Abstract
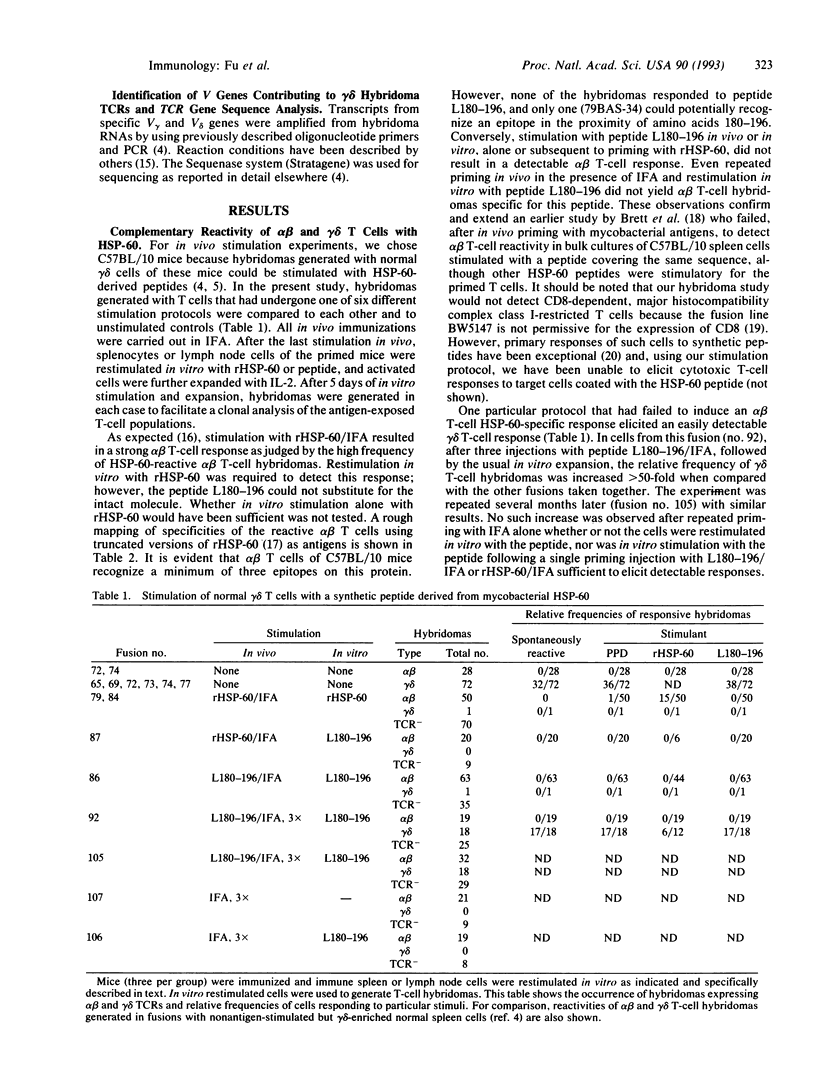
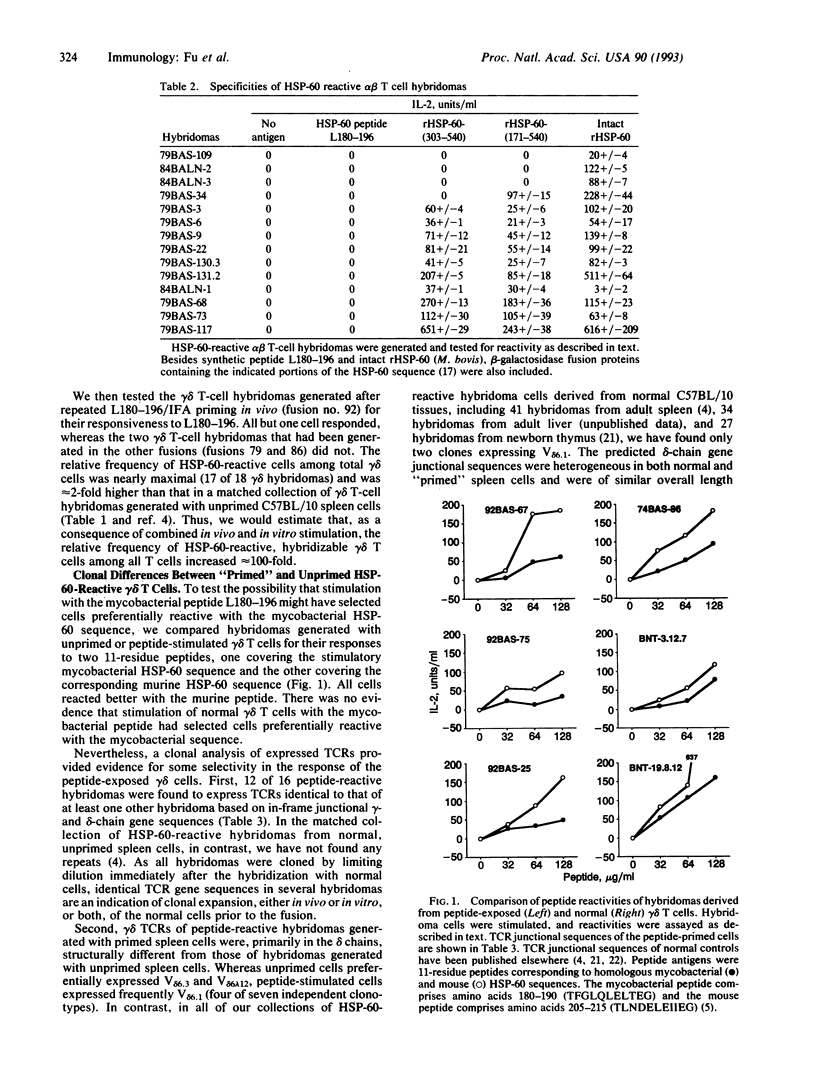
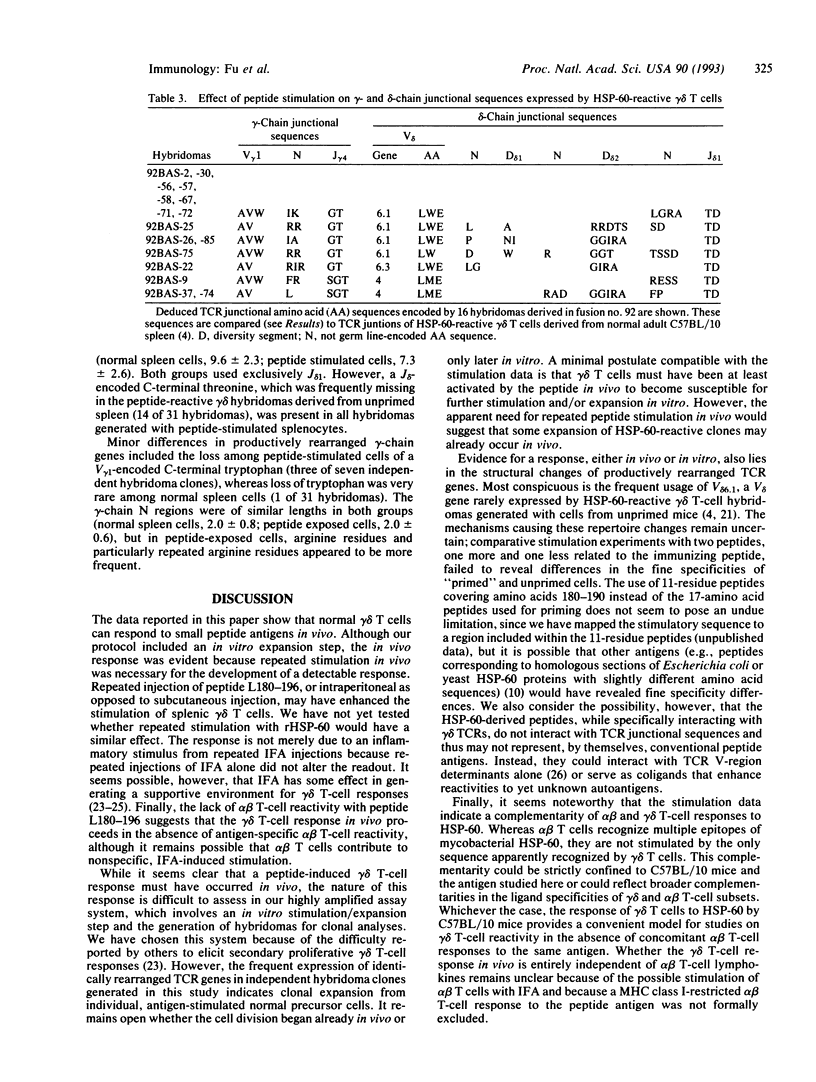
Recent results suggested that a large subset of heat shock protein HSP-60 reactive peripheral lymphoid gamma delta T cells preexists in normal adult mice, all members of which respond to a single segment of this common HSP. However, the experimental evidence supporting this idea involved in vitro peptide responses of gamma delta T-cell hybridomas generated from unprimed spleen cells. Here, we report an attempt to elicit a gamma delta T-cell response in vivo by stimulation of adult C57BL/10 mice with HSP-60 or an HSP-60-derived peptide fragment comprising amino acids 180-196 of mycobacterial HSP-60. Whereas no gamma delta T-cell response was detectable in mice injected with the intact protein, stimulation with the peptide altered the reactive gamma delta T-cell population in vivo. These changes were detected among hybridomas generated with cells restimulated in vitro and included a large increase in hybridizable gamma delta T cells, a nearly maximal increase in the relative frequency of HSP-60-reactive cells, and structural changes in expressed T-cell receptors of HSP-60-reactive cells. Interestingly, we failed to elicit a detectable alpha beta T-cell response to the particular peptide stimulatory for gamma delta T cells, although at least three other HSP-60 epitopes were recognized. Our data show that normal gamma delta T cells can respond in vivo to small peptide antigens. The gamma delta T-cell response to the HSP-60-derived peptide studied here is apparently independent of antigen-specific alpha beta T-cell reactivity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bill J., Yagüe J., Appel V. B., White J., Horn G., Erlich H. A., Palmer E. Molecular genetic analysis of 178 I-Abm12-reactive T cells. J Exp Med. 1989 Jan 1;169(1):115–133. doi: 10.1084/jem.169.