Abstract
Cervical cancer is a leading cause of cancer death among women in the world. The specific etiopathogenesis of cervical cancer is indeed complex. Even so, we should make arduous efforts to have a precise understanding of the complicate cellular/molecular mechanisms underlying initiation, progression and/or prevention of the cervical cancer. The high-risk human papillomavirus (hrHPV) is considered as the major causative agent of cervical cancer. But with the existence of hrHPV only is not sufficient, autophagy plays a vital character in the development of cervical cancer. Autophagy is the endogenous, tightly regulated cellular “housekeeping” process responsible for the degradation of damaged and dysfunctional cellular organelles and protein aggregates. Our aims in this review were (1) to provide a brief synopsis of process of autophagy (including an overview of the key molecular mediators of this catabolic process and its relationship with hrHPV infection) and (2) most importantly, summarize the current evidence for autophagy-mediated cervical carcinogenesis. One of the latest opinions about the etiopathogenesis is that hrHPV leads to the occurrence of cervical cancer via inhibiting the host’s autophagy. The infection of hrHPV will cause the autophagy of cancerous cells, resulting in autophagic cell death, which will suppress the further infection of HPV in return. But the autophagy would be knocked down by the hrHPV, which means the protecting action would end with failure. What’s worse, the negative denouement will enhance the infectivity of HPV ultimately, which leads to accelerate cervical carcinogenesis.
Keywords: Autophagy, HPV, cervical cancer, infectivity
Introduction
Autophagy means “self-eating”, which is first described by Christian de Duve in 1963 as a lysosome-mediated degradation process for non-essential or damaged cellular constituents [1,2]. According to recent studies, there are at least three types of autophagy existing: macroautophagy, microautophagy and chaperone-mediated autophagy and macroautophagy is the most extensively studied among the three [3]. After continuous exploration, scientists have found that autophagy plays a variety of physiological and pathophysiological roles, comprising cell survival, cell death, anti-aging, tumor suppression, pathogen clearance, antigen presentation, and neurodegeneration [3-5]. Autophagy occurs under certain stresses or inductions, such as nutrient starvation, radiation and cytotoxic compounds. Under such circumstances, cytoplasm components or organelles are delivered to a double-membrane vesicle called autophagosome and then they are fused with lysosomes for protein degradation by lysosomal hydrolases [6]. Many components including mTOR, PI3KC3 (class III phosphatidylinositol 3-kinases) complex, two ubiquitin-like conjugation systems, autophagy family members take part in this process, which can be introduced in our published review [7].
In order to have a more intuitionistic understanding, all the known autophagy associated genes are summarized on the Table 1, according to the published papers. Light chain 3 (LC3) protein, a kind of microtubule-associated protein and a mammalian homologue of yeast Atg8, is localized at autophagosomes and autolysosomes after processing and the amount of LC3-II cleaved product is related to the extent of autophagosome formation, providing the first molecular marker for the detection of autophagic activity. It has been identified that the amount of LC3-II increase significantly in HeLa cells cultured for 90 min under the starvation conditions, which induce autophagy [8,9]. PI3KC3 and Beclin-1 jointly exert significant impacts on autophagy induction, while Beclin-1 weighs more for its indispensable function in the formation of autophagic vacuoles [7,10]. The Atg12/Atg5 complex subsequently leads to the formation of larger protein complexes that are further transported onto the membrane, which is necessary for the formation of autophagic vesicles [8,9]. Phosphatidyl ethanolamine (PE) is a lipid molecule that considered to anchor Atg8/LC3-II to membranes, and Atg8/LC3- II is ligated to PE in a series of biochemical reactions assisted by Atg7, transforming into Atg8/LC3-II and finally leading to the formation of autophagic vacuoles [11]. From the above, we can classify those genes into four kinds according to their functions (Table 2).
Table 1.
