Abstract
Mucin4 (MUC4) is a secreted glycoprotein. Numerous studies had indicated that MUC4 was an attractive prognostic tumor biomarker. However, the results of different studies have been inconsistent. So we conducted this meta-analysis to explore the association between MUC4 expression and cancer prognosis. A systematically comprehensive search was performed through PubMed, EMBASE and CNKI (Chinese National Knowledge Infrastructure). Prognostic value of MUC4 expression in malignancy patients was evaluated by pooled hazard ratios (HRs) and their 95% confidence intervals (CIs). Meanwhile, pooled odds ratio (OR) with 95% CI was appropriate for the association between MUC4 expression and clinicopathological parameters. Eighteen studies including 1,933 patients were enrolled in this meta-analysis. Significant association was found between elevated MUC4 expression and poorer overall survival (OS) with pooled hazard ratio (HR) of 1.87 [95% confidence interval (CI): 1.58-2.23, P<0.001]. Significant associations were also detected in biliary tract carcinoma (HR: 2.41, 95% CI: 1.69-3.42, P<0.001), pancreatic cancer (HR: 2.01, 95% CI: 1.42-2.86, P<0.001) and colorectal cancer (HR: 1.73, 95% CI: 1.17-2.54, P=0.006). Moreover, combined odds ratio (OR) of MUC4 indicated that MUC4 overexpression was associated with tumor stage, tumor invasion and lymph node metastasis. Our results demonstrated that MUC4 may be exploited as a novel prognostic biomarker for cancer patients.
Keywords: MUC4, mucin, cancer, prognosis, meta-analysis
Introduction
Mucins are heavily glycosylated proteins that synthesized by epithelial cells and participate in the protection, repair and survival of the epithelia. To date, about 20 human mucins have been identified and categorized into two classes (secreted/gel forming mucins and transmembrane mucins) based on their structural characteristics and physiological functions.
As a critical member of transmembrane mucins, MUC4 was first identified in 1991 from a tracheobronchial cDNA library [1], and could be found expressed in various normal tissues [2-5]. Under normal conditions, MUC4 is localized at the apical surface of the epithelial cells. During cancer progression, MUC4 could act as an intramembrane ligand for receptor tyrosine kinase ErbB2 and thus participated in cancer cell signaling [6,7]. Furthermore, MUC4 was involved the regulation of p27 [8], which is a cyclin-dependent kinase inhibitor that regulates the G1 and S phases of the cell cycle [9].The association between MUC4 expression and malignancies had hitherto been indicated in amount of reports, and most of them suggested that overexpression of MUC4 was a potential predictor of poor outcome in cancer patients [10-24]. However, some researchers arrived at the opposite conclusions [25,26]. Thus, the prognostic value of hyper-expression of MUC4 remains inconclusive. Given these discrepancies of the results and the relatively small sample sizes of studies, we conducted this meta-analysis of all available studies to investigate the relationship between MUC4 expression and their prognosis effect in cancer patients.
Materials and methods
Search and selection process
A systematic literature search was conducted via the databases PubMed, EMBASE and CNKI (Chinese National Knowledge Infrastructure), covering all relevant studies published up to Apr 27, 2015, with a combination of the following keywords: “mucin 4” OR “MUC4” AND “prognosis” OR “survival” OR “outcome” AND “cancer” OR “carcinoma” OR “neoplasm”. Cited references in these papers had been surveyed as well to find additional eligible studies. Two investigators (Huang and Wang) performed the search independently.
Inclusion criteria
To be eligible for inclusion, studies have to meet the following criteria: (a) trials have to be published as a full paper in English or Chinese literature; (b) investigating the association between MUC4 and cancer prognosis; (c) sufficient data for estimating hazard ratio (HR) with 95% confidence interval (CI). The major reasons for exclusion of studies were:(a) overlapping data; (b) abstract, comment, and review; (c) studies without detailed data. The flow diagram was shown in Figure 1.
Figure 1.
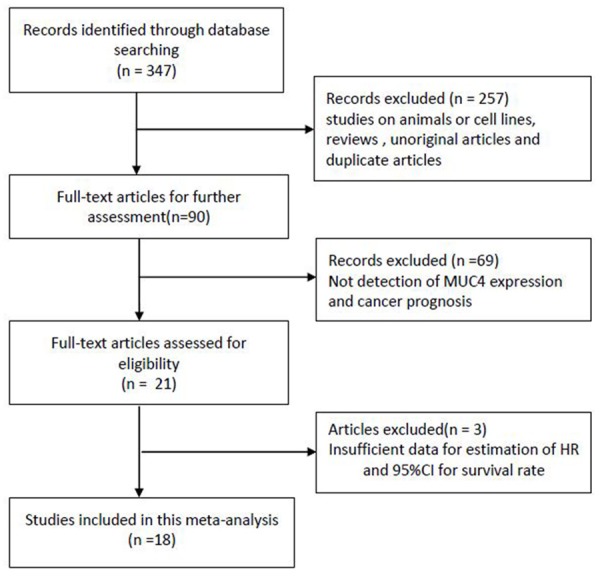
Study flow chart showing process for selecting eligible publications.