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W. K., Harshan K., Modlin R. L., O'Brien R. L. The role of gamma delta T lymphocytes in infection. Curr Opin Immunol. 1991 Aug;3(4):455–459. doi: 10.1016/0952-7915(91)90002-i. [DOI] [PubMed] [Google Scholar]
- Born W. K., O'Brien R. L., Modlin R. L. Antigen specificity of gamma delta T lymphocytes. FASEB J. 1991 Sep;5(12):2699–2705. doi: 10.1096/fasebj.5.12.1717333. [DOI] [PubMed] [Google Scholar]
- Born W., Fu Y. X., Kalataradi H., Ellis T., Reardon C., Heyborne K., O'Brien R. Germ-line-encoded recognition of certain short peptide antigens? Chem Immunol. 1992;55:185–195. [PubMed] [Google Scholar]
- Born W., Hall L., Dallas A., Boymel J., Shinnick T., Young D., Brennan P., O'Brien R. Recognition of a peptide antigen by heat shock--reactive gamma delta T lymphocytes. Science. 1990 Jul 6;249(4964):67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Born W., Miles C., White J., O'Brien R., Freed J. H., Marrack P., Kappler J., Kubo R. T. Peptide sequences of T-cell receptor delta and gamma chains are identical to predicted X and gamma proteins. Nature. 1987 Dec 10;330(6148):572–574. doi: 10.1038/330572a0. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Lamb J. R., Cox J. H., Rothbard J. B., Mehlert A., Ivanyi J. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol. 1989 Jul;19(7):1303–1310. doi: 10.1002/eji.1830190723. [DOI] [PubMed] [Google Scholar]