Autophagy genes
| Mammalian autophagy gene | Yeast gene old/new name | Function of the gene product |
|---|---|---|
| Beclin 1 | Apg6/Vps30/Atg6 | Forms a complex with class III PI3-kinase Vps34; A tumor suppressor gene in mammals |
| Apg3 | Apg3/Aut1/Atg3 | Regulates LC3 modification and conjugation of Apg5 to Apg12 |
| Apg4/autophagins | Apg4/Aut2/Atg4 | Supports LC3 modification by cleaving the C-terminus to expose glycine |
| Apg5 | Apg5/Atg5 | Localizes to isolation membranes that are forming new autophagosomes. Forms a complex with Apg12 |
| Apg7 | Apg7/Atg7 | Regulates conjugation of Apg5 to Apg12, and LC3 modification |
| MAP-LC3 | Aut7/Apg8 Atg8 | Localizes to the limiting membranes of autophagosomes |
| Apg10 | Apg10/Atg10 | Regulates conjugation of Apg5 to Apg12 and facilitates LC3 modification |
| Apg12 | Apg12/Atg12 | Localizes to isolation membranes that are forming new autophagosomes. Forms a complex with Apg 5 |
| Apg16L | Apg16/Atg16 | Links together Apg5-Apg12 complexes to form polymers |
| bif-1 (Endophilin B1) | A positive mediator of the class III PI3-kinase (PI3KC3) | |
| UVRAG | UVRAG directly interacts with the Beclin 1-PI3KC3 lipid kinase complex to activate autophagy; a bridge molecule for the Bif-1-Beclin 1 interaction | |
| VMP1 | VMP1 expression induces the formation of ultrastructural features of autophagy and recruitment of the microtubule-associated protein 1 light-chain 3 (LC3); VMP1 interacts with Beclin-1 through the VMP1-Atg domain, which is essential for autophagosome formation | |
| DRAM | Encoding a lysosomal protein that induces macroautophagy, as an effector of p53-mediated death | |
| TP53INP2 | TP53INP2 translocates from the nucleus to the autophagosome structures after activation of autophagy by rapamycin or starvation; TP53INP2 expression is necessary for autophagosome development because its small interfering RNA-mediated knockdown strongly decreases sensitivity of mammalian cells to autophagy |
Table 2.
Function of four kinds of autophagy genes
| Autophagy gene | Function |
|---|---|
| Atg1, Atg13, Atg17 | Responds to upstream signals such as TOR kinase |
| Atg6, Atg14, Vps34 and Vps15 | Mediates vesicle nucleation |
| the Atg8 and Atg12 systems | Mediate vesicle expansion |
| Atg2, Atg9, Atg18 | A recycling pathway that mediates the disassembly of Atg proteins from matured autophagosomes |
Uterine cervical cancer (UCC) is one of the most frequent cancers in women all the world and is associated with high-risk human papillomavirus (hrHPV) infection. In recent years, with the development of screening and treatment of UCC, the morbidity and mortality are declining, but there is a younger trend in the onset age [12].
HPV, which propagates only in differentiating keratinocytes [13], is composed of a double-stranded DNA genome (8 kb) and a non-enveloped icosahedral capsid consisted of two capsid proteins, L1 (major) and L2 (minor). HPVs-infected genital mucosal epithelia are divided into two groups: low-risk types (found mainly in benign condyloma) and 15 high-risk types (found in cervical cancer) [14]. HPV genotype 16 (HPV-16) is the most prevalent genotype associated with cervical cancer [15]. During the initial stage of infection, HPV anchors to heparin sulfate proteoglycans (HPSGs) on the epithelial cell surface and then enters the cell slowly [16,17]. Lots of virus particles are degraded during the entry process [18,19], suggesting induction of a host defense system.
Several kinds of HPV genotypes infecting the human anogenital area are known to be oncogenic, while most HPV infections are self-limiting and resolve within 24 months and reversely, persistent HPV infection with an oncogenic type is the predominant cause of UCC [20]. High risk HPV-16 and -18 account for approximately 70% of cervical cancer cases [21], while other oncogenic HPV types, including HPV-31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -66 and -68, account for the remainder [22]. As part of their carcinogenic mechanism, the two hrHPVs encode E6 and E7 viral oncoprotein that interfere with the function of the tumor suppressor proteins p53 and retinoblastoma, respectively [23]. However, viral infection alone is not sufficient for carcinogenesis [24]. Lower expression of autophagy is an important carcinogenic factors and autophagy is emerging as biological mechanism in targeting human cancers, including cervical cancer. In our review, our aims were (1) to provide a brief synopsis of process of autophagy (including an overview of the key molecular mediators of this catabolic process and its relationship with hrHPV infection) and (2) most importantly, summarize the current evidence for autophagy-mediated cervical carcinogenesis.
Autophagy plays an important role in both preventing and promoting cervical cancer
Autophagy is a tightly regulated cellular process responsible for eliminating damaged organelles, proteins and cell membrane via a lysosomal pathway and cell stress and diseases can also trigger this process [25]. In some ways, it can also be described as a self-degradation mechanism associated with tumor progression, including cervical cancer (Figure 1).
Figure 1.
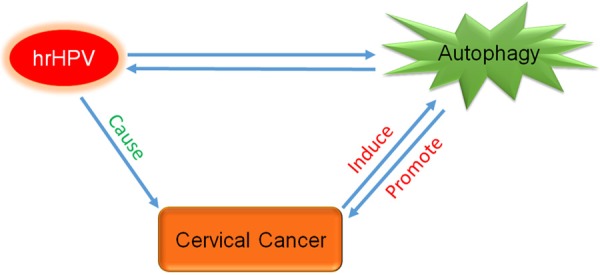
Relationship among HPV, autophagy and cervical cancer. During the cervical carcinogenesis, at the early stage, HPV infection induces an autophagy response; autophagy inhibits HPV infection in return. But the autophagy would be knocked down by the hrHPV, which means the protecting action would end with failure. On the contrary, inhibition of autophagy can enhance infectivity of HPV, which leads to accelerate cervical carcinogenesis. Autophagy is often regarded as a “double-edged sword”, both impeding or promoting initiation and progression of cancer. HrHPV is considered as the major causative agent of cervical cancer, which can induce autophagy, however, decrease of autophagy can promote cancer progress.