Data extraction and quality assessment
Two reviewers (Huang and Wang) did the search and identification independently using the standard approach [27]. The following items were collected from each eligible publication: first author’s name, publication year, nationality, geography (Asian or Western), cancer type, quantitative method (IHC or PCR or Others), cut-off value, follow-up months, hazard ratios (HR) with corresponding 95% confidence intervals (CI) for overall survival (OS), disease-free survival (DFS) and progression-free survival (PFS) and the total number of participants, respectively. In case of discrepancies, another investigator (Ren) was invited to discuss and check the original data until a consensus was reached. Quality assessment for each study included in final analysis was carried out by the same two reviewers according to the Newcastle-Ottawa quality assessment scale (NOS) [28]. NOS scores ranged from 0 to 9, and a score ≥7 indicates good quality in our present study.
Statistical analysis
Hazard ratio (HR) with a 95% confidence interval was calculated for the association between MUC4 expression and cancer prognosis (OS and DFS/PFS/DFS, respectively). Meanwhile, pooled odds ratio (OR) with 95% CI was appropriate for the association between MUC4 expression and clinicopathological parameters. When the statistical variables were described in text or tables, we obtained them directly. Otherwise, the methods reported by Tierney [29] was used to calculate data from Kaplan-Meier survival curves. The heterogeneity among these studies was checked using Chi-square based Q test and considered statistically significant when I2>50% or P<0.1. The fixed effects model (Mantel-Haenszel method) was picked if there was no significant heterogeneity; otherwise, the random effects model (the Der Simonian and Laird method) was utilized [16]. Sub-group analyses and logistic meta-regression analyses were conducted to explore the source of heterogeneity among variables, such as cancer types, geography, quantitative method, cut-off level and study quality. Sensitivity analysis was carried out to identify the effect of data from each study on pooled HRs. Publication bias was determined by Egger’s test and Begg’s funnel plots [17]. All statistical tests were conducted with STATA software version 12.0 (STATA Corporation, College Station, TX, USA) and P<0.05 was considered significant.
Results
Study characteristics
A total of 347 potentially relevant studies were identified after the initial database searches. After a rough review of the titles and abstracts of all studies, 257 studies were excluded; then, with a systematical review of the full texts by the same two reviewers, another 69 studies were excluded (Figure 1). Three studies were excluded because of insufficient data [30-32]. Eventually, 18 eligible studies containing 1,933 patients were included in this meta-analysis [10-26,33].
The main characteristics of the included studies are summarized in Tables 1 and 2. Of the 18 studies, 11 (1121 patients: 61.1%) were performed in Asian area [10-14,16,18,21,23,24,33], and the rest 7 studies (812 patients: 38.9%) were conducted in European or American areas [15,17,19,20,22,25,26]. All of these studies were retrospective in design. The malignant neoplasms assessed in these studies included biliary tract carcinoma [11,12,16,19,23,24], pancreatic cancer [14,18,20,33], colorectal cancer [10,15], lung cancer [21,25], oral squamous cell carcinoma (OSCC) [13], endometrial cancer [22], ovarian cancer [17] and Upper Aerodigestive Tract cancer [26]. Immunohistochemistry was used to detect the expression of MUC4 in all studies except one, which performed quantitative real-time PCR (qRT-PCR) [14].
Table 1.
Main characteristics of studies included in the meta-analysis
| First author | Publication year | Case nationality | Dominant geography | Sample size | Mean age | Malignant disease | Survival analysis | Source of HR | Follow-up months | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Higashi1 | 2015 | Japan | Asian | 114 | 67.4 | Pancreatic cancer | OS | Reported | 40 | 7 |
| Majhi | 2013 | USA | Western | 29 | NA | NSCLC | OS | SC | 144 | 5 |
| Khanh | 2013 | Japan | Asian | 206 | NA | Colorectal cancer | OS/RFS | Reported | 144 | 8 |
| Lee | 2012 | Korea | Asian | 63 | 66.9 | Gallbladder cancer | OS | Reported | 122 | 7 |
| Higashi2 | 2012 | Japan | Asian | 63 | 67.4 | Cholangio cancer | OS | Reported | NA | 8 |
| Hamada | 2012 | Japan | Asian | 150 | 64.5 | OSCC | OS/DFS | Reported/SC | 206 | 8 |
| Yi Zhu | 2011 | China | Asian | 57 | 61.7 | Pancreatic cancer | OS | Reported | 40 | 7 |
| Shanmugam | 2010 | England | Western | 132 | 65 | Colorectal cancer | OS | Reported | 300 | 8 |
| Aloysius | 2010 | England | Western | 104 | NA | Periampullary cancer | OS | Reported | 36 | 6 |
| Yeh CN | 2009 | China | Asian | 51 | 60 | Cholangio cancer | OS | Reported | 70.1 | 8 |
| Westgaard | 2009 | Norway | Western | 65 | 68 | Pancreatic cancer | OS | SC | 60 | 7 |
| Tsutsumida | 2007 | Japan | Asian | 185 | 67 | Lung cancer | OS/RFS | SC | 100 | 7 |
| Morrison | 2007 | USA | Western | 295 | NA | Endometrial cancer | OS/PFS | Reported | NA | 6 |
| Tamada | 2006 | Japan | Asian | 70 | 69.2 | Cholangio cancer | OS | Reported | 100 | 8 |
| Chauhan | 2006 | USA | Western | 38 | NA | Ovarian cancer | OS | SC | 108 | 7 |
| Saitou | 2005 | Japan | Asian | 135 | 65.8 | Pancreatic cancer | OS | Reported | 155 | 7 |
| Weed | 2004 | USA | Western | 149 | NA | Upper Aerodigestive Tract cancer | OS/RFS | Reported | 108 | 6 |
| Shibahara | 2004 | Japan | Asian | 27 | 65.3 | Cholangio cancer | OS | Reported | 60 | 7 |
OS, overall survival; DFS, disease-free survival; RFS, recurrence-free survival; PFS,progression-free survival; NSCLC, non-small cell lung cancer; OSCC, oral squamous cell carcinoma; NA, not available; SC, survival curve.
