Abstract
Various studies have evaluated the relationship between Caspase-3 expression and cancers of digestive tract. However, the prognostic value of Caspase-3 expression remains unclear. Hence, a meta-analysis was conducted with eligible studies which quantitatively evaluated the relationship between Caspase-3 expression and survival of patients in gastrointestinal tract cancers. Electronic databases updated to April 12th, 2015 were searched to obtain relevant primary articles. Twelve studies were finally included with 2402 patients of esophageal, gastric or colorectal cancers. The detection of Caspase-3 was performed by immunohistochemistry (IHC). Survival data were aggregated and quantitatively analyzed. Combined hazard ratios (HRs) suggested that Caspase-3 expression had no specific impact on the overall survival (OS) (HR=0.94, 95% CI (0.64-1.37), I2=79.6%, P<0.001) of cancers of digestive tract. When each subtype of gastrointestinal cancer was analyzed separately, the over-expression of Caspase-3 was associated with favorable prognosis of OS in esophageal cancer HR=0.31, 95% CI (0.09-1.09), I2=54.6%, P=0.138). On the contrary, high level of Caspase-3 was correlated with poor prognosis of OS in gastric cancer HR=1.53, 95% CI (0.93-2.50), I2=46.4%, P=0.172). However, the expression of Caspase-3 showed no relationship of OS for patients with colorectal cancer (HR=1.03, 95% CI (0.66-1.63), I2=0.0%, P=0.410). Furthermore, the Caspase-3 expression was an indicator of poor prognosis of recurrence-free survival (RFS) for digestive tract cancer (HR=1.65, 95% CI (1.12-2.41), I2=43.9%, P=0.148). More studies need to be carried out to certify the prognostic value of Caspase-3 expression for patients with digestive tract cancers.
Keywords: Caspase-3, immunohistochemistry, digestive tract cancers, prognosis, meta-analysis, systematic review
Introduction
Digestive tract cancers, which refer to malignant conditions of the gastrointestinal tract and comprise esophageal, gastric and colorectal cancers, are among the leading causes of cancer death in the world [1]. In recent years, the incidence of digestive tract cancers, has shown a marked increase and become a worldwide health burden, especially in developing countries like China. The survival rate of digestive tract cancers in China is far behind Europe and the United States [2]. An estimated 482,300 new esophageal cancer cases and 406,800 deaths occurred in 2008 worldwide and the incidence rates vary internationally by nearly 16-fold, with the highest rates found in Southern and Eastern Africa and Eastern Asia [3]. A total of 989,600 new gastric cancer cases and 738,000 deaths are estimated to have occurred in 2008, accounting for 8% of the total cases and 10% of total deaths and over 70% of new cases and deaths occur in developing countries [3]. As for colorectal cancer, it is the third most commonly diagnosed cancer in males and the second in females, with over 1.2 million new cases and 608,700 deaths estimated to have occurred in 2008 [3]. Altogether, digestive tract cancer is a heterogeneous, multi-factorial disease, while its initiation or development can be attributed to the cumulative effect of genetic predispositions, environmental factors, and their complex interplay.
Caspase-3 is one of the key factors in apoptosis and it is activated directly by Caspase-8, -9 and -10 in the apoptotic cells by extrinsic and intrinsic pathways to initiate apoptosis. And Caspase-3 is synthesized as an inactive 32 kDa proenzyme and is processed during apoptosis into its active form, which is composed of two subunits, p17-20 and p10-12. Activated Caspase-3 is responsible for the cleavage of poly ADP-ribose polymerase, actin and sterol regulatory element binding protein, which are associated with apoptosis [4-6]. Several reports have shown that Caspase-3 expression was associated with a favorable prognosis and was an independent prognostic factor for several cancers of digestive system [7,8,12,16,18,20-22]. However, the conclusions of these studies were controversial and to date, no meta-analysis on the relationship between Caspase-3 and survival of digestive tract cancers has been available. Therefore, we performed a meta-analysis by including the most recent and relevant publications to gain statistical evidence of the associations of Caspase-3 expression that have been investigated in cancers of gastrointestinal tract.