Autophagy is activated by cancerization
Autophagy occurs at low basal levels to maintain cellular homeostasis by eliminating damaged proteins and organelles, meanwhile autophagy is also strongly induced under starvation conditions to supply amino acids by degrading proteins [26]. With the development of cancer, cancer cells will suffer from a rugged microenvironment with hypoxia, starvation and accumulation of reactive oxygen species (ROS), which has a vital role in inducing autophagy. The progress has been figured out and described in this way: vascular insufficiency in tumors could lead to depletion of glucose/oxygen and conduce to increased ROS production, extracellular acidosis in tumor microenvironment which may eventually give rise to autophagy, thereby implicating glucose depletion and/or deprivation as a significant trigger for autophagy [27,28]. But the increase of autophagy is not easy to be detected, for the tendency of increasing autophagy will soon be suppressed by HPV, resulting in the level of autophagy will be even much lower than the normal value. The phenomenon may be misunderstood that cancerization would not activate autophagy. But Beclin-1 is a key regulator of autophagy formation and its overexpression is found in varieties of human cancers [29], which proves the autophagy is activated by cancerization indirectly.
Autophagy plays a cancer-suppressing role in UCC
In the vesicle nucleation process of autophagy, Beclin-1 plays a vital role and is a gene indispensable for the first phases of autophagy [30]. Beclin-1 is a specific gene involved in mammalian autophagy, and can control and regulate the occurrence and development of cancer via regulating the level of autophagy. To be specific, Beclin-1 mainly combines PI3KC3 into complexes, which adjust other Apg proteins located on autophagy precursor structure, thereby regulates the activity of autophagy [31]. The absence of Beclin-1 gene increases the incidence of cancer significantly [30,32], or it can be explained by defining that Beclin-1 is a kind of anti-oncogene of cervical cancer. What’s more, in other cancers such as gastric cancer, low Beclin-1 expression is strongly associated with decreased survival in gastric cancer patients and the intravascular embolus subset [29].
At present, it is known that autophagy play roles in survival against starvation, quality control of intracellular organelles and proteins, anti-aging, cancer suppression, antigen presentation and elimination of intracellular microbes [3]. Among those important functions, suppression of tumor formation is new-found and a hotspot discussed in recent years. There are several ways about autophagy suppress cancer. One way is that autophagy can affect the occurrence and development of cancer in multiple aspects such as cell cycle, angiogenesis and expression of autophagy-related factors [33-35]. Another way reported is that cells’ malignant transformation could be activated by the disorders of oncogenes or anti-oncogenes related to autophagy pathways in cells [30]. The third, autophagy could balance protein synthesis, maintain homeostasis stable and restrain the oncogenesis via adjusting the concentration of peroxide in the cell [36]. In the research of Zhu et al [29], the expression levels of both Beclin-1 and LC3 in cervical squamous cell carcinoma cells are distinctly lower than that in normal squamous epithelial cells, it can be inferred that the low expression levels of Beclin-1 and LC3 lead to the occurrence of cervical squamous cell carcinoma (SCC) and their expression may have prognostic significance in early stage cervical SCC. CaSki cells are a kind of cervical cancer cells. It has been proved that overexpression of Beclin-1 is associated with the inhibition of the proliferation of CaSki cells, which may be attributed to an imbalance among the expression of Ang-1, Ang-2 and Tie-2 [37]. Beclin-1 is the central regulator in the complex autophagic machinery, whose expression and/or activity levels may in turn tilt the malignant/cancerous cell’s fate towards apoptotic cell death or autophagy [38,39].
Tumor microenvironment is ATP and adenosine rich, suggesting a role for purinergic signaling in cancer cell growth and death. Although extracellular ATP itself has a small cytotoxic effect, while adenosine, formed from ATP degradation by ectonucleotidases, is mainly responsible for apoptosis induction. Additionally, if adenosine conversion into AMP is inhibited, it will also inhibit cell death, indicating that metabolism of intracellular adenosine originated from extracellular ATP account for the main effects the latter in human cervical cancer cells [40]. Briefly, adenosine conversion into AMP is a necessary factor in the process of autophagy.
By the way, autophagy is often regarded as a “double-edged sword”, either impeding or promoting initiation and progression of cancer [41].