Table 2.
HRs and 95% CIs for patient survival (OS) in association with MUC4 expression in enrolled studies
| First author | Publication year | Detecting method | Cut-off value | Case Number | HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| High expression | Low expression | OS | DFS/RFS/PFS | ||||
| Higashi1 | 2015 | IHC-8G7 | 10% | 106 | 8 | 1.00 (0.31-4.12) M | NA |
| Majhi | 2013 | NA | Score >12 | 16 | 13 | 0.32 (0.05-1.92) U* | NA |
| Khanh | 2013 | IHC-1G8 | 5% | 68 | 138 | 1.51 (0.91-2.53)M | 2.30 (1.21-4.36) M |
| Lee | 2012 | IHC-1G8 | 5% | 35 | 28 | 2.89 (0.884-9.451) M | NA |
| Higashi2 | 2012 | IHC-8G7 | 5% | 19 | 44 | 1.73 (0.83-3.60) M | NA |
| Hamada | 2012 | IHC-8G7 | 5% | 61 | 89 | 1.619 (1.115-2.409) M | 1.00 (0.97-1.03) U* |
| Yi Zhu | 2011 | qRT-PCR | 50% | 29 | 28 | 2.571 (1.277-5.177) M | NA |
| Shanmugam | 2010 | IHC-8G7 | 75% | 33 | 99 | 2.07 (1.14-3.75) M | NA |
| Aloysius | 2010 | IHC-1G8 | 5% | 53 | 51 | 1.79 (0.88-3.7) M | NA |
| Yeh CN | 2009 | IHC-1G8 | 1% | 13 | 38 | 3.40 (1.56-7.41) M | NA |
| Westgaard | 2009 | IHC-1G8 | 10% | 44 | 21 | 2.02 (1.02-3.98) U* | NA |
| Tsutsumida | 2007 | IHC-8G7 | 25% | 25 | 160 | 3.21 (1.39-7.42) U* | 1.00 (0.96-1.04) U* |
| Morrison | 2007 | IHC-1G8 | 5% | 69 | 226 | 2.15 (0.85-5.48) M | 1.44 (0.51-4.04) M |
| Tamada | 2006 | IHC-8G7 | 5% | 19 | 51 | 2.655 (1.125-6.625) M | NA |
| Chauhan | 2006 | IHC-8G7 | 25% | 23 | 15 | 1.61 (0.5-5.23) U* | NA |
| Saitou | 2005 | IHC-8G7 | 5% | 21 | 114 | 1.956 (1.13-3.384) M | NA |
| Weed | 2004 | IHC-1G8 | 10% | 19 | 130 | 0.27 (0.08-0.88) M | 0.27 (0.10-0.77) M |
| Shibahara | 2004 | IHC-8G7 | 5% | 10 | 17 | 4.560 (1.190-17.478) M | NA |
The source of HR and 95% CI is described as derived from univariate analysis (U) or multivariateanalysis (M).
HR and 95% CI calculated from survival curves.
OS, overall survival; DFS, disease-free survival; RFS, recurrence-free survival; PFS, progression-free survival; HR, HR (high vslow); qRT-PCR, quantitative real-time PCR; NA, not available; IHC-1G8: Immunohistochemistryusing 1G8 antibody; IHC-8G7: Immunohistochemistry using 8G7 antibody.
Meta-analysis
MUC4 expression and OS
There were 18 studies with a total of 1,933 patients providing survival results in the form of OS. Since the heterogeneity was not statistic significant (I2=28.0%, P=0.130), the fixed model was used to pool HRs. Our result showed that MUC4 overexpression was significantly associated with poor OS in various carcinomas, with the pooled HR of 1.87 (95% CI: 1.58-2.23, P<0.001) (Figure 2).
Figure 2.
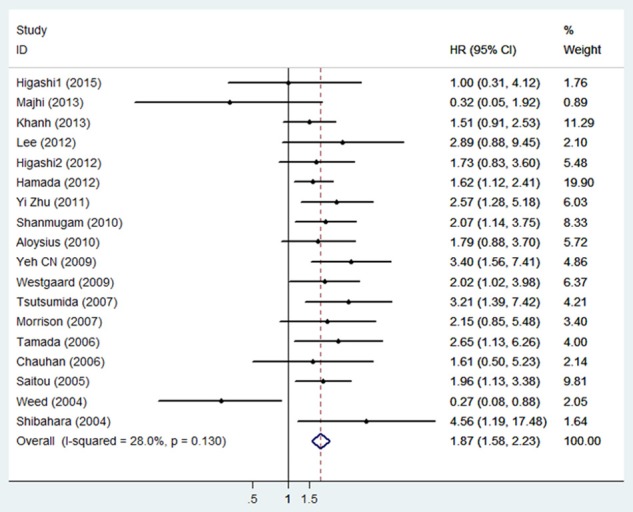
Forest plot of hazard ratio (HR) for the association between high MUC4 expression and overall survival in patients with malignant tumors.
