this diagnosis or with apoA-I deficiency.
This case has homozygous familial LCAT deficiency. LCAT is protein of molecular weight 63,000 Da, which catalyzes the transfer of a fatty acid (usually linoleic acid or 18:2n6) from the C-2 position of lecithin or phosphatidylcholine (the major phospholipid in plasma) to the 3 hydroxyl group of free cholesterol on the surface of an HDL particle to form cholesteryl ester, which moves into the core of HDL. In this process lysolecithin is formed, and is rapidly catabolized. The LCAT process allows for the formation of a spherical HDL particle converting small α 4 HDL that is discoidal to spherical medium-sized α 3 and large α 2 HDL particles. One needs apoA-I synthesis, ABCA1 function adding cellular free cholesterol to pre-β-1 HDL to form β 4 HDL, and then LCAT and lipoprotein lipase to form small spherical α 3 HDL containing both apoA-I and apoA-II.1,2
Familial LCAT deficiency was first described by Norum and Gjone in 1967 in a 33-year-old woman living in Norway, who presented with severe corneal opacities, hyperlipidemia, anemia, proteinuria, and normal kidney function.13–15 A kidney biopsy showed foam cells in the glomerular tufts. Plasma cholesterol and triglyceride levels were moderately increased, with most of the cholesterol in her plasma being unesterified. Two of her sisters were similarly affected, and all three were found to have a marked deficiency of LCAT activity, lacking the ability to transfer a fatty acid from phosphatidylcholine or lecithin to cholesterol to form cholesteryl ester and lysolecithin. Kindreds in other countries were subsequently described. These patients were found to not only have very low levels of HDL cholesterol, but also were noted to have elevations in free cholesterol-enriched VLDL, which had β instead of pre-β mobility on electrophoresis. Moreover, their LDL was found to be very large and heterogeneous and enriched in free cholesterol, phospholipids, and triglyceride, with a very low cholesteryl ester content. These LDL particles have also been found to be low in apoB and enriched in the C apolipoproteins. Because of their abnormal electrophoretic mobility they have been classed as lipoprotein X (LpX).13–16 These types of particles were present in the case reported here.
The two most common findings in patients with LCAT deficiency is corneal opacification and anemia, both present in this case.13–16 The opacification is especially marked near the limbal area, forming a circular band resembling arcus senilis. Surprisingly vision is not impaired usually, and that was true for the case reported here as well. The corneal opacification presents early in life and persists, it does not appear to result in decreased vision or other problems. The anemia in these patients is moderate with hemoglobin levels of approximately 10 g/dL and is associated with enhanced fractional clearance of red cells, probably secondary to hypersplenism.13–15 Sea blue histiocytes are found in the bone marrow and spleen of these patients, secondary to increased free cholesterol content.17 The spleen has been found to be enriched in both free cholesterol and phospholipids.18
The most clinically significant problem in these patients is renal insufficiency. Most of these patients are diagnosed after renal biopsy findings are reviewed. The renal disease presents as proteinuria and microscopic glomerular hematuria early in life, and increases in the fourth or fifth decades of life as renal function deteriorates.13–15 Light microscopy of renal biopsies has showed foam cells in the glomerular tufts, and arterioles have thickened intima and narrowed lumens. Subendothelial deposits of lipid material have been found in the renal arteries and arterioles. Lipid analysis of isolated glomeruli has shown significantly increased amounts of free cholesterol and phospholipids.13–15,18–20