Materials and methods
Search strategy and study selection
The electronic databases of PubMed, EMBASE and Cochrane library were searched for studies to include in the present meta-analysis. The deadline for publication of the included articles was April 12th, 2015. Searches included the terms “digestive system OR gastrointestinal OR esophageal OR gastric OR stomach OR intestinal OR colorectal”, “cancer OR carcinoma OR tumor OR neoplasm OR malignan*”, “Caspase-3 OR casp3 OR caspase 3 OR CC3” and “prognos* OR surviv* OR follow-up OR mortality OR predict OR outcome”. Meanwhile, reference lists of the relevant articles were also checked.
Studies eligible for inclusion in this meta-analysis met the following criteria: (1) the studies measured Caspase-3 expression in the primary digestive tract cancers; (2) they provided information on survival (overall survival (OS) and/or recurrence-free survival (RFS). Studies investigating response rates only were excluded); and (3) when the same author reported results obtained from the same patient population in more than one publication, only the most recent report, or the one with most complete data, was included in the analysis. Three reviewers independently determined the study eligibility. Disagreements were resolved by consensus.
Data extraction and quality assessment
Data retrieved from the reports included author, publication year, patient source, test method and survival data (Table 1). Differences in the data extraction were resolved together by 3 reviewers (Chen H, Yang X and Chen G). If data from any of the above categories were not reported in the primary study, items were treated as “not applicable”. We attempted to contact the authors of the primary study to request the original raw data of Caspase-3 expression and patient survival, however, no response was reached.
Table 1.
Main characteristics and results of the eligible studies
| First author | Year | Patients source | Tumor type | Method | Stage | Number | Caspase-3(+) | HR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|---|
| Jiang H [7] | 2010 | China | esophageal cancer | IHC | ND | 64 | 51/64 | OS 0.14 (0.03-0.6) | 0.001 |
| Hsia JY [8] | 2002 | Taiwan | esophageal squamous cell carcinoma | IHC | UICCI-III | 40 | 24/40 | OS 0.52 (0.22-1.26) | 0.02 |
| Cao FM [9] | 2005 | China | esophageal squamous cell carcinoma | flow cytometry | TNMI-III | 101 | 56/101 | ND | 0.014 |
| Parenti A [10] | 2005 | Italy | Barrett’s esophagus carcinogenesis | IHC | ND | 40 | 35/40 | ND | <0.001 |
| Isobe N [11] | 2004 | Japan | gastric carcinoma | IHC | ND | 151 | 78/151 | OS 2.22 (1.08-4.56) | 0.0301 |
| Kim MA [12] | 2011 | Korea | gastric carcinoma | IHC | TNMI-IV | 1162 | 730/1162 | OS 0.56 (0.47-0.68) | <0.001 |
| Xiao LJ [13] | 2013 | Japan | gastric carcinomas | IHC | TNM0-IV | 499 | 292/499 | OS 1.29 (0.96-1.74) | 0.031 |
| Li Y H [14] | 2004 | China | gastric carcinomas | IHC | UICCI-III | 80 | ND | ND | ND |
| Hector S [15] | 2012 | Ireland | colorectal cancer | IHC | Stage II-III | 224 | 58/224 | OS:II:1.55 (0.29-8.38) | 0.0662 |
| III:1.67 (0.77-3.66) | 0.7746 | ||||||||
| RFS:II:0.92 (0.23-3.74) | 0.0052 | ||||||||
| III:1.25 (0.65-2.41) | 0.9006 | ||||||||
| Meyer A [16] | 2009 | Germany | colon carcinomas | IHC | UICC:II-IV | 392 | 230/392 | OS 3.8 (1.2-12.4) | 0.047 |
| Dawson H [17] | 2013 | Switzerland | colorectal cancer | IHC | ND | 188 | ND | ND | 0.0262 |
| de Oca J [18] | 2008 | Spain | Colorectal cancer | IHC | ND | 54 | ND | OS 0.7 (0.31-1.55) | 0.013 |
| Guan JM [19] | 2012 | China | colorectal cancer | IHC | Duke A and B | 72 | 25/54 | ND | 0.018 |
| Koelink P J [20] | 2009 | Netherlands | colorectal cancer | IHC | Dukes’stage:A-D | 211 | 105/211 | OS:0.69 (0.49-0.97) | 0.04 |
| RFS:1.77 (1.05-3.01) | 0.02 | ||||||||
| DFS:0.69 (0.5-0.96) | 0.04 | ||||||||
| Noble P [21] | 2013 | UK | colorectal tumour | IHC | ND | 462 | 142/462 | OS 0.81 (0.34-1.92) | 0.629 |
| de Heer P [22] | 2007 | Netherlands | Rectal Cancer | IHC | Stage III | 117 | 59/117 | OS 7.5 (1.7-34.1) | 0.008 |
Statistical methods
The STATA 12.0 statistical package was used for the meta-analysis. For the quantitative aggregation of the survival results, we measured the impact of Caspase-3 expression on survival by HR between the two survival distributions. HRs and 95% confidence intervals (CIs) were applied to combine as the effective value. If the HRs and their 95% CIs were given explicitly in the articles, these data were extracted and used to calculate the summarized HR. When these variables were not provided explicitly, HR and 95% CI were estimated by calculating from Kaplan-Meier survival curves using the software Engauge Digitizer version 4.1 (http://digitizer.sourceforge.net/).