To determine the prognostic role of MUC4 in different cancers, studies were divided into subgroups by cancer types. The results indicated that high MUC4 expression was an unfavorable prognostic indicator in biliary tract carcinoma (HR: 2.41, 95% CI: 1.69-3.42, P<0.001), pancreatic cancer (HR: 2.01, 95% CI: 1.42-2.86, P<0.001) and colorectal cancer (HR: 1.73, 95% CI: 1.17-2.54, P=0.006), but not in lung cancer (HR: 1.18, 95% CI: 0.13-11.05, P=0.888) (Figure 3).
Figure 3.
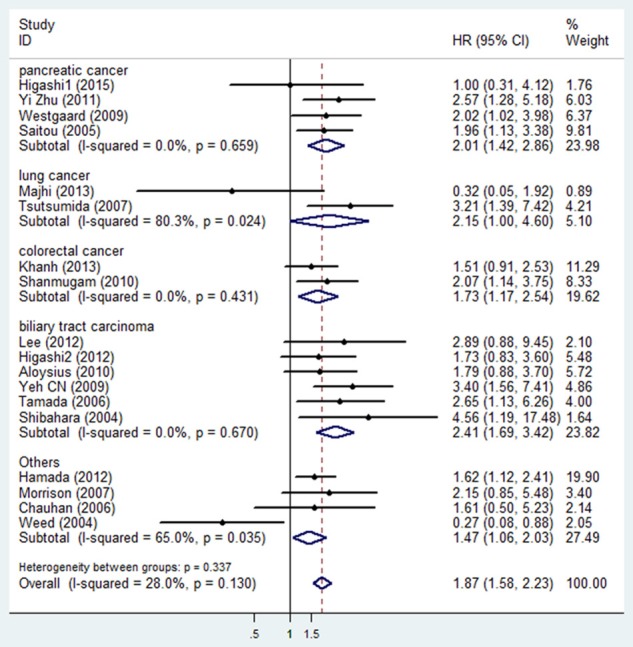
Meta-analysis (Forest plot) of the evaluable studies assessing MUC4 expression and overall survival stratified by cancer type.
We also performed subgroup analysis by geography, detecting methods, cut-off level and study quality. And the results indicated that a significant relationship between MUC4 overexpression and poor OS was also exhibited in studies with an Asian country (HR: 1.99, 95% CI: 1.63-2.44, P<0.001), IHC-1G8 (HR: 1.74, 95% CI: 1.11-2.75, P=0.017), IHC-8G7 (HR: 1.93, 95% CI: 1.54-2.42, P<0.001), the cut-off level >5% (HR: 1.83, 95% CI: 1.48-2.27, P<0.001) and the high quality study (HR: 1.99, 95% CI: 1.66-2.39, P<0.001) (Table 3).
Table 3.
Main results of meta-analysis
| Categories | Studies | Patients | MUC4+ | HRs | 95% CI | Heterogeneity | P | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| I-Square | Ph | |||||||
| Overall | 18 | 1933 | 663 | 1.87 | 1.58-2.23 | 28.00% | 0.13 | <0.001 |
| Cancer type | ||||||||
| Lung cancer | 2 | 214 | 41 | 1.18 | 0.13-11.05 | 80.30% | 0.024 | 0.888 |
| Colorectal cancer | 2 | 338 | 101 | 1.73 | 1.17-2.54 | 0.00% | 0.431 | 0.006 |
| Biliary tract carcinoma | 6 | 378 | 149 | 2.41 | 1.69-3.42 | 0.00% | 0.67 | <0.001 |
| Pancreatic cancer | 4 | 371 | 200 | 2.01 | 1.42-2.86 | 0.00% | 0.659 | <0.001 |
| Others | 4 | 632 | 172 | 1.22 | 0.59-2.55 | 65.00% | 0.035 | 0.589 |
| Geography | ||||||||
| Western | 7 | 812 | 257 | 1.4 | 0.84-2.33 | 54.20% | 0.041 | 0.191 |
| Asian | 11 | 1121 | 406 | 1.99 | 1.63-2.44 | 0.00% | 0.503 | <0.001 |
| Methods | ||||||||
| IHC-1G8 | 7 | 933 | 301 | 1.74 | 1.11-2.75 | 55.50% | 0.036 | 0.017 |
| IHC-8G7 | 9 | 914 | 317 | 1.93 | 1.54-2.42 | 0.00% | 0.698 | <0.001 |
| Others | 2 | 86 | 45 | 1.08 | 0.14-8.10 | 77.10% | 0.037 | 0.938 |
| Cut-off Value | ||||||||
| >5% | 9 | 1113 | 355 | 1.83 | 1.48-2.27 | 0.00% | 0.839 | <0.001 |
| Study quality | ||||||||
| High | 14 | 1333 | 506 | 1.99 | 1.66-2.39 | 0.00% | 0.738 | <0.001 |
| Low | 4 | 600 | 157 | 0.89 | 0.32-2.47 | 71.90% | 0.014 | 0.825 |
IHC-1G8: Immunohistochemistry using 1G8 clone antibody, IHC-8G7: Immunohistochemistry using 8G7 clone antibody, MUC4+: MUC4 positive patients number, Ph: PHeterogeneity; HR: hazard ratio, CI: confidence interval.