Electron microscopy has shown capillary lumens filled with a meshwork of membranes and particles filled with an amorphous mottled structure, with abnormal capillary walls and thickened basal lamina.13–15 Some of these patients have required renal transplantation with good results, but the disease can recur.13–15 Rarely patients have not had any proteinuria or kidney problems. In our case the diagnosis of LCAT deficiency was based on biochemical analysis and DNA sequencing, and therefore a renal biopsy was not felt to be justified because of potential risk to the patient. One research group has suggested that it is apoE containing lipoproteins that are actively taken up by mesangial cells in the kidney that are the cause of the excess lipid deposition in the kidney, and the resultant renal insufficiency in LCAT deficiency.20 ApoE can be found in all lipoprotein classes in controls and LCAT deficient patients.5 Jimi et al21 have carefully studied 4 patients with LCAT deficiency, all of whom had renal insufficiency. They have noted that the renal lesions begin with deposition of lipid in the glomerular basement membrane, and also accumulate in the mesangium and capillary subendothelium. In the glomerulus, vacuole structure and cross-striated membrane like structures have been found. The authors noted that oxidized phosphatidylcholine in the large abnormal LDL of these patients was markedly higher than in controls, and its concentration was linked to the severity of the kidney disease.21 Moreover, patients with fish-eye disease that have LCAT mutations only resulting in lack of α-LCAT activity affecting HDL, but not β-LCAT activity affecting apoB containing lipoproteins, do not seem to develop renal disease.15
It has been have reported that lipoprotein-X (Lp-x) stimulates monocyte chemoattractant protein-1 expression in mesangial cells via nuclear factor-κB.22 A mouse model has been developed with LCAT deficiency where elevated Lp-x levels have been linked to the development of nephropathy.23 In addition, inhibition of acylcholesterol acyltransferase (ACAT) in LCAT deficient rats improves their HDL levels and chronic renal failure.24 Recently a group at the National Institutes of Health has documented that human recombinant LCAT reverses LCAT deficiency in mice.25 Reductions in circulating Lp-x and use of recombinant LCAT may all be promising strategies to prevent or reduce the renal insufficiency that can be observed in LCAT deficient patients.
The case presented here may only have moderate renal impairment because he is on a very strict cholesterol lowering diet, and he has very low total cholesterol and non-HDL cholesterol levels of 71 and 64 mg/dL, whereas for other LCAT homozygotes these values are higher at 112 mg/dL and 103 mg/dL.5 Moreover, although this case is homozygous, he does have some LCAT activity, with a cholesterol esterification rate that was 8% of normal, rather than undetectable. Another factor is the presence of hypertension. It is imperative to keep the blood pressure under tight control to prevent any further deterioration of kidney function in this case as well as other cases.
Atherosclerosis has been reported in some patients with familial LCAT deficiency with aortic, carotid, and femoral atherosclerosis, but CHD before 60 years of age has not been reported. Atherosclerotic plaques found in the aorta and renal arteries of these patients have been reported to be rich in free cholesterol.13–15 These patients may be somewhat protected because they never form normal LDL particles, with very low LDL and small dense LDL cholesterol levels, as seen in this patient. It should also be mentioned that Chang et al26 have reported that the presence of albumin in the core of Lp-x some of the anti-oxidant properties in LDL, whereas others including Nagasaka et al27 have also documented this property of Lp-x.
A 62-year-old female patient with LCAT deficiency in Japan recently underwent mitral valve replacement, and no significant evidence of coronary atherosclerosis was noted.28 Another female patient (67 years of age) in Japan, with LCAT deficiency and diabetes, died of sepsis from foot ulcers after being on dialysis for 7 years, and on autopsy was noted to have severe diffuse atherosclerosis.29 The case described here has no evidence of clinically significant CHD, but did have a cardiac calcium score of 90 Agatston units, which places him at the 85th percentile for men of his age. Arrhythmias have not generally been a common feature in LCAT deficiency, even though they have altered membrane morphology.14 In our view, the arrhythmias seen in this kindred are not related to the LCAT deficiency, but possibly due to a familial conduction disorder.