Statistical heterogeneity was tested by Cochrane’s Q test (Chisquared test; Chi2) and by measuring inconsistency (I2) [23,24]. Since we had to assume that the data being analyzed consisted of different populations, HRs with 95% CI were pooled by random effects model. By convention, an observed HR>1 implied worse survival for the group with high Caspase-3 expression. The impact of Caspase-3 expression on survival was considered to be statistically significant if the 95% CI did not overlap 1. Horizontal lines in the forest plot represent 95% CIs. Each box represented the HR point estimate, and its area was proportional to the weight of the study. The diamond (and broken line) represented the overall summary estimate, with CI represented by its width. The unbroken vertical line was set at the null value (HR=1.0). Subgroup analyses were used according to the different location of tumor, e.g., esophageal, gastric and colorectal cancers. Sensitivity analysis was also tested by removing one study at a time to calculate the overall homogeneity and effect size.
Evidence of publication bias was sought using the methods of Egger et al [25] and of Begg et al [26]. Intercept significance was determined by the t test suggested by Egger (P<0.05 was considered representative of statistically significant publication bias).
Results
Study selection and characteristics
A total of 151 potentially relevant articles were found after initial searching in PubMed, EMBASE and Cochrane Library. Finally, the remaining 17 potential eligible studies published between 2002 and 2013 were obtained for full-text evaluation [7-22,27]. One study [30] was excluded due to the duplicate for the same population. The total number of patients included was 3857, ranging from 40 to 1162 patients per study (median 241). All of the studies reported the prognostic value of Caspase-3 expression status stained by immunohistochemistry for survival in patients with digestive tract cancers. Nine of the 16 studies identified Caspase-3 expression as an indicator of good prognosis, while the other 7 studies showed poor prognosis of Caspase-3 expression on survival. A HR on OS, RFS and DFS could be extracted or attained directly for twelve studies [7,8,11-13,15,16,18,20-22], respectively, of which were eligible for a meta-analysis. Since the HRs of the other 4 studies could not be obtained based on the data presented in the articles of Dawson et al [17], Cao et al [9], Parenti et al [10], Li et al [14], and Guan et al [19], these articles were excluded in the meta-analysis and remained in a systematic review. Figure 1 showed the flowchart of study inclusion. The major characteristics of the 16 eligible publications were reported in Table 1.
Figure 1.
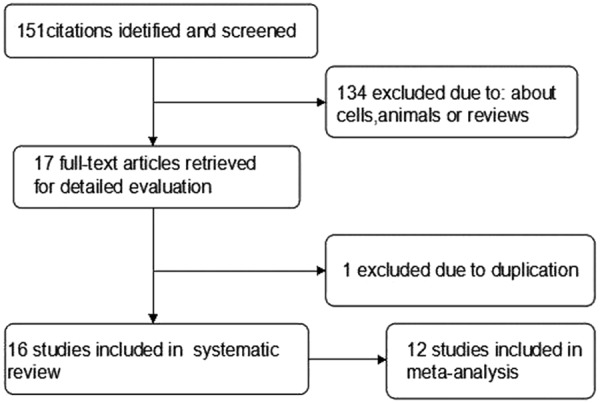
Flow chart of literature search and study selection.