Persistent hrHPV infection leads to UCC
ATPase family AAA domain contains 3A (ATAD3A), which has been proved to be an anti-autophagy factor in UCC. Chen et al [42] results showed that there is a significant correlation between ATAD3A expression and the presence of hrHPV, FIGO stage, lymph node involvement, c-MET expression, interleukin-8 and patient survival. Furthermore, silence of E6/E7 expression will decrease ATAD3A expression and cell survival. Subsequently, knockdown of ATAD3A expression increases cellular autophagy and apoptosis, and reduces drug resistance. By the way, in the ATAD3A knockdown cells, it is frequently found that resveratrol downregulates ATAD3A expression and increases abrasion of the mitochondrial outer membrane as well as numbers of autophagosomes. Above results demonstrated that HPV infection correlates with increased ATAD3A expression and drug resistance in UCC, persistent hrHPV infection may stabilize ATAD3A expression to inhibit cell autophagy and apoptosis as well as to increase drug resistance.
Resveratrol, a natural polyphenolic phytochemical, has received considerable interest on the basis of its potential as a chemopreventive agent against human cancer. After its treatment, the expression of p53 is decreased in HPV18-positive cell lines (CaLo and HeLa), however, the expression of p53 is increased in HPV16-positive cell lines (CaSki and SiHa) and C33A cells (with mutation in p53); the expression of NF-κB p65 is decreased in all cell lines except SiHa cells (Figure 2). These data indicated that resveratrol uses different mechanisms, mainly increasing the mitochondrial permeability in HPV16-positive cervical cancer cells and the lysosomal permeability in HPV18-positive and HPV-negative cancer cell line, to induce cell death in cell lines derived from cervical cancer [43].
Figure 2.
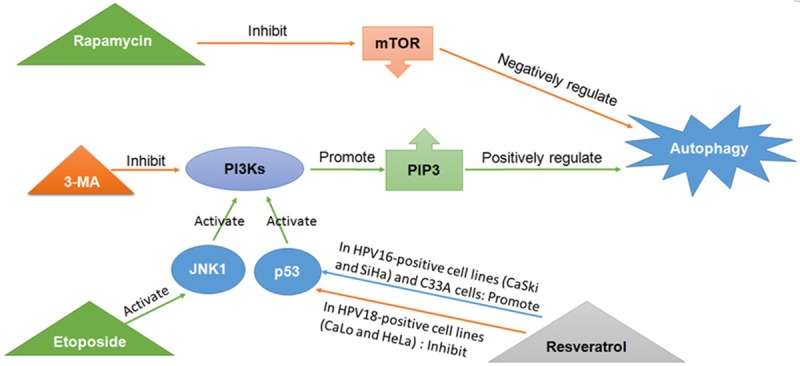
Different medicines act on different points of pathways leading to autophagy. Autophagy can be activated in the absence of starvation by the mTORC1 inhibitor, rapamycin. PI3K3Cs promote the formation of PIP3 from lipids and positively regulate the various stages of autophagosome development. 3-MA is often used to pharmacologically inhibit autophagy in cells for its inhibiting the kinase activity of PI3K3Cs. p53 and JNK1 can also activate the PI3K3Cs. While etoposide could activate JNK1 and induce autophagy [64,65]. After resveratrol’s treatment, the expression of p53 is decreased in HPV18-positive cell lines (CaLo and HeLa) and increased in HPV16-positive cell lines (CaSki and SiHa) and C33A cells. mTORC1: mammalian target of rapamycin complex 1; PI3K3C: class III phosphatidylinositol 3-kinase; PIP3: phosphatidylinositol 3-phosphate; 3-MA: 3-Methyladenine; JNK1: c-jun-N-terminal kinase 1.
In our previous study, the hrHPV infection signal is detected by QD-FISH using biotin-labeled DNA probes, the results showed hrHPV infection is closely correlated with cervical cancer [24,44]. Simultaneously, we also found that the expression levels of Beclin-1 and LC3B are significantly lower in cervical cancer cells, comparing with those of normal cervical squamous epithelial cells and are negatively correlated with hrHPV infection. The above data imply that the hrHPV-host cell interaction may inhibit autophagy, which may assist virus duplication and infection, as well as cervical cancer development [24].
Hanning et al [45] found that cervical neoplastic progression is characterized by dynamic changes in HPV16 transcript levels, in the same time, viral early gene expression is required for cell survival at all stages of carcinogenesis, regardless of viral physical state, levels of early gene expression or histology in organotypic tissue culture. In cervical carcinomas, hrHPV may be integrated into host chromosomes or remain extra-chromosomal (episomal), while HPV16 early gene depletion induces autophagy changes in both episome-containing and integrant-containing cervical cells. In conclusion, depleting HPV16 early genes could offer potential therapeutic benefits in all cervical carcinogenesis pathways, regardless of viral physical state.
A battle between HPV and autophagy, ending with HPV’s victory
Autophagy and/or autophagy genes act as both anti- and pro-viral roles in the life cycles of numerous virus families [46]. But in cervical cancer, the relationship between HPV and autophagy seems to be unique and complex (Figure 1).