Drayna et al30 at Genentech in collaboration with Fielding reported the complete cloning and sequencing of the LCAT gene in 1987. Since that time, multiple mutations have been reported.31 The LCAT gene consists of six exons, and is present on chromosome 16 (16q22).30,31 LCAT is synthesized mainly in the liver, and circulates mainly on HDL particles, with a small amount being found on apoB containing lipoproteins. The LCAT reaction accounts for almost all of the cholesteryl ester production in plasma31,32 LCAT mutations are spread from residue –13 to 399.19 At the present time, 78 mutations are listed in the Human Gene Mutation Database (http://www.hgmd.cf.ac/index.php), accessed on March 15th, 2011. There have been 59 missense/nonsense mutations, 1 splice site mutation, 10 small deletions, 1 gross deletion, 5 insertions, 1 small indel, and 1 gross insertion reported.33 This data is based on reference sequence NM_000229.1. The current report adds another missense mutation at the second nucleotide (ATG to AGG) of codon 293 resulting in the replacement of methionine by arginine at residue 293. Two prior reports have documented LCAT deficiency due to codon 293 mutations. Maeda et al9 reported a 56-year-old male Japanese patient with a G to A change (ATG to ATA) in the third nucleotide in codon 293 in exon 6 of the LCAT gene resulting in a replacement of methionine with isoleucine at position 293 of the amino acid sequence of the LCAT protein. Like our case, this Japanese patient had a history of corneal opacification since childhood, normochromic normocytic anemia, and an HDL cholesterol at 8 mg/dL. Moreover, this patient had measured LCAT activity of 10% of normal, whereas in our case it was 8% of normal. Renal insufficiency was not mentioned in the Japanese proband, however, but was found in our proband. The second case was heterozygous for two separate mutations, one of which was identical to the patient reported by Maeda et al9 and Gotoda et al.10 Although the mutation we report is novel, it is very similar to the LCAT mutation reported by Maeda et al.9
We have previously reported with Professor Laura Calabresi of Milano, Italy that homozygotes with complete LCAT deficiency have only very small pre-β-1 and α-4 HDL present in their plasma, whereas heterozygotes for LCAT deficiency have less than 50% of normal large α-1 HDL, but two-fold increases in very small pre-β-1 HDL.5 However the homozygote reported here has some LCAT activity present in his plasma, and consequently is able to make some larger α-2 (5% of normal), and α-1 HDL (30% of normal) particles, not found in homozygotes with complete LCAT deficiency.5,34 This finding may bode well for his future clinical course, in that he does not have complete LCAT deficiency. In addition he does not have the mild hyperlipidemia (especially hypertriglyceridemia) found in homozygotes with complete deficiency, which may also help his prognosis.5 Surprisingly despite not being on a statin, his lathosterol levels (a marker of cholesterol synthesis) were below detection limits, indicating that his cholesterol production is markedly suppressed.
The patient, however, does have evidence of proteinuria and renal insufficiency indicating that control of his hypertension is critical to preserve kidney function. The other issue in patients with LCAT deficiency is whether they develop premature cardiovascular disease. Some of these patients have developed atherosclerosis, so he will need to be monitored for any signs of clinical CHD because he already has cardiac calcification. Generally these patients have not been documented to have established CHD before 60 years of age. Their major clinical problem remains the renal insufficiency, and the anemia secondary to hypersplenism. Control of hypertension if present in these patients may be critical to prevent significant renal impairment, as well as minimizing lipoprotein-X (Lp-X) and oxidized phospholipid on Lp(x). In the future, the use of recombinant LCAT may be of value in patients who develop significant renal impairment.
Acknowledgments
Dr. Ganda's research is partially supported by a National Institutes of Health Diabetes Enrichment Core (P30DK36836). Drs. Schaefer and Asztalos are supported by research grants to the Lipid Metabolism Laboratory at Tufts University from the National Institutes of Health, Bethesda, MD (HL-60935, HL 74753, PO50HL083813) and the US Department of Agriculture, Washington DC (53-3K06-5-10). Drs. Polisecki, Asztalos, and Schaefer receive partial salary support from Boston Heart Laboratory, Framingham, MA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
There are no other conflicts of interest for the rest of the authors.