MUC4 expression and DFS /RFS/PFS
A total of five studies [10,13,21,22,26] were used for DFS/PFS/RFS analysis with a random-effects model due to significant heterogeneity (I2=69.9%, P=0.010). Our results failed to demonstrate any significant association between MUC4 expression and DFS/PFS/RFS (HR: 1.01, 95% CI: 0.93-1.09, P=0.869). Subgroup analysis, meta regression and sensitivity analysis were not applicable in analysis of the relationship between MUC4 expression and DFS/RFS/PFS because of the limited number of studies.
MUC4 expression and clinicopathological parameters
As shown in Table 4, overexpression of MUC4 was significantly associated with tumor stage (III/IV vs. I/II: OR 1.82, 95% CI 1.30-2.56) [10,13,15,21,26,33], tumor invasion (T3/T4 vs. T1/T2: OR 2.01, 95% CI 1.27-3.15) [10,11,13,23,33] and lymph node metastasis (positive vs. negative: OR 1.92, 95% CI 1.36-2.69) [10-13,18,21,23,24,33].
Table 4.
Meta-analysis of MUC4 expression and clinicopathological parameters intumor patients
| Categories | Studies | Patients | OR (95% CI) | I2% | Ph | P |
|---|---|---|---|---|---|---|
| Age (≥65 vs <65) | 9 | 928 | 1.38 (1.00, 1.91) | 0.00% | 0.483 | 0.05 |
| Gender (Male vs Female) | 11 | 1304 | 1.08 (0.82, 1.43) | 0.00% | 0.996 | 0.562 |
| Tumor stage (III/IV vs I/II) | 6 | 941 | 1.82 (1.30, 2.56) | 36.90% | 0.161 | 0.001 |
| Tumor invasion (T3/T4 vs T1/T2) | 5 | 603 | 2.01 (1.27, 3.15) | 39.90% | 0.155 | 0.003 |
| Histological grade (Moderate/Poor vs Well) | 9 | 1048 | 1.22 (0.88, 1.68) | 0.00% | 0.731 | 0.236 |
| Lymph node metastasis (Positive vs Negative) | 9 | 991 | 1.92 (1.36, 2.69) | 3.50% | 0.405 | <0.001 |
OR, odds ratio; 95% CI, 95% confidence interval; Ph: PHeterogeneity.
Sensitivity analysis
We adopted the “leave-one-out” scheme (i.e., analysis is conducted using all studies but one) to explore individual study’s influence on the pooled HRs. The results showed that pooled HRs was not materially altered which suggested that no individual study significantly affected the pooled results (Figure 4).
Figure 4.
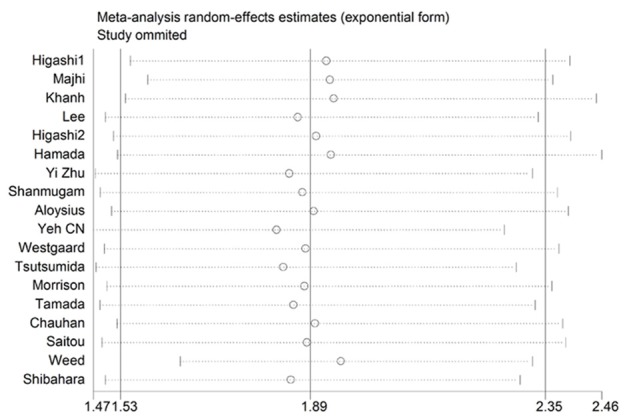
Sensitivity analysis for overall survival: effect of individual studies on pooled hazard ratios (HR) for cancer patients.
Publication bias
Begg’s funnel plot and the Egger’s linear regression test were conducted to evaluate the publication bias of the literature. In the pooled analyses of OS and DFS/RFS/PFS, the Egger’s test p values were 0.695 and 0.865, respectively, as shown by symmetric funnel plots (Figure 5). Therefore, no evidence of publication bias was noted.
Figure 5.
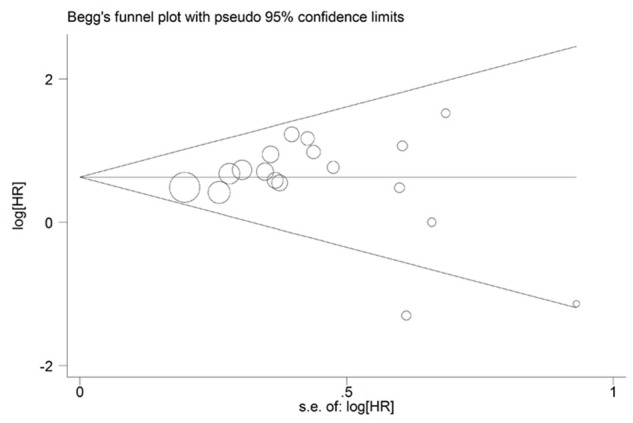
Begg’s funnel plot of MUC4 expression and OS in tumor patients.