Autophagy can be activated by HPV
Cell engagement by several viruses has been found to activate autophagy [47]. HPV is one of those viruses whose infection induces an autophagy response in host keratinocytes [48]. An electron microscopic analysis of autophagosomes are elicited by the entry of HPV pseudovirions (PsVs), and HeLa cells shows enhanced infectivity for PsVs of HPV 16 (16PsVs) when treated with an autophagy inhibitor, suggesting the involvement of autophagy in HPV infection [49]. When HeLa cells are inoculated with 16PsVs, transmission electron microscopy shows the presence of cup-shaped, double-membrane vesicles (phagophores) and double-membrane-bound vesicles, which are autophagosomes’ typical structures. The above results prove that autophagy is indeed induced during the HPV16 entry process and autophagy functions as a host cell defense. Moreover, the LC3 puncta are colocalized with the 16PsVs, which infers that the autophagosomes are in close proximity to the 16PsV particles during this process. There are more studies [25,48] supporting the opinion that HPV16 infection induces an autophagy response, including up-regulation of marker proteins for autophagy, in host keratinocytes.
Autophagy can fight with HPV
Recently, Surviladze et al reported that autophagy inhibits the early stage of HPV16 infection [25,48]. Their research shows that 3-MA enhances the early stage of HPV16 infection, while rapamycin has no effect and they also figure it out that PP242 is an mTOR inhibitor and lack of selectivity for mTOR, strongly inhibiting several kinase families. PP242 can decrease 16PsV infectivity [50], which implies that PP242 may inhibit cellular kinases other than mTOR, leading to decreased 16PsV infectivity [49]. Etoposide, a kind of cell cycle arrest reagents, inhibits 16PsV infection more effectively than other cell cycle arrest reagents [51], which is probably caused by induction of autophagy in addition to G2 arrest [49].
Beclin is a novel Bcl-2-interacting cellular protein. With the results that the brains of mice infected with SIN/beclin had fewer Sindbis virus RNA-positive cells, fewer apoptotic cells and lower viral titers than the brains of mice infected with SIN/beclinΔBcl-2BD or SIN/beclin [52] and conclude beclin may play a role in antiviral host defense. In another study, cell autophagy is known to capture and degrade intracellular pathogens, which is an important component of the host response against viral infections [53].
However, the specificity of genetic knockdowns of mTOR, Beclin-1 and Atg7 confirms that HPV-induced restraint of autophagy is important for early infection events [25]. Autophagy is finally inhibited by HPV.
Inhibition of autophagy can enhance infectivity of HPV
What’s more, if we let it alone that autophagy is inhibited by HPV, the situation is likely to be worse. The HPV-host cell interaction stimulates the PI3K/Akt/mTOR pathway and inhibits autophagy in the early stages of virus-host cell interaction, and in combination these events benefit virus infection [25]. More studies are stating that autophagy can inhibit cancer and the inhibition of autophagy would promote the growth of precancerous cells [54,55]. One of the possible mechanisms is inhibition of autophagy would add to oxidative stress, which would increase the accumulation of tumorigenicity mutation [36].
Host autophagy induced by HPV16 virions is found as a host defense mechanism in primary keratinocytes, inhibits HPV16 infection by rapidly degrading incoming virions; but the struggle seems in vain and HPV16 infectivity is dramatically enhanced by knockdown of essential autophagy genes as well as biochemical inhibition of autophagy, for example, an ~40-fold increase of HPV16 infectivity in primary keratinocytes in the presence of 3-MA [48]. Further, autophagy inhibition by 3-MA enhances HPV16 infectivity more significantly in primary keratinocytes with high levels of basal autophagy compared to immortalized keratinocytes with low levels of autophagy, showing an inverse correlation with basal HPV16 infectivity [48]. The absence of autophagy in combination with hrHPV infection may expedite the progress of cervical SCC [24]. In a word, infection levels are particularly enhanced upon inhibiting autophagy on account of 3-MA.
Zurab Surviladze’s study on the relationship among HPV16, autophagy and the phosphatidylinositol 3-Kinase/Akt/mTOR pathway [25] comes to results below: HPV16 rapidly activates mTOR and triggers mTORC1 substrates, and then quickly suppresses autophagy. Inversely, activation of PI3K/Akt/mTOR signaling is critical for HPV16 infection; PI3K and Src activation are required at early steps of HPV infection; so mTOR functions and autophagy are important for HPV infection. Together, rapid activation of PI3K/Akt/mTOR signaling and the impairment of autophagy both benefit HPV16 infection. This signaling cascade is primarily affected by activation via the EGFR, or stimulation of the MAPK (ERK1/2) pathway [56].