- Carbone A. M., Marrack P., Kappler J. W. Remethylation at sites 5' of the murine Lyt-2 gene in association with shutdown of Lyt-2 expression. J Immunol. 1988 Aug 15;141(4):1369–1375. [PubMed] [Google Scholar]
- Carbone A., Harbeck R., Dallas A., Nemazee D., Finkel T., O'Brien R., Kubo R., Born W. Alpha beta T-lymphocyte depleted mice, a model for gamma delta T-lymphocyte functional studies. Immunol Rev. 1991 Apr;120:35–50. doi: 10.1111/j.1600-065x.1991.tb00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carding S. R., Allan W., Kyes S., Hayday A., Bottomly K., Doherty P. C. Late dominance of the inflammatory process in murine influenza by gamma/delta + T cells. J Exp Med. 1990 Oct 1;172(4):1225–1231. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. P., Harshan K. V., Born W. K., Orme I. M. Kinetics of accumulation of gamma delta receptor-bearing T lymphocytes in mice infected with live mycobacteria. Infect Immun. 1991 Nov;59(11):4263–4265. doi: 10.1128/iai.59.11.4263-4265.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. B., Nielsen S. E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989 May 12;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Happ M. P., Kubo R. T., Palmer E., Born W. K., O'Brien R. L. Limited receptor repertoire in a mycobacteria-reactive subset of gamma delta T lymphocytes. Nature. 1989 Dec 7;342(6250):696–698. doi: 10.1038/342696a0. [DOI] [PubMed] [Google Scholar]
- Happ M. P., Palmer E. Thymocyte development: an analysis of T cell receptor gene expression in 519 newborn thymocyte hybridomas. Eur J Immunol. 1989 Jul;19(7):1317–1325. doi: 10.1002/eji.1830190725. [DOI] [PubMed] [Google Scholar]