Discussion
Cancer remains the major public health burden which counts for one in four deaths in the United States [34]. It is of great interest in identifying reliable and informative prognostic biomarkers for cancer patients to provide valuable information for clinical decision-making. Recently, many studies have suggested that mucins are potential biomarkers of cancer prognosis given their unique expression profiles in cancer patients compared with normal individuals [35,36]. Among them, MUC4 is considered to be a promising one. As a transmembrane glycoprotein, MUC4 has been considered a pivotal factor to regulate the cell proliferation and survival through interaction with ErbB2 family [6,7]. Moreover, MUC4 promotes tumor progression by repression apoptosis by both ErbB2 dependent and independent mechanisms [37]. Recently , several researches have reported that elevated expression of MUC4 might be a predictive factor for tumor prognosis, including bile duct carcinoma, colorectal cancer, oral squamous cell carcinoma, invasive ductal carcinoma of the pancreas, and small sized lung adenocarcinoma [10,11,13-15,18,23]. However, other researches arrived at the opposite conclusions [25,26]. Thus, the prognostic value of high MUC4 expression remained inconclusive. To address the prognostic value of MUC4 expression, we conducted this meta-analysis.
To the best of our knowledge, this is the first meta-analysis focused on the association between elevated MUC4 expression and the prognosis and clinicopathological characteristics of patients with various cancers. A total of 18 eligible studies [10-26,33], including 1,933 cases, were identified and analyzed in the present meta-analysis. The results revealed that elevated MUC4 expression was significantly associated with poor OS (HR 1.87, 95% CI 1.58-2.23, P<0.001) of tumor patients. Moreover, there seems to be a correlation between MUC4 overexpression and tumor stages, tumor invasion and lymph node metastasis. These results might be important for the understanding of cancer biology and help us to distinguish high-risk groups of patients and improve the clinical outcomes.
To determine the prognostic role of MUC4 in different cancers, we conducted subgroup analysis by cancer types. The results showed that elevated MUC4 expression was significantly associated with worse OS in patients with biliary tract carcinoma (HR 2.41, 95% CI 1.69-3.42, P<0.001), pancreatic cancer (HR 2.01, 95% CI 1.42-2.86, P<0.001), and colorectal cancer (HR 1.73, 95% CI 1.17-2.54, P=0.006). Thus, MUC4 could serve as a novel prognostic marker for carcinomas aforementioned. But in lung cancer, the prognostic role evidence of MUC4 is not powerful. We also conducted subgroup analysis by geography, detecting methods and study quality. In geography subgroup analysis, significant association was only found in Asian patients (HR 1.99, 95% CI 1.63-2.44, P<0.001), suggesting MUC4 had more prognostic value in Asian patients. When in terms of detecting methods, we found that IHC-1G8 (HR 1.74, 95% CI 1.112.75, P=0.017) and IHC-8G7 (HR 1.93, 95% CI 1.54-2.42, P<0.001) were both effective methods for detecting the expression of MUC4 in cancer patients. Besides, tumor patients had shorter OS only in high-quality studies (HR 1.99, 95% CI 1.66-2.39, P<0.001).
Several studies had indicated that the presence of MUC4 on the tumor cell can mask the surface epitopes to the cytotoxic immune cells such as cytotoxic-T lymphocytes or NK cells and, hence, escape from immune response [38,39]. But in this meta-analysis we failed to reveal any significant association between MUC4 expression and DFS/RFS/PFS (HR 1.01, 95% CI 0.93-1.09, P=0.869) with significant heterogeneity (I2=69.90%, P=0.010). Consider the small sample size (only five studies), it may be too early to reach a conclusion and more large size studies are needed to strengthen our conclusions.
Although the present study is the first meta-analysis on the association between MUC4 expression and patient survivals, some limitation should be noted. Firstly, our meta-analysis only encompassed a total of 18 studies, thus the results might be a fluke because sample error of eligible studies could lead to insufficient statistical power. Secondly, although most of the method for detecting MUC4 expression of all enrolled studies was IHC, the dyeing operation, antibody concentration and cutoff value of different tissues varied in different studies. Thirdly, not all of the HRs with 95% CIs was directly extracted from the studies, so we had to evaluate the HRs via Kaplan-Meier curves and these calculated HRs and 95% CIs might be less reliable than the directly given data. Finally, although no significant difference was detected according to the results of sensitivity analysis and publication bias assay, publication bias cannot be totally ruled out.
In conclusion, the present meta-analysis indicated that MUC4 overexpression may be positively correlated with poor prognosis in cancer patients. Therefore, MUC4 may be used as a prognostic marker and a novel potential therapeutic target for cancer patients. To strengthen our conclusion, standardized prospective studies with high quality are recommended to scoop the relationship between high MUC4 expression and prognosis for cancer patients.
Acknowledgements
This research was supported by the Jiangsu Provincial Special Program of Medical Science Funding (No. BL2012030), Jiangsu Provincial Six talent peak of Human affair Hall Funding (WSW-037) and National Science Foundation for Young Scholars (No. 81302013).