References
- 1.Santos RD, Miname L, Asztalos BF, et al. Clinical presentation, laboratory values, and coronary heart disease risk in marked high density lipoprotein deficiency states. J Clin Lipidol. 2008;2:237–247. doi: 10.1016/j.jacl.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaefer EJ, Santos RD, Asztalos BF. Marked HDL deficiency and premature coronary heart disease. Curr Opin Lipidol. 2010;21:289–297. doi: 10.1097/MOL.0b013e32833c1ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ai M, Otokozawa S, Asztalos BF, et al. Small dense low density lipoprotein cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem. 2010;6:967–976. doi: 10.1373/clinchem.2009.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asztalos BF, Cupples LA, Demissie S, et al. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants in the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004;24:2181–2187. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 5.Asztalos BF, Schaefer EJ, Horvath KV, et al. Role of LCAT in HDL remodeling: an investigation in LCAT deficiency states. J Lipid Res. 2007;48:592–599. doi: 10.1194/jlr.M600403-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Matthan NB, Pencina M, Larocquw JM, et al. Alterations in cholesterol absorption and synthesis characterize Framingham Offspring study participants with coronary disease. J Lipid Res. 2009;50:1927–1935. doi: 10.1194/jlr.P900039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Himbergen T, Otokozawa S, Matthan NR, et al. Familial combined hyperlipidemia is associated with alterations in the cholesterol synthesis pathway. Arterioscler Thromb Vasc Biol. 2010;30:1113–1120. doi: 10.1161/ATVBAHA.109.196550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asztalos BF, Swarbrick MM, Schaefer EJ, et al. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. J Lipid Res. 2010;51:2405–2412. doi: 10.1194/jlr.P900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda E, Naka Y, Matozaki T, et al. Lecithin-cholesterol acyl transfer-ase (LCAT) deficiency with a missense mutation in exon 6.of the LCAT gene. Biophys Res Commun. 1991;178:460–466. doi: 10.1016/0006-291x(91)90129-u. [DOI] [PubMed] [Google Scholar]
- 10.Gotoda T, Yamada N, Murase T, et al. Differential phenotypic expression by three mutant alleles in familial lecithin:cholesterol acyltransferase deficiency. Lancet. 1991;338:778–781. doi: 10.1016/0140-6736(91)90665-c. [DOI] [PubMed] [Google Scholar]
- 11.Jahn CE, Schaefer EJ, Taam L, et al. Lipoprotein abnormalities in primary biliary cirrhosis: association with hepatic lipase inhibition as well as altered cholesterol esterification. Gastroenterology. 1985;89:1266–1278. [PubMed] [Google Scholar]
- 12.Schaefer EJ, Heaton WH, Wetzel MG, et al. Plasma apolipoprotein A I absence associated with marked reduction of high density lipoproteins and premature coronary artery disease. Arteriosclerosis. 1982;2:16–26. doi: 10.1161/01.atv.2.1.16. [DOI] [PubMed] [Google Scholar]
- 13.Norum K, Gjone E. Familial plasma lecithin:cholesterol acyltransferase deficiency. Biochemical study of a new inborn error of metabolism. Scand J Clin Lab Med. 1967;20:231–240. [Google Scholar]
- 14.Gjone E, Norum K. Familial serum cholesteryl ester deficiency: clinical study of a patient with a new syndrome. Acta Med Scand. 1968;183:107–115. [PubMed] [Google Scholar]
- 15.Norum KR, Gjone E, Glomset JA. Familial lecithin: cholesterol acyltransferase deficiency, including fish eye disease. In: Scriver CR, Beaudet AL, Sly WS, et al., editors. The Metabolic Basic of Inherited Disease. McGraw-Hill; New York, NY: 1989. pp. 1181–1194. [Google Scholar]
- 16.Palmiero PM, Sbeity Z, Liebmann J, et al. In vivo imaging of the cornea in a patient with lecithin-cholesterol acyltransferase deficiency. Cornea. 2009;28:1061–1064. doi: 10.1097/ICO.0b013e31819839ae. [DOI] [PubMed] [Google Scholar]
- 17.Naghashpour M, Cualing H. Splenomegaly with sea-blue histiocytosis, dyslipidemia, and nephropathy in a patient with lecithincholesterol acyltransferase deficiency: a clinicopathologic correlation. Metabolism. 2009;58:1459–1464. doi: 10.1016/j.metabol.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Stokke KT, Bjerve KS, Blomhoff JP, et al. Familial lecithin:cholesterol acyltransferase deficiency: studies on lipid composition and morphology of tissues. Scand J Clin Lab Invest. 1973;33(Suppl):135–151. [PubMed] [Google Scholar]