Meta-analysis
The results of the meta-analysis were shown in Figures 2 and 3. Nine studies were evaluated for the effect of expression of Caspase-3 on OS. A random-effect model was used to combine HRs because of the heterogeneity present among the studies by chi-square Q-tests (χ2=49.07, P<0.001, I2=79.6%). Overall, the combined HR evaluating Caspase-3 expression on OS was 0.94 (95% CI 0.64-1.37), suggesting that Caspase-3 expression could not be an indicator of prognosis for digestive tract cancer. Significant heterogeneity was observed among the studies (I2=79.6%, P<0.001). Then, Sensitivity analyses were conducted. Sensitivity analyses showed that the studies by Kim et al [12], Koelink et al [20] and Meyer et al [16] were the top 3 with heterogeneity, and removal of these studies changed the effect into a significant one with lowest heterogeneity for each subgroup, respectively (esophageal: HR=0.31, 95% CI 0.09-1.09, I2=54.6%, P=0.138; gastric: HR=1.53, 95% CI 0.93-2.50, I2=46.4%, P=0.172; colorectal: HR=1.03, 95% CI 0.66-1.63, I2=0.0%, P=0.410). The result was shown in Figure 4. For RFS analysis with 4 studies included, statistically significant effect of Caspase-3 expression (HR=1.65, 95% CI 1.12-2.41) in patients with digestive tract cancer was observed. Meanwhile, no significant heterogeneity was observed among the studies (χ2=5.35, I2=43.9%, P=0.148).
Figure 2.
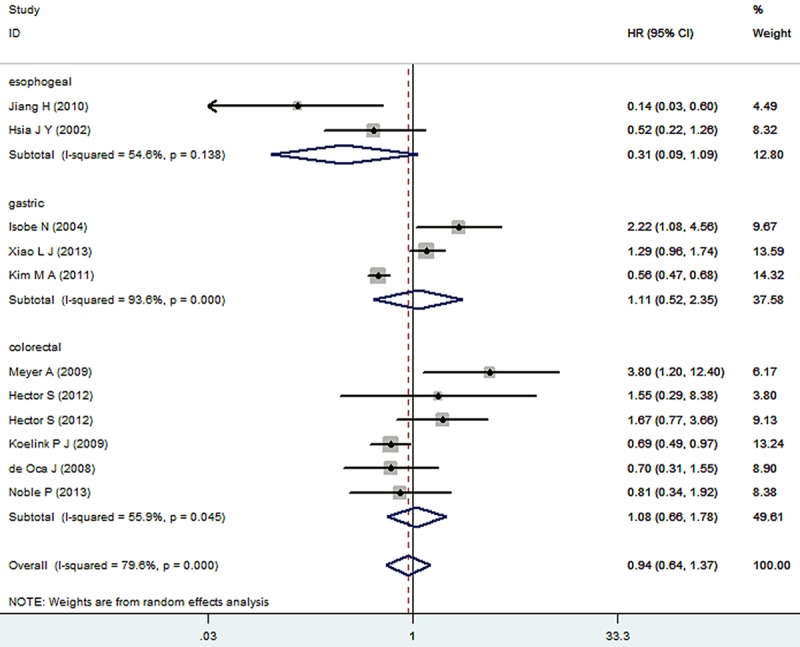
Meta-analysis (forest plot) of the 9 evaluable studies assessing Caspase-3 expression in digestive tract cancer for overall survival.
Figure 3.
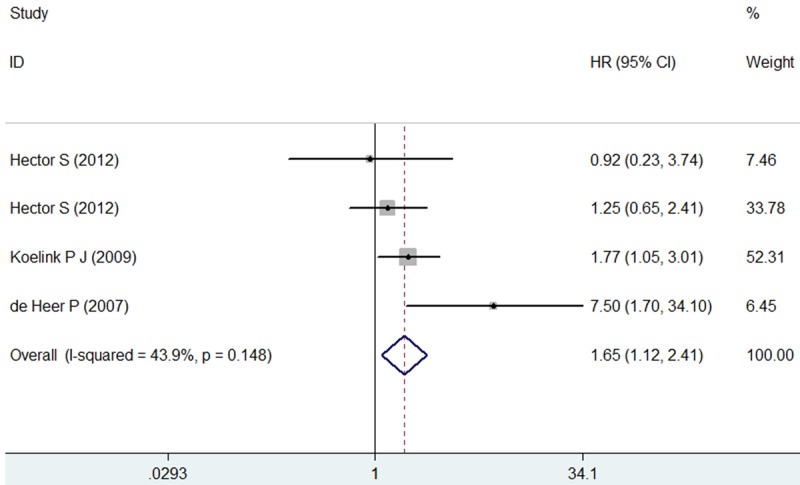
Meta-analysis (forest plot) of the 4 evaluable studies assessing Caspase-3 expression in digestive tract cancer for recurrence-free survival.