- Hermann E., Lohse A. W., Mayet W. J., van der Zee R., Van Eden W., Probst P., Poralla T., Meyer zum Büschenfelde K. H., Fleischer B. Stimulation of synovial fluid mononuclear cells with the human 65-kD heat shock protein or with live enterobacteria leads to preferential expansion of TCR-gamma delta+ lymphocytes. Clin Exp Immunol. 1992 Sep;89(3):427–433. doi: 10.1111/j.1365-2249.1992.tb06975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromatsu K., Yoshikai Y., Matsuzaki G., Ohga S., Muramori K., Matsumoto K., Bluestone J. A., Nomoto K. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992 Jan 1;175(1):49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- O'Brien R. L., Fu Y. X., Cranfill R., Dallas A., Ellis C., Reardon C., Lang J., Carding S. R., Kubo R., Born W. Heat shock protein Hsp60-reactive gamma delta cells: a large, diversified T-lymphocyte subset with highly focused specificity. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Picketts D. J., Mayanil C. S., Gupta R. S. Molecular cloning of a Chinese hamster mitochondrial protein related to the "chaperonin" family of bacterial and plant proteins. J Biol Chem. 1989 Jul 15;264(20):12001–12008. [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerz U. D., Zepp F., Schmid R., Hill M., Rothbard J. Recruitment of alloreactive cytotoxic T lymphocytes by an antigenic peptide. Eur J Immunol. 1989 Dec;19(12):2191–2196. doi: 10.1002/eji.1830191203. [DOI] [PubMed] [Google Scholar]
- White J., Blackman M., Bill J., Kappler J., Marrack P., Gold D. P., Born W. Two better cell lines for making hybridomas expressing specific T cell receptors. J Immunol. 1989 Sep 15;143(6):1822–1825. [PubMed] [Google Scholar]
- Young R. A. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]