Therapeutic implications
In the latest study, autophagy can fight with the HPV to block the development of tumors, which may be a promising therapeutic target for cervical cancer. Some medicines which induce autophagy or prevent HPV from inhibiting autophagy. For example, there are several kinds of cancer chemotherapeutic agents acting on different stages of autophagy (Figure 2): The mTORC1 (mammalian target of rapamycin complex 1) kinase, is a negative regulator of autophagy [57], and autophagy can be activated in the absence of starvation by the mTORC1 inhibitor, rapamycin [58]. PI3K3Cs promote the formation of phosphatidylinositol 3-phosphate (PIP3) from lipids and positively regulate the various stages of autophagosome development [59,60]. The drug 3-MA is often used to pharmacologically inhibit autophagy in cells for its inhibiting the kinase activity of PI3K3Cs [61]. p53 and c-jun-N-terminal kinase 1 (JNK1) can also activate the PI3K3Cs [62,63], while etoposide, a DNA-damaging agent used in anti-cancer chemotherapy, could activate JNK1 and induce autophagy [64,65]. It’s interesting that the expression of p53 is decreased in HPV18-positive cell lines and increased in HPV16-positive cell lines and C33A cells.
And if we can find or synthesize medicines involving the two aspects of effects, it would be more perfect. Moreover, it has been pointed out that if we do not inhibit autophagy, the infectivity of HPV will be enhanced, which later leads accelerates cervical cancer development, indicating that it’s vital to support autophagy.
Conclusion
The complex correlation among autophagy, HPV and UCC are explained in this way: the human body is a country, HPV is the enemy violating the country and starting a war with the country and the autophagy is one of the heroes who are born out to fight with the enemy, HPV. From subsistent studies, we can learn that the hero is born from the war created by the enemy, which could explain why overexpression of autophagy is found in a variety of human cancers. In this aspect, autophagy may be an “Ominous Signs” for tumor’s occurrence. But overexpression of autophagy may be just transient, for it will be inhibited by HPV. With the inhibition of autophagy, the infection of HPV will be enhanced obviously.
But we must notice its positive role in this war: apparently, autophagy fights with HPV to preventing the human body from the cervical cancer. As to the goal to knock down HPV and suppress the development of cervical cancer, we may achieve it by inducing autophagy. This is a fundamental thing we need to clear and bear in mind.
Acknowledgements
This research was supported by a grant from the Natural Science Foundation of Hubei Province (No: 2015CFB447).
Disclosure of conflict of interest
None.
References
- 1.De Duve C. The lysosome. Sci Am. 1963;208:64–72. doi: 10.1038/scientificamerican0563-64. [DOI] [PubMed] [Google Scholar]
- 2.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005;12(Suppl 2):1535–1541. doi: 10.1038/sj.cdd.4401728. [DOI] [PubMed] [Google Scholar]
- 5.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Wang F, Han L, Wu Y, Li S, Yang X, Wang Y, Ren F, Zhai Y, Wang D, Jia B, Xia Y, Chang Z. GABARAPL1 negatively regulates Wnt/beta-catenin signaling by mediating Dvl2 degradation through the autophagy pathway. Cell Physiol Biochem. 2011;27:503–512. doi: 10.1159/000329952. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, He Y, Chen H. Autophagic tumor stroma: mechanisms and roles in tumor growth and progression. Int J Cancer. 2013;132:1–8. doi: 10.1002/ijc.27664. [DOI] [PubMed] [Google Scholar]
- 8.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Klionsky DJ. The regulation of autophagy-unanswered questions. J Cell Sci. 2011;124:161–170. doi: 10.1242/jcs.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh CM, Jung KW, Won YJ, Shin A, Kong HJ, Jun JK, Park SY. Trends in the incidence of in situ and invasive cervical cancer by age group and histological type in Korea from 1993 to 2009. PLoS One. 2013;8:e72012. doi: 10.1371/journal.pone.0072012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 14.Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, Shah KV, Meijer CJ. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 15.Pillai MR, Hariharan R, Babu JM, Lakshmi S, Chiplunkar SV, Patkar M, Tongaonkar H, Dinshaw K, Jayshree RS, Reddy BK, Siddiqui M, Roychoudury S, Saha B, Abraham P, Gnanamony M, Peedicayil A, Subhashini J, Ram TS, Dey B, Sharma C, Jain SK, Singh N. Molecular variants of HPV-16 associated with cervical cancer in Indian population. Int J Cancer. 2009;125:91–103. doi: 10.1002/ijc.24322. [DOI] [PubMed] [Google Scholar]