Figure 4.
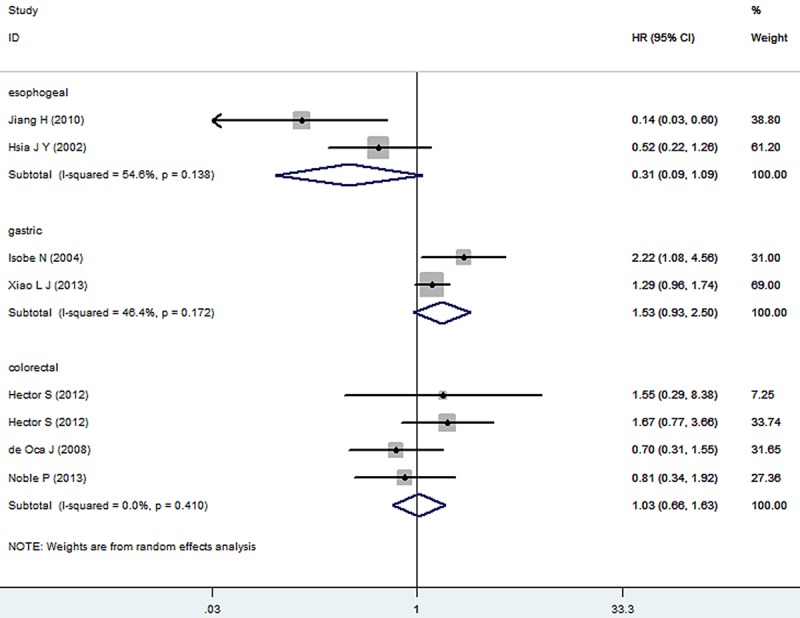
Sensitivity analyses of every study of expression of Caspase-3 on overall survival in patients with digestive tract cancer.
Publication bias
Begg’s funnel plot and Egger’s test were performed to assess the publication bias in the literatures. All 9 eligible studies investigating Caspase-3 expression on OS yielded a Begg’s test score of P=1.00 and an Egger’s test score of P=0.579. Meanwhile according to the funnel plot (Figure 5), the absence of publication bias was found. For RFS analysis, no publication bias was found for investigating Caspase-3 expression (a Begg’s test score of P=0.734 and an Egger’s test score of P=0.668) (Figure 6).
Figure 5.
Funnel plot of the 9 evaluable studies assessing Caspase-3 expression in digestive tract cancers for overall survival.
Figure 6.

Funnel plot of the 4 evaluable studies assessing Caspase-3 expression in digestive tract cancers for recurrence-free survival.
Systematic review
As 4 studies were excluded from meta-analysis, we summarized the information here. In the study of Kurabayashi et al [28] expression of Caspase-3 was not related to survival of patients with esophageal squamous cell carcinoma. In the studies of Parenti et al [10], Zhang et al [29] and Cao et al [9], the correlation of Caspase-3 with prognosis of esophageal carcinoma patients was reported. According to Parenti et al [10] and Zhang et al [29], patients with over-expression of Caspase-3 showed a tendency of progression of the Barrett’s esophagus carcinogenesis. According to Cao et al [9], high expression of Caspase-3 showed a significant association with a favorable prognosis. Li et al [14] and Sun et al [31] reported that high Caspase-3 in gastric carcinoma predicted favorable prognosis. Guan et al [19] found that colonic carcinomas and adenomas exhibited significantly higher Caspase-3 activity when compared with normal tissues, which suggested Caspase-3 expression was related to poor prognosis for colorectal carcinoma.