Disclosure of conflict of interest
None.
References
- 1.Porchet N, Nguyen VC, Dufosse J, Audie JP, Guyonnet-Duperat V, Gross MS, Denis C, Degand P, Bernheim A, Aubert JP. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem Biophys Res Commun. 1991;175:414–422. doi: 10.1016/0006-291x(91)91580-6. [DOI] [PubMed] [Google Scholar]
- 2.Audie JP, Janin A, Porchet N, Copin MC, Gosselin B, Aubert JP. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993;41:1479–1485. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- 3.Audie JP, Tetaert D, Pigny P, Buisine MP, Janin A, Aubert JP, Porchet N, Boersma A. Mucin gene expression in the human endocervix. Hum Reprod. 1995;10:98–102. doi: 10.1093/humrep/10.1.98. [DOI] [PubMed] [Google Scholar]
- 4.Gipson IK, Spurr-Michaud S, Moccia R, Zhan Q, Toribara N, Ho SB, Gargiulo AR, Hill JA 3rd. MUC4 and MUC5B transcripts are the prevalent mucin messenger ribonucleic acids of the human endocervix. Biol Reprod. 1999;60:58–64. doi: 10.1095/biolreprod60.1.58. [DOI] [PubMed] [Google Scholar]
- 5.Buisine MP, Devisme L, Copin MC, Durand-Reville M, Gosselin B, Aubert JP, Porchet N. Developmental mucin gene expression in the human respiratory tract. Am J Respir Cell Mol Biol. 1999;20:209–218. doi: 10.1165/ajrcmb.20.2.3259. [DOI] [PubMed] [Google Scholar]
- 6.Moniaux N, Nollet S, Porchet N, Degand P, Laine A, Aubert JP. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem J. 1999;338:325–333. [PMC free article] [PubMed] [Google Scholar]
- 7.Carraway KL 3rd, Rossi EA, Komatsu M, Price-Schiavi SA, Huang D, Guy PM, Carvajal ME, Fregien N, Carraway CA, Carraway KL. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem. 1999;274:5263–5266. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- 8.Jepson S, Komatsu M, Haq B, Arango ME, Huang D, Carraway CA, Carraway KL. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene. 2002;21:7524–7532. doi: 10.1038/sj.onc.1205970. [DOI] [PubMed] [Google Scholar]
- 9.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 10.Khanh do T, Mekata E, Mukaisho K, Sugihara H, Shimizu T, Shiomi H, Murata S, Naka S, Yamamoto H, Endo Y, Tani T. Transmembrane mucin MUC1 overexpression and its association with CD10(+) myeloid cells, transforming growth factor-beta1 expression, and tumor budding grade in colorectal cancer. Cancer Sci. 2013;104:958–964. doi: 10.1111/cas.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HK, Cho MS, Kim TH. Prognostic significance of muc4 expression in gallbladder carcinoma. World J Surg Oncol. 2012;10:224. doi: 10.1186/1477-7819-10-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higashi M, Yamada N, Yokoyama S, Kitamoto S, Tabata K, Koriyama C, Batra SK, Yonezawa S. Pathobiological implications of MUC16/CA125 expression in intrahepatic cholangiocarcinoma-mass forming type. Pathobiology. 2012;79:101–106. doi: 10.1159/000335164. [DOI] [PubMed] [Google Scholar]
- 13.Hamada T, Wakamatsu T, Miyahara M, Nagata S, Nomura M, Kamikawa Y, Yamada N, Batra SK, Yonezawa S, Sugihara K. MUC4: a novel prognostic factor of oral squamous cell carcinoma. Int J Cancer. 2012;130:1768–1776. doi: 10.1002/ijc.26187. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Zhang JJ, Zhu R, Zhu Y, Liang WB, Gao WT, Yu JB, Xu ZK, Miao Y. The increase in the expression and hypomethylation of MUC4 gene with the progression of pancreatic ductal adenocarcinoma. Med Oncol. 2011;28(Suppl 1):S175–184. doi: 10.1007/s12032-010-9683-0. [DOI] [PubMed] [Google Scholar]
- 15.Shanmugam C, Jhala NC, Katkoori VR, Wan W, Meleth S, Grizzle WE, Manne U. Prognostic value of mucin 4 expression in colorectal adenocarcinomas. Cancer. 2010;116:3577–3586. doi: 10.1002/cncr.25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeh CN, Pang ST, Wu RC, Chen TW, Jan YY, Chen MF. Prognostic value of MUC4 for mass-forming intrahepatic cholangiocarcinoma after hepatectomy. Oncol Rep. 2009;21:49–56. [PubMed] [Google Scholar]
- 17.Chauhan SC, Singh AP, Ruiz F, Johansson SL, Jain M, Smith LM, Moniaux N, Batra SK. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125) Mod Pathol. 2006;19:1386–1394. doi: 10.1038/modpathol.3800646. [DOI] [PubMed] [Google Scholar]
- 18.Saitou M, Goto M, Horinouchi M, Tamada S, Nagata K, Hamada T, Osako M, Takao S, Batra SK, Aikou T, Imai K, Yonezawa S. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845–852. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aloysius MM, Zaitoun AM, Awad S, Ilyas M, Rowlands BJ, Lobo DN. Mucins and CD56 as markers of tumour invasion and prognosis in periampullary cancer. Br J Surg. 2010;97:1269–1278. doi: 10.1002/bjs.7107. [DOI] [PubMed] [Google Scholar]