- 16.Giroglou T, Florin L, Schafer F, Streeck RE, Sapp M. Human papillomavirus infection requires cell surface heparan sulfate. J Virol. 2001;75:1565–1570. doi: 10.1128/JVI.75.3.1565-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joyce JG, Tung JS, Przysiecki CT, Cook JC, Lehman ED, Sands JA, Jansen KU, Keller PM. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J Biol Chem. 1999;274:5810–5822. doi: 10.1074/jbc.274.9.5810. [DOI] [PubMed] [Google Scholar]
- 18.Ishii Y, Kondo K, Matsumoto T, Tanaka K, Shinkai-Ouchi F, Hagiwara K, Kanda T. Thiol-reactive reagents inhibits intracellular trafficking of human papillomavirus type 16 pseudovirions by binding to cysteine residues of major capsid protein L1. Virol J. 2007;4:110. doi: 10.1186/1743-422X-4-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii Y, Tanaka K, Kondo K, Takeuchi T, Mori S, Kanda T. Inhibition of nuclear entry of HPV16 pseudovirus-packaged DNA by an anti-HPV16 L2 neutralizing antibody. Virology. 2010;406:181–188. doi: 10.1016/j.virol.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Al-Daraji WI, Smith JH. Infection and cervical neoplasia: facts and fiction. Int J Clin Exp Pathol. 2009;2:48–64. [PMC free article] [PubMed] [Google Scholar]
- 21.Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24(Suppl 3):S3/26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 23.Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 2007;98:1505–1511. doi: 10.1111/j.1349-7006.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HY, Yang GF, Huang YH, Huang QW, Gao J, Zhao XD, Huang LM, Chen HL. Reduced expression of autophagy markers correlates with high-risk human papillomavirus infection in human cervical squamous cell carcinoma. Oncol Lett. 2014;8:1492–1498. doi: 10.3892/ol.2014.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surviladze Z, Sterk RT, DeHaro SA, Ozbun MA. Cellular entry of human papillomavirus type 16 involves activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway and inhibition of autophagy. J Virol. 2013;87:2508–2517. doi: 10.1128/JVI.02319-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T, Takabatake Y, Takahashi A, Isaka Y. Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Res. 2013;73:3–7. doi: 10.1158/0008-5472.CAN-12-2464. [DOI] [PubMed] [Google Scholar]
- 27.Bensaad K, Cheung EC, Vousden KH. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 2009;28:3015–3026. doi: 10.1038/emboj.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dengjel J, Kristensen AR, Andersen JS. Ordered bulk degradation via autophagy. Autophagy. 2008;4:1057–1059. doi: 10.4161/auto.6824. [DOI] [PubMed] [Google Scholar]
- 29.Geng QR, Xu DZ, He LJ, Lu JB, Zhou ZW, Zhan YQ, Lu Y. Beclin-1 expression is a significant predictor of survival in patients with lymph node-positive gastric cancer. PLoS One. 2012;7:e45968. doi: 10.1371/journal.pone.0045968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edinger AL, Thompson CB. Defective autophagy leads to cancer. Cancer Cell. 2003;4:422–424. doi: 10.1016/s1535-6108(03)00306-4. [DOI] [PubMed] [Google Scholar]
- 32.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chau YP, Lin SY, Chen JH, Tai MH. Endostatin induces autophagic cell death in EAhy926 human endothelial cells. Histol Histopathol. 2003;18:715–726. doi: 10.14670/HH-18.715. [DOI] [PubMed] [Google Scholar]
- 34.Yanagisawa H, Miyashita T, Nakano Y, Yamamoto D. HSpin1, a transmembrane protein interacting with Bcl-2/Bcl-xL, induces a caspase-independent autophagic cell death. Cell Death Differ. 2003;10:798–807. doi: 10.1038/sj.cdd.4401246. [DOI] [PubMed] [Google Scholar]
- 35.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 36.Holm E, Hildebrandt W, Kinscherf R, Droge W. Low postabsorptive net protein degradation in male cancer patients: lack of sensitivity to regulatory amino acids? Oncol Rep. 2007;17:695–700. [PubMed] [Google Scholar]
- 37.Sun Y, Liu JH, Pan L, Jin L, Yang Y, Sui YX, Shi H. Modulatory effects of Beclin 1 on expression of angiopoietin and Tie-2 receptor in human cervical cancer cells. Asian Pac J Cancer Prev. 2011;12:2985–2990. [PubMed] [Google Scholar]
- 38.Zhu W, Pan X, Li F, Zhang Y, Lu X. Expression of Beclin 1 and LC3 in FIGO stage I-II cervical squamous cell carcinoma and relationship to survival. Tumour Biol. 2012;33:1653–1659. doi: 10.1007/s13277-012-0421-4. [DOI] [PubMed] [Google Scholar]
- 39.Pandey S, Chandravati Autophagy in cervical cancer: an emerging therapeutic target. Asian Pac J Cancer Prev. 2012;13:4867–4871. doi: 10.7314/apjcp.2012.13.10.4867. [DOI] [PubMed] [Google Scholar]