In the aggregate, among the 16 studies included, 10 studies identified high expression of Caspase-3 as an indicator of favorable prognosis, 5 studies showed inverse results, and 1 study showed no association of over-expression of caspase-3 with prognosis. Of 16 studies, there were 4 on esophageal cancer, 4 on gastric cancer and 8 on colorectal carcinoma. In the 4 studies on the association of Caspase-3 with esophageal cancer prognosis, 3 studies identified high level of Caspase-3 as favorable prognosis marker, 1 study showed contrary result. In the 4 studies on gastric carcinoma, 3 studies showed that high level of Caspase-3 was associated with good survival, 1 study showed with poor survival. In the 8 studies on colorectal carcinoma, 6 studies supported that Caspase-3 acted as favorable marker and 2 showed opposite result.
Discussion
The incidence of digestive tract cancers has shown a marked increase and become a worldwide health burden, the survival rate of digestive tract cancers in China is highly poor. Therefore, it is essential for clinicians and scientists to discover new methods to stratify patients for appropriate treatment. Traditionally, tumor staging system, tumor size and tumor type are used to predict the outcome of patients with digestive tract cancer, however, sometimes these clinical parameters cannot accurately value the prognosis of all patients. As far as we know, no meta-analysis has been undertaken to evaluate Caspase-3 as a prognostic marker in cases of malignancies of digestive system. The present meta-analysis has combined 12 publications including 2,402 patients to yield forecasting a statistical significance of Caspase-3 expression on OS and RFS in digestive tract cancers. Screening, study selection, and quality assessment were performed independently and reproducibly by three reviewers. We also explored heterogeneity and potential publication bias in accordance with published guidelines. In the analysis, significant heterogeneity was found among all studies. After removing 3 studies [12,16,20] contributing to heterogeneity from the analysis, we found that expression of Caspase-3 showed a favorable prognosis on esophageal cancer and on the contrary, a significant association with a poor prognosis on gastric cancer. However, it was not related to OS of patients with colorectal cancer, which indicated the possible variant role of Casepase-3 in different types of cancers of digestive tract. When concerning the relationship between Casepase-3 level and patient RFS, statistically significant effect of Caspase-3 expression with recurrence-free survival (HR=1.65, 95 % CI 1.12-2.41) was observed in patients with digestive tract cancers. Caspase-3 is the core member of caspase family. When activated, it can cleave the vast majority of polypeptides that undergo proteolysis in apoptotic cells. Its functional may be associated with survivin. Li et al [14] and Parenti et al [10] found that survivin expression was inversely associated with that of activated Caspase-3 and speculated that survivin mainly inhibits Caspase-3 activation, and then decreased apoptosis. This mechanism helps partly to explain the impact of Caspase-3 on prognosis of digestive tract cancer patients. However, more studies are required with larger patient size to investigate the potential prognostic role of Caspase-3 expression for digestive tract cancers patients.
As a meta-analysis, this study was restricted to some limitations that should be discussed for further consideration. Firstly, publication bias [27] was a major concern for all forms of meta-analysis, positive results tended to be accepted by journals, while negative results were often rejected or not even submitted. While, the present analysis does not support publication bias (for RFS analysis only 4 studies included may not offer strong evidence). Secondly, we conducted a literature search in PubMed, EMBASE and Cochrane Library and the study was restricted to papers published in English only, which probably introduced language bias. Third, due to the limited published studies on association of Caspase-3 with prognosis of esophageal carcinoma and gastric cancer, this meta-analysis cannot offer strong evidence on the overall of gastrointestinal cancer. Finally, extrapolating the survival rates from the survival curves may add inaccuracy in the extracted survival rates and led to potential bias.
In conclusion, despite the limitations described above, our study suggests that the expression of Caspase-3 has no relationship of OS for patients with colorectal cancer, but the expression of Caspase-3 is associated with poor prognosis in gastric cancer and with favorable prognosis of OS in esophageal cancer. Besides, the Caspase-3 expression was an indicator of poor prognosis of RFS for total digestive tract cancer, however, with only a small size of studies being involved. To strengthen our findings, well-designed prospective studies with better standardized assessment of prognostic markers should assist to explore the correlation between Caspase-3 expression and survival of digestive tract cancer patients.