- 19.Lager DJ, Rosenberg BF, Shapiro H, et al. Lecithin cholesterol acyltransferase deficiency: ultrastructural examination of sequential renal biopsies. Mod Pathol. 1991;4:331–335. [PubMed] [Google Scholar]
- 20.Groene EF, Walli AK, Groene HJ, et al. The role of lipids in nephro-sclerosis and glomerulosclerosis. Atherosclerosis. 1994;107:1–13. doi: 10.1016/0021-9150(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 21.Jimi S, Uesugi N, Saku K, et al. Possible induction of renal dysfunction in patients with lecithin:cholesterol acyltransferase deficiency by oxidized phosphatidylcholine in glomeruli. Arterioscler Thromb Vasc Biol. 1999;19:794–801. doi: 10.1161/01.atv.19.3.794. [DOI] [PubMed] [Google Scholar]
- 22.Lynn EG, Slow YL, Frohlich J, et al. Lipoprotein-X stimulates monocyte chemoattractant protein-1 expression in mesangial cells via nuclear factor-kappaB. Kidney Int. 2001;60:520–532. doi: 10.1046/j.1523-1755.2001.060002520.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Herzenberg AM, Eskandarian M, et al. A novel in vivo lecithin-cholesterol acyltransferase (LCAT)-deficient mouse expressing predominantly LpX is associated with spontaneous glomerulopathy. Am J Pathol. 2004;165:1269–1278. doi: 10.1016/S0002-9440(10)63386-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaziri ND, Liang K. ACAT inhibition reverses LCAT deficiency and improves plasma HDL in chronic renal failure. Am J Physiol Renal Physiol. 2004;287:F1038–F1043. doi: 10.1152/ajprenal.00150.2004. [DOI] [PubMed] [Google Scholar]
- 25.Rousset X, Vaisman B, Auerbach B, et al. Effect of recombinant human lecithin-cholesterol:acyltransferase infusion on lipoprotein metabolism in mice. J Pharmacol Exp Ther. 2010;335:140–148. doi: 10.1124/jpet.110.169540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang PY, Lu SC, Su TC, et al. Lipoprotein-X reduces LDL atherogenicity in primary biliary cirrhosis by preventing LDL oxidation. J Lipid Res. 2004;45:2116–2122. doi: 10.1194/jlr.M400229-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Nakasaka H, Yorifuji T, Kosugiyama K, et al. Resistance to parathyroihormone in two patients with familial intrahepatic cholestasis: possible involvement of the ATP8B1 gene in calcium regulation via parathyroid. J Pediatr Gastroenterol Nutr. 2004;39:404–409. doi: 10.1097/00005176-200410000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Miyatake T, Matsui Y, Koyama M, et al. Cardiac surgery for a patient with familial lecithin:cholesterol acyltransferase deficiency. J Thorac Cardiovasc Surg. 2005;53:317–319. doi: 10.1007/s11748-005-0136-1. [DOI] [PubMed] [Google Scholar]
- 29.Homma S, Murayama N, Yoshida I, et al. Marked atherosclerosis in a patient with familial lecithin:cholesterol acyltransferase deficiency associated with end-stage renal disease and diabetes mellitus. Am J Nephrol. 2001;21:415–419. doi: 10.1159/000046287. [DOI] [PubMed] [Google Scholar]
- 30.Drayna D, Jarnagin AS, McLean J, et al. Cloning and sequencing of human cholesteryl ester transfer protein cDNA. Nature. 1987;327:632–634. doi: 10.1038/327632a0. [DOI] [PubMed] [Google Scholar]
- 31.Calabresi L, Francheschini G. Genetic LCAT deficiency: molecular diagnosis, plasma lipids, and atherosclerosis. In: Schaefer EJ, editor. High Density Lipoproteins, Dyslipidemia, and Coronary Heart Disease. Springer; New York, NY: 2010. pp. 89–93. [Google Scholar]
- 32.Baass A, Wassef H, Tremblay M. Characterization of a new LCAT mutation causing familial LCAT deficiency (FLD) and the role of APOE as a modifier gene in FLD phenotype. Atherosclerosis. 2009;207:452–457. doi: 10.1016/j.atherosclerosis.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Human Gene Mutation Database. Available at: http://www.hgmd.cf.ac/index.php.
- 34.Asztalos BF, De la Llera-Moya M, Dallal GE, et al. Differential effects of HDL subpopulations on cellular ABCA1 and SRB1-mediated cholesterol efflux. J Lipid Res. 2005;46:2246–2253. doi: 10.1194/jlr.M500187-JLR200. [DOI] [PubMed] [Google Scholar]