- 20.Westgaard A, Schjolberg AR, Cvancarova M, Eide TJ, Clausen OP, Gladhaug IP. Differentiation markers in pancreatic head adenocarcinomas: MUC1 and MUC4 expression indicates poor prognosis in pancreatobiliary differentiated tumours. Histopathology. 2009;54:337–347. doi: 10.1111/j.1365-2559.2009.03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsutsumida H, Goto M, Kitajima S, Kubota I, Hirotsu Y, Wakimoto J, Batra SK, Imai K, Yonezawa S. MUC4 expression correlates with poor prognosis in small-sized lung adenocarcinoma. Lung Cancer. 2007;55:195–203. doi: 10.1016/j.lungcan.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Morrison C, Merati K, Marsh WL Jr, De Lott L, Cohn DE, Young G, Frankel WL. The mucin expression profile of endometrial carcinoma and correlation with clinical-pathologic parameters. Appl Immunohistochem Mol Morphol. 2007;15:426–431. doi: 10.1097/01.pai.0000213117.73720.89. [DOI] [PubMed] [Google Scholar]
- 23.Tamada S, Shibahara H, Higashi M, Goto M, Batra SK, Imai K, Yonezawa S. MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clin Cancer Res. 2006;12:4257–4264. doi: 10.1158/1078-0432.CCR-05-2814. [DOI] [PubMed] [Google Scholar]
- 24.Shibahara H, Tamada S, Higashi M, Goto M, Batra SK, Hollingsworth MA, Imai K, Yonezawa S. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39:220–229. doi: 10.1002/hep.20031. [DOI] [PubMed] [Google Scholar]
- 25.Majhi PD, Lakshmanan I, Ponnusamy MP, Jain M, Das S, Kaur S, Shimizu ST, West WW, Johansson SL, Smith LM, Yu F, Rolle CE, Sharma P, Carey GB, Batra SK, Ganti AK. Pathobiological implications of MUC4 in non-small-cell lung cancer. J Thorac Oncol. 2013;8:398–407. doi: 10.1097/JTO.0b013e3182829e06. [DOI] [PubMed] [Google Scholar]
- 26.Weed DT, Gomez-Fernandez C, Yasin M, Hamilton-Nelson K, Rodriguez M, Zhang J, Carraway KL. MUC4 and ErbB2 expression in squamous cell carcinoma of the upper aerodigestive tract: correlation with clinical outcomes. Laryngoscope. 2004;114:1–32. doi: 10.1097/00005537-200408001-00001. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 29.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon KY, Ro JY, Singhal N, Killen DE, Sienko A, Allen TC, Zander DS, Barrios R, Haque A, Cagle PT. MUC4 expression in non-small cell lung carcinomas: relationship to tumor histology and patient survival. Arch Pathol Lab Med. 2007;131:593–598. doi: 10.5858/2007-131-593-MEINCL. [DOI] [PubMed] [Google Scholar]
- 31.Alos L, Lujan B, Castillo M, Nadal A, Carreras M, Caballero M, de Bolos C, Cardesa A. Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am J Surg Pathol. 2005;29:806–813. doi: 10.1097/01.pas.0000155856.84553.c9. [DOI] [PubMed] [Google Scholar]
- 32.Giuntoli RL 2nd, Rodriguez GC, Whitaker RS, Dodge R, Voynow JA. Mucin gene expression in ovarian cancers. Cancer Res. 1998;58:5546–5550. [PubMed] [Google Scholar]
- 33.Higashi M, Yokoyama S, Yamamoto T, Goto Y, Kitazono I, Hiraki T, Taguchi H, Hashimoto S, Fukukura Y, Koriyama C, Mataki Y, Maemura K, Shinchi H, Jain M, Batra SK, Yonezawa S. Mucin Expression in Endoscopic Ultrasound-Guided Fine-Needle Aspiration Specimens Is a Useful Prognostic Factor in Pancreatic Ductal Adenocarcinoma. Pancreas. 2015;44:728–34. doi: 10.1097/MPA.0000000000000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 35.Williams KA, Terry KL, Tworoger SS, Vitonis AF, Titus LJ, Cramer DW. Polymorphisms of MUC16 (CA125) and MUC1 (CA15.3) in relation to ovarian cancer risk and survival. PLoS One. 2014;9:e88334. doi: 10.1371/journal.pone.0088334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Streppel MM, Vincent A, Mukherjee R, Campbell NR, Chen SH, Konstantopoulos K, Goggins MG, Van Seuningen I, Maitra A, Montgomery EA. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;43:1755–1763. doi: 10.1016/j.humpath.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carraway KL, Theodoropoulos G, Kozloski GA, Carothers Carraway CA. Muc4/MUC4 functions and regulation in cancer. Future Oncol. 2009;5:1631–1640. doi: 10.2217/fon.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komatsu M, Yee L, Carraway KL. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999;59:2229–2236. [PubMed] [Google Scholar]