Acknowledgements
The study was supported partly by the Fund of Guangxi Provincial Health Bureau Scientific Research Project (Z2014054), Youth Science Foundation of Guangxi Medical University (GXMUYSF201311), Guangxi University Science and Technology Research Projects (LX2014075), and the Fund of National Natural Science Foundation of China (NSFC 81360327). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Nikolopoulos D, Theocharis S, Kouraklis G. Ghrelin’s role on gastrointestinal tract cancer. Surg Oncol. 2010;19:e2–e10. doi: 10.1016/j.suronc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Chen WC, Pang XQ, Tian WY, Wang WP, Zhang XG. Combined effect of sCD40L and PI3K siRNA on transplanted tumours growth and microenvironment in nude mice with gastric cancer. Mol Biol Rep. 2012;39:8755–8761. doi: 10.1007/s11033-012-1736-3. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Zheng HC, Sun JM, Wei ZL, Yang XF, Zhang YC, Xin Y. Expression of Fas ligand and caspase-3 contributes to formation of immune escape in gastric cancer. World J Gastroenterol. 2003;9:1415–1420. doi: 10.3748/wjg.v9.i7.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S. The apoptotic cysteine protease CPP32. Int J Biochem Cell Biol. 1997;29:393–396. doi: 10.1016/s1357-2725(96)00146-x. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg MJ, Bates PA, Kelley LA, MacCallum RM. Progress in protein structure prediction: assessment of CASP3. Curr Opin Struct Biol. 1999;9:368–373. doi: 10.1016/S0959-440X(99)80050-5. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Gong M, Cui Y, Ma K, Chang D, Wang TY. Upregulation of caspase-3 expression in esophageal cancer correlates with favorable prognosis: an immunohistochemical study from a high incidence area in northern China. Dis Esophagus. 2010;23:487–492. doi: 10.1111/j.1442-2050.2009.01043.x. [DOI] [PubMed] [Google Scholar]
- 8.Hsia JY, Chen CY, Chen JT, Hsu CP, Shai SE, Yang SS, Chuang CY, Wang PY, Miaw J. Prognostic significance of caspase-3 expression in primary resected esophageal squamous cell carcinoma. Eur J Surg Oncol. 2003;29:44–48. doi: 10.1053/ejso.2002.1338. [DOI] [PubMed] [Google Scholar]
- 9.Cao FM, Zhang XH, Yan X, Wang JL, Xing LX, Wang XL, Shen HT, Wang FR. [Expression and prognostic significance of survivin and caspase-3 in esophageal squamous-cell carcinoma and their relationship with HSPs expression] . Zhonghua Zhong Liu Za Zhi. 2005;27:416–419. [PubMed] [Google Scholar]
- 10.Parenti A, Leo G, Porzionato A, Zaninotto G, Rosato A, Ninfo V. Expression of survivin, p53, and caspase 3 in Barrett’s esophagus carcinogenesis. Hum Pathol. 2006;37:16–22. doi: 10.1016/j.humpath.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Isobe N, Onodera H, Mori A, Shimada Y, Yang W, Yasuda S, Fujimoto A, Ooe H, Arii S, Kitaichi M, Imamura M. Caspase-3 expression in human gastric carcinoma and its clinical significance. Oncology. 2004;66:201–209. doi: 10.1159/000077996. [DOI] [PubMed] [Google Scholar]
- 12.Kim MA, Lee HE, Lee HS, Yang HK, Kim WH. Expression of apoptosis-related proteins and its clinical implication in surgically resected gastric carcinoma. Virchows Arch. 2011;459:503–510. doi: 10.1007/s00428-011-1150-6. [DOI] [PubMed] [Google Scholar]