- 40.Mello PD, Filippi-Chiela EC, Nascimento J, Beckenkamp A, Santana DB, Kipper F, Casali EA, Nejar Bruno A, Paccez JD, Zerbini LF, Wink MR, Lenz G, Buffon A. Adenosine Uptake is the Major Effector of Extracellular ATP Toxicity in Human Cervical Cancer Cells. Mol Biol Cell. 2014;25:2905–18. doi: 10.1091/mbc.E14-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldsmith J, Levine B, Debnath J. Autophagy and cancer metabolism. Methods Enzymol. 2014;542:25–57. doi: 10.1016/B978-0-12-416618-9.00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen TC, Hung YC, Lin TY, Chang HW, Chiang IP, Chen YY, Chow KC. Human papillomavirus infection and expression of ATPase family AAA domain containing 3A, a novel anti-autophagy factor, in uterine cervical cancer. Int J Mol Med. 2011;28:689–696. doi: 10.3892/ijmm.2011.743. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Zepeda SP, Garcia-Villa E, Diaz-Chavez J, Hernandez-Pando R, Gariglio P. Resveratrol induces cell death in cervical cancer cells through apoptosis and autophagy. Eur J Cancer Prev. 2013;22:577–584. doi: 10.1097/CEJ.0b013e328360345f. [DOI] [PubMed] [Google Scholar]
- 44.Sun J, Gao J, Hu JB, Fan LF, Zhu XB, Subahan R, Chen HL. Expression of Cav-1 in tumour cells, rather than in stromal tissue, may promote cervical squamous cell carcinoma proliferation, and correlates with high-risk HPV infection. Oncol Rep. 2012;27:1733–1740. doi: 10.3892/or.2012.1703. [DOI] [PubMed] [Google Scholar]
- 45.Hanning JE, Saini HK, Murray MJ, Caffarel MM, van Dongen S, Ward D, Barker EM, Scarpini CG, Groves IJ, Stanley MA, Enright AJ, Pett MR, Coleman N. Depletion of HPV16 early genes induces autophagy and senescence in a cervical carcinogenesis model, regardless of viral physical state. J Pathol. 2013;231:354–366. doi: 10.1002/path.4244. [DOI] [PubMed] [Google Scholar]
- 46.Kudchodkar SB, Levine B. Viruses and autophagy. Rev Med Virol. 2009;19:359–378. doi: 10.1002/rmv.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jordan TX, Randall G. Manipulation or capitulation: virus interactions with autophagy. Microbes Infect. 2012;14:126–139. doi: 10.1016/j.micinf.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffin LM, Cicchini L, Pyeon D. Human papillomavirus infection is inhibited by host autophagy in primary human keratinocytes. Virology. 2013;437:12–19. doi: 10.1016/j.virol.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishii Y. Electron microscopic visualization of autophagosomes induced by infection of human papillomavirus pseudovirions. Biochem Biophys Res Commun. 2013;433:385–389. doi: 10.1016/j.bbrc.2013.02.130. [DOI] [PubMed] [Google Scholar]
- 50.Liu Q, Kirubakaran S, Hur W, Niepel M, Westover K, Thoreen CC, Wang J, Ni J, Patricelli MP, Vogel K, Riddle S, Waller DL, Traynor R, Sanda T, Zhao Z, Kang SA, Zhao J, Look AT, Sorger PK, Sabatini DM, Gray NS. Kinome-wide selectivity profiling of ATP-competitive mammalian target of rapamycin (mTOR) inhibitors and characterization of their binding kinetics. J Biol Chem. 2012;287:9742–9752. doi: 10.1074/jbc.M111.304485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pyeon D, Pearce SM, Lank SM, Ahlquist P, Lambert PF. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009;5:e1000318. doi: 10.1371/journal.ppat.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 55.Ogier-Denis E, Codogno P. Autophagy: a barrier or an adaptive response to cancer. Biochim Biophys Acta. 2003;1603:113–128. doi: 10.1016/s0304-419x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 56.Surviladze Z, Dziduszko A, Ozbun MA. Essential roles for soluble virion-associated heparan sulfonated proteoglycans and growth factors in human papillomavirus infections. PLoS Pathog. 2012;8:e1002519. doi: 10.1371/journal.ppat.1002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 59.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3’-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275:992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- 61.Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D’Amelio M, Criollo A, Morselli E, Zhu C, Harper F, Nannmark U, Samara C, Pinton P, Vicencio JM, Carnuccio R, Moll UM, Madeo F, Paterlini-Brechot P, Rizzuto R, Szabadkai G, Pierron G, Blomgren K, Tavernarakis N, Codogno P, Cecconi F, Kroemer G. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10:676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimizu S, Konishi A, Nishida Y, Mizuta T, Nishina H, Yamamoto A, Tsujimoto Y. Involvement of JNK in the regulation of autophagic cell death. Oncogene. 2010;29:2070–2082. doi: 10.1038/onc.2009.487. [DOI] [PubMed] [Google Scholar]
- 65.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]