- 13.Xiao LJ, Zhao S, Zhao EH, Zheng X, Gou WF, Takano Y, Zheng HC. Clinicopathological and prognostic significance of Ki-67, caspase-3 and p53 expression in gastric carcinomas. Oncol Lett. 2013;6:1277–1284. doi: 10.3892/ol.2013.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YH, Wang C, Meng K, Chen LB, Zhou XJ. Influence of survivin and caspase-3 on cell apoptosis and prognosis in gastric carcinoma. World J Gastroenterol. 2004;10:1984–1988. doi: 10.3748/wjg.v10.i13.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hector S, Conlon S, Schmid J, Dicker P, Cummins RJ, Concannon CG, Johnston PG, Kay EW, Prehn JH. Apoptosome-dependent caspase activation proteins as prognostic markers in Stage II and III colorectal cancer. Br J Cancer. 2012;106:1499–1505. doi: 10.1038/bjc.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer A, Merkel S, Brückl W, Schellerer V, Schildberg C, Campean V, Hohenberger W, Croner RS. Cdc2 as prognostic marker in stage UICC II colon carcinomas. Eur J Cancer. 2009;45:1466–1473. doi: 10.1016/j.ejca.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Dawson H, Koelzer VH, Karamitopoulou E, Economou M, Hammer C, Muller DE, Lugli A, Zlobec I. The apoptotic and proliferation rate of tumour budding cells in colorectal cancer outlines a heterogeneous population of cells with various impacts on clinical outcome. Histopathology. 2014;64:577–584. doi: 10.1111/his.12294. [DOI] [PubMed] [Google Scholar]
- 18.de Oca J, Azuara D, Sanchez-Santos R, Navarro M, Capella G, Moreno V, Sola A, Hotter G, Biondo S, Osorio A, Martí-Ragué J, Rafecas A. Caspase-3 activity, response to chemotherapy and clinical outcome in patients with colon cancer. Int J Colorectal Dis. 2008;23:21–27. doi: 10.1007/s00384-007-0362-3. [DOI] [PubMed] [Google Scholar]
- 19.Guan J, Zhao R, Zhang X, Cheng Y, Guo Y, Wang L, Mi L, Liu F, Ma X, Li B. Chicken Skin Mucosa Surrounding Adult Colorectal Adenomas is a Risk Factor for Carcinogenesis. Am J Clin Oncol. 2012;35:527–532. doi: 10.1097/COC.0b013e31821dedf7. [DOI] [PubMed] [Google Scholar]
- 20.Koelink PJ, Sier CF, Hommes DW, Lamers CB, Verspaget HW. Clinical significance of stromal apoptosis in colorectal cancer. Br J Cancer. 2009;101:765–773. doi: 10.1038/sj.bjc.6605220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble P, Vyas M, Al-Attar A, Durrant S, Scholefield J, Durrant L. High levels of cleaved caspase-3 in colorectal tumour stroma predict good survival. Br J Cancer. 2013;108:2097–2105. doi: 10.1038/bjc.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Heer P, de Bruin EC, Klein-Kranenbarg E, Aalbers RI, Marijnen CA, Putter H, de Bont HJ, Nagelkerke JF, van Krieken JH, Verspaget HW, van de Velde CJ, Kuppen PJ Dutch Colorectal Cancer Group. Caspase-3 activity predicts local recurrence in rectal cancer. Clin Cancer Res. 2007;13:5810–5815. doi: 10.1158/1078-0432.CCR-07-0343. [DOI] [PubMed] [Google Scholar]
- 23.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Smith GD, Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 27.Begg CB, Berlin JA. Publication bias: a problem in interpreting medical data. J R Stat Soc A. 1988;151:419–463. [Google Scholar]
- 28.Kurabayashi A, Furihata M, Matsumoto M, Ohtsuki Y, Sasaguri S, Ogoshi S. Expression of Bax and apoptosis-related proteins in human esophageal squamous cell carcinoma including dysplasia. Modern Pathology. 2001;14:741–747. doi: 10.1038/modpathol.3880383. [DOI] [PubMed] [Google Scholar]
- 29.Zhang RG, Wang CS, Gao CF. Prevalence and Pathogenesis of Barrett’s Esophagus in Luoyang, China. Asian Pac J Cancer Prev. 2012;13:2185–2191. doi: 10.7314/apjcp.2012.13.5.2185. [DOI] [PubMed] [Google Scholar]
- 30.Jonges LE, Nagelkerke JF, Ensink NG, van der Velde EA, Tollenaar RA, Fleuren GJ, van de Velde CJ, Morreau H, Kuppen PJ. Caspase-3 activity as a prognostic factor in colorectal carcinoma. Lab Invest. 2001;81:681–688. doi: 10.1038/labinvest.3780277. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Chen XY, Liu J, Cheng XX, Wang XW, Kong QY, Li H. Differential caspase-3 expression in noncancerous, premalignant and cancer tissues of stomach and its clinical implication. Cancer Detect Prev. 2006;30:168–173. doi: 10.1016/j.cdp.2006.02.004. [DOI] [PubMed] [Google Scholar]


